|
1.
|
Gonzalez VM, Fuertes MA, Alonso C and
Perez JM: Is cisplatin-induced cell death always produced by
apoptosis? Mol Pharmacol. 59:657–663. 2001.PubMed/NCBI
|
|
2.
|
Kartalou M and Essigmann JM: Mechanisms of
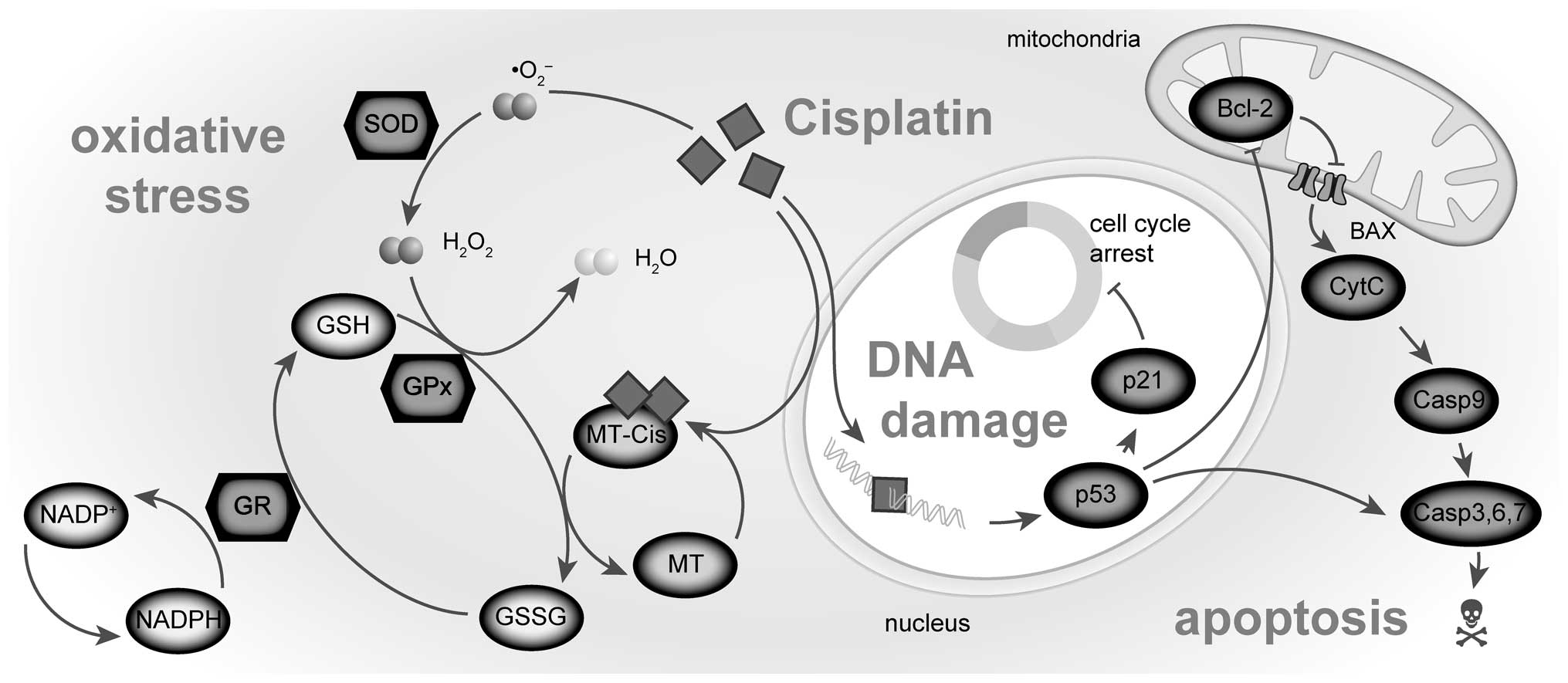
resistance to cisplatin. Mutat Res. 478:23–43. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
3.
|
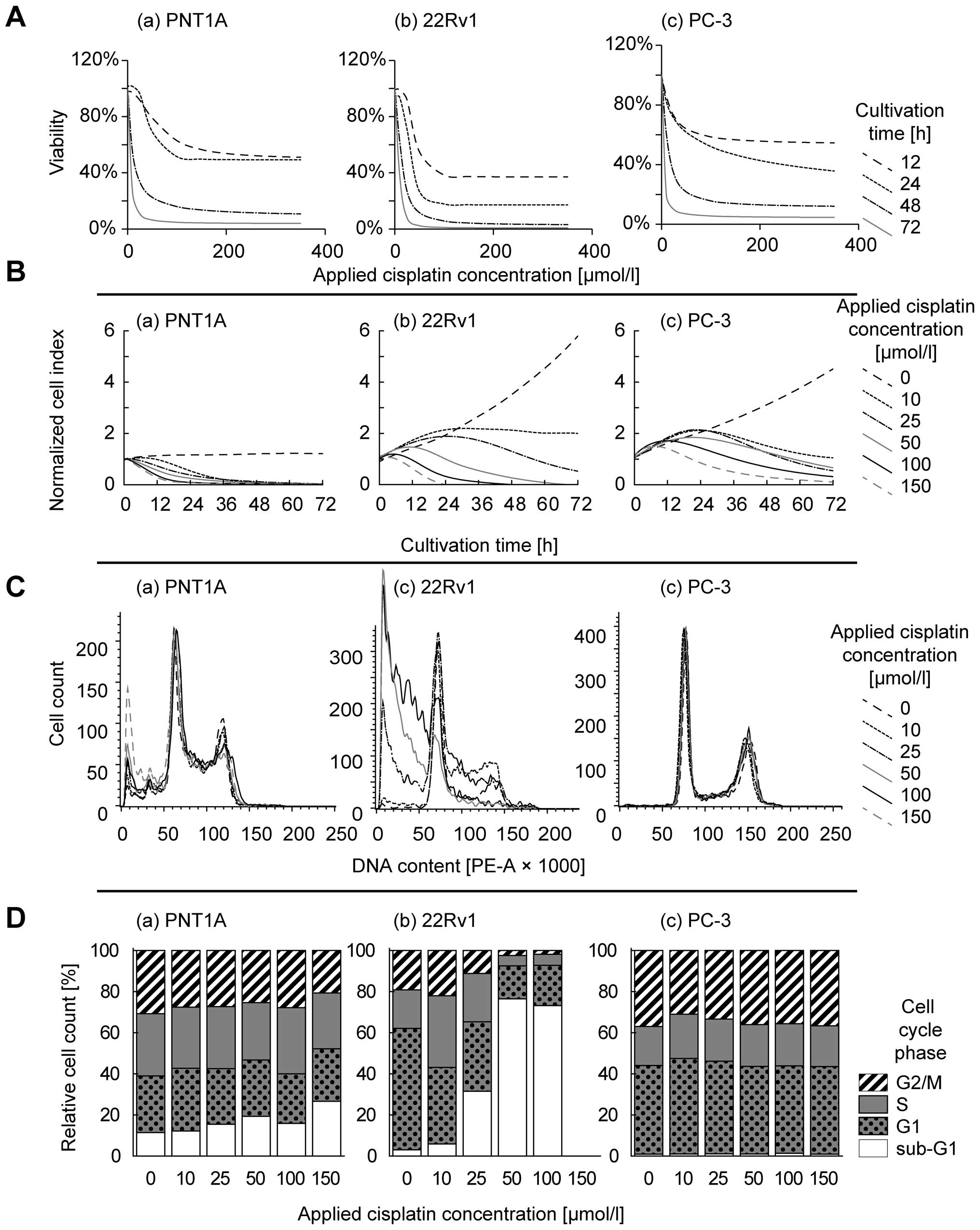
Niedner H, Christen R, Lin X, Kondo A and
Howell SB: Identification of genes that mediate sensitivity to
cisplatin. Mol Pharmacol. 60:1153–1160. 2001.PubMed/NCBI
|
|
4.
|
Herraez E, Gonzalez-Sanchez E, Vaquero J,
et al: Cisplatin-induced chemoresistance in colon cancer cells
involves FXR-dependent and FXR-independent up-regulation of ABC
proteins. Mol Pharm. 9:2565–2576. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
5.
|
Liu YB, Bernauer AM, Yingling CM and
Belinsky SA: HIF1 alpha regulated expression of XPA contributes to
cisplatin resistance in lung cancer. Carcinogenesis. 33:1187–1192.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
6.
|
Wu YC, Ling TY, Lu SH, et al:
Chemotherapeutic sensitivity of testicular germ cell tumors under
hypoxic conditions is negatively regulated by SENP1-controlled
sumoylation of OCT4. Cancer Res. 72:4963–4973. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
7.
|
Skjoth IHE and Issinger OG: Profiling of
signaling molecules in four different human prostate carcinoma cell
lines before and after induction of apoptosis. Int J Oncol.
28:217–229. 2006.PubMed/NCBI
|
|
8.
|
Faria MHG, Neves EHC, Alves MKS, Burbano
RMR, De Moraes MO and Rabenhorst SHB: TP53 mutations in astrocytic
gliomas: an association with histological grade, TP53 codon 72
polymorphism and p53 expression. APMIS. 120:882–889. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
9.
|
Fuertes MA, Alonso C and Perez JM:
Biochemical modulation of cisplatin mechanisms of action:
enhancement of antitumor activity and circumvention of drug
resistance. Chem Rev. 103:645–662. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
10.
|
Righetti SC, Perego P, Carenini N, et al:
Molecular alterations of cells resistant to platinum drugs: role of
PKC alpha. Biochim Biophys Acta. 1763:93–100. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
11.
|
Brozovic A, Ambriovic-Ristov A and Osmak
M: The relationship between cisplatin-induced reactive oxygen
species, glutathione, and BCL-2 and resistance to cisplatin. Crit
Rev Toxicol. 40:347–359. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
12.
|
Santos NAG, Catao CS, Martins NM, Curti C,
Bianchi MLP and Santos AC: Cisplatin-induced nephrotoxicity is
associated with oxidative stress, redox state unbalance, impairment
of energetic metabolism and apoptosis in rat kidney mitochondria.
Arch Toxicol. 81:495–504. 2007. View Article : Google Scholar
|
|
13.
|
Martins NM, Santos NAG, Curti C, Bianchi
MLP and Santos AC: Cisplatin induces mitochondrial oxidative stress
with resultant energetic metabolism impairment, membrane
rigidification and apoptosis in rat liver. J Appl Toxicol.
28:337–344. 2008. View
Article : Google Scholar
|
|
14.
|
Kizek R, Trnkova L and Palecek E:
Determination of metallothionein at the femtomole level by constant
current stripping chronopotentiometry. Anal Chem. 73:4801–4807.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
15.
|
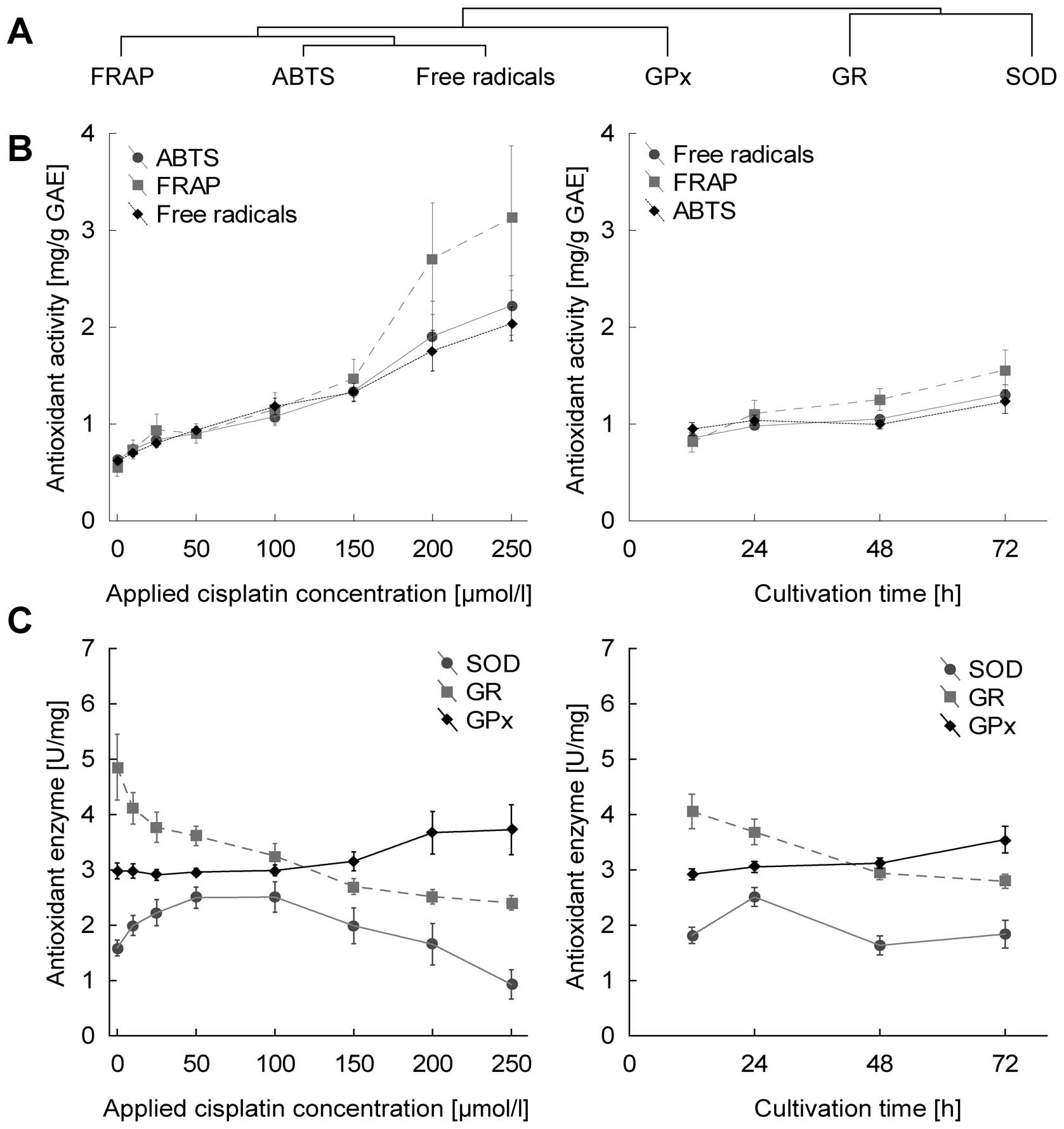
Sochor J, Ryvolova M, Krystofova O, et al:
Fully automated spectrometric protocols for determination of
antioxidant activity: advantages and disadvantages. Molecules.
15:8618–8640. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
16.
|
Quereda JJ, Martinez-Alarcon L, Mendoca L,
et al: Validation of xCELLigence real-time cell analyzer to assess
compatibility in xenotransplantation with pig-to-baboon model.
Transplant Proc. 42:3239–3243. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
17.
|
Vistejnova L, Dvorakova J, Hasova M, et
al: The comparison of impedance-based method of cell proliferation
monitoring with commonly used metabolic-based techniques.
Neuroendocrinol Lett. 30:121–127. 2009.PubMed/NCBI
|
|
18.
|
Carroll AG, Voeller HJ, Sugars L and
Gelmann EP: p53 oncogene mutations in 3 human prostate-cancer
cell-lines. Prostate. 23:123–134. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
19.
|
Rubin SJ, Hallahan DE, Ashman CR, et al: 2
prostate carcinoma cell-lines demonstrate abnormalities in tumor
suppressor genes. J Surg Oncol. 46:31–36. 1991. View Article : Google Scholar
|
|
20.
|
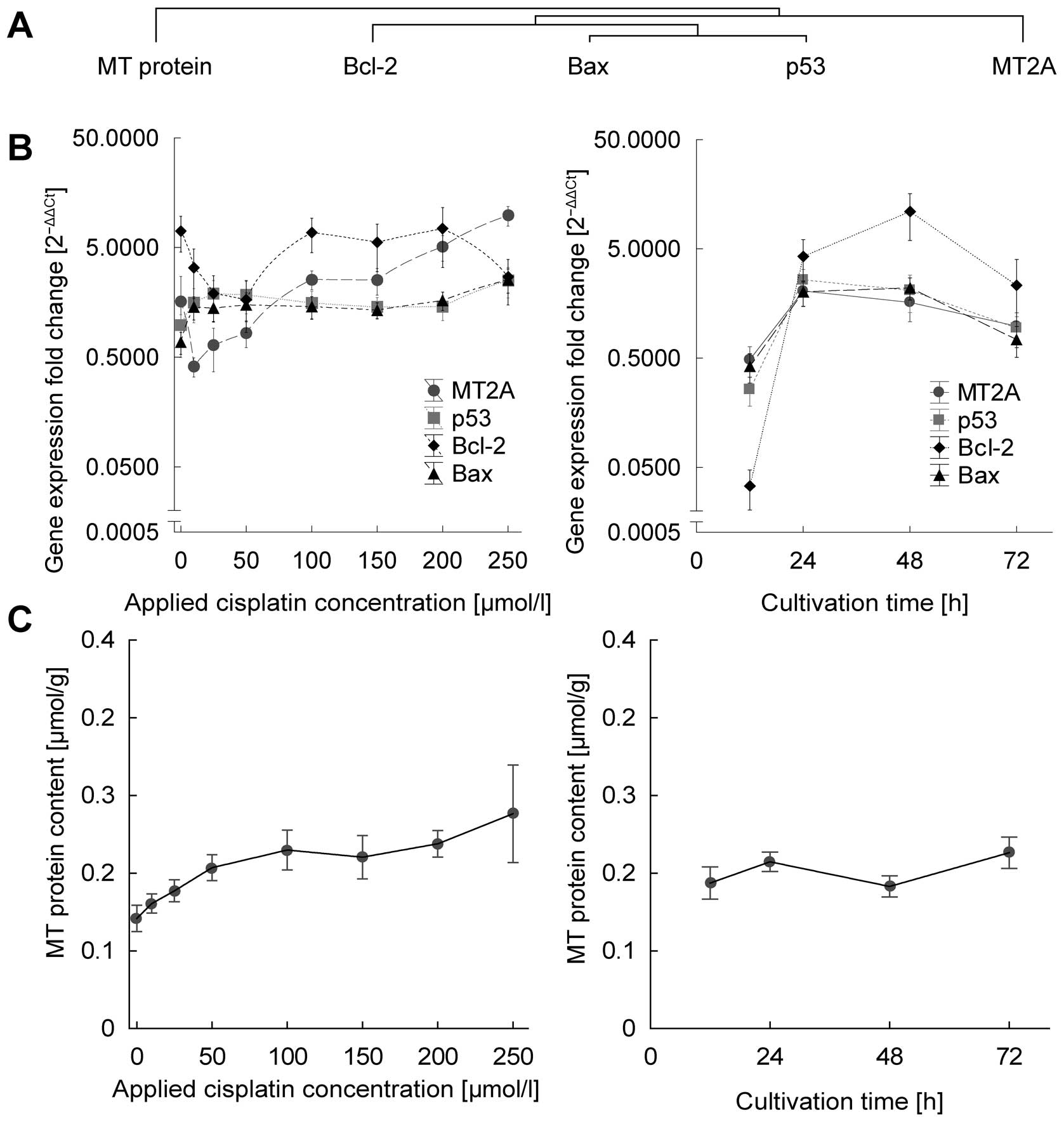
Sztalmachova M, Hlavna M, Gumulec J, et
al: Effect of zinc(II) ions on the expression of pro- and
anti-apoptotic factors in high-grade prostate carcinoma cells.
Oncol Rep. 28:806–814. 2012.PubMed/NCBI
|
|
21.
|
Schmieg FI and Simmons DT:
Characterization of the in vitro interaction between SV40 T-antigen
and p53: mapping the p53 binding-site. Virology. 164:132–140. 1988.
View Article : Google Scholar : PubMed/NCBI
|
|
22.
|
Gonin S, Diaz-Latoud C, Richard MJ, et al:
p53/T-antigen complex disruption in T-antigen transformed NIH3T3
fibroblasts exposed to oxidative stress: correlation with the
appearance of a Fas/APO-1/CD95 dependent, caspase independent,
necrotic pathway. Oncogene. 18:8011–8023. 1999. View Article : Google Scholar
|
|
23.
|
Barr MP, Gray SG, Hoffmann AC, et al:
Generation and characterisation of cisplatin-resistant non-small
cell lung cancer cell lines displaying a stem-like signature. Plos
One. 8:1–19. 2013.PubMed/NCBI
|
|
24.
|
Liu J, Liu YP, Habeebu SSM and Klaassen
CD: Metallothionein (MT)-null mice are sensitive to
cisplatin-induced hepatotoxicity. Toxicol Appl Pharmacol.
149:24–31. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
25.
|
Siddik ZH: Cisplatin: mode of cytotoxic
action and molecular basis of resistance. Oncogene. 22:7265–7279.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
26.
|
Wang D and Lippard SJ: Cellular processing
of platinum anti-cancer drugs. Nat Rev Drug Discov. 4:307–320.
2005. View Article : Google Scholar
|
|
27.
|
Gravina GL, Marampon F, Di Staso M, et al:
5-Azacitidine restores and amplifies the bicalutamide response on
preclinical models of androgen receptor expressing or deficient
prostate tumors. Prostate. 70:1166–1178. 2010. View Article : Google Scholar
|
|
28.
|
Aitken RJ, Whiting S, De Iuliis GN,
McClymont S, Mitchell LA and Baker MA: Electrophilic aldehydes
generated by sperm metabolism activate mitochondrial reactive
oxygen species generation and apoptosis by targeting succinate
dehydrogenase. J Biol Chem. 287:33048–33060. 2012. View Article : Google Scholar
|
|
29.
|
Komatsu M, Waguri S, Ueno T, et al:
Impairment of starvation-induced and constitutive autophagy in
Atg7-deficient mice. J Cell Biol. 169:425–434. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
30.
|
Cao C, Subhawong T, Albert JM, et al:
Inhibition of mammalian target of rapamycin or apoptotic pathway
induces autophagy and radiosensitizes PTEN null prostate cancer
cells. Cancer Res. 66:10040–10047. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
31.
|
Masarik M, Gumulec J, Hlavna M, et al:
Monitoring of the prostate tumour cells redox state and real-time
proliferation by novel biophysical techniques and fluorescent
staining. Integr Biol. 4:672–684. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
32.
|
Kuwana T and Newmeyer DD: Bcl-2-family
proteins and the role of mitochondria in apoptosis. Curr Opin Cell
Biol. 15:691–699. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
33.
|
Kharaziha P, Rodriguez P, Li Q, et al:
Targeting of distinct signaling cascades and cancer-associated
fibroblasts define the efficacy of Sorafenib against prostate
cancer cells. Cell Death Dis. 3:1–10. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
34.
|
Li GQ, Chen XG, Wu XP, et al: Effect of
dicycloplatin, a novel platinum chemotherapeutical drug, on
inhibiting cell growth and inducing cell apoptosis. Plos One.
7:1–13. 2012.PubMed/NCBI
|
|
35.
|
Kondo Y, Kuo SM, Watkins SC and Lazo JS:
Metallothionein localization and cisplatin resistance in human
hormone-independent prostatic tumor-cell lines. Cancer Res.
55:474–477. 1995.PubMed/NCBI
|
|
36.
|
Suzuki Y, Kondo Y, Himeno S, Nemoto K,
Akimoto M and Imura N: Role of antioxidant systems in human
androgen-independent prostate cancer cells. Prostate. 43:144–149.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
37.
|
Yamasaki M, Nomura T, Sato F and Mimata H:
Metallothionein is up-regulated under hypoxia and promotes the
survival of human prostate cancer cells. Oncol Rep. 18:1145–1153.
2007.PubMed/NCBI
|
|
38.
|
Costello LC, Fenselau CC and Franklin RB:
Evidence for operation of the direct zinc ligand exchange mechanism
for trafficking, transport, and reactivity of zinc in mammalian
cells. J Inorg Biochem. 105:589–599. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
39.
|
Bell SG and Vallee BL: The
metallothionein/thionein system: an oxidoreductive metabolic zinc
link. Chembiochem. 10:55–62. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
40.
|
Pratibha R, Sameer R, Rataboli PV,
Bhiwgade DA and Dhume CY: Enzymatic studies of cisplatin induced
oxidative stress in hepatic tissue of rats. Eur J Pharmacol.
532:290–293. 2006. View Article : Google Scholar : PubMed/NCBI
|