Introduction
Adenoid cystic carcinoma (ACC) is a generally
slow-growing, but highly malignant salivary gland neoplasm with
remarkable capacity for invasion and metastasis. Patients with ACC
of the salivary glands have a fair 5-year survival rate, but
long-term overall survival (10–20 years) remains poor (1,2)
owing to the development of late recurrence or metastases, even
with low-grade tumors (1,2). Distant metastases (to lung, bone and
soft tissues) develop in 40–60% of patients, despite good local
tumor control. Better control of lung metastases in particular is
expected to improve outcomes in patients with ACC.
Nitric oxide (NO) is a multifunctional gaseous
molecule synthesized from L-arginine by NO synthase (NOS). There
are three isoforms of NOS: neuronal NOS (nNOS), endothelial NOS
(eNOS) and inducible isoform of NOS (iNOS). nNOS and eNOS are
constitutively expressed and are thus referred to as constitutive
NOS. In contrast, iNOS is transcriptionally regulated and induced
by inflammatory cytokines, endotoxins, hypoxia and oxidative stress
(3,4). iNOS produces high, sustained
concentrations of NO, whereas the other two isoforms produce low,
transient concentrations of NO (5). Recent studies have shown positive
correlations between iNOS and poor outcomes in patients with breast
cancer and melanoma (6,7). These observations suggest that NO
generated by iNOS has multiple physiologic and pathologic effects.
Other recent studies report that eNOS can modulate cancer-related
events, such as angiogenesis, apoptosis, cell cycle dynamics, tumor
invasion and metastasis (8). We
previously confirmed the antitumor effects of NOS inhibitor and
iNOS inhibitor against a human KB carcinoma cell line in which
malignancy was increased by gene transfer of COX-2 cDNA (9).
CXCR4 is a receptor for stromal cell-derived
factor-1α (SDF-1α; the so called CXCL12), a chemokine expressed in
several tissues and organs, including skin, lymph nodes, lung,
liver and bone marrow (10–13).
SDF-1α stimulates cell adhesion, migration and activation (13–18).
CXCR4 is expressed in different tumor cell lines (10,19)
and the pulmonary metastatic potential of cells expressing CXCR4
was higher than that of their CXCR4-negative counterparts in a B16
murine melanoma model (20,21).
Furthermore, CXCR4 has been shown to play a key role in metastases
from breast cancer and melanoma (10,22).
We previously suggested that CXCR4 expression is closely related to
metastatic potential in surgical specimens of ACC (23).
We established a new human tumor cell line (ACCI)
derived from ACC of the oral floor. ACCI shows a cribriform pattern
histologically and is serially transplantable into nude mice. This
tumor is associated with spontaneous metastasis to the neck at the
second passage level and the histological features change from ACC
to undifferentiated carcinoma. The metastatic tumor, designated as
ACCIM, shows a high frequency of spontaneous metastasis to the lung
when transplanted subcutaneously in nude mice (24).
As mentioned above, NO is related to apoptosis,
angiogenesis and metastasis and CXCR4 plays a central role in cell
migration in metastases from cancer. The present study evaluated
the effectiveness of a NOS inhibitor and a CXCR4 antagonist, given
as single agents or in combination, in a xenotransplanted mouse
model of ACCIM.
Materials and methods
Animals
Five-week-old female nude mice (BALB/c nu/nu;
Oriental Yeast Ltd., Tokyo, Japan) were used as experimental
animals. Food was supplied ad libitum and the animals were
housed under sterile conditions. All animal experiments were
performed in compliance with the Guidelines for Experimental
Animals of Hyogo College of Medicine.
Agents
NG-nitro-L-arginine-methyl ester
(L-NAME), a NOS inhibitor and
1,1′-{1,4-phenylenebis(methylene)}bis-1,4,8,11-tetraazacyclotetradecane
octahydrochloride (AMD3100), a CXCR4 antagonist, were purchased
from Sigma-Aldrich Co. (St. Louis, MO, USA). Dihydrochloride
(1400W), a selective iNOS inhibitor, was purchased from Cayman
Chemical Co. (Ann Arbor, MI, USA). These agents were dissolved in
Mg2+ and Ca2+-free phosphate-buffered saline
(PBS[−]) before use.
Tumor tissue and heterotransplantation
into nude mice
The original tumor tissue was obtained from the
surgical specimens of a 72-year-old man with ACC of the oral floor.
Histologically, the tumor showed a cribriform pattern. Metastatic
tumor tissue was obtained from a lesion of spontaneous neck
metastasis from the above ACC transplanted into a second passage of
nude mice. The tumor was rinsed 3 times in PBS, cut into ~2×2-mm
pieces and transplanted into the flanks of the mice. At the second
passage level, ~1 year after ACC transplantation, another tumor
mass appeared in the neck, apart from the transplanted site of the
tissue fragment. Although this tumor was an undifferentiated
carcinoma histologically, it was considered a metastatic lesion and
designated as ACCIM. ACCIM produced multiple metastases to lymph
nodes and lungs 5 months after transplantation (24).
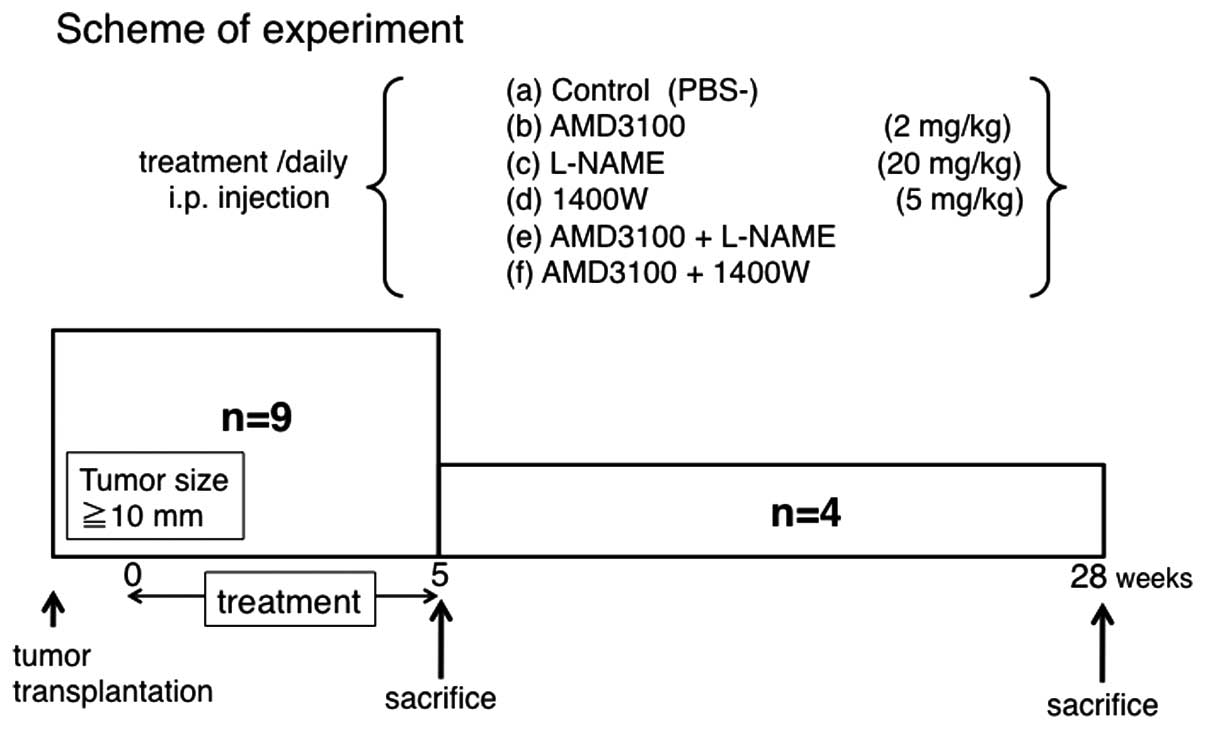
Treatment of agents on the subcutaneously
xenotransplanted tumor of ACCIM in nude mice
ACCIM was cut into ~2×2-mm pieces and implanted
subcutaneously in nude mice. Approximately 19 days after ACCIM
xenotransplantation, when the tumor reached 10 mm in diameter, mice
were subdivided into six groups and were assigned to receive one of
the following treatments by intraperitoneal injection every day for
5 weeks: a) vehicle (PBS[−]), b) AMD3100 (2 mg/kg), c) L-NAME (20
mg/kg), d) 1400W (5 mg/kg), e) both AMD3100 and L-NAME
(AMD3100+L-NAME), or f) both AMD3100 and 1400W (AMD3100+1400W).
Five weeks and 28 weeks after the start of treatment, 5 and 4 mice
from each group were randomly selected and sacrificed, respectively
(Fig. 1). The tumor volume was
calculated by the following formula: volume (mm3) =
a2xb/2, where a is the tumor
width in mm and b is the tumor length in mm (25). Necropsies were performed to
identify macro-metastases to the lung 28 weeks after treatment
began. The primary tumors and lungs were harvested, fixed in 10%
formalin, embedded in paraffin, cut into 4-μm-thick sections and
stained with hematoxylin and eosin (H&E) according to
conventional procedures.
Immunohistochemical study of
xenotransplanted tumors
Immunohistochemical examination was performed with
the use of the avidin-biotin-peroxidase complex (ABC) staining
method (26). Briefly, endogenous
peroxidase activity was blocked in tissue specimens by treatment
with 0.3% H2O2 in methanol for 5 min. The
specimens were washed and treated with 1% normal horse serum in PBS
for 15 min. After washing with PBS, rabbit polyclonal antibody for
human CXCR4 (Abcam, Cambridge, UK), rabbit polyclonal antibody for
human NOS3 (Santa Cruz Biotechnology, Santa Cruz, CA, USA), or
rabbit polyclonal antibody for human NOS2 (Santa Cruz), was applied
as primary antibody at 4°C overnight. After further washing with
PBS, the specimens were incubated with ABC complex solution
(Vectastain, Vector Lab., Burlingame, CA, USA) at room temperature
for 15 min. After washing with PBS, biotinylated goat anti-mouse
IgG (Vector) was applied to the sections, which were then incubated
for 30 min at room temperature. The specimens were treated for ~5
min with a substrate solution containing 3,3′-diaminobenzidine
tetrahydrochloride (Wako Pure Chemical Industries, Ltd., Osaka,
Japan) and H2O2. Finally, the specimens were
counterstained with hematoxylin, dehydrated and mounted with
glycerol gelatin.
The CXCR4, iNOS and eNOS labeling indexes (LI) were
obtained by calculating the ratio of positive cells to the total
number of tumor cells counted in well-labeled areas, as determined
by scanning twenty areas at ×200 magnification.
Western blot analysis
Tumor samples were lysed in a lysis buffer
consisting of PBS[−], supplemented with 20 mM Tris-HCl, pH 8.0, 1%
NP40, 150 mM NaCl, 1 mM EDTA, 10% glycerol, 0.1% β-mercaptoethanol,
0.5 mM dithiothreitol and a mixture of proteinase inhibitors
consisting of 1 mM phenylmethylsulfonyl fluoride, 10 μg/ml
aprotinin, 5 μg/ml leupeptin, 5 mM benzamidine, 1 μg/ml pepstatin,
2 μg/ml antipain hydrochloride (Boehringer, Mannheim, Germany), 50
μM 4-(2-aminoethyl)-benzenesulfonyl fluoride hydrochloride (Wako
Pure Chemical Industries), 2 mM sodium orthovanadate
(Sigma-Aldrich) and 20 U/ml ulinastatin (Mochida Pharmaceutical,
Tokyo, Japan). Lysates containing 15 μg protein were subjected to
electrophoresis in a 10–20% gradient SDS-PAGE mini gel (Bio-Rad,
Chicago, IL, USA) and blotted onto a PVDF membrane using Multiphor
II (Amersham Pharmacia Biotech, Buchinghamshire, UK) for 30 min.
The blotted membrane was blocked with 5% skim milk in 10 mM
Tris-HCl, pH 7.2, containing 150 mM NaCl and 0.5% Tween-20 and was
incubated with primary antibodies (0.1–1 μg/ml) at 4°C for 16 h as
described below. The membrane was then incubated with alkaline
phosphatase-conjugated secondary antibodies (0.02 μg/ml) for 4 h at
room temperature as described below. The membrane was rinsed and
then treated with nitroblue tetrazolium (Sigma-Aldrich) and
5-bromo-4-chloro-3-indolyl phosphate (Sigma-Aldrich) to visualize
the protein bands. The primary antibodies used were rabbit
polyclonal antibody for human CXCR4 (Abcam) and rabbit polyclonal
antibody against iNOS and eNOS (Santa Cruz). The secondary
antibodies used were anti-rabbit IgGs conjugated with alkaline
phosphatase (Santa Cruz). Actin was used as an internal
control.
Assessment of apoptosis in
xenotransplanted tumors by agents
To detect DNA breaks, in situ terminal
doxynucleotidyl transferase (TdT)-mediated dUTP-digoxigenin
nick-end labeling (TUNEL) was performed as described by Gavroieli
et al(27). Briefly, after
deparaffinization and blocking of endogenous peroxidase with 0.3%
H2O2 in methanol for 30 min at room
temperature, the sections were treated with 20 μg/ml proteinase K
(Dako Cytomention, Glostrup, Denmark) for 15 min at room
temperature. The sections were submitted to TdT reaction in the
presence of terminal transferase and biotin-16-dUTP for 60 min at
37°C. The sections were then incubated with diluted peroxidase
conjugated streptavidin for 30 min at room temperature to detect
biotin-16-dUTP labeling, followed by color development with a
solution containing 3,3′-diaminobenzidine and
H2O2. Methyl green was used for
counterstaining. TUNEL-positive cancer cells were counted in twenty
areas at high magnification (×400) that show the well-labeled
areas.
Assay for microvessel density (MVD)
Microvessels were detected by immunohistochemical
staining for CD31, a marker for vascular endothelial cells. After
pretreatment with 0.25% trypsin for 10 min, tissue sections were
immunostained with an anti-human CD31 mouse monoclonal antibody
(Novocastra, Newcastle Upon Tyne, UK) using the SABC method. MVD
was determined by counting the number of vessels at magnification
(×200) of the tumor stroma that contained the highest number of
capillaries.
Statistical analysis
Statistical analysis was done with Student's t-test.
Differences were considered statistically significant when the
p-value was <0.05.
Results
Effect of agents on the growth of
subcutaneously xenotransplanted tumors of ACCIM in nude mice
All agents were well tolerated by the mice, without
weight loss or signs of toxicity. These agents inhibited the
proliferation of the subcutaneously xenotransplanted tumors.
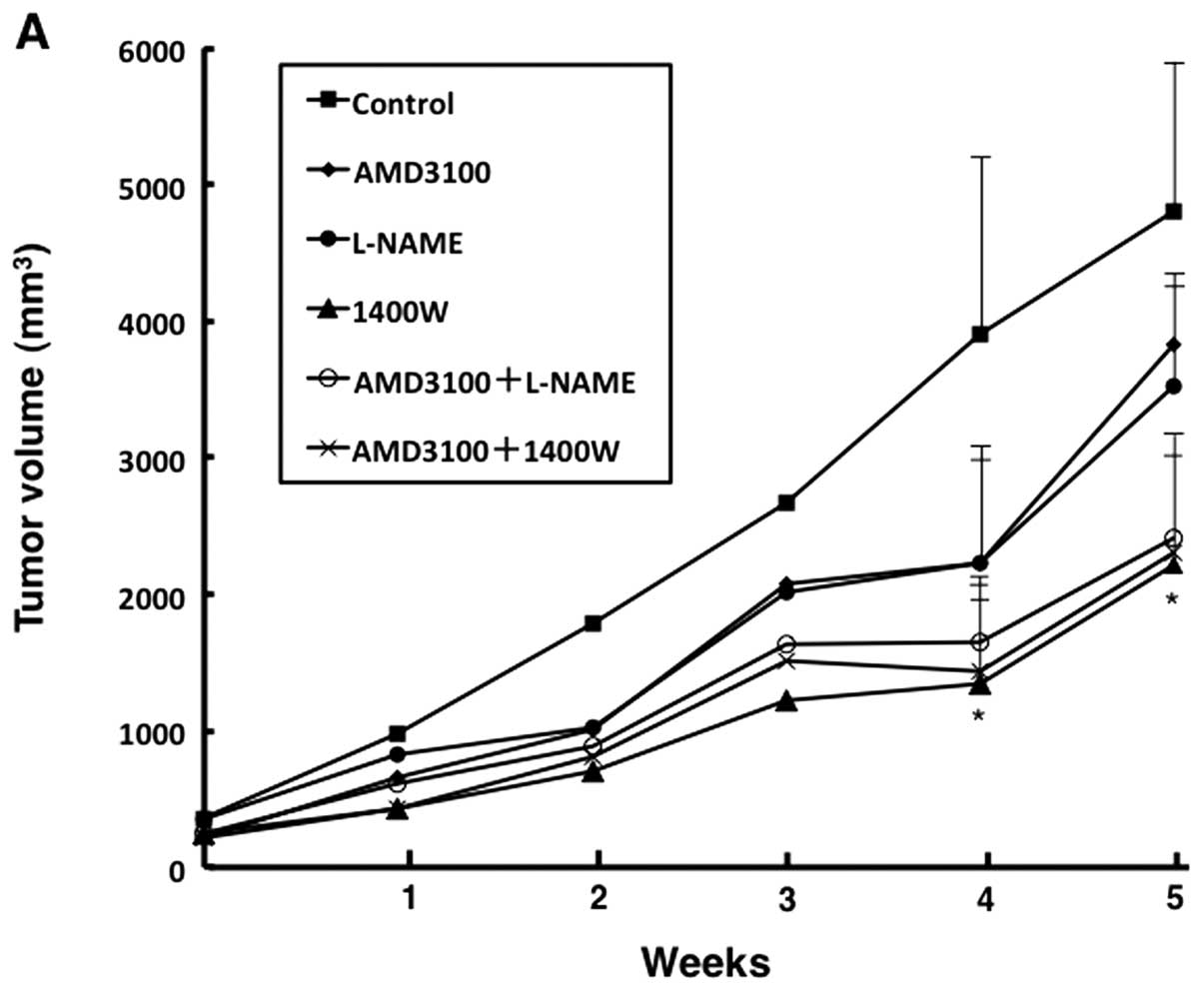
Treatment with 1400W for 4 and 5 weeks significantly inhibited
tumor growth as compared with treatment with vehicle (p<0.05)
(Fig. 2A). Combined treatment
(AMD3100+L-NAME and AMD3100+1400W) also significantly inhibited
tumor growth as compared with vehicle at the end of the observation
period (p<0.05). The reduction rate in tumor growth did not
differ significantly among these treatments (1400W, AMD3100+L-NAME
and AMD3100+1400W). The final mean tumor volume per mouse was
2234±556 mm3 (54.5% decrease) in 1400W-treated mice,
2554±612 mm3 (48.0% decrease) in AMD3100+L-NAME-treated
mice and 2443±602 mm3 (50.2% decrease) in
AMD3100+1400W-treated mice, as compared with 4910 mm3 in
vehicle-treated mice (Fig. 2B and
C). These tumors were undifferentiated carcinoma histologically
(Fig. 2C).
Immunohistochemical evaluation
The immunoreactivity of each specimen was evaluated
by light and transmission microscopy to assess the intensities of
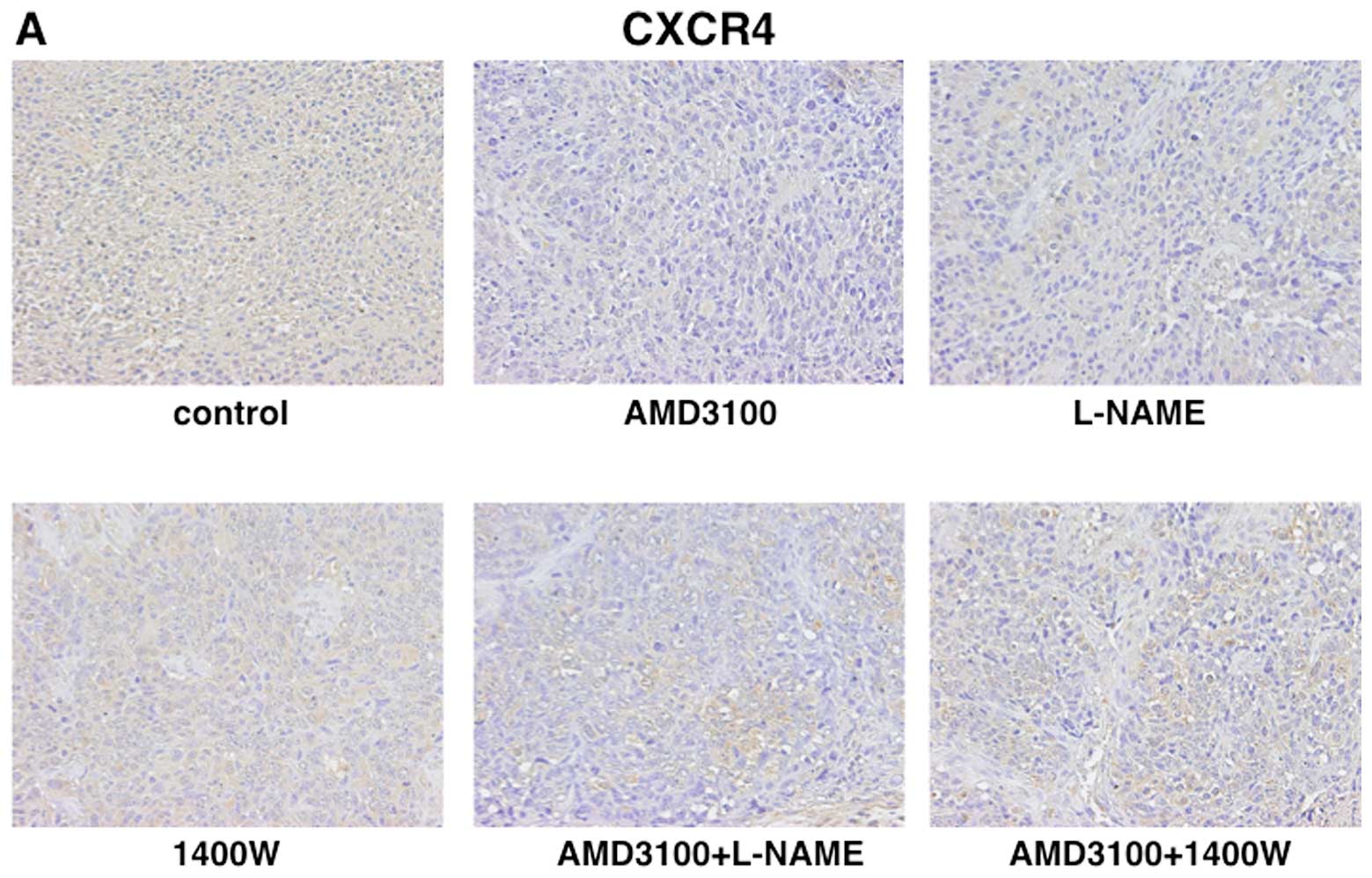
CXCR4, iNOS and eNOS expression. The labeling index (LI) of CXCR4
was significantly lower in tumors treated with AMD3100 (59.8%) than
in tumors treated with vehicle (89.3%). In contrast, there were no
significantly differences in the LI of CXCR4 in tumors treated with
L-NAME or 1400W as compared with tumors treated with vehicle
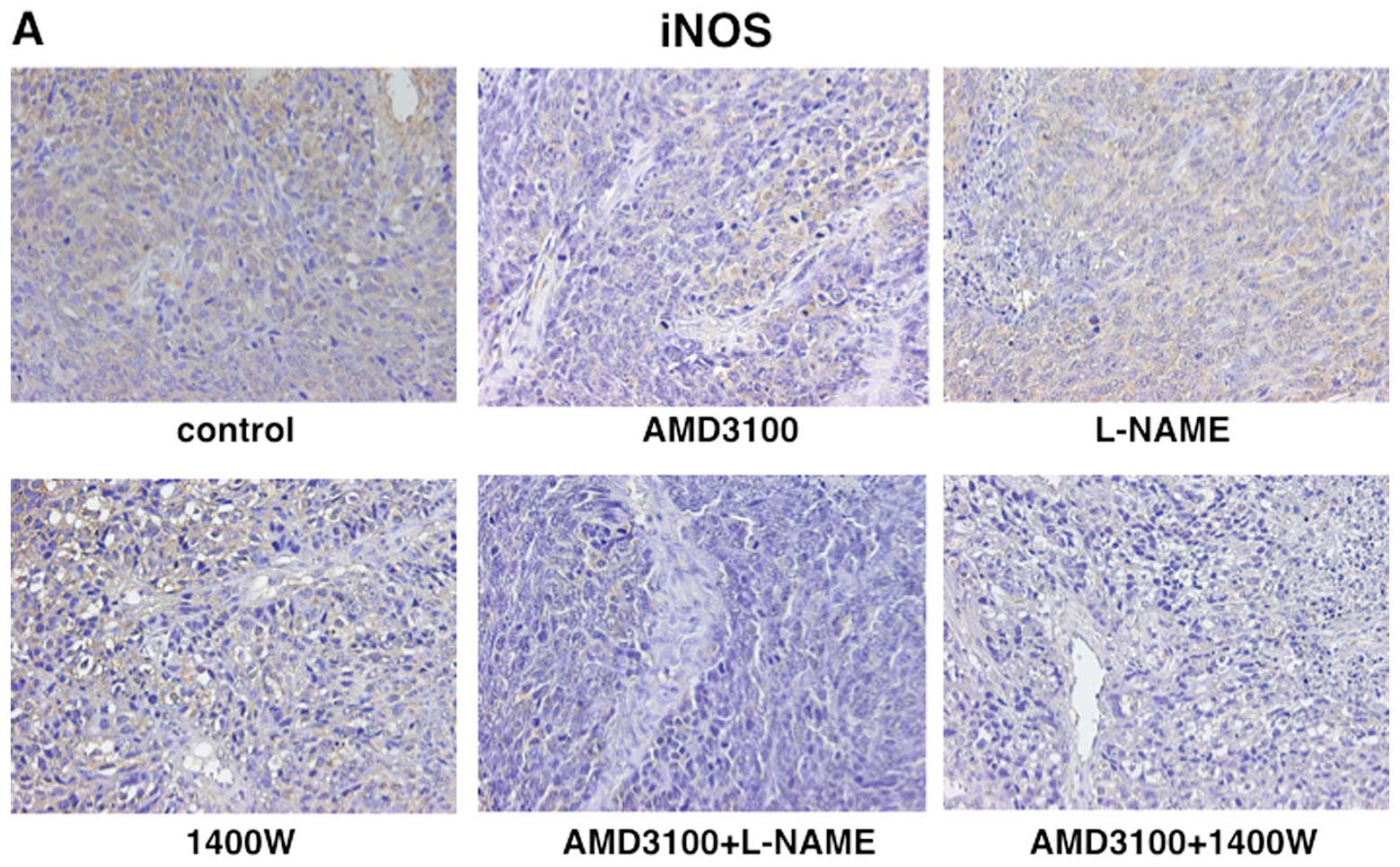
(Fig. 3). The LI of iNOS was 83.1%
in vehicle-treated tumors, 79.5% in L-NAME-treated tumors, 58.0% in
1400W-treated tumors and 61.5% in AMD3100-treated tumors. The LI of
iNOS was significantly lower in tumors treated with 1400W or with
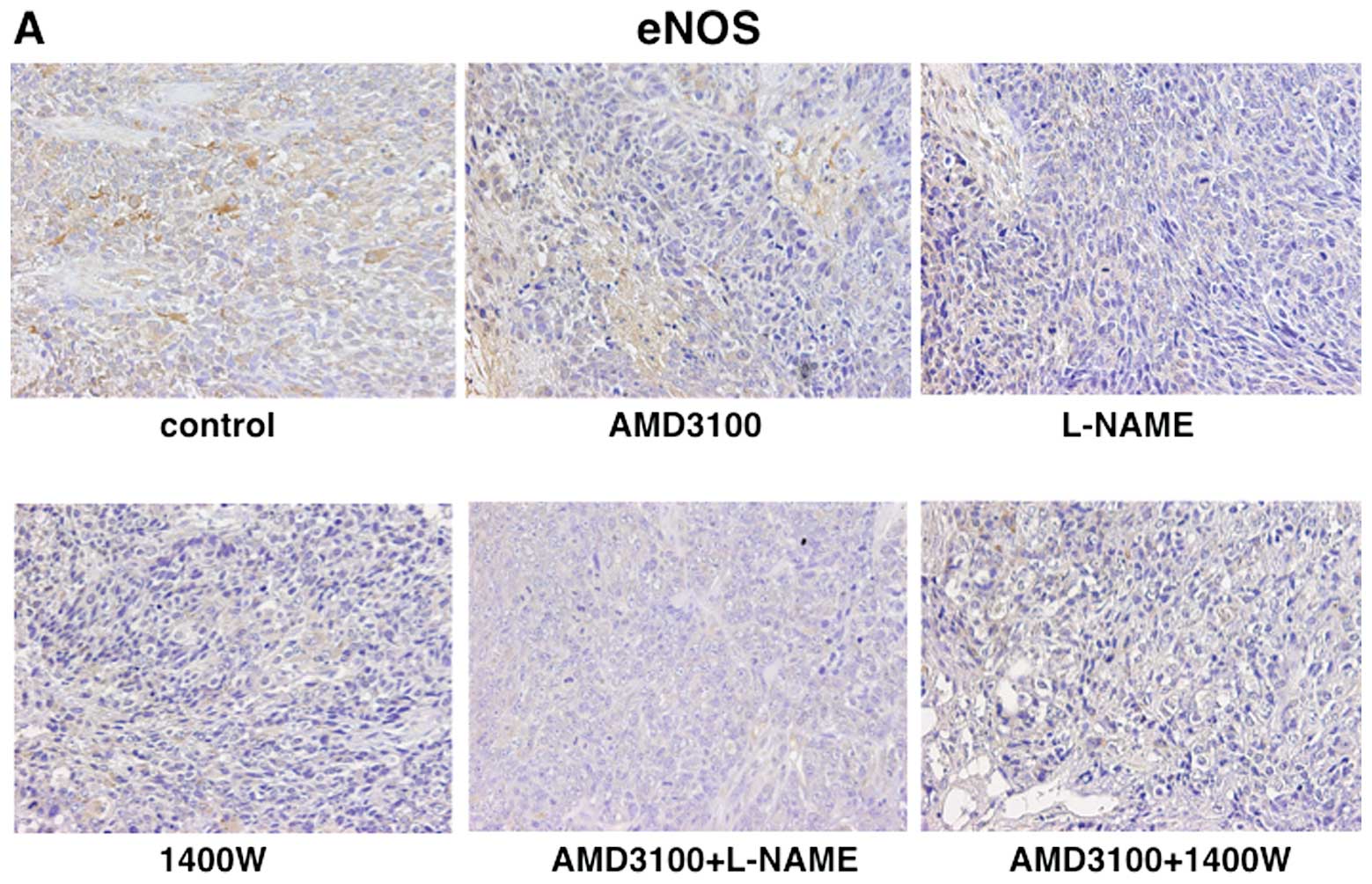
AMD3100 than in those treated with vehicle (Fig. 4). The LI of eNOS was 80.1% in
vehicle-treated tumors, 64.5% in L-NAME-treated tumors, 54.7% in
1400W-treated tumors and 75.3% in AMD3100-treated tumors. The LI of
eNOS was significantly lower in L-NAME- or 1400W-treated tumors
than in vehicle-treated tumors (Fig.
5). The decreases in the LI of iNOS and eNOS but not CXCR4 in
tumors subjected to combined treatment (AMD3100+L-NAME,
AMD3100+1400W) were significantly greater than those in L-NAME-,
1400W- or AMD3100-treated tumors (Figs. 3–5).
Effects of treatment with agents on
CXCR4, iNOS and eNOS protein expression
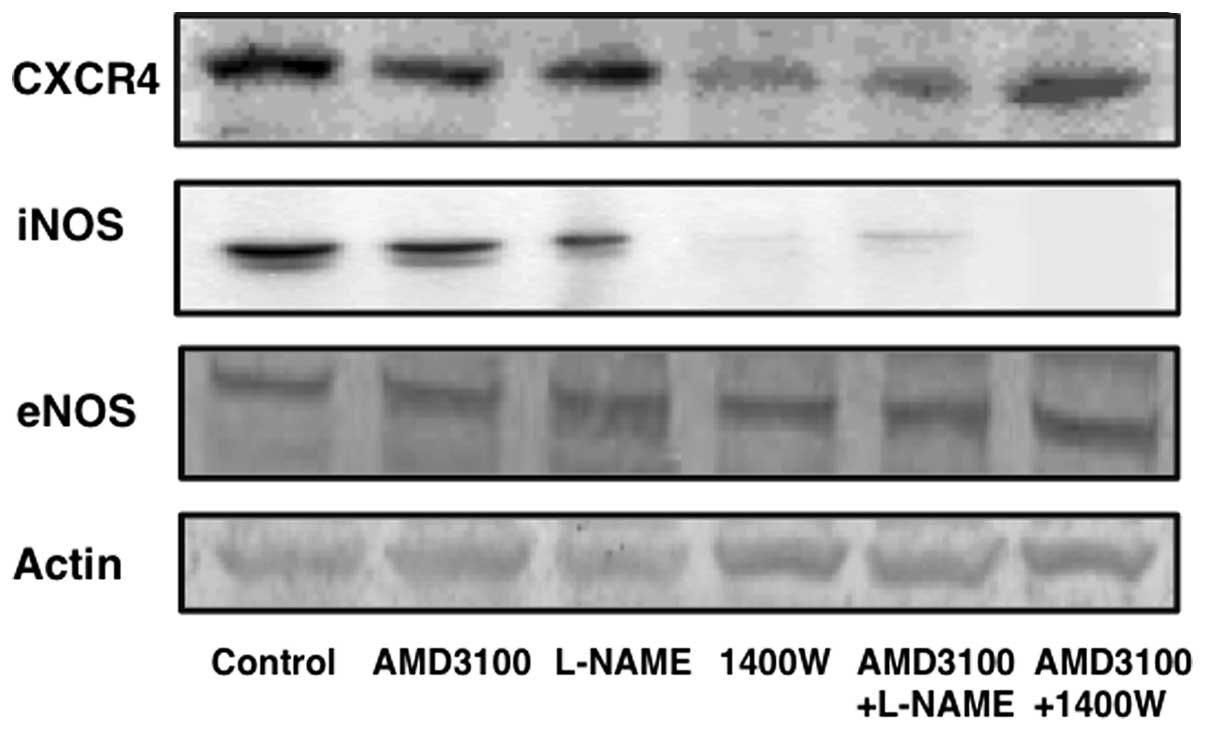
The expression levels of CXCR4, iNOS and eNOS in
vehicle-treated tumors on western blot analysis were compared with
those in tumors treated with each antagonist and inhibitor. CXCR4
expression decreased in tumors treated with 1400W, AMD3100+L-NAME,
or AMD3100+1400W. The decrease in iNOS expression was greatest in
tumors treated with 1400W. However, the expression of eNOS did not
differ significantly among the treatment groups (Fig. 6).
Increased apoptosis induction by
treatment with agents
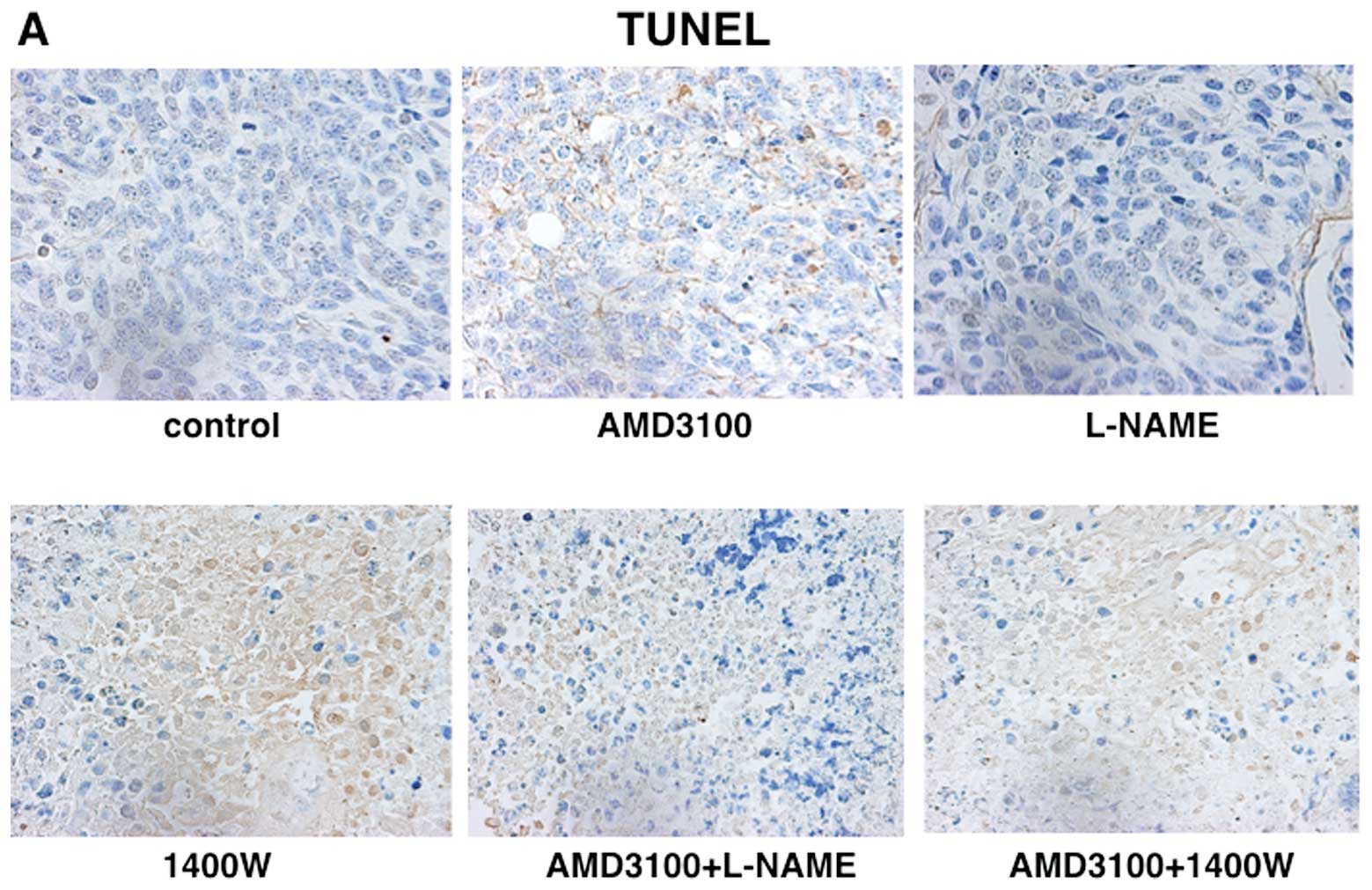
When apoptosis induction in tumor parenchyma was
examined by the TUNEL method, apoptotic cancer cells identified by
brown nuclear TUNEL signals were clearly observed in tumors treated
with 1400W, AMD3100+L-NAME, or AMD3100+1400W. The mean apoptosis
index was 0.9% in tumors treated with vehicle, but significantly
increased in tumors treated with 1400W, AMD3100+L-NAME, or
AMD3100+1400W (Fig. 7).
Inhibition of tumor angiogenesis by
treatment with agents
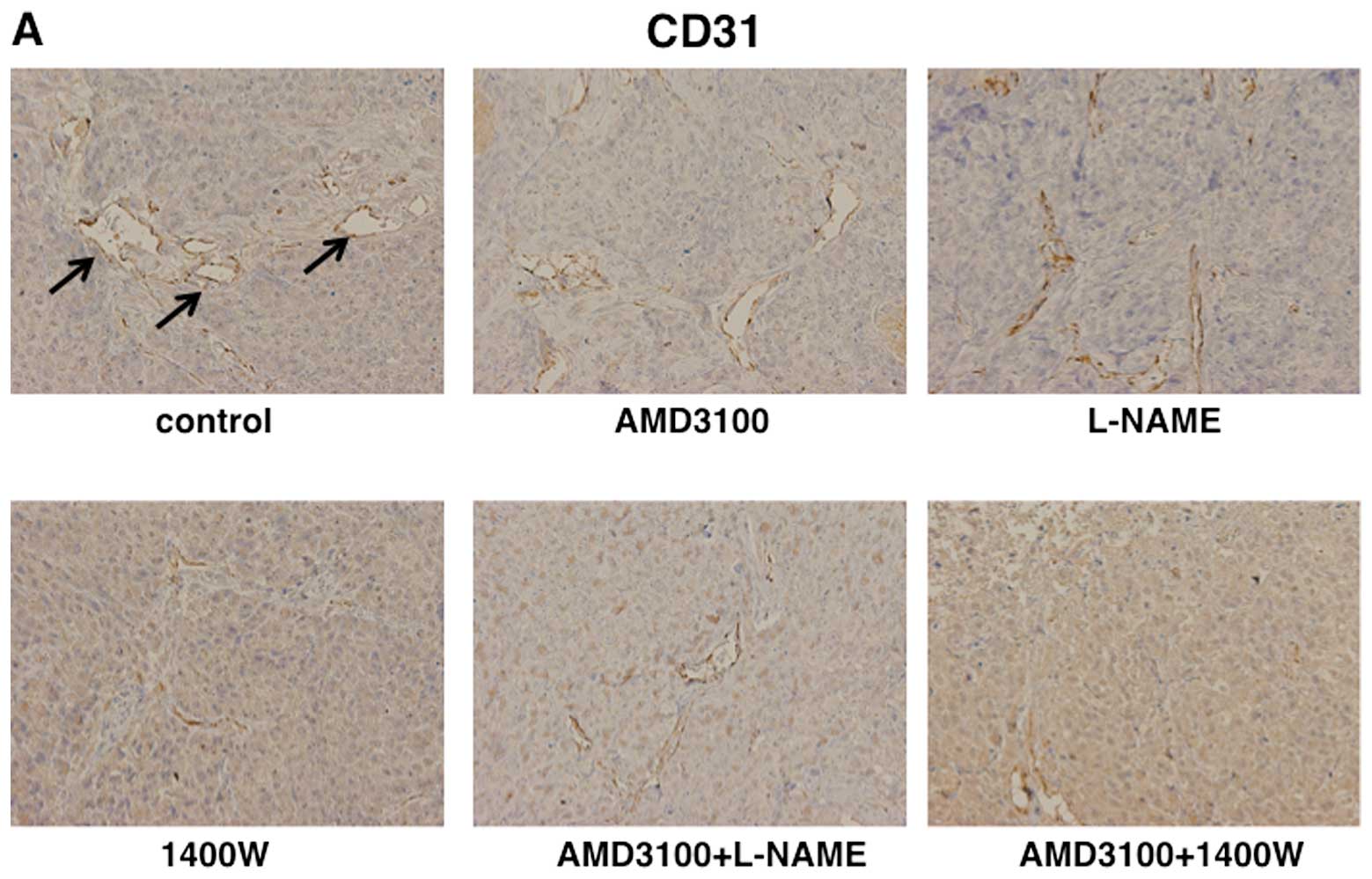
When the effects of treatment with agents on
tumor-induced angiogenesis in tumor stroma were examined
histologically, the MVD was significantly lower in tumors treated
with 1400W, AMD3100+L-NAME, or AMD3100+1400W than in
vehicle-treated tumors. There was no significant difference in the
MVD between 1400W-treated tumors and tumors treated with
AMD3100+L-NAME or AMD3100+1400W (Fig.
8).
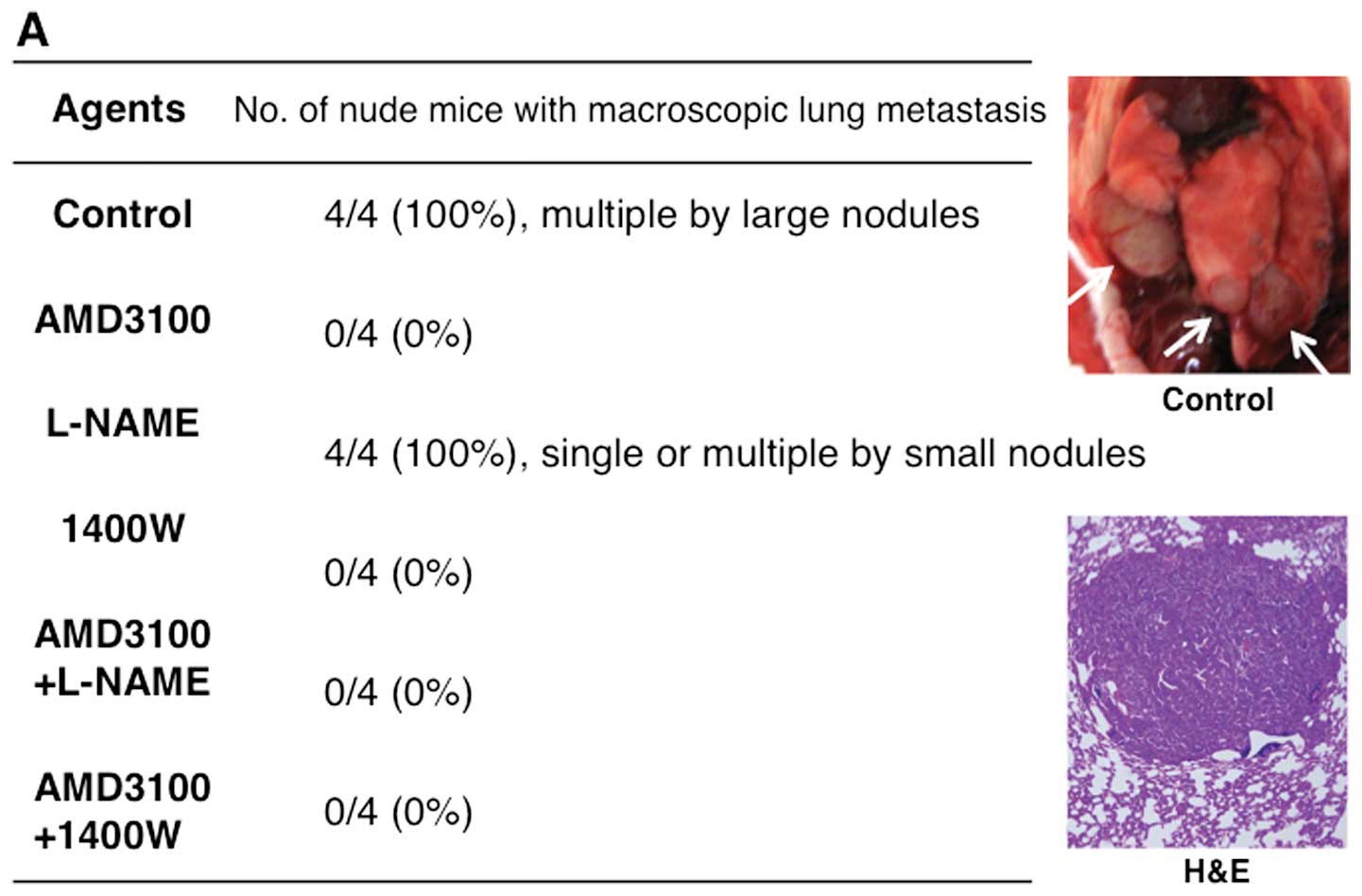
Inhibition of lung metastasis by
treatment with the agents
In vehicle- and L-NAME-treated mice, metastasis to
the lung occurred in all 4 mice (100%). After treatment with
AMD3100, 1400W, AMD3100+L-NAME, or AMD3100+1400W, no lung
metastasis was found (Fig. 9A).
The mean number of lung metastatic lesions per mouse was 5 in
vehicle-treated mice and two in L-NAME-treated mice. The diameters
of all lung metastatic lesions (20 lesions) in vehicle-treated mice
was ≥1.0 mm. Only one in eight metastatic lesions was >1.0 mm in
diameter in L-NAME-treated mice (Fig.
9B).
Discussion
A link between iNOS and cancer development and
progression has been proposed, based on both clinical and
experimental evidence. However, results have varied considerably
among studies, depending on the experimental model used (4,28,29).
Continuous inhibition of iNOS by the selective inhibitor 1400W has
been shown to suppress the growth of human colon cancers as well as
murine breast cancers that endogenously express iNOS (30). However, 1400W failed to inhibit
murine colon cancers that did not express iNOS at appreciable
levels (30). Thus, clinical as
well as in vitro and in vivo evidence supports the
hypothesis that targeted inhibition of iNOS and iNOS-derived NO may
be an effective therapeutic approach for tumors that express iNOS.
In the present study, treatment with 1400W much more effectively
inhibited the growth of subcutaneously xenotransplanted tumors than
did treatment with L-NAME, most likely because iNOS protein
expression reached appreciable levels and iNOS expression was
suppressed by treatment with 1400W.
There are several potential mechanisms by which iNOS
inhibitors can inhibit tumor growth, either directly or by
sensitizing cells to other forms of stress, such as hypoxia or
ROS-mediated stress. Interference with PI3K/AKT-mediated
overexpression of survivin is one mechanism by which iNOS
inhibition may affect the resistance of cancer cells to apoptosis
(31,32). Indeed, we observed that
TUNEL-positive cells increased in 1400W-treated tumors, suggesting
that iNOS inhibition might enhance the susceptibility of cancer
cells to apoptosis. NO has well-established pro-angiogenic
properties and stimulation of angiogenesis has been proposed as one
mechanism by which iNOS expression may support tumor growth
(33,34). We observed a nearly 3-fold decrease
in tumor microvessel density after treatment with 1400W. Since both
vascularization suppression and increased apoptosis can lead to a
diminished overall rate of tumor growth, these factors may have
acted additively or synergistically to cause the observed
significant decrease in tumor growth in vivo. Although
treatment with L-NAME or AMD3100 alone did not suppress tumor
growth, combination treatment with AMD3100+L-NAME or AMD3100+1400W
produced significant inhibition, comparable to that obtained by
treatment with 1400W alone. These results suggested that targeted
inhibition of iNOS and iNOS-derived NO by treatment with 1400W
alone and combination treatment with AMD3100+L-NAME or
AMD3100+1400W induce apoptosis and significantly inhibit
tumor-induced angiogenesis and proliferation of ACCIM in
vivo. L-NAME is a non-selective NOS inhibitor that inhibits not
only iNOS, but also eNOS. Ying and Hofseth (8) reported that although iNOS remains a
viable candidate for cancer prevention and treatment, targeting
eNOS might also be a viable strategy or at least deserves
attention. They also reported that eNOS inhibits apoptosis and
promotes angiogenesis, tumor cell proliferation, mobility and
invasiveness (8).
Metastasis occurs in an organ-specific and highly
organized manner. Tumors metastasize to preferred sites by diverse
determinants (35) and increasing
evidence has shown that the microenvironment can modulate
metastatic potential (36). In our
previous study, we immunohistochemically examined expression of
CXCR4 in surgical specimens of ACC and our results suggested that
CXCR4 and metastatic potential are closely related in ACC (23). Yasuoka et al(37,38)
reported that CXCR4 expression may be regulated by NO in breast
cancer and papillary thyroid carcinoma cell lines. Our present
study showed that CXCR4 expression decreased in tumors treated with
1400W on western blot analysis and that treatment with AMD3100 or
1400W significantly inhibited lung metastasis. Treatment with
L-NAME reduced the size and number of lung metastases as compared
with treatment with vehicle. Our results suggest that CXCR4 and
iNOS blockade inhibits lung metastases from ACCIM. CXCR4 and iNOS
may thus be important prognostic factors for long-term survival in
ACC.
Acknowledgements
This study was supported by JSPS KAKENHI grant no.
21592549 (to K.T.) and Grant-in-Aid for Young Scientists (B)
23792399 (to E.S.).
References
|
1
|
de Kerviler E, Bely N, Laccourreye O,
Clement O, Halimi P and Frija G: The aryepiglottic fold as a rare
location of adenoid cystic carcinoma. Am J Neuroradiol.
16:1375–1377. 1995.PubMed/NCBI
|
|
2
|
Fordice J, Kershaw C, El-Naggar A and
Goepfert H: Adenoid cystic carcinoma of the head and neck.
Predictors of morbidity and mortality. Arch Otolaryngol Head Neck
Surg. 125:149–152. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Nathan C and Xie QW: Nitric oxide
synthases: roles, tolls and controls. Cell. 78:915–918. 1994.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Fukumura D, Kashiwagi S and Jain RK: The
role of nitric oxide in tumor progression. Nat Rev Cancer.
6:521–534. 2006. View
Article : Google Scholar : PubMed/NCBI
|
|
5
|
Beckman JS, Beckman YW, Chen J, Marshall
PA and Freeman BA: Apparent hydroxyl radical production by
peroxynitrite: implications for endothelial injury from nitric and
superoxide. Proc Natl Acad Sci USA. 87:1620–1624. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Prueitt RL, Boersma BJ, Howe TM, et al:
Inflammation and IGF-1 activate the Akt pathway in breast cancer.
Int J Cancer. 120:796–805. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Ekmekcioglu S, Ellerhorst J, Prieto VG, et
al: Tumor iNOS predicts poor survival for stage III melanoma
patients. Int J Cancer. 119:861–866. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Ying L and Hofseth LJ: An emerging role
for endothelial nitric oxide synthase in chronic inflammation and
cancer. Cancer Res. 67:1407–1410. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Ohtsu N, Takaoka K, Segawa E, et al:
Antitumor effect of inhibitors of nitric oxide synthase or
cyclooxygenase-2 on human KB carcinoma cells overexpressing COX-2.
Oncol Rep. 24:31–36. 2010.PubMed/NCBI
|
|
10
|
Muller A, Homey B, Soto H, et al:
Involvement of chemokine receptors in breast cancer metastasis.
Nature. 410:50–56. 2001. View
Article : Google Scholar : PubMed/NCBI
|
|
11
|
Pablos JL, Amara A, Bouloc A, et al:
Stromal-cell derived factor is expressed by dendritic cells and
endothelium in human skin. Am J Pathol. 155:1577–1586. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Aiuti A, Webb IJ, Bleul C, Springer T and
Gutierrez-Ramos JC: The chemokine SDF-1 is a chemoattractant for
human CD34+ hematopoietic progenitor cells and provides
a new mechanism to explain the mobilization of CD34+
progenitors to peripheral blood. J Exp Med. 185:111–120.
1997.PubMed/NCBI
|
|
13
|
Tashiro K, Tada H, Heilker R, Shirozu M,
Nakano T and Honjo T: Signal sequence trap: a cloning strategy for
secreted proteins and type I membrane proteins. Science.
261:600–603. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Bleul CC, Fuhlbrigge RC, Cassasnovas JM,
Aiuti A and Springer TA: A high efficacious lymphocyte
chemoattractant, stromal cell-derived factor-1α (SDF-1α). J Exp
Med. 184:1101–1109. 1996.
|
|
15
|
Campbell JJ, Hedrick J, Zlotnik A, Siani
MA, Thompson DA and Butcher EC: Chemokines and the arrest of
lymphocytes rolling under flow conditions. Science. 279:381–384.
1998. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Grabovsky V, Feigelson S, Chen C, et al:
Subsecond induction of α4 integrin clustering by immobilized
chemokines stimulates leukocyte tethering and rolling on
endothelial vascular cell adhesion molecule 1 under flow
conditions. J Exp Med. 192:495–506. 2000.
|
|
17
|
Wright N, Hidalgo A, Rodriguez-Frade JM,
et al: The chemokine stromal cell-derived factor-1α modulates α4β7
integrin-mediated lymphocytes adhesion to mucosal addressin cell
adhesion molecule-1 and fibronectin. J Immunol. 168:5268–5277.
2002.
|
|
18
|
Ganju RK, Brubaker SA, Meyer J, et al: The
α-chemokine, stromal cell-derived factor-1α, binds to the
transmembrane G-protein-coupled CXCR-4 receptor and activates
multiple signal transduction pathways. J Biol Chem.
273:23169–23175. 1998.
|
|
19
|
Homey B, Muller A and Zlotnik A:
Chemokines: agents for the immunotherapy of cancer? Nat Rev
Immunol. 2:175–184. 2002. View
Article : Google Scholar : PubMed/NCBI
|
|
20
|
Cardones AR, Murakami T and Hwang ST:
CXCR4 enhances adhesion of B16 tumor cells to endothelial cells in
vitro and in vivo via β(1) integrin. Cancer Res. 63:6751–6757.
2003.PubMed/NCBI
|
|
21
|
Murakami T, Maki W, Cardones AR, et al:
Expression of CXC chemokine receptor-4 enhances the pulmonary
metastatic potential of murine B16 melanoma cells. Cancer Res.
62:7328–7234. 2002.PubMed/NCBI
|
|
22
|
Robledo MM, Bartolome RA, Longo N, et al:
Expression of functional chemokine receptor CXCR3 and CXCR4 on
human melanona cells. J Biol Chem. 276:45098–45105. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Zushi Y, Noguchi K, Hashitani S, et al:
Relations among expression of CXCR4, histological patterns and
metastatic potential in adenoid cystic carcinoma of head and neck.
Int J Oncol. 33:1133–1139. 2008.PubMed/NCBI
|
|
24
|
Hashitani S, Urade M, Zushi Y, Segawa E,
Okui S and Sakurai K: Establishment of nude mouse transplantable
model of a human adenoid cystic carcinoma of the oral floor showing
metastasis to the lymph node and lung. Oncol Rep. 17:67–72.
2007.PubMed/NCBI
|
|
25
|
Ovejera AA, Houchens DP and Baker AD:
Chemotherapy of human tumor xenografts in genetically athymic mice.
Ann Clin Lab Sci. 8:50–56. 1978.PubMed/NCBI
|
|
26
|
Hsu SM, Raine L and Franger H: Use of
avidin-biotin-peroxidase complex (ABC) in immunoperoxidase
techniques: a comparison between ABC and unlabeled antibody (PAP)
procedures. J Histochem Cytochem. 29:577–580. 1981. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Gavrieli Y, Sherman Y and Ben-Sasson SA:
Identification of programmed cell death in situ via specific
labeling of nuclear DNA fragmentation. J Cell Biol. 119:493–501.
1992. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Ekmekcioglu S, Tang C and Grimm EA: NO
news is not necessarily good news in cancer. Curr Cancer Drug
Targets. 5:103–115. 2005. View Article : Google Scholar
|
|
29
|
Wink DA, Ridnour LA, Hussain SP and Harris
CC: The reemergence of nitric oxide and cancer. Nitric Oxide.
19:65–67. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Li G, Yang T and Yan J: Cyclooxygenase-2
increased the angiogenic and metastatic potential of tumor cells.
Biochem Biophys Res Commun. 299:886–890. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Engels K, Knauer SK, Loibl S, et al: NO
signaling confers cytoprotectivity through the survivin network in
ovarian carcinomas. Cancer Res. 68:5159–5166. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Fetz V, Bier C, Habtemichael N, et al:
Inducible NO synthase confers chemoresistance in head and neck
cancer by modulating survivin. Int J Cancer. 124:2033–2041. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Roy HK, Wali RK, Kim Y, et al: Inducible
nitric oxide synthase (iNOS) mediates the early increase of blood
supply (EIBS) in colon carcinogenesis. FEBS Lett. 581:3857–3862.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Singh RP and Agarwal R: Inducible nitric
oxide synthase-vascular endothelial growth factor axis: a potential
target to inhibit tumor angiogenesis by dietary agents. Curr Cancer
Drug Targets. 7:475–483. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Nguyen DX, Bos PD and Massague J:
Metastasis: from dissemination to organ-specific colonization. Nat
Rev Cancer. 9:274–284. 2009. View
Article : Google Scholar : PubMed/NCBI
|
|
36
|
Joyce JA and Pollard JW:
Microenvironmental regulation of metastasis. Nat Rev Cancer.
9:239–252. 2009. View
Article : Google Scholar : PubMed/NCBI
|
|
37
|
Yasuoka H, Kodama R, Hirokawa M, et al:
CXCR4 expression in papillary thyroid carcinoma: induction by
nitric oxide and correlation with lymph node metastasis. BMC
Cancer. 8:2742008. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Yasuoka H, Tsujimoto M, Yoshidome K, et
al: Cytoplasmic CXCR4 expression in breast cancer: induction by
nitric oxide and correlation with lymph node metastasis and poor
prognosis. BMC Cancer. 8:3402008. View Article : Google Scholar : PubMed/NCBI
|