Introduction
Ubiquitin-proteasome system (UPS) is a well-known
specific protein degradation pathway. It plays major roles in
multiple biological processes such as proteins turnover, cell cycle
control, antigen processing, signal transduction, protein quality
control, cell differentiation and apoptosis (1,2). It
has been found that tumor cells are more sensitive to proteasome
inhibitors than normal cells. Today, the proteasome is one of the
most promising targets of anticancer drug, but the involved
molecular mechanisms are still unclear.
Gene expression is regulated by various mechanisms
in which RNA decay pathway is one of the most important regulators.
Nonsense mediated mRNA decay (NMD) is a highly conserved pathway
which degrades the nonsense mutation (also called premature
termination codon) containing mRNA selectively (3,4). In
addition to nonsense-mutation in genome, transcription error,
alternative splicing and RNA editing can also bear targets of NMD
(5). However, little was known
about the relationship between NMD pathway and UPS.
During the whole NMD pathway, Upf1, Upf2 and Upf3
proteins are essential for NMD (6). SMG1 is a member of PI3KK family and
phosphorylates Upf1 (7). SMG1 and
Upf1 form a complex with translation termination factors eRF1 and
eRF3 (SURF) and this complex formation is essential for NMD
(8,9). Upf2 is recruited to mRNA via binding
to N terminus of Upf3b that is a component of exon-exon junction
complex on mRNA molecule (10,11).
Then, SURF interacts with both Upf2 and stalled ribosome on
nonsense codon and induces the rapid degradation of aberrant mRNA
following decapping.
In the present study, we analyzed the effect of UPS
on NMD components which keep the genetic integrity. The results may
help us understand the mechanisms underlining the anticancer effect
of UPS inhibitors.
Materials and methods
Cell culture and siRNA transfection
A549 cells were cultured with Dulbecco’s modified
Eagle’s medium (Sigma-Aldrich, St. Louis, MO, USA) supplemented
with FBS (Sigma-Aldrich) and antibiotics (Penicillin, Wako Pure
Chemical Industries Ltd., Osaka, Japan). Cells
(0.8–2.0×105) were inoculated into 12-well plate one day
before transfection. RNAiFect transfection kit (Qiagen GmbH,
Hilden, Germany) was used following the manufacturer’s protocol.
The following were target sequences of siRNA: control luciferase,
CGUACGCGGAAU ACUUCGA; SMG1-1 (5032), AAGAUGAAUGGUGGA GAGUUA. SMG1-2
(2999), GCAGAAAGGUGGUUGACAA; control luciferase was from B-Bridge
Inc. (Tokyo, Japan). SMG1-1 (5032) was purchased from Dharmacon
(Thermo Fisher Scientific Inc., Waltham, MA, USA) and SMG1-2 (2999)
was purchased from Nippon EGT Co., Ltd. (Tokyo, Japan).
Chemical reagents
Cells were treated with 100 μg/ml
cycloheximide (Sigma-Aldrich), 20 to 30 μM MG132
(Calbiochem, Merck KGaA, Darmstadt, Germany), 20 μM
lactacystin (Calbiochem) or 10 μM wortmannin (Calbiochem)
for 12 h, and each was dissolved in DMSO as stock solution.
Western blot analysis
Cell lysates were prepared with lysis buffer (50 mM
Tris-HCl, 150 mM NaCl, 1.0% Nonidet P-40) supplemented with
protease inhibitor cocktail (Complete mini, EDTA-free, F.
Hoffmann-La Roche Ltd., Basel, Switzerland) on ice. NE-PER Nuclear
and Cytoplasmic Extraction Reagents (Pierce, Thermo Fisher
Scientific) were used for cellular fractionation. Lysates were
denatured by Laemmli sample buffer (Bio-Rad Laboratories Inc.,
Hercules, CA, USA) with 2-mercaptoethanol (Sigma-Aldrich). After
SDS-PAGE, the separated polypeptides were transferred to Immobilon
transfer membrane (Millipore, Merck KGaA). The first antibodies
used in the present study were: affinity purified anti-SMG1 rabbit
antiserum was obtained by immunization with synthesized peptide
GCAVSVWKRVKAKLEGRDVD; goat anti-Upf1 (P-14), goat anti-Upf2 (C-18),
rabbit anti-ubiquitin and mouse anti-lamin A/C (636) were purchased
from Santa Cruz Biotechnology Inc., (Santa Cruz, CA, USA) and
rabbit anti-caspase-3 was obtained from Cell Signaling Technology
Inc. (Danvers, MA, USA). Mouse anti-β-actin was purchased from
Sigma-Aldrich. Bound first antibodies were detected by adequate
HRP-conjugated second antibody (Pierce) and SuperSignal West Femto
maximum sensitivity substrate (Pierce) was used for detection. The
resultant chemiluminescence was captured by cooled CCD camera
system (Atto Corp., Tokyo, Japan) and quantified using Image
processing software (Image J).
Immunofluorescence analysis
The immunostaining method had been described
previously (13). For
anti-luciferase and SMG1 siRNA transfection, we subjected
exponentially growing A549 cells on Lab-Tek II chamber slide (Nalge
Nunc, Thermo Fisher Scientific). After one day’s culture, cells
were washed wish PBS and fixed with cold methanol at 4°C for 20
min. Then, after being permeated with 0.1% Triton X-100 and treated
with 10% fetal calf serum, cells were incubated with 1% bovine
serum albumin as negative control or one of the following first
antibodies overnight at 4°C: anti-SMG1, anti-Upf1 and anti-Upf2
antiserum. After washing to remove excess primary antibodies, cells
were incubated for 0.5 h at RT with Alexa Fluor 594 chicken
anti-rabbit or goat IgG (Invitrogen, Life Technologies, Carlsbad,
CA, USA). Finally cells were observed and images taken by
fluorescence microscopy.
Immunoprecipitation
Cell pellets were sonicated in NET-2 buffer (50 mM
Tris-HCl, 300 mM NaCl, 0.05% IGEPAL-CA630) and centrifuged.
Clarified supernatants were incubated with the primary antibodies
against Upf1, ubiquitin or normal IgG (Santa Cruz Biotechnology
Inc.), which were immobilized on protein A/G beads suspended in
NET-2 buffer. Following 3-h incubation at 4°C with gentle rotation,
beads were washed extensively five times with lysis buffer, boiled
in Laemmli sample buffer and microcentrifuged. Purified
polypeptides were separated in SDS-PAGE and detected with western
blot analysis with specific antibodies against Upf1 or
ubiquitin.
Statistical analysis
To evaluate the significance of average, we used
Student’s t-test. The level of statistical significance was set at
P<0.05.
Results
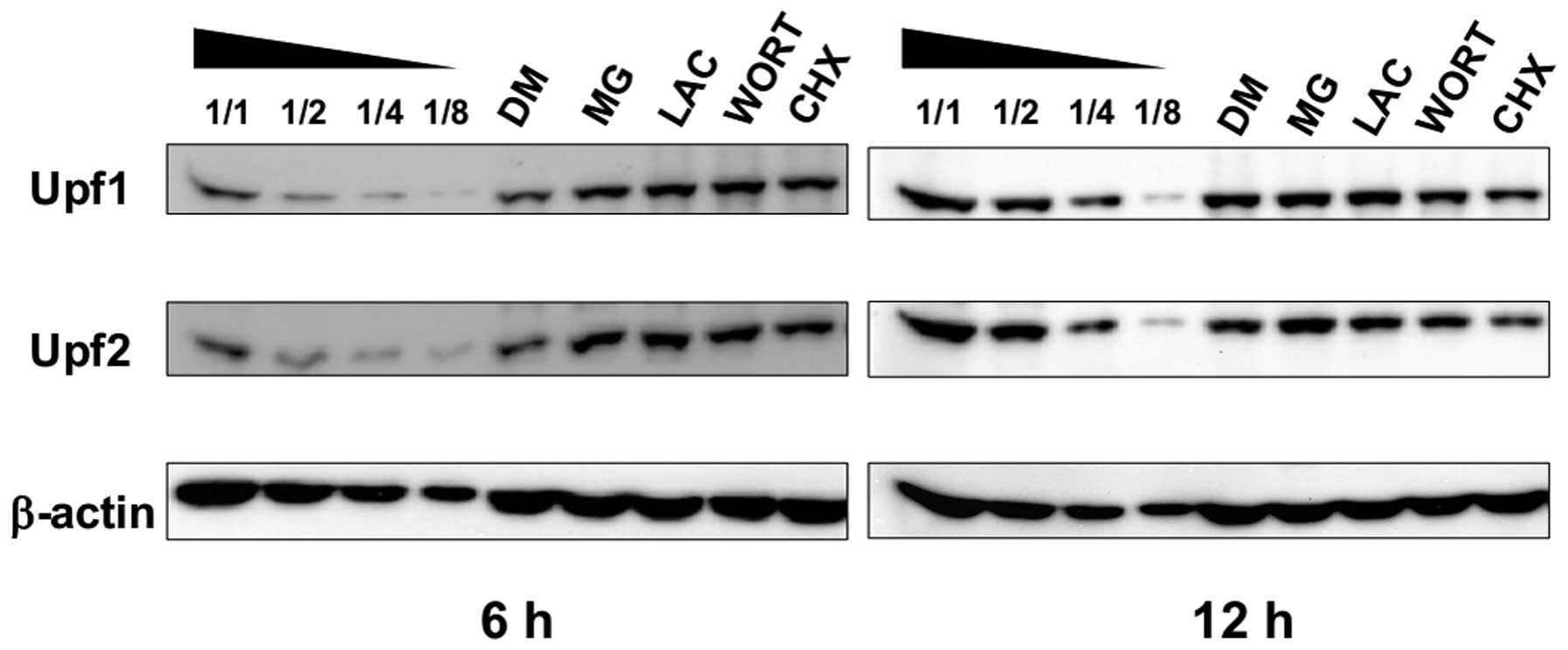
Proteasome inhibitors cause the
accumulation of Upf1 and Upf2 proteins
To reveal the relationship between UPS and NMD
pathway, we examined the effects of ubiquitin-proteasome
inhibitors, such as MG132 (MG) and lactacystin (LAC), on the
stabilization and accumulation of Upf proteins. Both Upf1 and 2 are
putative ubiquitin substrates that have been identified recently
(12). Cycloheximide (CHX), a
translation inhibitor, and wortmannin (WORT), a PI-3 kinase
inhibitor, are often used as NMD inhibitors and they can cause the
accumulation of nonsense-containing mRNAs. To identify the
underlying mechanisms of Upf1 and Upf2 accumulation, we compared
the effects of these inhibitors. As shown in Fig. 1, 6-h treatment with CHX or WORT caused
accumulation of Upf1 and Upf2 proteins. However, 12-h treatment
with CHX slightly reduced both of them, which might be caused by
spontaneous degradation with blocked protein synthesis. On the
other hand, MG or LAC treatment caused significant accumulation of
Upf1 and Upf2 proteins.
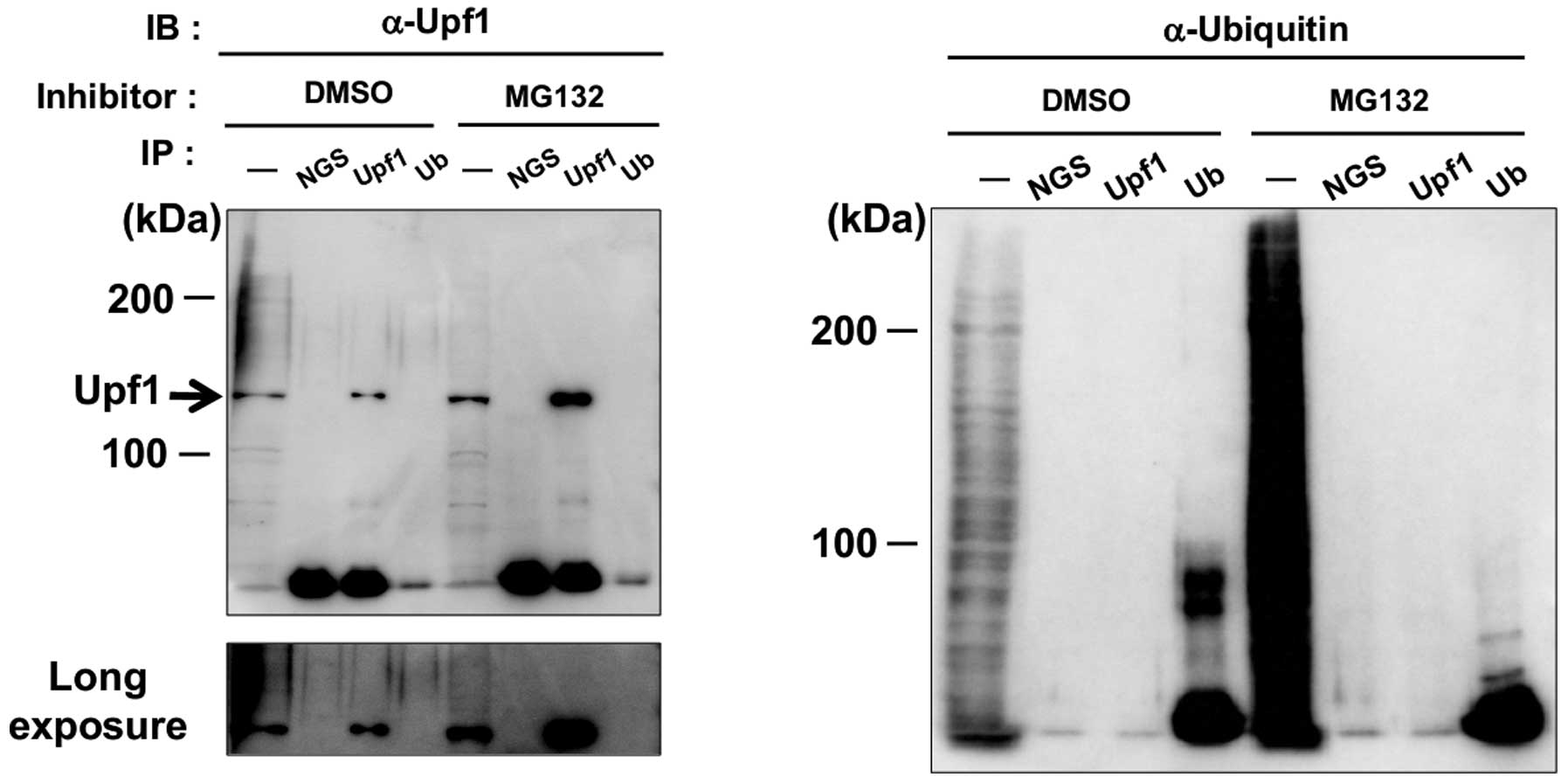
These results prompted us to speculate that Upf1 and
Upf2 would be target molecules of ubiquitination. To investigate
this possibility, we tried detection of higher band shift caused by
polyubiquitination of Upf1 proteins. However, we detected no band
shift of either unpurified Upf1 or immuno-precipitated Upf1, and no
signal of ubiquitin conjugation was detected even in
immuno-purified Upf1 (Fig. 2). The
accumulation of Upf1 could not be accounted for by the fraction of
a very small band shift in MG treated cells. In conclusion, our
experiments provided no clear evidence for polyubiquitination of
Upf1 and proposed that the target molecule(s) of
ubiquitin-proteasome might downregulate the amount of Upf1 directly
or indirectly.
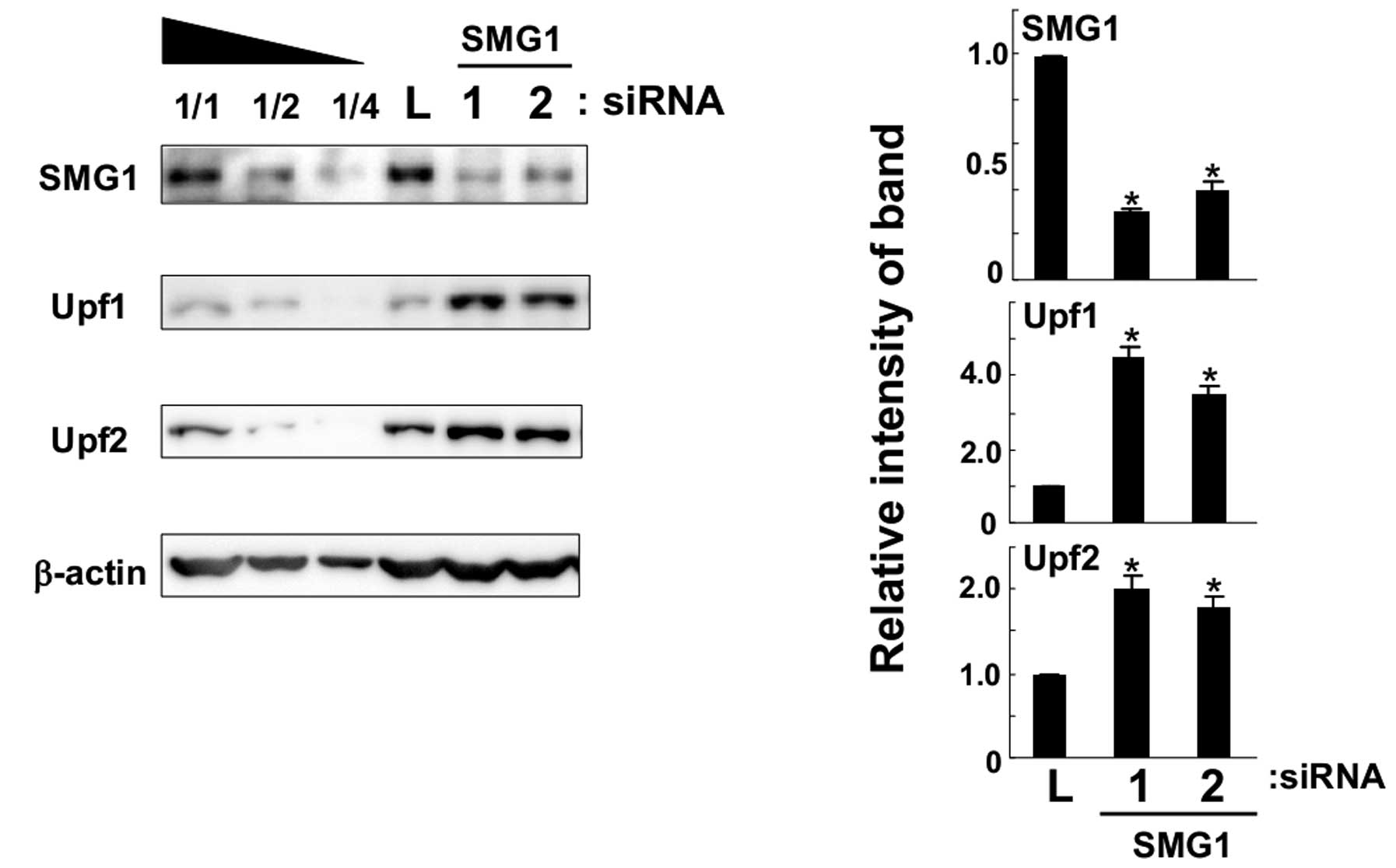
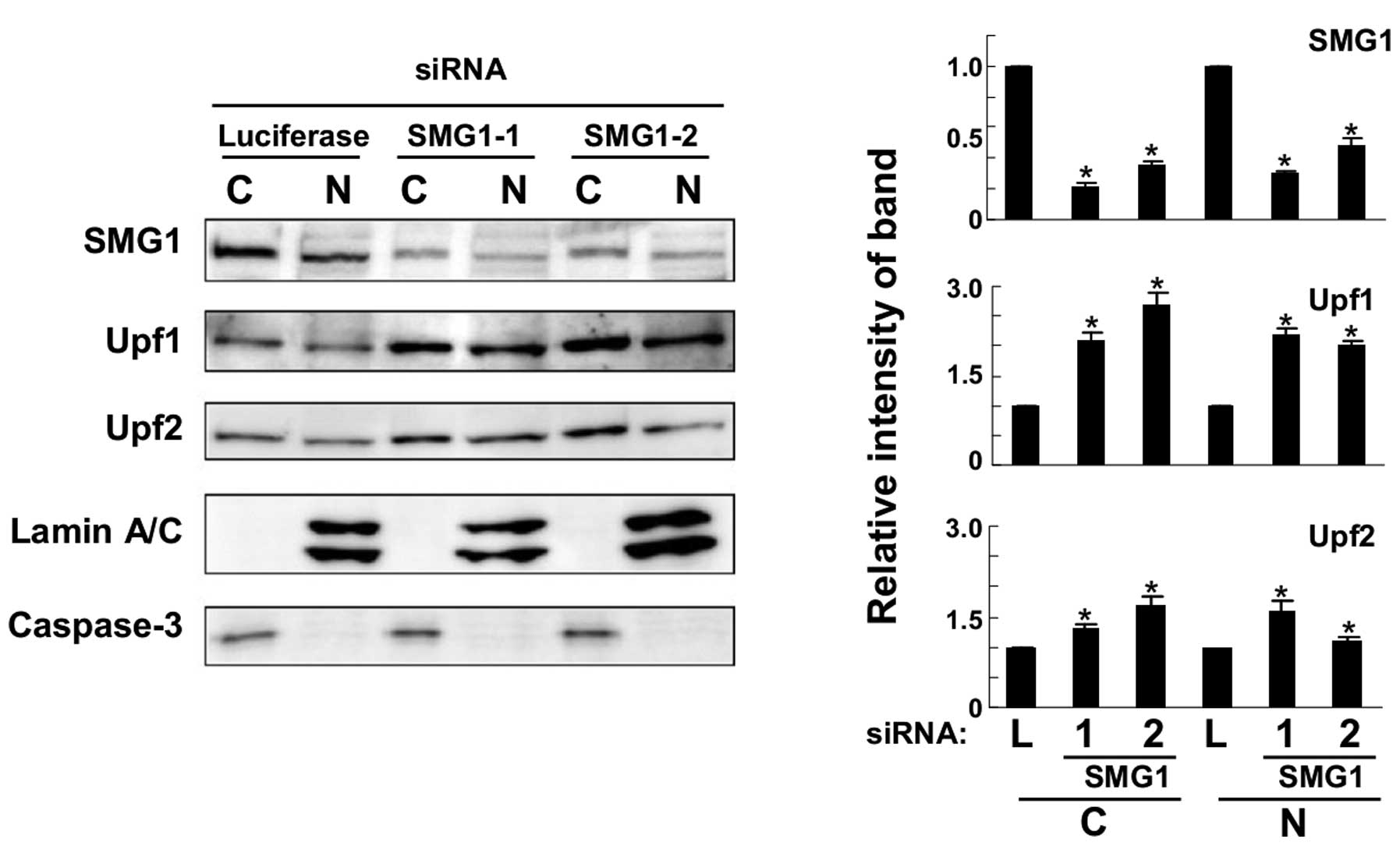
Knockdown of SMG1 causes the accumulation
of Upf1 and Upf2 proteins
A549 cells were transfected with SMG1-1 (5032) siRNA
against SMG1 mRNA and their lysates were analyzed by western
blotting with specific antibodies. As shown in Fig. 3, SMG1 was downregulated by siRNA
transfection successfully as compared to control cells transfected
with anti-luciferase siRNA. As mentioned in a previous publication
(7), short and long isoforms of
SMG1 were detected by our antiserum and both were knocked down by
siRNA in the present study. We found that both Upf1 and Upf2
proteins increased significantly in SMG1 knockdown cells. Next, we
used another SMG1-2 siRNA (2999) molecule and transfected it to
A549 cells. It caused similar accumulations of Upf1 and Upf2
proteins. From these results, we concluded that SMG1 knockdown
could cause the accumulation of Upf1 and Upf2 proteins in cultured
cells.
To determine the cellular fraction where Upf1 and
Upf2 accumulated, western blot analysis was performed with
fractionated cellular lysates. As shown in Fig. 4, the efficiency of fractionation
was confirmed by an enrichment of nuclear specific marker lamin A/C
and cytoplasmic marker, caspase-3. Specific bands were observed in
fractions. They were knocked down successfully in both fractions by
SMG1 siRNA transfection. In this condition, Upf1 and Upf2 proteins
increased in both nuclear and cytoplasmic fractions. It seemed that
SMG1 could downregulate the amounts of Upf1 and Upf2 proteins in
nuclear and cytoplasmic fraction.
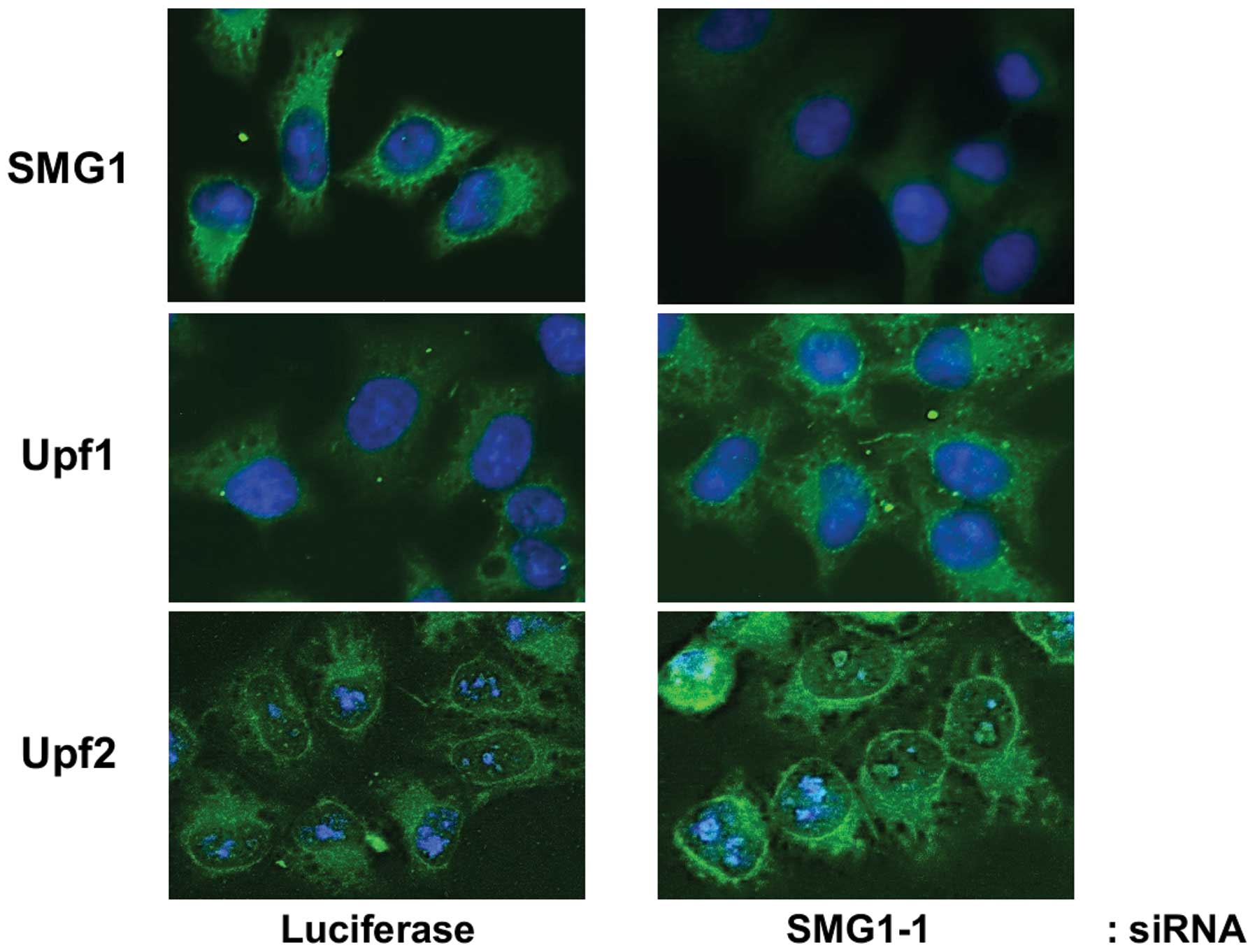
To get morphologic evidence, we performed
immunofluorescence staining to test the location of Upf1 and Upf2
proteins. By using specific antibodies, we observed that Upf1 was
evenly distributed throughout the cytoplasm, while Upf2 showed
strong perinuclear staining (Fig.
5). With anti-SMG1 siRNA knockdown, the fluorescence signals of
SMG1 in cells weakened significantly, which suggested successful
knock-down of SMG1. Moreover, we found the fluorescence signals of
Upf1 and Upf2 increased respectively, which confirmed our results
described in Fig. 3.
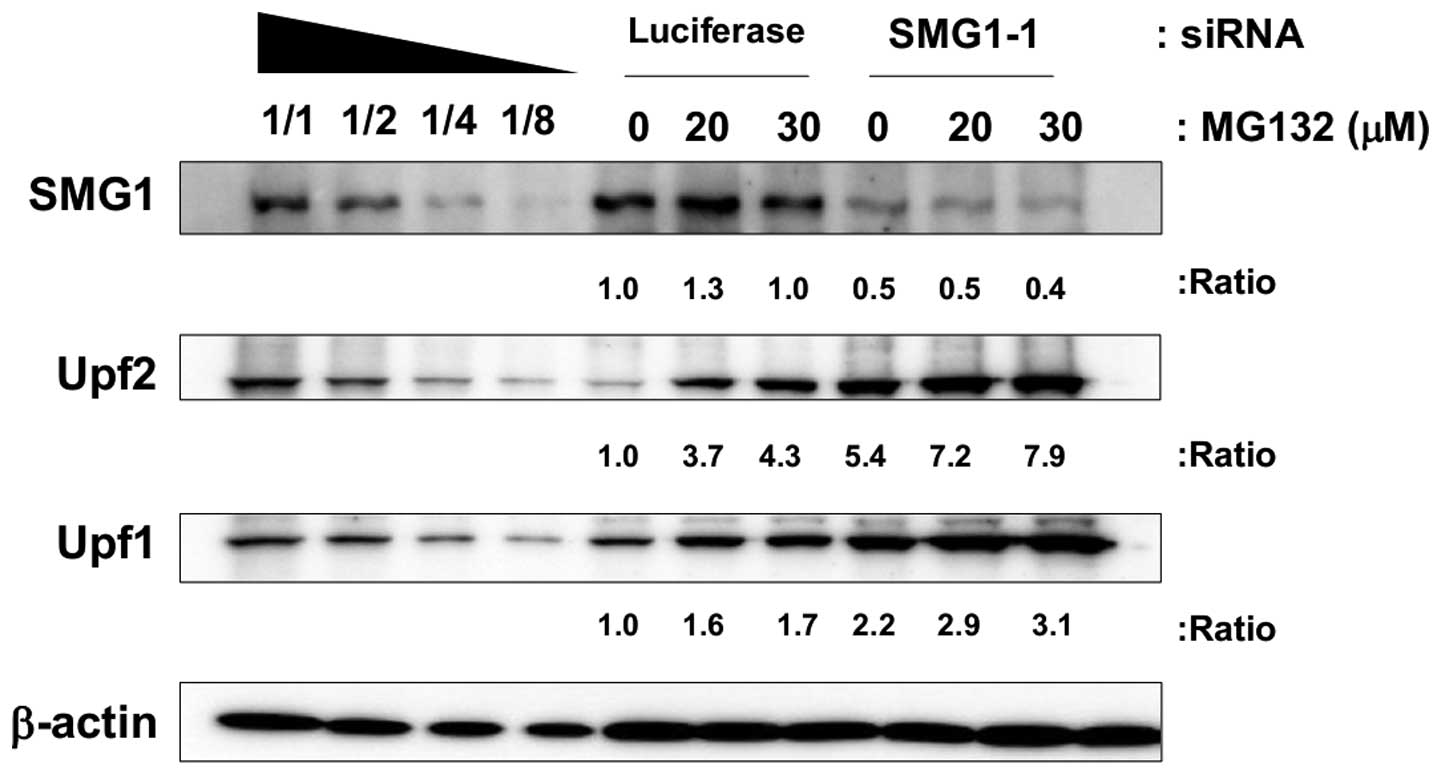
Additive effects of SMG1 knockdown and
proteasome inhibitors
To test whether SMG1 knockdown and
ubiquitinproteasome inhibitors share the same pathway to cause the
accumulation of Upf1 and Upf2, we treated SMG1-knocked down cells
with proteasome inhibitors and the lysates for immunoblot were
obtained. If they affected increments of Upf1 and Upf2
independently, an additional accumulation should be expected. As
shown in Fig. 6, SMG1 knockdown
and proteasome inhibitor MG caused accumulation of Upf1 and Upf2,
respectively. Moreover, in SMG1 knocked down cells, we detected
additive effects of Upf1 and Upf2 accumulation with proteasome
inhibitor treatment. In the real-time PCR results, we could not
observe any significant upregulation of mRNA levels of Upf1 and
Upf2 in proteasome inhibitor treated cells (data not shown). From
these results, we propose the possibility that SMG1 and
ubiquitin-proteasome system acted on different mechanisms that
regulated the amounts of Upf1 and Upf2 proteins.
Discussion
UPS is widely accepted as an attractive target for
drug development due to the tremendous potential for intervention
on multiple pathologies including cancer, neurodegenerative
diseases, immune diseases and multiple infections (14). The most common form of the
proteasome is known as 26S proteasome. Inhibition of the
proteolytic function of the 26S proteasome in malignant cells
provokes apoptosis, ER stress, cell cycle arrest and represses
angiogenesis as well as metastasis (15).
There is a wide variety of natural and synthetic
protea-some inhibitors that can be clustered into five groups:
peptide aldehydes, peptide vinyl sulfones, peptide boronates,
peptide epoxyketones and β-lactones (lactacystin and its
derivatives). MG is the best known molecule of peptide aldehyde
(16). Bortezomib
(Velcade®), the first FDA approved proteasome inhibitor,
belongs to the peptide boronate group. Bortezomib is now used as a
treatment for multiple myeloma (MM), mantle cell lymphoma (MCL),
non-small lung cancer and pancreatic cancer (17–19).
Recently, carfilzomib has become the second generation product of
this class of compounds. Several mechanisms have been revealed,
such as downregulation of NFκB and other anti-apoptotic proteins,
activation of the tumor suppressor protein p53 and modulation of
cell cycle proteins and other pro-apoptotic factors (20–25).
However, the cellular mechanisms for their clinical efficacy have
not been completely elucidated.
The biological and medical importance of NMD is
highlighted by its ability to suppress potential dominant-negative
effects of C-terminally truncated polypeptides (26). During the whole NMD pathway,
different kinds of proteins and enzymes are required, which
coordinate to induce the specific degradation of nonsense codon
containing aberrant transcripts. It was reported that Upf1
potentially acts as an E3 ubiquitin ligase by its association with
Upf3 in yeast (27). Kuroha et
al also reported that Upf1 was required for rapid
proteasome-mediated degradation of an aberrant protein (PTC
product) derived from a PTC-containing mRNA (28). These reports revealed a possible
correlation between NMD pathway and UPS. However, it is still
unclear whether there is a regulative relationship between
them.
In the present study, MG or LAC treatment caused the
accumulation of Upf1 and Upf2 proteins, indicating that UPS might
participate in the downregulation of the amount of Upf1 and Upf2.
However, our experiments provided no clear evidence for
polyubiquitination of Upf1 and proposed the target molecule(s) of
UPS might downregulate the amount of Upf1 directly or indirectly.
Furthermore, our results showed the accumulation of Upf1 and Upf2
proteins in SMG1-knocked down cells. One reasonable interpretation
of the upregulation of Upf1 and Upf2 is the accumulation of NMD
intermediates. Because SMG1 is essential for decap-ping the
nonsense containing mRNA, Upf1 and Upf2 form an incomplete complex
on mRNA in SMG1-knocked down cells. If this were true, Upf1 and
Upf2 would be downregulated or broken down after carrying out NMD.
In our studies, treatment with10 μM WORT, a PI-3 kinase
inhibitor, did not cause the accumulation of Upf1 and Upf2, which
suggested that their complex formation with SMG1 was required for
NMD rather than phosphorylation of Upf1. Additionally, treatment
with MG and SMG1 siRNA simultaneously caused an additive effect on
Upf1 and Upf2 amounts, which suggested the possibility that UPS and
SMG1 regulate the amounts of Upf1 and Upf2 in different manner.
Recent studies have revealed the in vivo role
of NMD factors. NMD reaction takes place on exon junction complex
on mRNA and it consists of various factors (29). Magoh, one of the complex members,
is the binding partner of RBM8A and the mutation of this gene
resulted in the microcephaly in mice (30,31).
RBM8A is related to TAR (thrombocytopenia-absent radii) syndrome in
human and anxiety behavior in mice (32,33).
The exon junction complex seems to be required for normal
development of mammals. On the other hand, Upf2 is required for
various developmental stages such as cell proliferation, liver
regeneration and synapse regulation (34–36).
The copy number variation of Upf2 was related to
neuro-developmental disorders in humans (37). The deficiency of Upf3b protein, a
binding partner of Upf2, also causes various mental illnesses in
human (38–41). Interestingly, the substantial part
of transcriptome in Upf2 deficient patients overlapped with the
data sets from Upf3b deficient patients (37). Therefore, NMD factors seem to be
required for the normal regulation of brain development and mental
health. Thus, it may be possible that the proteasome inhibitor
treatment could be used as care for neuro-development or mental
disorders via the upregulation of Upf2 proteins.
In conclusion, our results partly revealed the
quantitative regulation of Upf1 and Upf2 proteins by UPS and SMG1.
It may help us understand the mechanisms underlining the
anti-cancer effect of UPS inhibitors. The diverse involvement of
NMD factors make the relationship between them complex, and further
experiments are required.
Abbreviations:
|
CHX
|
cycloheximide;
|
|
LAC
|
lactacystin;
|
|
MG
|
MG132;
|
|
NMD
|
nonsense mediated mRNA decay;
|
|
SURF
|
SMG1-Upf1-eRF;
|
|
UPS
|
ubiquitin-proteasome system;
|
|
WORT
|
wortmannin
|
Acknowledgements
This study was supported by grants
from Kanazawa Medical University and Ministry of Education,
Culture, Sports, Science and Technology, Japan (S2013-2, H2012-16
[S1201022]).
References
|
1.
|
Groll M and Potts BC: Proteasome
structure, function, and lessons learned from beta-lactone
inhibitors. Curr Top Med Chem. 11:2850–2878. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
2.
|
Voges D, Zwickl P and Baumeister W: The
26S proteasome: a molecular machine designed for controlled
proteolysis. Annu Rev Biochem. 68:1015–1068. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
3.
|
Maquat LE and Carmichael GG: Quality
control of mRNA function. Cell. 104:173–176. 2011. View Article : Google Scholar
|
|
4.
|
Neu-Yilik G and Kulozik AE: NMD:
multitasking between mRNA surveillance and modulation of gene
expression. Adv Genet. 62:185–243. 2008.PubMed/NCBI
|
|
5.
|
Maquat LE: Nonsense-mediated mRNA decay:
splicing, translation and mRNP dynamics. Nat RevMol Cell Biol.
5:89–99. 2004. View
Article : Google Scholar : PubMed/NCBI
|
|
6.
|
Czaplinski K, Ruiz-Echevaria MJ, Gonzalez
CI and Peltz SW: Should we kill the messenger? The role of the
surveillance complex in translation termination and mRNA turnover.
Bioessays. 21:685–696. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
7.
|
Yamashita A, Ohnishi T, Kashima I, et al:
Human SMG-1, a novel phosphatidylinositol 3-kinase-related protein
kinase, associates with components of the mRNA surveillance complex
and is involved in the regulation of nonsense-mediated mRNA decay.
Genes Dev. 15:2215–2228. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
8.
|
Kashima I, Yamashita A, Izumi N, et al:
Binding of a novel SMG-1-Upf1-eRF1-eRF3 complex (SURF) to the exon
junction complex triggers Upf1 phosphorylation and
nonsense-mediated mRNA decay. Genes Dev. 20:355–367. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
9.
|
Silva AL and Romao L: The mammalian
nonsense-mediated mRNA decay pathway: to decay or not to decay!
Which players make the decision? FEBS Lett. 583:499–505. 2009.
|
|
10.
|
Lykke-Andersen J, Shu MD and Steitz JA:
Human Upf proteins target an mRNA for nonsense-mediated decay when
bound downstream of a termination codon. Cell. 103:1121–1131. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
11.
|
Chamieh H, Ballut L and Bonneau F: NMD
factors UPF2 and UPF3 bridge UPF1 to the exon junction complex and
stimulate its RNA helicase activity. Nat Stuct Mol Biol. 15:85–93.
2008. View
Article : Google Scholar : PubMed/NCBI
|
|
12.
|
Danielsen JM, Sylvestersen KB,
Bekker-Jensen S, et al: Mass spectrometric analysis of lysine
ubiquitylation reveals promiscuity at site level. Mol Cell
Proteomics. 10:M110.0035902011. View Article : Google Scholar : PubMed/NCBI
|
|
13.
|
Ishigaki Y, Nakamura Y, Takehara T, et al:
Scanning electron microscopy with an ionic liquid reveals the loss
of mitotic protrusions of cells during the epithelial-mesenchymal
transition. Microsc Res Tech. 74:1024–1031. 2011. View Article : Google Scholar
|
|
14.
|
Xolalpa W, Perez-Galan P, Rodríguez MS, et
al: Targeting the ubiquitin proteasome system: beyond proteasome
inhibition. Curr Pharm Des. 19:4053–4093. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
15.
|
Buac D, Shen M, Schmitt S, et al: From
Bortezomib to other inhibitors of the proteasome and beyond. Curr
Pharm Des. 19:4025–4038. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
16.
|
Kisselev AF and Goldberg AL: Proteasome
inhibitors: from research tools to drug candidates. Chem Biol.
8:739–758. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
17.
|
Aghajanian C, Soignet S, Dizon DS, et al:
A phase I trial of the novel proteasome inhibitor PS341 in advanced
solid tumor malignancies. Clin Cancer Res. 8:2505–2511.
2002.PubMed/NCBI
|
|
18.
|
Richardson PG, Barlogie B, Berenson J, et
al: A phase 2 study of bortezomib in relapsed, refractory myeloma.
N Engl J Med. 348:2609–2617. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
19.
|
Scagliotti G: Proteasome inhibitors in
lung cancer. Crit Rev Oncol Hematol. 58:177–189. 2006. View Article : Google Scholar
|
|
20.
|
Crawford LJ, Walker B and Irvine AE:
Proteasome inhibitors in cancer therapy. J Cell Commun Signal.
5:101–110. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
21.
|
Lopes UG, Erhardt P, Yao R, et al:
P53-dependent induction of apoptosis by proteasome inhibitors. J
Biol Chem. 272:12893–12896. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
22.
|
Pleban E, Bury M, Mlynarczuk I, et al:
Effects of proteasome inhibitor PSI on neoplastic and
non-transformed cell lines. Folia Histochem Cytobiol. 39:133–134.
2001.PubMed/NCBI
|
|
23.
|
Nakanishi C and Toi M: Nuclear
factor-kappa B inhibitors as sensitizers to anticancer drugs. Nat
Rev Cancer. 5:297–309. 2005. View
Article : Google Scholar : PubMed/NCBI
|
|
24.
|
Mitsiades N, Mitsiades CS, Poulaki V, et
al: Molecular sequelae of proteasome inhibition in human multiple
myeloma cell. Proc Natl Acad Sci USA. 99:14374–14379. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
25.
|
Russo A, Bronte G, Fulfaro F, et al:
Bortezomib: a new pro-apoptotic agent in cancer treatment. Curr
Cancer Drug Targets. 10:55–67. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
26.
|
Garneau NL, Wilusz J and Wilusz CJ: The
highways and byways of mRNA decay. Nat Rev Mol Cell Biol.
8:113–126. 2007. View
Article : Google Scholar : PubMed/NCBI
|
|
27.
|
Takahashi S, Araki Y, Ohya Y, et al: Upf1
potentially serves as a RING-related E3 ubiquitin ligase via its
association with Upf3 in yeast. RNA. 14:1950–1958. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
28.
|
Kuroha K, Tatematsu T and Inada T: Upf1
stimulates degradation of the product derived from aberrant
messenger RNA containing a specific nonsense mutation by the
proteasome. EMBO Rep. 10:1265–1271. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
29.
|
Melero R, Buchwald G, Castaño R, et al:
The cryo-EM structure of the UPF-EJC complex shows UPF1 poised
toward the RNA 3′ end. Nat Struct Mol Biol. 19:498–505.
2012.PubMed/NCBI
|
|
30.
|
Silver DL, Watkins-Chow DE, Schreck KC, et
al: The exon junction complex component Magoh controls brain size
by regulating neural stem cell division. Nat Neurosci. 13:551–558.
2010. View
Article : Google Scholar : PubMed/NCBI
|
|
31.
|
Silver DL, Leeds KE, Hwang HW, et al: The
EJC component Magoh regulates proliferation and expansion of neural
crest-derived melanocytes. Dev Biol. 375:172–181. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
32.
|
Albers CA, Paul DS, Schulze H, et al:
Compound inheritance of a low-frequency regulatory SNP and a rare
null mutation in exon-junction complex subunit RBM8A causes TAR
syndrome. Nat Genet. 44:435–439. 2012. View
Article : Google Scholar : PubMed/NCBI
|
|
33.
|
Alachkar A, Jiang D, Harrison M, et al: An
EJC factor RBM8a regulates anxiety behaviors. Curr Mol Med.
13:887–899. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
34.
|
Weischenfeldt J, Damgaard I, Bryder D, et
al: NMD is essential for hematopoietic stem and progenitor cells
and for eliminating by-products of programmed DNA rearrangements.
Genes Dev. 22:1381–1396. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
35.
|
Long AA, Mahapatra CT, Woodruff EA III, et
al: The nonsense-mediated decay pathway maintains synapse
architecture and synaptic vesicle cycle efficacy. J Cell Sci.
123:3303–3315. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
36.
|
Thoren LA, Nørgaard GA, Weischenfeldt J,
et al: UPF2 is a critical regulator of liver development, function
and regeneration. PLoS One. 5:e116502010. View Article : Google Scholar : PubMed/NCBI
|
|
37.
|
Nguyen LS, Kim HG, Rosenfeld JA, et al:
Contribution of copy number variants involving nonsense-mediated
mRNA decay pathway genes to neuro-developmental disorders. Hum Mol
Genet. 22:1816–1825. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
38.
|
Jolly LA, Homan C, Jacob R, et al: The
UPF3B Gene, implicated in intellectual disability, autism, ADHD and
childhood onset Schizophrenia regulates neural progenitor cell
behaviour and neuronal outgrowth. Hum Mol Genet. July 10–2013.(Epub
ahead of print).
|
|
39.
|
Nguyen LS, Jolly L, Shoubridge C, et al:
Transcriptome profiling of UPF3B/NMD-deficient lymphoblastoid cells
from patients with various forms of intellectual disability. Mol
Psychiatry. 17:1103–1115. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
40.
|
Szyszka P, Sharp SI, Dedman A, et al: A
nonconservative amino acid change in the UPF3B gene in a patient
with schizophrenia. Psychiatr Genet. 22:150–151. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
41.
|
Addington AM, Gauthier J, Piton A, et al:
A novel frame-shift mutation in UPF3B identified in brothers
affected with childhood onset schizophrenia and autism spectrum
disorders. Mol Psychiatry. 16:238–239. 2011. View Article : Google Scholar : PubMed/NCBI
|