Introduction
Lactic acid bacteria (LAB) have been used throughout
history to produce fermented food and milk products. In the early
1900s, biologist Eli Metchinkoff suggested that the intake of LAB
could increase the life span of humans (1). His findings have been verified by
many scientists who have found that ingesting LAB fermented
products provides many benefits for maintaining health (2,3). LAB
strains present in fermented milk are normal components of
intestinal microflora and help maintain a healthy balance of
probiotic bacteria while reducing pathogenic bacteria (4,5). In
addition to gut maintenance, LAB have more recently been tested to
treat a variety of diseases including Crohn’s disease, rheumatoid
arthritis and cancer (6).
The idea of treating cancers with LAB is not as
recent as one might think. Over a century ago, Metchnikoff
suggested that LAB had a protective effect against colorectal
cancer (7). However, it is the
recent resurgence of research concerning the microbiotic influence
on human health that has spurred researchers to confirm
Metchnikoff’s findings on the anti-cancer ability of LAB in animal
models and to expand understanding of LAB’s influence on the
cellular environment (8–12). Additional research has shown that
LAB also exerts anticancer activity against other types of cancer
such as breast and ovarian cancer (13–17).
Conventional treatments for cancer such as
chemotherapy aim to initiate apoptosis. However, these drugs can be
toxic and can decrease a cancer patient’s quality of life.
Therefore, there has been an effort to investigate alternative
treatments that have fewer side effects and improve the health of
the patient. A recent study has shown that Lactobacillus
reuteri enhanced tumor necrosis factor (TNF)-induced apoptosis
in human chronic myeloid leukemia-derived cells (18) and that bacterial soluble factors
secreted by Lactobacillus casei rhamnosus caused induction
of apoptosis in human monocytic leukemia-cell line, THP-1 (19). These data suggest that fermented
milk products and/or the fermentative bacteria themselves may have
chemoprotective effects without the toxic side effects of
conventional therapeutic drugs.
The product we use in this study is a symbiotic
microbe of lactic acid bacteria and yeasts known as Probiotics
Fermentation Technology (PFT) kefir grain product. PFT is separated
from kefir, a popular drink across Eastern and Northern Europe and
Russia. Kefir has been shown to have various health benefits, for
example, it protects the intestine against disease-causing bacteria
(20) and its kefiran component
was shown to lower high blood pressure and reduce serum cholesterol
levels in rats (21). PFT mainly
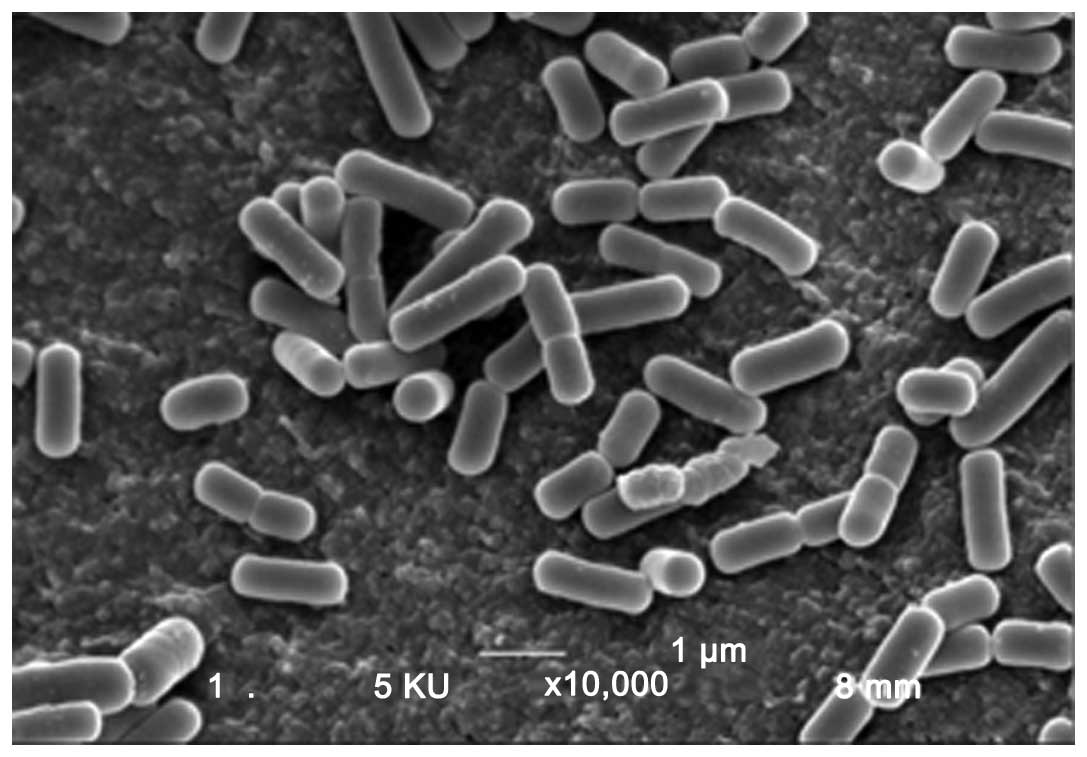
contains a unique Lactobacillus kefiri P-IF (L.
kefiri P-IF) strain that has a unique DNA sequence and shows a
99.6% homology with regular kefiries and similar 16S ribosome
sequence compared with other L. kefiri strains. Although
strain P-IF shares many common characteristics with other L.
kefiri strains, P-IF also has many distinct features that may
contribute to its effectiveness as an anticancer agent (Table I) (22). Unlike other L. kefiri
strains, P-IF utilizes galactose as a carbon source and produces
carbonic acid when its growth medium is agitated. Most L.
kefiri strains grow in a lengthwise-dimensional pattern,
however, P-IF grows three-dimensionally, which is attributed to the
unique carbohydrate chains on its surface (22).
 | Table I.L. kefiri P-IF strain
characteristics. |
Table I.
L. kefiri P-IF strain
characteristics.
| Test | P-IF
characteristic |
|---|
| Lactobacillis
kefiri | 99.6% sequence
homology |
| 16S ribosome
identification |
| Cell shape | Rod |
| Gram staining | + |
| Motility | Non-motile |
| Colony growth | 3D growtha |
| Carbon
utilization | Glucose |
| Fructose |
| Galactosea |
| L-arabinose |
| Ribose |
| Maltose |
| Lactose |
| Melibiose |
| Gluconate |
| Acid/gas
production | Carbonic acid
gasa |
| pH tolerance | >4.3 pHa |
The results of this study show that PFT possesses
the ability to pierce holes in MDR human myeloid leukemic (HL60/AR)
cells, which induces apoptosis in the cancer cells by intrinsic
(mitochondrial) pathway of apoptosis. This study suggests that PFT
may exert a therapeutic effect in treating MDR cancers.
Materials and methods
Tumor cell line and culture
conditions
Human multidrug-resistant (MDR) myeloid leukemia
(HL60/AR) cells were used in the present study. Cells were kindly
provided by Dr S. Gollapudi at the University of California,
Irvine, CA, USA. Tumor cells were maintained in our laboratory in a
complete medium (CM) that consisted of RPMI-1640, 10% fetal calf
serum (FCS), 2 mM glutamine and 100 μg/ml streptomycin and
penicillin.
Drugs and chemicals
3-[4,5-dimethylthiazol-2-yl]-2,5-diphenyltetrazolium
bromide (MTT) (Sigma-Aldrich, St. Louis, MO, USA), was
employed.
Probiotics Fermentation Technology (PFT)
kefir grain product
PFT is a mixture that mainly (∼90%) contains a
freeze-dried form of heat-killed L. kefiri P-IF; it is a
specific strain of LAB that has a unique DNA sequence and PET scans
show a 99.6% homology with regular kefiries. Characteristics of
L. kefiri P-IF are shown in Table I and Fig. 1. PFT also contains ∼2–3% each of
bacterial strain, Lactobacillus kefiri P-IF and
Lactobacillus kefiri P-B1 and three yeast strains,
Kazachstania turicensis, Kazachstania unispora and
Kluyveromyces marxianus (22). PFT was provided by Paitos Co., Ltd.
Yokohama, Kanagawa, Japan.
Detection of cancer cell viability using
propidium iodide
HL60/AR cells were cultured in the presence or
absence of PFT at different concentrations (0, 0.6, 1.25, 2.5 and 5
mg/ml) for 3 days and the percentage of dead cancer cells was
examined by the propidium iodide (PI) technique using a FACScan
flow cytometery. Briefly, PI was added to the cells
(1×106/ml) to give a final PI concentration of 50
μg/ml. The cells were stained for 30 min at room temperature
in the dark and analyzed by FACScan (Becton-Dickinson, San Jose,
CA, USA).
Expression of Bcl-2
For detection of Bcl-2, cells were first fixed and
permeabilized with ice-cold 70% methanol. Cells were then stained
with FITC-labeled anti-Bcl-2 or isotype control (Dako Corp.,
Carpinteria, CA, USA), washed and analyzed by FACScan. The
percentage of cells expressing Bcl-2 and mean fluorescent intensity
(an indicator of density of the molecules/cell) was determined.
Intracellular activity of caspase 3
The method for measuring intracellular activity of
caspase 3 is based on carboxyfluorescein labeled fluromethyl ketone
(FMK)-peptide inhibitors of caspases. These inhibitors are cell
permeable and non-toxic. Once inside the cells, these inhibitors
bind covalently to the active caspase. Caspase-positive (+) cells
are distinguished from caspase-negative (−) cells with the aid of
flow cytometry. Briefly, cells undergoing apoptosis were loaded
with fluorescein labeled FAM-DEVD-FMK for caspase 3 (Intergen Co.,
NY, USA). After 1-h incubation, the cells were washed to remove
unbound caspase and cells that contained bound inhibitor were
quantified using a FACScan flow cytometer.
Detection of mitochondrial membrane
potential (MMP)
Variations of the mitochondrial transmembrane
potential ΔΨm during apoptosis were studied using
tetramethylrhodamine ethylester (TMRE, Molecular Probes, Eugene,
OR, USA). Briefly, after treatment with PFT for 3 days, cancer
cells (5×105 cells/ml) were incubated with 50 nM TMRE
for 30 min at 37°C. The cells were washed with PBS and analyzed
with FACS Forward, the side scatters were used to gate and exclude
cellular debris using a FACScan. The cells were excited at 488 nm
and the emission was collected on the FL2 channel. Five thousand
cells were analyzed. The data were acquired and analyzed using
CellQuest software (Becton-Dickinson).
AFM imaging
HL60/AR cells (1×106 cells/ml) were
cultured with PFT (5 mg/ml) for 2 min and 24 h. Results were
compared to those of cells without treatment. Cytospin preparations
(Shandon Southern Inst., Sewickley, PA, USA) of cells were
air-dried, fixed in 100% MeOH for 5 min and prepared for AFM
studies. AFM studies were carried out to examine the morphological
changes associated with PFT treatment of HL60/AR cells such as hole
induction and membrane blebbing. Dimension 5000 AFM (Veeco) under
contact mode was used to image the HL60/AR cells with Bruker’s
Sharp Nitride Lever (SNL) silicon probes (Veeco). Topographic
height images were recorded at 512×512 pixels at a scan rate of 0.8
Hz. Image processing was performed using SPIPTM
Software. Usually an MLCT-AFM tip (with a ‘k’ value of 0.03N/m)
contributes to the broadening effect because of its specific
geometry (23).
Statistical analysis
Statistical significance for cell apoptosis in
Fig. 2 was determined by Student’s
t-test. Differences were considered significant at the p<0.05
level. Statistical analysis for flow cytometry was performed by the
Kolmogorov-Smirnov test using CellQuest Software system. A D-value
of >0.2 was considered statistically significant.
Results
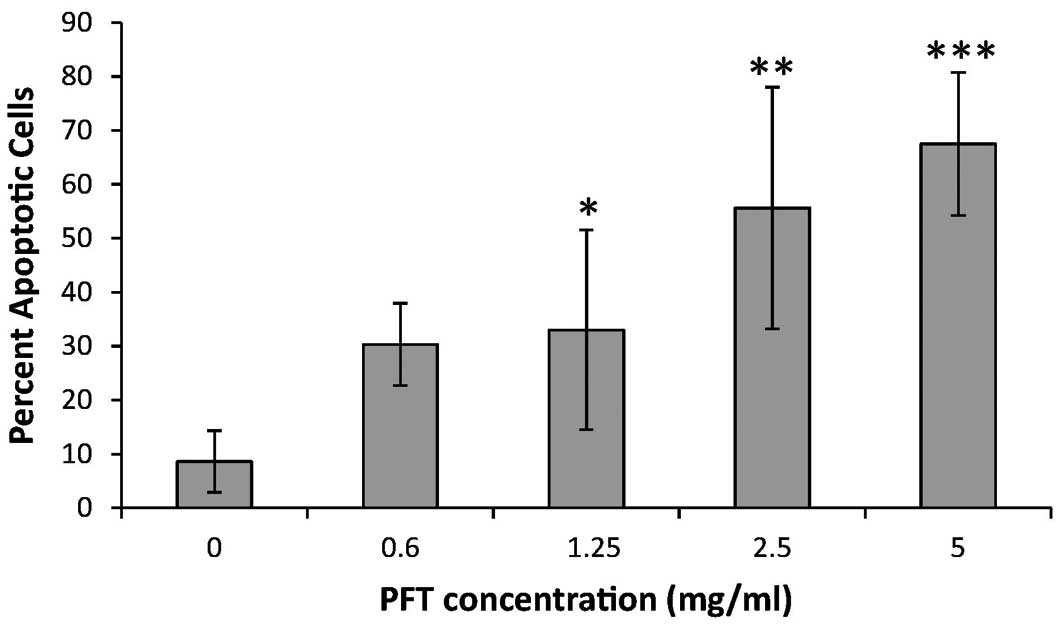
Effect of PFT on tumor cell survival
We examined the effect of PFT on tumor cell survival
using propidium iodide and FACScan flow cytometry. The data in
Fig. 2 demonstrate that treatment
with PFT increased apoptosis in the cancer cells in a
dose-dependent manner. Even at a lower concentration (0.6 mg/ml),
the PFT induced apoptosis in ∼30% of the HL60/AR cells. The
percentage of apoptosis continued to increase in conjunction with
higher concentrations of PFT reaching an average of 67.5% apoptosis
at 5 mg/ml.
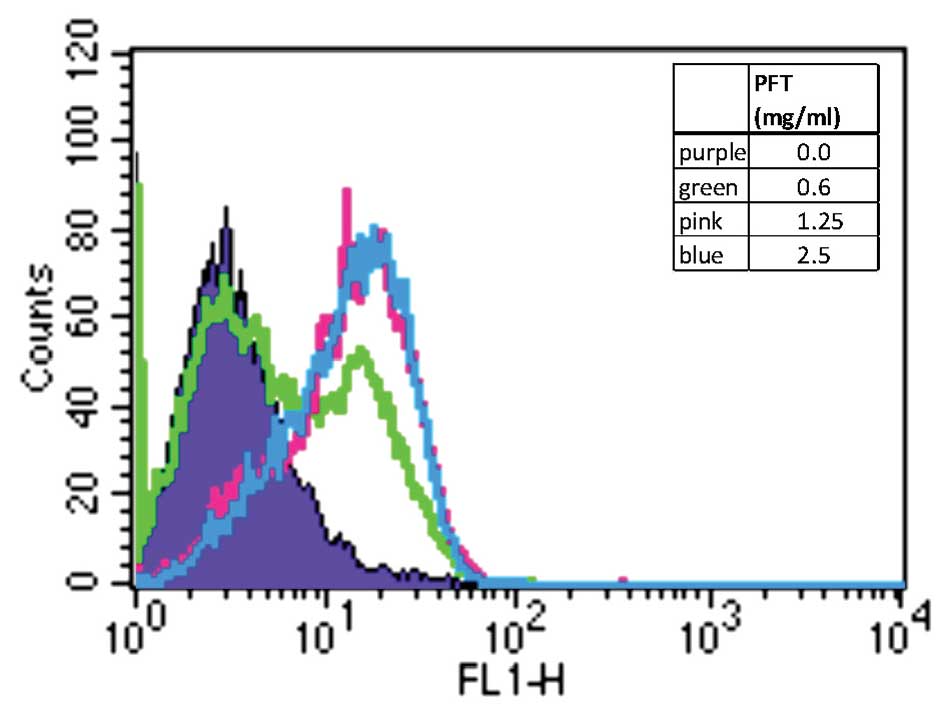
PFT induces activation of caspase 3
To verify activation of the apoptotic pathway, the
level of caspase 3 activation was investigated by treating HL60/AR
cells with PFT at varying concentrations for 24 h and analyzed
using flow cytometry. Data depicted in Fig. 3 show that the proportion of cancer
cells with increased active caspase 3 was higher in PFT-treated
cells than in control untreated cells. This would suggest that
pre-exposure of HL60/AR cells to PFT led to increased activation of
the executioner caspase 3.
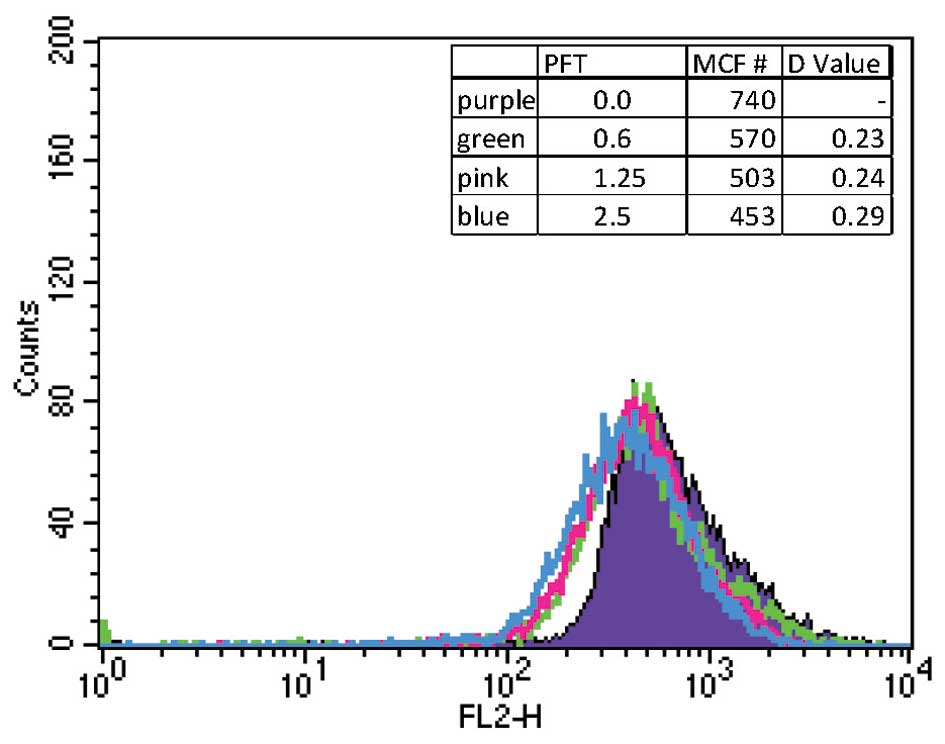
PFT depolarizes mitochondrial membrane
potential (MMP)
Experiments were carried out to examine the ability
of PFT to disrupt MMP. HL60/AR cells were treated in the presence
or absence of PFT at varying concentrations and MMP was determined
by flow cytometry using membrane potential sensitive TMRE dye. The
data in Fig. 4 show that treatment
of cells with PFT resulted in a significant decrease in the
mitochondrial polarization of cancer cells as compared to untreated
cells.
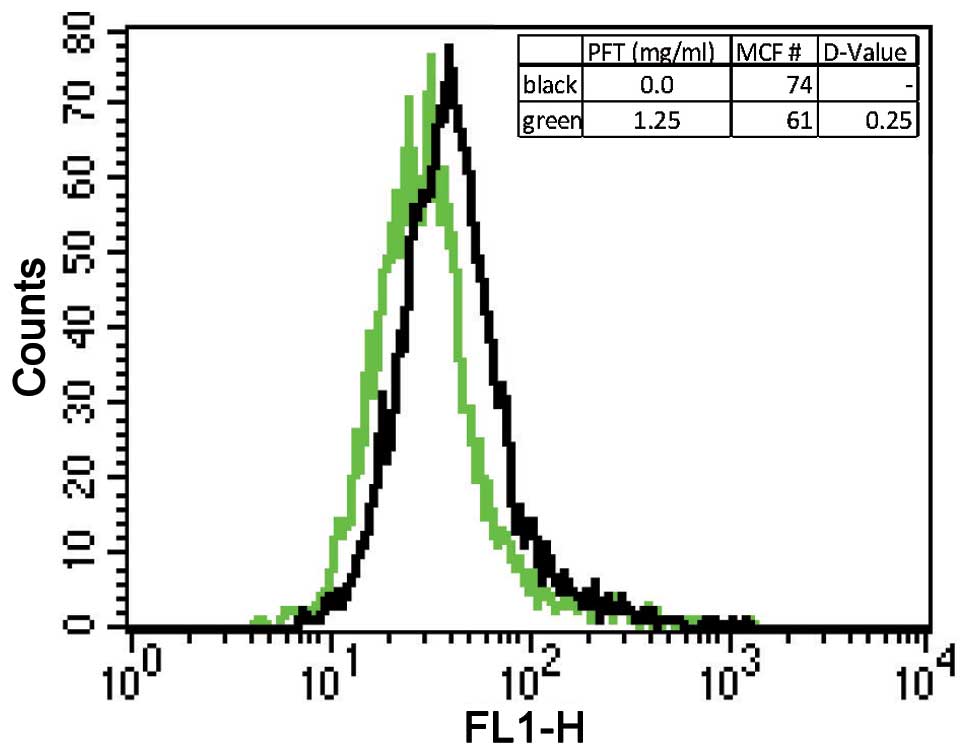
Expression of Bcl-2
The expression of Bcl-2 was examined to determine
the anti-apoptotic activity post-treatment of HL60/AR cells by PFT.
Results depicted in Fig. 5 show
that treatment with PFT caused a statistically significant decrease
in expression of Bcl-2 compared to control cancer cells without
treatment.
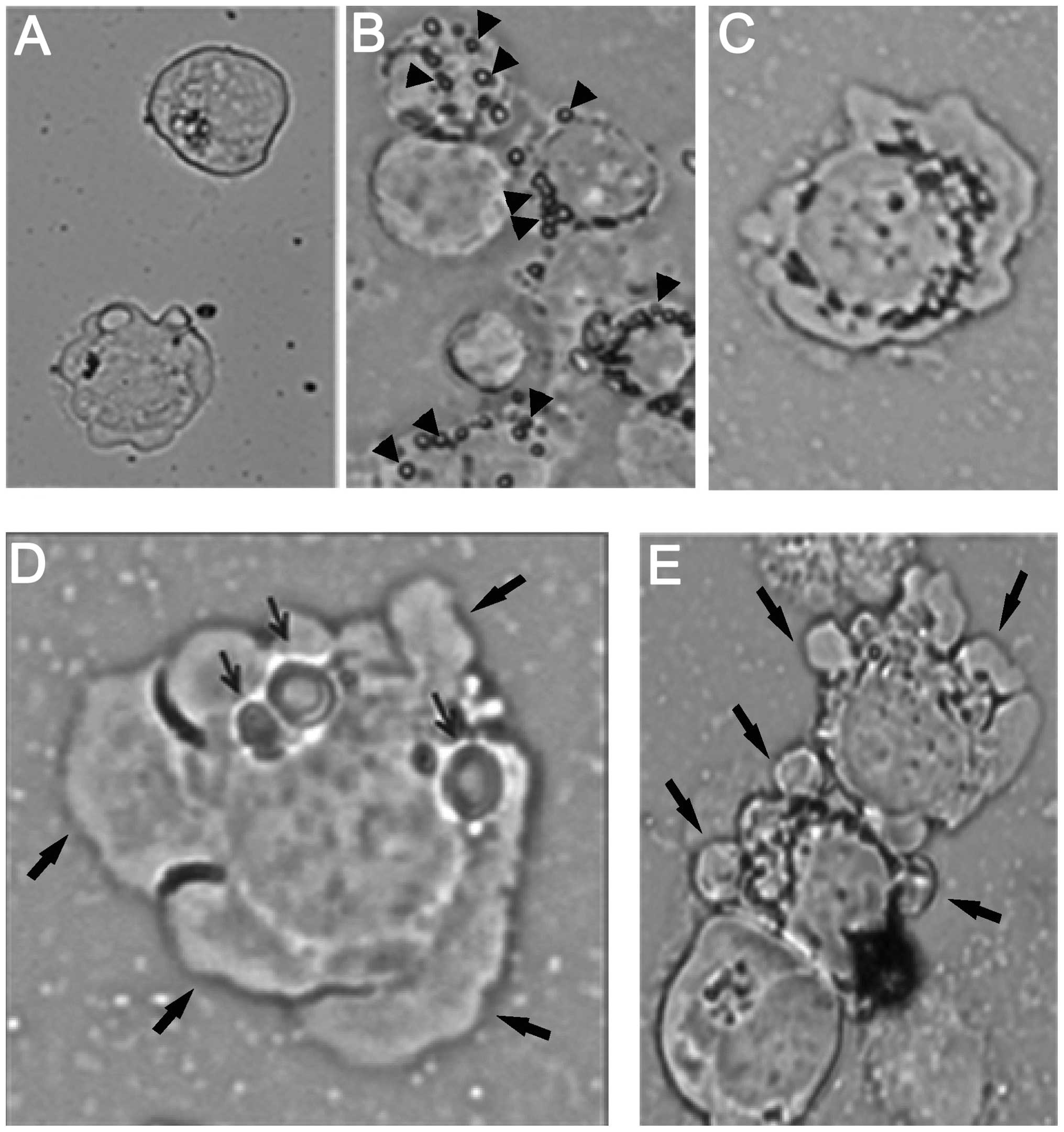
AFM studies
AFM studies were carried out to examine the
morphological changes associated with PFT treatment of HL60/AR
cells. Results showed a low percentage of the control untreated
HL60/AR cells with holes, as well as a small number of holes per
cell (Fig. 6A). When the cancer
cells were exposed to L. kefiri P-IF (5 mg/ml) for 2 min, we
observed an adherence of L. kefiri P-IF to the cancer cells
(Fig. 6B). Exposure of cancer
cells to L. kefiri P-IF for a longer time (24 h) resulted in
2.6-fold increase in the percentage of cancer cells with holes.
Control untreated cells showed 17.5% had single holes and 2% had
multiple holes. However, of the L. kefiri P-IF-treated
cells, 42% had single holes and 9.5% had multiple holes. Fig. 6C shows multiple holes surrounding
the nucleus. Some of the holes are highly enlarged, as can be seen
in Fig. 6D. The holes were
situated in the cytoplasm and the nucleus. Fig. 6D and E also shows that hole
induction was associated with membrane blebbing and a decrease in
the nuclear to cytoplasmic ratio. These morphological changes are
considered to be signs of cancer cell apoptosis. The holes were
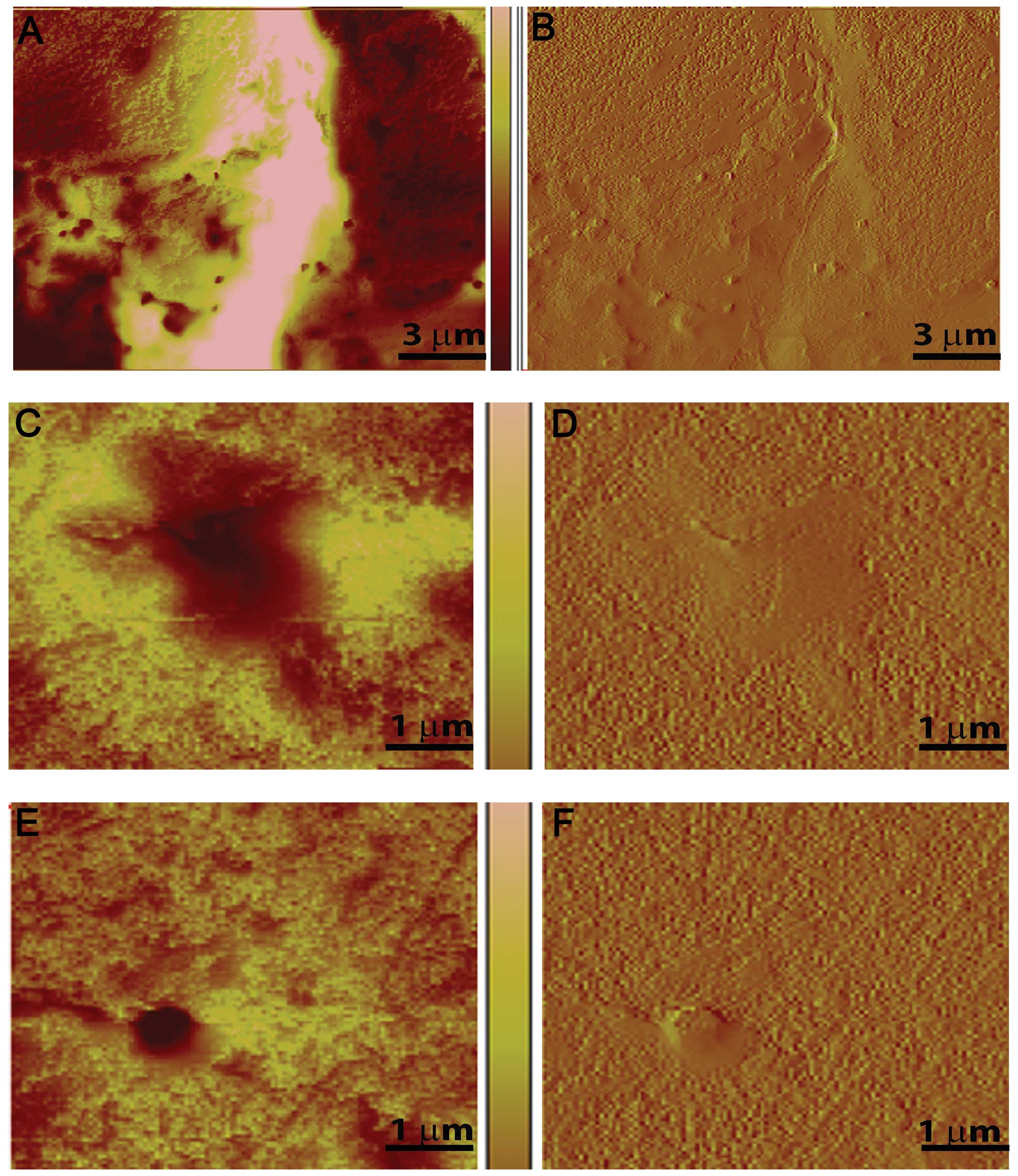
further characterized by peak force imaging (Figs. 7 and 8), where the variation in size, shape and
depth of the holes was measured. The color intensity indicates hole
depth, with lighter areas being shallow or flat and darker colors
indicating depth (Fig. 7). The
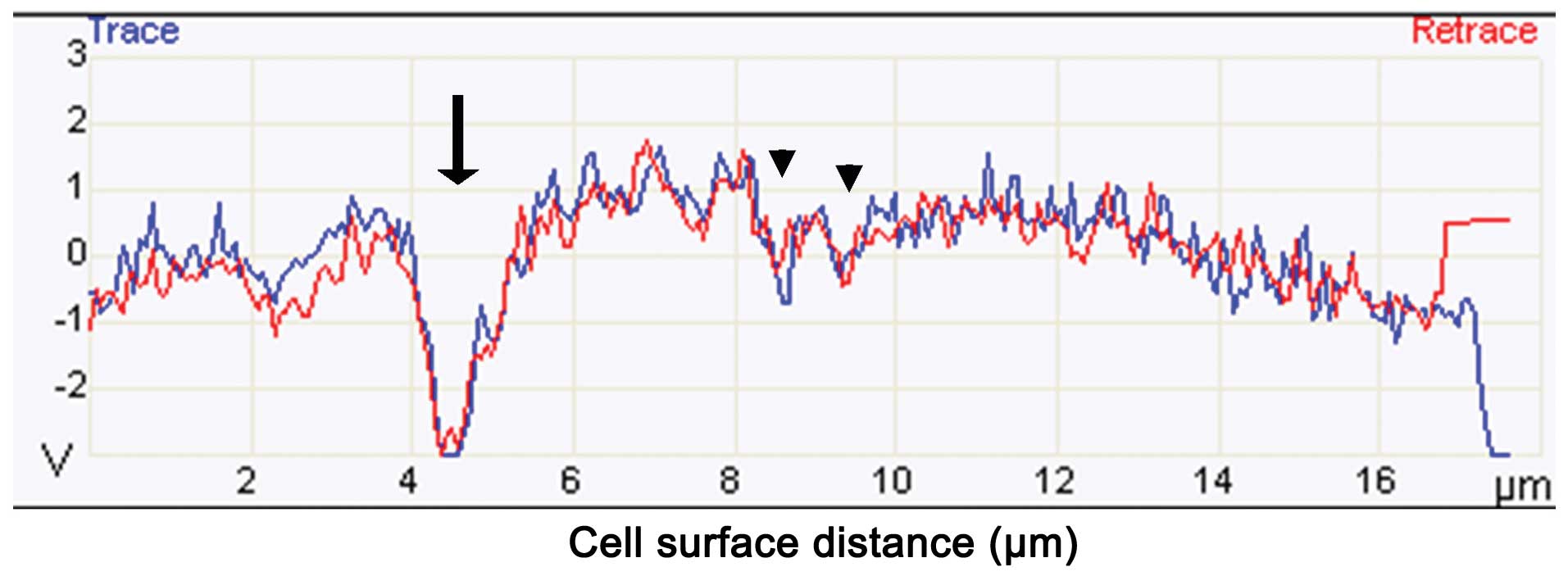
topography of the cell was traced using a cantilever tip. The
changes in the vertical movement of the SNL tip were recorded and
visualized in graphic form as seen in Fig. 8. A decrease along the y-axis
indicates the presence of a hole on the surface of the cell. During
PFT treatment, many holes were detected in HL60/AR cells using this
method of analysis (Fig. 8).
Discussion
In this study, we examined the apoptotic activity of
PFT, a mixture with the main constituent of L. kefiri P-IF.
While yogurt consumption and exposure to LAB has been shown to
exert an inhibitory effect on the growth of different types of
cancers (9,11,14–17),
no study has examined the apoptotic effects of LAB against MDR
cancer cells nor the possible mechanism underlying its effect on
these cells. Our results show that PFT has the ability to induce an
apoptotic effect on MDR cancer cells in a dose-dependent manner,
which is maximal at 67.5% at concentration of 5 mg/ml. This ability
of PFT holds great potential because the development of MDR to
chemotherapy by tumor cells presents great obstacles with the
current treatment options for cancer. Several drugs have been
developed as sensitizers for chemotherapeutics, such as the
membrane transporter P-glycoprotein (P-gp) (24,25),
12-deoxyphorbol 13-phenylacetate (26), probenecid (27) and magnetic iron oxide nanoparticles
(28,29) but these drugs are toxic.
Several studies have shown that LAB exerts antitumor
activity via several mechanisms. These include the ability of LAB
for binding mutagens and removing carcinogens from colon (30,31),
modulation of different arms of the immune system such as NK cells,
dendritic cells, B cells and T cells (32–34)
and induction of apoptosis in cancer cells (18,35,36).
Results of this study showed an interesting phenomenon whereby
L. kefiri P-IF was able to pierce holes in HL60/AR cells.
PFT induces apoptosis in HL60/AR cells associated with activation
of caspase 3, decreased expression of Bcl-2 and decrease in the
polarization of MMP. This suggests that these holes appear to be
responsible for the induction of apoptosis of cancer cells. In
addition, AFM studies show that cancer cells with holes are
correlated with signs of apoptosis such as membrane blebbing and
decrease in the nuclear to cytoplasmic ratio. The mechanism by
which uptake of L. kefiri P-IF induces an apoptotic effect
on cancer cells may involve [Ca2+]I. Our earlier studies
showed that human breast cancer MDA-MB-231 cells underwent
apoptosis following phagocytosis of heat-killed yeast S.
cerevisiae (37) by a
mechanism that involved the elevation of [Ca2+]I. This
was evidenced by an increase of [Ca2+]I post-uptake of
yeast and also in the inhibition of apoptosis post-addition of
2-aminoethoxydiphenyl borate (2APB), a pharmacological inhibitor of
Ca2+ release from the endoplasmic reticulum. We believe
that the uptake of L. kefiri P-IF by cancer cells may be
responsible for the apoptotic effect by this agent.
Hole piercing has been observed with nanoparticles
such as nickel (38) and DPV576, a
mixture of nanodiamond and nanoplatinum in liquid (39). It is noteworthy that the hole
piercing ability of DPV576 has been associated with reversing drug
resistance in HL60/AR cancer cells by increasing the accumulation
of chemotherapeautic drugs such as daunorubicin inside cancer cells
(39). Therefore, we believe that
hole induction by L. kefiri P-IF may likewise facilitate the
uptake and accumulation of chemotherapeutic drugs by MDR cancer
cells, in a manner similar to DPV576 nanoparticles. This is an area
for future study.
Vacuoles appear in the cancer cells apparently due
to the cells ability to phagocytize microorganisms, such as
bacteria and yeast (40–43) and other cells, including:
erythrocytes, lymphocytes and neutrophils (44–46).
Furthermore, research has also revealed the cannibalistic ability
of tumor cells to phagocytize other cancer cells (47). In the present study, we observed
that cancer cells treated with L. kefiri P-IF revealed a
2.6-fold increase in the percentage of cells with holes as compared
to control untreated HL60/AR cells. This increase was observed in
both cancer cells with single as well as multiple holes, which were
situated in the cytoplasm and in the nucleus.
The unique properties of the L. kefiri P-IF
strain may account for its ability to induce apoptosis in the MDR
HL60/AR leukemic cell line. The specific three-dimensional growth
of the P-IF colony indicates that the surface proteoglycans differ
from other L. kefiri strains (22). The cell surface of bacteria and
other microorganisms are composed of varying polysaccharide-peptide
complexes that have been shown to have specific stimulatory effects
on host cells that can be beneficial or inhibitory for cell growth
(48–50). Further investigation into the
unique cell wall components and attributes of L. kefiri P-IF
could contribute to a better understanding of the apoptotic
induction by cell surface proteoglycans. While not investigated in
this study, L. kefiri P-IF has the ability to survive at a
low pH, indicating that the P-IF strain remains viable as it
travels through the stomach into the intestine, allowing it to
continue to grow and provide a protective effect. Some of the
protective effects of the P-IF strain may come from its ability to
metabolize galactose. High levels of galactose can cause toxicity
which could lead to mutations and health problems (51). The presence of L. kefiri
P-IF in the gut may help to prevent damaging levels of galactose
(52). This property warrants
future investigation for prevention and maintenance of general
health.
L. kefiri P-IF has been shown to be a
non-toxic agent. Results of earlier studies in mice treated with
L. kefiri P-IF showed that no macroscopic or
histopathological abnormalities were detected in different organs
post-treatment and no changes in the body weight as compared with
control untreated mice (53).
In conclusion, L. kefiri P-IF represents a
novel symbiotic microbe culture that exerts an apoptotic effect on
HL60/AR cells by a mechanism that may involve piercing holes in the
cellular membrane of cancer cells. L. kefiri P-IF may
represent a new class of adjuvants that could be used to improve
the treatment of MDR leukemia.
Acknowledgements
The authors would like to thank Dr
Sastry Gollapudi, Professor of Immunology, Division of Basic and
Clinical Immunology, University of California, Irvine, CA, USA for
assistance in revision of the manuscript. The authors would also
like to thank Paitos Co., Ltd., Yokohama, Kanagawa, Japan; grant
no. T0099108. The authors thank Dr Peggy Vorwald for her
contributions. The authors acknowledge the use of resources
provided by Dr James Gimzewski and for the use of the SPM facility
and atomic force microscopy, at the Nano and Pico Characterization
Lab at the California NanoSystems Institute.
References
|
1.
|
Metchinkoff E and Metchinkoff II: The
Prolongation of Life: Optimistic Studies. Putnam; New York, NY: pp.
109–132. 1908
|
|
2.
|
Shiby VK and Mishra HN: Fermented milks
and milk products as functional foods - a review. Crit Rev Food Sci
Nutr. 53:482–496. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
3.
|
Ahmed Z, Wang Y, Ahmad A, Khan ST, Nisa M,
Ahmad H and Afreen A: Kefir and health: a contemporary perspective.
Crit Rev Food Sci Nutr. 53:422–434. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
4.
|
Bianchi-Salvadori B: Intestinal
microflora: the role of yoghurt in the equilibrium of the gut
ecosystem. Int J Immunother. 11(Suppl): 9–18. 1986.
|
|
5.
|
Kanmani P, Satish Kumar R, Yuvaraj N,
Paari KA, Pattukumar V and Arul V: Probiotics and its functionally
valuable products - a review. Crit Rev Food Sci Nutr. 53:641–658.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
6.
|
Marco ML, Pavan S and Kleerebezem M:
Towards understanding molecular modes of probiotic action. Cur Opin
Biotechnol. 17:204–210. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
7.
|
Metchnikoff E: Sur le flore du corps
humain (On the flora of the Human body). Manch Lit Philos Soc.
45:1–38. 1901.
|
|
8.
|
Bergonzelli GE, Blum S, Brussow H and
Corthesy-Theulaz I: Probiotics as a treatment strategy for
gastrointestinal diseases? Digestion. 72:57–68. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
9.
|
Pala V, Sieri S, Berrino F, Vineis P,
Sacerdote C, Palli D, Masala G, Panico S, Mattiello A, Tumino R,
Giurdanella MC, Agnoli C, Grioni S and Krogh V: Yogurt consumption
and risk of colorectal cancer in the Italian European prospective
investigation into cancer and nutrition cohort. Int J Cancer.
129:2712–2719. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
10.
|
Goldin BR, Gualtieri LJ and Moore RP: The
effect of Lactobacillus GG on the initiation and promotion
of DMH-induced intestinal tumors in the rat. Nutr Cancer.
25:197–204. 1996.
|
|
11.
|
Shackelford LD, Rao DR, Chawan CB and
Pulasani SR: Effect of feeding fermented milk on the incidence of
chemically induced colon tumors in rats. Nutr Cancer. 5:159–164.
1983. View Article : Google Scholar : PubMed/NCBI
|
|
12.
|
Bogdanov IG, Dalev PG, Gurevich LA,
Kolosov MN and Malkova VP: Antitumor effect of glycopeptides from
the cell wall of Lactobacillus bulgaricus. Biull Eksp Biol
Med. 84:709–712. 1977. View Article : Google Scholar : PubMed/NCBI
|
|
13.
|
Biffi A, Coradini D, Larsen R, Riva L and
Di Fronzo G: Antiproliferative effect of fermented milk on the
growth of a human breast cancer cell line. Nutr Cancer. 28:93–99.
1997. View Article : Google Scholar : PubMed/NCBI
|
|
14.
|
Van’t Veer P, Dekker JM, Lamars JWJ, Kok
FJ and Schouten EG: Consumption of fermented milk products and
breast cancer: a case-control study in The Netherlands. Cancer Res.
49:4020–4023. 1989.PubMed/NCBI
|
|
15.
|
Van’t Veer P, van Leer EM, Rietdijk A, Kok
FJ, Schouten EG, Hermus RJ and Sturmans F: Combination of dietary
factors in relation to breast-cancer occurrence. Int J Cancer.
47:649–653. 1991.PubMed/NCBI
|
|
16.
|
Le MG, Moulton LH, Hill C and Kramer A:
Consumption of dairy products and alcohol in a case control study
of breast cancer. J Natl Cancer Inst. 77:633–636. 1986.PubMed/NCBI
|
|
17.
|
Cramer DW, Harlow BL, Willett W, Welch WR,
Bell DA, Scully RE, Ng WG and Knapp RC: Galactose consumption and
metabolism in relation to the risk of ovarian cancer. Lancet.
2:66–71. 1989. View Article : Google Scholar : PubMed/NCBI
|
|
18.
|
Iyer C, Kosters A, Sethi G, Kunnumakkara
AB, Aggarwal BB and Versalovic J: Probiotic Lactobacillus
reuteri promotes TNF-induced apoptosis in human myeloid
leukemia-derived cells by modulation of NF-kappaB and MAPK
signalling. Cell Microbiol. 10:1442–1452. 2008.
|
|
19.
|
Chiu YH, Hsieh YJ, Liao KW and Peng KC:
Preferential promotion of apoptosis of monocytes by
Lactobacillus casei rhamnosus soluble factors. Clin Nutr.
29:131–140. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
20.
|
Hertzler SR and Clancy SM: Kefir improves
lactose digestion and tolerance in adults with lactose
maldigestion. J Am Diet Assoc. 103:582–587. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
21.
|
Maeda H, Zhu X, Omura K, Suzuki S and
Kitamura S: Effects of an exopolysaccharide (kefiran) on lipids,
blood pressure, blood glucose, and constipation. Biofactors.
22:197–200. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
22.
|
Suzuki K, Tani H, Yabumoto T, Yabumoto Y
and Yoshida Y: Novel fermented milk product and use thereof. US
Patent No. US 20110123640 A1. May;2011.
|
|
23.
|
Sharma S, Rasool HI, Palanisamy V,
Mathisen C, Schmidt M, Wong DT and Gimzewski JK:
Structural-mechanical characterization of nanoparticle exosomes in
human saliva, using correlative AFM, FESEM, and force spectroscopy.
ACS Nano. 4:1921–1926. 2010. View Article : Google Scholar
|
|
24.
|
Palmeira A, Sousa E, Vasconcelos MH and
Pinto MM: Three decades of P-gp inhibitors: skimming through
several generations and scaffolds. Curr Med Chem. 19:1946–2025.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
25.
|
Binkhathlan Z and Lavasanifar A:
P-glycoprotein inhibition as a therapeutic approach for overcoming
multidrug resistance in cancer: current status and future
perspectives. Curr Cancer Drug Targets. 13:326–346. 2013.
View Article : Google Scholar
|
|
26.
|
Gollapudi S, Singh H and Gupta S: Effect
of 12-deoxyphorbol 13-phenylacetate on daunorubicin resistance and
calcium-independent protein-kinase-C isozymes in drug-sensitive
murine leukemia p388 cells. Int J Oncol. 4:849–851. 1994.
|
|
27.
|
Gollapudi S, Kim CH, Tran BN, Sangha S and
Gupta S: Probenecid reverses multidrug resistance in multidrug
resistance-associated protein-overexpressing HL60/AR and H69/AR
cells but not in P-glycoprotein-overexpressing HL60/Tax and
P388/ADR cells. Cancer Chemother Pharmacol. 40:150–158. 1997.
View Article : Google Scholar
|
|
28.
|
Lai BB, Chen BA, Cheng J, Gao F, Xu WL,
Ding JH, Gao C, Sun XC, Li GH, Chen WJ, Liu LJ, Li XM and Wang XM:
Daunorubicin-loaded magnetic nanoparticles of Fe(3)O(4) greatly
enhance the responses of multidrug-resistant K562 leukemic cells in
a nude mouse xenograft model to chemotherapy. Zhongguo Shi Yan Xue
Ye Xue Za Zhi. 17:345–351. 2009.
|
|
29.
|
Cheng J, Wang J, Chen B, Xia G, Cai X, Liu
R, Ren Y, Bao W and Wang X: A promising strategy for overcoming MDR
in tumor by magnetic iron oxide nanoparticles co-loaded with
daunorubicin and 5-bromotetrandrin. Int J Nanomedicine.
6:2123–2131. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
30.
|
Tavan E, Cayuela C, Antoine JM, Trugnan G,
Chaugier C and Cassand P: Effects of dairy products on heterocyclic
aromatic amine induced rat colon carcinogenesis. Carcinogenesis.
23:477–483. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
31.
|
Lidbeck A, Nord CE, Gustafsson JA and
Rafter J: Lactobacilli, anticarcinogenic activities and
human intestinal microflora. Eur J Cancer Prev. 1:341–353. 1992.
View Article : Google Scholar
|
|
32.
|
Link-Amster H, Rochat F, Saudan KY, Mignot
O and Aeschlimann JM: Modulation of a specific humoral immune
response and changes in intestinal flora mediated through fermented
milk intakes. FEMS Immunol Med Microbiol. 10:55–64. 1994.
View Article : Google Scholar : PubMed/NCBI
|
|
33.
|
Tsai YT, Cheng PC and Pan TM: The
immunomodulatory effects of lactic acid bacteria for improving
immune functions and benefits. Appl Microbiol Biotechnol.
96:853–862. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
34.
|
Hong WS, Chen YP and Chen MJ: The
antiallergic effect of kefir Lactobacilli. J Food Sci.
75:H244–H253. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
35.
|
Perdigon G, de Moreno de LA, Valdez J and
Rachid M: Role of yoghurt in the prevention of colon cancer. Eur J
Clin Nutr. 56(Suppl 3): S65–S68. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
36.
|
Altonsy MO, Andrews SC and Tuohy KM:
Differential induction of apoptosis in human colonic carcinoma
cells (Caco-2) by Atopobium, and commensal, probiotic and
enteropathogenic bacteria: mediation by the mitochondrial pathway.
Int J Food Microbiol. 137:190–203. 2010. View Article : Google Scholar
|
|
37.
|
Ghoneum M, Matsuura M, Braga M and
Gollapudi S: S. cerevisiae induces apoptosis in human
metastatic breast cancer cells by altering intracellular
Ca2+and the ratio of Bax and Bcl-2. Int J Oncol.
33:533–539. 2008.
|
|
38.
|
Guo D, Wu C, Li J, et al: Synergistic
effect of functionalized nickel nanoparticles and Quercetin on
inhibition of the SMMC-7721 cells proliferation. Nanoscale Res
Lett. 4:1395–1402. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
39.
|
Ghoneum A, Sharma S and Gimzewski J:
Nano-hole induction by nanodiamond and nanoplatinum liquid, DPV576,
reverses multidrug resistance in human myeloid leukemia (HL60/AR).
Int J Nanomed. 8:2567–2573. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
40.
|
Vandenberghe J, Verheyen A, Lauwers S and
Geboes K: Spontaneous adenocarcinoma of the ascending colon in
Wistar rats: the intracytoplasmic presence of a Campylobacter-like
bacterium. J Comp Pathol. 95:45–55. 1985. View Article : Google Scholar : PubMed/NCBI
|
|
41.
|
Ghoneum M, Grewal I, Brown J, Osborne R,
Elembabi H and Gill G: Phagocytosis of candida albicans by
lymphatic tumour cells in vitro. Acta Histochem. 105:127–133.
2003.
|
|
42.
|
Ghoneum M and Gollapudi S: Phagocytosis of
Candida albicans by metastatic and non metastatic human
breast cancer cell lines in vitro. Cancer Detect Prev. 28:17–26.
2004.
|
|
43.
|
Ghoneum M, Hamilton J, Brown J and
Gollapudi S: Human squamous cell carcinoma of the tongue and colon
undergoes apoptosis upon phagocytosis of Saccharomyces
cerevisiae, the baker’s yeast, in vitro. Anticancer Res.
25:981–989. 2005.PubMed/NCBI
|
|
44.
|
Ghoneum M, Salem F, Shum SS, Perry L and
Gill G: In situ lymphophagocytosis by nonlymphoreticular neoplasms.
Nat Immun Cell Growth Regul. 6:77–87. 1987.PubMed/NCBI
|
|
45.
|
Ghoneum M, Salem F, Allen H and Gill G:
Phagocytosis of autologous lymphocytes by cervical preneoplastic
and neoplastic cells. Nat Immun Cell Growth Regul. 7:239–248.
1988.PubMed/NCBI
|
|
46.
|
Singhal N, Handa U, Bansal C and Mohan H:
Neutrophil phagocytosis by tumor cells - a cytological study. Diagn
Cytopathol. 39:553–555. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
47.
|
Overholtzer M, Mailleux AA, Mouneimne G,
et al: A non-apoptotic cell death process, entosis, that occurs by
cell-in-cell invasion. Cell. 131:966–979. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
48.
|
Kogan G, Pajtinka M, Babincova M,
Miadokova E, Rauko P, Slamenova D and Korolenko TA: Yeast cell wall
polysaccharides as antioxidants and antimutagens: can they fight
cancer? Neoplasma. 55:387–393. 2008.PubMed/NCBI
|
|
49.
|
Cattaruzza S, Nicolosi PA and Perris R:
Proteoglycans in the control of tumor growth and metastasis
formation. Connect Tissue Res. 49:225–229. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
50.
|
Oh JY, Baek YM, Kim SW, Hwang HJ, Hwang
HS, Lee SH and Yun JW: Apoptosis of human hepatocarcinoma (HepG2)
and neuroblastoma (SKN-SH) cells induced by polysaccharides-peptide
complexes produced by submerged mycelial culture of an
entomopathogenic fungus Cordyceps sphecocephala. J Microbiol
Biotechnol. 18:512–519. 2008.
|
|
51.
|
Lai K, Elsas LJ and Wierenga KJ: Galactose
toxicity in animals. IUBMB Life. 61:1063–1074. 2009. View Article : Google Scholar
|
|
52.
|
Davit-Spraul A, Pourci ML, Soni T and
Lemonnier A: Metabolic effects of galactose on human HepG2
hepatoblastoma cells. Metabolism. 43:945–952. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
53.
|
Paitos Co., Ltd. Yokohama, Kanagawa,
Japan: Increase the good bacteria held by nature, ideal AH21 is a
functional food, consider preventive medicine and food. http://www.bio-j.net/ken00.html.
Accessed June 14, 2013.
|