Contents
Introduction
Types of HPV head and neck cancers
HPV virology
Molecular mechanism of HPV carcinogenesis
Interaction of HPV E6 and p53 proteins
HPV E6/E7 proteins and zinc fingers
Metallothionein in head and neck cancer
Zinc, zinc fingers, p53 and metallothionein - is
there any connection with HPV?
Introduction
Human papillomaviruses (HPVs) are a large family of
small double-stranded DNA viruses infecting squamous epithelia
(1) and causing papillomas in most
mammals (2,3). The viruses are absolutely
species-specific (1) and they have
been detected in a variety of mammalian and avian species including
humans, parrots, canines and felines (4). In addition, HPV has been accepted as
an etiologic agent for cervical carcinoma, whereas the first
association with head and neck cancer was published in 1985
(5). HPV was also shown to play a
role in the pathogenesis of a subset of head and neck squamous cell
carcinomas (HNSCCs) (6). HNSCCs
belong to majority of head and neck malignancies (7–9). The
term head and neck cancer includes malignancy in an area that
comprises the skin, oral cavity, salivary glands, lip, pharynx,
larynx, nasal cavity, paranasal sinuses and soft tissues of the
neck and ear (7). Almost 650,000
patients worldwide are diagnosed with head or neck cancer each year
and 350,000 patients die of this disease (5) as this cancer is the sixth most
prevalent type of cancer worldwide. The ratio of males to females
is approximately 2:1 (7).
In head and neck cancer patients, two types of
clinical precancer lesions have been established: white lesions
(leukoplakia) and reddish lesions (erythroplakia) (10). Precancerous lesions of the oral
mucosa are epithelial changes that are able to undergo malignant
transformation more likely than normal tissue at other mucosal
sites. HPV is also a central causative agent in cervical
carcinogenesis (11). HPV
selectively infects the epithelium of the skin and mucous
membranes. Specific HPV types are associated with squamous cell
carcinoma, adenocarcinoma, and dysplasias of the cervix, penis,
anus, vagina and vulva (12). A
total of 150 HPV genotypes have been identified and fully sequenced
(13–15). Determination of HPV genotype is
based on the degree of homology within the L1 (major capsid
protein) ORF (13). If the DNA
sequence of the L1 ORF differs by more than 10% from the closest
related known type, it is regarded as a novel type. HPV types
associated with skin warts are for example HPV-1, -2 and -4
(16). A wide range of HPV types
including HPV-5, -8, -9, -23 and -47 cause epidermodysplasia
verruciformis lesions, which can be transformed to malignancy upon
exposure to ultraviolet light (17). The largest subgroup is represented
by HPV types infecting mainly mucosal surfaces of the genital and
respiratory tracts. More than 40 of the identified HPV types belong
to this group (13). HPV types are
often referred to as ‘low-risk’ or ‘high-risk’ based on their
potential for oncogenesis. The high-risk HPV types include HPV-16,
-18, -31, -33, -35, -39, -45, -51, -52, -56, -58, -59, -68, -73 and
-82. The low-risk HPV types cause especially benign lesions
affecting the anogenital areas, such as genital warts
(condylomata), low-grade squamous intraepithelial lesions (SILs) of
the cervix, and laryngeal papillomas. These low-risk types include
HPV-6, -11, -40, -42, -43, -44, -53,-54, -61, -72 and -81 (18).
Types of HPV head and neck cancers
There are different types of HPV head and neck
cancers according to the location in the human body (7). In the following section, several
types of HPV are defined and their characteristics are given.
Oral cavity, salivary glands and
lips
The verrucous carcinoma is a variant of HNSCCs found
in the oral cavity. This carcinoma has been recognized as a locally
invasive, non-metastasizing squamous cell carcinoma (SCC) with
locations in the oral cavity, lips and larynx as well as in the
genital tract (19). The most
common HPV types in oral carcinomas are HPV 16, 33 and 82 (20).
Laryngeal area
The number of copies of HPV DNA is low in head and
neck carcinomas excepting tonsillar carcinoma, which indicates a
non-clonal association of these tumors. The HPV detection rate of
51% in tonsillar carcinomas is among the highest of any
extragenital human malignancies (21,22).
Antibodies against HPV proteins E6 and E7 are present in 65% of HPV
DNA-positive cancers (oro-pharynx and tonsils), but only in 13% of
HPV-positive oral cancers (23).
The larynx is among the most significant anatomic sites in terms of
HPV involvement, exceeded perhaps only by the genital tract and
skin infections in clinical importance. This is because that HPV
infection is the etiological agent of a clinically significant
disease known as laryngeal papilloma (papillomatosis) (24,25).
Nasal cavity and paranasal sinuses
The HPV DNA in malignant lesions of the nasal cavity
and the paranasal sinuses produce polyps, inverted papillomas and
squamous cell carcinomas. Squamous cell carcinomas are the most
frequent malignant tumors in this region. Report on malignant
transformation of benign lesions to sinonasal carcinomas has been
published. The study focused on paranasal sinuses because this area
is independent of tobacco and especially alcohol exposure (26). The coexistence of two different
epithelia in sino-nasal papillomas and carcinomas (columnar cells
and stratified squamous epithelium) creates squamocolumnar
junctions (SCJs) at multiple sites in the respiratory tract,
entities that are thought to be a prerequisite for the spreading
HPV infections in this region (27–29).
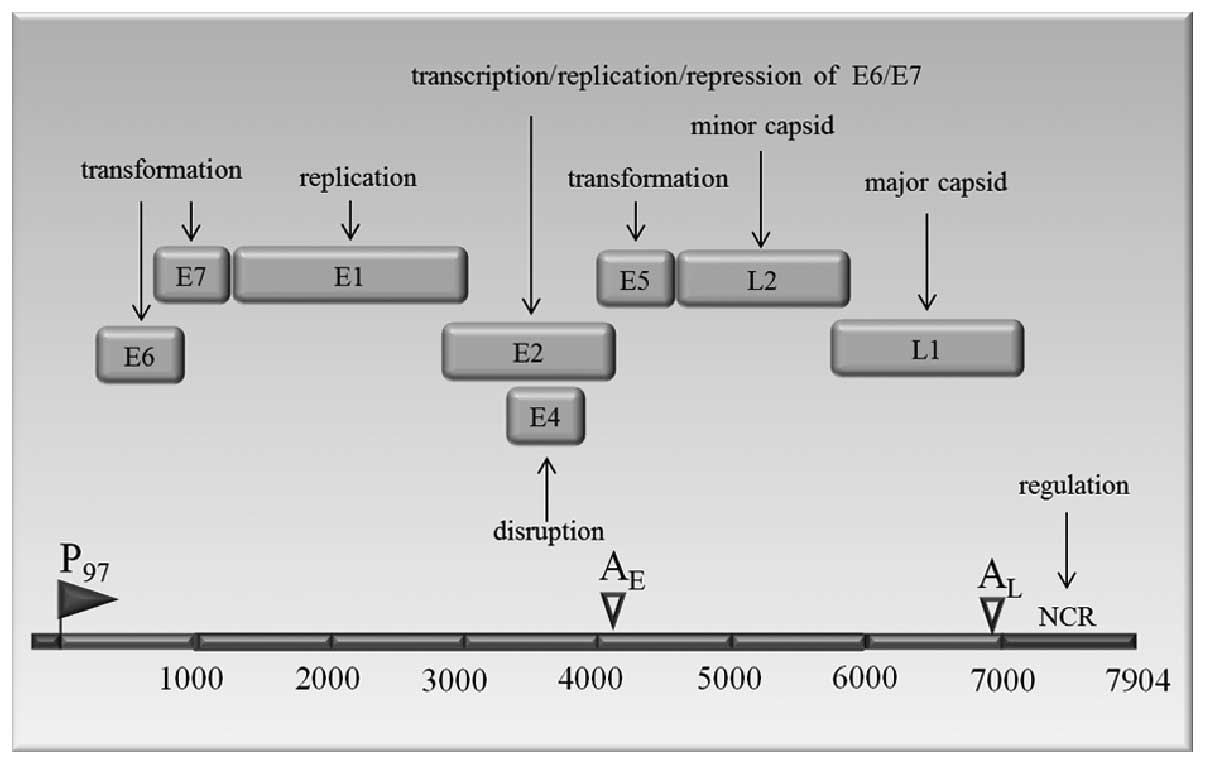
HPV virology
HPVs are small, non-enveloped double-stranded DNA
viruses. The HPV has a diameter of 55 nanometers and a genome
consisting of a double-stranded circular DNA of approximately 8,000
nucleotide base pairs associated with histones. This genome is
enclosed in an icosahedral capsid shell comprised of major and
minor capsid proteins (30). The
genome that can be divided into 3 domains: an early region with 6
open reading frames (ORFs) E6, E7, E1, E2, E4 and E5; a late region
with 2 ORFs, L1 (the major capsid protein) and L2 (the minor capsid
protein); and a non-coding regulatory region (NCR) of approximately
1 kb, is shown in Fig. 1. The
three regions are separated by polyadenylation sites, early AE and
late AL.
The E1 protein binds to the origin of replication
(31). The E2 ORF encodes protein
that act as a transcriptional activator of HPV gene expression in
both normal and immortalized keratinocytes (32,33).
The E2 proteins bind to E1 and stimulate viral DNA replication
(34). The E4 is expressed as a
late gene with a role in the productive infection. E5 protein
stimulates the transforming activity of the epidermal growth factor
receptor resulting in the increased cell proliferation (16,35).
The E6 protein of HPV-16 is a small polypeptide of approximately
150 amino acids that contains two zinc-binding domains (36). It is a transforming protein and
stimulates p53 degradation (37).
The HPV-16 E6 protein also activates telomerase, an enzyme that
maintains the telomeric DNA at the ends of linear chromosomes
(38,39). Without telomerase, telomeres
shorten upon each cell division, until they reach a critically
short length. Beyond this point further division induces damage in
the coding regions of the chromosome and causes cell senescence.
Almost all human cancers and immortalized cell lines have highly
active telomerase (40,41). The E7 protein of HPV-16 is a small,
nuclear polypeptide of 100 amino acids. Interestingly, the
carboxyl-terminus of E7 contains a similar zinc-binding domain as
does E6. E7 binds to retinoblastoma protein (pRb). Besides pRb, E7
also interacts with various other proteins, most of which are
important regulators of the cell growth (42). The E7 protein induces abnormal
centrosome duplication, resulting in multipolar, abnormal mitoses,
aneuploidy and genomic instability (43). Both the E6 and E7 proteins play a
role in the cell transformation and immortalisation (16,44,45).
The L1 and L2 late proteins form capsomers of the virus that
encapsidate the viral DNA. The L1 is the major capsid protein and
contains reactive epitopes for type-specific neutralisation. The L2
protein is a minor component of the viral capsid (24,46).
HPV infects cells in the basal layer (stratum basale), below the
surface of the epithelium and carries out an infection cycle that
is closely tied to the differentiation program of the host cells
(1).
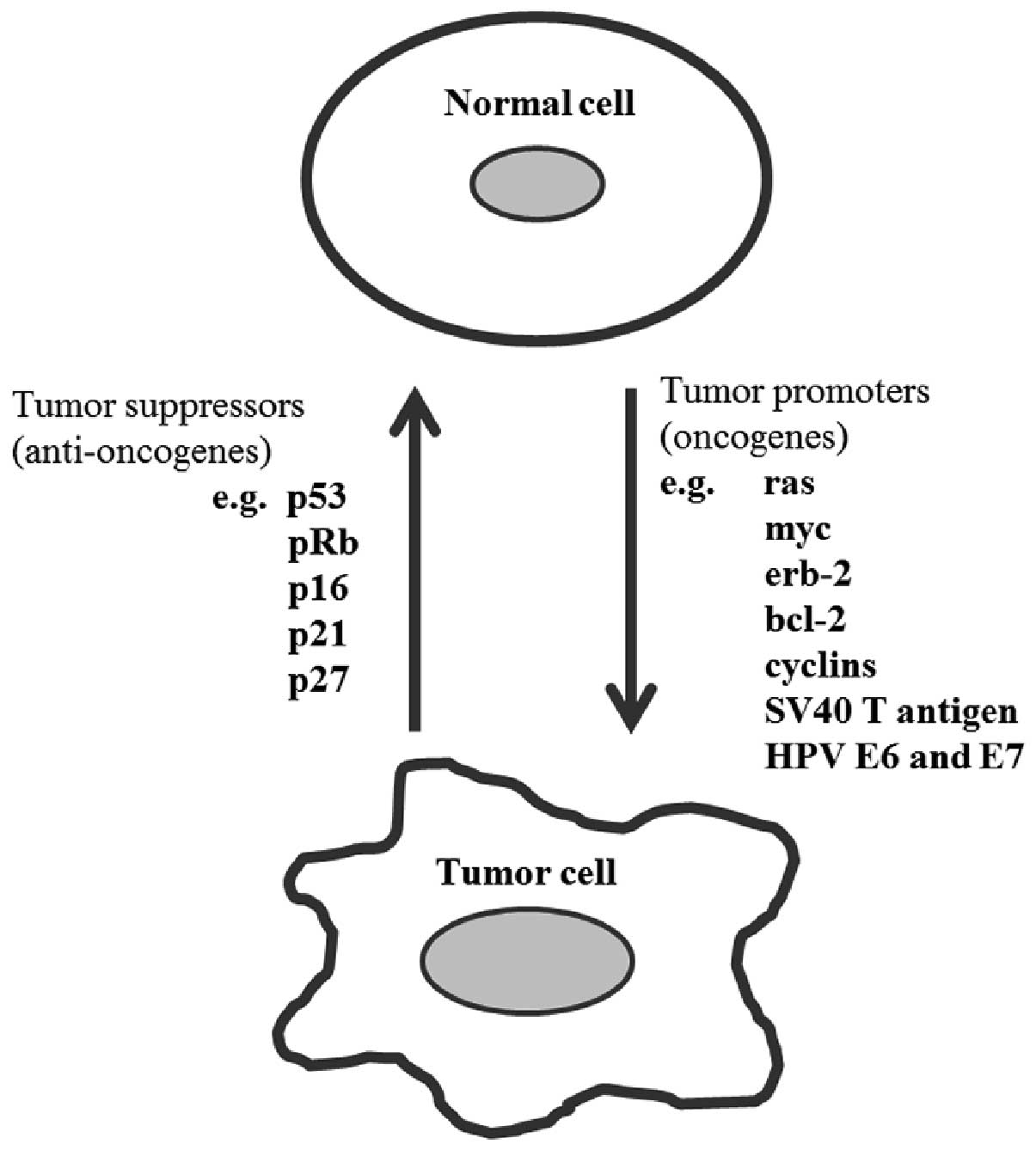
Molecular mechanism of HPV
carcinogenesis
Carcinogenesis is a multistep process associated
with the accumulation of genetic alterations in cells (47). The cancer results from the
accumulation of specific genetic mutations, many of which have now
been identified. These mutations can cause an activation of genes
that promote cellular proliferation or inhibit cell death
(oncogenes), or they may inactivate genes that inhibit
proliferation or promote cell death (tumor suppressor genes)
(47). The proteins derived from
various oncogenes, either cellular or viral, such as those of the
polyomavirus SV40 T gene or the oncogenic human papillomavirus
(HPV) E6 and E7 genes, will generally either increase the rate of
cell division or inhibit programmed cell death (Fig. 2), thereby they will increase the
risk of malignant transformation (48).
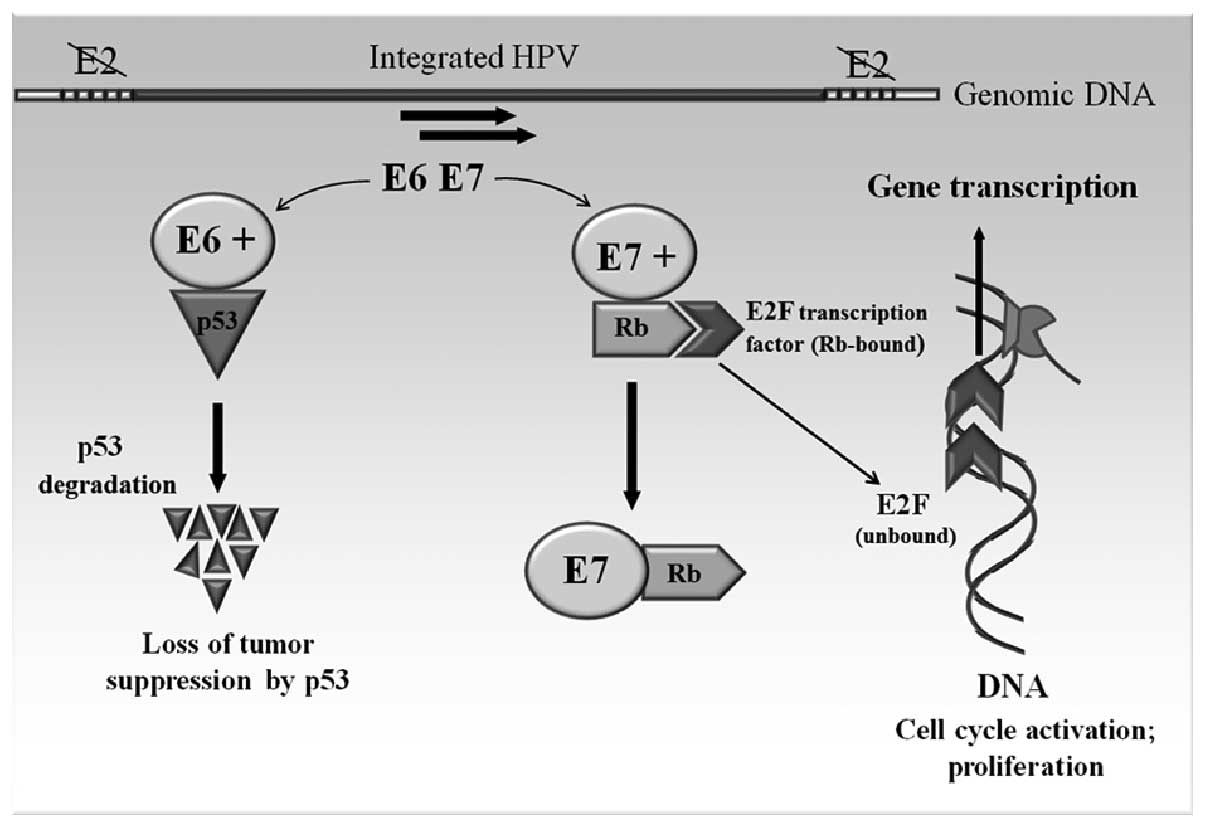
The molecular mechanism of HPV carcinogenesis can be
explained by the regulation and function of the two viral oncogenes
E6 and E7 (Fig. 3). These two
genes of HPV 18 have been shown to possess transforming ability
when transfecting into NIH 3T3 and Rat-1 cell lines (49). The E6 and E7 genes are under the
regulation of the E2 gene product. The E2 gene is often the site
for the integration, resulting in the disruption of the E2 gene and
subsequent derepression of the E6 and E7 (50). The E6 gene product binds to the p53
tumor suppressor gene. The association of E6 with p53 leads to the
specific ubiquitination and degradation of p53 protein (51). E7 targets another tumor suppressor
protein, the retinoblastoma gene product (pRb) (52). Binding of the E7 to pRb alters its
phosphorylation state and thereby functionally inactivates this
protein, which, like p53, functions in the control of the cell
cycle. Normally, pRb binds the transcription factor E2F, which
functions in the progression of the cell cycle from G1 to the S
phase. The binding of E7 to pRb results in creation of an inactive
E7-pRb complex; on the other hand, disrupted binding of E2F to pRb
allows E2F to bind DNA and induce the cell growth and proliferation
(53,54).
Many oncogenes encode growth factors that stimulate
proliferation of keratinocytes including transforming growth
factor-α (TGF-α) and epidermal growth factor (EGF). Both proteins
are frequently overexpressed in squamous cell carcinomas (SCC) of
the head and neck, particularly of the oral cavity. Overall,
approximately half of all the cancers of the mucosa of the head and
neck are believed to contain mutations in a specific region of the
p53 gene (55). Disruption to the
function of p53 appears to be an early event in the head and neck
oncogenesis and has been linked to exposure to mutagens, such as
benzopyrenes, in tobacco. The inactivation of the product of the
retinoblastoma gene (pRB), although it is less common than the p53,
malfunctions in SCC of the head and neck (44). Although the significance of the p53
and pRb has been known for many years, the recent finding that
malfunction of other genes controlling the cell cycle can lead to
malignant transformation represents an important advance in the
cancer biology (47).
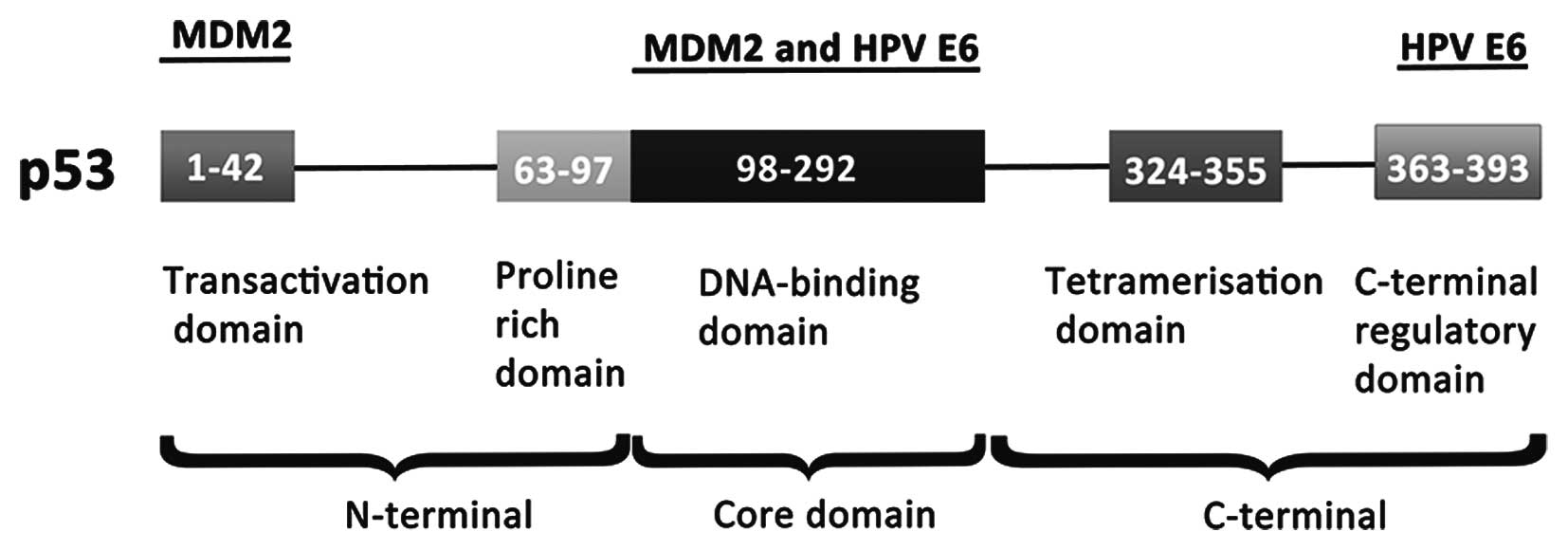
Interaction of HPV E6 and p53 proteins
The p53 is a tumor suppressor gene and a key
regulator of the cell proliferation. Due to its central role in the
regulation of the cell cycle, p53 is systematically dysregulated in
cancers (56). The p53 protein is
a multifunctional protein that consists of 393 amino acids
(Fig. 4). The cellular MDM2
protein, first known as a transcriptional target of p53, has been
found to act as an E3 ubiquitin-ligase, which transfers ubiquitin
(Ub) onto p53, thereby targeting it to proteasome-mediated
degradation (57,58). The p53 tumor suppressor is the
first described and best known target of HPV E6 (37). The presence of the E6 in the
high-risk types of HPV interferes with this process, because E6
binds to both p53 and E6-associated protein ligase (E6AP), causing
ubiquitinylation and the subsequent degradation of the p53. This
degradation then prevents p53 from inducing either the growth
arrest or apoptosis of infected cells (59). Papillomavirus E6 oncoproteins
interact with target cellular proteins through a conserved binding
motif containing the sequence LXXLL (60, 61). The cancer-associated human
papillomavirus type 16 protein E6 binds to the LXXLL motif (LQELL)
on the cellular E3 ubiquitin ligase E6AP (62).
Clinical correlations
Yu et al investigated the relation between
high-risk HPV 16/18 infection and p53 mutation in lung carcinomas
and its association with tumor behavior (63). The study indicated that mutation in
the p53 and HPV 16/18 infection might coordinate in the development
of lung squamous cell carcinomas, and their coexistence is
associated with poor prognosis. Within the group of lung squamous
cell carcinomas, the p53 mutation rate was significantly higher in
those with HPV infection (78.1%) than that of the non-infected
carcinomas (51.2%, P=0.004) (63).
Katori et al investigated the relationship between the
expression of p21 and p53 proteins, HPV infection and malignant
transformation in sinonasal-inverted papilloma (64). A significant decrease in expression
of p21 and p53 was observed in HPV 16/18 positive
sinonasal-inverted papilloma compared with HPV 16/18 negative
sinonasal-inverted papilloma, which could be caused by the
degradation of p53 in HPV infected sinonasal-inverted papilloma
(64). Similarly, Fujita et
al investigated HPV infection and the expression of p53 in
verrucous carcinoma (VC) (65).
The expression of p53 was correlated inversely with HPV infection.
Oral VC tumorigenesis may involve the inactivation of p53, which is
associated with HPV infection.
Therapy of HPV-related cancers
In order to develop a gene-specific therapy for
HPV-related cancers, Reschner et al investigated a potential
therapeutic strategy of the silencing of HPV16 E6 oncogene by using
an E6-antisense oligonucleotide (E6-ASO) in a polyazaaromatic
ruthenium (Ru-II) complex (E6-Ru-ASO) (66). This complex was able to crosslink
irreversibly the targeted sequence under visible illumination. They
demonstrated that E6-Ru-ASO induces a reactivation of p53, the most
important target of E6, as well as the inhibition of cell
proliferation with a selective repression of E6 at the protein
level after illumination in HPV16(+) SiHa cervical cancer cells
(66). High-risk types of HPV,
such as HPV16, have been detected in nearly all cases of cervical
cancer. Therapies targeted at blocking the HPV16 E6 protein and its
deleterious effects on the tumor suppressor pathways of the cell
can reverse the malignant phenotype of affected keratinocytes while
sparing uninfected cells. Togtema et al used sonoporation to
deliver the HPV16 E6 antibody into the HPV16 positive cervical
carcinoma derived cell lines (67). Delivery of the E6 antibody using
sonoporation significantly restored expression of p53 in these
cells, indicating that the antibody is able to enter the cells and
remains active. Cervical cancer develops via progression from
normal cervical epithelium through squamous intraepithelial lesions
(SIL) to invasive cancer. Cervical cancer is associated with
oncogenic human papillomavirus (HPV). The HPV E6 oncoprotein binds
to the tumor suppressor gene product p53 under promotion of its
degradation. The Arg allele of p53 containing Arg72 binds more
ardently with HPV E6 than the Pro72 variant. In individuals that
show HPV positivity, there was a significantly higher odds of
progression from SIL to cervical cancer with the p53 Arg allele
(68). Similarly, Chen et
al examined p53 codon 72 polymorphism and expression of HPV
oncoprotein in lung tumors from patients to determine the
polymorphism of p53 codon 72 (69). The presence of HPV 16/18 DNA and
the E6 protein was inversely associated with the expression of p53.
The frequency of degradation of p53 protein was also much higher in
HPV 16/18 E6-positive Arg/Arg (genotype at codon 72 of the p53
gene) lung tumors than in other groups (69).
HPV E6/E7 proteins and zinc fingers
The E6 protein of HPV16 consists of 158 amino acid
residues and contains two (Cys-X-X-Cys) zinc fingers (70–72).
This zinc finger sequence motif is unique for papillomavirus E6 and
E7 proteins and includes numerous specific amino acid residues,
highly conserved among all carcinogenic HPVs as well as among many
animal and human papillomaviruses associated with benign lesions
(73,74).
Beerheide et al (72) investigated 36 compounds selected
according to their structure for the ability to release zinc from
the E6 protein of HPV16. Nine of the 36 tested compounds released
zinc from E6, two of the nine compounds inhibited the interaction
of E6 with E6-associated protein and E6-binding protein, and one of
these two, 4,4′-dithiodimorpholine, selectively inhibited cell
viability and induced higher levels of p53 protein (associated with
the induction of apoptosis) in tumorigenic HPV-containing cells
(72). This assay system may be
useful in the development of drugs effective against cervical
cancer, genital warts, and asymptomatic infections by genital
HPVs.
Griffin et al (75) isolated an antibody fragment
(GTE6-1) that binds to the protein E6 of HPV16 in its first zinc
finger. The targeting of HPV16 oncogene may be an effective
anticancer therapy (75). HPV E6
proteins have two mechanisms to abolish the function of p53, the
repression of the transcriptional activity or eliminating the
protein through the proteosome degradation pathway. The
transcriptional coactivator CBP/p300 interacts with HPV-16 E6
protein in the C-terminal zinc finger to repress the p53-dependent
transcription (76).
The E6 oncoprotein of human papillomavirus (HPV) is
critical in the development of cervical cancer. Jong et al
have shown that HPV-16E6 (16E6) interacts with one of the DNA
fragmentation factors (DFFs), DFF40, which mediates the DNA
degradation during apoptosis (77). HPV-16 E6 interacts with DFF40
through its zinc finger motif 2 and a bridge section linking the
two zinc finger motifs.
The similar domains of E6 and E7 proteins allow the
formation of telomeric complexes that possess interesting
biological activities (78). The
mutations in the zinc finger domain of E7 do not affect the ability
to bind and deteriorate the pRB, but they do abrogate its ability
to immortalize cells (79). In the
human papillomavirus 83 (HPV83m) that produced cervical cancer,
five mutations were found in the second zinc finger domain of E6,
an important domain for protein-protein or protein-DNA
interactions. These mutations do not disrupt the p53 stability or
telomerase activity, and they act only as a specific modulator of
the transcriptional machinery. The mutation in the second zinc
finger can increase the oncogenic potential of HPV83 (80).
The pCAF acetyltransferase is a co-activator for a
variety of transcription factors including p53. An interaction of
the HPV 6, 16 and 18 E7 proteins with pCAF acetyltransferase has
been described (81). Mutation of
a highly conserved leucine residue within the zinc finger region of
HPV 16 E7 disrupts binding to pCAF and impairs transformation and
transcriptional activation (81).
Mino et al have constructed, as candidates
for new antiviral drugs, cell-permeable artificial zinc finger
proteins (AZPs), namely PTD-4 AZP, AZP-R9, AZPAla-R9 and E2C-R9,
for inhibition of HPV-18 DNA replication (82). The AZP used in this study is the
one that reduced the replication level of HPV 18 DNA to 12% of that
of a control. They confirmed that cell-permeable AZPs are effective
for inhibition of the HPV replication (82). Artificial zinc fingers (AZF) have
been designed as potent new inhibitors of HPV, thus, they block the
replication in episomal HPV infection, hindering the union of E2 to
origin of the viral replication (83). The avian papilloma-virus (PV) share
with the mammalian PV strictly conserved ORFs in E1, E2, L1 and L2
proteins (84). The differences of
avian PV from the mammalian PV is that avian E7 protein contain an
extended unfolded N-terminus and a zinc-binding domain of reduced
size, and the avian E6 proteins consist of a single zinc-binding
domain, whereas all mammalian E6 proteins always contain a pair of
zinc-binding domains (85). One
hypothesis suggests that it could be one common PV-single
zinc-binding domain ancestor and that the duplication event may
have taken place during the 310 million years separating birds and
mammals (86). A direct
interaction has been demonstrated between the HPV E2 and E7
proteins. It requires the hinge region of E2 and the zinc-binding
domain of E7. E2 is responsible for the stability of E7 and its
cellular location during mitosis (87). Mavromatis et al have shown
that the part carboxyl-terminus of human papillomavirus (HPV) E7
oncoprotein can be replaced by the zinc-binding domains of the HPV
E6 protein. This part is necessary for the functional and
structural integrity of the HPV (78).
Metallothionein in head and neck cancer
Metallothioneins (MT) are low-molecular weight
proteins involved in heavy metal detoxification, essential metal
ion homeostasis and cell protection against free radicals (88–92).
Several studies reported increased MT protein levels in malignant
tumors in head and neck area (93–95).
Sochor et al determined MT levels in tumor tissues of
patients suffering from head and neck tumors using differential
pulse voltammetry (94).
Fifty-five samples of tumor tissue were analyzed. The highest MT
level was determined in the tissues of oral tumors (170±70
μg/g) followed by hypopharynx (160±70 μg/g) and
larynx (160±70 μg/g). The relatively lowest MT level was
determined in tumors of oropharynx (130±50 μg/g). In the
following study, Krejcova et al analyzed MT levels in blood
of patients suffering from primary malignant tumor in head and neck
area also using differential pulse voltammetry (93). The tumor blood samples was
represented by patients suffering from oropharyngeal cancer,
laryngeal cancer, hypopharyngeal cancer, oral cavity cancer and
rarely occurring nasal cavity and paranasal sinus cancer. The
obtained data of MT level in blood of healthy human were from 0.2
to 0.8 μM. Determined MT levels in blood of oncological
patients varied from 1.08 to 6.39 μM (93). In the study of Dutsch-Wicherek
et al, tissue samples taken from patients with pharyngeal
squamous cell carcinoma were analyzed (96). An increased immunoreactivity levels
of MT was observed in the tissue samples from tonsillar squamous
cell carcinoma in comparison to reference group. Jayasurya et
al examined the relationship between MT expression and tissue
zinc levels in conjunction with cell proliferation in
nasopharyngeal cancer (NPC) (97).
Thirteen tumors displayed weak MT staining and the remaining 11
showed moderate to strong immunostaining. A linear relationship was
also observed between nuclear zinc levels and MT immunostaining. In
the next study Dutsch-Wicherek et al evaluated the MT
expression using immunohistochemistry in head and neck squamous
cells carcinoma and its histologically healthy adjacent tissue
(98). MT expression was revealed
in 85.7 % of head and neck cancers and MT expression was
statistically significantly higher in tumor adjacent tissue than in
cancer tissue in cases with the presence of lymph node metastases.
Therefore, it can be concluded that the increased MT expression is
observed in tumor tissues as well as in blood of patients with head
and neck cancers.
Zinc, zinc fingers, p53 and metallothionein
- is there any connection with HPV?
Zinc is an essential element that controls the
normal development of the cells, tissues, and organs via
zinc-containing proteins that orchestrate cell genesis,
differentiation and viability (99,100). Many transcriptional factors
contain zinc finger motifs. Zinc finger is able to form a complex
with DNA based on the interactions between α-helix of a zinc finger
and DNA-specific bases. The function of the zinc fingers consists
especially in the recognition of DNA and the activation of
transcriptional processes. Role of the zinc finger proteins in the
regulating the cell proliferation by HPV is described above. On the
other hand, p53 is a transcription factor encoded by the tumor
suppressor gene TP53 that binds DNA via structurally complex domain
stabilized by a zinc atom (101).
Genotoxic as well as non-genotoxic stress induce p53 and coordinate
pro-apoptotic (anti-proliferative) pathways to eliminate cells with
damaged DNA. The depletion of the intracellular zinc can induce a
change in the p53 protein conformation and the loss of DNA binding
capacity (101). The role of
metallothionein in the controlling the conformation and activity of
the p53 protein is still discussed (102,103). The intracellular level of
zinc(II) ions is strictly regulated in cells. Metallothionein (MT),
a family of proteins rich on cysteine residues, plays a key role in
the regulating the intracellular zinc levels and distribution
(88,89). MT is localized especially to the
membrane of the Golgi apparatus. On the other hand, Tohyama et
al (104) and Tsujikawa et
al (105) have shown presence
of MT in nuclei of hepatocytes, Nartey et al in fetal human
liver and kidney (106), and
Banerjee et al in nuclei of rat liver and kidney (107). Tohno et al have
characterized MT-binding chromatin after induction by
4-aminopyrazolo[3,4-d] pyrimidine (108). The MT-binding chromatin was
composed of supranucleosomal fibers. Localization of MT in myotomal
cell nuclei during somitogenesis of Xenopus laevis has been
described by Sunderman et al (109). These authors observed not only
presence of MT, but also its increasing after exposure to zinc(II)
ions. This fact indicates involvement of MT in the regulation of
the cell genesis, proliferation and viability. In light of these
facts, we must assume the role of metallothionein in the regulation
of transcription in HPV-infected cells via zinc(II) ions. Closer
information is unfortunately still lacking and further research to
determine the possible connection in the involvement of MT and
zinc(II) ions in HPV infection and regulation of the cell
proliferation and viability is necessary.
Abbreviations:
|
AZF
|
artificial zinc fingers
|
|
AZPs
|
artificial zinc finger proteins
|
|
BPV
|
bovine papillomavirus
|
|
EGF
|
epidermal growth factor
|
|
HNSCCs
|
head and neck squamous cell
carcinomas
|
|
HPV
|
human papillomavirus
|
|
MT
|
metallothionein
|
|
NPC
|
nasopharyngeal cancer
|
|
ORF
|
open reading frame
|
|
pRb
|
retinoblastoma protein
|
|
SILs
|
squamous intraepithelial lesions
|
|
SCCs
|
squamous cell carcinomas
|
|
SCJs
|
squamocolumnar junctions
|
|
TGF-α
|
transforming growth factor-α
|
Acknowledgements
Financial support from SPINCANCER
NT/14337 and CEITEC CZ.1.05/1.1.00/02.0068 is highly
acknowledged.
References
|
1.
|
Stanley M: Pathology and epidemiology of
HPV infection in females. Gynecol Oncol. 117:S5–S10. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
2.
|
Lowy DR, Strickland JE and Yuspa SH:
Efficient induction of papillomas by Harvey murine sarcoma-virus.
Clin Res. 34:A7641986.
|
|
3.
|
Joh J, Jenson AB, Proctor M, et al:
Molecular diagnosis of a laboratory mouse papillomavirus (MusPV).
Exp Mol Pathol. 93:416–421. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
4.
|
Mitsouras K, Faulhaber EA, Hui G, et al:
Development of a PCR assay to detect papillomavirus infection in
the snow leopard. BMC Vet Res. 7:1–11. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
5.
|
Badulescu F, Crisan A, Badulescu A and
Schenker M: Recent data about the role of human papillomavirus
(HPV) in oncogenesis of head and neck cancer. Rom J Morphol
Embryol. 51:437–440. 2010.PubMed/NCBI
|
|
6.
|
Fakhry C, Westra WH, Cmelak SLA, et al:
Improved survival of patients with human papillomavirus-positive
head and neck squamous cell carcinoma in a prospective clinical
trial. J Natl Cancer Inst. 100:261–269. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
7.
|
Syrjanen S: Human papillomavirus (HPV) in
head and neck cancer. J Clin Virol. 32:S59–S66. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
8.
|
Walden MJ and Aygun N: Head and neck
cancer. Semin Roentgenol. 48:75–86. 2013. View Article : Google Scholar
|
|
9.
|
Forte T, Niu J, Lockwood GA and Bryant HE:
Incidence trends in head and neck cancers and human papillomavirus
(HPV)-associated oropharyngeal cancer in Canada, 1992–2009. Cancer
Causes Control. 23:1343–1348. 2012.
|
|
10.
|
Axell T, Pindborg JJ, Smith CJ and van der
Waal I: Oral white lesions with special reference to precancerous
and tobacco related lesions: conclusions of an international
symposium held in Uppsala, Sweden, May 18–21 1994. J Oral Pathol
Med. 25:49–54. 1996.PubMed/NCBI
|
|
11.
|
Janicek MF and Averette HE: Cervical
cancer: prevention, diagnosis, and therapeutics. CA Cancer J Clin.
51:92–114. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
12.
|
Chen YC and Hunter DJ: Molecular
epidemiology of cancer. CA Cancer J Clin. 55:45–54. 2005.
View Article : Google Scholar
|
|
13.
|
De Villiers EM, Fauquet C, Broker TR,
Bernard HU and zur Hausen H: Classification of papillomaviruses.
Virology. 324:17–27. 2004.
|
|
14.
|
Bernard HU, Burk RD, Chen ZG, van
Doorslaer K, zur Hausen H and de Villiers EM: Classification of
papillomaviruses (PVs) based on 189 PV types and proposal of
taxonomic amendments. Virology. 401:70–79. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
15.
|
Doorbar J, Quint W, Banks L, et al: The
biology and life-cycle of human papillomaviruses. Vaccine.
30:F55–F70. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
16.
|
Chen RW, Aaltonen LM and Vaheri A: Human
papillomavirus type 16 in head and neck carcinogenesis. Rev Med
Virol. 15:351–363. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
17.
|
Pfister H: HPV and skin neoplasia.
Hautarzt. 59:26–30. 2008.(In German).
|
|
18.
|
Steben M and Duarte-Franco E: Human
papillomavirus infection: epidemiology and pathophysiology. Gynecol
Oncol. 107:S2–S5. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
19.
|
Chang KC, Su IJ, Tsai ST, Shieh DB and Jin
YT: Pathological features of betel quid-related oral epithelial
lesions in Taiwan with special emphasis on the tumor progression
and human papillomavirus association. Oncology. 63:362–369. 2002.
View Article : Google Scholar
|
|
20.
|
Kero K, Rautava J, Syrjanen K, Grenman S
and Syrjanen S: Oral mucosa as a reservoir of human papillomavirus:
point prevalence, genotype distribution, and incident infections
among males in a 7-year prospective study. Eur Urol. 62:1063–1070.
2012.
|
|
21.
|
Hong AM, Martin A, Armstrong BK, et al:
Human papillomavirus modifies the prognostic significance of T
stage and possibly N stage in tonsillar cancer. Ann Oncol.
24:215–219. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
22.
|
Milano MT, Peterson CR, Zhang H, Singh DP
and Chen Y: Second primary lung cancer after head and neck squamous
cell cancer: population-based study of risk factors. Head Neck.
34:1782–1788. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
23.
|
Ragin CCR and Taioli E: Survival of
squamous cell carcinoma of the head and neck in relation to human
papillomavirus infection: review and meta-analysis. Int J Cancer.
121:1813–1820. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
24.
|
Lin KY, Westra WH, Kashima HK, Mounts P
and Wu TC: Coinfection of HPV-11 and HPV-16 in a case of laryngeal
squamous papillomas with severe dysplasia. Laryngoscope.
107:942–947. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
25.
|
Syrjanen KJ, Chang F and Syrjanen SM: HPV
infections in etiology of benign and malignant sinonasal, bronchial
and oesophageal squamous cell lesions. Eurogin 2000: 4th
International Multidisciplinary Congress. Monsonego J: Medimond S R
L: 40128 Bologna; pp. 169–179. 2000
|
|
26.
|
Hoffmann M, Klose N, Gottschlich S, et al:
Detection of human papillomavirus DNA in benign and malignant
sinonasal neoplasms. Cancer Lett. 239:64–70. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
27.
|
Syrjanen S: Human papillomavirus
infections and oral tumors. Med Microbiol Immunol. 192:123–128.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
28.
|
Kashima HK, Kessis T, Hruban RH, Wu TC,
Zinreich SJ and Shah KV: Human papilloma virus in sinonasal
papillomas and squamous-cell carcinoma. Laryngoscope. 102:973–976.
1992. View Article : Google Scholar : PubMed/NCBI
|
|
29.
|
Mansell NJ and Bates GJ: The inverted
Schneiderian papilloma: a review and literature report of 43 new
cases. Rhinology. 38:97–101. 2000.PubMed/NCBI
|
|
30.
|
Zandberg DP, Bhargava R, Badin S and
Cullen KJ: The role of human papillomavirus in nongenital cancers.
CA Cancer J Clin. 63:57–81. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
31.
|
Ustav M, Ustav E, Szymanski P and Stenlund
A: Identification of the origin of replication of bovine
papillomavirus and characterization of the viral origin recognition
factor-E1. EMBO J. 10:4321–4329. 1991.PubMed/NCBI
|
|
32.
|
Baker CC, Phelps WC, Lindgren V, Braun MJ,
Gonda MA and Howley PM: Structural and transcriptional analysis of
human papillomavirus type-16 sequences in cervical-carcinoma
cell-lines. J Virol. 61:962–971. 1987.PubMed/NCBI
|
|
33.
|
Bouvard V, Storey A, Pim D and Banks L:
Characterization of the human papillomavirus E2 protein - evidence
of transactivation and transrepression in cervical keratinocytes.
EMBO J. 13:5451–5459. 1994.PubMed/NCBI
|
|
34.
|
Foguel D, Silva JL and de Prat-Gay G:
Characterization of a partially folded monomer of the DNA-binding
domain of human papillomavirus E2 protein obtained at high
pressure. J Biol Chem. 273:9050–9057. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
35.
|
Zur Hausen H: Cervical carcinoma and human
papillomavirus: on the road to preventing a major human cancer. J
Natl Cancer Inst. 93:252–253. 2001.
|
|
36.
|
Barbosa MS, Lowy DR and Schiller JT:
Papillomavirus polypeptide-E6 and polypeptide-E7 are zinc-binding
proteins. J Virol. 63:1404–1407. 1989.PubMed/NCBI
|
|
37.
|
Scheffner M, Werness BA, Huibregtse JM,
Levine AJ and Howley PM: The E6 oncoprotein encoded by human
papillomavirus type-16 and type-18 promotes the degradation of P53.
Cell. 63:1129–1136. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
38.
|
Vega-Pena A, Illades-Aguiar B,
Flores-Alfaro E, Lopez-Bayghen E, Reyes-Maldonado E and
Alarcon-Romero LD: Correlation between KI-67 and telomerase
expression with in situ hybridization for high-risk human
papillomavirus. Arch Biol Sci. 65:81–90. 2013. View Article : Google Scholar
|
|
39.
|
Li DS, Dong BL, Hu ZM, et al: A combined
assay of hTERT and E6 oncoprotein to identify virus-infected
keratinocytes with higher telomerase activity in human
papillomaviruses 16 and 18-related bowenoid papulosis. Am J
Dermatopathol. 34:813–817. 2012. View Article : Google Scholar
|
|
40.
|
Zhao YX, Qi L, Chen F, Zhao Y and Fan CH:
Highly sensitive detection of telomerase activity in tumor cells by
cascade isothermal signal amplification based on three-way junction
and base-stacking hybridization. Biosens Bioelectron. 41:764–770.
2013. View Article : Google Scholar
|
|
41.
|
Wilting SM, Verlaat W, Jaspers A, et al:
Methylation-mediated transcriptional repression of microRNAs during
cervical carcinogenesis. Epigenetics. 8:220–228. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
42.
|
Dayyani F, Etzel CJ, Liu M, Ho CH, Lippman
SM and Tsao AS: Meta-analysis of the impact of human papillomavirus
(HPV) on cancer risk and overall survival in head and neck squamous
cell carcinomas (HNSCC). Head Neck Oncol. 2:1–11. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
43.
|
Dyson N, Howley PM, Munger K and Harlow E:
The human papilloma virus-16 E7-oncoprotein is able to bind to the
retinoblastoma gene-product. Science. 243:934–937. 1989. View Article : Google Scholar : PubMed/NCBI
|
|
44.
|
Strati K, Pitot HC and Lambert PF:
Identification of biomarkers that distinguish human papillomavirus
(HPV)-positive versus HPV-negative head and neck cancers in a mouse
model. Proc Natl Acad Sci USA. 103:14152–14157. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
45.
|
Smith EM, Pawlita M, Rubenstein LM, Haugen
TH, Hamsikova E and Turek LP: Risk factors and survival by HPV-16
E6 and E7 antibody status in human papillomavirus positive head and
neck cancer. Int J Cancer. 127:111–117. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
46.
|
Doorbar J and Gallimore PH: Identification
of proteins encoded by the L1 and L2 open reading frames of human
papillomavirus 1a. J Virol. 61:2793–2799. 1987.PubMed/NCBI
|
|
47.
|
Rose BR, Thompson CH, Tattersall MH,
O’Brien CJ and Cossart YE: Squamous carcinoma of the head and neck:
molecular mechanisms and potential biomarkers. Aust N Z J Surg.
70:601–606. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
48.
|
Wiest T, Schwarz E, Enders C,
Flechtenmacher C and Bosch FX: Involvement of intact HPV16 E6/E7
gene expression in head and neck cancers with unaltered p53 status
and perturbed pRb cell cycle control. Oncogene. 21:1510–1517. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
49.
|
Bedell MA, Jones KH and Laimins LA: The
E6-E7 region of human papillomavirus type-18 is sufficient for
transformation of NIH-3T3 and RAT-1 cells. J Virol. 61:3635–3640.
1987.PubMed/NCBI
|
|
50.
|
Choo KB, Pan CC and Han SH: Integration of
human papillomavirus type-16 into cellular DNA of
cervical-carcinoma-preferential deletion of the E2 gene and
invariable retention of the long control region and the E6/E7 open
reading frames. Virology. 161:259–261. 1987. View Article : Google Scholar
|
|
51.
|
Huibregtse JM, Scheffner M and Howley PM:
Cloning and expression of the cDNA for E6-AP, a protein that
mediates the interaction of the human papillomavirus E6 oncoprotein
with P53. Mol Cell Biol. 13:775–784. 1993.PubMed/NCBI
|
|
52.
|
Chellappan S, Kraus VB, Kroger B, et al:
Adenovirus-E1A, simian virus-40 tumor-antigen, and human
papillomavirus-E7 protein share the capacity to disrupt the
interaction between transcription factor-E2F and the retinoblastoma
gene-product. Proc Natl Acad Sci USA. 89:4549–4553. 1992.
View Article : Google Scholar
|
|
53.
|
Cobrinik D, Dowdy SF, Hinds PW, Mittnacht
S and Weinberg RA: The retinoblastoma protein and the regulation of
cell cycling. Trends Biochem Sci. 17:312–315. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
54.
|
Nevins JR: E2F - a link between the Rb
tumor suppressor protein and viral oncoproteins. Science.
258:424–429. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
55.
|
Gillison ML, Koch WM, Capone RB, et al:
Evidence for a causal association between human papillomavirus and
a subset of head and neck cancers. J Natl Cancer Inst. 92:709–720.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
56.
|
May P and May E: Twenty years of p53
research: structural and functional aspects of the p53 protein.
Oncogene. 18:7621–7636. 1999.PubMed/NCBI
|
|
57.
|
Yu ZK, Geyer RK and Maki CG:
MDM2-dependent ubiquitination of nuclear and cytoplasmic P53.
Oncogene. 19:5892–5897. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
58.
|
Haupt Y, Maya R, Kazaz A and Oren M: Mdm2
promotes the rapid degradation of p53. Nature. 387:296–299. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
59.
|
Huibregtse JM, Scheffner M and Howley PM:
A cellular protein mediates association of P53 with the E6
oncoprotein of human papillomavirus type-16 or type-18. EMBO J.
10:4129–4135. 1991.PubMed/NCBI
|
|
60.
|
Chen JJ, Hong YH, Rustamzadeh E, Baleja JD
and Androphy EJ: Identification of an alpha helical motif
sufficient for association with papillomavirus E6. J Biol Chem.
273:13537–13544. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
61.
|
Elston RC, Napthine S and Doorbar J: The
identification of a conserved binding motif within human
papillomavirus type 16 E6 binding peptides, E6AP and E6BP. J Gen
Virol. 79:371–374. 1998.PubMed/NCBI
|
|
62.
|
Huibregtse JM, Scheffner M and Howley PM:
Localization of the E6-AP regions that direct human papillomavirus
E6 binding, association with P53, and ubiquitination of associated
proteins. Mol Cell Biol. 13:4918–4927. 1993.PubMed/NCBI
|
|
63.
|
Yu Y, Yang AM, Hu SK, Zhang JH and Yan H:
Significance of human papillomavirus 16/18 infection in association
with p53 mutation in lung carcinomas. Clin Respir J. 7:27–33. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
64.
|
Katori H, Nozawa A and Tsukuda M:
Relationship between p21 and p53 expression, human papilloma virus
infection and malignant transformation in sinonasal-inverted
papilloma. Clin Oncol. 18:300–305. 2006. View Article : Google Scholar
|
|
65.
|
Fujita S, Senba M, Kumatori A, Hayashi T,
Ikeda T and Toriyama K: Human papillomavirus infection in oral
verrucous carcinoma: genotyping analysis and inverse correlation
with p53 expression. Pathobiology. 75:257–264. 2008. View Article : Google Scholar
|
|
66.
|
Reschner A, Bontems S, Le Gac S, et al:
Ruthenium oligonucleotides, targeting HPV16 E6 oncogene, inhibit
the growth of cervical cancer cells under illumination by a
mechanism involving p53. Gene Ther. 20:435–443. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
67.
|
Togtema M, Pichardo S, Jackson R, Lambert
PF, Curiel L and Zehbe I: Sonoporation delivery of monoclonal
antibodies against human papillomavirus 16 E6 restores p53
expression in transformed cervical keratinocytes. PLoS One. 7:1–12.
2012. View Article : Google Scholar
|
|
68.
|
Habbous S, Pang V, Eng L, et al: p53
Arg72Pro polymorphism, HPV status and initiation, progression, and
development of cervical cancer: a systematic review and
meta-analysis. Clin Cancer Res. 18:6407–6415. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
69.
|
Chen SP, Hsu NY, Wu JY, et al: Association
of p53 codon 72 genotypes and clinical outcome in human
papillomavirus-infected lung cancer patients. Ann Thorac Surg.
95:1196–1203. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
70.
|
Grossman SR and Laimins LA: E6-protein of
human papillomavirus type-18 binds zinc. Oncogene. 4:1089–1093.
1989.PubMed/NCBI
|
|
71.
|
Kanda T, Watanabe S, Zanma S, Sato H,
Furuno A and Yoshiike K: Human papillomavirus type-16 E6 proteins
with glycine substitution for cysteine in the metal-binding motif.
Virology. 185:536–543. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
72.
|
Beerheide W, Bernard HU, Tan YJ, Ganesan
A, Rice WG and Ting AE: Potential drugs against cervical cancer:
zinc-ejecting inhibitors of the human papillomavirus type 16 E6
oncoprotein. J Natl Cancer Inst. 91:1211–1220. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
73.
|
Chan SY, Delius H, Halpern AL and Bernard
HU: Analysis of genomic sequences of 95 papillomavirus types -
uniting typing, phylogeny, and taxonomy. J Virol. 69:3074–3083.
1995.PubMed/NCBI
|
|
74.
|
Ullman CG, Haris PI, Galloway DA, Emery VC
and Perkins SJ: Predicted alpha-helix/beta-sheet secondary
structures for the zinc-binding motifs of human papillomavirus E7
and E6 proteins by consensus prediction averaging and spectroscopic
studies of E7. Biochem J. 319:229–239. 1996.
|
|
75.
|
Griffin H, Elston R, Jackson D, et al:
Inhibition of papillomavirus protein function in cervical cancer
cells by intrabody targeting. J Mol Biol. 355:360–378. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
76.
|
Zimmermann H, Degenkolbe R, Bernard HU and
O’Connor MJ: The human papillomavirus type 16 E6 oncoprotein can
down-regulate p53 activity by targeting the transcriptional
coactivator CBP/p300. J Virol. 73:6209–6219. 1999.PubMed/NCBI
|
|
77.
|
Jong JE, Jeong KW, Shin H, Hwang LR, Lee D
and Seo T: Human papillomavirus type 16 E6 protein inhibits DNA
fragmentation via interaction with DNA fragmentation factor 40.
Cancer Lett. 324:109–117. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
78.
|
Mavromatis KO, Jones DL, Mukherjee R, Yee
C, Grace M and Munger K: The carboxyl-terminal zinc-binding domain
of the human papillomavirus E7 protein can be functionally replaced
by the homologous sequences of the E6 protein. Virus Res.
52:109–118. 1997. View Article : Google Scholar
|
|
79.
|
Wayengera M: Zinc finger arrays binding
human papillomavirus types 16 and 18 genomic DNA: precursors of
gene-therapeutics for in-situ reversal of associated cervical
neoplasia. Theor Biol Med Model. 9:1–13. 2012. View Article : Google Scholar
|
|
80.
|
Cannavo I, Benchetrit M, Loubatier C,
Michel G, Lemichez E and Giordanengo V: Characterization of a
cluster of oncogenic mutations in E6 of a human papillomavirus 83
variant isolated from a high-grade squamous intraepithelial lesion.
J Gen Virol. 92:2428–2436. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
81.
|
Avvakumov N, Torchia J and Mymryk JS:
Interaction of the HPV E7 proteins with the pCAF acetyltransferase.
Oncogene. 22:3833–3841. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
82.
|
Mino T, Mori T, Aoyama Y and Sera T:
Cell-permeable artificial zinc-finger proteins as potent antiviral
drugs for human papillomaviruses. Arch Virol. 153:1291–1298. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
83.
|
Olthof NC, Straetmans J, Snoeck R,
Ramaekers FCS, Kremer B and Speel EJM: Next-generation treatment
strategies for human papillomavirus-related head and neck squamous
cell carcinoma: where do we go? Rev Med Virol. 22:88–105. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
84.
|
Garcia-Vallve S, Alonso A and Bravo IG:
Papillomaviruses: different genes have different histories. Trends
Microbiol. 13:514–521. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
85.
|
Van Doorslaer K, Sidi A, Zanier K, et al:
Identification of unusual E6 and E7 proteins within avian
papillomaviruses: cellular localization, biophysical
characterization, and phylogenetic analysis. J Virol. 83:8759–8770.
2009.
|
|
86.
|
Cole ST and Danos O: Nucleotide-sequence
and comparative-analysis of the human papillomavirus type 18
genome. Phylogeny of papillomaviruses and repeated structure of the
E6 and E7 gene products. J Mol Biol. 193:599–608. 1987. View Article : Google Scholar : PubMed/NCBI
|
|
87.
|
Gammoh N, Grm HS, Massimi P and Banks L:
Regulation of human papillomavirus type 16 E7 activity through
direct protein interaction with the E2 transcriptional activator. J
Virol. 80:1787–1797. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
88.
|
Ruttkay-Nedecky B, Nejdl L, Gumulec J, et
al: The role of metallothionein in oxidative stress. Int J Mol Sci.
14:6044–6066. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
89.
|
Krizkova S, Ryvolova M, Hrabeta J, et al:
Metallothioneins and zinc in cancer diagnosis and therapy. Drug
Metab Rev. 44:287–301. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
90.
|
Eckschlager T, Adam V, Hrabeta J, Figova K
and Kizek R: Metallothioneins and cancer. Curr Protein Pept Sci.
10:360–375. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
91.
|
Krizkova S, Fabrik I, Adam V, Hrabeta J,
Eckschlager T and Kizek R: Metallothionein - a promising tool for
cancer diagnostics. Bratisl Lek Listy. 110:93–97. 2009.PubMed/NCBI
|
|
92.
|
Babula P, Masarik M, Adam V, et al:
Mammalians’ metallothioneins and their properties and functions.
Metallomics. 4:739–750. 2012.
|
|
93.
|
Krejcova L, Fabrik I, Hynek D, et al:
Metallothionein electrochemically determined using Brdicka reaction
as a promising blood marker of head and neck malignant tumours. Int
J Electrochem Sci. 7:1767–1784. 2012.
|
|
94.
|
Sochor J, Hynek D, Krejcova L, et al:
Study of metallothionein role in spinocellular carcinoma tissues of
head and neck tumours using Brdicka reaction. Int J Electrochem
Sci. 7:2136–2152. 2012.
|
|
95.
|
Masarik M, Cernei N, Majzlik P, et al:
Level of metallothionein, glutathione and heat-stable proteins in
tumours from patients with head and neck cancer. Int J Mol Med.
26:S462010.
|
|
96.
|
Dutsch-Wicherek M, Lazar A, Tomaszewska R,
Kazmierczak W and Wicherek L: Analysis of metallothionein and
vimentin immunoreactivity in pharyngeal squamous cell carcinoma and
its microenvironment. Cell Tissue Res. 352:341–349. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
97.
|
Jayasurya A, Bay BH, Yap WM, Tan NG and
Tan BKH: Proliferative potential in nasopharyngeal carcinoma:
correlations with metallothionein expression and tissue zinc
levels. Carcinogenesis. 21:1809–1812. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
98.
|
Dutsch-Wicherek M, Popiela TJ, Klimek M,
et al: Metallothionein stroma reaction in tumor adjacent healthy
tissue in head and neck squamous cell carcinoma and breast
adenocarcinoma. Neuroendocrinol Lett. 26:567–574. 2005.PubMed/NCBI
|
|
99.
|
Babula P, Kohoutkova V, Opatrilova R,
Dankova I, Masarik M and Kizek R: Pharmaceutical importance of zinc
and metallothionein in cell signalling. Chim Oggi-Chem Today.
28:18–21. 2010.
|
|
100.
|
Gumulec J, Masarik M, Krizkova S, et al:
Insight to physiology and pathology of zinc(II) ions and their
actions in breast and prostate carcinoma. Curr Med Chem.
18:5041–5051. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
101.
|
Meplan C, Richard MJ and Hainaut P:
Metalloregulation of the tumor suppressor protein p53: zinc
mediates the renaturation of p53 after exposure to metal chelators
in vitro and in intact cells. Oncogene. 19:5227–5236. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
102.
|
Hainaut P and Mann K: Zinc binding and
redox control of p53 structure and function. Antioxid Redox Signal.
3:611–623. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
103.
|
Pintus SS, Ivanisenko NV, Demenkov PS, et
al: The substitutions G245C and G245D in the
Zn2+-binding pocket of the p53 protein result in
differences of conformational flexibility of the DNA-binding
domain. J Biomol Struct Dyn. 31:78–86. 2013.PubMed/NCBI
|
|
104.
|
Tohyama C, Suzuki JS, Hemelraad J,
Nishimura N and Nishimura H: Induction of metallothionein and its
localization in the nucleus of rat hepatocytes after
partial-hepatectomy. Hepatology. 18:1193–1201. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
105.
|
Tsujikawa K, Imai T, Kakutani M, et al:
Localization of metallothionein in nuclei of growing primary
cultured adult-rat hepatocytes. FEBS Lett. 283:239–242. 1991.
View Article : Google Scholar : PubMed/NCBI
|
|
106.
|
Nartey NO, Banerjee D and Cherian MG:
Immunohistochemical localization of metallothionein in cell-nucleus
and cytoplasm of fetal human-liver and kidney and its changes
during development. Pathology. 19:233–238. 1987. View Article : Google Scholar : PubMed/NCBI
|
|
107.
|
Banerjee D, Onosaka S and Cherian MG:
Immunohistochemical localization of metallothionein in cell-nucleus
and cytoplasm of rat-liver and kidney. Toxicology. 24:95–105. 1982.
View Article : Google Scholar : PubMed/NCBI
|
|
108.
|
Tohno Y, Tohno S, Minami T, et al:
Bindings of metallothionein to supranucleosomal fibers in mouse
pancreatic nuclei after induction by 4-aminopyrazolo [3,4-d]
pyrimidine. Cell Mol Biol. 42:1121–1127. 1996.PubMed/NCBI
|
|
109.
|
Sunderman FW, GrbacIvankovic S, Plowman MR
and Davis M: Zn2+-induction of metallothionein in
myotomal cell nuclei during somitogenesis of Xenopus laevis.
Mol Reprod Dev. 43:444–451. 1996.
|
|
110.
|
Bernard X, Robinson P, Nomine Y, et al:
Proteasomal degradation of p53 by human papillomavirus E6
oncoprotein relies on the structural integrity of p53 core domain.
PLoS One. 6:1–10. 2011. View Article : Google Scholar : PubMed/NCBI
|