Introduction
In May, 2011, the International Agency for Research
on Cancer (IARC) at WHO evaluated the carcinogenic effect to humans
from radiofrequency electromagnetic fields (RF-EMF). It included
radiation from mobile phones, and from other devices that emit
similar non-ionising electromagnetic fields. It was concluded that
RF-EMF is a group 2B, i.e. a ‘possible’ human carcinogen (1,2).
The IARC evaluation of mobile phones was based
mainly on case-control studies from the Hardell group in Sweden and
the IARC Interphone study. Both sets of studies provided
corroborative results, demonstrating an association between two
types of brain tumours, glioma and acoustic neuroma, with exposure
to RF-EMF from wireless phones. There was no consistent pattern of
an association within the studied latency period (time since first
exposure) with the most common benign brain tumour, meningioma,
suggesting specificity for these other tumour types. However, it
should be noted that in Interphone a reduced risk was found for
glioma among regular users of mobile phones but an increased risk
was found in the highest cumulative exposure group, >1,640 h
(3). Clearly an increased risk was
found using 1–1.9 years as reference entity (data not shown). The
pros and cons in the Interphone study have been discussed in
several articles, e.g. Hardell et al (4,5),
Cardis and Sadetzki (6).
We first provide some background to the development
of the wireless technology because of its relevance to
understanding the nature of exposures and exposure assessments.
The Nordic countries were among the first countries
in the world to widely adopt wireless telecommunications
technology. Analogue phones (NMT, Nordic Mobile Telephone System)
were introduced in the early 1980s using both 450 and 900 Megahertz
(MHz) frequencies. NMT 450 was used in Sweden from 1981, but closed
down on 31 December, 2007; NMT 900 operated during 1986–2000.
The digital system (GSM, Global System for Mobile
Communication) using dual band, 900 and 1,800 MHz, started to
operate in 1991, and it now dominates the market. The third
generation of mobile phones, 3G or UMTS (Universal Mobile
Telecommunication System), using 1,900/2,100 MHz RF fields has been
introduced worldwide in recent years, and in Sweden in 2003.
Currently, the fourth generation, 4G (Terrestrial 3G), operating at
800/2,600 MHz, and Trunked Radio Communication (TETRA 380–400 MHz)
are being established in Sweden and elsewhere in Europe. Nowadays
mobile phones are used more than landline phones in Sweden
(http://www.pts.se/upload/Rapporter/Tele/2011/sv-telemarknad-halvar-2011-pts-er-2011-21.pdf).
Worldwide, an estimate of 5.9 billion mobile phone subscriptions
was reported at the end of 2011 by the International
Telecommunication Union (http://www.itu.int/ITU-D/ict/facts/2011/material/ICTFactsFigures2011.pdf).
Desktop cordless phones (DECT) have been used in
Sweden since 1988, first using analogue 800–900 MHz RF fields, but
since the early 1990s using a digital 1,900 MHz system. They are
very common, overtaking phones connected to landlines. Also, these
devices emit RF-EMF radiation when used and should be equally
considered as mobile phones when human health risks are
evaluated.
The old analogue phones in Sweden, the so called
NMT, had an output power of 1 W and were very seldom down-regulated
giving lower RF-EMF emissions when used since the distance between
the base stations was several kilometers. The GSM phones are
transmitting in a pulsed mode, active 1/8 of the time, and with a
maximum output power of 2 W. This could be downregulated depending
on the distance to the base stations. A typical mean value for the
average output power is around 50–60 mW. The phone always starts
the call with the maximum power before going down in power. The
digital cordless phones operate in pulsed mode with a duty cycle of
1/24, the peak power is 250 mW. It is only the newer models that
have regulation of the output power. The old ones always stayed
with peak 250 mW, giving a time average of about 10 mW.
The absorption pattern, i.e. SAR values, associated
with the phones is very different between different phones; some
can give the peak value above the ear, some on the ear, and some
even below the ear, see for instance Wilén et al (7). There are no known measurements of SAR
for the cordless phones.
The first indication of an increased risk for brain
tumours associated with the use of mobile phones was published more
than 10 years ago (8). For tumours
located in the temporal, occipital or temporoparietal lobe areas of
the brain, an increased risk was found for ipsilateral mobile phone
use. Exposure to radiation from wireless phones (mobile and
cordless) is generally highest in the part of the brain that is
near to the ear, the temporal lobe, on the same side of the head as
the phone is generally held, ipsilateral exposure (9).
However, because these early results were based on
low numbers of exposed people and different histopathological types
of brain tumours, no firm conclusions could be drawn. Furthermore,
this first study did not include the use of cordless phones
(8,10). The next study from the Hardell
group included cases diagnosed in the period 1997–2003, and was
larger than the first study. This time, the use of cordless phones
was also assessed. Further details may be found in the various
publications that are based on the results from these studies
(11–16).
The Interphone study was conducted at 16 research
centres in 13 countries during varying time periods between 2000
and 2004. It was an international collaboration on brain tumour
risk and mobile phone use, conducted under the aegis of IARC. Cases
were diagnosed during 2000–2004, with slight variations in the
different study regions (3,17).
In contrast to the Hardell group studies, Interphone did not assess
or present results for cordless phone use. These are the only
studies to date that provide results for latency periods exceeding
10 years.
Exponential increases in access to and ownership of
wireless phones in most countries has occurred since the end of the
1990s. Because the technology is relatively recent, results on
health risks for long-term use, exceeding decades, are still
lacking. Moreover, in Sweden the major increase in use (duration in
minutes of calls) and exposure to radiation fields from these
phones (not merely access to or ownership of) in the general
population is most evident after 2003 (18).
To obtain results for longer exposure periods of
wireless phone use, we conducted an entirely new study on brain
tumours. In this article, we present the most recent results for
malignant brain tumours. Updated results and discussions of this
research area can be found elsewhere (5,19).
The study was approved by the ethics committee: Regional Ethics
Committee, Uppsala University; Uppsala, Sweden. DNR 2005:367.
Materials and methods
Case ascertainment
Sweden comprises six administrative medical regions
each having a cancer registry; annually, these registries are
linked to the national Swedish cancer register. The reporting to us
of newly diagnosed brain tumour cases varied between these six
regions, from once a month to once a year from one region (Umeå).
In our previous studies covering the time period 1997–2003, we
received reports on new cases as these arose, or one to two times
per month. For logistical reasons, this was not possible in the
present study for the different cancer registries.
Inclusion criteria
The inclusion criteria specified both men and women
aged 18–75 years at the time of brain tumour diagnosis (ICD-7 code
193.0) during the period 2007 to 2009. Furthermore, the diagnosis
had to be verified histopathology for all cases and only living
cases were included in the study. The cases were reported to us
from population-based cancer registries from across all regions of
Sweden. For administrative reasons, the Gothenburg region could be
included for only the years 2008 and 2009. All patients, both with
a malignant or a benign brain tumour, were included in the whole
study. Once the inclusion criteria were satisfied, the attending
physician was contacted for permission to include the case in the
study. The present publication presents results for cases with a
malignant brain tumour.
The Swedish Population Registry was used for
identification of controls. One control matched on gender and in
5-year age groups was used for each case, both malignant and benign
brain tumour cases. All controls were recruited from the same
source population (residential) as the cases. Controls were only
selected to the finally included living cases. They were assigned
the same year as the diagnosis of the respective case as the
cut-off in assessing exposure. Thus, the same methods were used as
in our previous studies (12,13).
Exposure assessment
Use of wireless phones, both mobile and cordless,
was assessed by a self-administered questionnaire supplemented over
the phone. Both cases and controls received an introduction letter
and were asked if they were willing to participate and answer the
included questionnaire. To get as high response rate as possible
two reminders were sent. All mobile phones in Sweden have had
either prefix 010 (analogue type) or prefix 07 (digital type). Thus
by asking for the prefix it was possible both to verify use of a
mobile phone and the type. The questionnaire also contained a
number of other questions on, for example, occupational history,
exposure to different agents, smoking habits, medical history
including hereditary risk factors, and exposure to ionizing
radiation. All questions were supplemented over the phone by the
interviewer at the same time. A structured protocol was used for
all questions as a prompt. The written questionnaire was evaluated
and further interviews were made according to the protocol. Most
subjects were also phone interviewed to clarify different aspects
in the questionnaire. There was no difference regarding
supplementary interviews according to being a case (75%
supplemented) or a control (70% supplemented). Adjusting for
whether or not a supplementary interview was performed did not
change the results of the logistic regression analysis.
The ear that had mostly been used during calls with
mobile and/or cordless phones was assessed by separate questions;
>50% of the time for one side, or equally much for both sides.
After informed consent from the patients, medical records including
computer tomography (CT) and/or magnetic resonance imaging (MRI)
were used to define tumour localization. The matched control was
assigned the same side as the tumour of the respective case using
the same method as in previous studies (3,12,13,17).
The whole procedure was blind to exposure status. Use of the
wireless phone was defined as ipsilateral (≥50% of the time), or
contralateral (<50% of the time) in relation to tumour side.
All questionnaires received a unique identity number
that did not indicate case or control status. Thus, the interviewer
was blind to case or control status throughout data processing. The
interviewers used a structured protocol that avoided questions that
could reveal if the interviewee was a case or a control. All
information was coded and entered into a database. A random sample
of the questionnaires was coded twice by two independent persons
with similar results. Being a case or control was revealed only
during the statistical analyses.
Statistical methods
All analyses were done using StataSE 12.1. Odds
ratios (OR) and 95% confidence intervals (CI) were calculated using
unconditional logistic regression analysis including the whole
control sample (i.e. matched to both malignant and benign cases) to
increase the power in the study. This was possible since
adjustment/stratification was made for the two matching variables
(gender, and age within 5 years).
The unexposed category consisted of people who
reported no use of mobile or cordless phones, or a latency period
≤1 year (amount of time between first use of the phone and year of
diagnosis). As noted earlier, the same year as for each case
diagnosis was used for the corresponding control as the cut-off for
exposure accumulation. Furthermore, because of the low number of
unexposed cases, a further criterion was used, i.e. regardless of
latency being ≤1 year, cumulative use ≤39 h (3rd percentile) of
wireless phones in total among the controls was also used as
cut-off for the referent group of ‘no exposure’ among cases and
controls. The 3rd percentile was chosen to approximately correspond
to one working week.
A latency period ≤1 year was used, as in our
previous studies, to make it possible to analyse a late effect
(promotion) in brain tumour genesis (12,13).
Note that latency (time since first use until date of diagnosis)
was calculated separately for the respective phone type or
combination of phones that were analysed.
Latency was analysed using six time periods, >1–5
years, >5–10 years, >10–15 years, >15–20 years, >20–25
years and >25 years. Cumulative use of the phone types was
analysed in quartiles based on use of wireless phones in total
among the controls (first quartile >39–405 h, second quartile
406–1,091 h, third quartile 1,092–2,376 h, fourth quartile
>2,376 h). Wald's test was performed to analyze the trend of the
ORs across the quartiles of the phone types. Latency and cumulative
use were also analysed as continuous variables (per year of
latency, per 100 h cumulative use) to further explore the
dose-response relations.
Adjustment was made for the matching variables
gender, age (as a continuous variable) and year of diagnosis. In
addition, adjustment was made for socio-economic index (SEI)
divided into four categories (blue-collar worker, white-collar
worker, self-employed, no work). Note that laterality of the tumour
was not available for all cases, e.g., for midline tumours, or for
tumours in both hemispheres (n=38). These were dropped from the
laterality analysis together with controls (n=306) matched to cases
without laterality data in the whole material. Laterality analysis
was not made for the whole group of wireless phone users since the
side differed for mobile phone and cordless phone for some of the
included persons using both phone types (8.3% of the cases, 8.9% of
the controls).
Restricted cubic splines were used to visualize the
relationship between cumulative use and latency of wireless phones
and malignant brain tumours. Adjustment was made for the same
variables as in the logistic regression. Four knots were used at
the 5th, 35th, 65th and 95th percentiles as suggested by Harrell
(20). A p-value for non-linearity
was estimated by testing if the coefficient of the second and third
spline was equal to zero (20).
Most of the participating cases with a benign brain
tumour (n=814) had meningioma (n=709). These results will be
presented in another publication. As a further step to evaluate
potential recall or observational bias the meningioma cases in the
same study were used as the reference entity to the cases with
malignant brain tumour, c.f. Hardell (21).
Results
In Table I, the
number of reported malignant cases from the regional cancer
registries is shown. The largest numbers of cases excluded from the
study were those who were ‘deceased’ (n=520), mostly with an
astrocytoma WHO grade IV (glioblastoma multiforme). The
implications of this exclusion are addressed below in the
discussion section. The second largest group excluded was that with
‘no permission from the treating physician’ (n=56). Thus, of the
1,334 cases with a malignant tumour, 683 (51%) remained eligible
for inclusion. Regarding cases with a benign brain tumour (n=920)
these results are presented in separate articles; one on meningioma
(22) one on acoustic neuroma
(23).
 | Table I.Descriptive data on the study sample
of malignant brain tumour cases diagnosed between 2007 and
2009. |
Table I.
Descriptive data on the study sample
of malignant brain tumour cases diagnosed between 2007 and
2009.
| Malignant |
|---|
| Reported from
cancer registries | 1,334 |
| Deceased | 520 |
| Wrong
diagnosis | 18 |
| Diagnosed other
years | 2 |
| No address
available | 6 |
| Language
problems | 2 |
| Not capable to
participate | 47 |
| No permission from
physician | 56 |
| Total included | 683 |
| Refused to
participate | 90 |
| Answered the
questionnaire | 593 |
Medical records and reports to the cancer registries
were used to classify tumour histopathology. Of the 683 cases of
malignancy, 593 (87%) answered the questionnaire; 350 were men and
243 women. In Table II, the
various diagnoses of malignant brain tumours are shown. Most of the
cases were diagnosed with a glioma (astrocytoma, oligodendroglioma,
other/mixed glioma; n=546; 92%) with astrocytoma being the most
common subtype (n=415; 76% of glioma).
 | Table II.Histopathology of all malignant brain
tumours. |
Table II.
Histopathology of all malignant brain
tumours.
| Men | Women | Total |
|---|
|
|
|
|---|
| Histopathology | n | % | n | % | n | % |
|---|
| Astrocytoma grade
I–II | 53 | 15.1 | 44 | 18.1 | 97 | 16.4 |
| Grade I | 6 | 1.7 | 5 | 2.1 | 11 | 1.9 |
| Grade II | 47 | 13.4 | 39 | 16.0 | 86 | 14.5 |
| Astrocytoma grade
III–IV | 205 | 58.6 | 113 | 46.5 | 318 | 53.6 |
| Grade III | 30 | 8.6 | 15 | 6.2 | 45 | 7.6 |
| Grade IV | 175 | 50.0 | 98 | 40.3 | 273 | 46.0 |
|
Medulloblastoma | 3 | 0.9 | 2 | 0.8 | 5 | 0.8 |
|
Oligodendroglioma | 32 | 9.1 | 37 | 15.2 | 69 | 11.6 |
| Ependymoma | 10 | 2.9 | 9 | 3.7 | 19 | 3.2 |
| Other/mixed
glioma | 39 | 11.1 | 23 | 9.5 | 62 | 10.5 |
| Other
malignant | 8 | 2.3 | 15 | 6.2 | 23 | 3.9 |
| All malignant | 350 | | 243 | | 593 | |
For the total sample of 1,601 cases, an equal number
of matched controls received a questionnaire. Note that one case
had two tumours, astrocytoma grade IV and meningioma and another
case had ependymoma and acoustic neuroma. Of the included controls,
1,368 (85%) answered the questionnaire, 564 were men and 804 women.
The mean age was 52 years for cases with malignant brain tumour
(median 55, range 18–75) and 55 years for all controls (median 58,
range 19–75). Of the cases with meningioma 200 were men and 509
were women. The mean age was 57 years (median 59, range 23–75
years).
In Table III, the
results are shown for all malignant brain tumours and use of
wireless phones. Analogue phones yielded OR=1.8, 95% CI=1.04–3.3
increasing to OR=3.3, 95% CI=1.6–6.9 in the latency group of >25
years. Note that the latency time was counted from the first use of
the specific telephone type; for instance, a 2G user may have used
an analogue phone before.
 | Table III.Odds ratio (OR) and 95% confidence
interval (CI) for malignant brain tumours (n=593). |
Table III.
Odds ratio (OR) and 95% confidence
interval (CI) for malignant brain tumours (n=593).
| Analogue | Digital (2G) | Digital (UMTS,
3G) | Mobile phone,
total | Cordless phone | Digital type | Wireless phone |
|---|
|
|
|
|
|
|
|
|---|
| Latency | OR | CI | Ca/Co | OR | CI | Ca/Co | OR | CI | Ca/Co | OR | CI | Ca/Co | OR | CI | Ca/Co | OR | CI | Ca/Co | OR | CI | Ca/Co |
|---|
| Total, >1
year | 1.8 | 1.04–3.3 | 144/260 | 1.6 | 0.996–2.7 | 546/1,208 | 1.2 | 0.6–2.4 | 67/140 | 1.6 | 0.99–2.7 | 548/1,217 | 1.7 | 1.1–2.9 | 461/1,015 | 1.7 | 1.04–2.8 | 571/1,261 | 1.7 | 1.04–2.8 | 571/1,261 |
| >1–5 years | - | | 0/0 | 1.8 | 1.01–3.4 | 42/109 | 1.2 | 0.6–2.4 | 55/126 | 1.8 | 1.002–3.4 | 41/108 | 2.0 | 1.1–3.4 | 102/209 | 2.6 | 1.4–4.9 | 33/63 | 2.6 | 1.4–5.0 | 32/61 |
| >5–10 years | 0.6 | 0.1–3.1 | 2/10 | 1.6 | 0.97–2.7 | 213/477 | 1.6 | 0.5–4.9 | 12/14 | 1.7 | 0.98–2.8 | 190/423 | 1.6 | 0.95–2.7 | 188/436 | 1.6 | 0.9–2.7 | 177/421 | 1.6 | 0.98–2.8 | 163/378 |
| >10–15
years | 1.4 | 0.7–3.0 | 25/51 | 1.3 | 0.8–2.2 | 187/453 | - | | 0/0 | 1.3 | 0.8–2.2 | 163/399 | 1.6 | 0.9–2.8 | 108/248 | 1.4 | 0.8–2.3 | 212/523 | 1.3 | 0.8–2.2 | 184/466 |
| >15–20
years | 1.4 | 0.7–2.7 | 39/86 | 2.1 | 1.2–3.6 | 104/169 | - | | 0/0 | 1.5 | 0.8–2.6 | 76/174 | 2.1 | 1.2–3.8 | 57/109 | 2.2 | 1.3–3.6 | 143/241 | 1.7 | 1.02–3.0 | 110/231 |
| >20–25
years | 2.1 | 1.1–4.0 | 48/80 | - | | 0/0 | - | | 0/0 | 1.9 | 1.1–3.5 | 48/80 | 1.5 | 0.5–4.6 | 6/13 | 1.5 | 0.5–4.6 | 6/13 | 1.9 | 1.04–3.4 | 52/92 |
| >25 years | 3.3 | 1.6–6.9 | 30/33 | - | | 0/0 | - | | 0/0 | 2.9 | 1.4–5.8 | 30/33 | - | | 0/0 | - | | 0/0 | 3.0 | 1.5–6.0 | 30/33 |
Use of digital 2G phones gave an overall OR=1.6, 95%
CI=0.996–2.7. In the latency group >1–5 years, an OR=1.8, 95%
CI=1.01–3.4 was calculated. Lower ORs were obtained in the latency
groups >5–10 years and >10–15 years increasing to an OR=2.1,
95% CI=1.2–3.6 with latency >15–20 years, which was the longest
latency interval.
The results for digital 3G phones showed highest
risk in the >5–10 years latency group, OR=1.6, 95% CI=0.5–4.9.
This result was based on low numbers and no long-term users existed
since this technology is new. One case and no control reported use
of only a 3G phone.
A similar pattern as for digital 2G phones was found
for use of cordless phones with increased risk in the shortest
latency period, then dropping off and again increasing in the
latency group >15–20 years to an OR=2.1, 95% CI=1.2–3.8. Only 6
cases and 13 controls reported use of cordless phone with latency
>20–25 years, so these results are less reliable.
In Table III we
also display results for all uses of digital phones (2G, 3G and/or
cordless phone; ‘digital type’). The pattern of an association was
similar to 2G and cordless phones, with a statistically significant
increased risk in the shortest latency period, then dropping off
and again statistically significant increased risk in the latency
group >15–20 years giving an OR=2.2, 95% CI=1.3–3.6.
We further show results for all wireless phone use
combined. An increased risk was found overall with an OR=1.7, 95%
CI=1.04–2.8, increasing in the shortest latency period >1–5
years to an OR=2.6, 95% CI=1.4–5.0, then decreasing somewhat with
increasing latency; but with the highest risk is in the longest
latency period >25 years with an OR=3.0, 95% CI=1.5–6.0.
In Table IV results
are displayed when patients with meningioma in the same study are
used as controls. The results were similar as in Table III using the population based
controls. Most ORs were somewhat higher using meningioma cases as
controls.
 | Table IV.Odds ratio (OR) and 95 % confidence
interval (CI) for malignant brain tumours (n=593) and meningioma
cases (n=708) as reference entity. |
Table IV.
Odds ratio (OR) and 95 % confidence
interval (CI) for malignant brain tumours (n=593) and meningioma
cases (n=708) as reference entity.
| Analogue | Digital (2G) | Digital (UMTS,
3G) | Mobile phone,
total | Cordless phone | Digital type | Wireless phone |
|---|
|
|
|
|
|
|
|
|---|
| Latency | OR | CI | Ca/Co | OR | CI | Ca/Co | OR | CI | Ca/Co | OR | CI | Ca/Co | OR | CI | Ca/Co | OR | CI | Ca/Co | OR | CI | Ca/Co |
|---|
| Total, >1
year | 2.2 | 1.1–4.1 | 144/108 | 1.8 | 1.1–3.2 | 545/592 | 2.3 | 0.9–5.7 | 67/47 | 1.8 | 1.1–3.2 | 547/593 | 1.8 | 1.03–3.1 | 460/521 | 1.8 | 1.1–3.1 | 570/640 | 1.8 | 1.1–3.1 | 570/640 |
| >1–5 years | - | | 0/0 | 1.7 | 0.9–3.4 | 42/70 | 2.4 | 0.96–6.1 | 55/40 | 1.7 | 0.9–3.4 | 41/69 | 2.0 | 1.1–3.7 | 102/109 | 2.1 | 1.05–4.3 | 33/43 | 2.1 | 1.04–4.3 | 32/42 |
| >5–10 years | 1.1 | 0.1–8.3 | 2/3 | 2.0 | 1.1–3.5 | 212/235 | 1.4 | 0.3–6.0 | 12/7 | 1.9 | 1.1–3.4 | 189/216 | 1.7 | 0.96–3.0 | 187/216 | 1.8 | 1.05–3.2 | 176/221 | 1.9 | 1.05–3.3 | 162/205 |
| >10–15
years | 2.0 | 0.8–4.9 | 25/21 | 1.5 | 0.9–2.7 | 187/212 | - | | 0/0 | 1.5 | 0.8–2.7 | 163/185 | 1.6 | 0.9–2.8 | 108/128 | 1.5 | 0.9–2.7 | 212/248 | 1.4 | 0.8–2.5 | 184/226 |
| >15–20
years | 1.8 | 0.8–3.7 | 39/39 | 2.3 | 1.2–4.3 | 104/75 | - | | 0/0 | 1.8 | 0.9–3.3 | 76/78 | 2.1 | 1.1–4.1 | 57/61 | 2.2 | 1.2–3.9 | 143/121 | 1.9 | 1.1–3.4 | 110/115 |
| >20–25
years | 2.4 | 1.1–5.2 | 48/29 | - | | 0/0 | - | | 0/0 | 2.5 | 1.2–5.2 | 48/29 | 1.0 | 0.3–3.6 | 6/7 | 1.1 | 0.3–3.8 | 6/7 | 2.1 | 1.05–4.2 | 52/36 |
| >25 years | 3.0 | 1.3–7.4 | 30/16 | - | | 0/0 | - | | 0/0 | 3.1 | 1.3–7.1 | 30/16 | - | | 0/0 | - | | 0/0 | 3.1 | 1.3–7.0 | 30/16 |
Overall, in Table
V, ipsilateral use of analogue phones was associated with a
higher risk, OR=2.3, 95% CI=1.2–4.5, than contralateral use,
yielding OR=1.4, 95% CI=0.7–2.9. Ipsilateral use of digital 2G
phones yielded a higher OR than contralateral use. Mobile phones
overall for ipsilateral use, resulted in an OR=1.7, 95%
CI=1.01–2.9; and for contralateral use, an OR=1.4, 95% CI=0.8–2.5.
Ipsilateral use of cordless phones yielded an OR=1.9, 95%
CI=1.1–3.2 compared with an OR=1.6, 95% CI=0.9–2.8 for
contralateral use.
 | Table V.Odds ratio (OR) and 95 % confidence
interval (CI) for malignant brain tumours, total, ipsilateral and
contralateral exposure. |
Table V.
Odds ratio (OR) and 95 % confidence
interval (CI) for malignant brain tumours, total, ipsilateral and
contralateral exposure.
| All | Ipsilateral | Contralateral |
|---|
|
|
|
|---|
| Ca/Co | OR | 95% CI | Ca/Co | OR | 95% CI | Ca/Co | OR | 95% CI |
|---|
| Analogue | 144/260 | 1.8 | 1.04–3.3 | 84/118 | 2.3 | 1.2–4.5 | 46/84 | 1.4 | 0.7–2.9 |
| Digital (2G) | 546/1,208 | 1.6 | 0.996–2.7 | 322/530 | 1.7 | 1.02–2.9 | 190/404 | 1.4 | 0.8–2.5 |
| Digital (UMTS,
3G) | 67/140 | 1.2 | 0.6–2.4 | 38/69 | 1.2 | 0.5–2.8 | 24/45 | 1.1 | 0.4–3.1 |
| Mobile phone,
total | 548/1,217 | 1.6 | 0.99–2.7 | 324/534 | 1.7 | 1.01–2.9 | 190/407 | 1.4 | 0.8–2.5 |
| Cordless phone | 461/1,015 | 1.7 | 1.1–2.9 | 272/454 | 1.9 | 1.1–3.2 | 156/327 | 1.6 | 0.9–2.8 |
Cumulative use of wireless phones was analysed in
quartiles based on use of wireless phones in total among the
controls, see Table VI. Note that
for the various phone types, the cumulative time was counted for
use of the specific phone, but for the category ‘mobile phones’ all
types of mobile phones were included, and for ‘wireless phones’
also use of cordless phones was included. For all phone types and
combinations thereof, the highest ORs were found in the fourth
quartile, see Table VI. Thus, for
analogue phones, an OR=7.7, 95% CI=2.5–24 (p-trend=0.01) was
calculated, although based on low numbers. The digital (2G) phone
yielded an OR=3.2, 95% CI=1.8–5.6 (p-trend <0.0001) in the same
category. Also, UMTS (3G) resulted in an increased risk with an
OR=5.1, 95% CI=0.8–32 (p-trend=0.28); but based on low numbers. The
fourth quartile of cumulative cordless phone use yielded an OR=3.1,
95% CI=1.8–5.5 (p-trend <0.0001). Wireless phone use overall
resulted in an OR=2.5, 95% CI=1.5–4.2 (p-trend=0.0001) in the
fourth quartile with >2,376 h of cumulative use.
 | Table VI.Malignant brain tumours (n=593). |
Table VI.
Malignant brain tumours (n=593).
| Analogue | Digital (2G) | Digital (UMTS,
3G) | Mobile phone,
total | Cordless phone | Digital type | Wireless phone |
|---|
|
|
|
|
|
|
|
|---|
| Quartile | OR | CI | Ca/Co | OR | CI | Ca/Co | OR | CI | Ca/Co | OR | CI | Ca/Co | OR | CI | Ca/Co | OR | CI | Ca/Co | OR | CI | Ca/Co |
|---|
| First | 1.7 | 0.9–3.0 | 90/184 | 1.4 | 0.8–2.3 | 202/620 | 1.1 | 0.5–2.4 | 35/87 | 1.4 | 0.8–2.3 | 190/587 | 1.3 | 0.8–2.2 | 164/434 | 1.5 | 0.9–2.5 | 113/327 | 1.5 | 0.9–2.5 | 108/317 |
| Second | 1.6 | 0.8–3.4 | 22/47 | 1.9 | 1.1–3.3 | 138/260 | 1.0 | 0.4–2.6 | 16/34 | 1.7 | 1.02–3.0 | 126/261 | 1.7 | 1.01–3.0 | 120/278 | 1.4 | 0.8–2.4 | 113/320 | 1.4 | 0.8–2.4 | 110/314 |
| Third | 2.6 | 1.2–6.0 | 18/23 | 1.4 | 0.8–2.5 | 84/199 | 1.7 | 0.6–4.8 | 11/17 | 1.5 | 0.9–2.7 | 95/210 | 2.1 | 1.2–3.7 | 98/194 | 1.7 | 1.01–2.9 | 139/317 | 1.7 | 1.003–2.9 | 137/315 |
| Fourth | 7.7 | 2.5–24 | 14/6 | 3.2 | 1.8–5.6 | 122/129 | 5.1 | 0.8–32 | 5/2 | 2.8 | 1.6–4.8 | 137/159 | 3.1 | 1.8–5.5 | 79/109 | 2.6 | 1.5–4.3 | 206/297 | 2.5 | 1.5–4.2 | 216/315 |
The ORs increased to statistically significant per
100 h of cumulative use for all types of phones except for UMTS
(3G) with borderline significance, see Table VII. In a multivariate analysis
including all phone types (i.e. analogue, 2G, 3G and cordless
phone) similar results were found although not statistically
significant for analogue phones (OR=1.015, 95% CI=0.996–1.034; data
not shown). Wireless phone use increased the risk with an OR=1.009,
95% CI=1.006–1.012 per 100 h of cumulative use, Table VII. The risk increased also per
additional year of latency, mostly for analogue phones, OR=1.044,
95% CI=1.019–1.070. These results did not change if years of use of
any mobile or cordless phone prior to the respective type was
included as a covariate in each analysis of the individual phone
types (data not shown). Wireless phones overall yielded OR=1.018,
95% CI=1.001–1.036.
 | Table VII.Odds ratio (OR) and 95% confidence
interval (CI) for malignant brain tumours per 100 h cumulative use
and per year of latency. |
Table VII.
Odds ratio (OR) and 95% confidence
interval (CI) for malignant brain tumours per 100 h cumulative use
and per year of latency.
| Per 100 h
cumulative use | Per year of
latency |
|---|
|
|
|---|
| OR | 95% CI | OR | 95% CI |
|---|
| Analogue | 1.037 | 1.014–1.060 | 1.044 | 1.019–1.070 |
| Digital (2G) | 1.012 | 1.007–1.017 | 1.013 | 0.989–1.037 |
| Digital (UMTS,
3G) | 1.031 | 0.988–1.076 | 1.043 | 0.894–1.216 |
| Mobile phone,
total | 1.011 | 1.006–1.015 | 1.016 | 0.999–1.034 |
| Cordless phone | 1.013 | 1.007–1.020 | 1.014 | 0.992–1.036 |
| Digital type | 1.010 | 1.006–1.013 | 1.016 | 0.994–1.039 |
| Wireless phone | 1.009 | 1.006–1.012 | 1.018 | 1.001–1.036 |
 | Table VIII.Odds ratio (OR) and 95%
confidenceinterval (CI) for malignant brain tumours located in
temporal (n=161) and overlapping lobes [temporofrontal (n=31),
temporoparietal (n=22), temporooccipital (n=13)]; in total
n=227. |
Table VIII.
Odds ratio (OR) and 95%
confidenceinterval (CI) for malignant brain tumours located in
temporal (n=161) and overlapping lobes [temporofrontal (n=31),
temporoparietal (n=22), temporooccipital (n=13)]; in total
n=227.
| Analogue | Digital (2G) | Digital (UMTS,
3G) | Mobile phone,
total | Cordless phone | Digital type | Wireless phone |
|---|
|
|
|
|
|
|
|
|---|
| Latency | OR | CI | Ca/Co | OR | CI | Ca/Co | OR | CI | Ca/Co | OR | CI | Ca/Co | OR | CI | Ca/Co | OR | CI | Ca/Co | OR | CI | Ca/Co |
|---|
| Total, >1
year | 2.4 | 0.9–6.1 | 67/260 | 2.4 | 0.99–5.6 | 211/1,208 | 1.7 | 0.5–5.9 | 17/140 | 2.3 | 0.99–5.6 | 212/1,217 | 2.5 | 1.04–6.0 | 175/1,015 | 2.5 | 1.05–5.9 | 221/1,261 | 2.5 | 1.05–5.9 | 221/1,261 |
| >1–5 years | - | | 0/0 | 3.3 | 1.2–8.7 | 19/109 | 1.6 | 0.5–5.9 | 14/126 | 3.1 | 1.2–8.4 | 18/108 | 3.0 | 1.2–7.6 | 41/209 | 4.4 | 1.6–12 | 15/64 | 4.5 | 1.6–13 | 14/61 |
| >5–10 years | 0.9 | 0.1–9.1 | 1/10 | 2.4 | 0.96–5.7 | 79/477 | 2.1 | 0.3–14 | 3/14 | 2.4 | 0.97–5.8 | 69/423 | 2.2 | 0.9–5.4 | 68/436 | 2.4 | 0.99–5.9 | 68/420 | 2.4 | 0.98–5.9 | 60/378 |
| >10–15
years | 1.6 | 0.5–5.3 | 11/51 | 1.8 | 0.7–4.3 | 69/453 | - | | 0/0 | 1.6 | 0.7–4.1 | 57/399 | 2.3 | 0.9–5.7 | 41/248 | 1.8 | 0.8–4.5 | 77/523 | 1.8 | 0.7–4.4 | 66/466 |
| >15–20
years | 1.7 | 0.6–5.0 | 18/86 | 3.0 | 1.2–7.4 | 44/169 | - | | 0/0 | 2.0 | 0.8–5.2 | 31/174 | 2.8 | 1.05–7.4 | 21/109 | 3.0 | 1.2–7.4 | 57/241 | 2.3 | 0.9–5.8 | 42/231 |
| >20–25
years | 2.6 | 0.9–7.2 | 21/80 | - | | 0/0 | - | | 0/0 | 2.7 | 1.02–7.3 | 21/80 | 3.3 | 0.8–14 | 4/13 | 3.4 | 0.8–14 | 4/13 | 2.7 | 1.04–7.2 | 23/92 |
| >25 years | 5.1 | 1.7–16 | 16/33 | - | | 0/0 | - | | 0/0 | 4.8 | 1.7–14 | 16/33 | - | | 0/0 | - | | 0/0 | 5.1 | 1.8–15 | 16/33 |
In Table VIII,
results are presented for malignant brain tumours localized in the
temporal lobe or overlapping temporal and adjacent lobe. Higher
risk estimates were obtained than for the overall results. Thus,
mobile phone use in the latency group >25 years resulted in an
OR=4.8, 95% CI=1.7–14 compared with an OR=2.9, 95% CI=1.4–5.8
overall (see Table III for
comparison). Cordless phone use in the group with the longest
latency, >20–25 years, resulted in an OR=3.3, 95% CI=0.8–14 in
the temporal lobe versus an OR=1.5, 95% CI=0.5–4.6 overall,
although based on low numbers. Also, for overall wireless phone
use, the highest OR was found among those with the longest latency,
>25 years, with an OR=5.1, 95% CI=1.8–15 for tumours in the
temporal lobe.
In Table IX,
results are displayed for use of only one type of wireless phone.
Regarding analogue phones, all cases and controls had also used
other phone types. Use of only digital 2G types resulted in the
highest risk in the shortest latency period >1–5 years with an
OR=3.4, 95% CI=1.2–9.5. The risk decreased somewhat with longer
latency, but increased again in the longest latency group >15–20
years to an OR=1.8, 95% CI=0.6–4.9. A similar risk pattern was
found for use of cordless phones only, with even higher risk
estimates, although based on low numbers in the longest latency
groups. Use of wireless phones of only the digital type (2G, 3G,
cordless phone) yielded an OR=1.7, 95% CI=1.01–2.7 overall,
increasing to an OR=2.7, 95% CI=1.4–5.3 in the latency group
>1–5 years. A decreased risk was seen with the longer latency
period, but, again, it increased with latency >15–20 years to an
OR=1.9, 95% CI=1.1–3.4.
 | Table IX.Odds ratio (OR) and 95% confidence
interval (CI) for malignant brain tumours (n=593) |
Table IX.
Odds ratio (OR) and 95% confidence
interval (CI) for malignant brain tumours (n=593)
| Analogue only | Digital (2G)
only | Digital (UMTS, 3G)
only | Cordless phone
only | Digital type
only |
|---|
|
|
|
|
|
|---|
| Latency | OR | CI | Ca/Co | OR | CI | Ca/Co | OR | CI | Ca/Co | OR | CI | Ca/Co | OR | CI | Ca/Co |
|---|
| Total, >1
year | - | | 0/0 | 1.6 | 0.9–2.9 | 78/176 | - | | 1/0 | 3.5 | 1.6–7.8 | 23/44 | 1.7 | 1.01–2.7 | 427/1,001 |
| >1–5 years | - | | 0/0 | 3.4 | 1.2–9.5 | 9/13 | - | | 1/0 | 5.8 | 2.0–17 | 10/14 | 2.7 | 1.4–5.3 | 32/61 |
| >5–10 years | - | | 0/0 | 1.6 | 0.8–3.2 | 33/79 | - | | 0/0 | 3.7 | 1.3–11 | 9/19 | 1.7 | 1.03–3.0 | 162/370 |
| >10–15
years | - | | 0/0 | 1.3 | 0.6–2.6 | 28/68 | - | | 0/0 | 2.0 | 0.4–9.4 | 3/8 | 1.3 | 0.8–2.2 | 163/418 |
| >15–20
years | - | | 0/0 | 1.8 | 0.6–4.9 | 8/16 | - | | 0/0 | 2.9 | 0.2–39 | 1/2 | 1.9 | 1.1–3.4 | 68/140 |
| >20–25
years | - | | 0/0 | - | | 0/0 | - | | 0/0 | - | | 0/1 | 0.6 | 0.1–2.7 | 2/12 |
| >25 years | - | | 0/0 | - | | 0/0 | - | | 0/0 | - | | 0/0 | - | | 0/0 |
Most types of malignant brain tumours were glioma
(n=546). Separate analysis of that group of tumours gave similar
results as for the whole group of malignant brain tumours. Mobile
phone use with latency >25 years resulted in an OR=2.8, 95%
CI=1.4–5.7 (data not shown). Also, for cordless phone use, the
results were similar as in the overall analysis. Thus, with a
latency >15–20 years, an OR=1.9, 95% CI=1.05–3.5 was found.
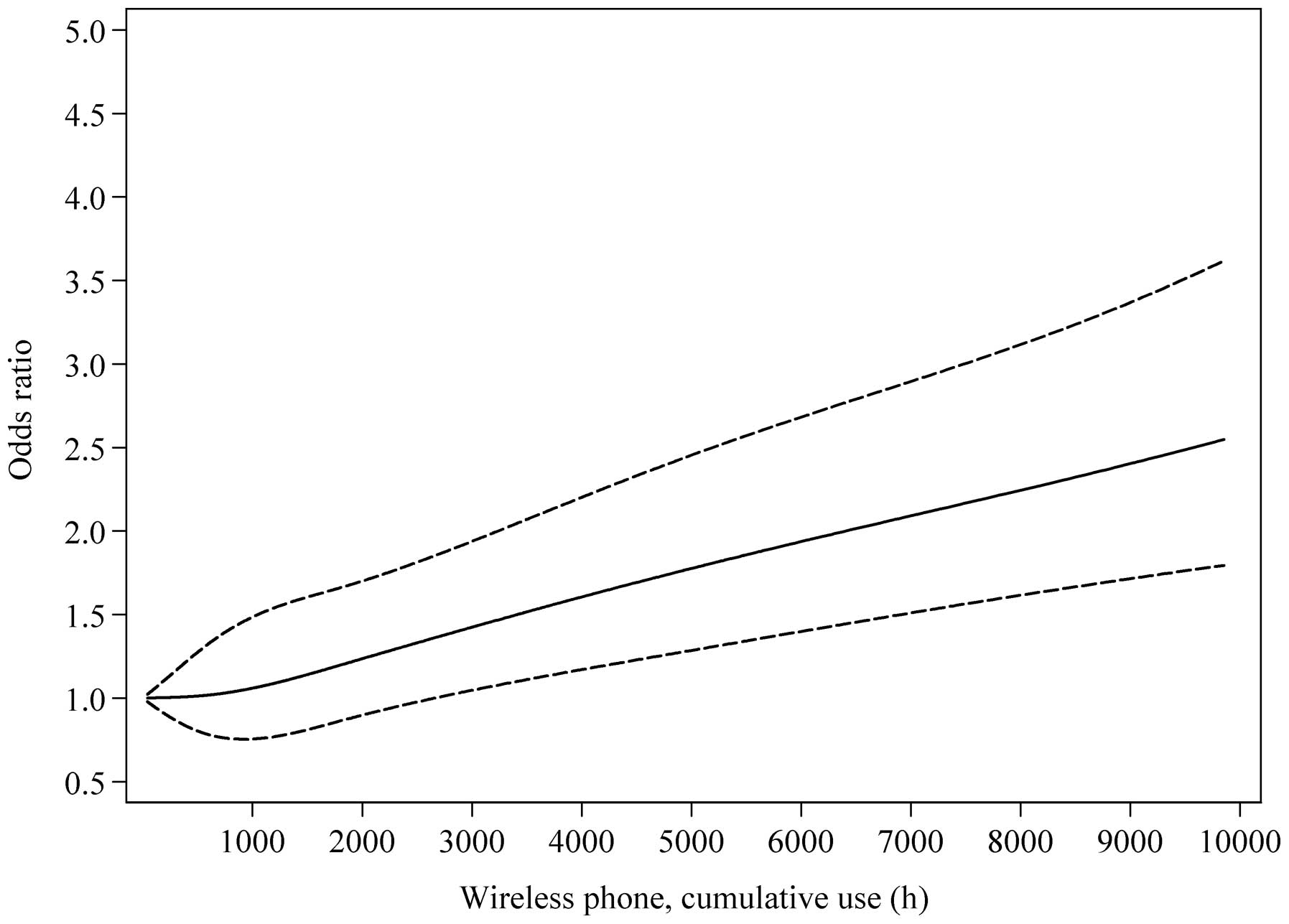
Fig. 1 illustrates
the results for cumulative use of wireless phones using the
restricted cubic splines method. There was a linear increasing
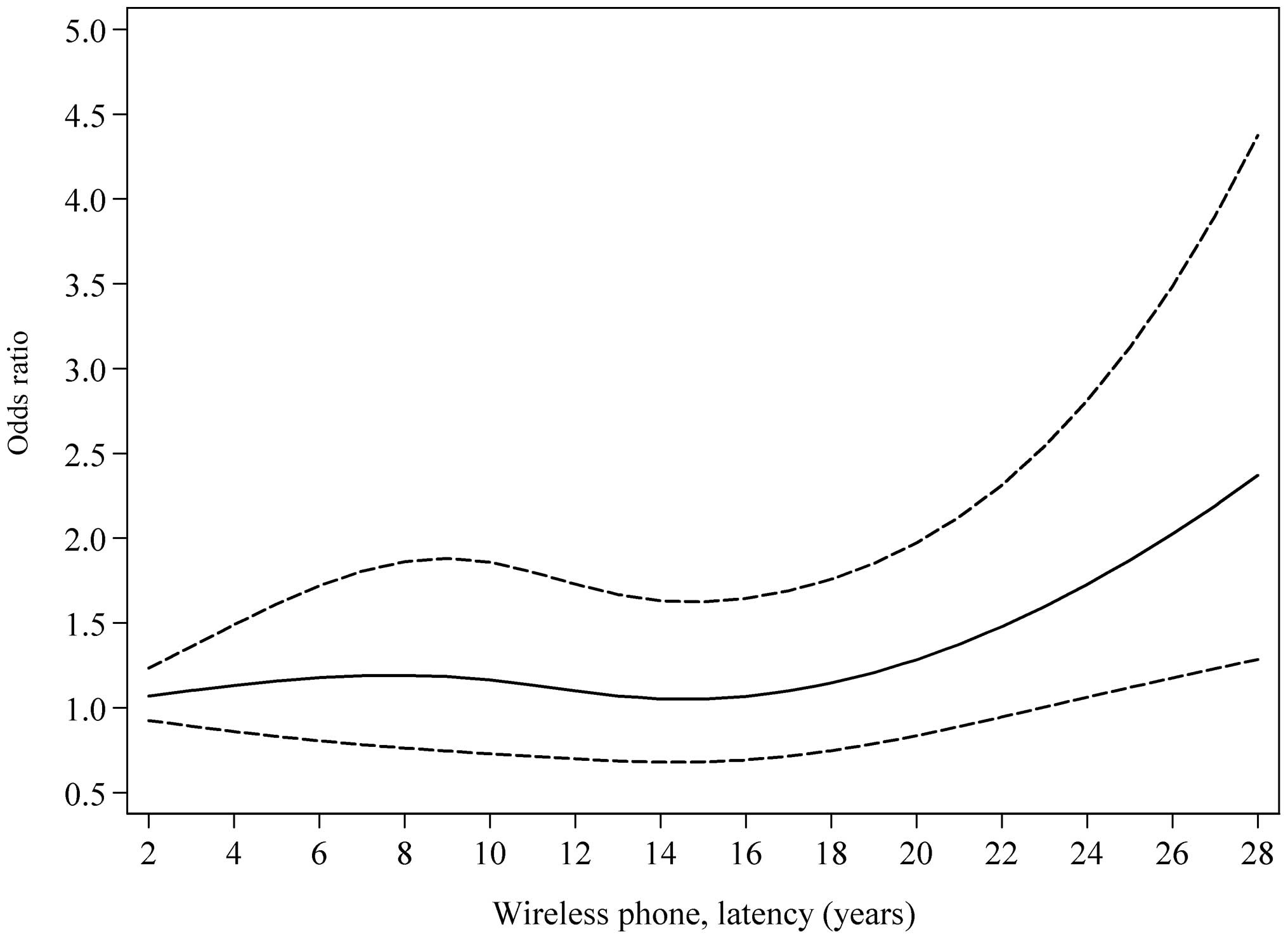
trend of the risk up to 10,000 h (p, non-linearity=0.52). Fig. 2 demonstrates a borderline
statistically significant non-linear relationship for the risk and
latency using data up to 28 years from first use of a wireless
phone before tumour diagnosis (p, non-linearity=0.05). Highest risk
was found with longest latency. This finding gives support for
RF-EMFs to play a role in the initiation and promotion stages of
carcinogenesis.
Discussion
Main results and latency (time since
first exposure) effects
The main result of this study was a statistically
significant increased risk for malignant brain tumours associated
with use of wireless phones, OR=1.7, 95% CI=1.04–2.8. The risk
increased further in the latency group >1–5 years, but lower ORs
were found in the latency groups >5–10 years and >10–15
years. With longer latency periods, the OR increased further with
highest risk in the latency group >25 years, OR=3.0, 95%
CI=1.5–6.0. From Table III,
analogue mobile phones produced a risk increasing with latency,
with the highest risk in the latency group >25 years. The OR
increased statistically significantly per year of latency, see
Table VII. A different pattern was
seen for digital wireless phones, both the mobile and cordless
types. The risk was higher in the short latency group >1–5
years, then dropped off and increased again with >15 years of
latency. Regarding digital 3G mobile phones no conclusions could be
drawn. The technique is new and no subject had latency >10
years.
No case or control had used a digital mobile phone
with latency >25 years. Only 6 cases and 13 controls had used a
cordless phone with latency >20–25 years, so the results for
cordless phones with longest latency time were less reliable. Only
one case had used only a 3G phone, so firm conclusions about the
risk with 3G mobile phone use are not possible from this study.
Regarding the use of digital 2G mobile and cordless phones, the OR
increased per year of latency with statistically borderline
significance. This was explained by the fact that the risk increase
was U-shaped in relation to latency period. A further illustration
is given in the restricted cubic spline plot showing a borderline
statistically significant non-linear relationship, see Fig. 2.
Regarding long-term use of wireless phones and the
association with brain tumours, it has not been possible to study
longer latency periods than >10 years previously since the
technology is too recent. This is the first study to examine
effects with a latency time >25 years. This was for analogue
phones. Regarding digital 2G mobile phones, the longest duration of
latency was >15–20 years. The longest latency for use of
cordless phones was >20–25 years with few subjects in that
category. The results in this study indicate an early effect in
brain tumour genesis seen both for analogue and digital phones, an
initiator. Regarding digital phones, we found also a late effect in
tumour development, a promoter.
Of interest is that we found that the risk was
elevated among those who reported using only digital 2G mobile
phones and only cordless phone, see Table IX. The risk was even higher for the
use of only cordless phones, a fact that is of importance since all
studies other than those from the Hardell group have not paid
attention to such use. Including the use of cordless phones in the
‘unexposed group’ would have biased risk estimates towards unity,
as discussed elsewhere (4,5).
Cumulative use
Cumulative use of wireless phones in our present
study was divided into quartiles based on cumulative use of
wireless phones overall among controls. For all phone types, the
highest risk was found in the fourth quartile >2,376 h of
cumulative use. This corresponds to about 40 min of wireless phone
use per day for 10 years. There was a statistically significant
trend for the different phone types, mobile phone use overall, and
wireless phones overall. An especially elevated OR was calculated
for analogue phone use, OR=7.7, 95% CI=2.5–24, in the fourth
quartile. Also, 3G mobile phone use resulted in increasing risk,
highest in the fourth quartile, but based on low numbers and no
statistically significant trend (p=0.28). These results are also
reflected in Table VII, with
statistically significant increasing risk per 100 h of cumulative
use for all phone types, except for 3G with borderline statistical
significance. A linear relationship between cumulative use of
wireless phones and the risk for malignant brain tumours is given
in Fig. 1.
Consistency with our previous
research
Clearly, digital mobile and cordless phones increase
the risk of malignant brain tumours in our present study, as well
as in our previous studies. For use of digital type wireless phones
only, we found an OR=1.7, 95% CI=1.01–2.7. This finding is
consistent with our previous result for the study period 1997–2003.
Use of digital mobile and cordless phones yielded an OR=1.4, 95%
CI=1.1–1.8 in that study (13).
Further analysis in our previous study on use of only mobile phones
yielded for glioma increased risk in the >10 year latency group,
OR=2.6, 95% CI=1.7–4.1. For use of only cordless phones, an
increased risk was found in the >5–10 years latency group,
OR=1.9, 95% CI=1.3–2.9, whereas the result for >10 year latency
was based on rather small numbers (5,15).
Furthermore, it should be noted that for the study
period 1997–2003, we found an increased risk of malignant brain
tumours in the latency period >5–10 years for users of wireless
phones of the digital type. Thus, digital 2G phones yielded an
OR=1.7, 95% CI=1.2–2.2, and for cordless phones, an OR=1.5, 95%
CI=1.1–2.0 in that latency group (13). These risks increased further in the
latency group >10 years, which was the longest time of wireless
phone use in that study. This pattern was different for use of
analogue phones, with statistically significant risk only in the
group with a latency >10 years, giving an OR=2.4, 95%
CI=1.6–3.4, a similar finding to that in the present study.
In summary, our results are consistent with an early
effect in carcinogenesis (initiator) by analogue mobile phones, and
both an early (initiator) and late (promoter) effect by wireless
phones of the digital type.
Comparison with other studies, e.g.
Interphone
In Interphone (data not shown), a statistically
significant increased risk for glioma was seen in the group 2–4
years for regular use, with 1–1.9 years use as reference category,
OR=1.68, 95% CI=1.16–2.41 (3). The
highest OR was found in the 10+ years category for regular use,
OR=2.18, 95% CI=1.43–3.31. Results were not presented according to
type of mobile phone used. Overall, cumulative use >1,640 h in
the shortest latency group of 1–4 years before reference date
resulted in an increased risk, OR=3.77, 95% CI=1.25–11.4.
The highest absorption of RF-EMF emissions from a
handheld phone is on the same side of the brain (ipsilateral) as
the phone is used (9). Highest
dose is absorbed in the temporal lobe of the brain. In previous
studies, we have found risk being highest for ipsilateral wireless
phone use (5,13). In Interphone, cumulative call time
of mobile phones >1,640 h, resulted in glioma in the temporal
lobe with an OR=1.87, 95% CI=1.09–3.22, and for ipsilateral mobile
phone use, an OR=1.96, 95% CI=1.22–3.16 (3). Likewise, in our present study, the OR
was higher for ipsilateral use of mobile or cordless phones, see
Table V, and for malignant brain
tumours in the temporal and overlapping lobes, see Table VIII.
A mean duration of mobile phone use of 2.8 years was
reported in a study from USA (24). Overall, no increased risk was found
for malignant brain tumours, except for a rare type,
neuroepithelioma with OR=2.1, 95% CI=0.9–4.7. The type of mobile
phone was not reported. No increased risk for glioma overall or in
different groups of duration of regular use, at most >5 years,
was reported in another study from USA (25). The type of mobile phone used was
not published. An increased risk for glioma with short duration of
analogue mobile phone use (1–2 years) was seen in a Finnish study,
whereas no increased risk was found for digital phones (26). These results were based on low
numbers. Cordless phone use was included in the ‘unexposed’
category in these studies, which is of interest to note since we
have found an association with such phone use as reported
above.
In a record linkage study from Denmark mobile phone
subscribers from January 1, 1982, until December 31, 1995, were
identified from the computerized files of the two Danish operating
companies, TeleDenmark Mobil and Sonofon, which partly also funded
the study. It has produced four articles that we have made a
thorough review of (27). We
concluded that its many limitations - embedded in the study design
from the very beginning and mainly related to poor exposure
assessment - cloud the findings of the four reports to such an
extent that render them uninformative, at best. The Danish cohort
study was included in the IARC evaluation of RF-EMF but the
conclusion was that ‘phone provider, as a surrogate for mobile
phone use, could have resulted in considerable misclassification in
exposure assessment’ (1). Thus,
the Danish cohort study is uninformative as to cancer risks from
mobile phone use.
Strengths and limitations
The present study included cases of malignant brain
tumours diagnosed during 2007–2009 from across Sweden. For the
cases diagnosed during 1997–2003 in our previous study (5), the prevalence of use of mobile phones
was highest in the age group 30–54 years for men, and 35–54 years
for women. Thus, we included the age group 18–75 years in this
study to allow for the longest possible latency time (28). This is in contrast to the
Interphone study, which included cases aged 30–59 years. Glioma is
the most common malignant brain tumour, and the most common glioma
subtype is astrocytoma. Glioblastoma multiforme (WHO grade IV)
accounts for 60–75% of all astrocytoma, in this study 66% of the
cases with astrocytoma. The peak incidence is between 45–75 years,
with a mean age of 61 years and with 80% older than 50 years
(29). Thus, limiting the upper
age to 59 years for cases as in Interphone (3) would diminish the possibility of
finding an increased risk for the long-term use of mobile
phones.
Recall and observational bias might be an issue in
case-control studies. We investigated in more detail the
possibility of that in one of our previous studies (11). Reporting a previous cancer or if a
relative helped to fill in the questionnaire did not change the
results. Potential observational bias during phone interviews was
analysed by comparing the results based on exposure assessment
before and after additional phone interviews. The results were
similar with no statistically significant differences, showing that
our results were unlikely to be explained by observational bias
(11).
To further validate exposure in the present study we
used meningioma cases as the referents, see Table IV. Thereby the results were similar
to those obtained using the population based controls with
consistency of the main findings for the main phone types, see
Table III. It should be mentioned
that a similar method was used previously on the controversy of
cancer risks from certain chemicals. Based on clinical observations
an increased risk for soft-tissue sarcoma (30) and malignant lymphoma (31) was postulated for exposure to
phenoxyacetic acids, chlorophenols and contaminating dioxins. These
bed-side observations were followed by case-control studies
confirming an association, e.g. Hardell and Sandström (32), Hardell et al (33). Using colon cancer cases as
referents yielded similar results as when population based controls
were used, that is the increased risks were unlikely to be
explained by recall or observational bias (21). Thus, similar conclusions can be
made in the present study.
In our previous studies, we included only living
cases so as to be able to solicit as good an assessment of exposure
as possible (10,13). Especially side of head mostly used
during phone calls would be difficult to assess using proxy
interviews. Excluding deceased cases might, theoretically, bias the
results, notably if there is no association between use of wireless
phones and brain tumour in that patient group or even a protective
effect. We, therefore, did a separate case-control study on
deceased cases diagnosed during 1997–2003 with a malignant brain
tumour in our previous studies (13) using deceased controls. Relatives of
both groups were interviewed and we were able to confirm an
increased risk for use of mobile phones (15,34).
Thus, inclusion of only living cases and controls in this study
would not likely bias the results away from unity.
In total, 1,334 cases were reported from the cancer
registries covering all of Sweden. From the Gothenburg region, it
was possible to get reports only of cases diagnosed during 2008 and
2009 for administrative reasons. However, exclusion of cases
diagnosed during 2007 could not conceivably have biased the
results. It has been published that the reporting of new brain
tumour cases to the Swedish cancer registry is insufficient
(35,36). It is, however, not likely that such
omission from our study of not reported cases would be related to
the status of being a user or not of wireless phones.
The majority of the cases with a histopathological
brain tumour diagnosis that were excluded from this study were
deceased (n=520; 39%). As mentioned above we have found an
association with use of wireless phones also among the deceased
cases (34). Furthermore, for
glioma we have found an increased hazard ratio (HR) for survival
(37). This was based on all
glioma cases, both alive and deceased at the time of the studies as
presented in Hardell et al (15). An increased hazard ratio was found
for >10 years latency for both mobile phone use, HR=1.3, 95%
CI=1.0005–1.6, and cordless phone use, HR=1.3, 95% CI=0.9–1.9. HR
increased also with the cumulative number of hours of mobile and
cordless phone use, with statistically significant trend for
tertiles (p=0.01) of use of both phone types. For glioblastoma
multiforme (WHO grade IV) use with >10 years latency for mobile
phones increased the ratio, HR=1.3, 95% CI=0.9–1.7, and cordless
phone, HR=1.8, 95% CI=1.2–2.8, indicating decreased survival for
long-term and high cumulative use of wireless phones.
Most of the deceased cases in the present study had
a diagnosis of glioblastoma multiforme, WHO grade IV. The median
survival in that patient group is less than one year (38). We have reported a higher risk for
mobile phone use for high grade glioma (WHO grades III–IV) than for
low grade glioma (WHO-grades I–II) (5). Hence, the exclusion of deceased cases
with glioblastoma multiforme with poor prognosis in this study
might actually have biased the risk estimates towards unity.
We included only cases with a histopathological
diagnosis of a brain tumour. We asked the six regional cancer
registries not to report cases with only a clinical diagnosis. The
reason was that we wanted to insure a confirmed diagnosis of the
brain tumour for separate analyses for each tumour type. If
necessary, we supplemented the histopathological reports with
records from pathology departments around the country after
informed consent from the respective case. Thus, we were able to
classify all brain tumours based on WHO codes, see Table II. It is not probable that
exclusion of cases with only a clinical diagnosis would have biased
the results. We checked the Swedish Cancer Registry for the total
number of patients with a brain tumour during the study period in
the relevant age group. In total, 2,553 patients aged 20–74 years
with a brain tumour were reported to the Swedish Cancer Registry
versus 2,310 aged 20–74 years with a diagnosis based on
histopathological diagnosis in our present study. This is in good
agreement with expected numbers since, during 2007–2009, roughly
90% of brain tumour diagnoses in the Swedish Cancer Register were
based on histology (http://www.socialstyrelsen.se/statistik/statistikdatabas).
An advantage of this study was the fairly high
response rate among both cases and controls. The response rate was
87% (n=593) among the eligible cases. Of the controls, 85%
(n=1,368) participated. These response rates were similar to those
in our previous studies on malignant brain tumours, 85% (n=1,251)
among cases and 84% (n=2,438) among controls (5). Lower response rates were obtained in
the Interphone study, namely 64%, range by centre 36–92%, (n=2,765)
for glioma cases, and 53%, range 42–74%, (n=7,658) for controls
(3). To obtain the most valid
results possible, it is always necessary to have the highest
possible response rate. In fact, not responding controls in
Interphone tended to be less frequent users of mobile phones than
participating controls, leading to an underestimation of the risk
(4,39,40).
Our study was not designed to include a
mini-interview on the use of wireless phones among non-responding
cases and controls as done in parts of the Interphone study; we had
no ethics clearance for that. Certainly, it would have been of
value to verify the use of mobile phones by operator data on the
phone traffic. We had no possibility to do this since we did not
obtain valid information on the operator used over the years in
spite of asking. Furthermore, use of cordless phones, an important
source of RF-EMF exposure, is not possible to validate by operator
data.
Statistical considerations
In view of the fact that practically everybody is
using a wireless phone of some type today, it is not possible to
obtain a large enough ‘unexposed’ group for meaningful statistical
calculations. We, therefore, in addition to a latency ≤1 year, used
the 3rd percentile (39 h) of cumulative time as a cut-off. Another
option to obtain more ‘unexposed’ individuals would have been to
change the cut-off for latency. However, doing this would limit the
possibility of studying a late effect (promotion) in brain tumour
genesis. Furthermore, it is difficult to find users that have been
using only one single technology, i.e. NMT, GSM, UMTS, etc. Most
users have used several technologies, and those with 3G phones who
reported such use may have been unaware that the phone might have
been operating on a 2G net for voice, if that was available. The
analysis must be viewed with these facts in mind.
In the unconditional logistic regression analysis,
all controls, both to cases with malignant and benign brain
tumours, were used so as to maximise the statistical power.
Analysis using conditional logistic regression yielded overall for
wireless phones OR=2.1, 95% CI=1.1–3.7 versus OR=1.7, 95%
CI=1.04–2.8 using unconditional logistic regression, see Table III. Using unconditional logistic
regression only with controls matched to the malignant cases
yielded overall for wireless phones OR=2.0, 95% CI=1.1–3.5. Similar
differences were seen for the different phone types i.e. slightly
higher risk estimates using conditional logistic regression or
unconditional logistic regression with matched controls, although
with wider confidence intervals. The latter was due to the fact
that only controls matched to malignant cases could be included and
also because only discordant matched pairs are considered in a
conditional logistic regression analysis. The considerably smaller
material would limit the possibility of performing several of the
subgroup analyses in this article using this method. Using
unconditional logistic regression analysis was possible since
adjustment was made for the matching variables of age, gender and
year of diagnosis. In addition, adjustment was made for
socio-economic index since an association between white-collar work
and brain tumours has been reported (41). Not adjusting for any of these
variables yielded for wireless phone overall crude OR=2.2, 95%
CI=1.4–3.5. No statistically significant interactions were found
between the adjustment factors and wireless phone use. In our
previous study, we found that heredity and previous X-ray
investigations of the head increased the risk for glioma. However,
these were independent risk factors with no interaction with use of
wireless phones (16). Thus, it
was not necessary to adjust for these risk factors in the present
study.
More women than men were included as controls. This
was because all controls in the study were included in the
analysis. Among the cases with benign brain tumour, meningioma was
about 2.5 times more common among women than men, an expected
number. Thus, adjustment for gender was necessary.
Biological mechanisms
There is no generally accepted mechanism by which
RF-EMF exposure produces changes in DNA. The energy level
associated with exposure is too low to cause direct DNA strand
breaks and DNA crosslinks. However, DNA damage can be caused by
cellular biochemical activities such as free radicals. Several
studies indicate that RF-EMFs increase free radical activity in
cells (42,43). This process is probably mediated
via the Fenton reaction. Hydrogen peroxide is converted into
hydroxyl free radicals that are potent cytotoxic molecules. This
reaction is catalyzed by iron. High levels of iron are found in
metabolic active cells such as cancer cells as well as in cells
undergoing abnormal proliferation, but also in brain cells. Glia
cells might turn cancerous from DNA damage.
In a recently published study, it was demonstrated
that RF-EMF exposure induced the formation of oxidative base damage
in a mouse spermatocyte-derived cell line (44). This was mediated by reactive oxygen
species (ROS) production. To further elucidate the central role of
ROS in RF-EMF exposure-induced DNA base damage, the authors used
α-tocopherol pretreatment to antagonize the oxidation of ROS;
α-tocopherol is an important lipophilic chain-breaking antioxidant
that can inactivate harmful ROS. The protective role of
α-tocopherol pretreatment confirmed that ROS are involved in RF-EMF
exposure-induced DNA base damage (44). These findings support the idea that
low energy RF-EMF that is insufficient to directly induce DNA
strand breaks may nonetheless produce genotoxic effects in the form
of DNA base damage.
We know little about the earliest events in the
genesis of glioma in humans for obvious reasons. However,
progression of glioma has been studied in a large series of tumours
of different malignancy grades. Patients with low-grade glioma have
been followed with later progression to high-grade glioma (45). Thus, since the natural history of
most glioma cases, from earliest events to clinical manifestation,
is unknown but, most likely requires several decades, the exposure
duration has in most studies been incompatible with a tumour
initiating effect. This is the first study with long-term use of
wireless phones. Interestingly, the most elevated OR was found in
the latency group >25 years use. We also found results
indicating a late effect on tumour development (promotion).
Initiation and promotion have different effects on
the incidence of brain tumours. An initiating effect would have the
most direct effect on the incidence. Our results indicate that such
an effect would be apparent after more than a 20-year use of mobile
phones, and thus be too early to be found in cancer registries. On
the other hand, if the exposure acts as a promoter, this would
decrease latency time for already existing tumours, giving a
temporary, but not a continuous, increase in incidence. In
addition, it must be noted that any such effect on tumour
development is limited by the magnitude of the shift of the
age-incidence function and its slope for the respective tumour type
(28).
In conclusion, this study confirmed previous results
of an association between use of mobile and cordless phones and
malignant brain tumours. The risk was highest for ipsilateral use
and tumours in the temporal lobe. The results are consistent with
initiation carcinogenesis for analogue phones, and both initiation
and promotion carcinogenesis for digital wireless phones.
Acknowledgements
We thank Ms. Iréne Larsson and Mrs.
Margaretha Sjölund, for assistance in the data collection and Mr.
Brian Stein for general support. Colin L. Soskolne, Professor
Emeritus (Epidemiology), University of Alberta, Canada, and Adjunct
Professor, Faculty of Health, University of Canberra, Australia,
provided helpful editorial assistance in the writing of this
article. The study was supported by grants from Cancer- och
Allergifonden, Cancerhjälpen, Örebro University Hospital Cancer
Fund, Pandora-Foundation for Independent Research, Berlin, Germany,
and Gigaherz.ch, Schweizerische Interessengemeinschaft
Elektrosmog-Betroffener, https://www.gigaherz.ch.
The reporting of cancer patients from the Oncology Centres in
Sweden is acknowledged.
References
|
1.
|
Baan R, Grosse Y, Lauby-Secretan B, et al:
Carcinogenicity of radiofrequency electromagnetic fields. Lancet
Oncol. 12:624–626. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
2.
|
IARC Monographs on the Evaluation of
Carcinogenic Risks to Humans. Non-ionizing radiation, Part 2:
Radiofrequency electromagnetic fields. 102:IARCPress; Lyon: 2013,
http://monographs.iarc.fr/ENG/Monographs/vol102/mono102.pdf.
Accessed July 11, 2013.
|
|
3.
|
Interphone Study Group: Brain tumour risk
in relation to mobile telephone use: results of the INTERPHONE
international case-control study. Int J Epidemiol. 39:675–694.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
4.
|
Hardell L, Carlberg M and Hansson Mild K:
Methodological aspects of epidemiological studies on the use of
mobile phones and their association with brain tumors. Open Env
Sciences. 2:54–61. 2008. View Article : Google Scholar
|
|
5.
|
Hardell L, Carlberg M and Hansson Mild K:
Use of mobile phones and cordless phones is associated with
increased risk for glioma and acoustic neuroma. Pathophysiology.
20:85–110. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
6.
|
Cardis E and Sadetzki S: Indications of
possible brain tumour risk in mobile phone studies: should we be
concerned? Occup Environ Med. 68:169–171. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
7.
|
Wilén J, Sandström M and Hansson Mild K:
Subjective symptoms among mobile phone users - a consequence of
absorption of radiofrequency fields? Bioelectromagnetics.
24:152–159. 2003.PubMed/NCBI
|
|
8.
|
Hardell L, Hansson Mild K, Påhlson A and
Hallquist A: Ionizing radiation, cellular telephones and the risk
for brain tumours. Eur J Cancer Prev. 10:523–529. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
9.
|
Cardis E, Deltour I, Mann S, et al:
Distribution of RF energy emitted by mobile phones in anatomical
structures of the brain. Phys Med Biol. 53:2771–2783. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
10.
|
Hardell L, Näsman Å, Påhlson A, Hallquist
A and Hansson Mild K: Use of cellular telephones and the risk for
brain tumours: A case-control study. Int J Oncol. 15:113–116.
1999.PubMed/NCBI
|
|
11.
|
Hardell L, Hallquist A, Hansson Mild K,
Carlberg M, Påhlson A and Lilja A: Cellular and cordless telephones
and the risk for brain tumours. Eur J Cancer Prev. 11:377–386.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
12.
|
Hardell L, Carlberg M and Hansson Mild K:
Case-control study of the association between the use of cellular
and cordless telephones and malignant brain tumors diagnosed during
2000–2003. Environ Res. 100:232–241. 2006.
|
|
13.
|
Hardell L, Carlberg M and Hansson Mild K:
Pooled analysis of two case-control studies on use of cellular and
cordless telephones and the risk for malignant brain tumours
diagnosed in 1997–2003. Int Arch Occup Environ Health. 79:630–639.
2006.
|
|
14.
|
Hardell L, Carlberg M and Hansson Mild K:
Epidemiological evidence for an association between use of wireless
phones and tumor diseases. Pathophysiology. 16:113–122. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
15.
|
Hardell L, Carlberg M and Hansson Mild K:
Pooled analysis of case-control studies on malignant brain tumours
and the use of mobile and cordless phones including living and
deceased subjects. Int J Oncol. 38:1465–1474. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
16.
|
Carlberg M and Hardell L: On the
association between glioma, wireless phones, heredity and ionising
radiation. Pathophysiology. 19:243–252. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
17.
|
Interphone Study Group: Acoustic neuroma
risk in relation to mobile telephone use: results of the INTERPHONE
international case-control study. Cancer Epidemiol. 35:453–464.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
18.
|
Post-och Telestyrelsen: Svensk Telemarknad
2010. http://www.statistik.pts.se/pts2011/FILES/RAPPORTER/2010/Telemarknad_2010.pdf.
Accessed July 11, 2013.
|
|
19.
|
Hardell L, Carlberg M and Gee D: Mobile
phone use and brain tumour risk: early warnings, early actions?
Late Lessons from Early Warnings, Part 2. European Environment
Agency; Copenhagen: 2013, http://www.eea.europa.eu/publications/late-lessons-2/late-lessons-chapters/late-lessons-ii-chapter-21/at_download/file.
Accessed July 11, 2013.
|
|
20.
|
Harrell FE Jr: Regression Modeling
Strategies With Application to Linear Models, Logistic Regression
and Survival Analysis. Springer; New York, NY: pp. 20–26. 2001
|
|
21.
|
Hardell L: Relation of soft tissue
sarcoma, malignant lymphoma, and colon cancer to phenoxy acids,
chlorophenols and other agents. Scand J Work Environ Health.
7:119–130. 1981. View Article : Google Scholar : PubMed/NCBI
|
|
22.
|
Carlberg M, Söderqvist F, Hansson Mild K
and Hardell L: Meningioma patients diagnosed 2007–2009 and the
association with use of mobile and cordless phones: a case-control
study. Environ Health. 12:602013.
|
|
23.
|
Hardell L, Carlberg M, Söderqvist F and
Hansson Mild K: Pooled analysis of case-control studies on acoustic
neuroma diagnosed 1997–2003 and 2007–2009 and use of mobile and
cordless phones. Int J Oncol. 43:1036–1044. 2013.
|
|
24.
|
Muscat JE, Malkin MG, Thompson S, et al:
Handheld cellular telephone use and risk of brain cancer. JAMA.
284:3001–3007. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
25.
|
Inskip PD, Tarone RE, Hatch EE, et al:
Cellular-telephone use and brain tumors. N Engl J Med. 344:79–86.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
26.
|
Auvinen A, Hietanen M, Luukkonen R and
Koskela RS: Brain tumors and salivary gland cancers among cellular
telephone users. Epidemiology. 13:356–359. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
27.
|
Söderqvist F, Carlberg M and Hardell L:
Review of four publications on the Danish cohort study on mobile
phone subscribers and risk of brain tumours. Rev Environ Health.
27:51–58. 2012.PubMed/NCBI
|
|
28.
|
Kundi M: Essential problems in the
interpretation of epidemiologic evidence for an association between
mobile phone use and brain tumours. C R Physique. 11:556–563. 2010.
View Article : Google Scholar
|
|
29.
|
Ohgaki H, Dessen P, Jourde B, et al:
Genetic pathways of glioblastoma: a population-based study. Cancer
Res. 64:6892–6899. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
30.
|
Hardell L: Soft-tissue sarcoma and
exposure to phenoxy acids - A clinical observation. Läkartidningen.
74:2853–2854. 1977.(In Swedish).
|
|
31.
|
Hardell L: Malignant lymphoma of
histiocytic type and exposure to phenoxyacetic acids or
chlorophenols. Lancet. 1:55–56. 1979. View Article : Google Scholar : PubMed/NCBI
|
|
32.
|
Hardell L and Sandström A: Case-control
study: soft-tissue sarcomas and exposure to phenoxyacetic acids or
chlorophenols. Br J Cancer. 39:711–717. 1979. View Article : Google Scholar : PubMed/NCBI
|
|
33.
|
Hardell L, Eriksson M, Lenner P and
Lundgren E: Malignant lymphoma and exposure to chemicals,
especially organic solvents, chlorophenols and phenoxy acids: a
case-control study. Br J Cancer. 43:169–176. 1981. View Article : Google Scholar : PubMed/NCBI
|
|
34.
|
Hardell L, Carlberg M and Hansson Mild K:
Mobile phone use and the risk for malignant brain tumors: a
case-control study on deceased cases and controls.
Neuroepidemiology. 35:109–114. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
35.
|
Bergenheim T, Malmström A, Bolander H, et
al: Registration on regional basis of patients with primary brain
tumors. Regional differences disclosed. Läkartidningen.
104:332–341. 2007.(In Swedish).
|
|
36.
|
Barlow L, Westergren K, Holmberg L and
Talbäck M: The completeness of the Swedish Cancer Register: a
sample survey for year 1998. Acta Oncol. 48:27–33. 2009.PubMed/NCBI
|
|
37.
|
Hardell L and Carlberg M: Use of mobile
and cordless phones and survival of patients with glioma.
Neuroepidemiology. 40:101–108. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
38.
|
Ohgaki H and Kleihues P: Population-based
studies on incidence, survival rates, and genetic alterations in
astrocytic and oligodendroglial gliomas. J Neuropathol Exp Neurol.
64:479–489. 2005.PubMed/NCBI
|
|
39.
|
Vrijheid M, Cardis E, Armstrong BK, et al:
Validation of short term recall of mobile phone use for the
Interphone study. Occup Environ Med. 63:237–243. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
40.
|
Vrijheid M, Deltour I, Krewski D, Sanchez
M and Cardis E: The effects of recall errors and of selection bias
in epidemiologic studies of mobile phone use and cancer risk. J
Expo Sci Environ Epidemiol. 16:371–384. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
41.
|
Preston-Martin S and Mack W: Neoplasms of
the nervous system. Cancer Epidemiology and Prevention.
Schottenfeld D and Fraumeni JF Jr: Oxford University Press; New
York, NY: pp. 1231–1281. 1996
|
|
42.
|
Lai H and Singh NP: Melatonin and a
spin-trap compound block radiofrequency electromagnetic
radiation-induced DNA strand breaks in rat brain cells.
Bioelectromagnetics. 18:446–454. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
43.
|
Phillips JL, Singh NP and Lai H:
Electromagnetic fields and DNA damage. Pathophysiology. 16:79–88.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
44.
|
Liu C, Duan W, Xu S, et al: Exposure to
1800 MHz radiofrequency electromagnetic radiation induces oxidative
DNA base damage in a mouse spermatocyte-derived cell line. Toxicol
Lett. 218:2–9. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
45.
|
Collins VP: Gliomas. Cancer Surv.
32:37–51. 1998.
|