Introduction
Metastasis occurs when tumor cells disseminate from
primary tumor and invade distal organs by the circulation of
ascities in ovarian cancer (1).
The capacity to detach from adjacent cells in a primary location,
degrade extracellular matrix (ECM) and invade omentum and
peritoneum are necessary for cancer cells to be capable of
metastasizing (2). Migration and
invasion are two of the most important processes in the metastasis
of cancer cells and the full understanding of these processes are
essential to the prevention and eradication the metastasis of
cancer.
E-cadherin and N-cadherin are two important adhesion
molecules which are involved in epithelial-mesenchymal transition
(EMT), playing a pivotal role in the pathogenesis, development and
metastasis of cancer. E-cadherin is regarded as a tumor suppressor
factor whereas N-cadherin is related to a poor prognosis in cancer
patients (3). The switching
between E-cadherin and N-cadherin determines the behavior and
characteristics of tumor cells and impacts the progression of
cancer and reversing the expression of E-cadherin and N-cadherin is
a potential treatment approach. Matrix metal-loproteinases (MMPs)
are a family of zinc-dependent proteins which have the capacity to
degrade ECM and whose function can be inhibited by the tissue
inhibitor of metalloproteinases (TIMPs), the role of MMPs and TIMPs
in tumor growth, invasion, metastasis and angiogenesis have been
investigated in different cancer cells (4). MMP-2 and MMP-9 are enzymes degrade
type IV collagen which are strongly expressed in ovarian cancer and
are prognostic markers of survival for ovarian cancer patients
(5–7).
Tumor spheroids are found in ascites of ovarian
cancer patients and exhibit aggressive features which promote
adhesion and invasion of cancer cells into peritoneum and omentum,
resistant to chemotherapy and radiation and are thought to be key
contributors to progression of ovarian cancer (8). Ovarian cancer spheroids degrade ECM
and facilitate invasion mainly mediated by MMPs, β-integrin and
serine protease (9). A recent
study found that ovarian cancer spheroids use α5β1 integrin to bind
to the fibronectin presented by mesothelial cells and mediate
displacement of mesothelium allowing attachment and invasion
(10). Due to the important role
of spheroids in ovarian cancer metastasis, prevention of spheroid
formation and growth is an attractive treatment strategy for
ovarian cancer.
TSA is a potent histone deacetylase inhibitor with
antitumor effect in a mouse model of breast cancer through
promotion of histone acetylation which is the basis for
anti-neoplastic activity (11).
Decitabine, a cytosine analog is used in combination or
sequentially with conventional chemotherapeutic agents is able to
resensitize chemoresistant ovarian cancer cell lines to platinum or
taxane (12). We focused on
markers of apoptosis and cell cycle because a recent study reported
that decitabine in combination with HDAC inhibitor SAHA
(suberoylanilide hydroxamic acid, Vorinostat) suppressed the
tumorigenicity of the ovarian cancer cell line SKOV3 and HEY
primarily mediated through an increase in apoptosis, additional
effects included decreased cellular proliferation, altered cell
cycle, increased autophagy, stimulated re-expression of inhibitory
imprinted tumor suppressor gene ARHI and PEG3 (13). Demethylating agents and histone
deacetylase inhibitors also inhibited proliferation of cells from
primary ovarian cancer patient ascites and enhanced cytotoxicity of
carboplatin and paclitaxel (14–16).
We hypothesized that the combination of TSA and
decitabine has increased anticancer activity on human ovarian
ascites cells (SKOV3 cell line) compared to either drug alone. We
measured the effect of these drugs on mouse model in vivo,
migration and invasion capacity of ovarian cancer cells by
transwell and matrigel assays, on spheroid formation, on epigenetic
regulation, including the expression of epigenetic associated
enzymes DNMTs, HDACs, LSD1 and on the acetylation and methylation
of histones.
Materials and methods
Cell lines and agents
SKOV3 cell line was purchased from American Type
Culture Collection (ATCC). Cells were cultured in DMEM/F-12 (1:1)
(Gibco) with 10% FBS (Cellgro) and 1% penicillin/streptomycin
(Gibco). Decitabine (5-aza-2′-deoxycytidine, DAC, 10 mg) and TSA
(trichostatin A 5 mM/200 μl) were purchased from
Sigma-Aldrich. Decitabine was dissolved in DMSO at a concentration
of 1 μM/l. Spheroid culture medium MammoCult™ Basic Medium
(450 ml) was used for spheroid culture along with MammoCult™
proliferation supplements (50 ml), hydrocortisone (1 μM/l,
500 μl), Antibiotic-antimycotic (5 ml) and heparin solution
(0.2%, 0.5 ml) (StemCell Technology). Transwell®
Permeable Supports system (Corning), BD BioCoat™ BD Matrigel™
invasion chamber (BD Bioscience), 0.25% trypsin-EDTA (Gibco),
CellTiter® 96 Aqueous Non-Radioactive Cell Proliferation
assay (Promega) were employed.
Cell viability measurement
Promega Cell Proliferation assay was used for
detection of cell viability. The Chou-Talalay median effect and
combination index (CI) model was used to determine the effect of
combination drug treatments (17).
Briefly, SKOV3 cells were treated with each drug individually at
multiples (1.0, 2.0 and 3.0) and fraction (0.5) of the
IC50 concentration in a fixed ratio (1:50). Combination
index was calculated as:
CI=(D)1/(DX)1+(D)2/(DX)2.
(DX)1 and (DX)2 are the concentration of single drugs
required to inhibit x% of cells and (D)1 and
(D)2 are drugs concentration in combination treatments
which also inhibit x% of cells. CI<1, CI=1 and CI>1 indicate
synergism, additive and antagonism effect, respectively.
Tumorigenicity of SKOV3 cells
Four- to five-week C3H.CPrkdc/SCID mice were used in
this study and the protocol was approved by the Institutional
Animal Care and Use Committee of The University of Connecticut
Health Center (IACUC). Briefly, SKOV3 cells were pretreated with
TSA 0.1 μM or the combination of TSA 0.1 μM and
decitabine 5 μM for 48 h in vitro and then treated
cells were incubated in drug-free medium for 4 h for recovery.
Cells were trypsinized and suspended in fresh medium, SKOV3
(1×107) cells were injected into the flank of mice
subcutaneously and the mice were carefully observed. Tumor size was
measured by caliper every 5 days and mice were sacrificed at day
30. Tumor volumes were calculated according to the formula
described previously (18).
Cell migration and invasion assay
Transwells were used to measure the migration
capacity of cancer cells. In cell migration assay, SKOV3 cells were
treated with 0.1 μM TSA, 5 μM decitabine and TSA plus
decitabine for 48 h, then replaced with fresh medium and incubated
4 h for recovery, then digested with trypsin-EDTA and cell numbers
were counted by hemocytometer. SKOV3 cell suspension (0.5 ml)
(5×104) were seeded into the inserts of transwells and
incubated in 37°C incubator for 48 h. All the transwell inserts
were then washed with fresh 1X PBS, then upper surface of the
transwell insert were scraped by cell scraper and washed with fresh
1X PBS, stained with hematoxylin and eosin and migrated cells in
the lower side of the membranes were counted under Olympus IX71
microscope and three fields of triplicate membranes of each group
were selected. In cell invasion assay, the protocol of drug
treatment was the same as the migration assay, when the cells were
harvested and then the cells were seeded into BD Matrigel invasion
chamber. After incubation, the non-invading cells were moved from
the upper surface of the membrane by cell scraper. The cells on the
lower surface of the membrane were stained with hematoxylin and
eosin and the number of invaded cells was counted by Olympus IX71
microscope. Three fields of triplicate membranes of each group were
selected.
Spheroid formation
Suspended single cells (500 cells/ml) were mixed
with combined drugs and seeded in 24-well ultra-low attachment
plates (Corning) in spheroid culture medium and incubated at 37°C
for 15 days and images were recorded every 5 days. To further
investigate the spheroid formation ability (spheroid number),
1×103 suspended cells were seeded simultaneously with
drugs in 96-well ultra-low attachment plates (Corning) and
incubated at 37°C for 15 days. The number of spheroids were counted
and the difference between untreated and combined treated group was
compared using Student’s t-test.
In vivo implantation assay
SKOV3 (1×107) cells were mixed with TSA
(0.1 mg/kg) and decitabine (5 mg/kg) and then injected into
4–5-week C3H.C-Prkdc/SCID mice intraperitoneally for total
treatment of 5 days. Mice were sacrificed on day 30 from the first
day of inoculation. The number and volume of implanted xenograft
nodules were assessed and expression of apoptosis related proteins
Bcl-2, Bcl-xL, p21, p27 of tumors were detected by western
blotting.
Western blotting
Pretreated SKOV3 cells or cancer cells from
implanted xenograft nodules were digested and total protein was
quantified by Bradford Protein assay (Bio-Rad), protein was
denatured in 2X laminin sample buffer (Sigma-Aldrich), 10–30
μg/lane samples were loaded and separated on 7–10% SDS-PAGE
gel, the gels were transferred to nitrocellulose membrane
(Whatman), membranes were blocked in 5% non-fat milk (Lab
Scientific) for 1 h and then primary antibodies were added and
incubated at 4°C overnight. The antibodies and dilution were:
E-cadherin, N-cadherin, Twist, DNMT3A, DNMT3B, HDAC1, HDAC2, LSD1
(1:1000, Cell Signaling Technology), Bcl-2, Bcl-xL, p21, p27,
MMP-2, MMP-9 (1:500 Santa Cruz Biotechnology) and β-actin (1:10,000
Sigma-Aldrich). Then, the membranes were washed with fresh 1X TBST
solution three times for 10 min and incubated with secondary
antibodies (Santa Cruz Biotechnology) for 1 h at room temperature,
after being washed with fresh 1X TBST solution three times for 10
min. SuperSignal® West Femto Maximum chemiluminescent
substrate (Thermo Scientific) was added to membranes and detected
by luminescent detection system (Syngene system) and densitometric
values of western blotting were assessed by ImageJ software
(NIH.gov).
Histone immunoblots
Histone protein of treated SKOV3 cells was extracted
and purified by the Epiquick total histone extraction kit
(Epigenetek), the extraction was quantified and then diluted in 1X
NuPAGE/LDS loading buffer (Invitrogen), heated at 95°C in a dry
heater for 5 min, 10–30 μg/lane samples were loaded in 10%
NuPAGE/Bus/Tris gel (Invitrogen), run in NuPAGE/MES/SDS buffer
(Invitrogen), transferred into nitrocellulose membranes, blocked in
5% BSA/TBST solution for 1 h and incubated with primary antibodies
at 4°C overnight. Membranes were washed by 1X TBST solution and
incubated with secondary antibodies for 1 h at room temperature.
The subsequent protocol was the same as described in the western
blotting. The antibodies and dilution were: H3K4me2, H3K9me2 and
histone H3 (1:1,000, Cell Signaling Technology).
Statistical analysis
SPSS 16.0 (IMB, Armonk, NY) was used to analysis the
data, GraphPad Prism 5 (GraphPad Software, San Diego, CA) was used
for making graphs and ImageJ software (NIH.gov) was used to assess
the densitometric readings of western blotting, the comparison of
more than two groups was evaluated by one-way ANOVA and the
difference between two groups was evaluated by independent
Student’s t-test, P<0.05 was considered statistically
significant.
Results
Effect of TSA and decitabine on
tumorigenicity of SKOV3 cells in a mouse xenograft model
DNA demethylation agents and histone deacetylase
inhibitor reported to suppress tumor formation when used alone or
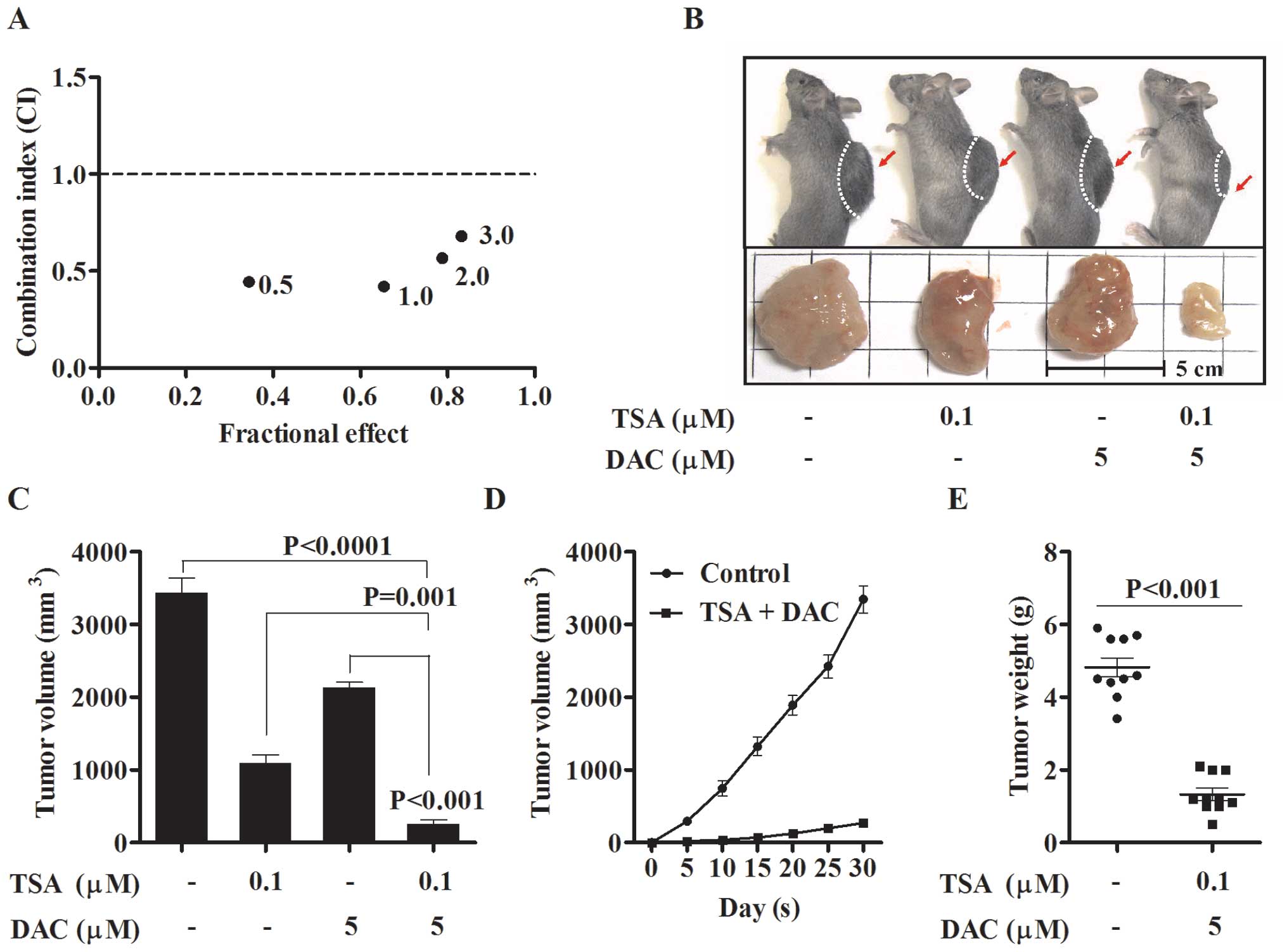
combined with conventional cytotoxic drugs in cancers (12). We first assessed the effect of
fixed ratio combination of TSA and decitabine on the cell viability
and the results indicated that the combination of TSA 0.1 μM
and decitabine 5 μM achieved the ultimate synergistic effect
(CI=0.421) and this combination was selected for subsequent
experiments (Fig. 1A). To
determine if the combination of TSA and decitabine was better than
either drug alone in suppressing xenograft tumor formation with
SKOV3 cells, we evaluated the tumorigenicity of pretreated SKOV3
cells in mouse xenograft models. We found that the combination
suppressed tumor formation greater than either agent used alone
(Fig. 1B). There was a
statistically significant difference between tumor volume and
weight from the cells pretreated with single drugs compared to the
combination (Fig. 1C–E),
suggesting a profound effect on tumorigenesis only by pretreating
cells with the combination of agents.
Effect of TSA and decitabine on the
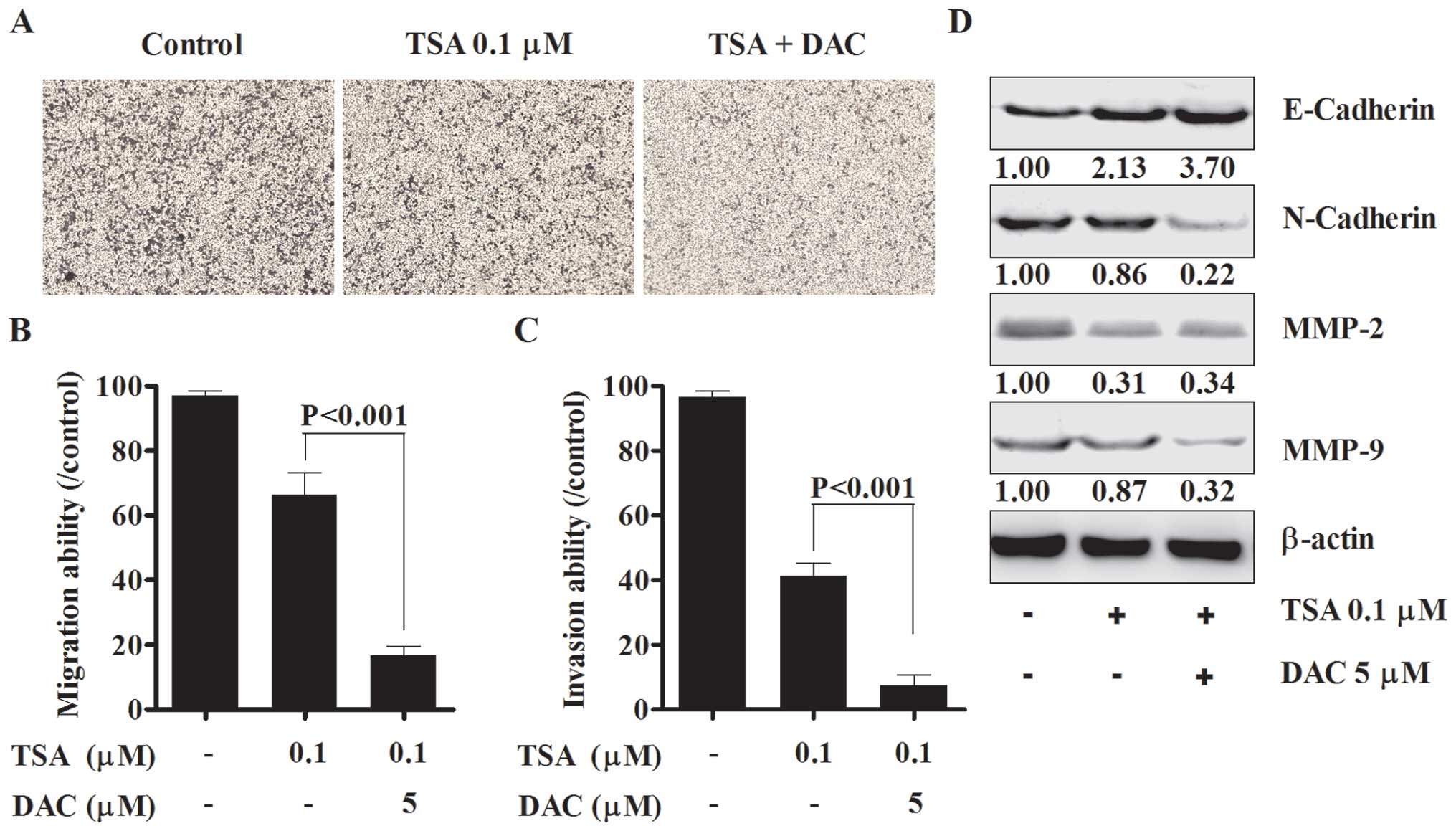
migration and invasion of SKOV3 cells in vitro
Migration and invasion play an important role in the
pathogenesis, development and metastasis of ovarian cancer
(2). Migration capacity was
measured in SKOV3 cells which were treated with TSA alone or the
combination of TSA and decitabine. TSA/decitabine suppressed
migration more than either drug alone (Fig. 2A and B) and induced reversal of
epithelial to mesenchymal transition (EMT) promoting a switch from
N-cadherin to E-cadherin (Fig.
2D). We also found that invasion capacity of SKOV3 cells was
almost totally suppressed by the combination of TSA and decitabine
(Fig. 2C), the expression levels
of MMP-2 and MMP-9 were significantly suppressed by the combined
treatment (Fig. 2D). The
upregulation of E-cadherin, downregulation of N-cadherin and
suppression of MMP-2 and MMP-9 may suppress metastasis by
inhibiting migration and ECM degradation.
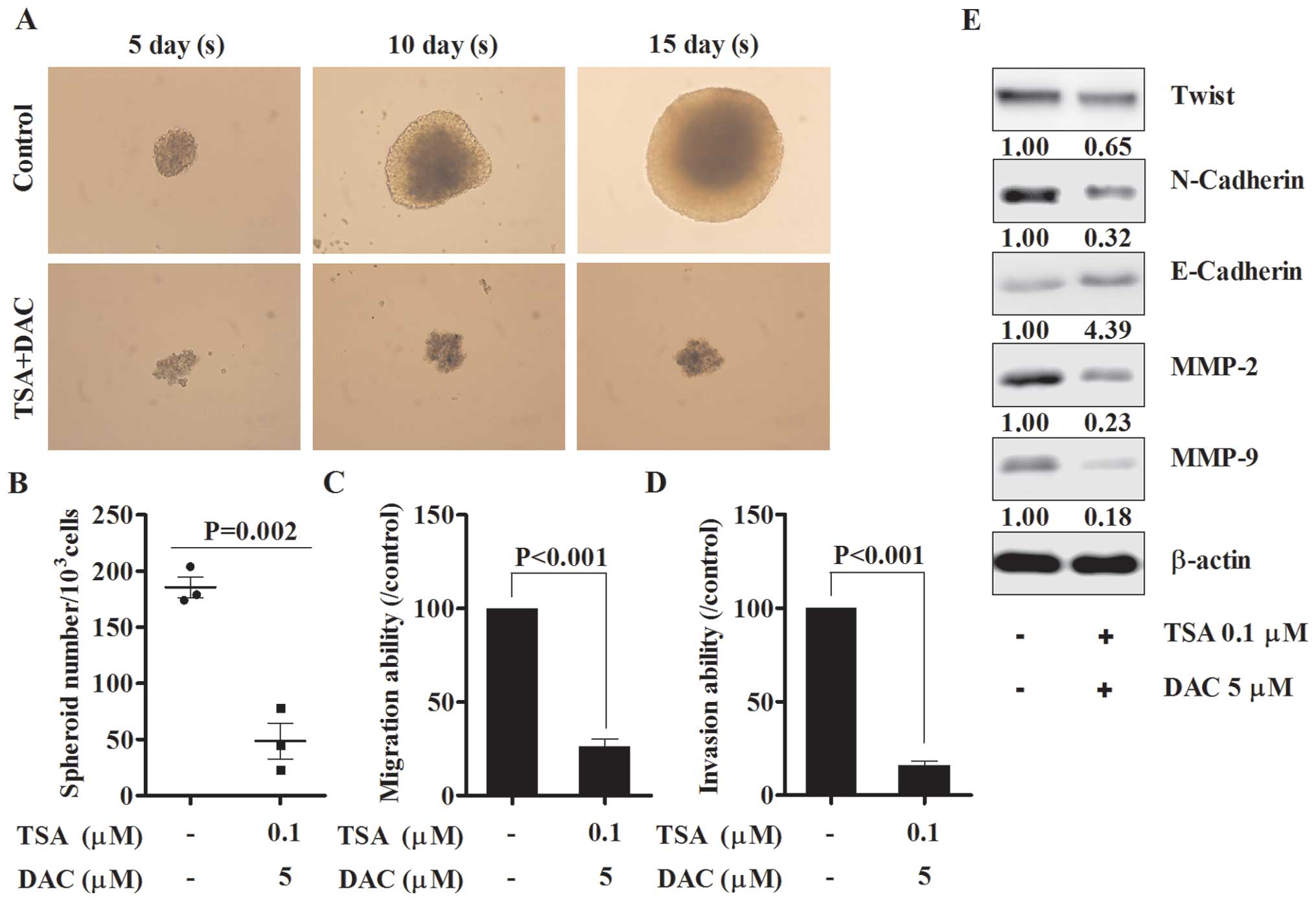
The combination of TSA and decitabine
suppresses spheroid formation via regulation of EMT in vitro
Spheroids, normally occurring in ovarian cancer
patients, show enhanced migration, invasion and motility and are
thought to play an important role in the metastasis of ovarian
cancer (19). When grown in
serum-free medium, SKOV3 cells formed round, tightly adhered
spheroids which were inhibited with the combination of drugs
(Fig. 3A and B). Migration and
invasion ability of spheroid derived cells were then assessed and
both of these functions were impaired when compared with untreated
group (Fig. 3C and D). The
expression of several EMT markers was detected and the results
indicated that Twist and N-cadherin were significantly suppressed,
E-cadherin was markedly induced and both MMP-2 and MMP-9 were
markedly suppressed by the combined drugs (Fig. 3E).
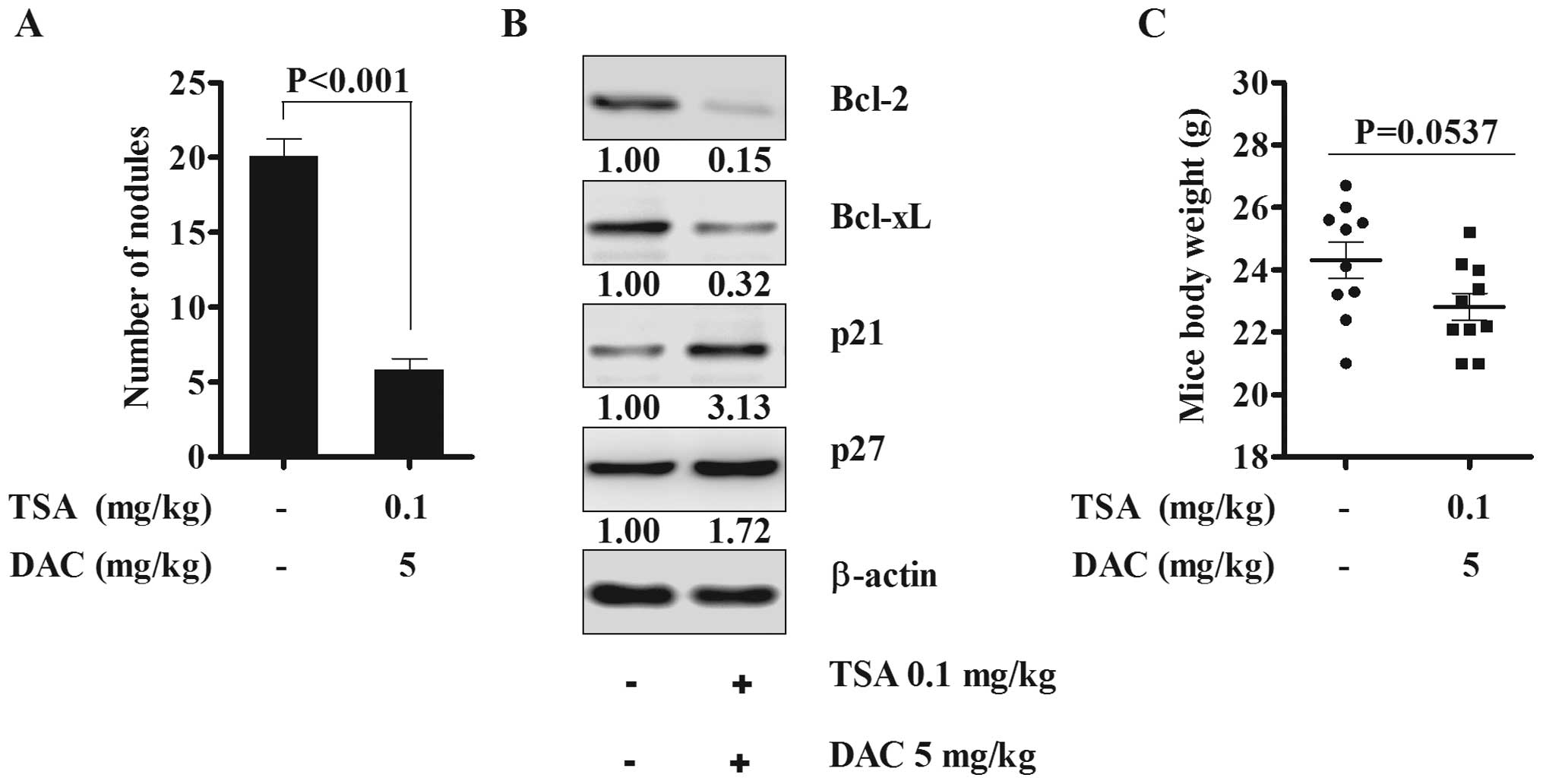
The combination of TSA and decitabine
suppresses tumor metastases and implantation through apoptosis
pathway in vivo
Based on the spheroid assay results, in order to
further assess if the combination of TSA and decitabine could have
anticancer effect in abdominal cavity in mouse models, we performed
in vivo treatment assay with the combined drugs. There was a
significant reduction in peritoneal cavity cancer cell implants
when TSA and decitabine were administered intraperitoneally for 5
days following IP injection of SKOV3 cells (Fig. 4A). Bcl-2 and Bcl-xL were
significantly suppressed and p21 and p27 were slightly induced in
implanted tumor nodules in animals treated with the combination
drugs (Fig. 4B). Body weight of
untreated and treated mice were measured and there was no
significant weight loss or adverse side effects although body
weight difference was P=0.0537 (Fig.
4C). The animal results suggested that continuous combined
treatment suppress tumor implantation through inhibiting spheroid
formation by activation of the apoptosis pathway without any
apparent drug toxicity.
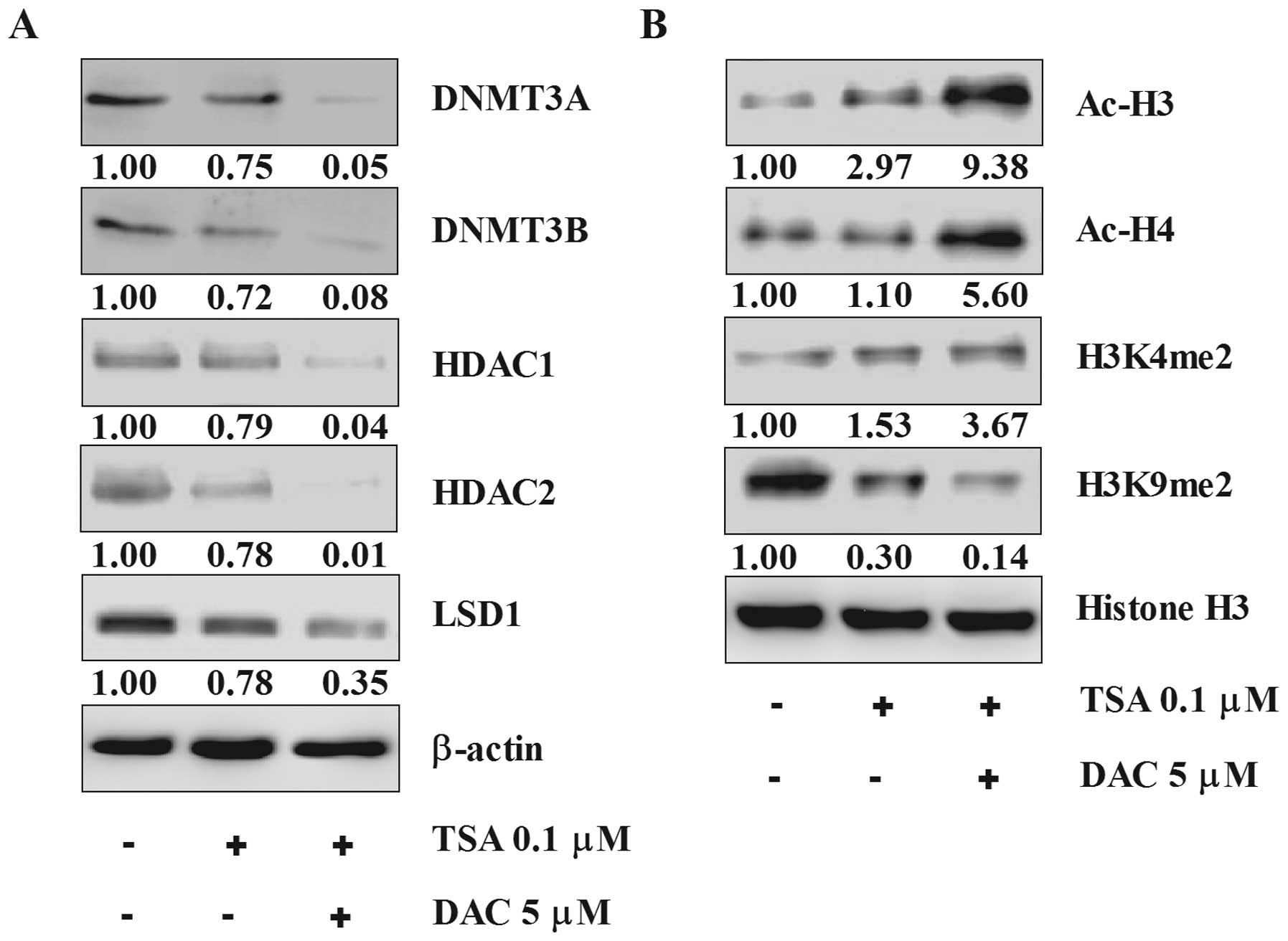
Effect of TSA and decitabine on the
expression of DNMTs/HDACs/LSD1 and the expression of acetylation of
histone H3, -H4 and H3K4me2, H3K9me2
Essential epigenetic associated enzymes DNMT3A/3B,
HDAC1/2 and LSD1 were significantly suppressed by the combination
whereas TSA alone moderately decreased expression (Fig. 5A). Acetylation status of histone H3
and H4, were significantly stimulated and H3K4me2 was significantly
induced but H3K9me2 was suppressed by TSA alone and the
combination, with the combination having more effect than TSA alone
(Fig. 5B). These results suggest
that tumor suppression effect of SKOV3 xenografts by the
combination of TSA and decitabine are at least partially
epigenetically regulated and the combination of multi-target
epigenetic inhibitors achieves a synergistic effect.
Discussion
In order to metastasize, ovarian cancer cells must
have the ability to detach from their primary location, invade and
migrate into ECM, reattach to the omentum and peritoneum, and these
processes are mainly mediated by downregulation of cell-cell
adhesion and upregulation of ECM degradation.
The expression of E-cadherin have been shown to be
regulated at multiple levels, transcriptionally, translationally
and post-translationally. Several important epigenetic associated
regulators have been found to be involved in the regulation of
E-cadherin both at the epigenetic and transcription levels. A
recent study reported that the presence of LDS1 is a prerequisite
for the repression of E-cadherin by Snail through demethylation of
dimethylated H3K4 and suggested that BRAF-HDAC complex could be a
potential target for the prevention of EMT-associated tumor
invasion (20). Snail mediated the
expression of E-cadherin by interacting with HDACs and the addition
of TSA is sufficient to block the repressive effect of Snail on
E-cadherin (21). Twist is a
transcriptional factor, which is able to promote metastasis of
epithelial cancer cells through regulating the process of EMT,
inhibiting the expression of E-cadherin by binding to E-cadherin
promoter and activating expression of the mesenchymal marker
N-cadherin (22,23). E-cadherin is epigenetically
regulated and upregulation of E-cadherin suppressed migration and
invasion. E-cadherin and γ-catenin could be markedly induced by the
simultaneous inhibition of DNMT3A and DNMT3B with the addition of
decitabine in choriocarcinoma cells (24). Our findings are consistent with
these data and suggest that the inhibition of migration and
invasion of ovarian cancer cells with the combination of TSA and
decitabine is mediated through inhibition of HDACs and LSD1, which
in turn, blocks the suppression of E-cadherin and activation of
Twist and N-cadherin.
The role of MMPs is far more than cleavage of
basement membrane to facilitate invasion into the omentum and the
peritoneal cavity in ovarian cancer. Studies have found that MMP-9
triggers angiogenesis and the stromal MMP-9 promotes blood vessel
formation and pericyte recruitment to angiogenesis in neuroblastoma
(25). Recent reports demonstrated
that down-regulation of MMP-2 mRNA or inhibition of the proteolytic
activity of MMP-2 in both human ovarian primary cells and cell
lines reduced the attachment of ovarian cancer cells to the
peritoneum and omentum in vitro and in vivo. The
cleavage of vitronectin and fibronectin, which is highly expressed
in the mesothelial cells of the lining of peritoneal cavity, is
mediated by MMP-2 which is considered to be the iniating event in
ovarian cancer metastasis (6,7).
Inhibiting the secretion and proteolytic activity of MMP-2 would be
vital to the prevention of cancer cell metastasis and colonization
and our data show that the combination of TSA and decitabine
significantly inhibited the expression of MMP-2 and MMP-9 and
inhibited ovarian cancer invasion. If ovarian cancer in women could
be induced to undergo the reverse of EMT with upregulation of
E-cadherin and downregulation of N-cadherin as well as modulation
of the MMPs necessary for invasion, this could be a major
breakthrough to prevent recurrence of this cancer.
Epigenetic alterations, including DNA methylation
and histone modification, contribute to ovarian cancer progression
and drug resistance (26).
Intimate crosstalk exists between histone modification and DNA
methylation. DNA demethylating agents and HDAC inhibitors are two
classes of epigenetic modifiers which have been extensively studied
and show promising anticancer efficacy in chemoresistant ovarian
cancers (27). DNMTs are thought
to be important in the pathogenesis and progression of ovarian
cancers. The expression of DNMTs is suppressed by the histone
deacetylase inhibitor TSA, with the effect of HDAC inhibitor on
DNMTs mainly associated with the spatiotemporal regulation of DNA
methylation and histone modification (28). Our data suggest that the
combination of a DNMT inhibitor and HDAC inhibitor could achieve
significant epigenetic regulation effect via the stimulation of
acetylation of histone H3/4 and suppression of epigenetic related
enzymes, such as HDACs and DNMTs.
Histone methylation is involved in gene
transcription, whether it functions to activate or repress mainly
depends on the number of the methyl groups. Two of the best studied
methylation markers are transcription activation markers H3K4me2
and repression markers H3K9me2 (29). The demethylated-H3K4 could be
demethylated by LSD1 and demethylated-H3K9 can also be demethylated
by LSD1 in presence of the androgen receptor (30). Our study demonstrated the LSD1 was
inhibited by TSA and decitabine and suggested that interactive
regulation exists among DNA methylation, histone deacetylation and
histone methylation. Moreover, our previous data showed that TSA or
decitabine in combination of cisplatin could significantly suppress
the expression of LSD1 and HDAC1/2 in SKOV3 cells (data not
published). We thus hypothesize that combining epigenetic modifiers
potentiate their anticancer effect.
Based on our results, suppression of tumorigenicity
in vivo and the inhibitory effect on migration and invasion
in vitro, we hypothesized that the combination of TSA and
decitabine could also affect the implantation of cancer cells in
vivo. The suppression of spheroid formation and viability could
significantly impact the ability of ovarian cancer to recur with
metastasis and invasion. The major application of this approach may
lie in prolonging remission in women in clinical remission
following chemotherapy or treating recurrent disease.
Acknowledgements
This study was supported by The Carole
and Ray Neag Comprehensive Cancer Center, University of Connecticut
Health Center.
References
|
1.
|
Bast RC Jr, Hennessy B and Mills GB: The
biology of ovarian cancer: new opportunities for translation. Nat
Rev Cancer. 9:415–428. 2009. View
Article : Google Scholar : PubMed/NCBI
|
|
2.
|
Lengyel E: Ovarian cancer development and
metastasis. Am J Pathol. 177:1053–1064. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
3.
|
Ahmed N, Thompson EW and Quinn MA:
Epithelialmesenchymal interconversions in normal ovarian surface
epithelium and ovarian carcinomas: an exception to the norm. J Cell
Physiol. 13:581–588. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
4.
|
Roy R, Yang J and Moses MA: Matrix
metalloproteinases as novel biomarkers and potential therapeutic
targets in human cancer. J Clin Oncol. 27:5287–5297. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
5.
|
Sillanpää S, Anttila M, Voutilainen K,
Ropponen K, Turpeenniemi-Hujanen T, Puistola U, Tammi R, Tammi M,
Sironen R, Saarikoski S and Kosma VM: Prognostic significance of
matrix metalloproteinase-9 (MMP-9) in epithelial ovarian cancer.
Gynecol Oncol. 104:296–303. 2007.PubMed/NCBI
|
|
6.
|
Kenny HA, Kaur S, Coussens LM and Lengyel
E: The initial steps of ovarian cancer cell metastasis are mediated
by MMP-2 cleavage of vitronectin and fibronectin. J Clin Invest.
118:1367–1379. 2008. View
Article : Google Scholar : PubMed/NCBI
|
|
7.
|
Kenny HA and Lengyel E: MMP-2 functions as
an early response protein in ovarian cancer metastasis. Cell Cycle.
8:683–688. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
8.
|
Burleson KM, Casey RC, Skubitz KM,
Pambuccian SE, Oegema TR Jr and Skubitz AP: Ovarian carcinoma
ascites spheroids adhere to extracellular matrix components and
mesothelial cell monolayers. Gynecol Oncol. 93:170–181. 2004.
View Article : Google Scholar
|
|
9.
|
Burleson KM, Hansen LK and Skubitz AP:
Ovarian carcinoma spheroids disaggregate on type I collagen and
invade live human mesothelial cell monolayers. Clin Exp Metastasis.
21:685–697. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
10.
|
Iwanicki MP, Davidowitz RA, Ng MR, Besser
A, Muranen T, Merritt M, Danuser G, Ince TA and Brugge JS: Ovarian
cancer spheroids use myosin-generated force to clear the
mesothelium. Cancer Discov. 1:144–157. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
11.
|
Vigushin DM, Ali S, Pace PE, Mirsaidi N,
Ito K, Adcock I and Coombes RC: Trichostatin A is a histone
deacetylase inhibitor with potent antitumor activity against breast
cancer in vivo. Clin Cancer Res. 7:971–976. 2001.PubMed/NCBI
|
|
12.
|
Kristensen LS, Nielsen HM and Hansen LL:
Epigenetics and cancer treatment. Eur J Pharmacol. 625:131–142.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
13.
|
Chen MY, Liao WS, Lu Z, Bornmann WG,
Hennessey V, Washington MN, Rosner GL, Yu Y, Ahmed AA and Bast RC
Jr: Decitabine and suberoylanilide hydroxamic acid (SAHA) inhibit
growth of ovarian cancer cell lines and xenografts while inducing
expression of imprinted tumor suppressor genes, apoptosis, G2/M
arrest and autophagy. Cancer. 117:4424–4438. 2011. View Article : Google Scholar
|
|
14.
|
Li Y, Hu W, Shen DY, Kavanagh JJ and Fu S:
Azacitidine enhances sensitivity of platinum-resistant ovarian
cancer cells to carboplatin through induction of apoptosis. Am J
Obstet Gynecol. 200:177 e1:–e9. 2009.PubMed/NCBI
|
|
15.
|
Sonnemann J, Gänge J, Pilz S, Stötzer C,
Ohlinger R, Belau A, Lorenz G and Beck JF: Comparative evaluation
of the treatment efficacy of suberoylanilide hydroxamic acid (SAHA)
and paclitaxel in ovarian cancer cell lines and primary ovarian
cancer cells from patients. BMC Cancer. 6:1832006. View Article : Google Scholar : PubMed/NCBI
|
|
16.
|
Dietrich CS III, Greenberg VL, DeSimone
CP, Modesitt SC, van Nagell JR, Craven R and Zimmer SG:
Suberoylanilide hydroxamic acid (SAHA) potentiates paclitaxel
induced apoptosis in ovarian cancer cell lines. Gynecol Oncol.
116:126–130. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
17.
|
Chou TC: Drug combination studies and
their synergy quantification using the Chou-Talalay method. Cancer
Res. 70:440–446. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
18.
|
Baba T, Convery PA, Matsumura N, Whitaker
RS, Kondoh E, Perry T, Huang Z, Bentley RC, Mori S, Fujii S, Marks
JR, Berchuck A and Murphy SK: Epigenetic regulation of CD133 and
tumorigenicity of CD133+ ovarian cancer cells. Oncogene.
28:209–218. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
19.
|
Shield K, Ackland ML, Ahmed N and Rice GE:
Multicellular spheroids in ovarian cancer metastases: biology and
pathology. Gynecol Oncol. 113:143–148. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
20.
|
Lin T, Ponn A, Hu X, Law BK and Lu J:
Requirement of the histone demethylase LSD1 in Snai1-mediated
transcriptional repression during epithelial-mesenchymal
transition. Oncogene. 29:4896–4904. 2010. View Article : Google Scholar
|
|
21.
|
Peinado H, Ballestar E, Esteller M and
Cano A: Snail mediates E-cadherin repression by the recruitment of
the Sin3A/histone deacetylase 1 (HDAC1)/HDAC2 complex. Mol Cell
Biol. 24:306–319. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
22.
|
Yang J, Mani SA, Donaher JL, Ramaswamy S,
Itzykson RA, Come C, Savagner P, Gitelman I, Richardson A and
Weinberg RA: Twist, a master regulator of morphogenesis, plays an
essential role in tumor metastasis. Cell. 117:927–939. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
23.
|
Vesuna F, van Diest P, Chen JH and Raman
V: Twist is a transcriptional repressor of E-cadherin gene
expression in breast cancer. Biochem Biophys Res Commun.
367:235–241. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
24.
|
Rahnama F, Shafiei F, Gluckman PD,
Mitchell MD and Lobie PE: Epigenetic regulation of human
trophoblastic cell migration and invasion. Endocrinology.
147:5275–5283. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
25.
|
Bergers G, Brekken R, McMahon G, Vu TH,
Itoh T, Tamaki K, Tanzawa K, Thorpe P, Itohara S, Werb Z and
Hanahan D: Matrix metalloproteinase-9 triggers the angiogenic
switch during carcinogenesis. Nat Cell Biol. 2:737–744. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
26.
|
Chen H, Hardy TM and Tollefsbol TO:
Epigenomics of ovarian cancer and its chemoprevention. Front Genet.
2:672011. View Article : Google Scholar : PubMed/NCBI
|
|
27.
|
Balch C, Huang TH, Brown R and Nephew KP:
The epigenetics of ovarian cancer drug resistance and
resensitization. Am J Obstet Gynecol. 191:1552–1572. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
28.
|
Balch C, Fang F, Matei DE, Huang TH and
Nephew KP: Minireview: epigenetic changes in ovarian cancer.
Endocrinology. 150:4003–4011. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
29.
|
Yoo CB and Jones PA: Epigenetic therapy of
cancer: past, present and future. Nat Rev Drug Discov. 5:37–50.
2006. View
Article : Google Scholar : PubMed/NCBI
|
|
30.
|
Metzger E, Wissmann M, Yin N, Müller JM,
Schneider R, Peters AH, Günther T, Buettner R and Schüle R: LSD1
demethylates repressive histone marks to promote androgen-receptor
dependent transcription. Nature. 437:436–439. 2005.PubMed/NCBI
|