Introduction
Peptide vaccine treatment has attracted attention in
recent years as a new therapy option for chemotherapy resistant,
advanced, unresectable cancer (1).
We conducted a phase I clinical trial for gastric cancer treatment
with a peptide vaccine using an URLC10 origin
HLA-A*2402-restrictive epitope peptide and a new blood
vessel antigen epitope peptide of VEGFR1. From comprehensive
genomic studies, these epitopes were determined to be tumor
antigens.
In recent years, combined therapy using together an
anti-neoplastic drug with a molecular-targeted drug has led to an
improved prognosis for chemotherapy-resistant, advanced,
unresectable gastric cancer. The results of the Trastuzumab for
Gastric Cancer (ToGA) Study showed that the prognosis of
HER2-positive gastric cancer was improved by chemotherapy with
trastuzumab, which is a human epidermal growth factor receptor
(HER) 2 antibody to chemotherapy of cytotoxic agent, in combination
with fluorouracil (5-FU) and CDDP (2).
However, the efficacy of existing medical treatments
for advanced and recurrent gastric cancer is limited, and new more
effective treatments are needed. Cancer vaccine development has
advanced by the identification of various cancer-related antigens.
The vaccine Sipuleucel-T (Provenge®) was approved as a
treatment for prostate cancer by the US Food and Drug
Administration (FDA) in 2010 (3).
Standard treatments for gastric cancer include surgery,
chemotherapy and radiotherapy; however, there has been interest
recently in developing a vaccine-based therapy with fewer
side-effects. Vaccine therapies are now being evaluated in clinical
trials of pancreatic, esophageal, liver cell, colorectal and bile
duct cancer (4–10).
Vaccination with peptides derived from vascular
endothelial growth factor receptor (VEFGR) 1 has been shown to
inhibit tumor growth in mice (11,12).
VEGFR1 peptides established CTL clones in vitro from human
peripheral blood mononuclear cells with HLA-A*2402.
These CTL clones were shown to have potent cytotoxicity in an HLA
class I-restricted manner not only against peptide-pulsed target
cells but also against target cells endogenously expressing VEGFR1.
These results strongly suggest that VEGFR1 is a promising target
for an anti-angiogenic cancer vaccine.
Vaccination with upregulated lung cancer (URLC10)
epitope peptide in patients with esophageal cancer was recently
demonstrated to be well tolerated (13–15).
Fujiwara et al reported that URLC10 is highly expressed in
gastric cancer tissue (16).
In the present study, we employed a combination of 2
peptides: URLC10 peptide, which is highly expressed in gastric
cancers, and VEGFR1 peptide. The safety of vaccination with
HLA-A*2402-restricted URLC10 and VEGFR1 epitope-peptides
was examined in patients with advanced gastric cancer refractory to
chemotherapy.
Materials and methods
Patient eligibility
Patients diagnosed with unresectable gastric cancer
refractory to chemotherapy were enrolled in this trial from August,
2009, to January, 2011, at Juntendo University Hospital, Tokyo,
Japan.
Patient inclusion criteria
The criteria for patient inclusion were as follows:
unresectable/recurrent gastric cancer refractory to chemotherapy or
that the treatment could not be continued because of adverse
events; Eastern Cooperative Oncology Group (ECOG) performance
status 0–2; age over 20 years but less than 85 years; presence or
absence of measurable or evaluable lesions by Response Evaluation
Criteria in Solid Tumors (RECIST) was not taken into account;
surgery performed and recovery achieved or 2 weeks or more had
passed since previous treatment; survival of 3 months or longer
expected; white blood cell count >3,000/mm3 but
<15,000/mm3, platelet count
>75,000/mm3, aspartate aminotransferase (AST) and
alanine aminotransferase (ALT) <150 IU/l, total bilirubin
<3.0 mg/dl and creatinine <2.0 mg/dl; and written informed
consent provided prior to the trial.
Patient exclusion criteria
The criteria for exclusion of patients were:
pregnancy or lactation; uncontrollable severe infectious diseases;
receiving treatment with steroid or immunotherapy at the time of
the clinical trial; presence of 2 or more uncontrollable
malignancies; presence of severe trauma; insufficient recovery from
an injury; and being judged inappropriate as a participant by
doctors.
Study design and treatment schedule
This study was a phase I clinical trial in patients
with advanced gastric cancer that became chemotherapy resistant.
The HLA-A*2402 restrictive epitope peptides URLC10 and
VEGFR1 were prepared in incomplete Freund’s adjuvant (IFA) and
injected subcutaneously, each at a dose of 2 mg, in the inguinal
region or an axillary region of the patients on days 1, 8, 15 and
22 of the 28-day treatment cycle. Safety was evaluated weekly for
up to 2 weeks after the last dose of medication. Patients were
considered to have completed therapy if they received one or more
courses. The curative outcome was analyzed approximately 29 days
from the start of treatment. The administration of peptide
vaccination was continued as long as possible until disease
progression.
No report exists on whether there is any effect to
non-HLA-A24 group vaccine so far. Therefore, patients were enrolled
regardless of the HLA genotype. To assess the safety of the vaccine
and to compare the efficacy without any bias, we disclosed the HLA
genotypes in all cases after the completion, and the results were
compared. The data were held by an evaluation committee and both
patients and investigators were blinded as to the results until
completion of the study. Data of the study end-points were compared
between the HLA-A*2402-positive group and the
HLA-A*2402-negative group.
End-points
The primary end-point was the safety of the peptide
vaccination, which was evaluated according to the National Cancer
Institute Common Toxicity Criteria (Ver.3.0). Immunological
reaction at the injection site (RAI) was defined by erythema and/or
induration. Toxicity was evaluated 2 weeks from the last
administration. The secondary end-points were immunological
responses and clinical outcomes in patients who received at least
one course of vaccination. Clinical outcomes included assessment by
CT scanning in accordance with RECIST criteria, time to progression
(TTP), and overall survival (OS). Tumor reduction was evaluated at
the end of 1 course of therapy according to RECIST criteria. CT
scanning was performed after the first and second cycles, and after
every cycle thereafter. TTP was determined as the time from the
date of the initial vaccination until the documentation of clear
disease progression. OS was calculated from the date of the initial
vaccination to the date of death from any cause.
Definition of dose-limiting toxicity
Dose-limiting toxicity (DLT) was defined as
hematological toxicity of grade 4 or non-hematological toxicity of
grade 3 or greater (excluding nausea and vomiting) when it could
not be ruled out that peptide vaccination was the cause. DLT
evaluation period was until 2 weeks after the last
administration.
Peptides
VEGFR1-peptide was manufactured in accordance with
Good Manufacturing Practice by the American Peptide Company Inc.
(Sunnyvale, CA, USA). URLC10-derived from LY6K-177 (RYCNLEGPPI)
that bound to HLA-A molecule was synthesized using
metallopanstimulin (MPS).
Statistical analysis
TTP and OS curves were estimated using Kaplan-Meier
methodology.
Results
Patient characteristics
A total of 14 patients was enrolled in this study.
We analyzed adverse events as primary end-point in all 14 cases.
Two cases were excluded from secondary end-point because they were
not able to complete one course of vaccination. Among them, one
case found it too difficult to come to the hospital due to
exacerbation of cancer pain and another case with PS 2 from the
beginning, was admitted to a hospital near his home. These cases
were not related to the vaccination, or due to progression of
primary disease. Number of administrations of vaccine during this
study was twice the minimum number of times, 44 times the maximum
number of times, 11 times the average number of times. The
characteristic of the 14 patients (10 males, 4 females: average age
60.3 years) are shown in Table I.
ECOG PS was 0 in 6 patients, 1 in 6 patients, and 2 in 2 patients.
All 14 patients had previously received chemotherapy, with 7
patients also undergoing surgery. A total of 11 patients had liver
metastasis, 7 had peritoneal dissemination, 7 had lymph node
metastasis, 2 had esophagus invasion, and 1 had lung metastasis.
Eight of 12 patients who were able to complete the course belonged
to the HLA-A*2402-positive group.
 | Table I.Patient characteristics. |
Table I.
Patient characteristics.
| Patient no. | Gender | Age | PS | Primary tumor
site | HLA | Prior therapy
|
|---|
| Surgery |
Chemotherapy/Radiation (RT) | Metastatic sites |
|---|
| 1 | M | 43 | 1 | C | A2402 | − | S-1/CDDP, CPT-11,
PTX, RT | Lung, lymph node |
| 2 | F | 58 | 0 | M | A2402 | − | S-1/CDDP,
CPT-11/CDDP, CPT-11, PTX, DOC | Liver |
| 3 | M | 69 | 1 | M | A2402 | − | S-1/CDDP, PTX,
CPT-11 | Liver, lymph
node |
| 4 | M | 57 | 1 | M | A2402 | + | S-1/CDDP, PTX | Liver,
peritoneum |
| 5 | M | 70 | 0 | A | A2402 | + | S-1/CDDP, PTX,
CPT-11, 5-FU/MTX | Liver, lymph node,
peritoneum |
| 6 | M | 59 | 0 | M | A2402 | + | S-1/CDDP, CPT-11,
PTX | Liver, lymph
node |
| 7 | F | 45 | 0 | C | Non-A2402 | − | S-1/CDDP, CPT-11,
PTX | Liver, lymph node,
peritoneum |
| 8 | M | 53 | 2 | C | A2402 | − | S-1/CDDP, CPT-11,
PTX, RT(bone) | Liver, esophagus
invasion |
| 9 | M | 69 | 0 | C | A2402 | + | S-1, PTX,
CPT-11/CDDP, 5-FU/MTX | Liver,
peritoneum |
| 10 | M | 70 | 1 | A | Non-A2402 | − | By pass, S-1/CDDP,
CPT-11, PTX | Peritoneum |
| 11 | F | 50 | 1 | C | Non-A2402 | − | S-1/CDDP,
CPT-11/PTX | Liver, lymph node,
did not complete the course |
| 12 | M | 78 | 2 | M | A2402 | + | S-1, CPT-11/CDDP,
PTX, 5-FU/MTX, S-1/MMC | Liver, lymph node did
not complete the course |
| 13 | M | 56 | 0 | | Non-A2402 | + | S-1, CPT-11/CDDP,
PXT | Peritoneum |
| 14 | F | 68 | 1 | UM | Non-A2402 | + | S-1, S-1/PTX, PTX,
CPT/CDDP | Liver,
peritoneum |
Toxicity
All 14 patients were evaluated for adverse events
(Table II). No patients had a
severe adverse event in relation to the vaccine treatment. Five of
14 patients had RAI; i.e., erythema, induration or pruritus
(Table III).
 | Table II.Summary of toxicity and
dermatology. |
Table II.
Summary of toxicity and
dermatology.
| Grade 1 | Grade 2 | Grade 3 | Grade 4 | Total patients n=14
(%) |
|---|
| Blood/bone
marrow | | | | | |
| Anemia | 0 | 2 | 3 | 2 | 7 (50.0) |
| Elevated ALP | 2 | 0 | 2 | 1 | 5 (35.7) |
| Elevated ALT | 1 | 0 | 0 | 0 | 1 (8) |
|
Hypoalbuminemia | 0 | 1 | 2 | 0 | 3 (21.4) |
| Creatinine | 2 | 1 | 1 | 0 | 4 (33.3) |
|
Hyperuricemia | 0 | 0 | 0 | 2 | 2 (17) |
| Hyponatremia | 0 | 1 | 0 | 0 | 1 (8) |
| Constitutional
symptoms | | | | | |
| Fatigue | 3 | 1 | 0 | 0 | 4 (33.3) |
| Anorexia | 2 | 0 | 3 | 0 | 5 (35.7) |
| Edema | 2 | 0 | 0 | 0 | 2 (17) |
|
Nausea/vomiting | 1 | 0 | 0 | 0 | 1 (8) |
| Diarrhea | 1 | 0 | 0 | 0 | 1 (8) |
| Alopecia | 1 | 0 | 0 | 0 | 1 (8) |
| Dermatology | | | | | |
| Rash | 3 | 0 | 0 | 0 | 3 (21.4) |
| Induration | 2 | 0 | 0 | 0 | 2 (17) |
| Pruritus | 2 | 0 | 0 | 0 | 2 (17) |
 | Table III.Clinical outcomes. |
Table III.
Clinical outcomes.
| Patient no. |
HLA-A*2402 | Injection times of
peptid | Course
| PFS (day) | OS (day) |
|---|
| 1 | 2 | 3 | 4 |
|---|
| 1 | A2402 | 4th | PD | | | | 24 | 29 |
| 2 | A2402 | 12th | PD | | | | 28 | 117 |
| 3 | A2402 | 16th | PD | | | | 24 | 133 |
| 4 | A2402 | 4th | SD | SD | PD | | 28 | 122 |
| 5 | A2402 | 8th | PD | | | | 24 | 86 |
| 6 | A2402 | 8th | SD | | | | 56 | 150 |
| 7 | Non-A2402 | 12th | PD | | | | 27 | 109 |
| 8 | A2402 | 19th | PD | | | | 27 | 175 |
| 9 | A2402 | 16th | SD | | | | 63 | 226 |
| 10 | Non-A2402 | 6th | PD | | | | 26 | 168 |
| 11 | Non-A2402 | 2th | | | | | NA |
| 12 | A2402 | 3th | | | | | NA |
| 13 | Non-A2402 | 44th | SD | SD | SD | PD | 126 | 384 |
| 14 | Non-A2402 | 12th | PD | | | | 28 | 242 |
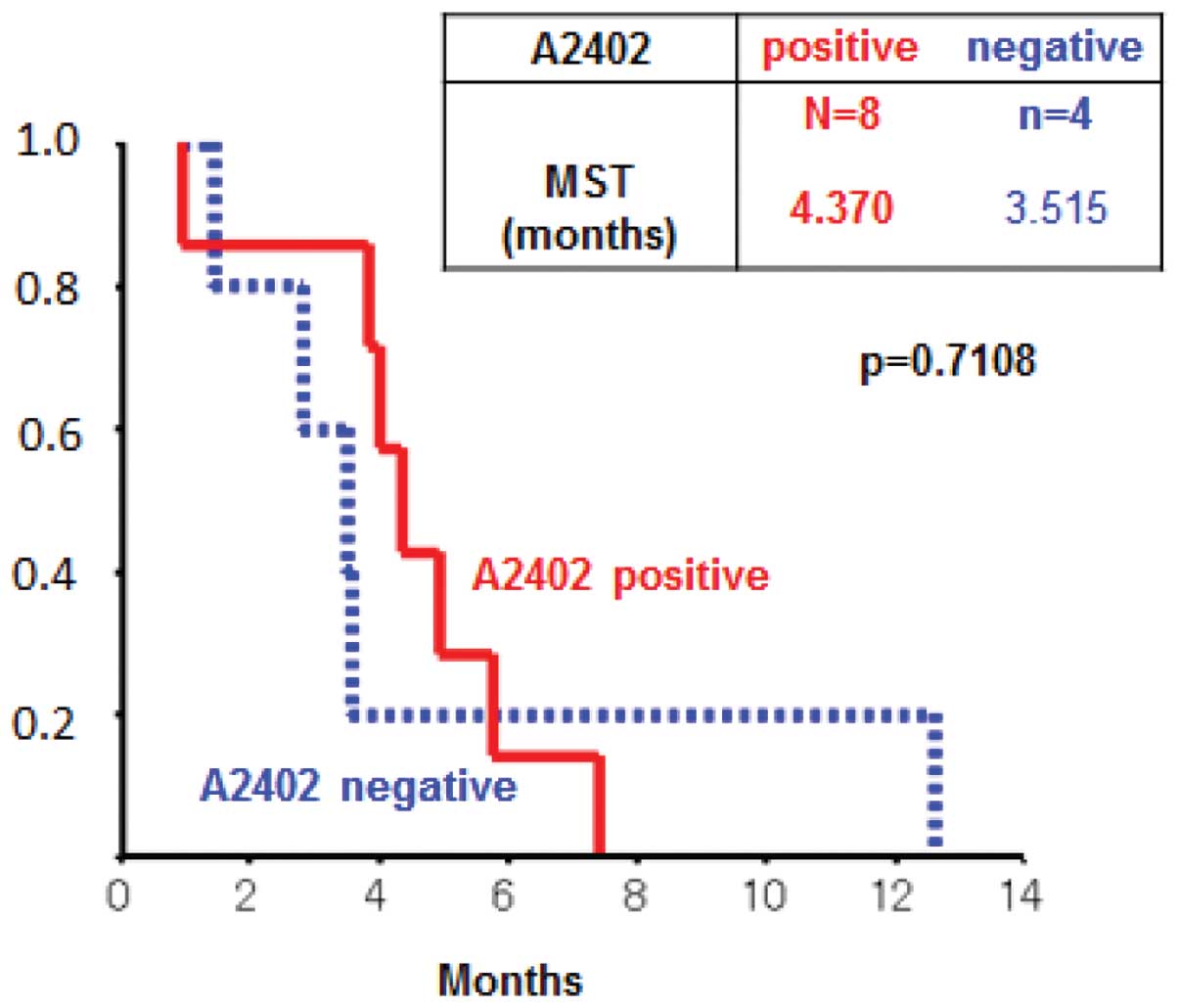
Clinical outcomes
The evaluation after 1 course showed stable disease
in 4 cases and progressive disease in 8 cases (Table III). The 3 cases with stable
disease were HLA-A*2402-positive. The MST was 3.9 months
when all 12 patients were analyzed. The MSTs in the
HLA-A*2402-positive group and the
HLA-A*2402-negative group were 4.2 and 3.6 months
(p=0.9164), respectively (Fig.
1).
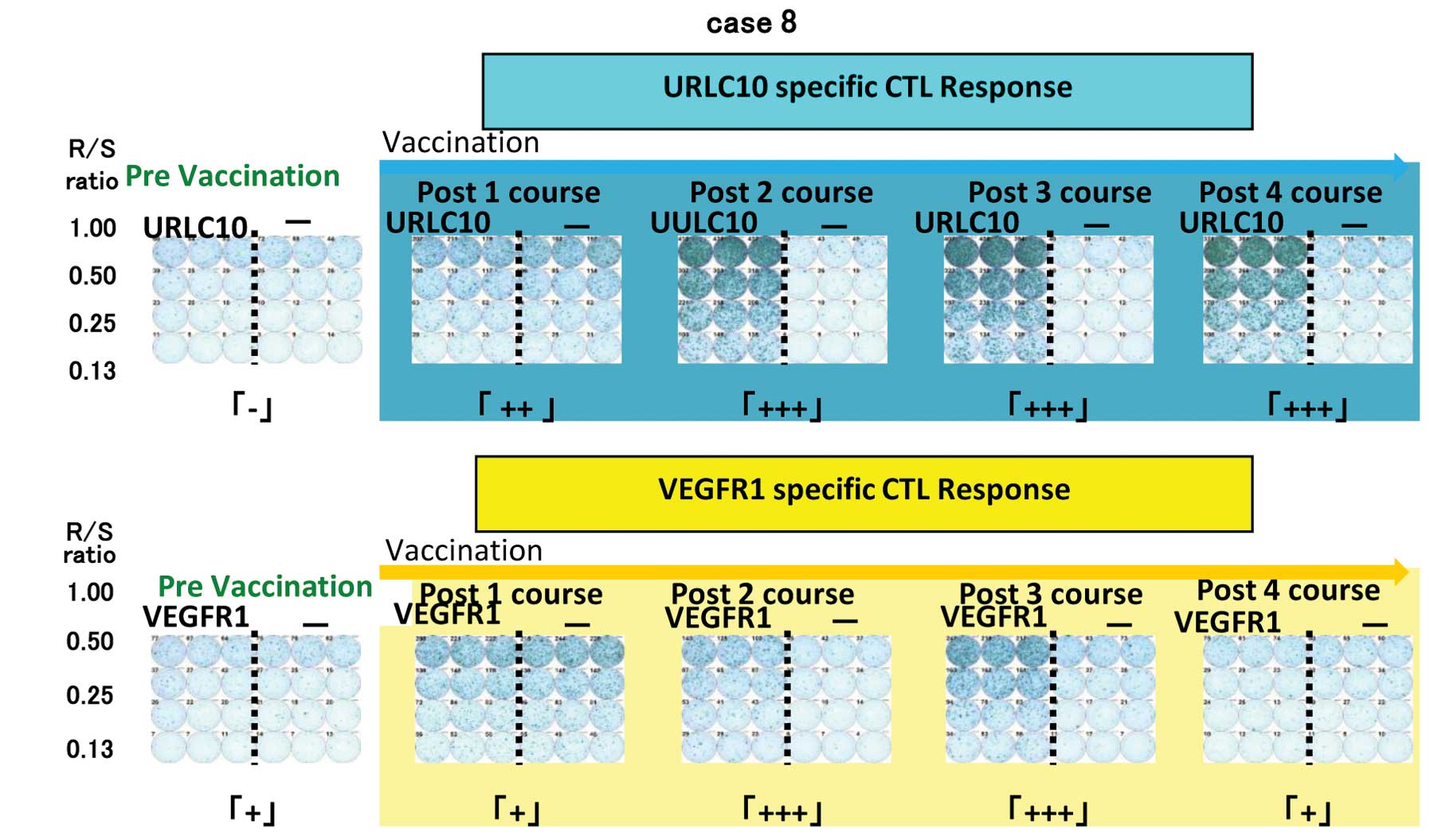
CTL response
An INF-γ ELISPOT assay was conducted using
peripheral blood monocytes periodically obtained from patients to
assess the cellular immune responses to URLC10 and VEGFR1. Positive
CTL responses specific to the vaccinated peptide were determined as
previously described. Positive CTL responses were seen in 5 of 8
patients (62.5%) for URLC10 and 4 patients (50%) for VEGFR1
(Table IV, Fig. 2).
 | Table IV.CTL response. |
Table IV.
CTL response.
| Patient no. | Course | CTL response
|
|---|
| URLC10 CTL response
co-culture | VEGFR1 CTL response
co-culture |
|---|
| 1 | Pre | + | + |
| Post1 | − | − |
| 2 | Pre | + | + |
| Post1 | − | − |
| Post2 | + | + |
| 3 | Pre | − | − |
| Post1 | − | − |
| Post2 | − | + |
| Post3 | − | − |
| Post4 | + | − |
| 4 | Pre | − | − |
| Post1 | + | + |
| 5 | Pre | + | − |
| Post1 | + | − |
| 6 | Pre | + | − |
| Post1 | − | − |
| Post2 | + | + |
| 8 | Pre | − | + |
| Post1 | ++ | + |
| Post2 | +++ | +++ |
| Post3 | +++ | +++ |
| Post4 | +++ | + |
| 9 | Pre | − | + |
| Post1 | NA | NA |
| Post2 | − | + |
| Post3 | +++ | − |
Discussion
Tumor antigens have been identified in a variety of
carcinomas, and many clinical trials have been conducted to prove
the efficacy of cancer vaccinations, since the recognition of
cancer-associated antigen by cytotoxic T cells (CTL) was first
reported by Van der Brungeen et al in 1991 (17). In 2010, the US FDA subsequently
approved an autologous cellular vaccine (sipuleucel-T;
Provenge®) for the treatment of prostate cancer
(3).
Clinical trials are now underway to evaluate the
safety and efficacy of the vaccination for the treatment of
malignant melanoma and cancers of lung, kidney, pancreas, bile
duct, colon and esophagus (5,17–20).
We previously reported that vaccination with KIF20A and VEGFR1
epitope peptides was safe and feasible for the treatment of
patients with advanced pancreatic cancer (6). In 2010, Masuzawa et al
reported the results of a phase I/II clinical trial in which the
safety of vaccination with VEGFR1 and VEGFR2 peptide combined with
S-1 and cisplatin was demonstrated in patients with advanced
gastric cancer (21). However, to
date, there have been no reports demonstrating the efficacy of the
vaccination for the treatment of gastric cancer. In the present
study, we investigated the safety of peptide vaccination with
HLA-A*2402-restricted URLA10 and VEGFR1 epitope peptides
in patients with advanced gastric cancer refractory to
chemotherapy. In many of the clinical cancer vaccine trials,
maximum tolerated dose (MTD) has not been observed. Many kinds of
peptides which were employed in previous studies had a fixed dose
(5,6,22).
Further, dose escalation was not recognized in a previous colon
cancer trial (23). Therefore, we
did not examine dose escalation and fixed dose of peptide was used
in this study. In our study, grade 3 or 4 anemia was observed in
approximately 33% of the patients, and anorexia of grade 2 or more
was reported for 25% of the patients, although these events were
associated with progression of primary disease, and a total of 4
patients developed RAI. No severe adverse effects caused by the
vaccine therapy were observed. Masuzawa et al (21) reported on adverse effects occurring
during the first 2 cycles of the combination therapy. Grade 3 or 4
neutropenia and anemia were observed in approximately 20% of the
patients, while anorexia of grade 2 or more was reported for 70% of
the patients. The results were almost the same as those of the
SPIRITS trial, a phase III trial of S-1 plus cisplatin for
first-line treatment of advanced gastric cancer (24). A total of 6 patients developed RAI
and 2 patients developed an ulcer at the injection sites. No severe
adverse effects caused by the vaccine therapy were reported. In the
study by Masuzawa et al (21), the percentage of patients with
anorexia was higher than in the current study, presumably because
in their study cytotoxic chemotherapy with cisplatin and S1 was
combined with vaccination, and the percentages of patients with
physical symptoms and blood toxicity were similar to those reported
in the SPIRITS trial, indicating that vaccination can be used
safely with chemotherapy. In the present study, the MST was 3.9
months when all 12 patients were analyzed. The MSTs in the
HLA-A*2402-positive group and
HLA-A*2402-negative group were 4.2 and 3.6 months, and
these values did not differ significantly from one another (p=
0.9164), respectively. Among the 8HLA-A2402-positive patients, 3
(37.5%) had stable disease after the end of 1 treatment course.
Masuzawa et al (21)
demonstrated that the disease control rate (i.e., the percentage of
patients with a partial response or stable disease) was 100% (54.5%
partial response and 45.5% stable disease) after 2 cycles of
combination therapy. The median time to progression was 9.6 months
and the median overall survival time was 14.2 months in patients
who showed a CTL response to VEGFR2 peptide. In our study, of the 8
patients who were HLA-A*2402-positive, 5 (62.5%) showed
a CTL-positive response to URLC10 and 4 (50%) showed a CTL-positive
response to VEGFR1. Two cases had an MST of 6.6 months, indicating
a strong CTL response. The effectiveness of treatment in patients
with strong CTL response has been suggested. Patients with advanced
cancer may not be able to secure the time needed until an antitumor
immune response can be obtained because of the short time between
the start of treatment until disease progression. It is believed
that lymphocyte immune response may be low in patients receiving
intensive chemotherapy. In our study, most patients received four
or more agents; thus, it is possible that the desired immune
response was not achieved in all cases. There was no significant
difference in MST between the HLA-A*2402-positive group
and HLA-A*2402-negative group. However, in the future,
the effectiveness of this therapy will be confirmed in additional
cases.
In conclusion, this study demonstrates that
vaccination with URLC10 and VEGFR1 epitope peptides for advanced
gastric cancer can be safely performed.
Acknowledgements
The authors would like to thank
Professor Hideki Ogawa, Chairman of the Board of Directors,
Juntendo University, Professor Eiki Kominami, President of Juntendo
University, Professor Yoshinari Takasaki, Director of Jutendo
University Hospital, Professor Hajime Arai, Dean of Juntendo
University, Professor Kazuhiro Sase of Juntendo University, and
Juntendo University Hospital for excellent lead and suggestion.
References
|
1.
|
Noguchi M, Mine T, Komatsu N, Suekane S,
Moriya F, Matsuoka K, Yutani S, et al: Assessment of immunological
biomarkers in patients with advanced cancer treated by personalized
peptide vaccination. Cancer Biol Ther. 10:1266–1279. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
2.
|
Bang YJ, Van Cutsem E, Feyereislova A,
Chung HC, Shen L, Sawaki A, Lordic F, et al: Trastuzumab in
combination with chemotherapy versus chemotherapy alone for
treatment of HER2-positive advanced gastric or gastro-esophageal
junction cancer (ToGA): a phase 3, open-label, randomized control
trial. Lancet. 376:687–697. 2010. View Article : Google Scholar
|
|
3.
|
Cheever MA and Higano CS: PROVENGE
(Sipuleusel-T) in prostate cancer: the first FDA-approved
therapeutic cancer vaccine. Clin Cancer Res. 17:3520–3526. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
4.
|
Kono K, Mizukami Y, Daigo Y, Takano A,
Masuda K, Yoshida K, Tsunoda T, et al: Vaccination with multiple
peptides derived from novel cancer-testis antigens can induce
specific T-cell esophageal cancer. Cancer Sci. 100:1502–1509. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
5.
|
Miyazawa M, Ohsawa R, Tsunoda T, Hirono S,
Kawai M, Tani M, Nakamura Y, et al: Phase I clinical trial using
peptide vaccine for human vascular endothelial growth factor
receptor 2 in combination with gemcitabine for patients with
advanced pancreatic cancer. Cancer Sci. 101:433–439. 2010.
View Article : Google Scholar
|
|
6.
|
Kato J, Nagahara A, Kotani T, Higashihara
Y, Matsumura Y, Osada T, Yoshizawa T, et al: Phase I clinical trial
of peptide vaccination with KIF20 and VEGFR1 epitope peptides in
patients with advanced pancreatic cancer. Pancreatic Dis Ther.
2:1022012. View Article : Google Scholar
|
|
7.
|
Kawada J, Wada H, Isobe M, Gnjatic S,
Nishikawa H, Jungbluth AA, Okazaki N, et al: Heteroclitic
serological response in esophageal and prostate cancer patient
after NY-ESO-1 protein vaccination. Int J Cancer. 130:584–592.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
8.
|
Watanabe T, Suda T, Tsunoda T, Uchida N,
Ura K, Kato T, Hasegawa S, et al: Identification of immunoglobulin
super-family 11 (IGSF11) as a novel target for cancer immunotherapy
of gastrointestinal and hepatocellular carcinoma. Cancer Sci.
96:498–506. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
9.
|
Komori H, Nakahara T, Senju S, Yoshitake
Y, Motomura Y, Ikuta Y, Fukuma D, et al: Identification of HLA-A2-
or HLA-A24-restricted CTL epitopes possibly useful for
glypican-3-specific immunotherapy of hepatocellular carcinoma. Clin
Cancer Res. 12:2689–2697. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
10.
|
Imai K, Hirata S, Irie A, Senju S, Ikuta
Y, Yokomine K, Harao M, et al: Identification of a novel
tumor-associated antigen, cadherin 3/P-cadherin, as a possible
target for immunotherapy of pancreatic, gastric, and colorectal
cancer. Clin Cancer Res. 14:6487–6495. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
11.
|
Nakatsura T, Komori H, Kubo T, Yoshitake
Y, Senju S, Kataguri T, Fukukawa Y, et al: Mouse homologue of a
novel human oncofetal antigen, glypican-3, evokes T-cell-mediated
tumor rejection without autoimmune reactions in mice. Clin Cancer
Res. 10:8630–8640. 2004. View Article : Google Scholar
|
|
12.
|
Ishizaki H, Tsynoda T, Wada S, Yamauchi M,
Shibuya M and Tahara H: Inhibition of tumor growth with
antiangiogenic cancer vaccine using epitope peptides derived from
human vascular endothelial growth factor receptor 1. Clin Cancer
Res. 12:5841–5849. 2006. View Article : Google Scholar
|
|
13.
|
Mizukami Y, Kono K, Daigo Y, Takano A,
Tsunoda T, Kawaguchi Y, Nakamura Y, et al: Detection of novel
cancer-testis antigen-specific T-cell responses in TIL, regional
lymph nodes, and PBL in patients with esophageal squamous cell
carcinoma. Cancer Sci. 99:1448–1454. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
14.
|
Kono K, Iinuma H, Akutsu Y, Tanaka H,
Hayashi N, Uchikado Y, Noguchi T, et al: Multicenter, phase II
clinical trial of cancer vaccination for advanced esophageal cancer
with three peptides derived from novel cancer-testis antigens. J
Transl Med. 10:1412012. View Article : Google Scholar
|
|
15.
|
Suda T, Tsunoda T, Daigo Y, Nakamura Y and
Tahara H: Identification of human leukocyte antigen-A24-restricted
epitope peptides derived from gene products upregulated in lung and
esophageal cancer as novel targets for immunotherapy. Cancer Sci.
98:1803–1808. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
16.
|
Fujiwara Y, Kishi K, Yano M, Mototsugu M,
Ishikawa O, Okada K, Masuzawa T, et al: Peptide vaccination for
gastric cancer. J Gastrointest Res. 20:120–127. 2012.
|
|
17.
|
Van der Brunggen P, Traversari C, Chomez
P, Lurquin C, De Plaen E, Van den Eynde, Kunth A, et al: A gene
encoding an antigen recognized by cytolytic T lymphocytes on a
human melanoma. Science. 254:1643–1647. 1991.
|
|
18.
|
Correale P, Cusi MG, Tsang KY, Del Vecchio
MT, Marsili S, Placa ML, Intrivici C, et al: Chemo-immunotherapy of
metastatic colorectal carcinoma with gemcitabine plus FOLFOX4
followed by subcutaneous granulocyte macrophage colony-stimulating
factor and interleukin-2 induces strong immunologic and antitumor
activity in metastatic colon cancer patients. J Clin Oncol.
23:8950–8958. 2005.
|
|
19.
|
Hattori T, Mine T, Komatsu N, Yamada A,
Itoh K, Shiozaki H and Okumo K: Immunological evaluation of
personalized peptide vaccination in combination with UFT and UZEL
for metastatic colorectal carcinoma patients. Cancer Immunol
Immunother. 58:1843–1852. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
20.
|
Shimokawa T, Matsushima S, Tsunoda T,
Tahara H, Nakamura Y and Furukawa Y: Identification of TOMMO34,
which shows elevated expression in the majority of human colon
cancer, as a novel drug target. Int J Oncol. 29:381–386.
2006.PubMed/NCBI
|
|
21.
|
Masuzawa T, Fujiwara Y, Okada K, Nakamura
A, Takiguchi S, Nakajima K, Miyata H, et al: Phase I/II study of
S-1 plus cisplatin combined with peptide vaccines for human
vascular endothelial growth factor receptor 1 and 2 in patients
with advanced gastric cancer. Int J Oncol. 41:1297–1304. 2012.
|
|
22.
|
Sato Y, Shomura H, Maeda Y, Mine T, Ueno
Y, Akasaka Y, Kondo M, et al: Immunological evaluation of peptide
vaccination for patients with gastric cancer based on pre-existing
cellular response to peptide. Cancer Sci. 94:802–808. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
23.
|
Miyagi Y, Imai N, Sasatomi T, Yamada A,
Mine T, Katagiri K, Nakagawa M, et al: Induction of cellular immune
responses to tumor cells and peptides in colorectal cancer patients
by vaccination with SART3 peptides. Clin Cancer Res. 7:3950–3962.
2001.PubMed/NCBI
|
|
24.
|
Koizumi W, Nakahara H, Hara T, Takigane A,
Akiya T, Takagi M, Miyashita K, et al: S-1 plus cisplatin versus
S-1 alone for first-line treatment of advanced gastric cancer
(SPIRITS trial): a phase III trial. Lancet Oncol. 9:215–221. 2008.
View Article : Google Scholar : PubMed/NCBI
|