Introduction
Cervical cancer is a major problem in women’s health
worldwide and is the second leading cause of cancer deaths in women
(1). Clinical, molecular and
epidemiological investigations identify the high-risk human
papillomavirus (HPV) as the major cause of cervical cancer, with
the high-risk types HPV16 and 18 accounting for nearly 70% of
cervical cancer cases (2–5). The E6 and E7 oncoproteins encoded by
these two viruses, are crucial for the productive viral life cycle
in the transformation and maintenance of the malignant phenotype
(6). Therefore, various forms of
HPV vaccines targeting the two proteins are designed for cervical
cancer immunotherapy, including plasmid DNA, viral or bacterial
vectors, chimeric virus-like particles (VLP), synthetic peptides
and recombinant proteins (3).
Cervical cancer therapeutic vaccines face many challenges,
including immunocompromised state of cancer patients, difficulty in
immune system stimulation, immune escape and tolerance mechanisms
used by tumors and virally infected cells, as well as safety
concerns (2,7). Thus, an emerging need for the
treatment of cervical cancer and other HPV infection-associated
diseases is required.
Dendritic cells (DCs) are the most potent
professional antigen-presenting cells (APC) with key regulatory
roles in immune response. It maintains tolerance to self-antigens
and activates innate and adaptive immunity against viral infection
by producing various proinflammatory cytokines, which is involved
in processing and presenting antigens to T cells (6,8).
Recent studies on dendritic cell (DC)-based tumor vaccine
demonstrated that enhancing DC maturation/co-stimulation and
antigen presentation can greatly enhance human CTL and Th immune
response in cancer therapy, suggesting a promising way to treat HPV
and other cancers (8–13). Hence, activation of the full
immunostimulatory potential of DCs to achieve an effective immune
response provides a promising way to prevent or control many viral
infections.
Recently, a major immunosuppressive, the
intracellular signal regulator suppressors of cytokine signaling 1
(SOCS1) was found to play an important role in blocking the
constitutive activation of the immune response (14). SOCS1 functions as an inducible
negative feedback inhibitor of the JAk/STAT signal pathway
(14) and negatively regulates
various cytokines, such as interferon (IFN)-γ, the interleukin
(IL)-2, IL-6, IL-7, IL-12, or IL-15 in T cells, DCs and other cells
(15).
Several studies demonstrate that ex vivo
SOCS1 silencing in antigen-presenting DCs can strongly enhance
antigen-specific antitumor and antivirus immunity (8,13,16,17).
Hanada et al (18) and
Guenterberg et al (19)
showed that the antitumor activity of exogenous IFN-α was enhanced
by CD4+ and CD8+ T cells in SOCS1−/−
transgenic mice and T and B cells were restored via exogenous SOCS1
expression. SOCS1−/− DCs were also hyper-responsive to
LPS and IFN-γ, which triggers an allogeneic T-cell expansion. In
addition, studies in macrophages demonstrated that silencing SOCS1
can improve Mycobacterium tuberculosis control and suppress
tumor development by enhancing antitumor inflammation (20). All these findings indicate that
SOCS1 possessed an inhibitory role in controlling antigen
presentation by APCs and magnitude of the adaptive immunity. The
regulation of antigen presentation and development of more
effective tumor vaccines can therefore be achieved by silencing the
crucial ‘natural inhibitor’ in antigen-presenting DCs.
DC vaccines combined with SOCS1 silencing has been
previously reported for antitumor and antivirus purpose (13,16).
In this study, we sought to find a safer and more effective
approach to treat cervical cancer and other HPV
infection-associated malignancies by constructing an HPV16 E7
mutant, the HPV16mE7, to reduce transformation activity and enhance
antigenicity (21–23). The immunotherapeutic effect of
mE7-pulsed DC vaccine with adenovirus-mediated SOCS1 silencing on
the allografted tumor mouse models which express HPV16E6E7
oncoproteins, was investigated.
Materials and methods
Peptide synthesis of E7.49-57 and HBV
surface antigen (HBsAg) R187
The synthetic peptides E7.49-57 (RAHYNIVTF)
corresponding to the HPV16-E7 H2-Db restricted epitope (24) and HBV surface antigen (HBsAg) R187
(aa183-191) (FLLTRILTI) (25) were
synthesized and purified to >95% purity by Sangon Inc.
(Shanghai, China) via high-performance liquid chromatography
(HPLC).
Construction of modified HPV16 E7
gene, protein expression and purification
The HPV16 E7 mutant gene (HPV16mE7) was designed to
reduce its transformation activity and enhance its antigenicity
(21–23,26).
The three arbitrarily designated regions, a, b and c, of the native
98-amino acid HPV16 E7 protein were mutated as follows: in region
a, amino acids 21, 24 and 26 were mutated from DLYCYEQ to GLYGYGQ.
In region c, two zinc finger-binding motifs were disrupted by
mutating C61→G61 and C94→G94 using a site-directed mutagenesis kit
according to the manufacturer’s instructions (Promega Co., Madison,
WI, USA). The HPV16mE7 was cloned into the plasmid pET-28a vector
(Invitrogen, Carlsbad, CA, USA) and then transformed into the
Escherichia coli strain BL21γ (DE3) (Novagen). The
recombinant protein containing His-Tag was expressed upon induction
with 0.5 mM isopropyl-β-D-thiogalactopyranoside (IPTG, Sigma, St.
Louis, MO, USA) and purified on Ni-NTA resin (Qiagen, Hilden,
Germany). Isopropanol washes were used to remove lipopolysaccharide
(LPS) contamination. HPV16mE7 protein was confirmed by western blot
analysis probed either with mouse monoclonal anti-HPV16-E7 antibody
(Zymed, San Francisco, CA, USA) or mouse anti-His polyclonal
antibody. Immune complexes were detected with goat HRP-conjugated
anti-mouse immunoglobulins (Chemicon, Temecula, CA, USA) and the
result was revealed via electrochemical luminescence (ECL, Pierce
Biotechnology, Inc., Rockford, IL, USA). HPV16 E7 concentration was
measured by Bradford assay.
Adenovirus vector production
The shRNA-SOCS1 and its mutant form shRNA-mSOCS1
were constructed according to http://www.bioon.com.cn. Primers used are as follows:
shRNASOCS1 (F): 5′-GATCC CTA CCT GAG TTC CTT CCC
CT TCAAGAG AG GGG AAG GAA CTC AGG TAGTTTTTT G-3′ (BamHI and
EcoRI); shRNA-SOCS1 (R): 5′-AATTC AAAAAACTA CCT GAG TTC CTT
CCC CT CTCTTGA AG GGG AAG GAA CTC AGG TAG G-3′; shRNA-mSOCS1 (F):
5′-GATCC ACT ATC
TAA GTT ACT ACC CCT TCAAGAG AGG GGT AGT AAC TTA GAT AGT
TTTTTTG-3′;
shRNA-mSOCS1 (R): 5′-AATTC AAAAAAACT ATC TAA GTT ACT
ACC CCT CTCTTGA AGG GGT AGT AAC TTA GAT AGT G-3′. The shRNA-SOCS1 and
shRNA-mSOCS1 were cloned into the plasmid vector
RNAi-SOCS1-pShuttle (BD Clontech) and then inserted into the
replication-deficient pAdeno-X vector (BD Clontech). The
recombinant adenovirus plasmids were generated according to
manufacturer’s instructions and verified by PCR and sequencing and
titrated using Adeno-X Rapid Titer kits (BD Bioscience).
Transduction of BM-derived DCs with
adenoviral vectors
Recombinant adenoviral vectors (ad-shRNA-SOCS1 and
ad-shRNA-mSOCS1) were produced and titrated as described above.
Mouse bone marrow (BM)-derived DCs (MBDDs) were prepared by Shen
et al method (16). In
brief, mouse BM was flushed from the hind limbs, passed through a
nylon mesh and depleted of red cells using ammonium chloride. After
extensive washing with RPMI-1640, the cells were cultured with
RPMI-1640 supplemented with 10% FBS, recombinant mouse
granulocyte-macrophage colony-stimulating factor (GM-CSF, 20 ng/ml;
PeproTech) and recombinant mouse IL-4 (20 ng/ml; PeproTech). On the
2nd and 4th day of culture, the supernatant was removed and
replaced with fresh medium containing mGM-CSF and mIL-4. All
cultures were incubated at 37°C in 5% humidified CO2.
Non-adherent granulocytes were removed after 48 h and fresh medium
was added. After 7 days of culture, flow cytometric analysis showed
that >80% of the cells expressed characteristic DC-specific
markers. The DCs were washed and placed in 12-well plates at a
concentration of 105 cells/well in 400 μl
RPMI-1640. The cells were exposed to ad-shRNA-mSOCS1 or
ad-shRNA-SOCS1 at 50 MOI. After 8–12-h transduction, the cells were
washed with PBS and further incubated in fresh tissue culture
medium. The immature BMDCs were then pulsed with the HPVm16E7
protein (25 μg/ml) at 37°C for 6 h, followed by stimulation
with LPS (0.5 μg/ml) for 24 h to develop mature BMDCs. The
harvested DCs were tested with CD80-flurescein isothiocyanate
(FITC), CD-83-FITC and CD86-FITC to confirm DC maturation. Flow
cytometry acquisition and analysis were performed via a FACS using
the Cell Quest software (Becton-Dickinson).
Analysis of SOCS1
Quantitative real-time PCR analysis of
SOCS1
Total RNA was extracted from DCs using the TRIzol
reagent (Invitrogen) with 1.0 μg for each sample and was
reverse-transcribed with random hexamer primers using Super Script
First-Strand Synthesis kits (Invitrogen). The expression level of
GAPDH was evaluated as an internal control. The target mRNAs were
then assessed using the following primers: GAPDH: ACAGTC
CATGCCATCACTGCC (sense) and GCCTGCTTCACCACCTTCTTG (anti-sense);
SOCS1: TGGTTGTAGCAGCTTGTGTCTGG (sense) and CCTGGTTTGTGCAAAGATACTGGG
(anti-sense). Real-time PCR analysis was performed using an ABI
7900HT Sequence Detection System (Applied Biosystems, Foster City,
CA, USA) in 20 μl quadruplicate reactions, using the
equivalent of 5 ng of the starting RNA material per reaction as the
template.
Western blot analysis of SOCS1
silencing
Since SOCS1 expression in DCs was difficult for
western blot analysis, the inhibitory effect of ad-shRNA-SOCS1 and
ad-shRNA-mSOCS1 were conducted in B16 cells (ATCC, Manassas, VA,
USA). The cells were cultured in DMEM and infected by the
recombinant adenovirus plasmids for 48 h at 50 and 100 MOI and then
lysed with cell lysis buffer [0.3% NP40, 1 mM EDTA, 50 mM Tris-Cl
(pH 7.4), 2 mM EGTA, 1% Triton X-100, 150 mM NaCl, 25 mM NaF, 1 mM
Na3VO3, 10 μg/ml phenylmethylsulfonyl
fluoride] for 30 min on ice and then centrifuged at 12,000 × g for
15 min at 4°C. The protein samples were separated via SDS-PAGE and
transferred onto immobilon membranes (Millipore, MA, USA). SOCS1
and β-actin proteins were identified using anti-SOCS1 polyclonal
and anti-β-actin monoclonal antibodies (Santa Cruz Biotechnology,
Santa Cruz, CA, USA), respectively.
Cytokine ELISA analysis
Levels of various cytokines (IL-12p70, IL-6, TNF-α)
induced by HPV16mE7-pulsed DCs in response to LPS stimulation (100
ng/ml) for 24 h were quantitated using the supernatant of DC
cultures at the time-points and with the stimulus by ELISA analysis
(Dakewe Biotech Co. Ltd., China) according to the manufacturer’s
instructions. Data are representative of three independent
assays.
Tumor models
Female C57BL/6 (H-2b) mice were purchased
from Guangzhou Traditional Chinese Medicine University (Guangzhou,
China). The mice were housed in filter top cages under specific
pathogen-free conditions and used in accordance with the guidelines
of Tsinghua University Council on Animal Care. C57BL/6 mice (10 per
group) in therapy experiments received a subcutaneous (s.c.) tumor
injection of 5×106 cells/ml of TC-1 cells (ATCC)
constitutively expressing wild-type HPV16E6E7 in 100 μl
Hank’s buffered salt solution (HBSS, Sigma-Aldrich) for nine days
prior to immunization. At day 9, all mice had palpable tumors and
then were treated with PBS and various forms of DCs vaccines.
DCs immunization for tumor
therapy
Immature BMDCs infected with ad-shRNA-mSOCS1 or
ad-shRNA-SOCS1 at 50 MOI were pulsed with the HPVm16E7 protein (25
μg/ml) at 37°C for 6 h, followed by stimulation with LPS
(0.5 μg/ml) for 6 h to develop mature BMDCs. The cells
(2×105) were then washed with PBS to remove
extracellular LPS and injected into the hind footpads of C57BL/6
mice inoculated with TC-1 cells expressing HPV16E6E7. The immunized
mice were intraperitoneally treated with LPS three times on days 1,
3 and 5 after DC vaccine immunization. The tumor volume was
calculated using the following formula: (major axis × minor
axis2) × 0.52. The tumor-bearing mice were euthanized
when the tumor volume reached ∼2,500 mm3.
ELISA for HPV16mE7 antibodies
Enzyme-linked immunosorbent assays (ELISA) were
performed by immobilizing 0.5 μg HPV16mE7 protein in 50 mM
NaHCO3 (pH 9.6) per microtiter well plate overnight at
4°C. Blocking was done by 1% phosphate-buffered saline (PBS) (pH
7.5) containing 1% gelatin for 2 h at 37°C. Each protein ligand was
incubated in PBS (pH 7.5) with 0.2% gelatin within the appropriate
wells for 1 h at room temperature at a concentration of 5
μg/ml, then anti-HPV16 E7 monoclonal antibody (Santa Cruz
Biotechnology) or sera from the immunized mice were added. In the
control experiments, the first antigen was omitted from the
immobilization step. Incubation with each antibody was carried out
in the same solution overnight at 4°C.
CTL assay
C57BL/6 mice (four per group) were immunized twice
in one-week interval with subcutaneous injections of different DCs
vaccines at the hind footpads. Control mice received a sham
injection of Dulbecco’s phosphate-buffered saline (DPBS). Two weeks
after DCs inoculation, the mice were euthanized via cervical
dislocation. Single-cell suspensions of pooled spleens from each
group were prepared in a CTL medium composed of RPMI-1640
(Gibco/BRL, Carlsbad, CA, USA) supplemented with 10% FCS, 2 mM
L-glutamine, 1 mM sodium pyruvate (Gibco/BRL), 50 μM
2-mercaptoethanol (Sigma-Aldrich,) and 50 μg/ml gentamicin
sulfate (Gibco/BRL). The splenocytes were restimulated in 10 ml CTL
medium by incubating 3×107 viable lymphoid cells in 1
μM E7.49-53 peptide at an upright T25 tissue culture flask.
The effector cells were harvested after a 7-day incubation and then
analyzed for cytolytic activity and intracellular γ interferon
(IFN-γ) production.
LDH release assay
The cytolytic activity was assayed in triplicate in
96-well culture plates by culturing the effector cells with target
TC-1 cells expressing HPV16 E6 and E7 proteins at effector/target
(E/T) ratios of 100:1, 33:1 or 11:1. After a 4-h incubation at
37°C/5% CO2, the 96-well culture plates were centrifuged
at 200 × g for 5 min using a Beckman G5-6R centrifuge (Beckman
Coulter Canada, Mississauga, ON, Canada). The culture supernatant
(100 μl) was collected from each well and transferred into
Beckman ready caps and the released LDH activity was determined via
biochemical analysis using the Beckman Biochemical analyzer. The
target cells cultured without effector cells in either the medium
or Triton X-100 (1% wt/vol) were used to determine the spontaneous
or total release of the label. The results were expressed as
percent specific lysis, calculated by [(LDHtest ×
LDHspont)/(LDHtotal − LDHspont)] ×
100. The control target LDH values were consistently <10% and
were subtracted from the obtained results.
IFN-γ ELISA and ELISPOT assay
The intracellular γ interferon (IFN-γ) production of
the activated antigen-specific cytotoxic T cells in splenocytes
harvested from immunized C57BL/6 mice were detected using the ELISA
kit and ELISPOT kit (eBioscience Co.).
IFN-γ ELISA assay. C57BL/6 mice (four per
group) were immunized twice with one week interval with PBS, DCs,
DC-ad-shRNA-SOCS1, or DC-ad-shRNA-mSOCS1. The DCs were HPV16mE7
pulsed. After 7 days post-immunization, pooled splenocytes from
each group were re-stimulated with E7.49-57 for 7 days. The
effector cells was cultured with target TC-1 cells which express
HPV16 E6 and E7 proteins at effector/target (E/T) ratios of 100:1,
33:1 or 11:1. The IFN-γ ELISA assay was performed following the
instruction manual.
IFN-γ ELISPOT assay. In brief, on day 9 of
post-immunization, the 96-well nitrocellulose-base plates were
coated overnight at 4°C with purified anti-mouse IFN-γ antibodies
and then blocked with the complete media. Splenocytes (100
μl/well) cultured in RPMI-1640 (Gibco/BRL) were seeded in
the wells at an initial concentration of 106 cells/well
and a row of serial dilutions either unstimulated or stimulated
with E7.49-57 or R187 peptide (1 μg/ml) or buffer were
prepared. PMA (5 ng/ml, Sigma) served as a positive control and the
R187 peptide or media served as a negative control. The plate was
incubated overnight at 37°C/5% CO2 and then detected by
detection antibody (a biotinylated anti-mouse IFN- γ antibody) for
2 h at room temperature. After removing the unbound detection
antibody, the enzyme conjugate (streptavidin-HRP) was added. The
unbound streptavidin-HRP was washed off after a 1-h incubation at
room temperature and the plate was stained with an AEC substrate
solution for 20 min. Further, the plate was washed and air-dried
overnight. Foci of staining were counted using a magnifying
lens.
Statistical analysis
All data were expressed as means ± standard
deviation (SD) and were representative of at least two different
experiments. The statistical significance of group differences was
measured by Student’s t-test. p-values <0.05 were considered to
be significant. The mouse survival rates were analyzed using the
Kaplan-Meier method (log-rank test).
Results
Construction and characterization of
recombinant HPV16mE7
HPV16mE7 was cloned into pET-28a vector, expressed
in BL21γ (DE3) E. coli and purified to homogeneity to allow
in vitro/in vivo assays (Fig.
1A). An LPS elimination procedure was introduced in the
purification protocol. A series of diluted imidazole elution
buffer, 60, 100, 150 and 200 mM, respectively, were used to
determine the best elution concentration. The presence of the E7
protein in HPV16mE7 was confirmed via western blot analysis using
an anti-HPV16 E7 monoclonal antibody or anti-His monoclonal
antibody (Fig. 1B). HPV16 E7
concentration was measured by BCA kit (Beyotime, China) according
to the instruction manual for the following assays.
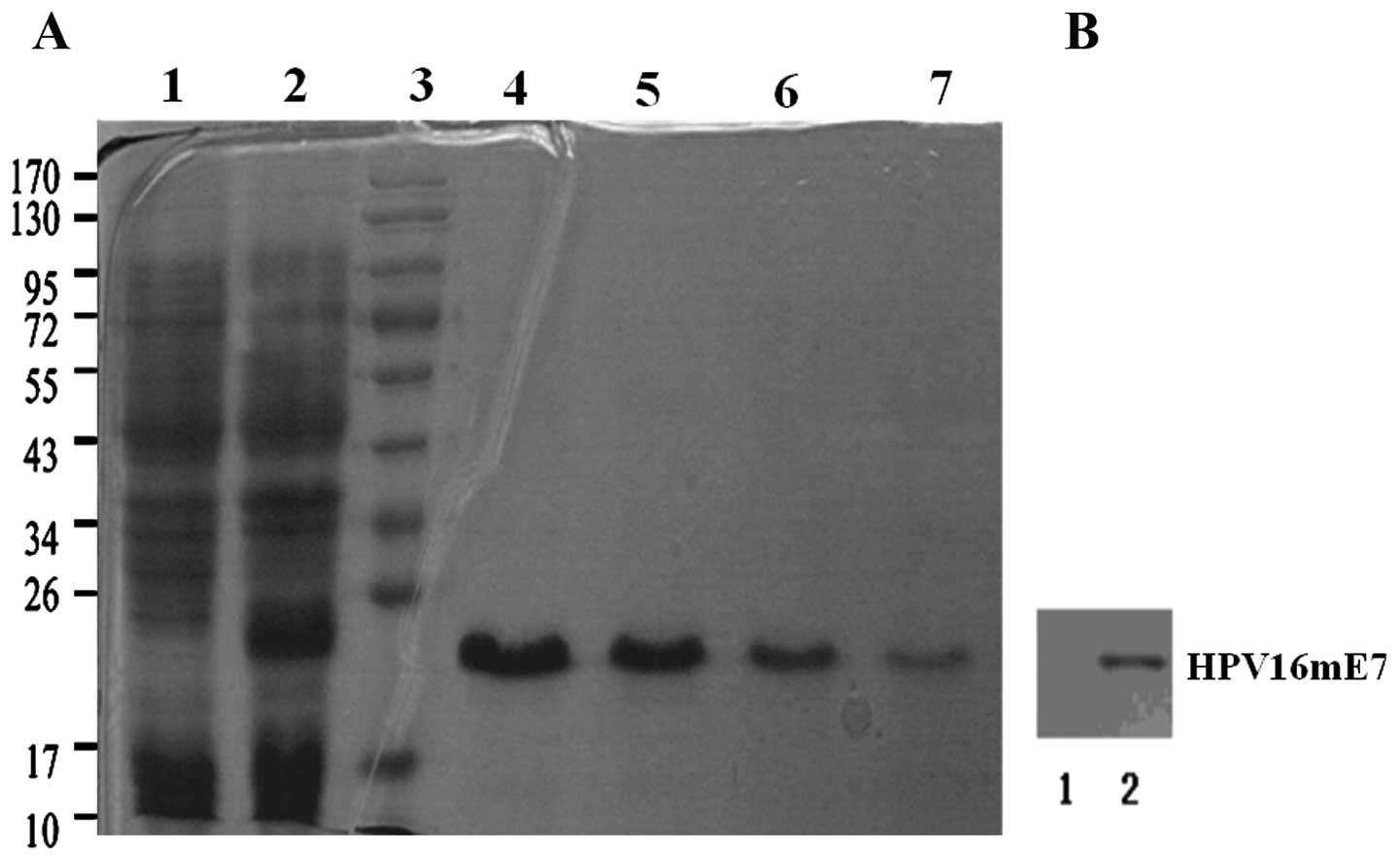 | Figure 1.(A) Expression, purification and
characterization of HPV16mE7. Lane 1, without IPTG induction; lane
2, with IPTG induction; lane 3, marker; lanes 4–7, 60, 100, 150 and
200 mmol/l imidazole elution buffer, respectively. (B) Western blot
analysis for HPV16mE7 using anti-HPV16 E7 monoclonal antibodies.
Lane 1, control; lane 2, HPV16mE7. |
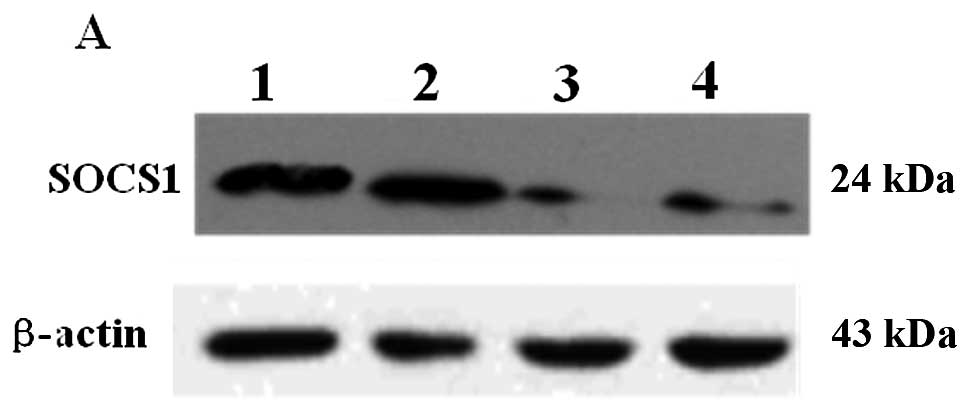
Inhibition of SOCS1 expression
Constructs of ad-shRNASOCS1 and ad-shRNA-mSOCS1 were
verified in B16 cells (Fig. 2A).
Western blot analysis showed that ad-shRNASOCS1 (MOI, 50 or 100)
infection greatly reduced SOCS1 expression compared with infection
by ad-shRNA-mSOCS1 (MOI, 50). Real-time RT-PCR analysis also
confirmed the inhibition of SOCS1 expression in DCs (Fig. 2B). The SOCS1 mRNA level was reduced
significantly by ad-shRNA-SOCS1 but not by ad-shRNA-mSOCS1.
Ad-shRNA-SOCS1 inhibited the SOCS1 mRNA level, however,
ad-shRNA-mSOCS1 failed. Furthermore, the inhibition by
ad-shRNA-SOCS1 at MOI 100 was better than that at MOI 50.
Analysis of DC maturation
Immature DCs (iDCs) were produced from mouse bone
marrow via stimulation with GM-CSF and IL-4 for 7 days and further
stimulation with ad-shRNA-SOCS1. The expression of the surface
markers CD80, CD83 and CD86 were analyzed using flow cytometry
before and after 24 h LPS stimulation and analyzed by SPSS one-way
ANOVA (Table I). Results in
Table I showed that the expression
levels of all three antigens significantly increased by 2–4-fold
during maturation in both ad-shRNA-SOCS1 and ad-shRNA-mSOCS1
infected DCs with respect to control. The former increased 10% more
than the latter. In addition, although LPS greatly induced the
expression of these CD markers in BMDC, it was lower than that of
the ad-shRNASOCS1, and the ad-shRNA-mSOCS1 group.
 | Table I.Percentage of DCs expressing CD80,
CD83 and CD86. |
Table I.
Percentage of DCs expressing CD80,
CD83 and CD86.
| CD80 | CD83 | CD86 |
|---|
| Control | 27.27±6.3 | 38.8±6.5 | 26.5±5.5 |
| LPS | 72.5±8.3a | 78±9.2a | 73.3±7.2a |
|
Ad-shRNA-mSOCS1 |
75.2±8.7a,b |
80.3±6.2a,b |
78.3±4.5a,b |
| Ad-shRNA-SOCS1 |
88.6±7.1a,c |
92.3±4.8a,c |
95.5±2.8a,d |
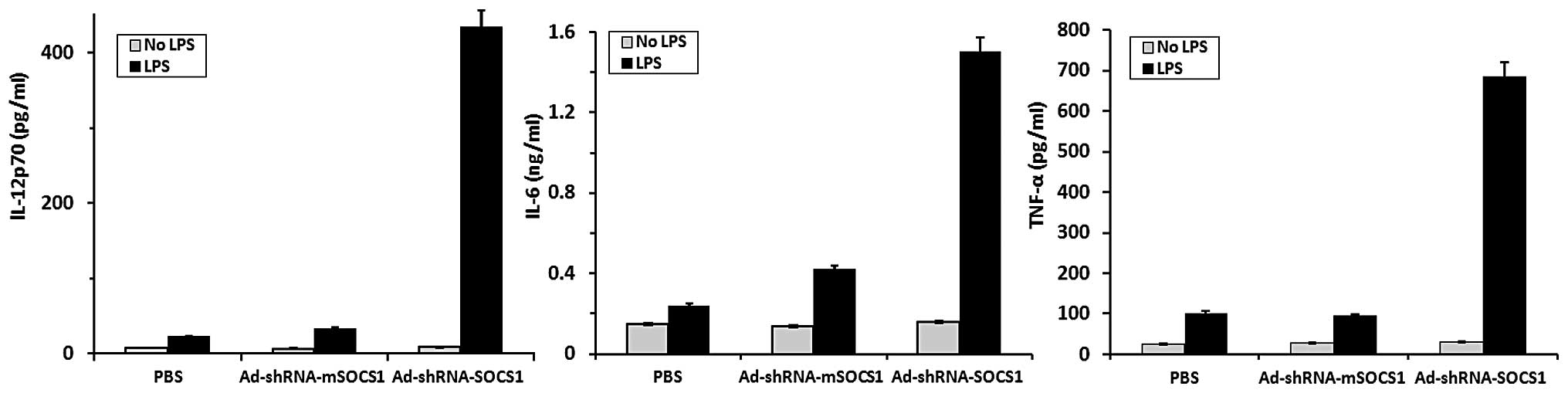
Cytokine ELISA analysis in DCs
Levels of various proinflammatory cytokines such as
IL-12, IL-6 and tumor necrosis factor (TNF)-α (IL-12p70, IL-6,
TNF-α) induced by HPV16mE7-pulsed DCs with or without LPS
stimulation were quantitated by ELISA analysis kits (Fig. 3). Silencing SOCS1 in
HPV16mE7-pulsed DCs drastically enhanced the production of
cytokines, such as IL-12p70, IL-6 and TNF-α with LPS stimulation,
but not in the absence of LPS stimulation. By contrast, the PBS
control group and the ad-shRNA-mSOCS1 group expressed far less
cytokines with or without LPS stimulation.
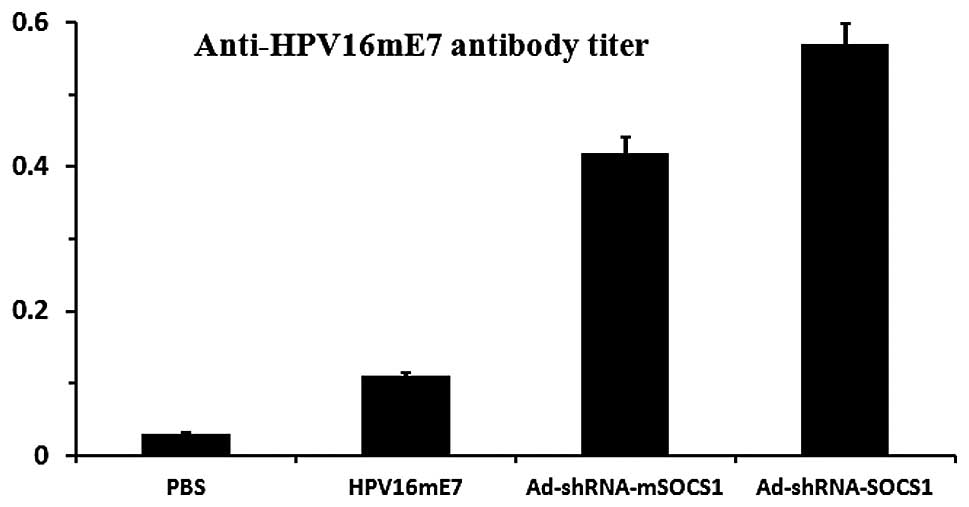
Immune antibodies induced by different
DC vaccines
Four mice from each group were immunized using
105 DC-treated vaccine or 5 μg HPV16 E7 in order
to determine the HPV16 E7 antibody level induced by different DC
vaccines. After two weeks, the HPV16 E7 antibody level in the serum
was measured by ELISA. Antibody responses which were induced by
different vaccines were compared with PBS controls.
DCs-ad-shRNA-SOCS1 induced the strongest response, with antibody
titers 5- and 1.5-fold higher than those of the HPV16mE7 vaccine
and DC-ad-sh-mSOCS1, respectively (Fig. 4). Our results suggest that
SOCS1-silenced HPV16mE7-pulsed DCs promoted the production of
specific antibodies.
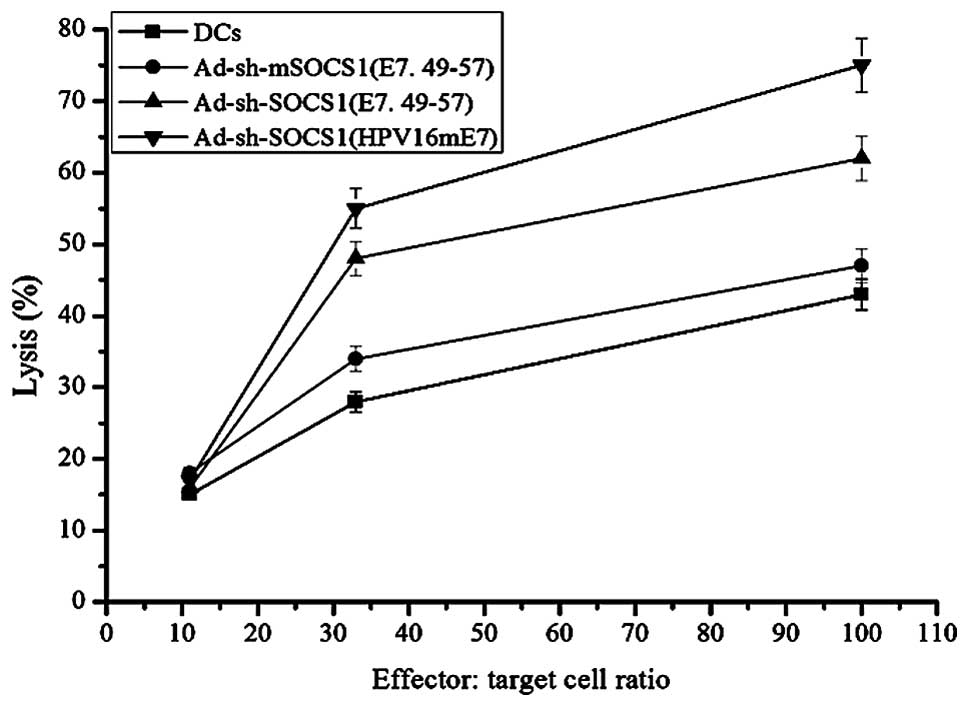
LDH release assay
C57BL/6 mice (four per group) were immunized at the
footpads with DCs, ad-shRNA-mSOCS1 pulsed with E7.49-57,
ad-shRNA-SOCS1 pulsed with E7.49-57 and ad-shRNA-SOCS1 pulsed with
HPVmE7, respectively, to determine whether DC vaccines could induce
CTL responses against HPV16 E7. After 7 days of post-immunization,
pooled splenocytes from each group were restimulated with 1
μg/ml E7.49-57 for 7 days. Their specific lytic activities
against TC-1 cells at 100:1, 33:1 and 11:1 E/T ratios were assayed
using an LDH release assay (Fig.
5). Immunization of C57BL/6 mice with DC-ad-shRNA-SOCS1
(HPVmE7) induced the strongest specific CTL responses to TC-1 cells
than that pulsed with E7.49-57 and controls (DCs and
ad-shRNA-mSOCS1 pulsed with E7.49-57), respectively. The lysis
effect induced by ad-shRNA-SOCS1 (E7.49-57) and ad-shRNA-SOCS1
(HPVmE7) were not linear, but it increased with effector:target
ratio compared with the linear increase of the controls.
ELISA analysis and ELISPOT of IFN-γ
expression
ELISA analysis was used for the detection of IFN-γ
in the supernatant of the same restimulated splenocytes. C57BL/6
mice (four per group) were immunized twice in 1-week interval with
PBS, DCs, DC-ad-shRNA-SOCS1, or DC-ad-shRNA-mSOCS1, respectively.
The DCs were all pulsed by HPV16mE7. Seven days post-immunization,
pooled splenocytes from each group were re-stimulated with E7.49-57
for 7 days. The released IFN-γ in the supernatant at 100:1, 33:1
and 11:1 E/T ratio was assayed (Fig.
6A). Results showed that the expression level of IFN-γ was
greatly enhanced by silencing SOCS1, whereas similar effect was
observed in ad-shRNA-mSOCS1 and non-treated DC cells. ELISPOT of
IFN-γ in response to in vitro stimulation with HPV16mE7 was
quantified to estimate the frequencies of HPV16-E7-specific
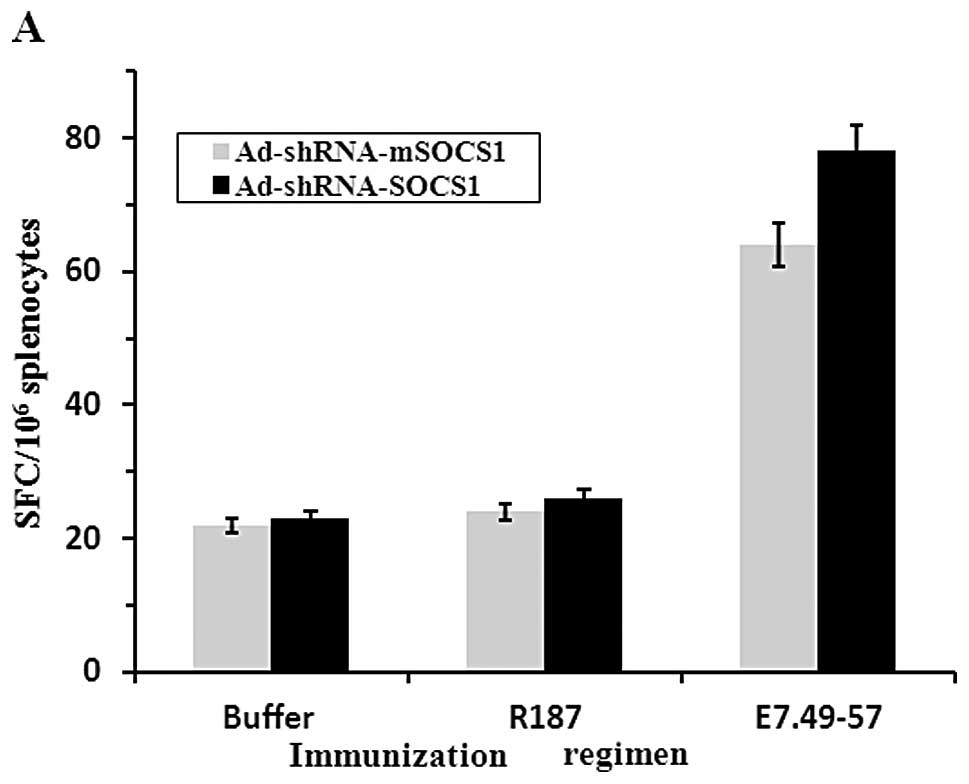
splenocytes in mice immunized with DC vaccines (Fig. 6B). The detection efficiency of the
positive control, PMA (5 ng/ml) was 95% (data not shown). Our
results also showed that ad-shRNA-SOCS1 group had a significant
effect in inducing IFN-γ expression when stimulated by E7.49-57
peptide. Further, Ad-shRNA-mSOCS1 group expressed high level of
IFN-γ, compared with the buffer group, and the R187 group.
Efficacy of the DC vaccine in treating
mice bearing tumors
The ability of the DC-ad-shRNA-SOCS1 vaccine to
treat mice bearing tumors was investigated in the groups of 10
C57BL/6 mice which received 5×105 TC-1 tumor cells. At
day 9, all mice had palpable tumors and therapeutic treatments were
initiated with DC-ad-shRNA-SOCS1, DC-ad-shRNA-mSOCS1, DCs and PBS,
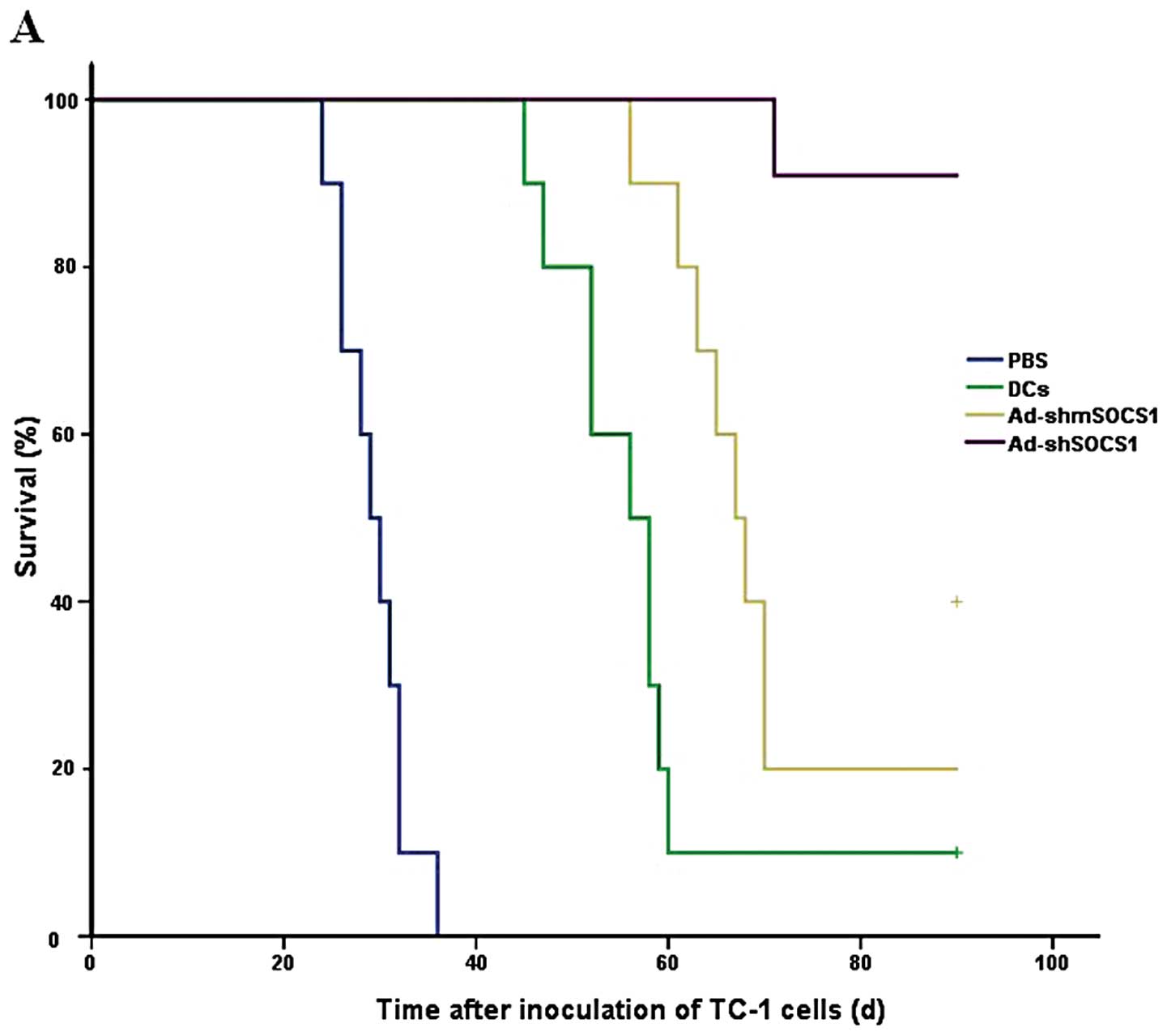
respectively. The vaccination with DC-ad-shRNASOCS1 (Fig. 7A) showed 100% survival rate until
day 68, while all mice died on days 36, 62 and 72 in the PBS, DCs
and DC-ad-shRNA-mSOCS1 group, respectively. Tumor volume and time
experiment showed that DCs-ad-shRNASOCS1 vaccination significantly
decreased the tumor volume compared with vaccination with controls
(Fig. 7B). The experiments were
repeated, producing a composite p-value (p<0.01). Although
therapeutic vaccination could not always eradicate the tumor
completely, the survival rate was significantly enhanced in mice
for >80 days (Fig. 7).
Discussion
In the present study, the HPV16mE7 protein-pulsed DC
vaccine was based on the E7 oncogene of HPV16, which is thought as
the gold standard for cervical cancer immune therapy, with some
therapeutic vaccines under development in preclinical models
(27). Experiments on the DNA
vaccination with E7 oncogene, however, still harbor the risk of
transformation for the cells that receive and express the oncogene
(28,29). In this study, HPV-16E7 gene was
modified to resolve the interference with binding to the host cell
Rb protein and by E7 gene mutation to reduce the transformation
capacity (22). Western blot
analysis (Fig. 1B) and anti-HPV16
E7 antibody titer test (Fig. 4)
demonstrated that the mutated HPV16 E7 possessed antigenicity of
wild-type HPV16 E7. Our results (Figs.
3 and 7) confirmed that the
HPV16mE7 based vaccine strategies dramatically enhanced the
expression levels of various cytokines, retarded the tumor growth
and prolonged the survival time of the mouse models in therapeutic
experiments and improved CTL mediated lysis compared with the
controls. In addition, vaccination with the modified HPV16 E7
showed obvious advantages in inducing immune responses compared to
vaccination with the wild-type as described in a previous study
(23). Thus, the modified HPV16 E7
protein-pulsed DC vaccine could be a safe and effective vaccine in
treating HPV-associated diseases.
The antigen-presenting DCs were able to induce
robust cell-mediated immunity capable of attacking and eliminating
abnormal antigen-bearing cells. DC vaccine pulsed with the HPV16 E7
oncoprotein exhibits significant advantages of potentially
presenting multiple immunogenic CTL and antibody responses
(30–33). Clinical studies in patients with
human papillomavirus cervical cancers demonstrated by vaccinating
with HPV E7-pulsed dendritic cells that HPVE7-loaded DC vaccination
is safe and immunogenic for stage IB or IIA cervical cancer
patients (32). DC vaccination was
well tolerated and no significant toxicities were recorded. Similar
results were obtained by Ferrara et al (33). How to fully activate DCs as a
vaccine is the key to cancer immunotherapy. One strategy to achieve
immunotherapy is to inhibit the negative regulatory pathways during
DC activation which enhances cancer immunity and breaks
self-tolerance (8). In this study,
we knocked down SOCS1, a negative signaling regulator of various
cytokines in DCs. The efficiency of SOCS1 silencing was verified in
B16 and DCs (Fig. 2) and the
inhibition by ad-shRNA-SOCS1 at MOI 100 was better than that at MOI
50, indicating a dosage-dependent manner. Silencing SOCS1 also
enhanced the expression levels of IL-12p, IL-6, TNF-α and IFN-γ
(Figs. 3 and 6). Our finding is consistent with
previous reports that SOCS1 represents an inhibitory mechanism for
qualitatively and quantitatively controlling antigen presentation
by DCs and the magnitude of adaptive immunity (11,12,16–18).
Hence, this study underscores the critical role of SOCS1 silencing
in the stimulation of DC immune response and indicates that SOCS1
silencing can be applied for a large number of diseases in addition
to HPV diseases.
An important finding of this study is that
DCs-ad-shRNASOCS1 induced specific anti-HPV16mE7 antibody and CTL
responses. Although the mechanism on how the SOCS1 silencing
induced the priming of antigen-specific CTLs is not clear,
SOCS1-restricted DCs greatly enhanced the secretion of
pro-inflammatory cytokines, such as IL-12, IL-6 and TNF-α (Fig. 3) and promoted the production of
specific antibodies (Fig. 4).
Animal experiments demonstrated that the vaccine with SOCS1
silencing possessed a high ability to induce CTLs (Fig. 5) and enhanced IFN-γ expression
(Fig. 6B). These data are
consistent with observations of Hashimoto et al in mouse DCs
(34) and the findings of the CTL
response generated against HIV (12). Song et al found that SOCS1
silencing in DCs enhanced production of a mixed pattern of Th1-and
Th2-polarizing cytokines (12).
Further, Kelvin et al pointed out that persistent antigen
presentation of DCs to induce pathological autoimmune responses
against normal tissues and tumor could be achieved by silencing
SOCS1 to unleash the signaling of IL-12 (11). Previous studies by Hong et
al showed that the addition of anti-IL-6 antibody into the
DC:T-cell co-culture also blocked the immuno-stimulatory function
of siSOCS1 DCs, albeit with a lower efficiency (17). Interestingly, the study also found
that the control vector Ad-shRNA-mSOCS1 showed high levels of DC
markers, anti-HPV antibody and IFN-γ-producing splenocytes compared
with DCs-ad-shRNASOCS1 (Table I,
Figs. 4 and 6A), indicating a non-specific stimulatory
effect of siRNA molecules via the activation of TLR signaling and
some cellular genes (35–37) or possibly due to the toxicity of
the replication-incomplete adenovirus vector-specific response
(38,39), or both. However, the level of
non-specific immune caused by SOCS1 silencing was low compared with
that by CTLA-4 (another immunosuppressive) (16) and could achieve a more
antigen-specific antitumor response. Results in Fig. 6 indicate that SOCS1 silencing had
no obvious function in inducing IFN-γ-producing splenocytes, but
significantly enhanced IFN-γ expression in these cells. The
possible explanation for the marginal difference of IFN-γ-producing
cells between DCs-ad-shRNA-SOCS1 and DCs-ad-shRNA-mSOCS1 in
Fig. 6A was that silencing SOCS1
has limited functions in promoting antigen presentation and
splenocyte activation, which was in accordance with the results in
Table I. However, there is no
direct evidence that SOCS1 is involved in antigen presentation and
splenocytes activation or what part it takes. More research is
needed for further explanation. In addition, our study confirmed
that DCs with SOCS1 silencing were hyper-responsive to
lipopolysaccharide (LPS) (Fig. 3),
which was thought to interact with Toll-like receptor (TLR) 4 for
signaling and involved in the stimulation of DC maturation and
proinflammatory cytokine expression. Thus, the immune responses
induced by DC-adshRNA-SOCS1 may be a collective result of specific
and non-specific immunity as well as the enhanced sensitivity to
LPS as discussed by Song et al (12).
This study, to our knowledge, first underscores the
immunotherapeutic effect by combining the HPV16mE7 protein and
silencing SOCS1 in DCs as a vaccine in treating HPV. The vaccine
showed significant immune effect by inducing complex immune
responses against HPV and greatly prolonged the lifetime of the
mouse models. Thus, the strategy of silencing SOCS1 in HPV16mE7
protein-pulsed DCs may open a new and alternative avenue to develop
safe and effective therapeutic HPV and other infectious disease
vaccines.
Acknowledgements
We would like to thank Dr Xiaosong
Song and other members of Huang Lab for suggestions on the study
and assistance in revising the manuscript. This work was supported
in part by funding from Shenzhen Municipal Government and Bureau of
Science, Technology and Information (grants 2006464, 200712,
SG200810150043A, CXB201005260070A, and CXB201104220043A to L.H.;
and JC200903180532A to Y.Z.), RFDP (20090002120055 to Y.Z.), and
Nanshan District Bureau of Science and Technology.
References
|
1.
|
Jemal A, Bray F and Center MM: Global
cancer statistics. CA Cancer J Clin. 61:69–90. 2011. View Article : Google Scholar
|
|
2.
|
Berzofsky JA, Terabe M, Oh SK, Belyakov
IM, Ahlers JD, Janik JE and Morris JC: Progress on new vaccine
strategies for the immunotherapy and prevention of cancer. J Clin
Invest. 113:1515–1525. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
3.
|
Ali M and Monk BJ: Vaccines against human
papillomavirus and cervical cancer: promises and challenges.
Oncologist. 10:528–538. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
4.
|
Kanodia S, Da Silva DM and Kast WM: Recent
advances in strategies for immunotherapy of human
papillomavirus-induced lesions. Int J Cancer. 122:247–259. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
5.
|
Psyrri A and Daniel D: Human
papillomavirus in cervical and head-and-neck cancer. Nat Clin Pract
Oncol. 5:24–31. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
6.
|
Brinton LA: Epidemiology of cervical
cancer: overview. IARC Sci Publ. 119:3–23. 1992.PubMed/NCBI
|
|
7.
|
Zur Hausen H: Papillomaviruses and cancer:
from basic studies to clinical application. Nat Rev Cancer.
2:342–350. 2002.PubMed/NCBI
|
|
8.
|
Kobayashi T and Yoshimura A: Keeping DCs
awake by putting SOCS1 to sleep. Trends Immunol. 26:177–179. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
9.
|
Santin AD, Hermonat PL, Ravaggi A,
Chiriva-Internati M, Zhan D and Pecorelli S: Induction of human
papillomavirus-specific CD4(+) and CD8(+) lymphocytes by E7-pulsed
autologous dendritic cells in patients with human papillomavirus
type 16- and 18-positive cervical cancer. J Virol. 73:5402–5410.
1999.PubMed/NCBI
|
|
10.
|
Kim TW, Lee JH, He L, Boyd DA, Hardwick JM
and Hung CF: Modification of professional antigen-presenting cells
with small interfering RNA in vivo to enhance cancer vaccine
potency. Cancer Res. 65:309–316. 2005.PubMed/NCBI
|
|
11.
|
Kelvin EK, Song XT, Aldrich M, Huang XF
and Chen SY: SOCS1 restricts dendritic cells’ ability to break self
tolerance and induce antitumor immunity by regulating IL-12
production and signaling. J Clin Invest. 116:90–100. 2006.
|
|
12.
|
Song XT, Evel-Kabler K, Rollins L, Aldrich
M, Gao F, Huang XF and Chen SY: An alternative and effective HIV
vaccination approach based on inhibition of antigen presentation
attenuators in dendritic cells. PLoS Med. 3:76–93. 2006.PubMed/NCBI
|
|
13.
|
Akita H, Kogure K, Moriguchi R, Nakamura Y
and Higashi T: Nanoparticles for ex vivo siRNA delivery to
dendritic cells for cancer vaccines: Programmed endosomal escape
and dissociation. J Control Release. 143:311–317. 2010. View Article : Google Scholar
|
|
14.
|
Kubo M, Hanada T and Yoshimura A:
Suppressors of cytokine signaling and immunity. Nat Immunol.
4:1169–1176. 2003. View
Article : Google Scholar : PubMed/NCBI
|
|
15.
|
Alexander WS and Hilton DJ: The role of
suppressors of cytokine signaling (SOCS) proteins in regulation of
the immune response. Annu Rev Immunol. 22:503–529. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
16.
|
Shen L, Evel-Kabler K, Strube R and Chen
SY: Silencing of SOCS1 enhances antigen presentation by dendritic
cells and antigen-specific anti-tumor immunity. Nat Biotechnol.
22:1546–1553. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
17.
|
Hong B, Ren W, Song XT and Evel-Kabler K:
Human suppressor of cytokine signaling 1 controls immunostimulatory
activity of monocyte-derived dendritic cells. Cancer Res.
69:8076–8084. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
18.
|
Hanada T, Yoshida H, Kato S, Tanaka K and
Masutani K: Suppressor of cytokine signaling-1 is essential for
suppressing dendritic cell activation and systemic autoimmunity.
Immunity. 19:437–450. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
19.
|
Guenterberg KD, Lesinski GB, Mundy-Bosse
BL and Karpa VI: Enhanced anti-tumor activity of interferon-alpha
in SOCS1-deficient mice is mediated by CD4+ and
CD8+ T cells. Cancer Immunol Immunother. 60:1281–1288.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
20.
|
Carow B, Ye Xq, Gavier-Widén D, Bhuju S
and Oehlmann W: Silencing suppressor of cytokine signaling-1
(SOCS1) in macrophages improves Mycobacterium tuberculosis
control in an IFN-γ-dependent. J Biol Chem. 286:26873–26887. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
21.
|
Münger K, Basile JR, Duensing S, Eichten A
and Gonzalez SL: Biological activities and molecular targets of the
human papillomavirus E7 oncoprotein. Oncogene. 20:7888–7898.
2001.PubMed/NCBI
|
|
22.
|
Cassetti MC, McElhiney SP, Shahabi V,
Pullen JK and Le Poole IC: Antitumor efficacy of Venezuelan equine
encephalitis virus replicon particles encoding mutated HPV16E6 and
E7 genes. Vaccine. 22:520–527. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
23.
|
Brinkman JA, Xu XM and Kast WM: The
efficacy a DNA vaccine containing inserted and replicated regions
of the E7 gene for treatment of HPV 16 induced tumors. Vaccine.
25:3437–3444. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
24.
|
Feltkamp MC, Smits HL, Vierboom MP,
Minnaar RP, de Jongh BM and Drijfhout JW: Vaccination with
cytotoxic T lymphocyte epitope containing peptide protects against
a tumor induced by human papillomavirus type 16-transformed cells.
Eur J Immunol. 23:2242–2249. 1993. View Article : Google Scholar
|
|
25.
|
Liu HG, Fan ZP, Chen WW, Yang HY, Liu QF
and Zhang H: A mutant HBs antigen (HbsAg) 183–191 epitope elicits
specific cytotoxic T lymphocytes in acute hepatitis B patients.
Clin Exp Immunol. 151:441–447. 2008.PubMed/NCBI
|
|
26.
|
Zheng Y, Zhang Y, Ma Y, Wan J and Shi C:
Enhancement of immunotherapeutic effects of HPV16E7 on cervical
cancer by fusion with CTLA4 extracellular region. J Microbiol.
46:728–736. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
27.
|
Brinkman JA, Caffrey AS and Muderspach LI:
The impact of anti HPV vaccination on cervical cancer incidence and
HPV induced cervical lesions: consequences for clinical management.
Eur J Gynaecol Oncol. 26:129–142. 2005.PubMed/NCBI
|
|
28.
|
Münger K, Phelps WC, Bubb V and Howley PM:
The E6 and E7 genes of the human papillomavirus type 16 together
are necessary and sufficient for transformation of primary human
keratinocytes. J Virol. 63:4417–4421. 1989.PubMed/NCBI
|
|
29.
|
Phelps WC, Yee CL and Munger K: The human
papillomavirus type 16 E7 gene encodes transactivation and
transformation functions similar to those of adenovirus E1A. Cell.
53:539–547. 1988. View Article : Google Scholar
|
|
30.
|
Nonn M, Schinz M, Zumbach K, Pawlita M and
Schneider A: Dendritic cell-based tumor vaccine for cervical cancer
I: in vitro vaccination with recombinant protein pulsed
dendritic cells induces specific T cells to HPV16 E7 and HPV18 E7.
J Cancer Res Clin Oncol. 129:511–520. 2003.PubMed/NCBI
|
|
31.
|
Santin AD, Bellone S, Gokden M and Cannon
MJ: Vaccination with HPV-18 E7-pulsed dendritic cells in a patient
with meta-static cervical cancer. N Engl J Med. 346:1752–1753.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
32.
|
Santin AD, Bellone S, Palmieri M, Zanolini
A, Ravaggi A and Siegel ER: Human papillomavirus type 16 and 18
E7-pulsed dendritic cell vaccination of stage IB or IIA cervical
cancer patients: a phase I escalating-dose trial. J Virol.
82:1968–1979. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
33.
|
Ferrara A, Nonn M, Sehr P, Schreckenberger
C and Pawlita M: Dendritic cell-based tumor vaccine for cervical
cancer II: results of a clinical pilot study in 15 individual
patients. J Cancer Res Clin Oncol. 129:521–530. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
34.
|
Hashimoto M, Ayada T, Kinjyo I, Hiwatashi
K and Yoshida H: Silencing of SOCS1 in macrophages suppresses tumor
development by enhancing antitumor inflammation. Cancer Sci.
100:730–736. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
35.
|
Judge AD, Sood V, Shaw JR, Fang D and
McClintock K: Sequence-dependent stimulation of the mammalian
innate immune response by synthetic siRNA. Nat Biotechnol.
23:457–462. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
36.
|
Hornung V, Guenthner-Biller M, Bourquin C
and Ablasser A: Sequence-specific potent induction of IFN-alpha by
short interfering RNA in plasmacytoid dendritic cells through TLR7.
Nat Med. 11:263–270. 2005. View
Article : Google Scholar : PubMed/NCBI
|
|
37.
|
Sledz CA, Holko M, de Veer MJ and
Silverman RH: Activation of the interferon system by
short-interfering RNAs. Nat Cell Biol. 5:834–839. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
38.
|
Basak SK, Kiertscher SM and Harui A:
Modifying adenoviral vectors for use as gene-based cancer vaccines.
Viral Immunol. 17:182–196. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
39.
|
Benihoud K, Yeh P and Perricaudet M:
Adenovirusvectors for gene delivery. Biotechnology. 10:440–447.
1999.PubMed/NCBI
|