Introduction
Hepatocellular carcinoma (HCC) is the fifth most
common malignant disorder worldwide and causes nearly one million
deaths a year (1). Regardless of
the etiological agent, malignant transformation of HCC is known to
occur, in a context of inflammation and oxidative DNA damage
(2). Inflammation and oxidative
stress caused by hepatitis virus (HBV or HCV) infection play
important roles in liver parenchyma fibrogenesis and
carcinogenesis. It has been found that acute intracellular
oxidative stress is usually elevated in chronic HCV patients who
are at high risk of HCC (2), while
transgenic mice expressing HCV core protein show an increased
production of reactive oxygen species (ROS) (3,4). A
correlation between oxidative stress and the transformation of HCC
has also been suggested (5–7). All
these studies lead to the hypothesis that an agent with anti-viral,
anti-inflammatory and anti-oxidation attributes could contribute to
prevention against HCC.
Curcuma aromatica, with multiple ingredients,
has been identified as having anti-inflammatory, anti-oxidative
stress and anticancer properties (8,9).
Regarding the use of medicinal phytochemicals, the principal
biological active components of Curcuma aromatica are
consistent with two fractions: crystallization and volatile oil.
The crystallization fraction is normally obtained by alcohol
extraction, which mainly contains these related curcuminoids:
curcumin, bisdesmethoxycurcumin and desmethoxycurcumin. The
volatile fraction, extracted by steam distillation, contains a
spectrum of related sesquiterpenoids including turmerone,
germacrone and β-elemene (10).
At present, numerousin vitro studies have
shown that curcumin exhibits a broad range of biological
activities, including anti-carcinogenic effects (11); however, poor absorption and rapid
elimination from the body raise questions about whether these
biological activities actually occur in vivo (12). The other phytochemicals, especially
the sesquiterpenoids in curcuma oil, in fact, have been
demonstrated to show anticancer effect in some studies (13,14).
Recently, turmerone, a major sesquiterpenoid in curcuma oil, has
demonstrated both anti-inflammation and anticancer effects.
Turmerone can not only suppress cyclooxygenase (COX-2) and nitric
oxide synthase (iNOS), but also induce apoptosis in various cancer
cell lines (15,16). In addition, turmerone showed
immunomodulatory activities which could contribute to the
anticancer effect (17). We have
found that turmerone is rich in curcuma oil; however, studies are
largely missing regarding the hepatopreventive and anti-HCC effects
these substances. In this study, we investigated the
hepatopreventive effect and anticancer effect of curcuma oil.
Materials and methods
Animals, cell line and materials
A University of Louisville Institutional Animal Care
and Use Committee protocol #09101 was used for this study. Male
25–30-g C57 mice (Jackson Labs, Bar Harbor, ME, USA) were used in
the Concanavalin A (Con A) liver injury model to evaluate
properties of curcuma oil for anti-inflammation and anti-oxidative
stress similar to our prior studies (18,19).
Male 20–25-g, 8-week-old C57L/J mice (Jackson Labs) were used in
the study of orthotopic inoculated hepatoma model to evaluate the
possible anticancer effect of curcuma oil in vivo. Mouse
hepatocellular carcinoma cell line Hepa1-6 was purchased from ATCC
(Manassas, VA, USA). Curcuma essential oil (curcuma oil) was
obtained from New Directions Aromatics Inc. (San Ramon, CA, USA).
The ApopTag® In Situ Apoptosis Detection kit was
purchased from Intergen Co. (Purchase, NY, USA). Dako
EnVision™+System kit was purchased from Dako Corp. (Carpinteria,
CA, USA). OXItek TBARS assay kit was purchased from ZeptoMetrix
Corp. (Buffalo, NY, USA). SOD assay kit-WST was purchased from
Dojindo Molecular Technologies, Inc., (Gaithersburg, MD, USA). ALT
(GPT) reagent kit was purchased from Thermo Electron Corp.
(Waltham, MA, USA).
The ingredient analysis of curcuma oil by
GCxGC/TOF-MS
The molecular composition of curcuma oil was
analyzed on a two-dimensional gas chromatography time-of-flight
mass spectrometer (GCxGC/TOF-MS). Stock solutions
were then transferred to GC vials waiting for analysis. The
methoxymation and derivatization were prepared immediately before
the GCxGC/TOF-MS analysis.
The Pegasus 4D GCxGC/TOF-MS instrument (LECO Corp.,
St. Joseph, MI, USA) was equipped with an Agilent 6890 gas
chromatograph featuring a two-stage cryogenic modulator and
secondary oven, 60 m × 0.25 mm i.d. × 0.25 μm film
thicknesses, DB-5ms GC capillary column
[(5%-phenyl)-dimethylpolysiloxane, Agilent Technologies J&W]
was used as the primary column for the GCxGC/TOF-MS analysis. A
second GC column of 1 m × 0.25 mm i.d. × 0.25 μm film
thickness, DB-17ms [(50%-phenyl)-methylpolysiloxane, Agilent
Technologies J&W] was placed inside the secondary GC oven after
the thermal modulator.
LECO’s ChromaTOF software package (version 4.21)
equipped with the National Institute of Standards and Technology
(NIST) MS database (NIST MS Search 2.0, NIST/EPA/NIH Mass Spectral
Library; NIST 2008) was used for instrument control, spectrum
deconvolution and metabolite identification. We used the
manufacturer’s recommended parameters for ChromaTOF to reduce the
raw instrument data into a metabolite peak list. These parameters
are: baseline offset, 1; smoothing, Auto; peak width in first
dimension, 15 sec; peak width in the second dimension, 0.1 sec;
signal-to-noise ratio (S/N), 10; match required to combine peaks,
700; R.T. shift, 0.08 sec; minimum forward similarity match before
name is assigned, 600. The peak true spectrum was also exported as
part of the information for each peak in absolute format of
intensity values.
Con A-induced hepatic injury model
Con A is a polyclonal mitogen which can induce T
lymphocyte activation and a cytokine secretion syndrome that
progressively destroys the liver parenchyma leading to
organ-specific immune liver injury. We used this Con A liver injury
model to evaluate the potential hepatoprotective effect of curcuma
oil. Male C57 mice were divided into 3 groups. Con A + curcuma oil
group was pretreated with curcuma oil at 100 mg/kg (i.p.) for 3
days followed by a treatment of Con A at 30 mg/kg (i.v., tail
vein); Con A group was pretreated with saline (same volume as
curcuma oil, i.p.) for 3 days and then treated with Con A at 30
mg/kg (i.v., tail vein); the control group was treated with saline
only. Twelve hours after the administration of Con A, the mice were
euthanized and liver tissue was harvested for the measurements of
histology, ALT, apoptosis, lipid peroxidation and MnSOD enzymatic
activity.
Serum alanine aminotransferase
activity
Alanine aminotransferase (ALT) enzymatic activity of
serum samples was determined in liver tissues of all mice using an
ALT (GPT) reagent kit according to the instructions provided.
Orthotopic mouse liver cancer model
The orthotopic liver cancer model was established to
evaluate the cancer incidence and the possible anti-HCC effect of
curcuma oil. Hepa1-6 cell line is derivative of the BW7756 mouse
hepatoma and shows reliable growth in the syngeneic host C57L/J
mouse (17). Eight-week-old C57L/J
mice were housed five per cage, given standard commercial chow
(20) and tap water and maintained
on a 12-h light/dark cycle. Hepa1-6 cells were maintained at 37°C,
95% air and 5% CO2 in Dulbecco’s modified Eagle’s medium
(DMEM), supplemented with 10% FBS. The Hepa1-6 cells were injected
into the right flank of C57L/J mice at 1×107 in 100
μl. When the tumors were reached a size of ∼1,000–2,000
mm3, the tumor cells were isolated for cell culture. The
3–4 generation of subcultured Hepa1-6 cells were used to
established orthotopic growth liver cancer in C57L/J mice. The
cells were directly inoculated into the left liver lobe of mice at
1×106 through a 27-gauge needle. To prevent tumor cell
leakage from the injection point, a gelatin sponge patch 2 mm in
diameter was attached onto the site of injection for a few minutes
after withdrawal of the needle. Twenty-five mice were divided into
three groups, control group; tumor inoculation group; and tumor
inoculation + curcuma oil group, rendomly. The mice were pretreated
with curcuma oil every 3 day for 1 week before Hepa1-6 cell
inoculation and treated curcuma oil every 3 day after Hepa1-6 cell
inoculation, at does 100 mg/kg (i.p.). The same volume saline was
administrated as controls. The curcuma dose was utilized as per our
previous manuscripts and previous bioavailability studies (18,21).
Histopathology, cancer incidence and
tumor weight
The animals were sacrificed and entire liver were
isolated and weighted. The entire liver lobes were removed to
examine for gross abnormalities cancer incidence and histological
studies in the Con A model and HCC model. Hematoxylin and eosin
(H&E) and immunohistochemical staining were performed.
Regarding the HCC model, appearance of tumor, intrahepatic
invasion, peritoneal metastasis and tumor weight were evaluated.
Tumor weight/liver weight was also calculated.
Terminal transferase-mediated dUTP nick-end-labeling
(TUNEL) assay, immunohistochemical and immunofluorescent assays,
thiobarbituric acid reactive substances (TBARS) assay and MnSOD
enzymatic activity assay were all performed as per our prior
studies.
Assays for in vitro cell viability and
apoptosis
Hepa1-6 cells were seeded in 96-well plates at a
density of 2×105 cells per well. Three days after cell
seeding, the cells reached ∼80% confluence and were followed by
serum-free medium for 12 h. The cells were then treated with
curcuma oil that was dissolved in DMSO at the concentrations of 50,
100, 200 and 500 μg for 2, 6, 12 and 24 h, respectively.
After the treatment, the cells were evaluated for growth inhibition
and apoptosis by curcuma oil.
Cell viability was evaluated by
3-(4,5-dimethyl-thiazol-2-yl)-2, 5-diphenyltetrazolium bromide
(MTT) reduction assay. The mean optical density (OD) values from
triplicate wells for each treatment were used as the index of cell
viability.
For the TUNEL assay, plastic slide covers were
precoated with a mixture of 0.01 mg/ml fibronectin, 0.03 mg/ml
bovine collagen type I and 0.01 mg/ml bovine serum albumin and then
inserted into 24-well plates. The cells were seeded on the slide
cover in 24-well plates at a density of 5×105 cells per
well and then treated with curcuma oil at the same concentrations
as the MTT reduction assay. The TUNEL-positive epithelial cells
were counted against negative cells at a magnification, ×40. An
apoptotic index was calculated.
Immunofluorescent and immunohistochemical
assays
Immunofluorescent assay was performed on the frozen
section of liver tissues. The slides were dried and blocked with
10% serum in PBS-T. After washing, CD4 was stained by incubation
with monoclonal anti-CD4 antibody labeled with fluorescein
isothiocyanate (FITC). Interferon-γ (IFN-γ) was stained by
monoclonal anti-IFN-γ antibody and rhodamine-conjugated donkey
anti-mouse IgG. A negative control was included in each run.
FITC-labeled CD4 and rhodamine-labeled IFN-γ were examined under a
fluorescence microscope at the oil objective ×100
magnification.
Western blot analysis
Western blot analysis was performed to determine the
protein expression in the liver tissues as well as the cells. In
brief, total protein of tissue or cells is isolated with a cold
lysis buffer and total protein is determined spectrophotometrically
(12).
Statistical analysis
Student’s t-tests assuming unequal variance were
performed. The results are expressed as mean values ± standard
deviation. Comparisons were made among the treated and untreated
control groups by analysis of variance. A p-value of <0.05 was
considered statistically significant.
Results
The potential active ingredients and
hepatic protection of curcuma oil
In total 909 ingredients were identified from the
curcuma oil. Of the 909 ingredients, 105 are acids and most of them
are organic acids. There are 252 kinds of alkenes, 243 alcohols,
133 ketos, 24 acetates and 9 oximes. Further analysis shows that 22
ingredients are considered as biologically active compounds. These
ingredients include furanone, adamantaneacetic acid, fluorobenzoic
acid, pyridinecarboxaldehyde, indazolinone, chlorobicyclo,
acetamide, ascaridole epoxide, benzofuran, turmerone, etc. The
biological activities of these compounds are various, for example,
synthetic furanones have been shown to inhibit quorum-sensing and
enhancing bacterial clearance in Pseudomonas aeruginosa lung
infection in mice (22). Among
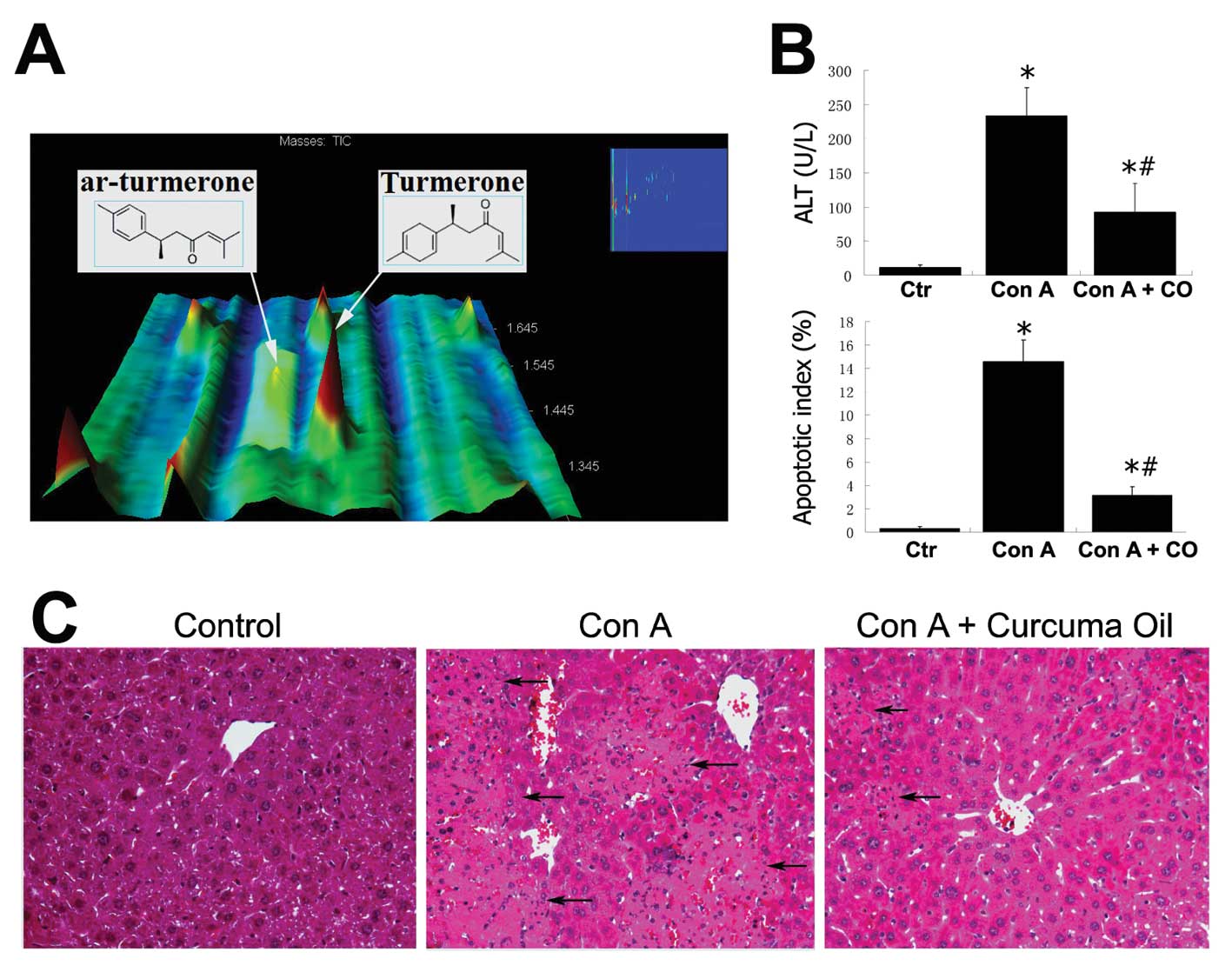
these ingredients, turmerone is rich (Fig. 1A). Turmerone and ar-turmerone elute
at different times from the two-dimensional GC because of the
difference in their chemical structures.
Con A treatment induced severe hepatocytic injury
and markedly increased serum ALT values at 233 U/l (p<0.01
compared to control) detected in Con A treated mice. However,
pretreatment with curcuma oil attenuated the hepatocytic injury by
Con A. The ALT level in the Con A + curcuma oil group was
significantly decreased to 93 U/l (p<0.01 compared to Con A
group) even though it was still elevated compared to 11 U/l in the
control group (p<0.05). The increased apoptotic hepatocytes were
also observed in the liver tissues by Con A challenge. The
apoptotic index in the Con A group significantly increased in
comparison to the controls (p<0.05). In the Con A + curcuma oil
group, however, the apoptotic index was decreased statistically
compared to that in the Con A challenged mice (p<0.05). The ALT
levels and apoptotic index are shown in Fig. 1B. Microscopic examination by
H&E stained liver sections revealed severe and extensive
necrosis, acompanied by leukocyte infiltration and massive presence
of red blood cells in the livers from Con A treated mice. This Con
A induced hepatic injury was attenuated by the curcuma oil
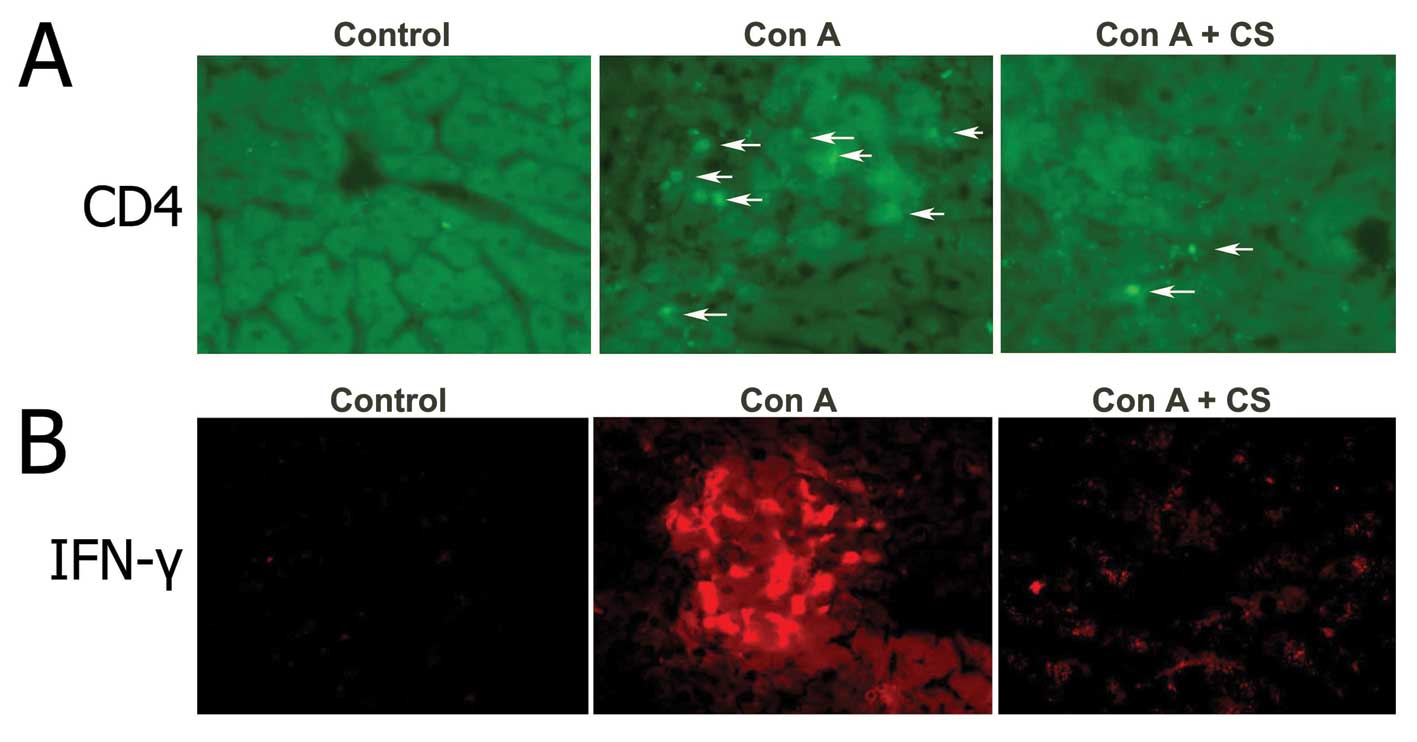
(Fig. 1C). Immunofluorescent
detection revealed high CD4 positive staining in hepatic sinusoids
and increased the early production of IFN-γ 12 h after Con A
injection. While pretreatment with curcuma oil significantly
attenuated the CD4 positive staining and production of IFN-γ in Con
A challenged livers.
Oxidative damage and hepatic protection
of curcuma oil
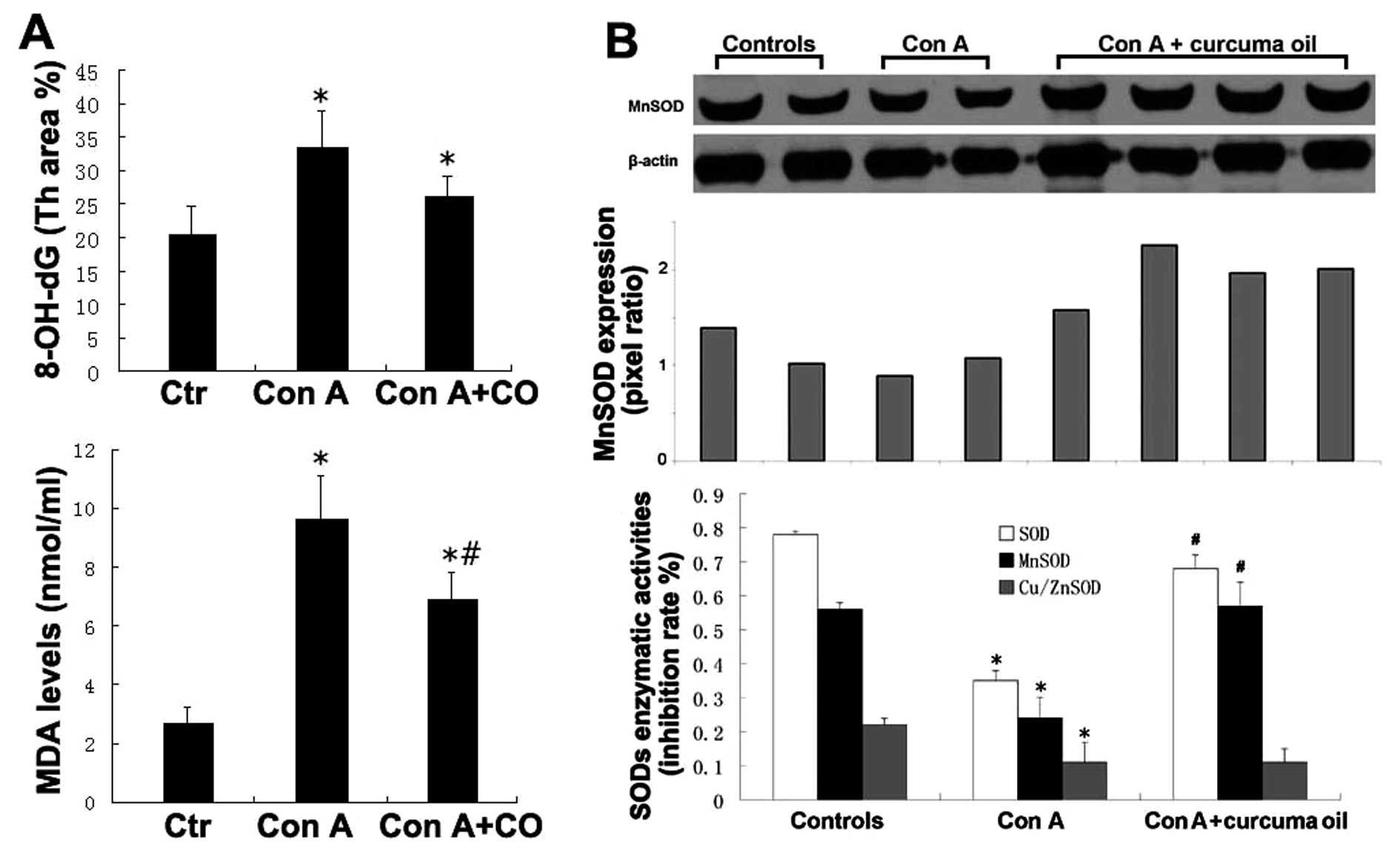
Oxidative damage is implicated in the progression of
acute inflammatory liver injury to chronic inflammatory liver
disease. In this study, we performed the measurements regarding
oxidative damage by Con A challenged liver injury. 8-OH-dG was
measured for DNA oxidative damage and TBARS was measured for lipid
peroxidation. The levels of 8-OH-dG in the hepatic tissues were
evaluated by immunohistochemical staining along with computer image
analysis. As compared with the controls, the level of 8-OH-dG was
significantly increased in the hepatic tissues by Con A challenge.
However, the level of 8-OH-dG was significantly decreased in the
hepatic tissues of mice pretreated with curcuma oil. 8-OH-dG levels
were not significantly different between the curcuma oil + Con A
group and the saline controls. The TBARS results indicated that the
level of MDA in the hepatic tissues of Con A challenged mice was
considerably increased compared to saline controls. However,
pretreatment with curcuma oil resulted in a significantly decreased
lipid peroxidation product in the hepatic tissues after Con A
challenge. The results of 8-OH-dG and TBARS are shown in Fig. 2A.
MnSOD expression and SODs enzymatic activity were
also determined in the hepatic tissues of each sample. The results
indicated that the level of MnSOD expression decreased after Con A
administration. Pretreatment with curcuma oil attenuated the
decreased level of MnSOD in the Con A affected hepatic tissue.
Regarding SOD enzymatic activities, there was a significant loss of
total SOD activity and MnSOD activity in the hepatic tissues by Con
A challenging compared to the controls. The loss of both MnSOD
activity and Cu/ZnSOD contributes to the decreased level of total
SOD. Treatment with curcuma oil can significantly increase MnSOD
activity, but not the Cu/ZnSOD level. The results of MnSOD
expression and MnSOD enzymatic activities are shown in Fig. 2B.
Curcuma oil inhibits hepatoma cell growth
in the orthotopic inoculated model
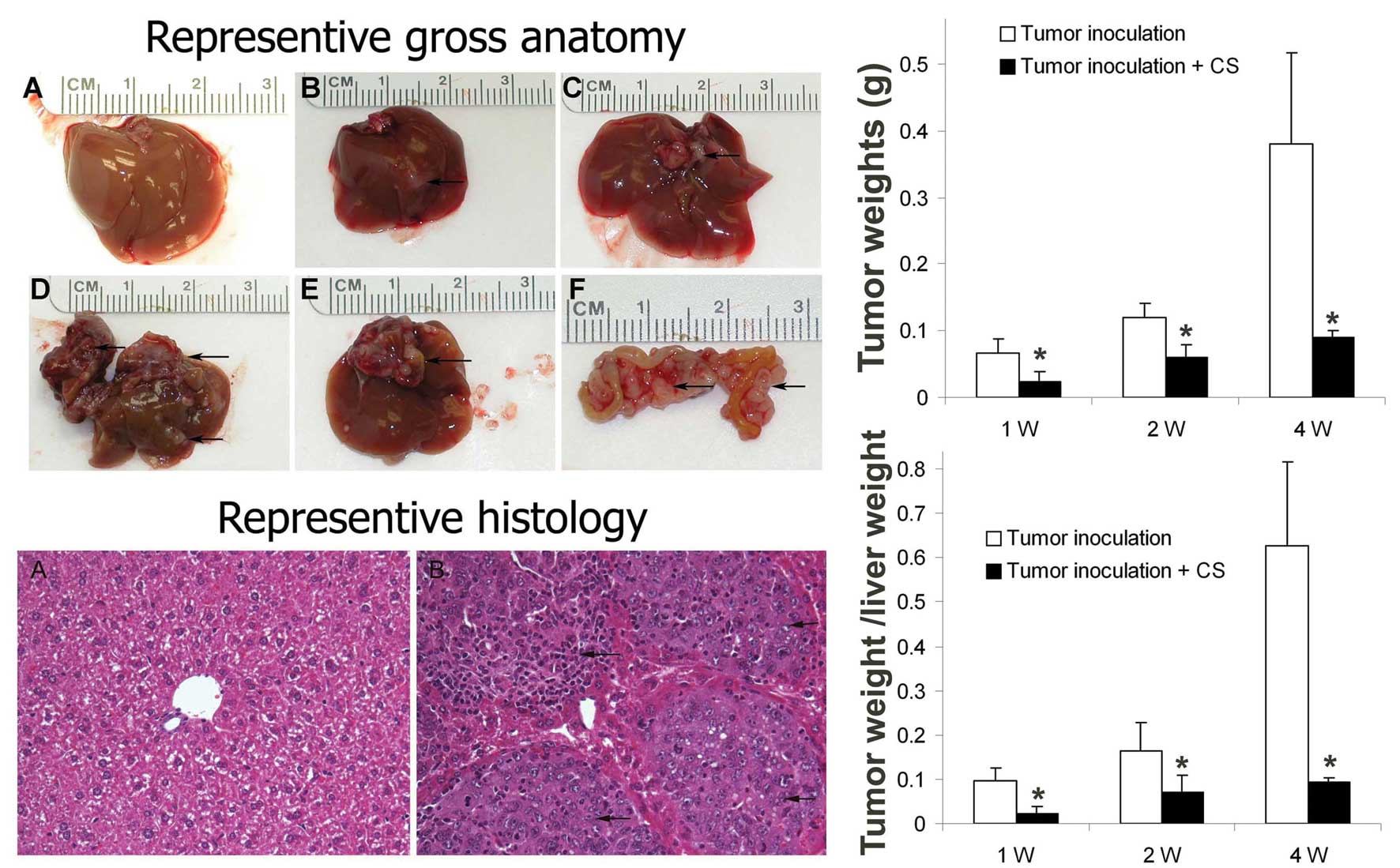
We evaluated the effect of curcuma oil on the
inoculated tumor cell growth in an orthotopic HCC mouse model.
Tumor weight, tumor intrahepatic distribution and tumor peritoneal
metastasis were evaluated. The results indicated that all animals
with a hepatoma cell inoculation were found with liver tumor,
however the tumor weight was reduced in the hepatoma cell
inoculated mice pretreated with curcuma oil. The intrahepatic
metastasis and peritoneal metastasis were also inhibited in the
curcuma oil treated group. The intrahepatic distribution and
peritoneal metastasis in the tumor cell inoculation group and in
the curcuma oil treated group at 1, 2 and 4 weeks are shown in
Table I. Representive images of
gross anatomy, histology by H&E staining, tumor weight and
tumor weight/liver weight are shown in Fig. 3.
 | Table I.Incidence of HCC in mice with Hepa1-6
cell liver orthotopic implantation. |
Table I.
Incidence of HCC in mice with Hepa1-6
cell liver orthotopic implantation.
| | 1 week | 2 weeks | 3 weeks |
|---|
|
|
|
|---|
| n | TN | HD | PM | TN | HD | PM | TN | HD | PM |
|---|
| Controls | 5 | | | | | | | 0 (5) | 0 (5) | 0 (5) |
| Tumor
inoculation | 10 | 3 (3) | 1 (3) | 0 (3) | 3 (3) | 2 (3) | 2 (3) | 4 (4) | 4 (4) | 4 (4) |
| Tumor inoculation +
CS | 10 | 3 (3) | 0 (3) | 0 (3) | 3 (3) | 1 (3) | 1 (3) | 4 (4) | 2 (4) | 2 (4) |
The effects of curcuma oil on hepatoma
cells in vitro
The cell viability results indicated that curcuma
oil can significantly inhibit the Hepl-6 cell growth. The
inhibition of Hepa1-6 cell growth by curcuma oil showed a
dose/time-effect relationship. We also investigated the effect of
curcuma oil on cell apoptosis using a TUNEL assay. The results
indicated that curcuma oil can also induce Hepal-6 cell apoptosis
in a dose/time-effect manner (Fig.
4A).
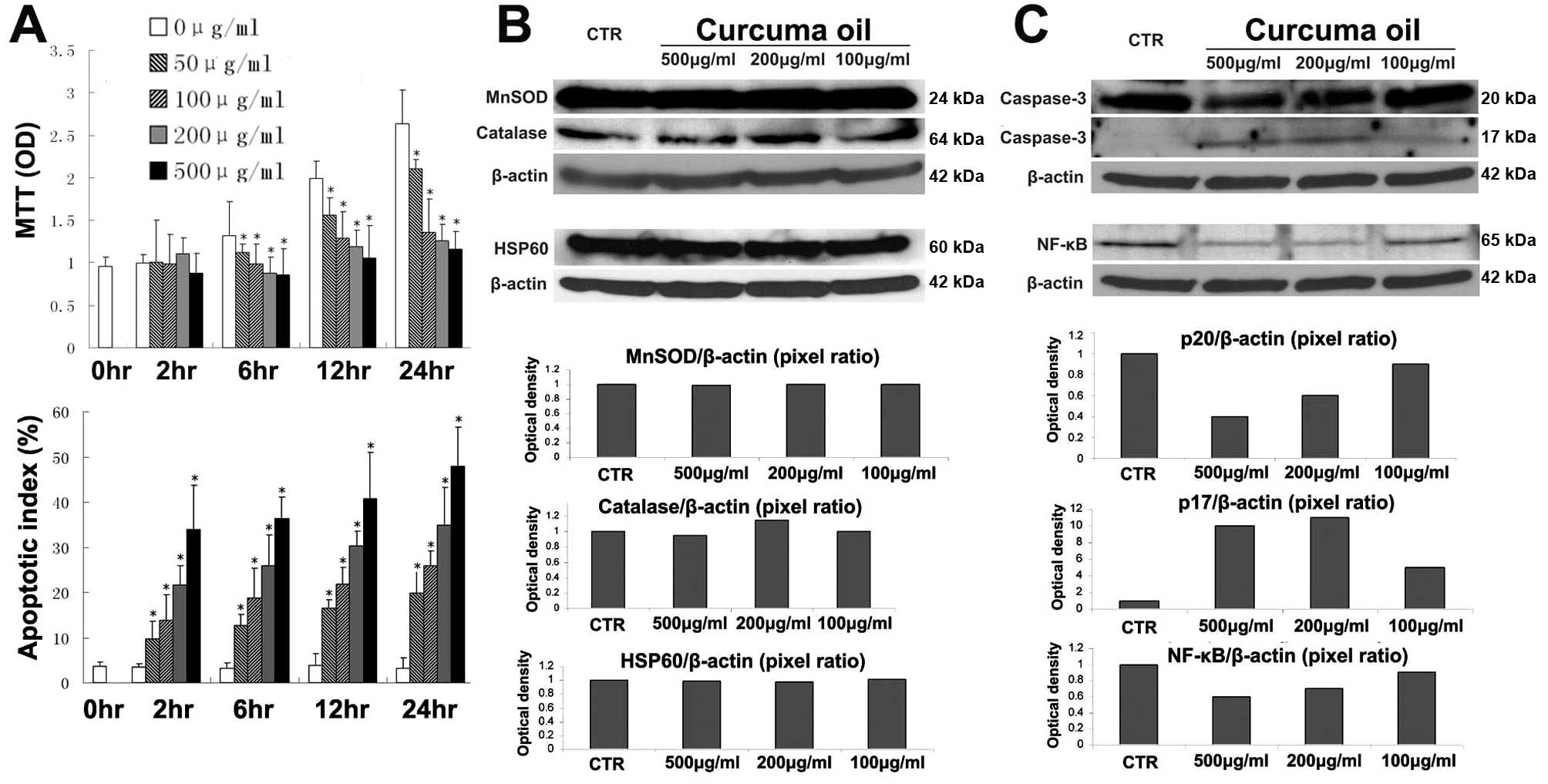 | Figure 4.The effects of curcuma oil on
hepatoma cells in vitro. (A) Curcuma oil inhibits growth and
induces apoptosis in hepatoma cells. Hepa1-6 cells were seeded in a
96-well plate at 2×105 cells/well. Curcuma
sesquiterpenoids were added at the concentrations 50, 100, 200 and
500 μg/ml for 2, 6, 12 and 24 h. MTT assay was used to
determine cell viability. Spectrophotometric OD value was used as
the cell growth index. TUNEL assay was used to determine cell
apoptosis. Results are presented as the percentage of apoptotic
cells over total cells. The values are expressed as means ± SD.
*p<0.05 vs untreated cells (0 μg/ml). (B and
C) Effect of curcuma oil on MnSOD, catalase, HSP60, caspase-3 and
NF-κB in Hepa1-6 cells. Hepa1-6 cells were seeded in
100-mm dish at 1×106 cells and reached 95% confluence.
The cells were treated with curcuma oil at the concentrations 100,
200 and 500 μg/ml for 2 h. After treatment, total protein
was extracted to perform the western blotting. The optical density
was further quantified by computer imaging software and the pixel
ratio was used as the expression levels. CTR, control
(untreated). |
We have found that curcuma oil can increase MnSOD
protein level and enzymatic activity against Con A insults. If the
MnSOD protein level and enzymatic activity were enhanced by curcuma
oil in the cancer cells, the increased oxidative defense may favor
cell growth. Therefore, we further investigated oxidative defense
related enzymes such as MnSOD and catalase and stress response
proteins such as heat shock protein 60 (HSP60). As opposed to
findings in the normal hepatocytes, curcuma oil did not alter the
MnSOD, catalase or HS60 in the Hepa1-6 cells (Fig. 4B).
We found that curcuma oil can induce apoptosis in
Hepa1-6 cells. The measurement of a critical apoptotic effector,
caspase-3, indicated that curcuma oil can significantly affect the
apoptotic effector protein level. The caspase-3 precursor, p20
peptide, is decreased while the active caspase-3 enzyme, p17
subunit is generated after curcuma oil treatment. The NF-κB protein
in response to the curcuma oil showed that NF-κB protein was
decreased after curcuma oil administration (Fig. 4C).
To investigate whether curcuma oil could affect the
Con A caused inflammatory changes, we performed measurements of
CD4+ T-cells and IFN-γ. The results indicated that
curcuma oil can decrease the CD4+ T-cell infiltration in
hepatic parenchyma and the production of IFN-γ in the Con A
challenged hepatic tissue (Fig.
5).
Discussion
Two animal models are used in this study are: i)
hepatitis by Con A and ii) liver cancer by hepatoma cell
implantation. Con A induced hepatic tissue injury through
activation of T lymphocytes and related cytokines is similar to
tissue injury in chronic hepatitis of viral origin (HVB and HVC)
and cirrhosis, which are high risk factors for HCC carcinogenesis.
The established orthotopic mouse HCC model is feasible because
Hepa1-6 cells is a mouse hepatoma cell line and it has been
demonstrated that Hepa1-6 cells can grow in C57L/J mouse with the
potential of metastasis (23). We
used these two models to study curcuma oil regarding
anti-inflammation, anti-oxidation and anticancer properties. In
addition to animal models, we also performed an in vitro
study of Hepa1-6 cells to investigate the effects of growth
inhibition and apoptosis by curcuma oil.
Curcuma oil, consisting of multiple
sesquiterpenoids, is steam-distilled from the rhizome of Curcuma
longa. Although curcumin has been found as an important
bioactive compound in Curcuma longa, poor systemic
absorption of curcumin was also reported (24,25).
Recently, curcuma sesquiterpenoids from the volatile oil fraction
have been found with similar bioactivities to curcumin. Some
sesquiterpenoids with anticancer activity have been identified such
as turmerone and β-elemene (26,27).
Unlike curcumin, sesquiterpenoids receive less attention and the
precise molecular mechanisms underlying their bioactivities are
largely unknown. We investigated the effects of curcuma oil
(multiple sesquiterpenoids) on hepatic parenchyma protection and
HCC chemoprevention.
Intravenous administration of Con A is an excellent
model because it would resemble hepatic injury, with inflammatory
cells infiltrating into the hepatic parenchyma and triggering
hepatocyte death. We used a Con A model to study the
hepatoprotective effect of curcuma oil. Our result indicated that
Con A induces severe liver injury including elevation of ALT,
apoptosis and necrosis, which is similar to other previous reports
(28,29). Pretreatment with curcuma oil can
attenuate Con A-induced ALT elevation and cell death. There are
several lines of evidence showing anti-inflammatory properties of
curcuma sesquiterpenoids. These include inhibition of nitric oxide
(NO) production in LPS-activated macrophages by sesquiterpenoids
from curcuma (30); inhibition of
chemokines, COX-2 and receptor activator of nuclear factor-κB
(NF-κB) ligand (RANKL)(31); and
inhibition of paw swelling, COX-2 activity and serum haptoglobin in
an adjuvant arthritic mouse model using curcuma extract (32). Our previous study showed that
curcuma oil can significantly inhibit esophagitis induced by
duodenogastroesophageal reflux (11). Therefore, the hepatic protection by
curcuma oil could be through anti-inflammatory properties.
The results of CD4+ T-cells and IFN-γ
(Fig. 5) indicated that curcuma
oil can decrease the CD4+ T-cell infiltration in hepatic
parenchyma and the production of IFN-γ in the Con A challenged
hepatic tissue. As well known, Con A-induced liver injury is
mediated by the activation of innate and adaptive immune cells,
including NK cells, Kupffer cells and CD4+ T-cells and
their production of inflammatory cytokines such as IFN-γ. It has
been demonstrated the IFN-γ plays a critical role in
T-cell-dependent liver injury initiated by Con A. The decreased
levels of CD4+ T cells and IFN-γ might be a potential
protective mechanism of curcuma oil against Con A-induced hepatic
injury. This hypothesis is supported by the following observations.
i) Pretreatment with monoclonal anti-mouse CD4 antibodies fully
protected mice against Con A-induced hepatic injury (33); ii) Mice with severe combined
immunodeficiency syndrome as well as athymic nude mice were
resistant against Con A (34); and
iii) IFN-γ knockout mice are protected from hepatic injury by Con A
(35). Taken collectively, the
anti-inflammatory effect of curcuma oil could be attributable, at
least partly, to inhibition of CD4+ T lymphocytes
migration and the production of IFN-γ, thereby protecting the
hepatic cells against Con A insults.
The protective effect of curcuma oil against Con A
could be also the potential activity of free radical scavenging,
which has been demonstrated in other studies (14,36).
We further explored the role of curcuma oil against oxidative
damage. The results indicated that curcuma oil can not only
decrease the levels of 8-OH-dG and lipid peroxidation, but also
increase the protein level and enzymatic activity of MnSOD in the
Con A challenged hepatic tissues. It has been demonstrated that
sesquiterpenoids in curcuma species play important role as potent
anti-oxidant (37). Interestingly,
curcumin is poorly absorbed following ingestion, however diet
supplementation with Curcuma longa or the whole extract of
Curcuma longa to animals still showed antioxidant activity
(9,38,39).
This implicated that the sesquiterpenoids existing in Curcuma
longa could possess the anti-oxidative activities and
sesquiterpenoids could be more bioavailable than curcumin. We have
demonstrated that curcuma oil can significantly decrease the levels
of both lipid peroxidation and 8-OH-dG in the esophageal epithelium
damaged by esophagoduodenal anastomosis induced reflux (11). Evidence is also provided by other
studies, which include: i) supplementation with Curcuma
longa reduces oxidative stress and lowers plasma lipid peroxide
in rabbits fed a high cholesterol diet (9); ii) a significant reversal in lipid
peroxidation, brain lipids and produced enhancement of glutathione
by Curcuma aromatica was observed in model of brain injury
with ethanol intoxicated rats (40). Therefore, the antioxidant activity
could be from the sesquiterpenoids of curcuma. The potential
antioxidant activity of curcuma oil could contribute to the
hepatoprotection against oxidative stress-mediated destruction of
hepatic parenchymal cells.
Actually, the active sesquiterpenoids in curcuma
have been studied, while a spectrum of anticancer ingredients such
as turmerone and β-elemene has enhanced our knowledge on the
biologic functions of curcuma oil against cancer (41). Curcuma oil was found to exhibit an
anti-proliferative effect in HepG2 cells by inducing apoptosis
(13). This growth inhibition of
curcuma oil is associated with cell cycle arrest, cytochrome C
translocation, caspase-3 activation, poly-ADP-ribose polymerase
(PARP) degradation and loss of mitochondrial membrane potential
(13). The inhibitory effects of
curcuma oil on hepatoma in vivo were also found and the
growth inhibition by curcuma oil on hepatoma in mice might be
associated with its depression on cellular proliferative activity
(42). Our recent study showed
that curcuma oil can prevent the carcinogenetic transformation of
esophageal adenocarcinoma (11).
The result in this study is in agreement with the above
observations. We found that curcuma oil can inhibit the tumor cell
growth both in vivo and in vitro. Interestingly, we
found that treatment with curcuma oil did not change the protein
levels of MnSOD, catalase and HSP60 in vitro, but the
apoptotic effector (caspase-3) was activated. As we know, the
caspase-3 precursor is first cleaved at Asp 175-Ser 176 to produce
the p11 subunit and the p20 peptide. Subsequently, the p20 peptide
is cleaved at Asp 28-Ser 29 to generate the mature p17 subunit. Our
result indicated a decreased p20 peptide but increased p17 subunit.
The p17 peptide is an important active caspase-3 subunit during the
execution of the apoptotic cascade. The increased p17 subunit level
of caspase-3 implied that curcuma oil contributes to cell
apoptosis. The mechanism needs to be further explored. NF-κB has
been proposed to be involved in the regulation of genes which
control apoptotic cell death. Activation of NF-κB in cancer cells
is correlated with the inhibition of apoptosis that leads to
increased expression of anti-apoptotic proteins (43). We found that curcuma oil decreased
NF-κB expression in Hepa1-6 cells. Although decreased NF-κB
expression is consistent with increased apoptosis, the NF-κB DNA
binding activity and the potential signaling of the regulation for
anti-apoptotic proteins need to be further investigated.
Two important issues of curcuma oil addressed in
this study are hepatoprotection and inhibition of HCC. However, it
should be noted that some limitations exist. Con A-induced
hepatocyte injury and liver lobe inoculation of hepatoma cells are
adequate for evaluation of the bioactivities of curcuma oil
regarding anti-inflammation, anti-oxidation and antitumor effects,
but use of these two animal models is limited. A model of
transformation from normal to hepatitis to HCC, such as
diethylnitrosamine-induced liver cancer, is needed to provide a
connection between hepatic injury and HCC carcinogenesis, thereby
to determine the chemopreventive and chemotherapeutic effects of
curcuma sesquiterpenoids. A study of combination of
diethylnitrosamine and partial hepatectomy-induced liver cancer is
underway to provide further insights into the effects of curcuma
oil on HCC transformation. In this study, we only focus on the
effects of hepatoprotection and HCC inhibition by curcuma oil in
vivo; the mechanisms for hepatoprotection and HCC inhibition,
especially the pharmaceutical targets of curcuma oil, need to be
further investigated both in vivo and in vitro.
In conclusion, curcuma oil shows promising
properties for hepatoprotection in Con A-induced injury and for
chemotherapeutic effect against inoculated hepatoma in mice. It
retains anti-inflammation, anti-oxidation and antitumor properties
while adding potential advantages: multiple target effects and
fewer side effects. Furthermore, it may be useful for
hepatoprotection, HCC chemoprevention and for cancer patients as a
long-term maintenance drug to prevent tumor recurrence.
Acknowledgements
This study was supported partly by
Award Number R03CA137801 from the National Cancer Institute and
supported partly by The Clinical & Translational Science Pilot
Grant Program’s Basic Award and Innovative Award at University of
Louisville, 2010.
References
|
1.
|
Farazi PA and DePinho RA: Hepatocellular
carcinoma pathogenesis: from genes to environment. Nat Rev Cancer.
6:674–687. 2006. View
Article : Google Scholar : PubMed/NCBI
|
|
2.
|
Levrero M: Viral hepatitis and liver
cancer: the case of hepatitis C. Oncogene. 25:3834–3847. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
3.
|
Moriya K, Nakagawa K, Santa T, et al:
Oxidative stress in the absence of inflammation in a mouse model
for hepatitis C virus-associated hepatocarcinogenesis. Cancer Res.
61:4365–4370. 2001.PubMed/NCBI
|
|
4.
|
Moriya K, Fujie H, Shintani Y, et al: The
core protein of hepatitis C virus induces hepatocellular carcinoma
in transgenic mice. Nat Med. 4:1065–1067. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
5.
|
Koike K, Tsutsumi T, Miyoshi H, et al:
Molecular basis for the synergy between alcohol and hepatitis C
virus in hepatocarcinogenesis. J Gastroenterol Hepatol 23. (Suppl
1): S87–S91. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
6.
|
Vali L, Hahn O, Kupcsulik P, et al:
Oxidative stress with altered element content and decreased ATP
level of erythrocytes in hepatocellular carcinoma and colorectal
liver metastases. Eur J Gastroenterol Hepatol. 20:393–398. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
7.
|
Zhao J, Chen J, Lu B, et al: TIP30 induces
apoptosis under oxidative stress through stabilization of p53
messenger RNA in human hepatocellular carcinoma. Cancer Res.
68:4133–4141. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
8.
|
Jee SH, Shen SC, Tseng CR, Chiu HC and Kuo
ML: Curcumin induces a p53-dependent apoptosis in human basal cell
carcinoma cells. J Invest Dermatol. 111:656–661. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
9.
|
Quiles JL, Mesa MD, Ramirez-Tortosa CL, et
al: Curcuma longa extract supplementation reduces oxidative
stress and attenuates aortic fatty streak development in rabbits.
Arterioscler Thromb Vasc Biol. 22:1225–1231. 2002. View Article : Google Scholar
|
|
10.
|
Zhou X, Li Z, Liang G, Zhu J, Wang D and
Cai Z: Analysis of volatile components of Curcuma
sichuanensis X. X. Chen by gas chromatography-mass
spectrometry. J Pharm Biomed Anal. 43:440–444. 2007.
|
|
11.
|
Li Y, Wo JM, Liu Q, Li X and Martin RC:
Chemoprotective effects of Curcuma aromatica on esophageal
carcinogenesis. Ann Surg Oncol. 16:515–523. 2009.
|
|
12.
|
Li Y, Wo JM, Ellis S, Ray MB, Jones W and
Martin RC: Morphological transformation in esophageal submucosa by
bone marrow cells: esophageal implantation under external
esophageal perfusion. Stem Cells Dev. 15:697–705. 2006. View Article : Google Scholar
|
|
13.
|
Xiao Y, Yang FQ, Li SP, Hu G, Lee SM and
Wang YT: Essential oil of Curcuma wenyujin induces apoptosis
in human hepatoma cells. World J Gastroenterol. 14:4309–4318.
2008.
|
|
14.
|
Hastak K, Lubri N, Jakhi SD, et al: Effect
of turmeric oil and turmeric oleoresin on cytogenetic damage in
patients suffering from oral submucous fibrosis. Cancer Lett.
116:265–269. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
15.
|
Lee SK, Hong CH, Huh SK, et al:
Suppressive effect of natural sesquiterpenoids on inducible
cyclooxygenase (COX-2) and nitric oxide synthase (iNOS) activity in
mouse macrophage cells. J Environ Pathol Toxicol Oncol. 21:141–148.
2002.PubMed/NCBI
|
|
16.
|
Ji M, Choi J, Lee J and Lee Y: Induction
of apoptosis by ar-turmerone on various cell lines. Int J Mol Med.
14:253–256. 2004.PubMed/NCBI
|
|
17.
|
Yue GG, Chan BC, Hon PM, et al:
Immunostimulatory activities of polysaccharide extract isolated
fromCurcuma longa. Int J Biol Macromol. 47:342–347. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
18.
|
Bower MR, Aiyer HS, Li Y and Martin RC:
Chemoprotective effects of curcumin in esophageal epithelial cells
exposed to bile acids. World J Gastroenterol. 16:4152–4158. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
19.
|
Schiffman SC, Li Y and Martin RC: The
association of manganese superoxide dismutase expression in
Barrett’s esophageal progression with MnTBAP and curcumin oil
therapy. J Surg Res. 176:535–541. 2012.
|
|
20.
|
Aiyer HS, Li Y and Martin RC: Diet
composition affects surgery-associated weight loss in rats with a
compromised alimentary tract. J Surg Res. 168:42–48. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
21.
|
Martin RC, Aiyer HS, Malik D and Li Y:
Effect on pro-inflammatory and antioxidant genes and bioavailable
distribution of whole turmeric vs curcumin: Similar root but
different effects. Food Chem Toxicol. 50:227–231. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
22.
|
Wu H, Song Z, Hentzer M, et al: Synthetic
furanones inhibit quorum-sensing and enhance bacterial clearance in
Pseudomonas aeruginosa lung infection in mice. J Antimicrob
Chemother. 53:1054–1061. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
23.
|
Kroger A, Ortmann D, Krohne TU, et al:
Growth suppression of the hepatocellular carcinoma cell line
Hepa1-6 by an activatable interferon regulatory factor-1 in mice.
Cancer Res. 61:2609–2617. 2001.PubMed/NCBI
|
|
24.
|
Ravindranath V and Chandrasekhara N: In
vitro studies on the intestinal absorption of curcumin in rats.
Toxicology. 20:251–257. 1981. View Article : Google Scholar : PubMed/NCBI
|
|
25.
|
Fang JY, Hung CF, Chiu HC, Wang JJ and
Chan TF: Efficacy and irritancy of enhancers on the in-vitro and
in-vivo percutaneous absorption of curcumin. J Pharm Pharmacol.
55:11752003. View Article : Google Scholar
|
|
26.
|
Wu W, Deng R and Ou Y: Therapeutic
efficacy of microsphere-entrapped Curcuma aromatica oil
infused via hepatic artery against transplanted hepatoma in rats.
Zhonghua Gan Zang Bing Za Zhi. 8:24–26. 2000.In Chinese.
|
|
27.
|
Deng SG, Wu ZF, Li WY, et al: Safety of
Curcuma aromatica oil gelatin microspheres administered via
hepatic artery. World J Gastroenterol. 10:2637–2642. 2004.
|
|
28.
|
Luo Q, Wang Y, Feng D, Xu Y and Xu L:
Vasoactive intestinal peptide attenuates concanavalin A-mediated
liver injury. Eur J Pharmacol. 607:226–233. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
29.
|
Jiang W, Sun R, Zhou R, Wei H and Tian Z:
TLR-9 activation aggravates concanavalin A-induced hepatitis via
promoting accumulation and activation of liver CD4+NKT
cells. J Immunol. 182:3768–3774. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
30.
|
Jang MK, Lee HJ, Kim JS and Ryu JH: A
curcuminoid and two sesquiterpenoids from Curcuma zedoaria
as inhibitors of nitric oxide synthesis in activated macrophages.
Arch Pharm Res. 27:1220–1225. 2004.PubMed/NCBI
|
|
31.
|
Funk JL, Frye JB, Oyarzo JN, et al:
Efficacy and mechanism of action of turmeric supplements in the
treatment of experimental arthritis. Arthritis Rheum. 54:3452–3464.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
32.
|
Tohda C, Nakayama N, Hatanaka F and
Komatsu K: Comparison of anti-inflammatory activities of six
Curcuma rhizomes: a possible curcuminoid-independent pathway
mediated by Curcuma phaeocaulis extract. Evid Based
Complement Alternat Med. 3:255–260. 2006.PubMed/NCBI
|
|
33.
|
Tiegs G, Hentschel J and Wendel A: A T
cell-dependent experimental liver injury in mice inducible by
concanavalin A. J Clin Invest. 90:196–203. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
34.
|
Tiegs G: Cellular and cytokine-mediated
mechanisms of inflammation and its modulation in immune-mediated
liver injury. Z Gastroenterol. 45:63–70. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
35.
|
Hong F, Jaruga B, Kim WH, et al: Opposing
roles of STAT1 and STAT3 in T cell-mediated hepatitis: regulation
by SOCS. J Clin Invest. 110:1503–1513. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
36.
|
Rathore P, Dohare P, Varma S, et al:
Curcuma oil: reduces early accumulation of oxidative product and is
anti-apoptogenic in transient focal ischemia in rat brain.
Neurochem Res. 33:1672–1682. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
37.
|
Ramirez-Tortosa MC, Mesa MD, Aguilera MC,
et al: Oral administration of a turmeric extract inhibits LDL
oxidation and has hypocholesterolemic effects in rabbits with
experimental atherosclerosis. Atherosclerosis. 147:371–378. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
38.
|
Asai A, Nakagawa K and Miyazawa T:
Antioxidative effects of turmeric, rosemary and capsicum extracts
on membrane phospholipid peroxidation and liver lipid metabolism in
mice. Biosci Biotechnol Biochem. 63:2118–2122. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
39.
|
Miquel J, Bernd A, Sempere JM, Diaz-Alperi
J and Ramirez A: The curcuma antioxidants: pharmacological effects
and prospects for future clinical use. A review. Arch Gerontol
Geriatr. 34:37–46. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
40.
|
Rajakrishnan V, Viswanathan P,
Rajasekharan KN and Menon VP: Neuroprotective role of curcumin from
Curcuma longa on ethanol-induced brain damage. Phytother
Res. 13:571–574. 1999. View Article : Google Scholar
|
|
41.
|
Cao J, Qi M, Fang L, Zhou S, Fu R and
Zhang P: Solid-phase microextraction-gas chromatographic-mass
spectrometric analysis of volatile compounds from Curcuma
wenyujin Y.H. Chen et C. Ling. J Pharm Biomed Anal. 40:552–558.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
42.
|
Wu WY, Xu Q, Shi LC and Zhang WB:
Inhibitory effects of Curcuma aromatica oil on proliferation
of hepatoma in mice. World J Gastroenterol. 6:216–219. 2000.
|
|
43.
|
Auyeung KK, Law PC and Ko JK: Astragalus
saponins induce apoptosis via an ERK-independent NF-kappaB
signaling pathway in the human hepatocellular HepG2 cell line. Int
J Mol Med. 23:189–196. 2009.PubMed/NCBI
|