Introduction
Deep-sea water (DSW) defined as sea water from a
depth of more than 200 meters is rich in minerals such as calcium
(Ca), magnesium (Mg), potassium (K), sodium (Na), and zinc (Zn).
Hence, DSW has been widely utilized in the fields of aqua-culture,
agriculture, food processing and cosmetics (1). More recently, the scientific
community has begun to establish the health benefits of DSW which
include lowering of blood cholesterol and preventing obesity and
diabetics (2–4). However, these studies are preliminary
yet and applications of DSW in medical fields require more
scientific evidence for its biological activities. Previously, we
showed the inhibitory effects of DSW on carcinogen-induced
expression of cyclooxygenase-2 (COX-2), transforming growth
factor-β (TGF-β), and urokinase plasminogen activator (uPA) in
HT-29 colorectal cancer cells (5).
It is presumed that these antitumor activities of DSW may be
derived from the combined ionic action of several minerals such as
calcium, magnesium and potassium in DSW. Among these minerals,
calcium and magnesium may play important roles in mediating the
inhibition of metastasis because they are the main mineral ions
present in DSW and it was found that deficiency of magnesium and/or
calcium levels has been linked to increased risks of cancer and
metastasis (6–10).
Metastasis is a major cause of lethality in cancer
patients rather than the primary tumors. Recurrent metastatic
disease is estimated to develop in 30–75% of patients undergoing
surgery and adjuvant treatment. The median survival of patients
with metastatic breast cancer is about 2 years after metastasis has
been detected (11). Thus,
understanding the mechanism involved in the regulation of
metastasis is of major importance for improving cancer survival.
The mechanism underlying tumor metastasis remains unclear but
considerable focus has been directed towards characterizing
metastasis genes in the context of relevant signaling pathways.
Several studies have demonstrated the implication of
TGF-β signaling in cell invasion and metastasis. Although TGF-β has
a complex role in tumor progression by acting as a tumor suppressor
or tumor promoter depending on the tumor type and the tumor stage,
the clinical and experimental evidence implies the involvement of
TGF-β in the metastatic processes (12). Moreover, elevated levels of plasma
TGF-β have been detected in patients with cancer and predicts early
metastasis (13,14). It has been shown that activation of
TGF-β signaling accelerates tumor growth, migration and metastasis
whereas blockade of TGF-β signaling reduces the development of bone
metastasis in animal models (15,16).
Recent studies identified that alterations in TGF-β
signaling regulated Wingless-related mouse mammary tumor virus
(MMTV) integration site 5A (Wnt5a) gene expression. Wnt5a is a
non-canonical signaling member of the Wnt family, activation of
which is independent of β-catenin. In contrast, Wnt1 and Wnt3a are
canonical Wnt signaling members, that bind to Wnt receptors such as
Frizzled (FZD) and low-density lipoprotein receptors 5/6 (LRP5/6)
to promote stabilization and nuclear translocation of β-catenin,
resulting in the activation of target genes (17). It is found that TGF-β directly
upregulated Wnt5a expression through the Smad complex and the Wnt5a
promoter reserved Smad binding sites (18). Wnt5a is also associated with poor
prognosis and early invasive breast cancer, suggesting the
involvement of Wnt5a in malignant transformation and tumor
progression (19,20). Its overexpression is observed in a
variety of cancers in comparison to the respective benign tissues.
Moreover, a study analyzing gene expression profiles with cutaneous
melanomas revealed that Wnt5a expression was one of the most robust
markers for highly aggressive tumors, while it was underexpressed
in the less motile tumors (21).
Transfection of Wnt5a in non-invasive melanoma cells expressing low
levels of Wnt5a resulted in increased invasiveness via PKC
activation (22). Although a role
for Wnt5a in mediating motility is not entirely clear, it is found
that the treatment with recombinant Wnt5a upregulates expression of
CD44 (23), which is a tumor
homing and metastasis antigen associated with tumor cell invasion
(24).
Expression of a gene other than CD44 is also
involved in Wnt5a-dependent invasion and metastasis. Recently,
binding of Wnt5a to Ror2, a member of the Ror-family of
receptor-tyrosine kinases, was reported to regulate the expression
of matrix metalloproteinases (MMPs) (25). MMPs belong to a family of
zinc-dependent enzymes capable of degrading extracellular matrix,
and thus mediate the physiological processes involving
extracellular matrix remodeling, such as wound healing,
angiogenesis and tumor progression (26). Direct evidence for involvement of
MMPs in tumor invasion was revealed through studies in which MMP-9
knockout mice had reduced melanoma tumor progression and
angiogenesis (27).
In this study, we have evaluated the potential
health benefits of DSW on the inhibition of the metastatic
potential in relation to tumor cell migration, TGF-β and its
relevant signaling pathway, such as Wnt5a, CD44 and MMPs, by using
two human breast cancer cell lines (MDA-MB-231 and MCF-7).
MDA-MB-231 cells are representative of triple negative breast tumor
characterized by the absence of estrogen receptor (ER),
progesterone receptor (PR), and Her2. Recent studies analyzing gene
expression profiles reveal that the triple negative tumor subtypes
highly express genes regulating tumor migration, invasion, and
differentiation, including the TGF-β signaling pathway and the
Wnt-signaling pathway (28,29).
This triple negative tumor subtype is mainly correlated to poor
outcomes, showing the worst overall and disease-free survival rates
due to its metastatic and invasive features. MDA-MB-231 cells
exhibited invasive/metastatic tumor features with rapid migratory
ability and relatively high endogenous expression of TGF-β and
Wnt5a (30). In contrast to
MDA-MB-231 cells, MCF-7 cells are non-invasive ER-positive breast
cancer cells, representing the ER/PR positive luminal subtype.
Despite the fact that ER/PR positive luminal subtypes have been
found to be associated with the most favorable outcomes, tumor
recurrent disease in patients undergoing chemotherapy exhibited
metastatic tumor features. Thus, we used MDA-MB-231 cells to
evaluate whether DSW directly inhibits the invasive behavior of
tumor features in breast cancer cells. Besides, the non-invasive
MCF-7 cells were used with treatment of
12-O-tetradecanoylphorbol-13-acetate (TPA) to examine the
preventive effects of DSW on TPA-induced invasive/metastatic tumor
characteristics.
Materials and methods
Preparation of deep-sea water
Marine Deep Ocean Water Application Center in Korea
Institute of Ocean Science and Technology (Goseong, Korea) provided
desalinated water and deep-sea water of hardness 4,000. The ratio
of magnesium to calcium was 3:1 and the hardness of DSW was
determined from the concentration of calcium and magnesium ions
within DSW. The following equation was used to calculate the
hardness of DSW in this study: Hardness (mg/l) = magnesium (mg/l) ×
4.1 + calcium (mg/l) × 2.5.
To prepare DSW containing media, DMEM powder was
dissolved in hardness 4,000 DSW and diluted with distilled water to
obtain hardness 1,500 DSW media. It was further serially diluted
with desalinated media to prepare hardness 200–800 media.
Cell culture
MCF-7 and MDA-MB-231 human breast cancer cell lines
were purchased from the Korean Cell Line Bank (Seoul, Korea). MCF-7
cells were cultured in DMEM (Gibco-BRL, Rockville, MD, USA)
supplemented with 10% fetal bovine serum and 10 μg/ml
insulin, while MDA-MB-231 cells were cultured in DMEM supplemented
with 10% fetal bovine serum without insulin.
RNA isolation and reverse transcriptase
polymerase chain reaction (RT-PCR)
MCF-7 cells were seeded on 6-well plates and
cultured for 24 h. After 24 h serum starvation with serum-free
media, cells were treated with conditioned media containing various
hardness of DSW for 2 h prior to adding 100 nM TPA (Sigma, St.
Louis, MO, USA). Cells were further incubated for 24 h and
harvested for RNA isolation. For treatment of MDA-MB-231 cells with
DSW, cells were cultured for 3 days in the presence of conditioned
media containing various hardness of DSW and 2% fetal bovine serum
and harvested for RNA isolation. Total RNA was isolated using
easy-BLUE™ Total RNA Extraction kit (iNtRON Biotechnology Inc.,
Sungnam, Korea). cDNA synthesis and PCR reactions were performed as
previously described (5). Primer
sequences and PCR conditions for target genes are presented in
Table I. Densitometric analysis
was performed using Scion Image (Scion Corporation, Frederick, MD,
USA).
 | Table I.Primer sequences and annealing
temperatures for RT-PCR. |
Table I.
Primer sequences and annealing
temperatures for RT-PCR.
| Gene | Primer | Annealing
temperature (°C) |
Authors/(Refs.) |
|---|
| MMP-9 | Forward:
5′-TTCATCTTCCAAGGCCAATC-3′
Reverse: 5′-CTTGTCGCTGTCAAAGTTCG-3′ | 50 | Jang et al
(45) |
| MMP-2 | Forward:
5′-TCGCCCATCATCAAGTTC-3′
Reverse: 5′-GTGATCTGGTTCTTGTCC-3′ | 52 | Kang et al
(46) |
| TGF-β | Forward:
5′-CGTCTGCTGAGGAGGCTCAAGTTA-3′
Reverse: 5′-CAGCCGAGGTCCTTGCGGAA-3′ | 55 | |
| uPA | Forward:
5′-CCAATTAGGAAGTGTAACAGC-3′
Reverse: 5′-GCCAAGAAAGGGACATCTATG-3′ | 55 | Kang et al
(46) |
| uPAR | Forward:
5′-CACAAAACTGCCTCCTTCCTA-3′
Reverse: 5′-AATCCCCGTTGGTCTTACAC-3′ | 58 | Li and Sarkar
(47) |
| Wnt5a | Forward:
5′-GGGAGGTTGGCTTGAACATG-3′
Reverse: 5′-GAATGGCACGCAATTACCTT-3′ | 58 | Suzuki et al
(48) |
| Wnt3a | Forward:
5′-TGAACAAGCACAACAACGAG-3′
Reverse: 5′-CAGTGGCATTTTTCCTTCC-3′ | 55 | Suzuki et al
(48) |
| β-actin | Forward:
5′-CAAGAGATGGCCACGGCTGCT-3′
Reverse: 5′-TCCTTCTGCATCCTGTCGGCA-3′ | 58 | Takeuchi et
al (49) |
| GAPDH | Forward:
5′-ATCCCATCACCATCTTCCAG-3′
Reverse: 5′-TTCTAGACGGCAGGTCAGGT-3′ | 58 | |
Wound healing assay
MCF-7 cells were seeded on 6-well plates coated with
collagen IV and grown to reach confluence. Monolayers of cells were
scratched with a 200 μl yellow pipette tip to create wounds.
After washing cells with PBS to remove cell debris, cells were
treated with conditioned media containing various hardness of DSW
for 1 h prior to addition of 100 nM TPA, and pictures were taken at
time 0, and at indicated time points. The wound healing assay for
MDA-MB-231 cells was performed as described above, but stimulation
of migration by 100 nM TPA was omitted due to the inherent invasive
properties of the cells.
Gelatin zymography assay
Conditioned medium was electrophoresed in an 8%
sodium dodecyl sulfate (SDS)-polyacrylamide gel containing 0.1%
(v/v) gelatin. The gel was then washed at room temperature for 1 h
with 0.25% Triton X-100 and subsequently incubated at room
temperature for 24 h in reaction buffer containing 5 mM
CaCl2, 0.04% NaN3 and 50 mM Tris-HCl. The gel
was stained with 0.2% Coomassie Brilliant Blue and proteolysis was
detected as a white zone in a dark blue field.
Flow cytometry analysis
Cells were trypsinized and washed in 2% FBS in PBS.
Cells were incubated with CD44-FITC (BD Biosciences, San Diego, CA,
USA) for 30 min on ice and washed with 2% FBS in PBS. Cells were
resuspended in a final volume of 500 μl buffer for analysis.
FACS analysis was performed on a FACSCalibur II (BD Biosciences).
Unstained and single color-labeled samples were used to calibrate
the analyzer for each experiment.
Results
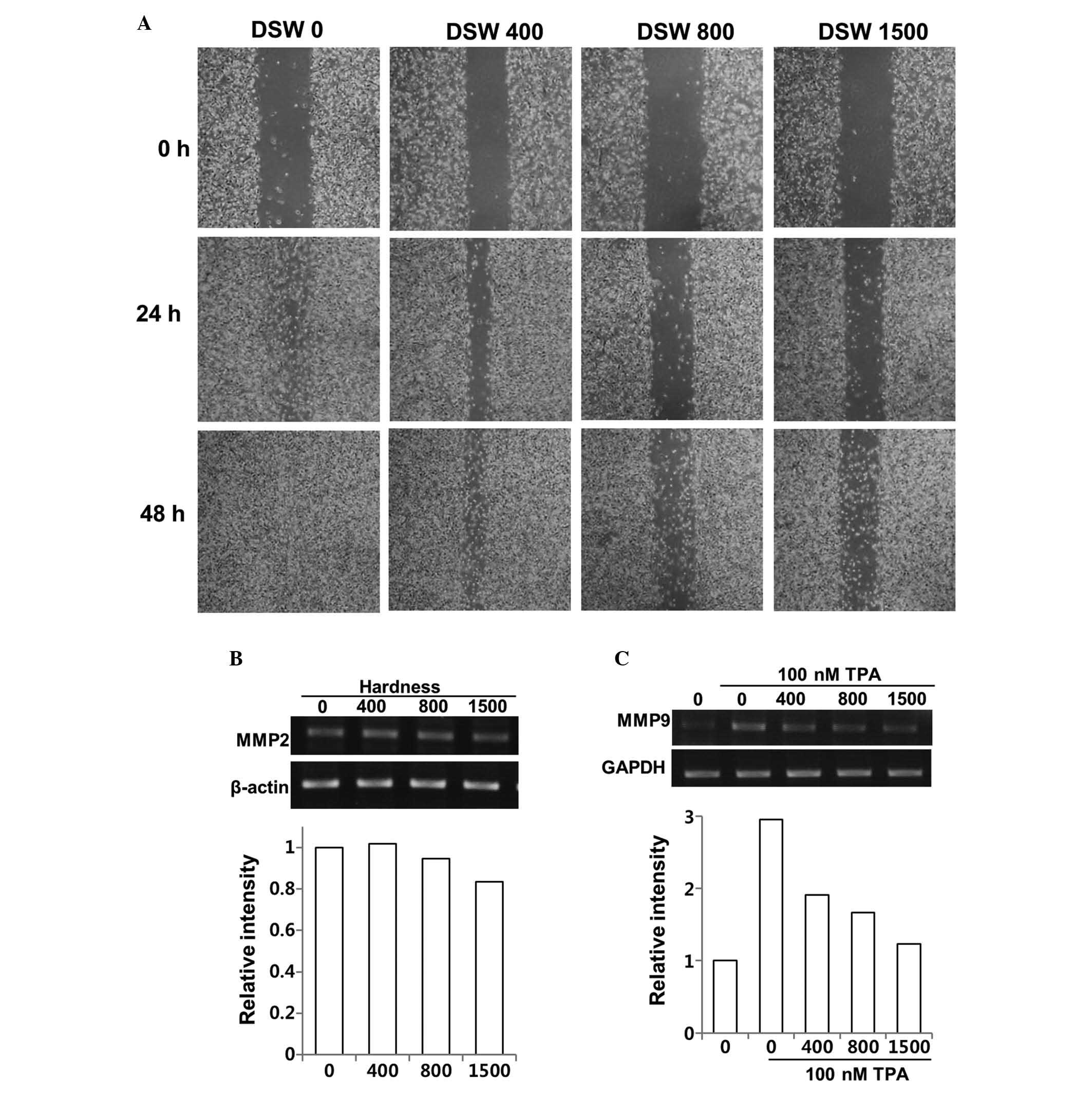
Effect of DSW on MDA-MB-231 invasive
human breast cancer cells
Since MDA-MB-231 cells provide a model of aggressive
human breast cancer, we tested whether DSW inhibits their migratory
ability in an in vitro wound healing assay. MDA-MB-231 cells
showed rapid wound closure at all time points, but treatment of
cells with different hardness of DSW for up to 2 days significantly
reduced migration of MDA-MB-231 cells in a dose-dependent manner
(Fig. 1A). The role of MMPs in
cancer invasion and metastasis is crucial due to their ability to
degrade extracellular matrix proteins, which is an essential
process for cancer invasion and metastasis that allows the cancer
cells to be released from site of the primary tumor and to
penetrate to the nearby tissues or spread to other parts of the
body (26). Therefore, we
evaluated the effects of DSW on the expression of MMPs. MMP-2 is
usually expressed constitutively, while the synthesis and secretion
of MMP-9 are induced by a variety of stimuli including cytokines
and TPA. Since the relative levels of MMP-2 are higher in the
highly invasive and metastatic MDA-MB-231 cell line, we evaluated
the inhibitory effect of DSW on both the endogenous gene expression
of MMP-2 of MDA-MB-231 cells and TPA-induced MMP-9 gene expression.
Although our data suggested that DSW had mild inhibitory effects on
the MMP-2 gene expression, exhibiting approximately 20% of
inhibition at 1,500 hardness (Fig.
1B), TPA-induced MMP-9 expression was significantly inhibited
by the treatment with DSW (Fig.
1C). The endogenous level of MMP-9 gene expression was barely
detectable in MDA-MB-231 cells, while treatment with TPA for 24 h
induced MMP-9 gene expression by about 3-fold. However, treatment
with DSW showed clear inhibitory effects on MMP-9 gene expression;
in particular, cells treated with conditioned media containing
1,500 hardness DSW showed almost basal level expression of MMP-9.
Therefore, our data suggested that DSW may have inhibitory effects
on the mechanisms underlying cancer invasion and metastasis.
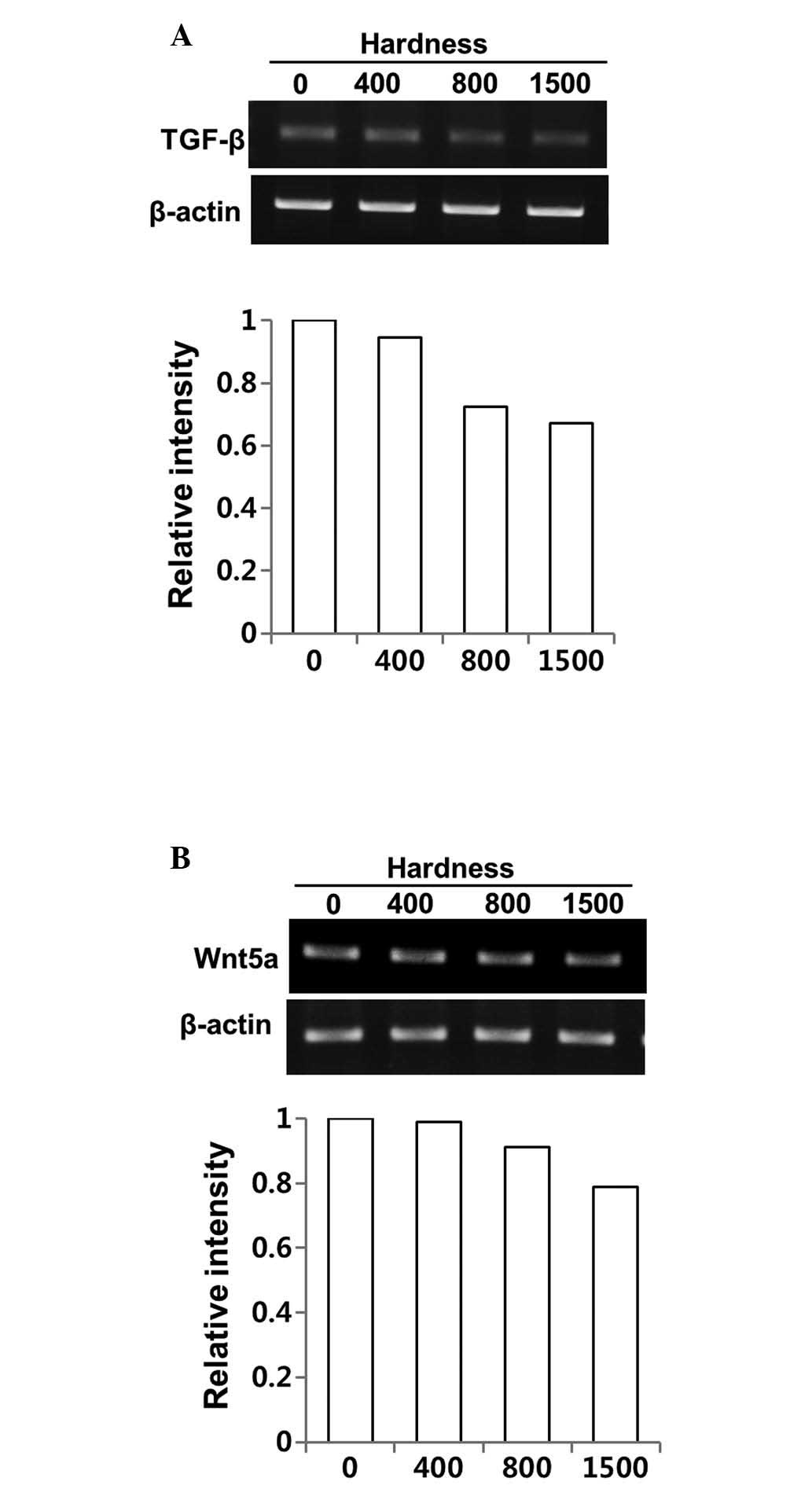
Regulation of the expression of TGF-β and
its direct mediator, Wnt5a, by DSW in MDA-MB-231 cells
Cell migration and invasion are complex requiring
coordination of many signaling pathways. We first evaluated whether
the inhibitory effects of DSW on migratory ability of MDA-MB-231
cells occurred from altered TGF-β signaling upon treatment with
DSW. As shown in Fig. 2A,
treatment with DSW for 3 days inhibited the TGF-β gene expression
of MDA-MB-231 cells; the cells treated with the conditioned media
containing 800 or 1,500 hardness DSW exhibited about 40% inhibition
(Fig. 2A). The expression of
Wnt5a, a direct mediator of TGF-β, was also affected by DSW
(Fig. 2B) but the inhibitory
effect of DSW on Wnt5a gene expression was relatively mild compared
to its effect on the TGF-β gene expression.
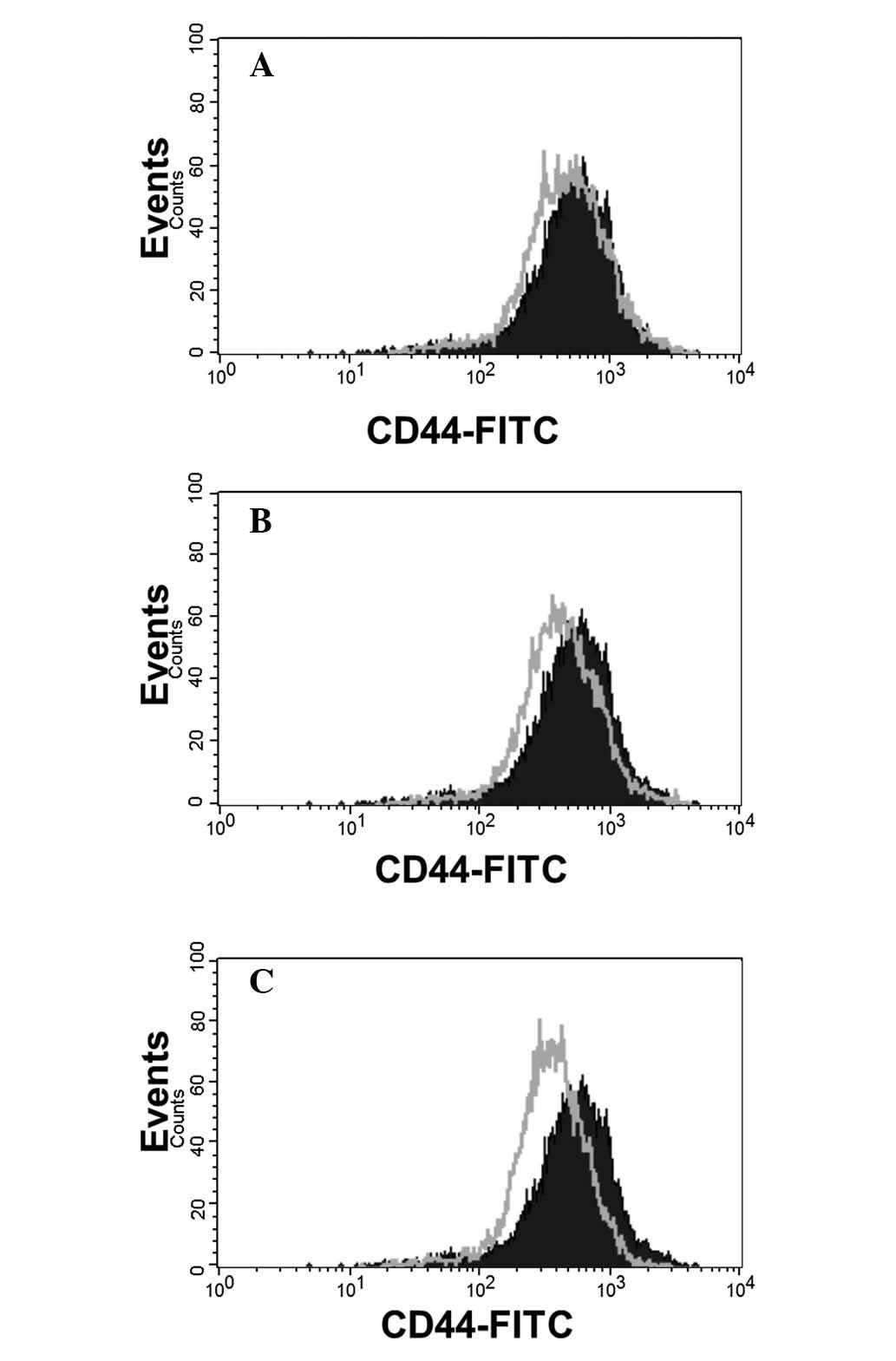
Attenuated CD44 expression in MDA-MB-231
cells cultured in DMEM with different hardness of DSW
Wnt5a overexpression or knockdown using siRNA
identified CD44 as one of the Wnt5a target genes (23). Studies analyzing gene expression
profiles with 52 breast cancer cell lines revealed that CD44
amplification is correlated to triple negative breast cancer cell
lines including MDA-MB-231 cells (31). Since CD44 is identified as a Wnt5a
target gene and is highly expressed in MDA-MB-231 cells, we tested
whether DSW can affect the expression of CD44 in MDA-MB-231 cells.
Flow cytometric analysis was performed to measure CD44 expression
in MDA-MB-231 cells treated with different hardness of DSW for 3
days. CD44 was highly expressed in MDA-MB-231 cells but treatment
with DSW attenuated overall expression of CD44, suggesting that the
inhibitory effect of DSW on Wnt5a expression subsequently affected
the expression of CD44 in MDA-MB-231 cells (Fig. 3).
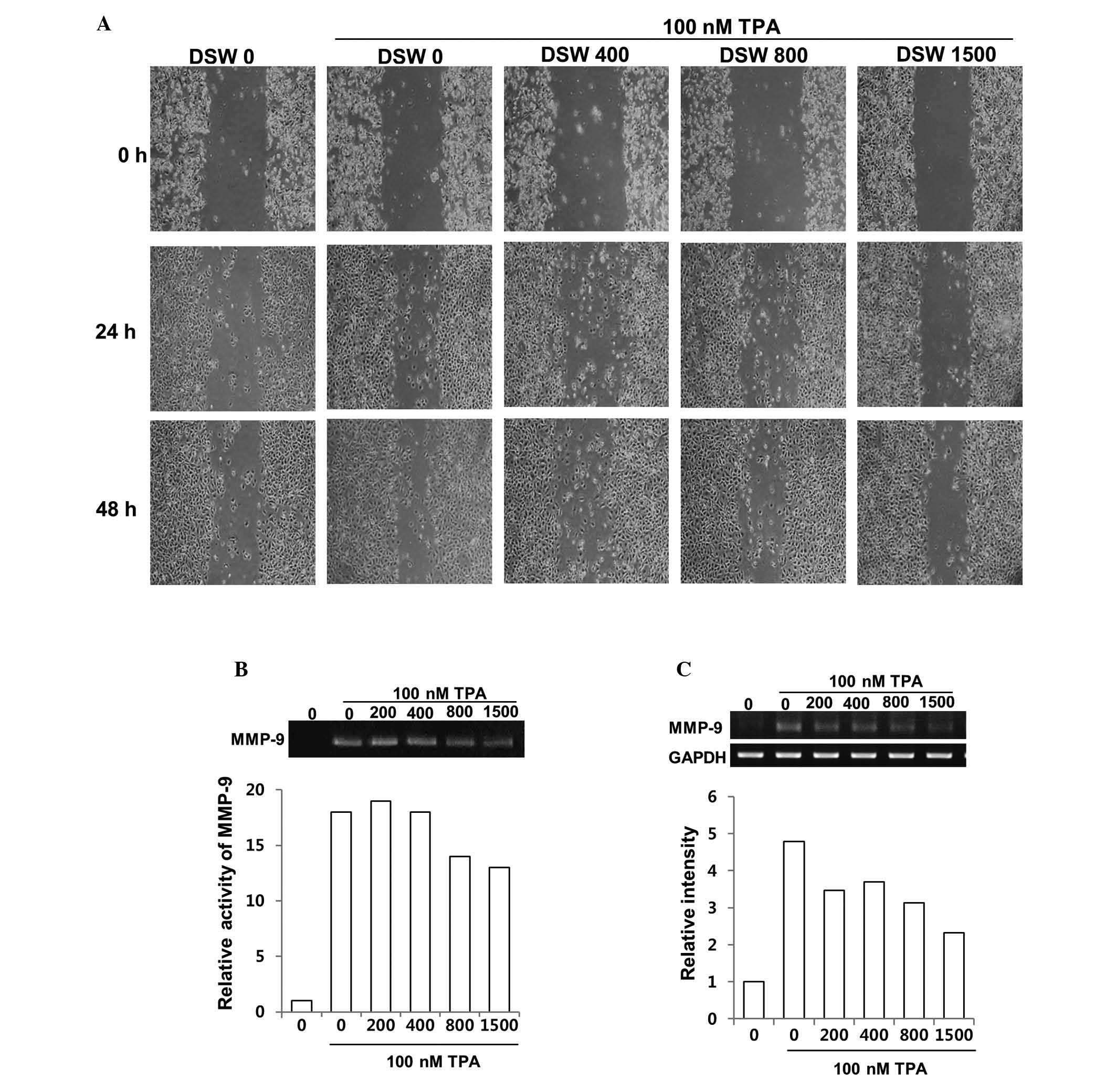
Effect of DSW on TPA-induced migration in
non-invasive human breast cancer cells
To evaluate the preventive effect of DSW on
TPA-induced invasive/metastatic tumor features, the migratory
ability of TPA-treated MCF7 cells was evaluated using an in
vitro wound healing assay. MCF-7 cells are weakly invasive
in vitro, but treatment with TPA resulted in more rapid
wound closure at all-time points (Fig.
4A). Treatment of the cells with different hardness of DSW for
up to 2 days attenuated the TPA-induced migration in a
dose-dependent manner compared to that in TPA-treated control cells
(Fig. 4A). We further investigated
the effects of DSW on the activity and expression of MMP-9 in MCF-7
cells. Since the endogenous level of MMP-2 is undetectable in
non-invasive MCF-7 breast cancer cells, we evaluated the inhibitory
effect of DSW on MMP-9 activity in a gelatin zymography assay after
inducing the MMP-9 expression by treatment with TPA. The media from
control cells showed barely detectable proteolytic activity, while
treatment with TPA for 24 h induced MMP-9 secretion by about
18-fold compared to control cells. However, the MMP-9 activity from
cells treated with conditioned media containing 800 or 1,500
hardness DSW was about 30% less than the TPA-induced MMP-9 activity
(Fig. 4B).
Inhibitory effect of DSW on MMP-9
activity is via suppressed MMP-9 gene expression rather than
uPA/uPAR system
To understand the mechanism underlying the
suppression of MMP-9 activity by DSW, we first examined whether
treatment with DSW directly affects the gene expression of MMP-9.
Cells were treated with different hardness of DSW. After 2 h, cells
were treated with TPA, and then continuously cultured for another
24 h before isolating the total cellular mRNA for RT-PCR. As shown
in Fig. 4C, gene expression of
MMP-9 was induced in cells treated with TPA. However, treatment
with DSW clearly inhibited MMP-9 gene expression (Fig. 4C). It is well known that the
activation of MMP-9 is mediated by active uPA, which catalyzes the
cleavage of plasminogen to generate active plasmin. In turn,
plasmin facilitates the release of several proteolytic enzymes,
including MMPs. Thus, we tested whether the inhibitory effects of
DSW on MMP-9 activity result from suppressed expression of uPA and
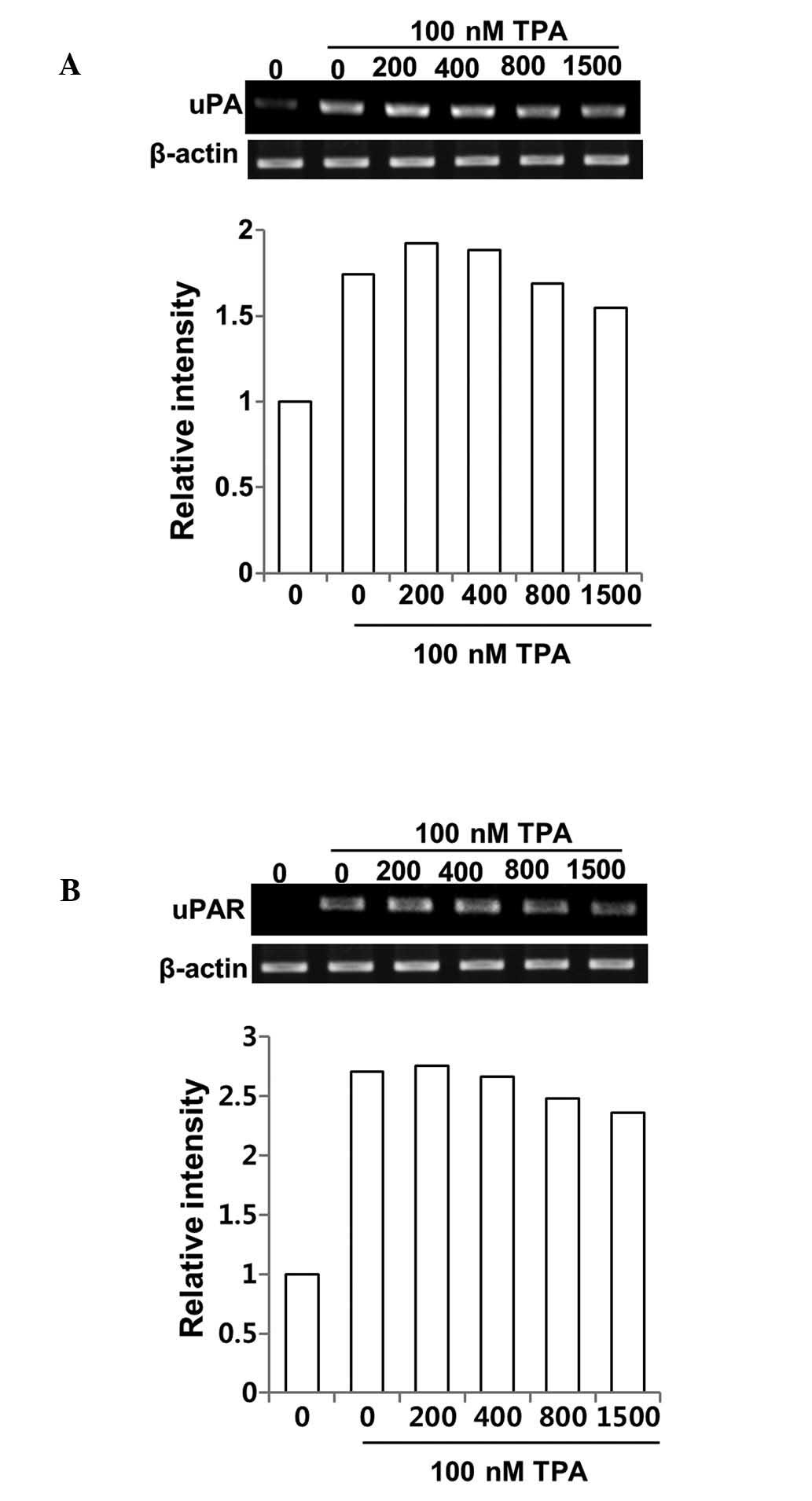
its receptor, uPAR, in DSW-treated MCF-7 cells. The effect of TPA
in inducing uPA and uPAR expression was significant, but DSW
exhibited only mild inhibitory effects on uPA and uPAR expression
(Fig. 5A and B). Taken together,
our data suggest that DSW inhibits TPA-induced MMP-9 activity
mainly through the inhibition of MMP-9 gene expression rather than
by inhibition of uPA/uPAR expression.
Regulation of the expression of TGF-β and
Wnt ligands by DSW in MCF-7 cells
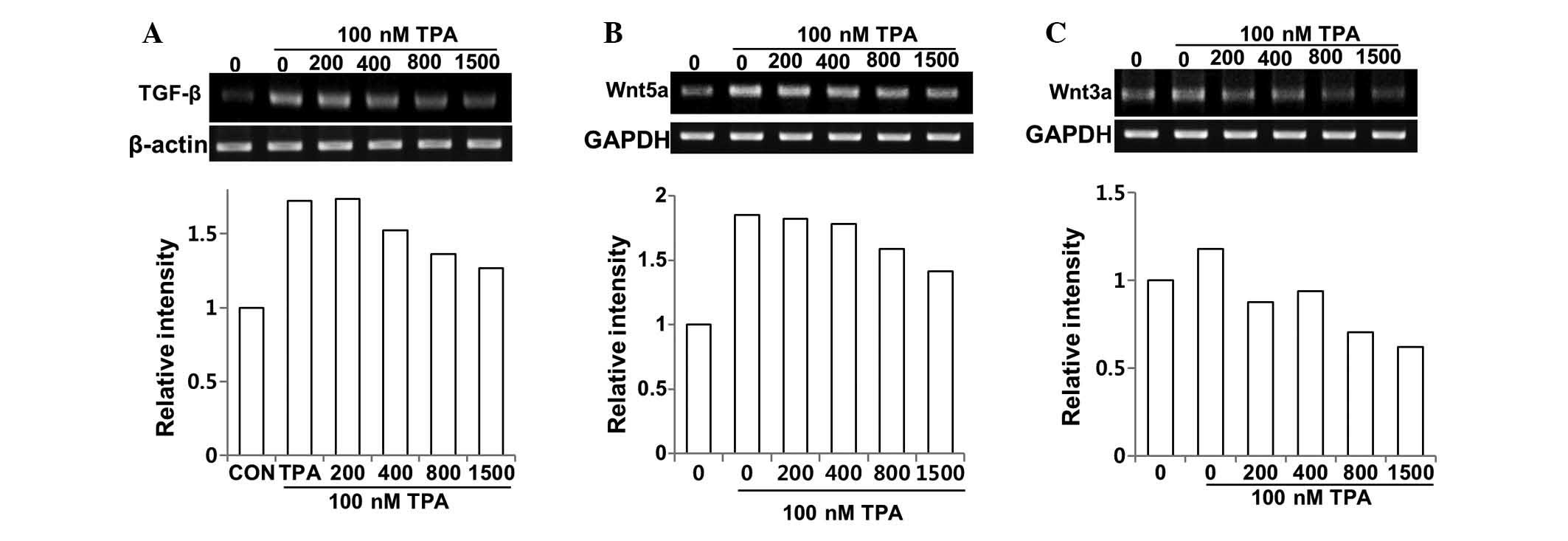
We evaluated the effects of DSW on the mRNA
expression of TGF-β. The control cells showed barely detectable
mRNA expression of TGF-β, while treatment with TPA for 24 h showed
significant induction of TGF-β expression. However, treatment with
DSW inhibited the TGF-β gene expression of MCF-7 cells; in
particular, conditioned media containing 800 or 1,500 hardness DSW
inhibited the TPA-induced TGF-β expression by approximately 60%
(Fig. 6A). The expression of Wnt5a
was also affected by treatment with DSW (Fig. 6B). Similar to the mRNA expression
of TGF-β, the endogenous expression of Wnt5a was very low, but
treatment with TPA efficiently induced the expression of Wnt5a.
Inhibitory effect of DSW on the TPA-induced Wnt5a expression was
significant thereby exhibiting over 50% inhibition in cells treated
with 800 or 1,500 hardness DSW (Fig.
6B). We further investigated whether DSW can alter the
expression of the Wnt-canonical signaling ligand, Wnt3a. Its
binding to Wnt receptors such as FZD and LRP5/6 promoted
stabilization and nuclear translocation of β-catenin, resulting in
the activation of target genes with cancer-promoting roles
(17). TPA mildly induced the
expression of Wnt3a, but DSW efficiently inhibited TPA-induced
Wnt3a expression (Fig. 6C),
implying that DSW may also inhibit the Wnt signaling-involved tumor
migration and metastasis.
Discussion
In this study, we have evaluated the potential
health benefit of DSW on the inhibition of metastatic potential of
human breast cancer cells. We showed that treatment with DSW
efficiently inhibited the migratory ability of highly invasive
MDA-MB-231 cells in a wound healing assay. This effect of DSW
appears to be mediated through TGF-β and Wnt5a signaling,
subsequently resulting in the attenuated expression of CD44. TGF-β
is one of the well known signaling pathways that are involved in
the survival of breast cancer cells and cell migration (32). Moreover, abrogation of autocrine
TGF-β signaling in MDA-MB-231 cells with transfection of a
kinase-inactive type II TGF-β receptor impaired the basal migratory
potential by blocking both Smad-dependent and -independent
signaling pathways (33). The
inhibitory effect of DSW on the Wnt5a gene expression was
relatively mild compared to its effect on TGF-β gene expression.
Since TGF-β directly upregulates Wnt5a expression through the Smad
complex, the mild inhibitory effects of DSW on Wnt5a gene
expression may indicate that the relative contribution of
Smad-dependent TGF-β signaling is moderate in mediating motility
compared to that of Smad-independent TGF-β signaling. The
inhibitory effects of DSW on Smad-independent TGF-β signaling need
to be clarified in future studies.
CD44 is also known to be involved in Wnt5a-dependent
invasion and metastasis. CD44 is a transmembrane glycoprotein that
participates in many cellular processes including regulation of
cell-cell interactions, migration and adhesion. It is aberrantly
expressed in breast cancers and has been implicated in the
metastatic process as well as in the putative cancer stem cell
compartment (24). In this study,
we showed that treatment with DSW attenuated the overall expression
of CD44, suggesting that the inhibitory effect of DSW on Wnt5a
expression subsequently affected the expression of CD44 in
MDA-MB-231 cells. Besides CD44, the expression of MMPs is also
regulated by Wnt5a via binding to Ror2 (25).
We further investigated the preventive effect of DSW
on TPA-induced invasive/metastatic tumor features in non-invasive
MCF-7 cells. Similar to the inhibitory effects shown in MDA-MB-231
cells, DSW treatment inhibited TPA-induced migration and MMP-9
activity with a concomitant decrease in mRNA levels of MMP-9, TGF-β
and Wnt5a. Interestingly, we observed that DSW can alter the
expression of Wnt-canonical signaling ligand, Wnt3a. Aberrant
activation of Wnt signaling was observed in a variety of cancers,
implying the importance of Wnt signaling in carcinogenesis
(34). Wnt signaling also mediates
the metastatic progression by enhancing the migratory ability of
cancer cells. On top of that, blockade of Wnt signaling reduces
motility and lung metastasis as well as tumor outgrowth, suggesting
the involvement of Wnt signaling in tumor invasion and migration
(35). Thus, the inhibitory effect
of DSW on Wnt3a expression implies that the Wnt signaling pathway
mediating the cell migration and invasion may be affected by
treatment with DSW.
Although it is not clear which component of DSW
affects the metastatic potential of human breast cancer cells, it
is presumed that the combined ionic action of several minerals such
as calcium, magnesium and zinc, may play important roles in
mediating the inhibition of metastasis. Among these, calcium and
magnesium are the chief mineral ions present in DSW. The
concentration of calcium is approximately 100 mg/l in 1,500
hardness DSW, while the amount of magnesium is approximately 300
mg/l.
Calcium, a key cellular mediator, has been
implicated in the induction of apoptosis and regulation of
apoptosis signaling pathway in human breast cancer cells via the
activation of Ca2+-dependent proapoptotic protease
calpain and caspase-12 (36).
Epidemiologic studies also suggest that intake of calcium appear to
reduce the risk of breast cancer (37,38),
as well as colon cancer (7,10).
Individuals with a calcium intake of more than 700 mg per day had a
35 to 45% reduced risk of cancer of the distal part of the colon
than those who had a calcium intake of 500 mg or less per day.
However, there was no incremental benefit of additional calcium
intake beyond 700 mg/day (10).
Furthermore, improving calcium and vitamin D nutritional status
substantially reduced all-cancer risk in postmenopausal women
(7). Although some studies do not
support a benefit of calcium (39,40),
the majority of studies suggest calcium supplements with vitamin D
have shown some promise in cancer risk reduction.
Magnesium deficiency is also known to be associated
with advancement of malignancy and metastasis. Decreased serum
levels of magnesium are frequently observed in patients with tumors
(6,9), and low magnesium status was observed
in cancer patients undergoing several courses of chemotherapy
(41). In contrast, daily intake
of an additional 100 mg of magnesium reduced the risk of colorectal
cancer by 12% (42), suggesting
beneficial effects of magnesium against multiple cancers.
Nevertheless, the deficiency of extracellular magnesium ceased
tumor cell growth, its deficiency caused development of more lung
metastasis than controls despite smaller size of the primary tumor
in mice (8).
The exact mechanism underlying metastasis with
regard to magnesium deficiency is unclear, but a gene expression
array of tumors from magnesium deficient mice suggested that
magnesium influences the remodeling of extracellular matrix via
upregulation of several proteases such as metalloproteinase,
calpain and others (43).
Therefore, the inhibitory effects of DSW on migration and MMP-9
activity may have occurred from a high concentration of magnesium
within DSW. Nevertheless, mineral water containing only exogenous
magnesium as 1,500 hardness did not show significant inhibitory
effects against metastatic potential of human breast cancer cells
compared to effects of DSW (data not shown). Thus, the presence of
the proper ratio of magnesium to calcium in DSW may provide
synergetic effects via the inter-relationship between magnesium and
calcium. In this regard, recent studies have pointed out the
importance of the ratio of calcium to magnesium, which can modify
their effects on carcinogenesis (44). Both high magnesium and calcium
levels have been linked to reduced risks of cancer, but studies
have also shown that high calcium levels inhibit the absorption of
magnesium. In fact, the results from a large clinical trial found
that supplementation of calcium reduced the risk of cancer
recurrence only if the ratio of calcium to magnesium was low,
suggesting a need for both minerals in reducing the risk of cancer
(44). Thus, DSW may be a
beneficial source that provides the proper ratio of calcium to
magnesium.
Our data showed substantial inhibitory effects of
DSW on the metastatic potential of human breast cancer cells. The
mechanism underlying DSW-mediated suppression on tumor migration
and invasion were not fully elucidated in this study, but we
believe that this is the first study to provide an important clue
of a possible mechanism for the inhibitory effects of DSW on the
Wnt signaling pathway, which mediates cell migration and invasion
in coordination with the TGF-β signaling pathway. Taken together,
our data showed that DSW has inhibitory effects on breast cancer
invasion/metastasis, suggesting that DSW shows some promise in
improving cancer survival by preventing tumor metastasis.
Abbreviations:
|
DSW
|
deep-sea water
|
|
TPA
|
12-O-tetradecanoylphorbol-13-acetate
|
|
COX-2
|
cyclooxygenase-2
|
|
TGF-β
|
transforming growth factor-β
|
|
uPA
|
urokinase plasminogen activator
|
|
uPAR
|
urokinase plasminogen activator
receptor
|
|
ER
|
estrogen receptor
|
|
PR
|
progesterone receptor
|
|
FZD
|
Frizzled
|
|
LRP5/6
|
low-density lipoprotein receptors
5/6
|
Acknowledgements
This study was supported by the
project entitled ‘Development of Technology for support of deep-sea
water industry (PJT200014)’ from the Ministry of Land, Transport
and Maritime Affairs, Korea.
References
|
1.
|
Nakasone T and Akeda S: The application of
deep sea water in Japan. UJNR Technical Report. 28:69–75. 1999.
|
|
2.
|
Shon YH, Kim JS and Nam KS: Inhibitory
effect of Deep-sea water on cytochrome P450 1A1, aromatase, and
MMP-9. J Life Sci. 18:503–508. 2008. View Article : Google Scholar
|
|
3.
|
Hwang HS, Kim SH, Yoo YG, et al:
Inhibitory effect of deep-sea water on differentiation of 3T3-L1
adipocytes. Mar Biotechnol (NY). 11:161–168. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
4.
|
Radhakrishnan G, Yamamoto M, Maeda H, et
al: Intake of dissolved organic matter from deep seawater inhibits
atherosclerosis progression. Biochem Biophys Res Commun. 387:25–30.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
5.
|
Lee KS, Shin JS, Kwon YS, Moon DS and Nam
KS: Suppression of cancer progression and metastasis in HT-29 human
colorectal adenocarcinoma by deep sea water. Biotechnol Bioproc
Eng. 18:194–200. 2013. View Article : Google Scholar
|
|
6.
|
Kohli GS, Bhargava A, Goel H, et al: Serum
magnesium levels in patients with head and neck cancer. Magnesium.
8:77–86. 1989.PubMed/NCBI
|
|
7.
|
Lappe JM, Travers-Gustafson D, Davies KM,
Recker RR and Heaney RP: Vitamin D and calcium supplementation
reduces cancer risk: results of a randomized trial. Am J Clin Nutr.
85:1586–1591. 2007.PubMed/NCBI
|
|
8.
|
Nasulewicz A, Wietrzyk J, Wolf FI, et al:
Magnesium deficiency inhibits primary tumor growth but favors
metastasis in mice. Biochim Biophys Acta. 1739:26–32. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
9.
|
Sartori S, Nielsen I, Tassinari D, et al:
Serum and erythrocyte magnesium concentrations in solid tumours:
relationship with stage of malignancy. Magnes Res. 5:189–192.
1992.PubMed/NCBI
|
|
10.
|
Wu K, Willett WC, Fuchs CS, Colditz GA and
Giovannucci EL: Calcium intake and risk of colon cancer in women
and men. J Natl Cancer Inst. 94:437–446. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
11.
|
Gamucci T, D’Ottavio AM, Magnolfi E, et
al: Weekly epirubicin plus docetaxel as first-line treatment in
metastatic breast cancer. Br J Cancer. 97:1040–1045. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
12.
|
Padua D and Massague J: Roles of TGFbeta
in metastasis. Cell Res. 19:89–102. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
13.
|
Shariat SF, Shalev M, Menesses-Diaz A, et
al: Preoperative plasma levels of transforming growth factor
beta(1) (TGF-beta(1)) strongly predict progression in patients
undergoing radical prostatectomy. J Clin Oncol. 19:2856–2864.
2001.
|
|
14.
|
Tsushima H, Ito N, Tamura S, et al:
Circulating transforming growth factor beta 1 as a predictor of
liver metastasis after resection in colorectal cancer. Clin Cancer
Res. 7:1258–1262. 2001.PubMed/NCBI
|
|
15.
|
Yang YA, Dukhanina O, Tang B, et al:
Lifetime exposure to a soluble TGF-beta antagonist protects mice
against metastasis without adverse side effects. J Clin Invest.
109:1607–1615. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
16.
|
Yin JJ, Selander K, Chirgwin JM, et al:
TGF-beta signaling blockade inhibits PTHrP secretion by breast
cancer cells and bone metastases development. J Clin Invest.
103:197–206. 1999. View
Article : Google Scholar : PubMed/NCBI
|
|
17.
|
Howe LR and Brown AM: Wnt signaling and
breast cancer. Cancer Biol Ther. 3:36–41. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
18.
|
Katoh M: Transcriptional mechanisms of
WNT5A based on NF-kappaB, Hedgehog, TGFbeta, and Notch signaling
cascades. Int J Mol Med. 23:763–769. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
19.
|
Lejeune S, Huguet EL, Hamby A, Poulsom R
and Harris AL: Wnt5a cloning, expression, and up-regulation in
human primary breast cancers. Clin Cancer Res. 1:215–222.
1995.PubMed/NCBI
|
|
20.
|
Smith K, Bui TD, Poulsom R, Kaklamanis L,
Williams G and Harris AL: Up-regulation of macrophage wnt gene
expression in adenoma-carcinoma progression of human colorectal
cancer. Br J Cancer. 81:496–502. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
21.
|
Bittner M, Meltzer P, Chen Y, et al:
Molecular classification of cutaneous malignant melanoma by gene
expression profiling. Nature. 406:536–540. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
22.
|
Weeraratna AT, Jiang Y, Hostetter G, et
al: Wnt5a signaling directly affects cell motility and invasion of
metastatic melanoma. Cancer Cell. 1:279–288. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
23.
|
Dissanayake SK, Wade M, Johnson CE, et al:
The Wnt5A/protein kinase C pathway mediates motility in melanoma
cells via the inhibition of metastasis suppressors and initiation
of an epithelial to mesenchymal transition. J Biol Chem.
282:17259–17271. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
24.
|
Dietrich A, Tanczos E, Vanscheidt W,
Schopf E and Simon JC: High CD44 surface expression on primary
tumours of malignant melanoma correlates with increased metastatic
risk and reduced survival. Eur J Cancer. 33:926–930. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
25.
|
O’Connell MP, Fiori JL, Xu M, et al: The
orphan tyrosine kinase receptor, ROR2, mediates Wnt5A signaling in
metastatic melanoma. Oncogene. 29:34–44. 2010.PubMed/NCBI
|
|
26.
|
Nabeshima K, Inoue T, Shimao Y and
Sameshima T: Matrix metalloproteinases in tumor invasion: role for
cell migration. Pathol Int. 52:255–264. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
27.
|
Itoh T, Tanioka M, Yoshida H, Yoshioka T,
Nishimoto H and Itohara S: Reduced angiogenesis and tumor
progression in gelatinase A-deficient mice. Cancer Res.
58:1048–1051. 1998.PubMed/NCBI
|
|
28.
|
Perou CM, Sorlie T, Eisen MB, et al:
Molecular portraits of human breast tumours. Nature. 406:747–752.
2000. View
Article : Google Scholar : PubMed/NCBI
|
|
29.
|
Subramaniam DS and Isaacs C: Utilizing
prognostic and predictive factors in breast cancer. Curr Treat
Options Oncol. 6:147–159. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
30.
|
Klemm F, Bleckmann A, Siam L, et al:
beta-catenin-independent WNT signaling in basal-like breast cancer
and brain metastasis. Carcinogenesis. 32:434–442. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
31.
|
Kao J, Salari K, Bocanegra M, et al:
Molecular profiling of breast cancer cell lines defines relevant
tumor models and provides a resource for cancer gene discovery.
PLoS One. 4:e61462009.PubMed/NCBI
|
|
32.
|
Lei X, Yang J, Nichols RW and Sun LZ:
Abrogation of TGFbeta signaling induces apoptosis through the
modulation of MAP kinase pathways in breast cancer cells. Exp Cell
Res. 313:1687–1695. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
33.
|
Dumont N, Bakin AV and Arteaga CL:
Autocrine transforming growth factor-beta signaling mediates
Smad-independent motility in human cancer cells. J Biol Chem.
278:3275–3285. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
34.
|
Anastas JN and Moon RT: WNT signalling
pathways as therapeutic targets in cancer. Nat Rev Cancer.
13:11–26. 2013. View
Article : Google Scholar : PubMed/NCBI
|
|
35.
|
Matsuda Y, Schlange T, Oakeley EJ, Boulay
A and Hynes NE: WNT signaling enhances breast cancer cell motility
and blockade of the WNT pathway by sFRP1 suppresses MDA-MB-231
xenograft growth. Breast Cancer Res. 11:R322009. View Article : Google Scholar : PubMed/NCBI
|
|
36.
|
Mathiasen IS, Sergeev IN, Bastholm L,
Elling F, Norman AW and Jaattela M: Calcium and calpain as key
mediators of apoptosis-like death induced by vitamin D compounds in
breast cancer cells. J Biol Chem. 277:30738–30745. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
37.
|
Cui Y and Rohan TE: Vitamin D, calcium,
and breast cancer risk: a review. Cancer Epidemiol Biomarkers Prev.
15:1427–1437. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
38.
|
Shin MH, Holmes MD, Hankinson SE, Wu K,
Colditz GA and Willett WC: Intake of dairy products, calcium, and
vitamin d and risk of breast cancer. J Natl Cancer Inst.
94:1301–1311. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
39.
|
Benito E, Stiggelbout A, Bosch FX, et al:
Nutritional factors in colorectal cancer risk: a case-control study
in Majorca. Int J Cancer. 49:161–167. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
40.
|
Negri E, La Vecchia C, D’Avanzo B and
Franceschi S: Calcium, dairy products, and colorectal cancer. Nutr
Cancer. 13:255–262. 1990. View Article : Google Scholar
|
|
41.
|
Lajer H and Daugaard G: Cisplatin and
hypomagnesemia. Cancer Treat Rev. 25:47–58. 1999. View Article : Google Scholar
|
|
42.
|
Wark PA, Lau R, Norat T and Kampman E:
Magnesium intake and colorectal tumor risk: a case-control study
and meta-analysis. Am J Clin Nutr. 96:622–631. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
43.
|
Maier JA, Nasulewicz-Goldeman A, Simonacci
M, Boninsegna A, Mazur A and Wolf FI: Insights into the mechanisms
involved in magnesium-dependent inhibition of primary tumor growth.
Nutr Cancer. 59:192–198. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
44.
|
Dai Q, Shrubsole MJ, Ness RM, et al: The
relation of magnesium and calcium intakes and a genetic
polymorphism in the magnesium transporter to colorectal neoplasia
risk. Am J Clin Nutr. 86:743–751. 2007.PubMed/NCBI
|
|
45.
|
Jang JY, Jeon YK and Kim CW: Degradation
of HER2/neu by ANT2 shRNA suppresses migration and invasiveness of
breast cancer cells. BMC Cancer. 10:3912010. View Article : Google Scholar : PubMed/NCBI
|
|
46.
|
Kang JH, Song KH, Jeong KC, et al:
Involvement of Cox-2 in the metastatic potential of
chemotherapy-resistant breast cancer cells. BMC Cancer. 11:3342011.
View Article : Google Scholar : PubMed/NCBI
|
|
47.
|
Li Y and Sarkar FH: Gene expression
profiles of genistein-treated PC3 prostate cancer cells. J Nutr.
132:3623–3631. 2002.PubMed/NCBI
|
|
48.
|
Suzuki A, Ozono K, Kubota T, Kondou H,
Tachikawa K and Michigami T: PTH/cAMP/PKA signaling facilitates
canonical Wnt signaling via inactivation of glycogen synthase
kinase-3beta in osteoblastic Saos-2 cells. J Cell Biochem.
104:304–317. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
49.
|
Takeuchi E, Tanaka T, Umemoto E, et al:
VLA-4-dependent and -independent pathways in cell contact-induced
proinflammatory cytokine production by synovial nurse-like cells
from rheumatoid arthritis patients. Arthritis Res. 4:R102002.
View Article : Google Scholar
|