Introduction
Adenoid cystic carcinoma (AdCC) is among the most
common malignant tumors of the salivary glands and is characterized
by unique clinical features and behavior. Although slow growing,
AdCC spreads relentlessly into adjacent tissues. It carries a high
risk of recurrence and distant metastases, with 40–60% of afflicted
patients developing distant metastases to the lungs, bone, and soft
tissues (1,2). AdCC is resistant to chemotherapy and
radiotherapy. Therefore, treatment-resistant distant metastases
remain a significant obstacle to the long-term cure of patients
with AdCC, emphasizing the need for anti-metastasis therapy for
AdCC.
We previously established 3 AdCC cell lines that
express green fluorescent protein (GFP) from the ACCS cell line
using orthotopic transplantation and in vivo selection in
the nude mouse. These 3 lines include the parental ACCS GFP, the
highly tumorigenic ACCS-T GFP, and the metastatic ACCS-M GFP line.
We demonstrated that ACCS-M GFP cells exhibited a loss of
E-cadherin and integrins and a gain in vimentin, suggesting that
the epithelial-mesenchymal transition (EMT) is a key event in AdCC
metastasis that induces tumor cell dissemination from the primary
tumor site (3). We also showed a
direct correlation between EMT and prevalence of cancer stem
cell-like cells in AdCC (4).
The EMT program triggered during tumor progression
appears to be controlled by expression of early embryonic genes,
including Twist, Snail, Slug, Goosecoid and SIP1
(5,6). The transcription factors encoded by
these genes impart mesenchymal traits to tumor cells, including
motility and invasiveness. For example, expression of Twist
is elevated in various cancers, including breast, prostate, gastric
and melanoma (7). In addition, the
T-box transcription factor Brachyury, a protein required for
mesoderm formation during development (8–10),
reportedly promotes EMT in human carcinoma cell lines (11). The latter study also showed that
Brachyury overexpression in human carcinoma cells induced
changes characteristic of EMT. These findings suggest that the EMT
in cancer cells is controlled by mechanisms similar to the EMT
during normal human development.
Other studies using neoplastic tissue have
identified self-renewing, stem-like cells within tumors, referred
to as cancer stem cells (CSCs). CSCs constitute a minority of
neoplastic cells within a tumor and are defined operationally by
their ability to seed new tumors. For this reason, they have also
been termed tumor-initiating cells (12). During the process of tumor
metastasis, which is often enabled by EMT (13), disseminated cancer cells are
thought to require self-renewal properties similar to those
exhibited by stem cells in order to spawn macroscopic metastases.
This raises the possibility that the EMT process, which enables
cancer cell dissemination, may also impart self-renewal to
disseminating cancer cells. Indeed, emerging evidence of a direct
interaction between EMT and CSCs (11,14,15).
Similarly to normal stem cells, CSCs are regulated by key genes,
such as Oct4, Nanog, c-Myc, Sox2, and Klf4, which are
similar to EMT-regulator genes (16,17).
CSCs are resistant to chemotherapy and radiotherapy (18,19),
suggesting a new therapeutic principle for targeting CSCs (20,21).
We have confirmed a direct interaction between the
EMT and CSCs in the highly metastatic AdCC subclone ACCS-M GFP. We
also reported that the T-box transcription factor Brachyury, which
is also a marker of mesoderm differentiation (22,23),
regulates CSC and the EMT in AdCC cells. Brachyury knockdown
exerted a stronger effect on cancer sternness and EMT phenotype
than did knockdown of the conventional CSC regulator gene,
Sox2. By reducing the sternness of CSCs, Brachyury
knockdown significantly inhibited tumorigenicity and metastasis
in vivo (4). This
hypothesis has been supported by recent evidence linking Brachyury
to CSCs in colon cancer (24).
These observations suggest that knocking down
Brachyury can control CSC and EMT, thus inducing CSC
differentiation and sensitization to conventional chemotherapy and
radiotherapy. In this study, we validated that Brachyury
knockdown suppresses chemo- and radioresistance in vitro as
a first step in establishing its therapeutic potential against
CSCs.
Materials and methods
Chemicals
Standard anticancer drug kits were provided by
Scientific Support Programs for Cancer Research, Grant-in-Aid for
Scientific Research on Innovative Areas from the Ministry of
Education, Culture, Sports, Science and Technology of Japan.
Docetaxel, 5-fluorouracil (5-FU), pacli-taxel, cisplatin (CDDP),
mitomycin C, bestatin hydrochloride, bleomycin sulfate and
etoposide were purchased from Sigma-Aldrich (St. Louis, MO, USA).
Actinomycin D and streptomycin-SP were purchased from Calbiochem
(Merck, Darmstadt, Germany).
Cells and cell culture
The human cell lines ACCS, ACCS GFP and ACCS-M GFP
were established in our laboratory as previously described
(3). Briefly, the parental cell
line ACCS and the GFP-transfected sub-line ACCS GFP displayed
similar morphology, growth rate and tumorigenicity in vitro
and in vivo. Similar to the parental ACCS cells, ACCS GFP
cells had low tumorigenicity (22.2% incidence). Using ACCS GFP
cells injected into the tongue of nude mice, tumor formation was
observed under the excitation wavelength. Green fluorescence was
not observed in the absence of tumors. We performed in vivo
selection of clones with higher tumorigenicity by repeatedly
recovering cells in vitro and transplanting them into the
tongue of nude mice. This selection process yielded a subline
exhibiting high tumorigenicity (100% incidence) and high frequency
of metastasis to submandibular lymph nodes (100% incidence); these
cells were termed ACCS-M GFP. The histological and
immunohistochemical features of ACCS-M GFP tumors were similar to
the solid pattern of AdCC. The cell lines were maintained as a
monolayer culture in Dulbecco’s modified Eagle’s medium (DMEM;
Sigma-Aldrich) supplemented with 10% fetal bovine serum (FBS; ICN
Biomedicals, Aurora, OH, USA), 2 mM 1-glutamine, penicillin G, and
streptomycin in a humidified incubator under an atmosphere of 5%
CO2 at 37°C (3).
Transfection of Brachyury and SOX2
shRNA
Cultured ACCS cells were transfected with short
hairpin RNA (shRNA) lentiviral plasmids (pLKO.l-puro;
Sigma-Aldrich) using Lipofectamine LTX (Invitrogen Life
Technologies, Carlsbad, CA, USA) according to the manufacturer’s
instructions as previously described (4). ACCS-M sh cont. cells were generated
by transfecting ACCS-M GFP cells with pLKO.l-puro Control shRNA
Vector (Sigma-Aldrich). ACCS-M shBr and ACCS-M shSOX2 cells were
generated by transfecting ACCS GFP and ACCS-M GFP cells with
pLKO.l-puro/sh. Brachyury or pLKO.l-puro/sh. SOX2 (Sigma-Aldrich),
respectively. Colonies resistant to puromycin (Sigma) were pooled
from the individual transfection experiments. The expression level
of Brachyury in shRNA-transfected ACCS cells was monitored
by real-time reverse transcription-PCR (RT-PCR) (4). All transfected cells were maintained
in DMEM containing 10% FBS and 2μg/ml puromycin
(Sigma-Aldrich).
Wound healing assay
Cells (3×l05) were seeded on a 6-well
plate. After 24 h, ‘wounds’ were scratched with a 200-μl
pipette tip, washed with medium and observed under a fluorescence
microscope (BZ-8000; Keyence, Osaka, Japan). The wound regions were
photographed again after 8, 16 and 24 h, and the wound areas were
measured. Wound area was calculated using the following formula:
wound area (% of control) = (wound area after the indicated period
× l00)/initial wound area.
Evaluation of tumor dissemination from
the primary cancer nest
Evaluation of tumor dissemination from the primary
cancer nest was performed as previously described (25). Briefly, living ACCS cell lines were
fluorescently labeled using Vybrant DiO and DiD cell-labeling
solutions (Molecular Probes, Eugene, OR, USA) according to the
manufacturer’s instructions. Then, l×l06 labeled cells
were pelleted and resuspended in 10 μl collagen type I gel
to form a solid cell cluster. The collagen-embedded tumor cell
pellets were allowed to solidify for 30 min at 37°C in a
100-μl microcentrifuge tube; the pellets were then embedded
in non-labeled fibroblasts containing collagen type I gel
(1×105 cells/ml) and solidified. Growth medium was
placed over the collagen gels and cultured. Tumor dissemination was
observed under a fluorescence microscope (BZ-8000; Keyence). The
grade of tumor dissemination from the tumor cell pellet (modeling
the primary tumor nest) was evaluated by measuring the distance of
all cells from the edge of the nest in 5 randomly selected,
standardized rectangular light fields (500×100 μm), and the
values were summed. The evaluation was conducted twice daily for 7
days.
MTT assay
ACCS cell lines were seeded into CellTiter 96
Aqueous Non-radioactive Cell Proliferation Assay G4000 plates
(Promega, Madison, WI, USA) at a density of 5×l03 cells
per well and incubated in DMEM containing 10% FBS for 8 h. The
medium was replaced with serum-free DMEM after 3 washes with PBS.
For chemosensitivity analysis, a dilutional series of anticancer
drugs was applied at final drug concentrations of 0, 0.001, 0.01,
0.1, 1, 10, 100 and 1,000 μM and incubated for 24 h in a
humidified incubator under an atmosphere of 5% CO2 at
37°C. For radiosensitivity analysis, in vitro gamma-ray
irradiation was administered at 5,10,15, or 30 Gy with a Gammacell
40® Exactor Low Dose-Rate Research Irradiator (Best
Theratronics, Ottawa, Canada), and cells were then incubated for 48
or 72 h in a humidified incubator under an atmosphere of 5%
CO2 at 37°C. After incubation, ACCS cells were analyzed
by CellTiter 96 Aqueous Non-radioactive Cell Proliferation Assay
G4000 (Promega) according to the manufacturer’s instructions. The
absorbance of samples at 590 nm (A590) was measured with a
microplate reader (Model 680, Bio-Rad, USA). All experiments were
carried out in triplicate and repeated 3 times.
Data were normalized to the untreated controls and
reported as % viability. The IC50 (μM) values for
cytotoxicity of the anticancer drug represents the concentration
yielding 50% viability, which was determined from the
concentration-viability curve. The concentration-viability curve
was generated using a non-linear regression model with the Solver
function of Microsoft Excel as previously described (26).
Real-time RT-PCR
Total RNA was extracted from ACCS GFP cells using
the RNeasy Mini kit (Qiagen, Chats worth, CA, USA) and used for
first-strand cDNA synthesis. The mRNA levels were quantified in
triplicate using a real-time PCR system with the Brilliant SYBR
Green qPCR kit (Stratagene, La Jolla, CA, USA) for Brachyury
and Sox2 or the RT2 Profiler PCR Array (96-well
format) for human drug transporters (Qiagen). Specific primers for
Brachyury and Sox2 were: hBrachyury (F)
5’-TGCTGCAATCCCATGACA-3’, (R) 5’-CGTTGCTCACAGACCACA-3’; hSOX2, (F)
5’-TGG GTTCGGTGGTCAAGT-3’, (R) 5’-CTCTGGTAGTGCTG GGACA-3’. The PCR
cycling conditions were 10 min at 95°C followed by 47 cycles at
95°C for 30 sec, 60°C for 30 sec, and 72°C for 60 sec. Dissociation
curve analyses confirmed that the signals corresponded to unique
amplicons. Expression levels were normalized to (β-actin mRNA
levels for each sample obtained from parallel assays and analyzed
using the LightCycler software package version 3.5 (Roche
Diagnostics, Mannheim, Germany) for hBrachyury and hSOX2 and Mx
3000P QPCR system (Agilent Technologies, CO, USA) for the
RT2 Profiler PCR Array.
Statistical analysis
All data are represented as mean ± SD, as analyzed
via analysis of variance and Student’s t-test, and processed using
the statistical software SPSS 13.0. Statistical significance was
defined as P<0.05.
Results
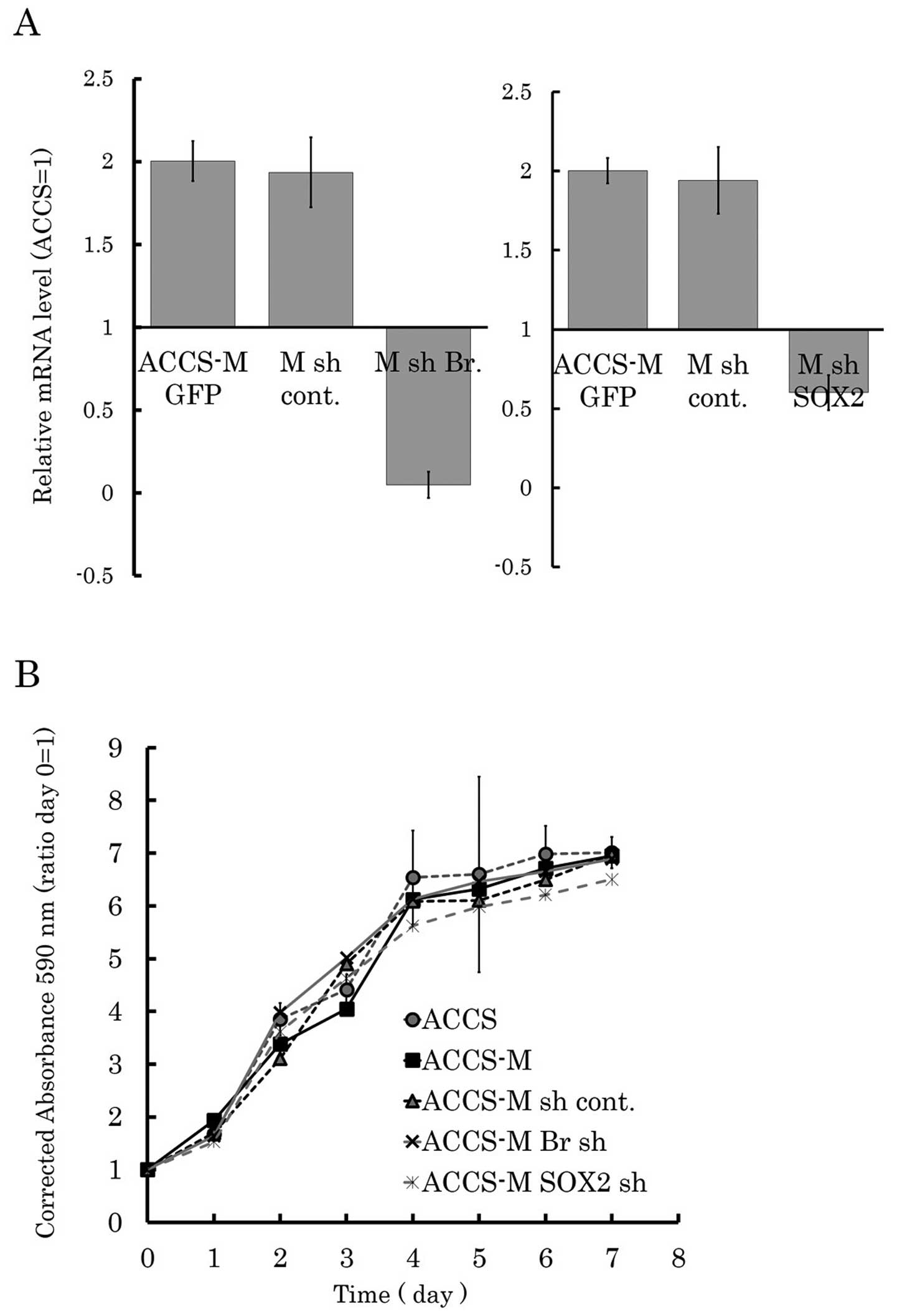
Brachyury and SOX2 shRNA do not influence
growth of ACCS cell lines
We established ACCS GFP and ACCS-M GFP-derived cell
lines by stable transfection of Brachyury or SOX2
shRNA lentiviral plasmids. The expression level of Brachyury
or SOX2 in shRNA-transfected ACCS cells was monitored by
real-time RT-PCR to confirm silencing of the target genes (Fig. 1A). We first analyzed the effect of
Brachyury or SOX2 knockdown on cell growth in
vitro by MTT assay. Cancer stem cell-like ACCS-M GFP cells
demonstrated a similar growth pattern to parental ACCS GFP cells.
Stable transfection of shRNA did not affect cell growth (ACCS-M sh
cont. GFP). Neither Brachyury shRNA nor SOX2 shRNA affected cell
growth (ACCS-M shBr GFP and ACCS-M shSOX2 GFP, respectively;
Fig. 1B).
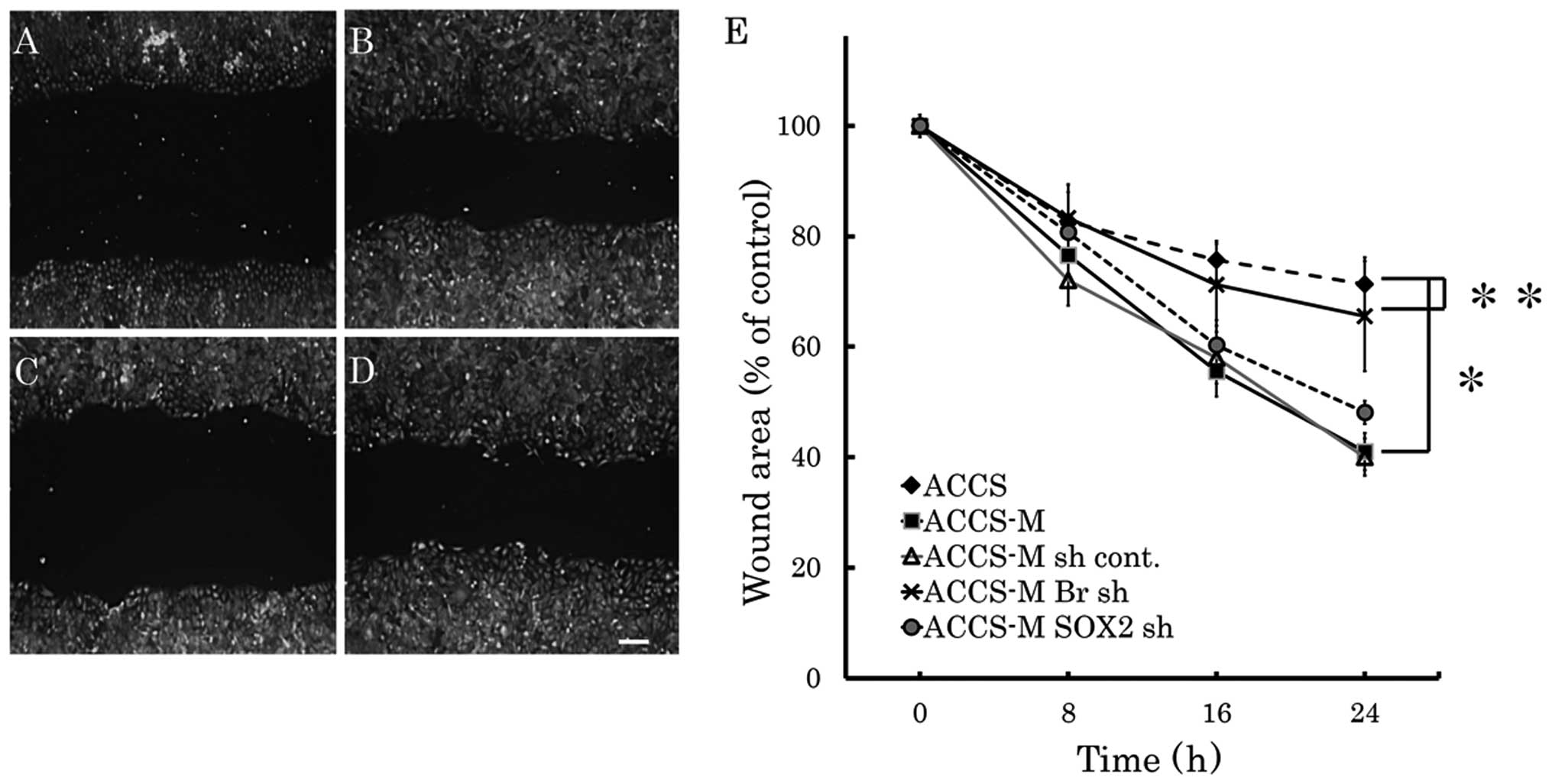
Brachyury shRNA inhibits cell
migration
The effect of Brachyury or SOX2
knockdown on cell migration in vitro was analyzed by the
wound healing assay (Fig. 2). Cell
migration of ACCS-M GFP cells was approximately twice as fast as
that of ACCS GFP cells. Brachyury knockdown significantly
inhibited migration of ACCS-M GFP cells to the level of parental
ACCS GFP (P=0.001). By contrast, SOX2 knockdown had no
effect on ACCS-M GFP cell migration.
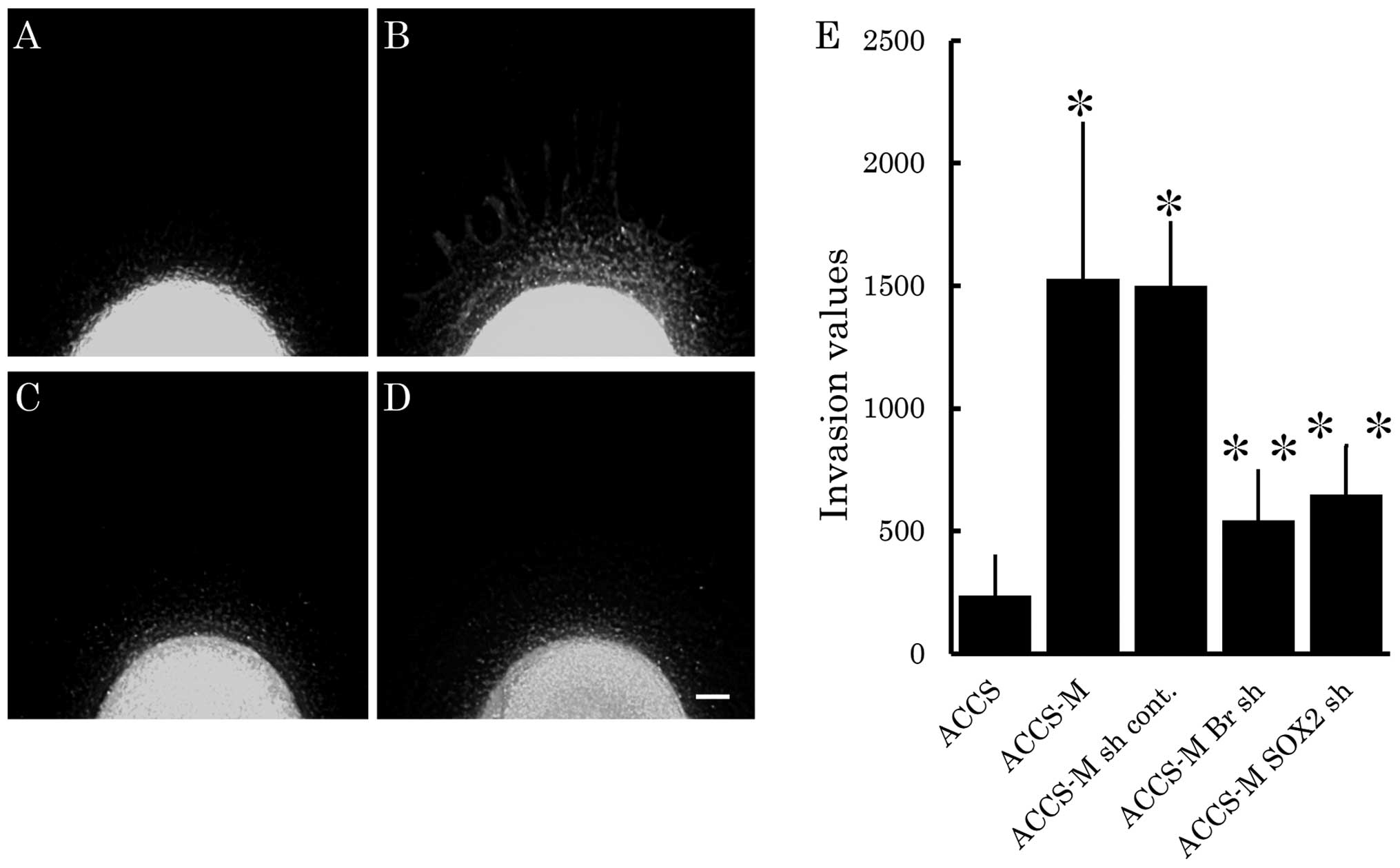
Brachyury and SOX2 shRNA inhibit cell
invasion
We next analyzed the effect of Brachyury or
SOX2 knockdown on cell invasiveness in vitro using
our previously reported tumor-cell dissemination assay (25). In this assay, invasion of carcinoma
cells is visualized as small green fluorescent spots escaping from
a cell pellet that models the primary cancer nest. Therefore, we
evaluated cancer cell invasion by the number of invasive cells and
their distance from the artificial primary cancer nest. As shown in
Fig. 3B, ACCS-M GFP cells
demonstrated aggressive cell invasion into artificial stromal
tissue. Invasiveness of ACCS-M GFP cells was strongly inhibited by
knockdown of Brachyury (Fig.
3C) or SOX2 (Fig. 3D).
Fig. 3E compares invasiveness
among ACCS cell lines. Relative invasiveness values (ACCS GFP = 1)
were 6.4 (ACCS-M GFP), 2.3 (ACCS-M shBr GFP), and 3.2 (ACCS-M
shSOX2 GFP).
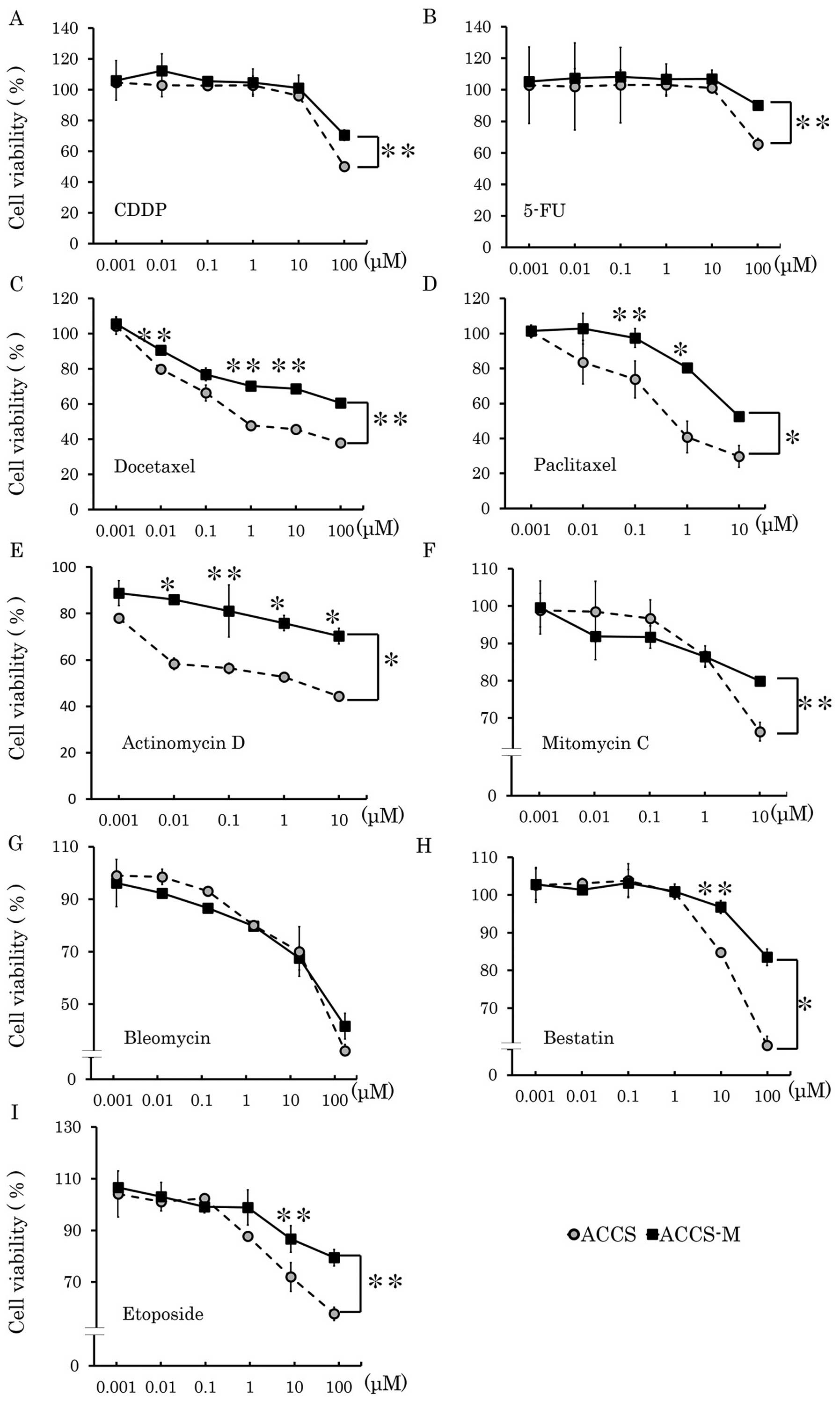
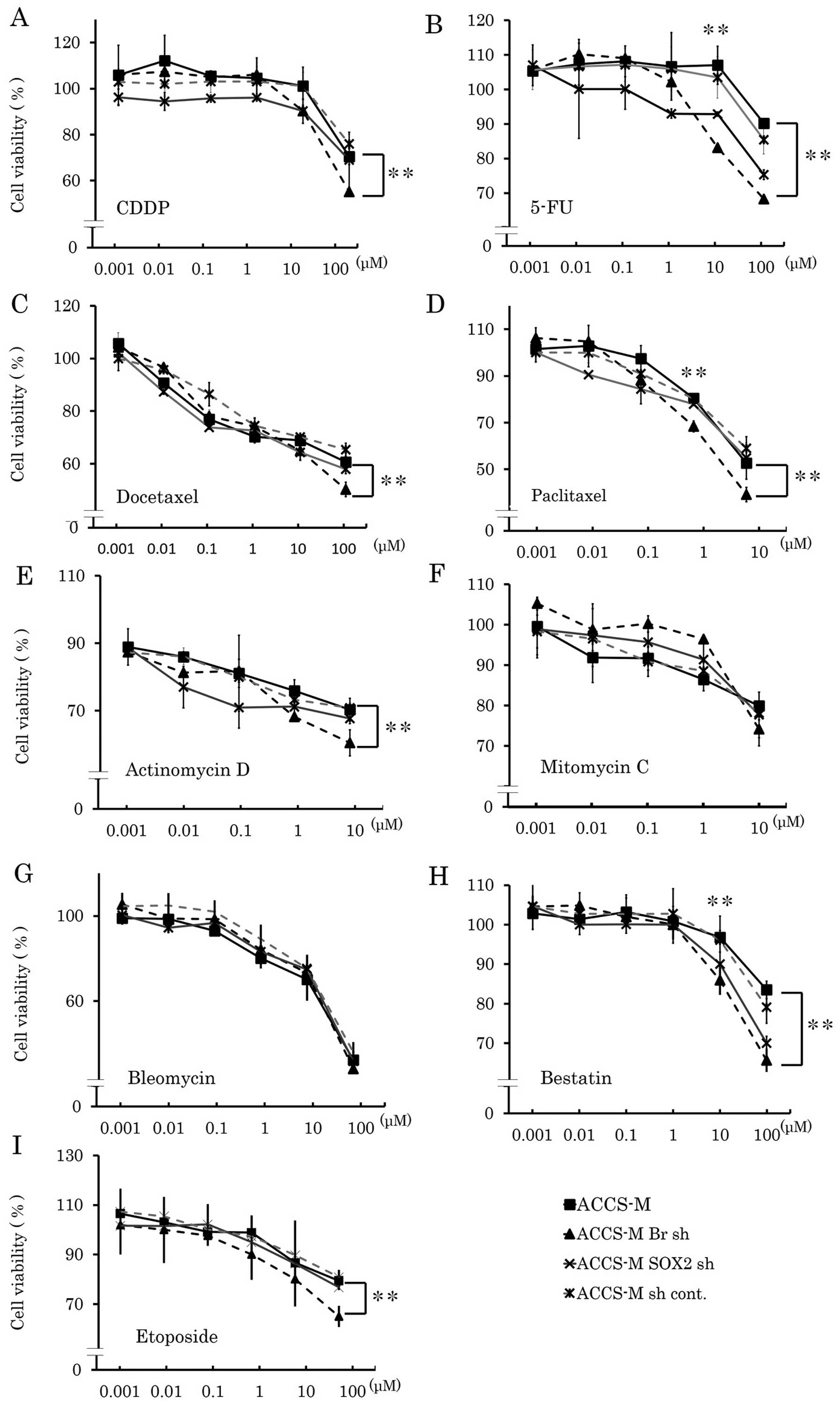
Brachyury and SOX2 shRNA induce
chemosensitivity in vitro
Cancer stem cells are known to resist various types
of anticancer drugs. Therefore, we next assessed whether knockdown
of cancer stem cell regulators could change their sensitivity to
anticancer drugs. ACCS-M GFP cells demonstrated chemoresistance to
CDDP, docetaxel, actinomycin D, etoposide, 5-FU, paclitaxel,
mitomycin C and bestatin (Fig. 4).
Table I shows the IC50
values of each anticancer drug in each ACCS cell line. The
IC50 values of ACCS-M GFP cells were higher than those
of ACCS GFP cells for each anticancer agent (range: 1.2–355-fold).
In particular, resistance to taxane drugs, docetaxel and
paclitaxel, was very high (355- and 23-fold of ACCS GFP
IC50 values, respectively). Brachyury shRNA and SOX2
shRNA reduced chemoresistance of ACCS-M GFP cells, but the effect
of Brachyury shRNA was greater than that of SOX2 shRNA (Fig. 1 and Table I). Relative IC50 values
(ACCS-M GFP=1) of Brachyury shRNA were ∼0.33 (docetaxel) to 0.85
(mitomycin C and bleomycin), and those of SOX2 shRNA were 0.59
(bestatin) to 1 (paclitaxel, mytomycin C, and bleomycin). However,
with the exception of bestatin, the degree of reduction did not
reach parental ACCS GFP levels (Table
I). We could not determine an IC50 for etoposide,
because ACCS cell lines showed strong resistance, and the maximum
dose of etoposide (1,000 μM) did not reduce cell viability
to 50%.
 | Table I.Measured IC50 (μM)
of each anticancer drug. |
Table I.
Measured IC50 (μM)
of each anticancer drug.
| IC50
(μM)
|
|---|
| Anticancer
drug | ACCS | ACCS-M | ACCS-M sh
cont. | Br sh | SOX2 sh |
|---|
| Cisplatin | 353.4 | 527.6 | 529.1 | 360.2 | 403.4 |
| 5-Fluorouracil | 463.5 | 842.9 | 835.1 | 623.7 | 673.6 |
| Docetaxel | 0.9 | 320.5 | 315.8 | 104.5 | 248.5 |
| Paclitaxel | 0.75 | 17.3 | 18.5 | 6.7 | 18.8 |
| Actinomycin D | 3.9 | 43.6 | 42.1 | 27.5 | 35.5 |
| Mitomycin C | 33.8 | 49.8 | 47.1 | 40.2 | 48.2 |
| Bleomycin | 41.1 | 57.8 | 58.6 | 50.2 | 55.8 |
| Bestatin | 459 | 558.8 | 549.3 | 306.7 | 325 |
| Etoposide | N.D. | N.D. | N.D. | N.D. | N.D. |
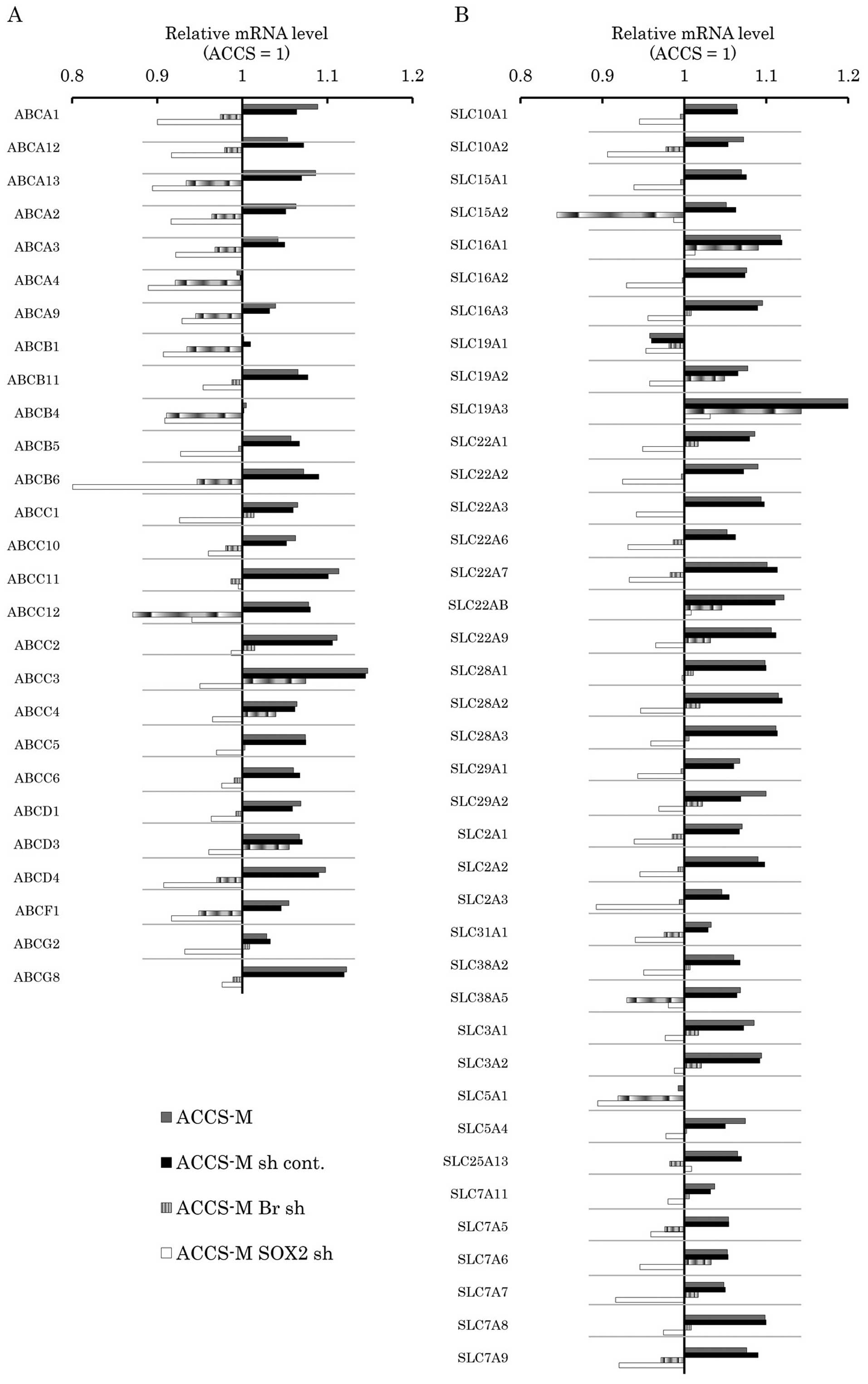
Brachyury and SOX2 shRNA modify
expression of drug-transporter genes in vitro
Multidrug resistance in cancer is heavily dependent
on 2 major super families of membrane transporter proteins that
influence the pharmacokinetics of drugs, ATP-binding cassette (ABC)
transporters and solute-carrier (SLC) transporters. Therefore, we
analyzed the effect of Brachyury and SOX2 knockdown
on the expression levels of these membrane transporters by
real-time PCR array. The differences between ACCS-M GFP and ACCS
GFP in expression of these membrane transporters were not
significant (1.05–1.2-fold). Notably, ACCS-M GFP cells generally
expressed higher levels of ABC transporter genes than ACCS GFP
cells, and this expression was reduced by Brachyury or
SOX2 knockdown (Fig. 6A).
SLC transporter genes were also generally expressed at higher
levels in ACCS-M GFP cells with the exception of SLC19A1 and
SLC5A1. Brachyury knockdown increased the expression of only
one SLC transporter gene, SLC19A1 (Fig. 6B).
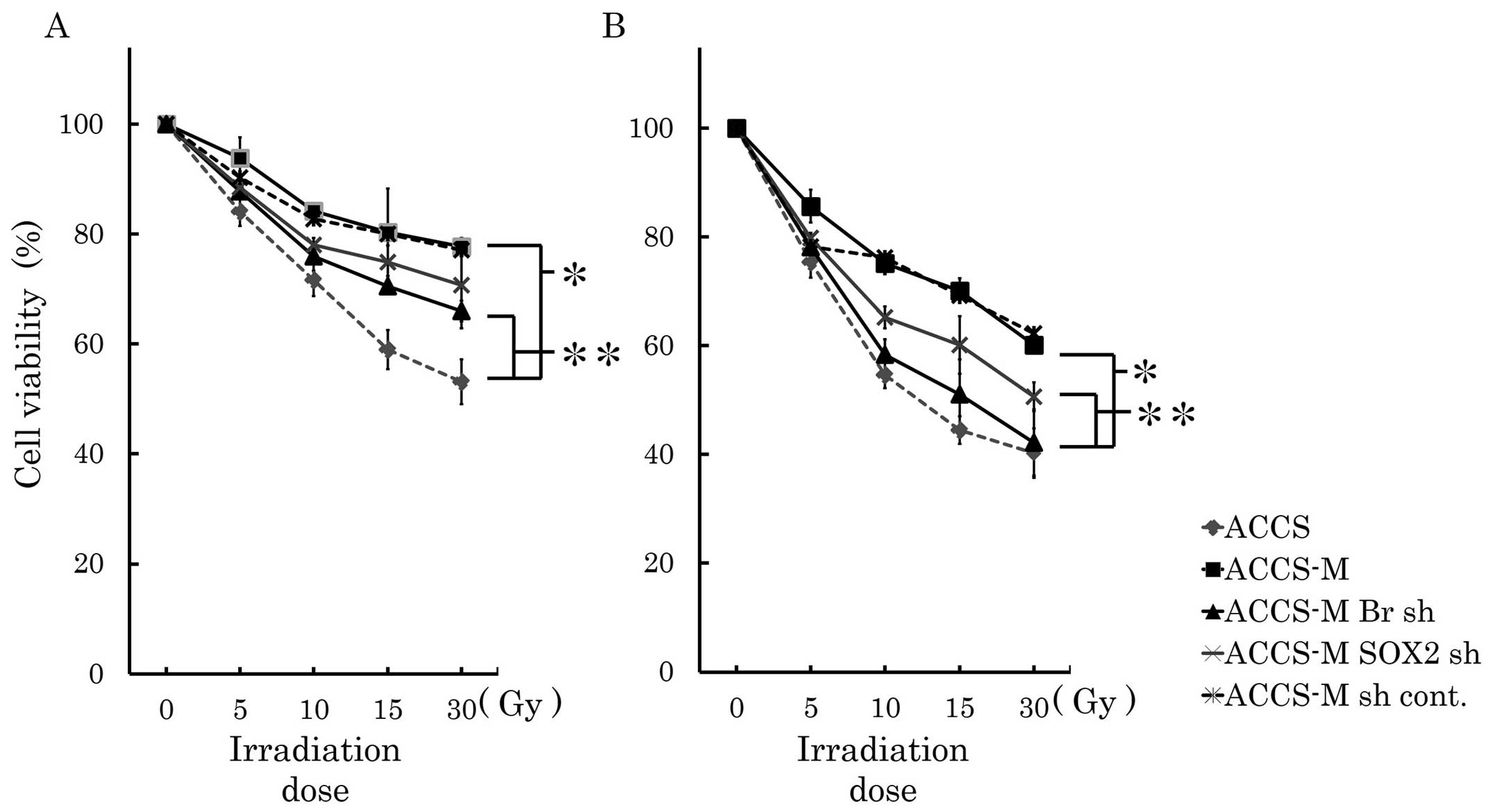
Brachyury and SOX2 shRNA induce radio
sensitivity in vitro
Cancer stem cells are insensitive to radiation. We
analyzed sensitivity to radiation treatment in vitro and
found that ACCS-M GFP cells were significantly more resistant to
irradiation than ACCS GFP cells (P<0.001). The viability of ACCS
GFP and ACCS-M GFP cells was 53 and 77%, respectively, 48 h after
30-Gy irradiation and 40 and 60% 72 h after 30-Gy irradiation,
respectively. Brachyury and SOX2 knockdown reduced
cell viability 72 h after 30-Gy irradiation. Brachyury
knockdown reduced cell viability upon irradiation significantly
more than SOX2 knockdown (P<0.05) and increased the
radiosensitivity of ACCS-M GFP to the level of ACCS GFP cells
(Fig. 7).
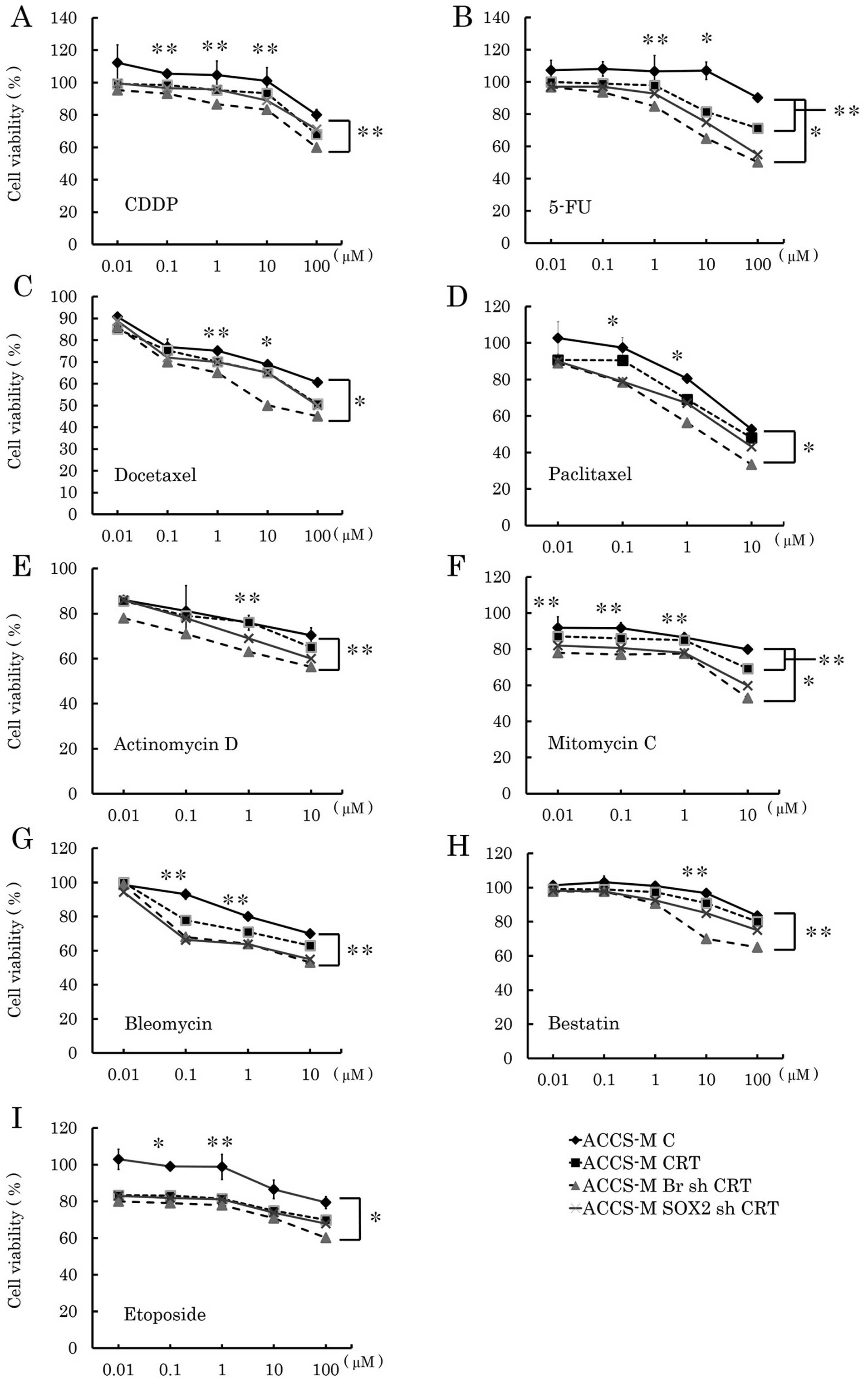
Brachyury and SOX2 shRNA enhance the
cytotoxicity of concurrent chemoradiation treatment in vitro
We analyzed the effect of Brachyury and SOX2 shRNA
on concurrent chemoradiation treatment (CRT) in vitro to
assess the clinical potential of Brachyury and SOX2 shRNA. Fig. 8 compares anticancer drugs with or
without radiation treatment. Brachyury and SOX2 shRNA demonstrated
significantly affected CRT. Brachyury shRNA was more effective than
SOX2 shRNA and significantly affected 5-FU, docetaxel, paclitaxel,
mitomycin C, and etoposide CRT (P<0.001). Notably, Brachyury
shRNA was also significantly effective for CRT with bleomycin and
mitomycin C (both P<0.05), while Brachyury shRNA did not affect
any single chemotherapy treatment.
Discussion
Clinically, oral AdCC is resistant to chemotherapy
and radiotherapy, which poses a major obstacle to treatment. CSCs
may contribute to chemo- and radioresistance, and new cancer
therapies targeting CSCs are under investigation (27–29).
Recently, we reported that T-box transcription factor Brachyury is
a putative factor underlying the EMT and CSC sternness of AdCC
in vitro and metastasis in vivo and that
Brachyury knockdown inhibits AdCC tumorigenicity and
metastasis (4). Therefore,
clinical application of Brachyury knockdown may be effective
for inhibiting cancer metastasis. It remains to be determined
whether Brachyury knockdown in pre-existing cancer reduces
the invasiveness of CSCs in the primary nest and increases their
sensitivity to chemo- and radiotherapy.
Cellular invasiveness and migration were markedly
higher in ACCS-M GFP than in ACCS GFP cells. Brachyury
knockdown completely inhibited cellular invasiveness and migration,
while SOX2 knockdown did not. Activation of cellular
invasiveness is an important characteristic of EMT. Matrix
metalloproteinases (MMPs) are upregulated in EMT and induce
cellular invasion of cancer cells (30–32).
Moreover, EMT-related MMP-9 upregulation degrades cell-surface
E-cadherin (33), an important
phenotype of EMT. Thus, Brachyury knockdown inhibited not
only tumorigenicity and metastasis, but also cancer cell invasion
at the primary site. This finding suggests that Brachyury knockdown
can inhibit cancer cell invasiveness of pre-existing cancers.
CSCs are chemoresistant. Similarly, ACCS-M GFP cells
demonstrated resistance to all tested anticancer drugs except
bleomycin. The mechanism underlying chemoresistance involves drug
transporters. Genetic variations in efflux transporters of the ABC
family, such as ABCB1 (MDR1, P-glycoprotein), ABCC1
(MRP1), ABCC2 (MRP2), and ABCG2 (BCRP), and uptake
transporters of the SLC family, such as SLC19A1 (RFC1) and
SLC01B1 (SLC21A6), are implicated in resistance to
chemotherapy (34,35). We found that nearly all ABC family
genes were expressed at higher levels in ACCS-M GFP than in ACCS
GFP cells, and this difference in expression may underlie the
chemoresistance of ACCS-M GFP cells. However, the degree of
upregulation of these genes was not large (∼5–15%), suggesting that
another crucial factor underlies the chemoresistance of ACCS-M
GFP.
Brachyury and SOX2 knockdown inhibited
the expression of ABC family genes. The SOX2 gene enhances
ABCC3 and ABCC6 expression through direct
transcriptional regulation (36).
Brachyury knockdown inhibited SOX2 expression.
Therefore, Brachyury may indirectly inhibit ABC transporter
genes thorough SOX2 downregulation. By contrast, SLC family
genes were upregulated in ACCS-M GFP cells, suggesting that drug
uptake into cancer cells is induced in ACCS-M GFP cells. This
finding contradicts the observed chemoresistance of ACCS-M GFP.
However, only SLC19A1 (RFC1), the most relevant gene in
chemoresistance (37,38), was decreased in ACCS-M GFP cells,
which indicates that drug uptake was inhibited. Furthermore,
Brachyury knockdown recovered expression of SLC19A1
(RFC1) to its level in ACCS GFP cells, indicating that
Brachyury knockdown induced drug uptake.
Another possible explanation for the drug resistance
of CSCs is the characteristic proportion of CSCs at each stage of
the cell cycle, because various anticancer drugs are cell
cycle-dependent. CSCs have a significantly higher proportion of
cells in the G2-phase of the cell cycle (39). Bleomycin, a glycopeptide antibiotic
with a unique mechanism of antitumor activity, has
G2-phase-specific cytotoxicity (40). Our results showed that only the
cytotoxicity of bleomycin was unchanged by Brachyury
knockdown in ACCS-M GFP cells. By contrast, ACCS-M GFP cells
demonstrated resistance to the cell cycle-specific anticancer drugs
5-FU [S-phase (41)], etoposide
[S/G2-phase (42)], and taxanes
[docetaxel and paclitaxel, G2/M-phase (43)], which was reduced by
Brachyury knockdown. These results suggest that CSC
resistance to cell cycle-specific anticancer agents is partially
regulated by G2-phase elongation in CSCs and that Brachyury
knockdown can break the cell cycle arrest in CSCs.
Cancer stem-like cells are relatively radioresistant
owing to intrinsic and extrinsic factors, including quiescence,
radiation-response mechanisms (e.g., enhanced DNA repair,
upregulated cell cycle-control mechanisms, and increased
free-radical scavengers), and a microenvironment that enhances cell
survival mechanisms (e.g., hypoxia and interaction with stromal
elements) (44). Therefore, the
same mechanisms of cell cycle regulation underlying chemoresistance
of ACCS-M GFP or CSCs may contribute to radioresistance and the
radiosensitizing effect of Brachyury knockdown. In radiation
biology, cells in the late S-phase are especially resistant, and
cells in the G2/M-phase are the most sensitive, to ionizing
radiation (45). CSCs in the
breast cancer cell line MDA-MB231 are shifted to the S- and
G2-phases and are radioresistant. Cyclin D and E protein levels are
consistent with this profile, suggesting the involvement of
homologous recombination repair in the radioresistant phenotype
(46). Therefore, cell cycle
regulation in ACCS-M GFP cells may be a key factor underlying
radioresistance. This mechanism is an important area of future
investigation.
Clinically, CRT and multidrug chemotherapy reduce
cancer cell viability by complementarily targeting cellular
vulnerabilities. However, CSCs survive these treatments, because
they do not target CSC cell cycle regulation. As shown in Fig. 8, Brachyury knockdown
significantly enhanced the effect of CRT in vitro for all
tested anticancer drugs to which cells were resistant as a single
drug. These data support the conclusion from our previous study
that Brachyury knockdown forcibly differentiates CSCs,
causing them to lose their sternness. Furthermore, the effect of
Brachyury knockdown was significantly stronger than that of
SOX2, a conventional stem cell regulatory gene. Multiple
regulatory genes are believed to regulate cell sternness. However,
we have shown that knockdown of a single gene, Brachyury,
silenced multiple regulatory genes simultaneously. Hence,
Brachyury knockdown may be an important therapeutic approach
and should be further investigated for clinical use.
In conclusion, this study presents evidence that
Brachyury knockdown reduces the invasiveness and chemo- and
radio-resistance of CSCs in vivo and suggests that
Brachyury knockdown is a useful therapeutic tool for
sensitizing CSCs to conventional chemoradiotherapy.
Acknowledgements
This study was supported by
Grants-in-Aid from the Ministry of Education, Culture, Sports,
Science, and Technology of Japan (KAKEN no. 23390465 to T. Sugiura
and 25861958 to Y. Kobayashi). We would like to thank Dr Manabu
Kawata (Institute of Microbial Chemistry, Numazu Microbial
Chemistry Research Foundation) for providing standard anticancer
drug kits.
References
|
1.
|
Rapidis AD, Givalos N, Gakiopoulou H, et
al: Adenoid cystic carcinoma of the head and neck.
Clinicopathological analysis of 23 patients and review of the
literature. Oral Oncol. 41:328–335. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
2.
|
Ampil FL and Misra RP: Factors influencing
survival of patients with adenoid cystic carcinoma of the salivary
glands. J Oral Maxillofac Surg. 45:1005–1010. 1987. View Article : Google Scholar : PubMed/NCBI
|
|
3.
|
Ishii K, Shimoda M, Sugiura T, et al:
Involvement of epithelial-mesenchymal transition in adenoid cystic
carcinoma metastasis. Int J Oncol. 38:921–931. 2011.PubMed/NCBI
|
|
4.
|
Shimoda M, Sugiura T, Imajyo I, et al: The
T-box transcription factor Brachyury regulates
epithelial-mesenchymal transitions in association with cancer
stem-like cells in adenoid cystic carcinoma cells. BMC Cancer.
12:3772012. View Article : Google Scholar
|
|
5.
|
Grunert S, Jechlinger M and Beug H:
Diverse cellular and molecular mechanisms contribute to epithelial
plasticity and metastasis. Nat Rev Mol Cell Biol. 4:657–665. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
6.
|
Cano A, Perez-Moreno MA, Rodrigo I, et al:
The transcription factor snail controls epithelial-mesenchymal
transitions by repressing E-cadherin expression. Nat Cell Biol.
2:76–83. 2000. View
Article : Google Scholar : PubMed/NCBI
|
|
7.
|
Yang MH, Hsu DS, Wang HW, et al: Bmil is
essential in Twistl-induced epithelial-mesenchymal transition. Nat
Cell Biol. 12:982–992. 2010. View
Article : Google Scholar
|
|
8.
|
Kispert A, Herrmann BG, Leptin M and
Reuter R: Homologs of the mouse Brachyury gene are involved in the
specification of posterior terminal structures in Drosophila,
Tribolium, and Locusta. Genes Dev. 8:2137–2150. 1994.
View Article : Google Scholar : PubMed/NCBI
|
|
9.
|
Behr R, Heneweer C, Viebahn C, Denker HW
and Thie M: Epithelial-mesenchymal transition in colonies of rhesus
monkey embryonic stem cells: a model for processes involved in
gastrulation. Stem Cells. 23:805–816. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
10.
|
Vidricaire G, Jardine K and McBurney MW:
Expression of the Brachyury gene during mesoderm development in
differentiating embryonal carcinoma cell cultures. Development.
120:115–122. 1994.PubMed/NCBI
|
|
11.
|
Fernando RI, Litzinger M, Trono P,
Hamilton DH, Schlom J and Palena C: The T-box transcription factor
Brachyury promotes epithelial-mesenchymal transition in human tumor
cells. J Clin Invest. 120:533–544. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
12.
|
Reya T, Morrison SJ, Clarke MF and
Weissman IL: Stem cells, cancer, and cancer stem cells. Nature.
414:105–111. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
13.
|
Thiery JP: Epithelial-mesenchymal
transitions in development and pathologies. Curr Opin Cell Biol.
15:740–746. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
14.
|
Mani SA, Guo W, Liao MJ, et al: The
epithelial-mesenchymal transition generates cells with properties
of stem cells. Cell. 133:704–715. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
15.
|
Blick T, Hugo H, Widodo E, et al:
Epithelial mesenchymal transition traits in human breast cancer
cell lines parallel the CD44(hi/)CD24 (lo/-) stem cell phenotype in
human breast cancer. J Mammary Gland Biol Neoplasia. 15:235–252.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
16.
|
Huang HP, Chen PH, Yu CY, et al:
Epithelial cell adhesion molecule (EpCAM) complex proteins promote
transcription factor-mediated pluripotency reprogramming. J Biol
Chem. 286:33520–33532. 2011. View Article : Google Scholar
|
|
17.
|
Utikal J, Maherali N, Kulalert W and
Hochedlinger K: Sox2 is dispensable for the reprogramming of
melanocytes and melanoma cells into induced pluripotent stem cells.
J Cell Sci. 122:3502–3510. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
18.
|
Ahmed N, Abubaker K, Findlay J and Quinn
M: Epithelial mesenchymal transition and cancer stem cell-like
phenotypes facilitate chemoresistance in recurrent ovarian cancer.
Curr Cancer Drug Targets. 10:268–278. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
19.
|
Alison MR, Lim SM and Nicholson LJ: Cancer
stem cells: problems for therapy? J Pathol. 223:147–161.
2010.PubMed/NCBI
|
|
20.
|
Wang Z, Li Y, Ahmad A, Azmi AS, Kong D,
Banerjee S and Sarkar FH: Targeting miRNAs involved in cancer stem
cell and EMT regulation: an emerging concept in overcoming drug
resistance. Drug Resist Updat. 13:109–118. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
21.
|
Raimondi C, Gianni W, Cortesi E and
Gazzaniga P: Cancer stem cells and epithelial-mesenchymal
transition: revisiting minimal residual disease. Curr Cancer Drug
Targets. 10:496–508. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
22.
|
Herrmann BG, Labeit S, Poustka A, King TR
and Lehrach H: Cloning of the T gene required in mesoderm formation
in the mouse. Nature. 343:617–622. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
23.
|
Edwards YH, Putt W, Lekoape KM, Stott D,
Fox M, Hopkinson DA and Sowden J: The human homolog T of the mouse
T(Brachyury) gene; gene structure, cDNA sequence, and assignment to
chromosome 6q27. Genome Res. 6:226–233. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
24.
|
Sarkar D, Shields B, Davies ML, Muller J
and Wakeman JA: BRACHYURY confers cancer stem cell characteristics
on colorectal cancer cells. Int J Cancer. 130:328–337. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
25.
|
Abe M, Sugiura T, Takahashi M, Ishii K,
Shimoda M and Shirasuna K: A novel function of CD82/KAI-1 on
E-cadherin-mediated homophilic cellular adhesion of cancer cells.
Cancer Lett. 266:163–170. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
26.
|
Brown AM: A step-by-step guide to
non-linear regression analysis of experimental data using a
Microsoft Excel spreadsheet. Comput Methods Programs Biomed.
65:191–200. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
27.
|
Hsu HS, Huang PI and Chang YL: et al
Cucurbitacin I inhibits tumorigenic ability and enhances
radiochemosensitivity in nonsmall cell lung cancer-derived
CD133-positive cells. Cancer. 117:2970–2985. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
28.
|
Cheung ST, Cheung PF, Cheng CK, Wong NC
and Fan ST: Granulin-epithelin precursor and ATP-dependent binding
cassette (ABC)B5 regulate liver cancer cell chemoresistance.
Gastroenterology. 140:344–355. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
29.
|
Chuthapisith S, Eremin J, El-Sheemey M and
Eremin O: Breast cancer chemoresistance: emerging importance of
cancer stem cells. Surg Oncol. 19:27–32. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
30.
|
Hayashi Y, Osanai M and Lee GH: Fascin-1
expression correlates with repression of E-cadherin expression in
hepatocellular carcinoma cells and augments their invasiveness in
combination with matrix metalloproteinases. Cancer Sci.
102:1228–1235. 2011. View Article : Google Scholar
|
|
31.
|
Lin CY, Tsai PH, Kandaswami CC, Lee PP,
Huang CJ, Hwang JJ and Lee MT: Matrix metalloproteinase-9
cooperates with transcription factor Snail to induce
epithelial-mesenchymal transition. Cancer Sci. 102:815–827. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
32.
|
Zhang K, Chen D, Jiao X, et al: Slug
enhances invasion ability of pancreatic cancer cells through
upregulation of matrix metalloproteinase-9 and actin cytoskeleton
remodeling. Lab Invest. 91:426–438. 2011. View Article : Google Scholar
|
|
33.
|
Zuo JH, Zhu W, Li MY, et al: Activation of
EGFR promotes squamous carcinoma SCC10A cell migration and invasion
via inducing EMT-like phenotype change and MMP-9-mediated
degradation of E-cadherin. J Cell Biochem. 112:2508–2517. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
34.
|
Huang Y and Sadee W: Membrane transporters
and channels in chemoresistance and -sensitivity of tumor cells.
Cancer Lett. 239:168–182. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
35.
|
Huang Y: Pharmacogenetics/genomics of
membrane transporters in cancer chemotherapy. Cancer Metastasis
Rev. 26:183–201. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
36.
|
Jeon HM, Sohn YW, Oh SY, Kim SH, Beck S,
Kim S and Kim H: ID4 imparts chemoresistance and cancer sternness
to glioma cells by derepressing miR-9*-mediated suppression of
SOX2. Cancer Res. 71:3410–3421. 2011.PubMed/NCBI
|
|
37.
|
Yang R, Li WW, Hoang BH, et al:
Quantitative correlation between promoter methylation and messenger
RNA levels of the reduced folate carrier. BMC Cancer. 8:1242008.
View Article : Google Scholar : PubMed/NCBI
|
|
38.
|
Matherly LH, Hou Z and Deng Y: Human
reduced folate carrier: translation of basic biology to cancer
etiology and therapy. Cancer Metastasis Rev. 26:111–128.
2007.PubMed/NCBI
|
|
39.
|
Harper LJ, Costea DE, Gammon L, Fazil B,
Biddle A and Mackenzie IC: Normal and malignant epithelial cells
with stem-like properties have an extended G2 cell cycle phase that
is associated with apoptotic resistance. BMC Cancer.
10:1662010.PubMed/NCBI
|
|
40.
|
Dorr RT: Bleomycin pharmacology: mechanism
of action and resistance, and clinical pharmacokinetics. Semin
Oncol. 19:3–8. 1992.PubMed/NCBI
|
|
41.
|
Song B, Wang Y, Xi Y, et al: Mechanism of
chemoresistance mediated by miR-140 in human osteosarcoma and colon
cancer cells. Oncogene. 28:4065–4074. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
42.
|
Lin CK, Nguyen TT, Morgan TL, et al:
Apoptosis may be either suppressed or enhanced with strategic
combinations of antineoplastic drugs or anti-IgM. Exp Cell Res.
244:1–13. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
43.
|
O’Leary J, Volm M, Wasserheit C and Muggia
F: Taxanes in adjuvant and neoadjuvant therapies for breast cancer.
Oncology (Williston Park). 12:23–27. 1998.
|
|
44.
|
Hittelman WN, Liao Y, Wang L and Milas L:
Are cancer stem cells radioresistant? Future Oncol. 6:1563–1576.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
45.
|
Pajonk F, Vlashi E and McBride WH:
Radiation resistance of cancer stem cells: the 4 R’s of
radiobiology revisited. Stem Cells. 28:639–648. 2010.
|
|
46.
|
Al-Assar O, Mantoni T, Lunardi S, Kingham
G, Helleday T and Brunner TB: Breast cancer stem-like cells show
dominant homologous recombination due to a larger S-G2 fraction.
Cancer BiolTher. 11:1028–1035. 2011.PubMed/NCBI
|