Introduction
Although continuous efforts to exploit efficient
therapeutic approaches including molecular target-based drugs for
hematopoietic malignancies are ongoing, there is still a growing
concern about treatment resistance, disease relapse and side
effects of drugs clinically used. Of note, numerous components of
edible plants, collectively termed phytochemicals that have
beneficial effects for health, are being reported increasingly in
the scientific literature and these compounds are now widely
recognized as potential therapeutic compounds (1,2).
Vitex agnus-castus is a shrub of the
Lamiaceae family (previously known as Verbenaceae
family) and found naturally in the Middle East and Southern Europe
and China. Ripe fruit of V. agnus-castus has been used to
treat patients with various obstetric and gynecological disorders
in Europe as well as in China (3,4).
Furthermore, we have previously demonstrated that an extract from
dried ripe V. agnus-castus (Vitex) exhibits cytotoxic
activities against various types of solid tumor cells, such as
KATO-III, COLO 201 and MCF-7 (5,6). We
further demonstrated that the levels of cytotoxic activity of Vitex
were attributed to growth rate of respective cell line, in which
cell lines with faster growth rate were more susceptible to Vitex
cytotoxicity (5). Casticin has
been demonstrated to be one of major components of Vitex (7). Shen et al recently reported
that casticin exhibited proliferation inhibitory effect on leukemia
cells including K562, Kasumi-1 and HL-60, consequently induced cell
death through apoptosis and mitotic catastrophe (8). However, to date, the effects of Vitex
on hematopoietic cell line has not been evaluated.
Leukemia is a group of malignant diseases with poor
prognosis. Recent studies have demonstrated that less
differentiated cancer cells, referred to as leukemia stem cells
(LSCs), acquired limitless self-renewal through oncogenic
transformation, and the incomplete eradication of primary LSCs is
closely linked to chemotherapy resistance, and consequently
contribute to eventual disease relapse (9). It is well known that there are
obvious differences in the degree of differentiation of
hematopoietic cells including normal and malignant cells. These
findings thus suggest that it is necessary to clarify responses of
hematopoietic cell with different degree of differentiation to
Vitex and casticin in order to provide fundamental insight into
future clinical application of them for leukemia. Since human
promyelocytic leukemia HL-60 cell line can be induced to
differentiate along either granulocytic or monocytic pathway when
treated with respective inducers, it has been shown to be a good
model for leukemogenesis research and differentiation study in
vitro (10–12).
In the current study, we investigated first the
effects of Vitex and casticin on leukemic cell lines HL-60 and
U-937, since it is well documented that HL-60 is more immature than
U-937 cells although both cells are belong to the monocyte/
macrophage lineage (13). We
further evaluated the cytocidal effects of Vitex and casticin
against undifferentiated and differentiated HL-60 cells after
treatment with phorbol 12-myristate 13-acetate (PMA) and
1,25-dihydroxyvitamin D3 (calcitriol, VD3).
It is well known that differentiation is accompanied with cell
adhesion through adhesion molecules such as integrins,
immunoglobulin (Ig)-related molecules, which in turn contribute to
acquired resistance to cytocidal reagents (14,15).
It has been demonstrated that intercellular adhesion molecule-1
(ICAM-1, also known as CD54), a member of Ig-related molecules,
together with leukocyte function-associated antigen-1 (LFA-1), a
member of integrins, plays an essential role in cellular adhesion
and/or aggregation in PMA-treated HL-60 cells (16). Therefore, contribution of ICAM-1 to
the cell adhesion and an alteration of cytocidal effect of Vitex
against PMA-treated HL-60 cells was also investigated.
Materials and methods
General
PMA and calcitriol (VD3) were purchased
from Wako Pure Chemical Industries (Osaka, Japan) and dissolved in
ethanol. Anti-ICAM-1 mouse monoclonal antibody (anti-ICAM-1 mAb)
and casticin were purchased from Calbiochem (La Jolla, CA, USA) and
ChromaDex (Irvine, CA, USA), respectively.
2,3-Bis(2-methoxy-4-nitro-5-sulfophenyl)-5-[(phenylamino)carbonyl]-2H-tetrazolium
hydroxide (XTT) was purchased from Sigma-Aldrich (St. Louis, MO,
USA). α-Naphthyl Acetate (Non-Specific Esterase) kit was purchased
from Muto Pure Chemicals Co. (Tokyo, Japan). Phenazine methosulfate
(PMS) and an RNA extraction kit, ISOGEN were obtained from Wako
Pure Chemical Industries. Column chromatography was performed on
silica gel 60 (Nacalai Tesque, 70-230 mesh) using the indicated
solvents. TLC was performed using pre-coated silica gel 60
F254 plates (Merck KGaA) using the indicated solvents.
Melting point (mp) was determined with an AS ONE melting point
apparatus ATM-02 without correction. 1H NMR spectra were
recorded on a Bruker Avance™ III 600 (600 MHz) with
tetramethylsilane (0 ppm) as an internal standard. 13C
NMR spectra were recorded on a Bruker Avance III 600 (150 MHz) with
dimethylsulfoxide (DMSO) (39.7 ppm) as an internal standard.
Preparation of an ethanol extract from
dried ripened Vitex agnus-castus fruits (Vitex)
Preparation of Vitex was carried out according to
the methods previously described (6). Briefly, dried ripened V.
agnus-castus fruit from Israel was gently triturated. The
extract was prepared from 1 g of the triturate with 10 ml of
ethanol under reflux for 2 h. The extract was then cooled,
filtered, evaporated and dried in a vacuum desiccator, a product of
which was designated as Vitex. The yield of Vitex was 0.08-0.1 g
from 1 g of dried fruit.
Isolation and identification of
casticin in Vitex
Vitex (1 g) was subjected to flash column
chromatography using silica gel 60 (51.6 g) eluted with
chloroform-methanol (250:1). The obtained eleven fractions (F1-F11:
25 ml per one fraction) were analyzed by thin-layer chromatography
(TLC) [eluents: chloroform-methanol (20:1)] using commercial
casticin as a standard sample. Based on the analysis, five
fractions (F5–F9) containing casticin were concentrated under
reduced pressure to give a brown oil residue. Then, the residue was
further purified by recrystallization from n-hexane-EtOAc (3:1) to
afford casticin as pale yellow crystals. mp 185–188°C
(n-hexane-EtOAc); 1H NMR (600 MHz, CDCl3)
3.88 (3H, s), 3.92 (3H, s), 3.96 (3H, s), 3.99 (3H, s), 5.71 (1H,
s), 6.51 (1H, s), 6.97 (1H, d, J=8.4 Hz), 7.69 (1H, d, J=1.2 Hz),
7.73 (1H, dd, J=8.4, 1.2 Hz), 12.60 (1H, s); 13C NMR
(150 MHz, DMSO-d6) δ 55.9, 56.7, 59.9, 60.2, 91.5, 105.8, 112.1,
115.3, 120.6, 122.4, 131.8, 138.2, 146.6, 150.5, 151.8, 152.0,
155.8, 158.9, 178.5. Melting point, 1H and
13C NMR spectroscopic data of the crystal were
comparable with the reported value of casticin (17).
Cell cultures and Vitex/casticin
treatment
Leukemic cell lines HL-60 and U-937 were purchased
from the Health Science Research Resources Bank (Tokyo, Japan).
Peripheral blood mononuclear cells (PBMNC) were isolated from three
healthy volunteers (Vitex treatment; 26±1, and casticin treatment;
26.3±3.21 years of age), as previously described (18). Briefly, 10 ml of heparinized blood
was loaded on 3.5 ml of Ficoll Hypaque (Nakalai Tesque, Kyoto,
Japan) and centrifuged at 2,000 rpm for 20 min, and PBMNC were
separated. Both the cell lines and PBMNC were cultured in RPMI-1640
medium (Gibco, Grand Island, NY, USA) supplemented with 10%
heat-inactivated fetal bovine serum (Invitrogen, Carlsbad, CA, USA)
and antibiotics [100 U/ml penicillin and 100 μg/ml
streptomycin (Invitrogen)] at 37°C in a humidified atmosphere (5%
CO2 in air). Vitex and casticin were dissolved in DMSO.
As for unstimulated HL-60 and U-937 control cells, and PBMNC, 2X
105 cells/ml were precultured for 12 h, followed by the
treatment with Vitex or casticin at various concentrations
indicated at 37°C for a designated time. Control samples were
prepared by treating cells with culture medium containing vehicle
reagent, DMSO alone (final concentration: 0.1%). This study was
approved by the IRB committee of Tokyo University of Pharmacy and
Life Sciences. An informed consent was obtained from all healthy
volunteers.
Induction of differentiation and
Vitex/casticin treatment
To induce differentiation, cells (3×105
cells/ml) were exposed to 10 ng/ml PMA for 48 h, or 100 nM
VD3 for 96 h with medium change at 48-h post-exposure,
respectively. As for control groups, cells were exposed to vehicle
reagent, ethanol alone, at a final concentration of 0.1%.
Morphological changes of HL-60 cells were observed using
phase-contrast microscope (CK2, Olympus, Japan). After exposure to
PMA, non-adherent cells were discarded, and tightly adherent cells
were designated as PMA-treated HL-60 cells and harvested for cell
counting. The number of PMA-treated HL-60 cells was
∼3×106 in 5 ml medium (i.e. at the density of
6×105 cells/ml) each time. Moreover, after exposure to
VD3, loosely adherent cells were designated as
VD3-treated HL-60 cells. After the cell density of
VD3-treated HL-60 and control group cells were also
adjusted to 6×105 cells/ml in fresh medium, the three
kinds of cells were treated with Vitex or casticin at various
concentrations indicated at 37°C for a designated time.
Furthermore, in order to elucidate the involvement of ICAM-1 in the
cytotoxicity of Vitex against differentiated HL-60 cells, HL-60
cells were treated with 10 ng/ml of PMA in the presence or absence
of 1 μg/ml of anti-ICAM-1 mAb for 48 h, followed by the
treatment with 50 μg/ml Vitex for 24 h.
Cell viability assay
Cell viability was determined by XTT dye-reduction
assay according to the method previously described (19). Briefly, after treatment with
various concentrations of Vitex or casticin for a designated time,
cells were washed with PBS twice and resuspended in appropriate
volume of PBS. An aliquot (0.2 ml) of cell suspension was
inoculated into 96-well micro-plates followed by the addition of 50
μl XTT/PMS mixed solution [1.5 mM XTT and 0.025 mM PMS].
After incubation at 37°C for 4 h, plates were mixed on a mechanical
plate shaker, and absorbance at 450 nm was measured with a
microplate reader (Safire, Tecan, Switzerland). The relative cell
viability was expressed as the ratio of the absorbance of each
treatment group against those of the corresponding untreated
control group. The IC50 value of Vitex and casticin was
calculated from the cell proliferation inhibition curve after 24 h
of treatment.
Nonspecific esterase activity
To identify nonspecific esterase (NSE) activity, a
commercially available kit for NSE staining was used. Briefly,
smear preparations of stimulated cells were fixed with
formalin-acetone fixative solution for 30 sec at 4°C, then washed
with running water, followed by incubation with reaction agent [10
mg of fast garnet GBC salt, 10 μl of naphthyl butyrate, 0.5
ml of ethylene glycol monomethyl ether (EGME) and 9.5 ml of
phosphate buffer (1/15 M, pH 6.3)] for 30 min at 37°C. After
washing with running water, these preparations were stained with
Karachi’s hematoxylin for 10 min at 37°C, then washed with running
water for achieving proper color intensity. After drying, smear
preparations were enclosed with glycerol-gelatin, followed by
observation with inverted microscope (Axiovert 200, Carl Zeiss,
Germany) and photographed with the software of AxioVision 4.5 (Carl
Zeiss). Furthermore, to conduct the sodium fluoride (NaF)
inhibition test, the above-mentioned reaction agent was replaced
with reaction agent containing 4.5 mg of NaF.
Determination of apoptosis
DNA gel electrophoresis
DNA preparation and agarose gel electrophoresis were
carried out according to a method previously reported (19). Extracted DNA was dissolved in TE
buffer (10 mM Tris-HCl, pH 8.0, 1 mM EDTA). DNA samples (∼20
μg/20 μl) and a Tracklt™ 100 bp DNA Ladder
(Invitrogen) as a DNA size marker were electrophoresed on a 2%
agarose gel (Agarose X, Nippon Gene, Tokyo, Japan) using TBE buffer
(89 mM Tris, 89 mM boric acid, 2 mM EDTA). Gels were stained with
ethidium bromide and viewed under printgraph (ATTO Corp., Tokyo,
Japan).
Hoechst 33342 staining
After treatment with 30 and 70 μg/ml Vitex,
respectively, for 24 h, cells were washed twice with PBS and fixed
with 1% glutaraldehyde/PBS at 4°C for at least 2 h. Then, cell were
washed twice with PBS and resuspended in 10 μl of 200
μM Hoechst 33342 (Calbiochem)/PBS for 15 min. The stained
cells were enclosed in FluorSave™ reagent in a mounting medium and
then viewed using a fluorescence microscopy, Axiovert 200.
Reverse transcription-polymerase chain
reaction (RT-PCR) analysis
Total RNA isolation and complementary DNA were
prepared according to methods previously described with
modifications (20). Total RNA was
extracted from cells using an RNA extraction kit, ISOGEN.
Complementary DNA was synthesized from 1 μg of RNA using 100
pmol random primer and 50 units of moloney murine leukemia virus
reverse transcriptase (Invitrogen) in a total volume of 20
μl, according to the manufacturer’s instructions. PCR was
performed according to the methods previously described (20) using a Takara Thermal Cycler MP
(Takara Shuzo Co., Osaka, Japan). DNA sequence of PCR primers and
optimal conditions for PCR are shown in Table I. PCR primers were purchased from
Sigma-Aldrich (Hokkaido, Japan). PCR products and a Tracklt™ 100 bp
DNA Ladder as a DNA size marker were electrophoresed on a 2%
UltraPure™ agarose gel (Invitrogen), respectively, and visualized
by ethidium bromide staining, followed by viewing under UV Light
Printgraph.
 | Table I.PCR primers and conditions used in
the present study. |
Table I.
PCR primers and conditions used in
the present study.
| A, PCR primers and
optimal numbers of PCR cycle |
|---|
|
|---|
| Target mRNA | DNA sequence of PCR
primer | Optimal cycles |
|---|
| CD11b | Sense:
5′-CCCCCAGGTCACCTTCTCCG-3′ | 36 |
| Antisense:
5′-GCTCTGTCGGGAAGGAGCCG-3′ |
| ICAM-1 | Sense:
5′-GCAATGTGCAAGAAGATAGCCAACC-3′ | 36 |
| Antisense:
5′-ACACTTCACTGTCACCTCGGTCCCT-3′ |
| β-actin | Sense:
5′-CCTTCCTGGGCATGGAGTCCTG-3′ | 24 |
| Antisense:
5′-GGAGCAATGATCTTGATCTTC-3′ |
| B, Conditions for
PCR |
|---|
|
|---|
| Target mRNA | Denaturation
reaction | Annealing
reaction | Extension
reaction |
|---|
|
|
|
|---|
| Temp. (°C) | Time (sec) | Temp. (°C) | Time (sec) | Temp. (°C) | Time (sec) |
|---|
| CD11b | 94 | 60 | 62 | 60 | 72 | 600 |
| ICAM-1 | 94 | 60 | 60 | 120 | 72 | 180 |
| β-actin | 94 | 45 | 60 | 45 | 72 | 120 |
Statistical analysis
Experiments were independently repeated three times,
and the results are shown as the mean ± standard deviation (SD) of
three assays. Student’s t-test was applied, and p<0.05 was
considered as significant.
Results
Identification and quantitation of
casticin in Vitex
The above-mentioned F5–F9 fractions containing
casticin obtained by chromatographic separation from Vitex (1 g)
were concentrated under reduced pressure to give a brown oil
residue (100.3 mg). 1H NMR analysis of the residue using
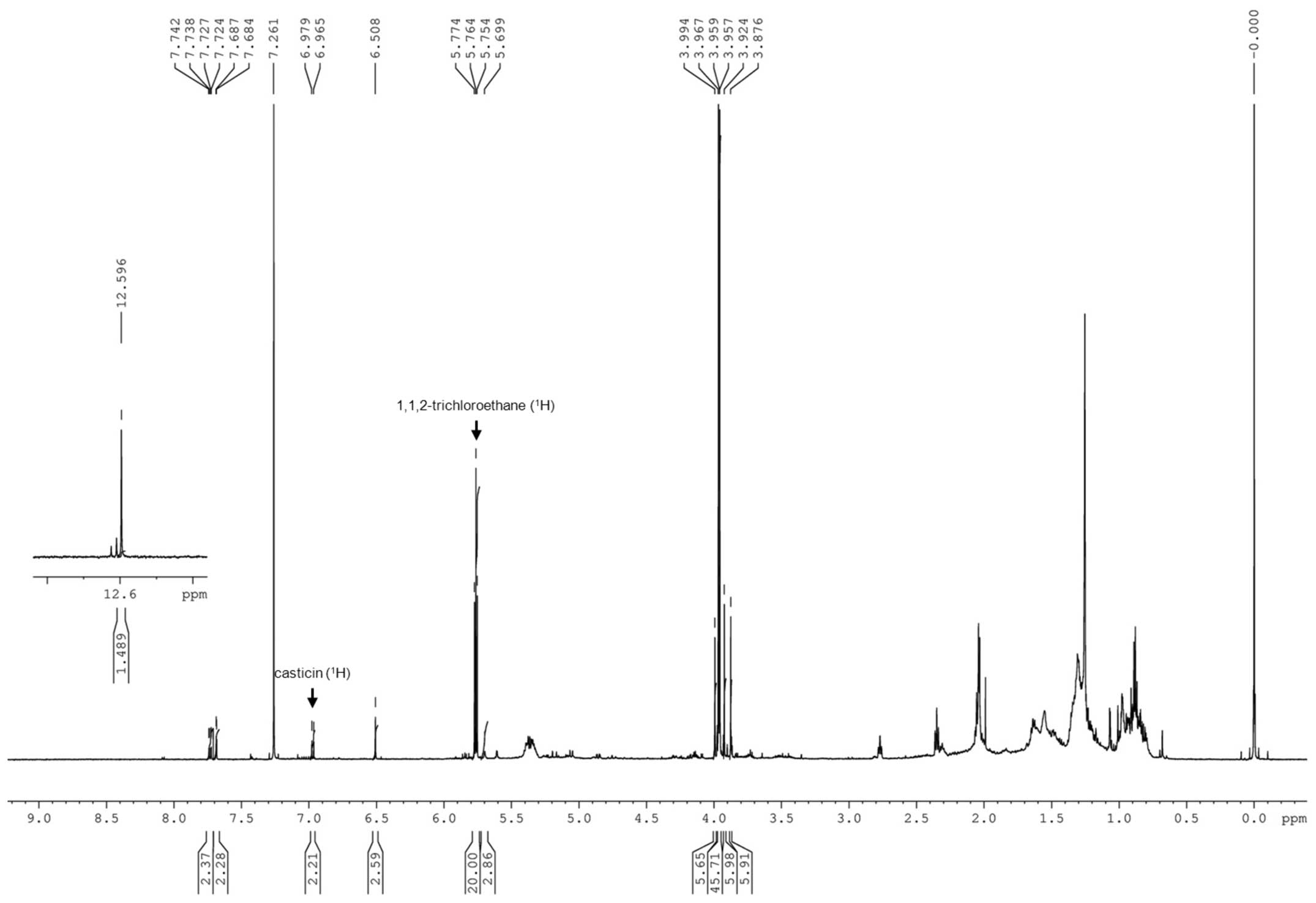
1,1,2-trichloroethane (0.25 mmol, 46 μl) as an internal
standard indicated that the amount of casticin in the residue was
estimated to be 0.0275 mmol (10.3 mg) (Fig. 1). The analysis indicated that
casticin accounted for approximate 1% weight of Vitex.
Cytocidal effects of Vitex and
casticin on HL-60 and U-937 cells
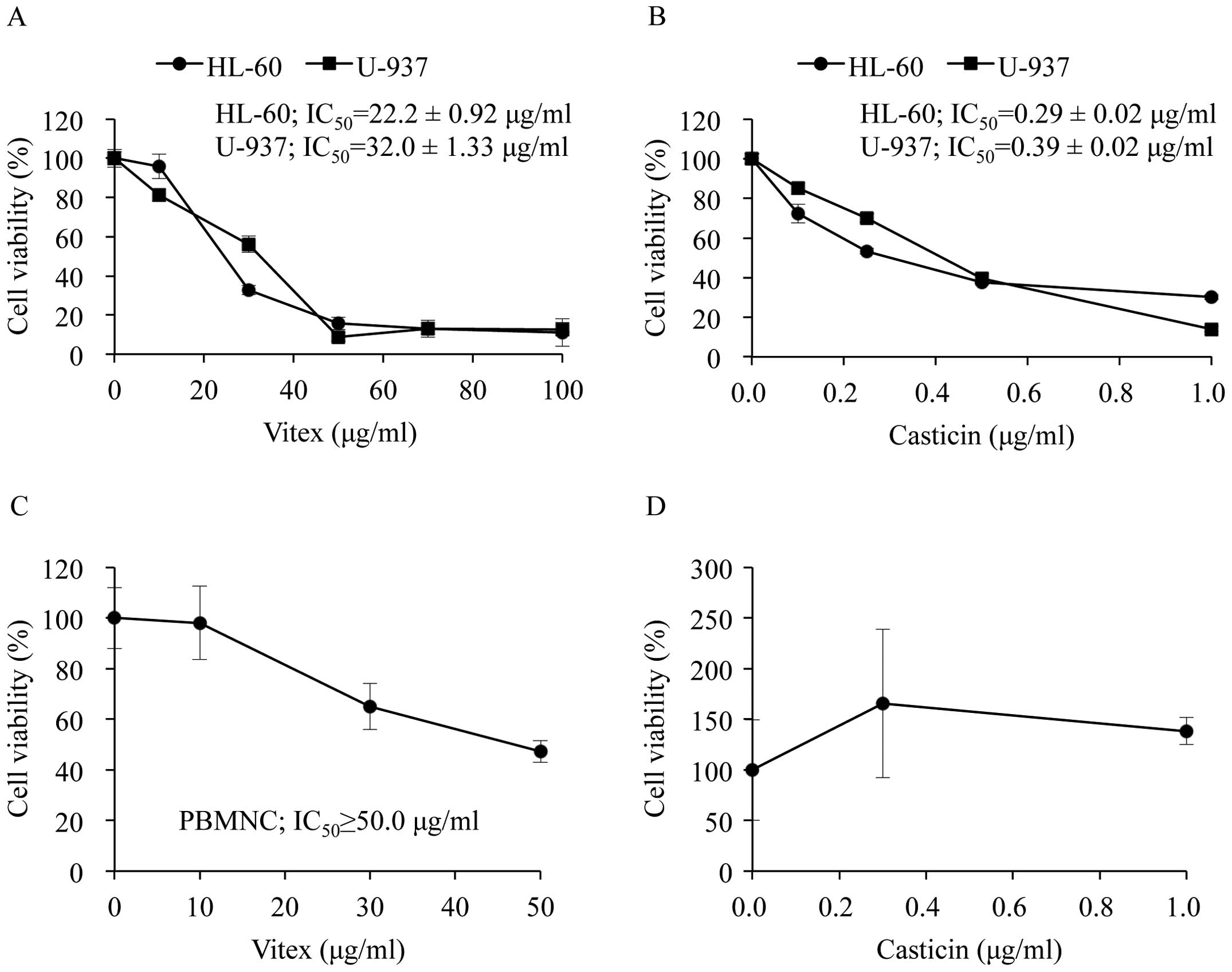
After 24 h of cultivation, the ratio of the
absorbance at 450 nm of both HL-60 and U-937 cells compared to that
at 0 h increased by ∼2.5-fold, indicating cell growth rate of the
two cells was almost same. Although Vitex exhibited cytotoxic
activities against both cells in a dose-dependent manner, a
significant difference in the IC50 value was observed
between HL-60 and U-937 cells (22.2±0.92 μg/ml in HL-60;
32.0±1.33 μg/ml in U-937; p<0.01) (Fig. 2A). Furthermore, similar phenomena
were observed in both cells when treated with casticin (0.29±0.02
μg/ml in HL-60; 0.39±0.02 μg/ml in U-937; p<0.01)
(Fig. 2B), indicating HL-60 is
more sensitive to the cytotoxicity of Vitex and casticin compared
to U-937. Although a relatively high concentration of Vitex
exhibited cytocidal effect against PBMNC, the IC50 value
was more than 50 μg/ml and was ∼2 times higher than that in
both leukemic cell lines (Fig.
2C). Of note, no apparent cytotoxicity of casticin was observed
in PBMNC when treated with concentrations showing significant
cytotoxicity in both leukemic cell lines (Fig. 2D).
Identification of differentiation
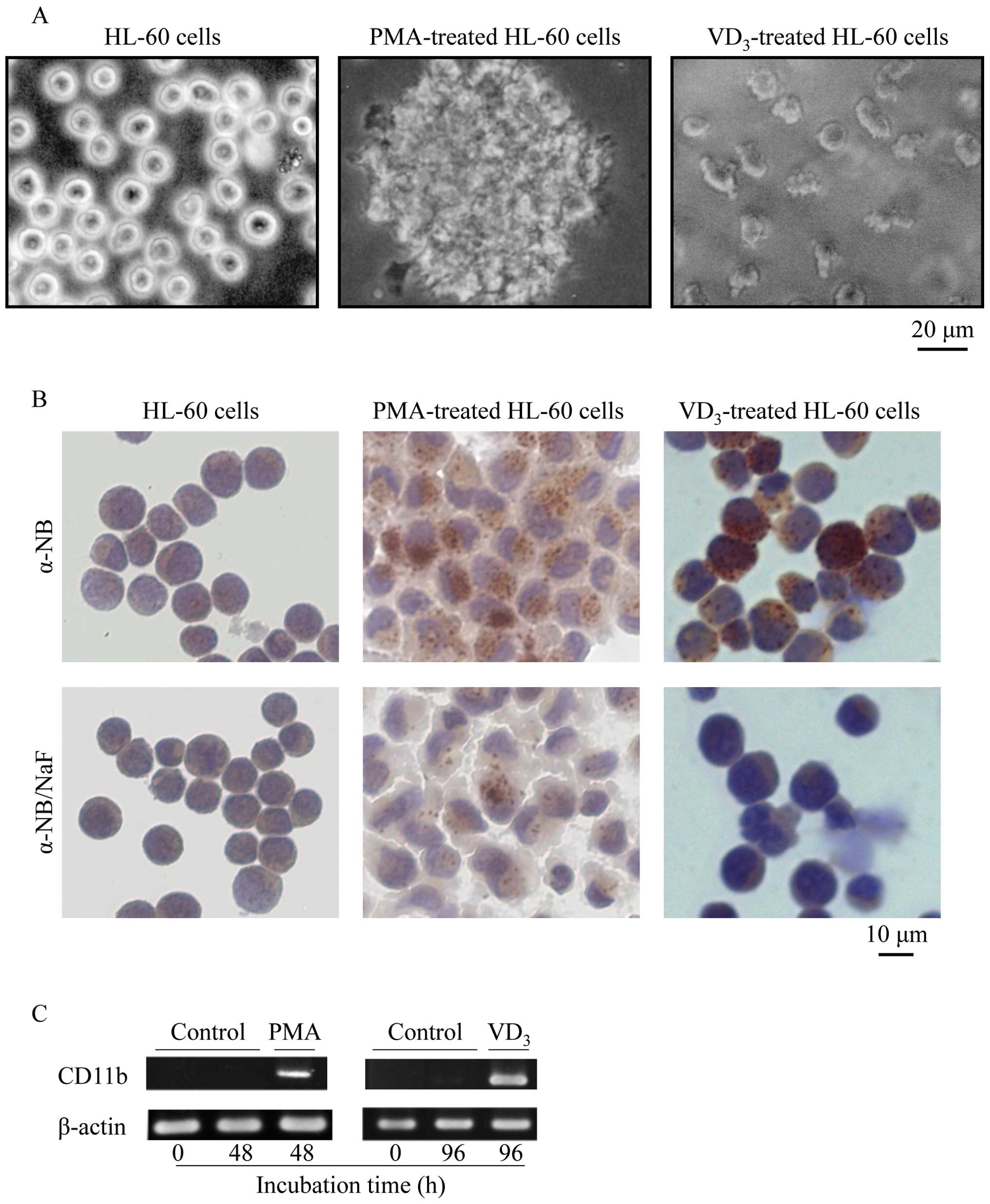
induced by PMA and VD3 in HL-60 cells
After exposure to 10 ng/ml PMA and 100 nM
VD3 for 48 h and 96 h, respectively, the mature
phenotype was confirmed by cell attachment, NSE activity, and
manifestation of a maturation surface marker, CD11b. Whereas
unstimulated HL-60 cells grew as single cell suspension cultures,
PMA-treated HL-60 cells adhered tightly to plastic culture plate
and showed apparent cellular aggregation, and morphology became
macrophage-like (Fig. 3A). On the
other hand, VD3-treated HL-60 cells adhered loosely to
plastic culture plate and its morphology became monocytoid
(Fig. 3A). The NSE is a well-known
selective cytochemical marker for the monocyte/macrophage lineage
(21). Consistent with these
previous reports, NSE activity (brownish-red granulation) was
observed in PMA- and VD3-treated HL-60 cells, but not in
untreated HL-60 cells (Fig. 3B).
Furthermore, NaF abolished NSE activity as expected (Fig. 3B). A predominant increase in CD11b
mRNA was coincidentally observed in both PMA- and
VD3-treated HL-60 cells (Fig. 3C).
Acquisition of resistance to Vitex and
casticin in PMA- and VD3-treated HL-60 cells
Compared to unstimulated control HL-60 cells, PMA-
and VD3-treated HL-60 cells exhibited acquired
resistance to both Vitex and casticin treatment (Table II), although the IC50
value of unstimulated control HL-60 cells indicated in Table II was different from that in
Fig. 2 due to different
cell-density at the point of treatment. In the case of Vitex
treatment, the IC50 value was >100 μg/ml and
39.3±0.55 μg/ml in PMA- and VD3-treated HL-60
cells, respectively, and significantly higher than that in
unstimulated control HL-60 cells (28.3±0.17 μg/ml;
p<0.05). As for casticin treatment, the IC50 value
increased more than two times in both PMA- and
VD3-treated HL-60 cells compared to that in unstimulated
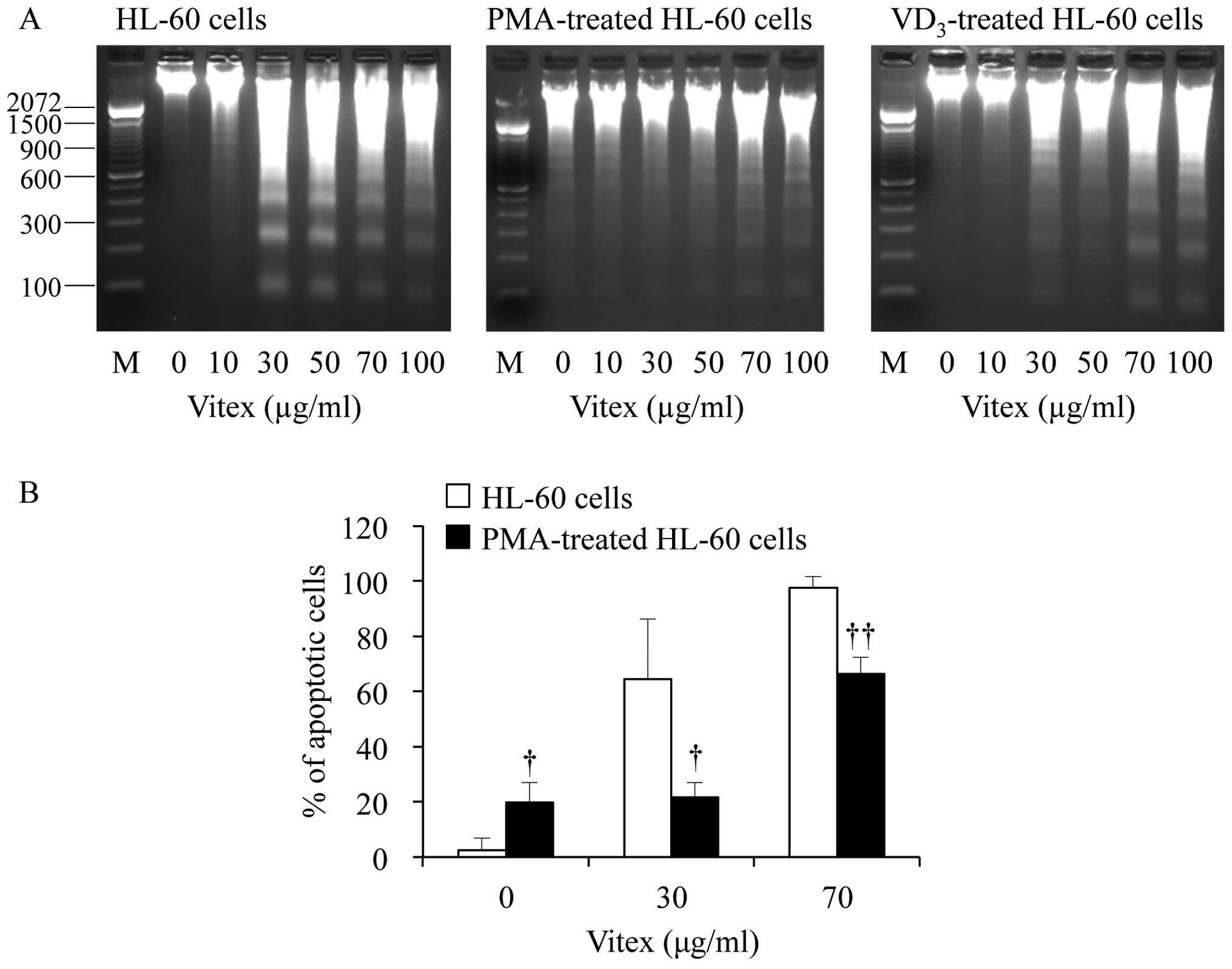
control cells. Furthermore, an evident distinct increase of DNA
fragmentation ladder representing apoptosis induction was observed
at concentrations starting from 30 μg/ml Vitex in
unstimulated control HL-60 cells (Fig.
4A). However, the DNA fragmentation was clearly abrogated in
both differentiated HL-60 cells (Fig.
4A). Hoechst 33342 fluorescent staining also demonstrated that
DNA fragmentation was significantly abrogated in PMA-stimulated
cells even when treated with as high as 70 μg/ml Vitex
(Fig. 4B). On the other hand, we
also observed that a certain degree of apoptotic population was
observed in the flasks that were not treated with Vitex after 24
additional hours of culture (Fig.
4B), similar to a previous study showing that a population of
PMA-treated HL-60 cells spontaneously detached and became dead
cells (14,22).
 | Table II.Acquisition of resistance to Vitex
and casticin in HL-60, PMA-treated HL-60 and VD3-treated
HL-60 cells. |
Table II.
Acquisition of resistance to Vitex
and casticin in HL-60, PMA-treated HL-60 and VD3-treated
HL-60 cells.
| Vitex | Casticin |
|---|
|
|
|---|
| Cells | IC50
value (μg/ml) | IC50
value (μg/ml) |
|---|
| HL-60 | 28.3±0.17 | 0.46±0.01 |
| PMA-treated
HL-60 | >100a | >1.0a |
|
VD3-treated HL-60 | 39.3±0.55a | >1.0a |
Contribution of ICAM-1 to acquired
resistance against Vitex cytotoxicity in PMA-treated HL-60
cells
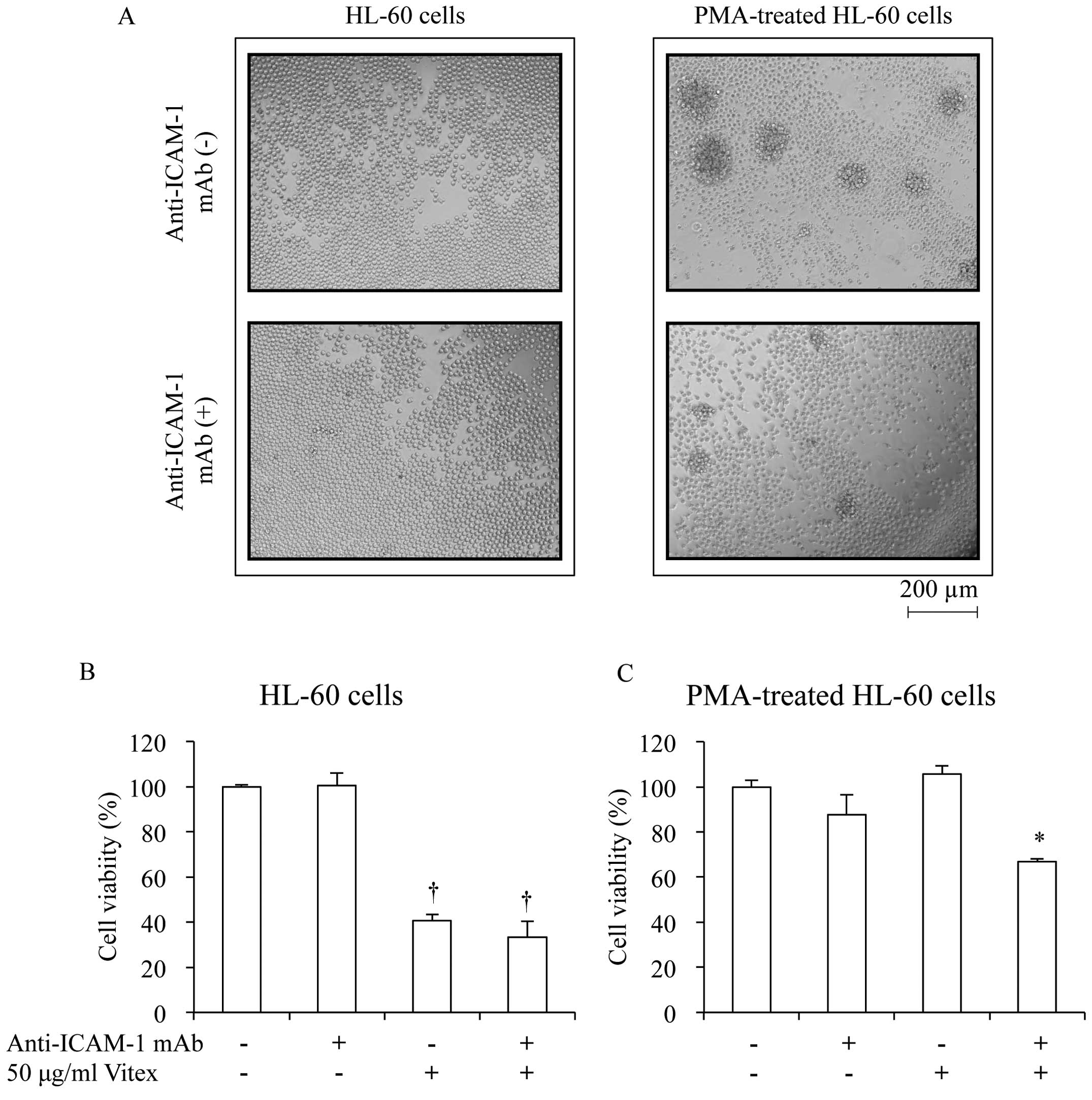
As shown in Fig.
5A, PMA induced cellular aggregation and adhesion within 48 h
after stimulation. However, the addition of 1 μg/ml
anti-ICAM-1 mAb significantly abrogated the effects of PMA. In
agreement with results presented in Table II, no cytotoxicity was observed in
PMA-treated HL-60 when treated with 50 μg/ml Vitex
exhibiting apparent cytocidal effect against unstimulated control
HL-60 cells (Fig. 5B and C).
Again, the addition of anti-ICAM-1 mAb abrogated the acquired
resistance to Vitex (Fig. 5C).
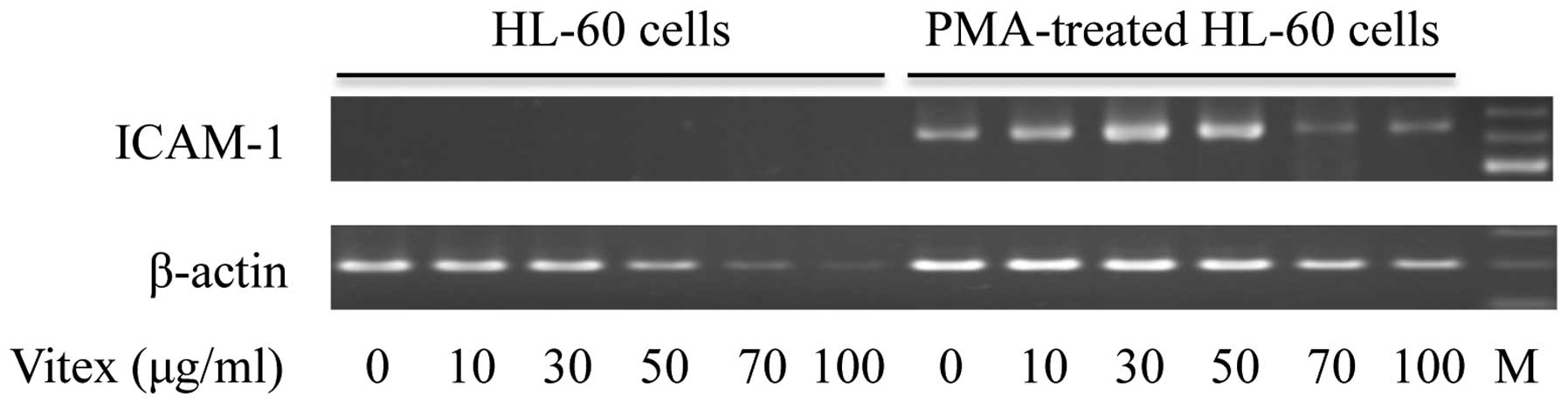
Alterations of ICAM-1 gene expression
in PMA-treated HL-60 cells treated with Vitex
The expression of ICAM-1 mRNA was observed in
PMA-treated HL-60 cells, but not in unstimulated control cells
(Fig. 6), indicating treatment
with PMA induced ICAM-1 gene expression associated with cellular
adhesion and aggregation. Consistent with apparent cytotoxicity
induced by Vitex in unstimulated control cells, the expression
level of β-actin was clearly downregulated when treated with >50
μg/ml Vitex. However, only a slight downregulation of
β-actin expression level was observed in PMA-treated HL-60 cells
even when treated with as high as 70 and 100 μg/ml Vitex.
Noteworthy, a clear upregulation of ICAM-1 mRNA was observed in
PMA-treated HL-60 cells treated with Vitex ranging from 10 to 50
μg/ml, which exhibited no cytocidal effect against the
cells. Furthermore, a prominent decrease in the expression level of
ICAM-1 mRNA was observed in PMA-treated HL-60 when treated with
>70 μg/ml Vitex.
Discussion
In the current study, we demonstrated that HL-60 is
more sensitive to the cytotoxicity of Vitex/casticin compared to
U-937, although growth rates of two cell lines were almost the
same. Based on the fact that HL-60 is more immature than U-937
(13), we hypothesized that unlike
solid tumor cells, the levels of cytotoxic activity of
Vitex/casticin were largely attributed to degree of differentiation
of leukemic cells. Furthermore, similar to our previous studies
showing no apparent cytotoxicity against non-tumor cells, such as
human uterine cervical canal fibroblasts and embryo fibroblasts
(5), much less cytotoxicity was
observed in PBMNC when treated with concentrations of
Vitex/casticin showing significant cytotoxicity in both leukemic
cell lines. These results suggest that Vitex/casticin possess a
selective cytotoxic activity against tumor cells.
In order to verify our hypothesis, the cytocidal
effects of Vitex/casticin were investigated in both
undifferentiated and differentiated HL-60 cells. Morphology
analysis, NSE activity and CD11b expression profiles demonstrated
that HL-60 cells successfully differentiated into
monocyte/macrophage lineage after stimulation by PMA and
VD3, respectively, as demonstrated in previous studies
(12,23). As expected, differentiated HL-60
cells by both PMA and VD3 exhibited acquired resistance
to both Vitex and casticin compared to unstimulated control HL-60.
We demonstrated that a contribution of apoptosis induction was
linked to Vitex-induced cytocidal effects in KATO-III and COLO 201
(6,19,24).
The current study demonstrated that not only solid tumor cells, but
also leukemic cell line HL-60 underwent apoptosis after treatment
with Vitex. Again, apoptosis induction was clearly abrogated in
PMA/VD3-stimulated HL-60 cells when treated with Vitex, even if at
the concentrations as high as 70 μg/ml. These results thus
strongly support our hypothesis that the degree of differentiation
contributed to the sensitivity of leukemic cells to cytotoxic
activity of Vitex.
Cell adhesion molecules, such as integrins,
Ig-related molecules, and selectins, are well known to be involved
in cell-cell/cell-substrate adhesion, which plays a major role in
regulating various cellular processes including the maintenance of
cell survival (15,25). ICAM-1 is one of Ig-related
molecules and recognized by integrins such as LFA-1, consequently
contributing to cell adherence (25). Previous studies have demonstrated
that ICAM-1 together with LFA-1 plays an essential role in cellular
adhesion and/or aggregation in HL-60 cells induced by PMA or TNF-α
(16,26). Furthermore, Solary et al
have previously demonstrated a strong inhibition of apoptosis
induced by camptothecin, and vinblastine in PMA-differentiated
HL-60 cells (14). They further
suggested that inactivation of a cytoplasmic activity (e.g.
inhibition of endonuclease or its activation pathway) resulted from
PMA-induced differentiation contributed to the inhibitory effects,
whereas the correlation of the cell adhesion molecules was not
clarified (14).
In the current study, we demonstrated that
anti-ICAM-1 mAb not only abrogated PMA-induced aggregation and
adhesion of HL-60 cells, but also restored its sensitivity to
Vitex. We also demonstrated that a clear upregulation of ICAM-1
mRNA was observed in PMA-treated HL-60 cells when treated with less
than 50 μg/ml Vitex. Although it was not clarified whether
ICAM-1 contributed to cell-cell or cell-substrate adhesion,
respectively, or both, in our experimental system, and the detailed
experimental studies for the upregulation of ICAM-1 mRNA in
PMA-treated HL-60 are needed, there is little doubt that ICAM-1
plays a crucial role in the acquisition of resistance to Vitex. It
is well established that anti-apoptotic pathways initiated by cell
adhesion are operative in both solid and hematopoietic tumor cells
and, further cause resistance to various cytotoxic drugs with
different mechanisms (27,28). Therefore, it is suggested that
therapeutical efficacy could be achieved by the reversal of drug
resistance resulted from cell adhesion.
In conclusion, we demonstrated for the first time
that the levels of cytotoxicity of Vitex/casticin were largely
attributed to degree of differentiation of hematopoietic cell
lines, in which cell lines with less differentiated phenotype were
more susceptible than the differentiated ones. These results
suggest a potential future application of Vitex/casticin in
combination with clinically used anticancer drugs in view of
developing more efficient strategy to eradicate primary LSCs, which
contribute to eventual disease relapse. We revealed that
administration of Vitex significantly suppressed tumor growth in
COLO 201 xenograft mice (24).
Furthermore, we recently reported that 5-FU in combination with
Vitex achieved an enhanced cytocidal effect on COLO 201 cells
(29). We also clarified that
ICAM-1 resulted from PMA-induced differentiation plays a crucial
role in the acquisition of resistance to Vitex in PMA-treated HL-60
cells, supporting the opinion that therapeutically beneficial
outcomes can be achieved through reversal of the cell adhesion. It
is interesting to note that Vitexins, which is isolated from the
seed of Chinese herb Vitex Negundo and bears a basic flavonoid
structure, shows cytotoxic and antitumor effects against breast,
prostate and ovarian cancer cells through apoptosis induction via
an intrinsic pathway based on in vitro and in vivo
xenograft tumor models (30).
Therefore, our results provide new insight into the clinical use of
Vitex for not only solid tumors but also hematopoietic
malignancy.
Acknowledgements
This work was supported in part by
grants from Japan China Medical Association to Bo Yuan. This work
was also supported in part by grants from the Ministry of
Education, Culture, Sports, Science and Technology and by the
Promotion and Mutual Aid Corporation for Private Schools of
Japan.
References
|
1.
|
Fimognari C, Lenzi M and Hrelia P:
Chemoprevention of cancer by isothiocyanates and anthocyanins:
mechanisms of action and structure-activity relationship. Curr Med
Chem. 15:440–447. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
2.
|
Yuan B, Imai M, Kikuchi H, Fukushima S,
Hazama S, Akaike T, Yoshino Y, Ohyama K, Hu X, Pei X and Toyoda H:
Cytocidal effects of polyphenolic compounds, alone or in
combination with, anticancer drugs against cancer cells: potential
future application of the combinatory therapy. Apoptosis and
Medicine. Ntuli TM: InTech; Croatia: pp. 155–174. 2012
|
|
3.
|
Schellenberg R: Treatment for the
premenstrual syndrome with agnus castus fruit extract: prospective,
randomised, placebo controlled study. BMJ. 322:134–137. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
4.
|
Ma L, Lin S, Chen R, et al: Treatment of
moderate to severe premenstrual syndrome with Vitex agnus castus
(BNO 1095) in Chinese women. Gynecol Endocrinol. 26:612–616. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
5.
|
Ohyama K, Akaike T, Hirobe C, et al:
Cytotoxicity and apoptotic inducibility of Vitex
agnus-castus fruit extract in cultured human normal and cancer
cells and effect on growth. Biol Pharm Bull. 26:10–18.
2003.PubMed/NCBI
|
|
6.
|
Ohyama K, Akaike T, Imai M, et al: Human
gastric signet ring carcinoma (KATO-III) cell apoptosis induced by
Vitex agnuscastus fruit extract through intracellular
oxidative stress. Int J Biochem Cell Biol. 37:1496–1510. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
7.
|
Chen SN, Friesen JB, Webster D, et al:
Phytoconstituents from Vitex agnus-castus fruits.
Fitoterapia. 82:528–533. 2011.
|
|
8.
|
Shen JK, Du HP, Yang M, et al: Casticin
induces leukemic cell death through apoptosis and mitotic
catastrophe. Ann Hematol. 88:743–752. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
9.
|
Lane SW, Scadden DT and Gilliland DG: The
leukemic stem cell niche: current concepts and therapeutic
opportunities. Blood. 114:1150–1157. 2009. View Article : Google Scholar
|
|
10.
|
Collins SJ: The HL-60 promyelocytic
leukemia cell line: proliferation, differentiation, and cellular
oncogene expression. Blood. 70:1233–1244. 1987.PubMed/NCBI
|
|
11.
|
Tanaka H, Abe E, Miyaura C, et al: 1
alpha, 25-dihydroxyvitamin D3 induces differentiation of human
promyelocytic leukemia cells (HL-60) into monocyte-macrophages, but
not into granulocytes. Biochem Biophys Res Commun. 117:86–92. 1983.
View Article : Google Scholar : PubMed/NCBI
|
|
12.
|
Rovera G, Santoli D and Damsky C: Human
promyelocytic leukemia cells in culture differentiate into
macrophage-like cells when treated with a phorbol diester. Proc
Natl Acad Sci USA. 76:2779–2783. 1979. View Article : Google Scholar : PubMed/NCBI
|
|
13.
|
Drexler HG and Minowada J: History and
classification of human leukemia-lymphoma cell lines. Leuk
Lymphoma. 31:305–316. 1998.PubMed/NCBI
|
|
14.
|
Solary E, Bertrand R, Kohn KW, et al:
Differential induction of apoptosis in undifferentiated and
differentiated HL-60 cells by DNA topoisomerase I and II
inhibitors. Blood. 81:1359–1368. 1993.PubMed/NCBI
|
|
15.
|
Vachon PH: Integrin signaling, cell
survival, and anoikis: distinctions, differences, and
differentiation. J Signal Transduct. 2011:7381372011. View Article : Google Scholar : PubMed/NCBI
|
|
16.
|
Katagiri K, Yokosawa H, Kinashi T, et al:
Ubiquitin-proteasome system is involved in induction of
LFA-1/ICAM-1-dependent adhesion of HL-60 cells. J Leukoc Biol.
65:778–785. 1999.PubMed/NCBI
|
|
17.
|
Lewin G, Maciuk A, Thoret S, et al:
Semisynthesis of natural flavones inhibiting tubulin
polymerization, from hesperidin. J Nat Prod. 73:702–706. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
18.
|
Fukushima H, Hirano T and Oka K:
Staphylococcus aureus-superantigen decreases FKBP51 mRNA
expression and cell-response to suppressive efficacy of a
glucocorticoid in human peripheral blood mononuclear cells:
possible implication of mitogen-activated protein kinase pathways.
Eur J Pharmacol. 570:222–228. 2007. View Article : Google Scholar
|
|
19.
|
Imai M, Kikuchi H, Denda T, et al:
Cytotoxic effects of flavonoids against a human colon cancer
derived cell line, COLO 201: a potential natural anti-cancer
substance. Cancer Lett. 276:74–80. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
20.
|
Yuan B, Ohyama K, Bessho T, et al:
Contribution of inducible nitric oxide synthase and
cyclooxygenase-2 to apoptosis induction in smooth chorion
trophoblast cells of human fetal membrane tissues. Biochem Biophys
Res Commun. 341:822–827. 2006. View Article : Google Scholar
|
|
21.
|
Yourno J, Burkart P, Mastropaolo W, et al:
Monocyte nonspecific esterase. Enzymologic characterization of a
neutral serine esterase associated with myeloid cells. J Histochem
Cytochem. 34:727–733. 1986. View Article : Google Scholar
|
|
22.
|
Solary E, Bertrand R and Pommier Y:
Apoptosis of human leukemic HL-60 cells induced to differentiate by
phorbol ester treatment. Leukemia. 8:792–797. 1994.PubMed/NCBI
|
|
23.
|
Murao S, Gemmell MA, Callaham MF, et al:
Control of macrophage cell differentiation in human promyelocytic
HL-60 leukemia cells by 1,25-dihydroxyvitamin D3 and
phorbol-12-myristate-13-acetate. Cancer Res. 43:4989–4996.
1983.PubMed/NCBI
|
|
24.
|
Imai M, Yuan B, Kikuchi H, et al: Growth
inhibition of a human colon carcinoma cell, COLO 201, by a natural
product, Vitex agnus-castus fruits extract, in vivo and in
vitro. Adv Biol Chem. 2:20–28. 2012. View Article : Google Scholar
|
|
25.
|
Prieto J, Eklund A and Patarroyo M:
Regulated expression of integrins and other adhesion molecules
during differentiation of monocytes into macrophages. Cell Immunol.
156:191–211. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
26.
|
Lee SM, Lee YJ, Kim YC, et al: Vascular
protective role of vitexicarpin isolated from Vitex
rotundifolia in human umbilical vein endothelial cells.
Inflammation. 35:584–593. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
27.
|
Hazlehurst LA and Dalton WS: Mechanisms
associated with cell adhesion mediated drug resistance (CAM-DR) in
hematopoietic malignancies. Cancer Metastasis Rev. 20:43–50. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
28.
|
Li ZW and Dalton WS: Tumor
microenvironment and drug resistance in hematologic malignancies.
Blood Rev. 20:333–342. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
29.
|
Imai M, Kikuchi H, Yuan B, et al: Enhanced
growth inhibitory effect of 5-fluorouracil in combination with
Vitex agnus-castus fruits extract against a human colon
adenocarcinoma cell line, COLO 201. J Clin Clin Med. 6:14–19.
2011.
|
|
30.
|
Zhou Y, Liu YE, Cao J, et al: Vitexins,
nature-derived lignan compounds, induce apoptosis and suppress
tumor growth. Clin Cancer Res. 15:5161–5169. 2009. View Article : Google Scholar : PubMed/NCBI
|