Introduction
Despite a declining trend worldwide, colorectal
cancer still remains as the third most common cancer among men and
the second in women (1,2). More than one million new cases of
colorectal cancer are diagnosed every year (3). A wide variety of natural compounds
derived from edible plants have been shown to prevent colon
carcinogenesis (4). Carnosol, a
diterpene present in rosemary (Rosmarinus officinalis), has
been reported to prevent the development of intestinal tumors in
APC (adenomatous polyposis coli)min+/+ mice (5) and to induce apoptosis in human colon
cancer (COLO 205) cells (6).
However, the molecular mechanisms underlying the chemopreventive
and/or chemotherapeutic effects of carnosol in colon cancer are yet
to be fully elucidated. We, therefore, attempted to investigate the
effects of carnosol on human colon cancer (HCT116) cells and to
elucidate its underlying mechanisms.
One of the hallmarks of cancer is the evasion of
tumor cells from apoptosis (7).
Numerous naturally occurring polyphenols inhibit proliferation and
induce apoptosis in various cancer cells (8). Apoptosis is induced by two cellular
mechanisms: intrinsic (mitochondria-dependent) and extrinsic (death
receptor-mediated) signaling (9).
The intrinsic pathway of apoptosis involves the depolarization of
mitochondrial membrane, release of cytochrome c, sequential
activation of caspase-9, -7 and -3, and the cleavage of
poly-(ADP-ribose) polymerase (PARP). The extrinsic pathway, on the
other hand, is mediated through the activation of cell
membrane-bound death receptors, followed by the activation of
pro-caspase-8, which then execute cell death by triggering the
activity of caspase-3 (9,10).
Cancer cells are characterized by constitutively
elevated expression of a variety of anti-apoptotic proteins and the
diminished expression of pro-apoptotic proteins (11). The balance between the expression
of pro- versus anti-apoptotic proteins determines the cell fate.
Signal transducer and activator of transcription-3 (STAT3) is a
transcription factor that transactivates the genes encoding various
cell survival proteins, such as cyclins, survivin, Bcl-2 and Bcl-xl
(12,13). STAT3 is consitutively active in
cancer cells, and the aberrant activation of STAT3-mediated
signaling have been implicated in colon carcinogenesis (14–16).
In response to diverse growth stimulatory signals, STAT3 gets
activated through phosphorylation by upstream kinases, such as
janus-activated kinase-2 (Jak2) (16) and Src tyrosine kinase (17) followed by STAT3 dimerization and
nuclear localization. The blockade of STAT3 activation inhibits
cell proliferation and induces apoptosis. Thus, STAT3 is a prime
target of many anticancer agents (12). Herein we report, for the first
time, that carnosol induces apoptosis in HCT116 cells through
inactivation of STAT3 signaling pathway.
Materials and methods
Materials
Carnosol (purity 99%), N-acetyl cysteine
(NAC), etoposide, and β-actin antibody were purchased from
Sigma-Aldrich (St. Louis, MO, USA). AG490 and PP2 were purchased
from Cayman Chemical Co. (Ann Arbor, MI, USA). Antibodies against
cleaved caspase-9, and -3, PARP, Bcl-2, Bcl-xl, Bax, STAT3,
p-STAT3(Y705), Jak2, p-Jak2, Src, p-Src, cyclin-D1, -D2 and -D3,
and survivin were procured from Cell Signaling Technology Inc.
(Beverly, MA, USA). Primary antibody against each of p53, murine
double minute-2 (Mdm2), Lamin-A, and horseradish
peroxidase-conjugated secondary antibodies were purchased from
Santa Cruz Biotechnology, Inc. (Santa Cruz, CA, USA). The 2’-7’
dichlorofluorescin diacetate (DCF-DA) was procured from Invitrogen
(Carlsbad, CA, USA). Hank’s balanced salt solution (HBSS) was
purchased from Meditech (Herndon, VA, USA). All other chemicals
were of analytical or highest purity grade available.
Cell culture and treatment
HCT116 cells were obtained from American Type
Culture Collections and maintained in Dulbecco’s modified Eagle’s
medium (DMEM) supplemented with 10% fetal bovine serum and
antibiotics (100 U/ml penicillin G and 100 μg/ml
streptomycin) at 37°C in a humidified incubator containing 5%
CO2 and 95% air. In all the experiments, cells were
seeded at 2×105 cells/ml and incubated with carnosol at
50–60% confluence. All chemicals were dissolved in ethanol and the
final ethanol concentration was <1%.
Cell proliferation assay
The anti-proliferative effect of carnosol against
HCT116 cells was measured by using a solution of tetrazolium
compound
[3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium,
inner salt (MTS) (Promega, WI, USA). Briefly, cells
(2×103) were incubated in triplicate in a 96-well plate
in presence or absence of carnosol in a final volume of 0.1 ml for
different time intervals at 37°C. Thereafter, 20 μl of MTS
solution was added to each well and incubated for 60 min. The
number of viable cells was measured in a 96-well plate at an
optical density of 492 nm on a microplate reader (Tecan Trading AG,
Männedorf, Switzerland). Cell viability is described as the
relative percentage of control.
Annexin V staining
Annexin V staining was performed using FITC-Annexin
V staining kit (BD Biosciences, San Jose, CA, USA) following the
manufacturer’s instructions. Briefly, carnosol-treated cells were
washed with PBS and resuspended in binding buffer containing
Annexin V and propidium iodide (P.I.). Fluorescence intensity was
measured using flow cytometry (BD Biosciences).
Western blot analysis
Cells were harvested and lysed with RIPA buffer, and
collected protein samples were quantified by using bichinconinic
acid protein assay kit (Pierce Biotechnology, Rockford, IL, USA).
The protein samples were separated by sodium dodecyl
sulfate-polyacrylamide gel electrophoresis and immunoblot analysis
was done according to the protocol described earlier (18). Immunoblot membranes were incubated
with Super-signal pico-chemiluminescent substrate or dura-luminol
substrate (Thermo Scientific, MA, USA) according to manufacturer’s
instructions and visualized with ImageQuant™ LAS 4000 (Fujifilm
Life Science, Japan).
Caspase-3 activity assay
The activity of caspase-3 was detected using
Caspase-3 Colorimetric Activity Assay kit (Millipore, MA, USA). The
assay was performed in 96-well plates by incubating cell lysates
(50 μg) in 100 μl reaction buffer containing
caspase-3 substrate Ac-DEVD-pNA at 37°C for 2 h 30 min following
the method described earlier (18).
STAT3-Luciferase reporter gene assay
Cells were seeded into 12-well plates at a density
of 5×104 cells per well prior to transfection. Cells
were transfected with p-STAT3-TA-luc (Clontech, CA, USA) or control
vector using Genefectin transfection reagent (Genetrone Biotech,
Korea). After 24 h of trans fection, cells were treated with
carnosol for additional 24 h and cell lysis was carried out with 1X
reporter lysis buffer. After mixing the cell lysates with
luciferase substrate (Promega), the luciferase activity was
measured by a luminometer. The β-galactosidase assay was performed
according to the supplier’s instructions (Promega Enzyme Assay
System) for normalizing the luciferase activity and the results are
expressed as fold transactivation.
Preparation of cytosolic and nuclear
extracts
The cytosolic and nuclear extracts were prepared by
using NE-PER Nuclear and Cytoplasmic Extraction Reagent kit (Thermo
Scientific). Cells were washed with ice cold PBS, collected and
centrifuged at 1,600 × g for 15 min at 4°C. Pellets were suspended
in 50 μl of Cytoplasmic Extraction Reagent (CER)-I for 15
min, then CERII was added for 2 min. The mixture was centrifuged
for 10 min at 16,000 × g. Supernatant was collected as cytosolic
extract. The pellets were washed with Nuclear Extraction Reagent
and incubated on ice for 1 h and centrifuged at 16,000 × g for 15
min. The supernatant containing nuclear proteins was collected and
stored at −70°C after determination of protein concentration by
using Bradford Reagent (Bio-Rad Laboratories, Hercules, CA,
USA).
Electrophoretic mobility gel shift assay
(EMSA)
The EMSA for STAT3 DNA binding was performed using a
DNA-protein binding detection kit, according to the manufacturer’s
protocol (Gibco BRL, Grand Island, NY, USA). The nuclear extract
was prepared from cells incubated with or without carnosol. The
STAT3 oligonucleotide probe 5′-AGC TTC ATT TCC CGT AAA TCC CTA-3′
(Bioneer, Korea) was labeled with [γ-32P]-ATP and the
EMSA was performed according to the protocol described earlier
(19).
Measurement of the accumulation of
reactive oxygen species (ROS)
Cells were treated with carnosol in the presence or
absence of NAC for 24 h and then loaded with 25 μM of
DCF-DA. After incubation for 30 min at 37°C in a 5% CO2
incubator, cells were washed twice with HBSS solution, suspended in
the complete media and were examined under a fluorescence
microscope to detect the intracellular accumulation of ROS.
Statistical analysis
When necessary, data were expressed as mean ± SD of
at least three independent experiments, and statistical analysis
for single comparison was performed using the Student’s t-test and
a p-value <0.05 was considered as statistically significant.
Results
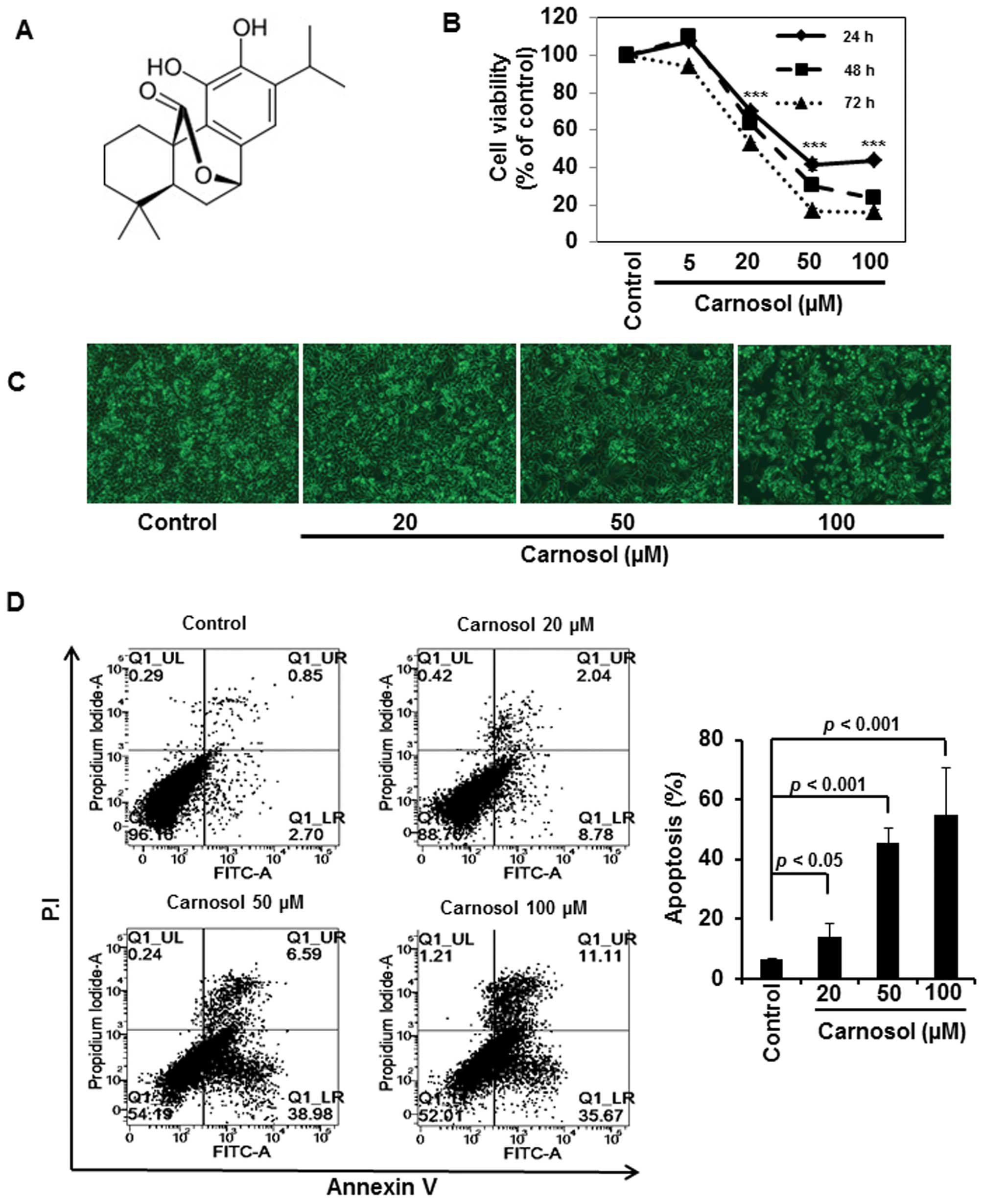
Carnosol induces apoptosis in HCT116
cells
We initially examined the effect of carnosol on the
viability of HCT116 cells. Incubation of cells with carnosol (5,
20, 50 or 100 μM) reduced the cell viability in a time- and
concentration-dependent manner (Fig.
1B). Analysis of cell morphology showed that carnosol treatment
induced apoptotic cell death (Fig.
1C), which was further verified by Annexin V and P.I. staining
of cells treated with indicated concentrations of carnosol. As
shown in Fig. 1D, carnosol induced
apoptosis in a concentration-dependent manner.
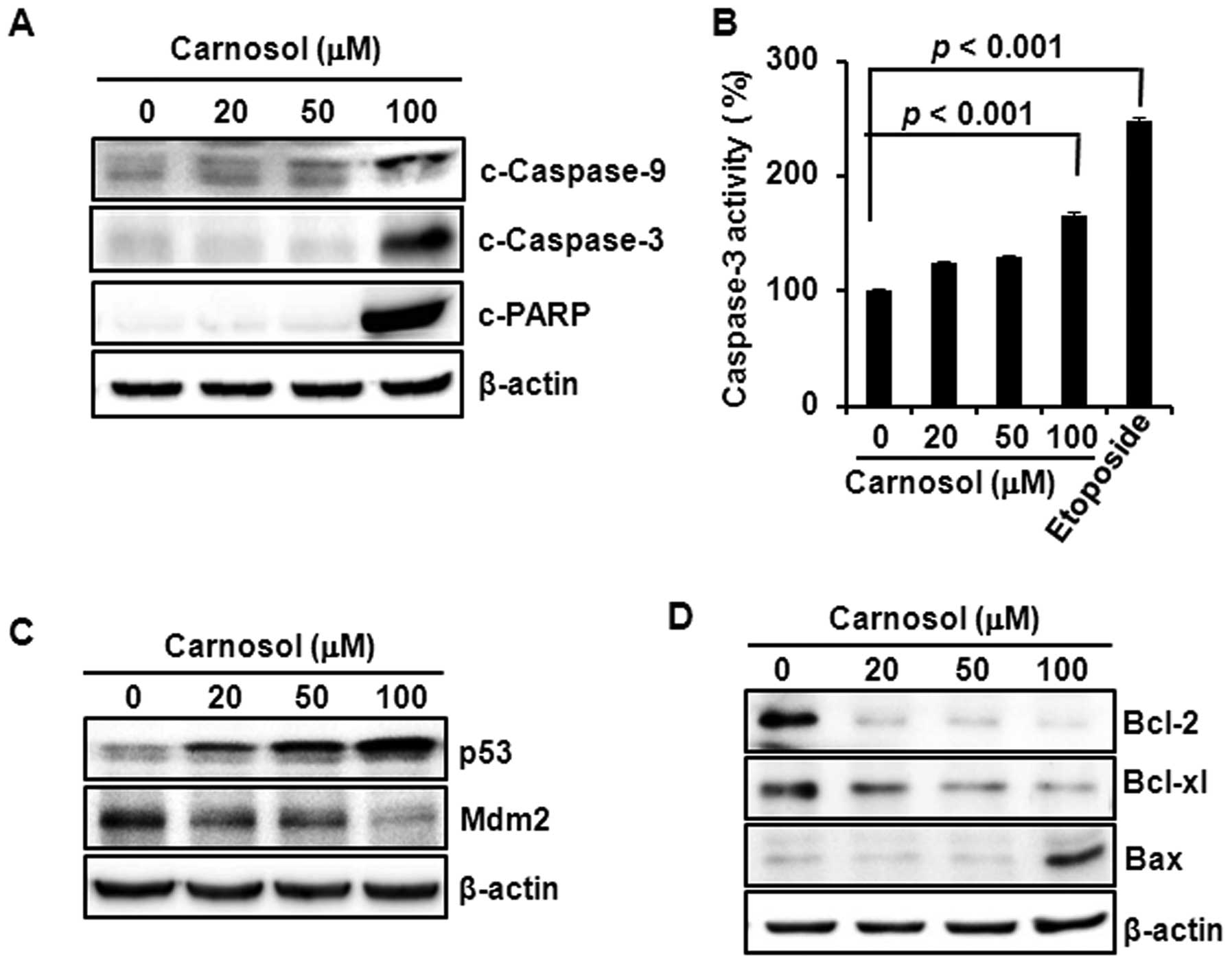
Carnosol-induced apoptosis is mediated
through caspase activation, p53 induction and the modulation of
Bcl-2/Bax expression
Treatment of HCT116 cells with carnosol induced the
cleavage of caspase-9, -3 and PARP (Fig. 2A) and increased the caspase-3
activity, which was comparable to that of the reference compound
etoposide (Fig. 2B). Incubation of
cells with carnosol increased the expression of p53 and diminished
the expression of Mdm2, that promotes p53 degradation through
proteasomal degradation, in a concentration-dependent manner
(Fig. 2C). Moreover, carnosol
reduced the expression of anti-apoptotic protein Bcl-2, while the
compound increased expression of pro-apoptotic protein Bax in
HCT116 cells (Fig. 2D).
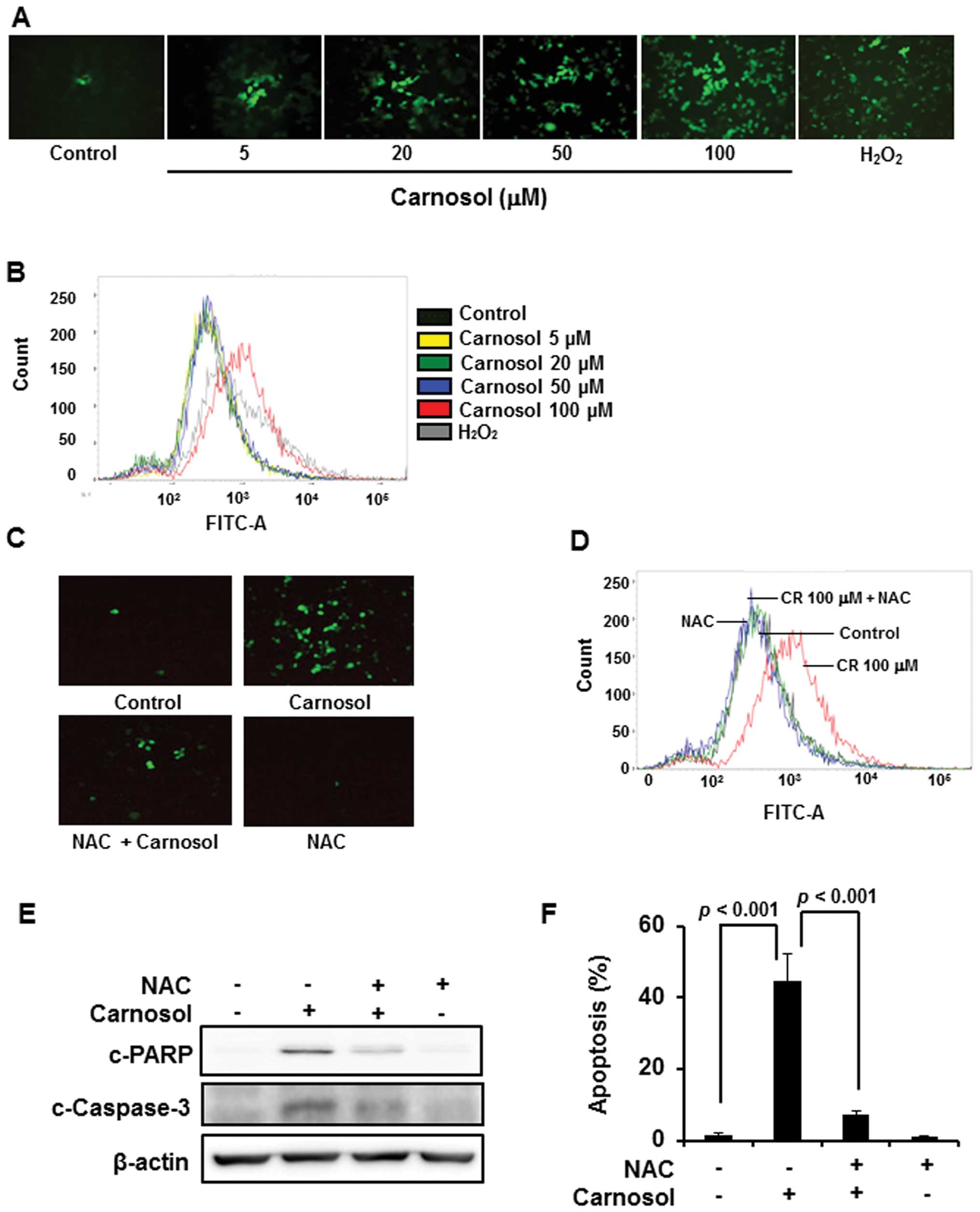
Involvement of ROS in carnosol-induced
apoptosis in HCT116 cells
Since accumulation of intracellular ROS induces cell
death, we examined the effect of carnosol on ROS generation.
Treatment of cells with carnosol (100 μM) generated ROS as
revealed by immunofluorescence analysis upon DCF-DA staining
(Fig. 3A) as well as by FACS
analysis (Fig. 3B), which was
abrogated by pretreatment of cells with NAC (Fig. 3C and D). Treatment of cells with
ROS scavenger NAC abrogated carnosol-induced cleavage of caspase-3
and PARP (Fig. 3E), and the
induction of apoptosis (Fig.
3F).
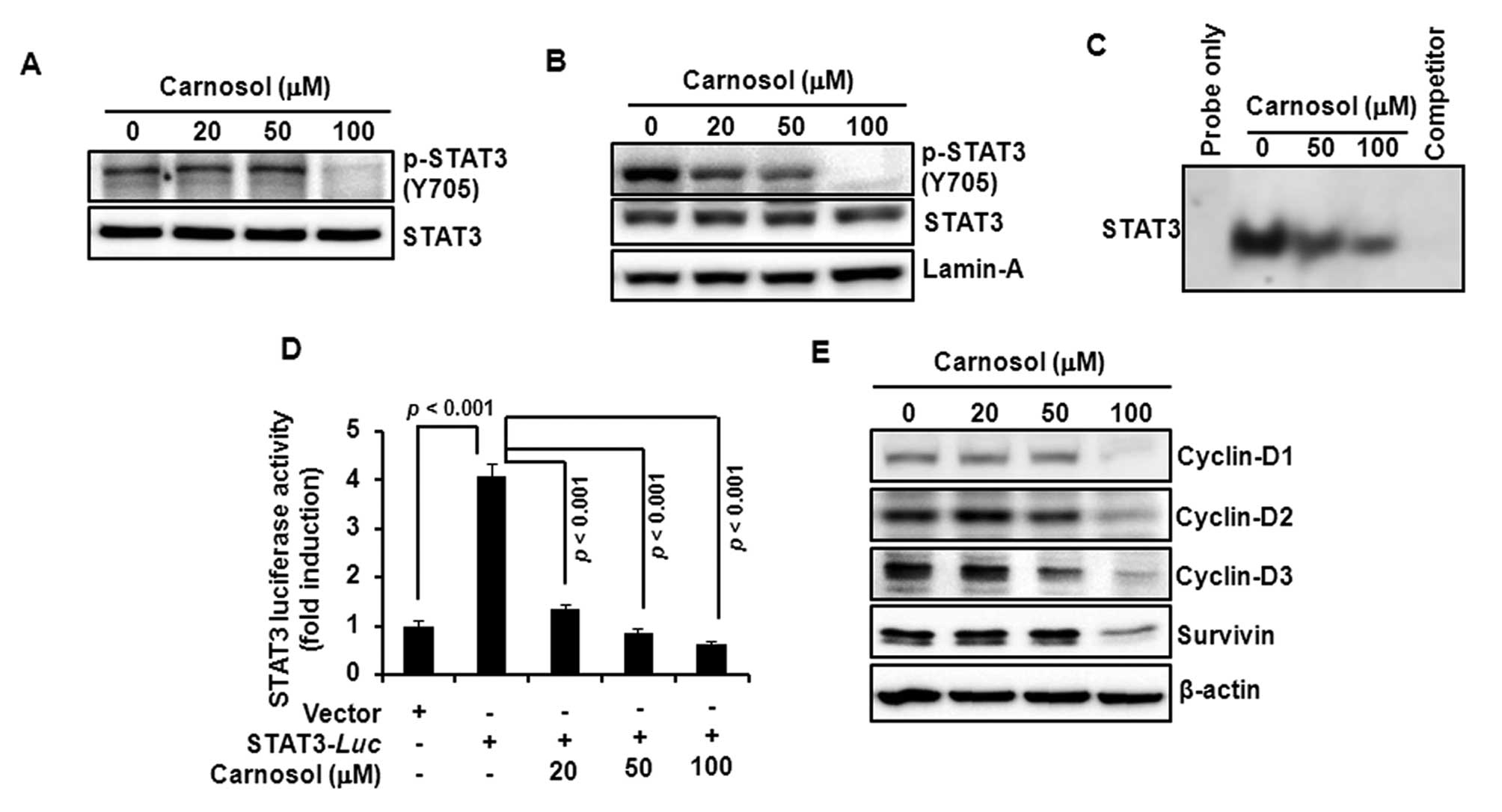
Carnosol attenuates the activation of
STAT3 and expression of its target gene products in HCT116
cells
Since STAT3 plays a key role in cell proliferation
through transcriptional activation of pro-survival genes, we
examined the effect of carnosol on STAT3 activation in HCT116
cells. Incubation with carnosol inhibited constitutive
phosphorylation of STAT3 at tyrosine705 residue (Fig. 4A) and decreased the nuclear
localization of phosphorylated STAT3 (Fig. 4B). Carnosol also reduced the
constitutive STAT3 DNA-binding activity (Fig. 4C) and the STAT3 reporter gene
activity in cells transiently transfected with STAT3-luc vector
(Fig. 4D). Moreover, carnosol
attenuated the expression of STAT3 target gene products, such as
cyclin-D1, -D2, -D3 and survivin (Fig.
4E).
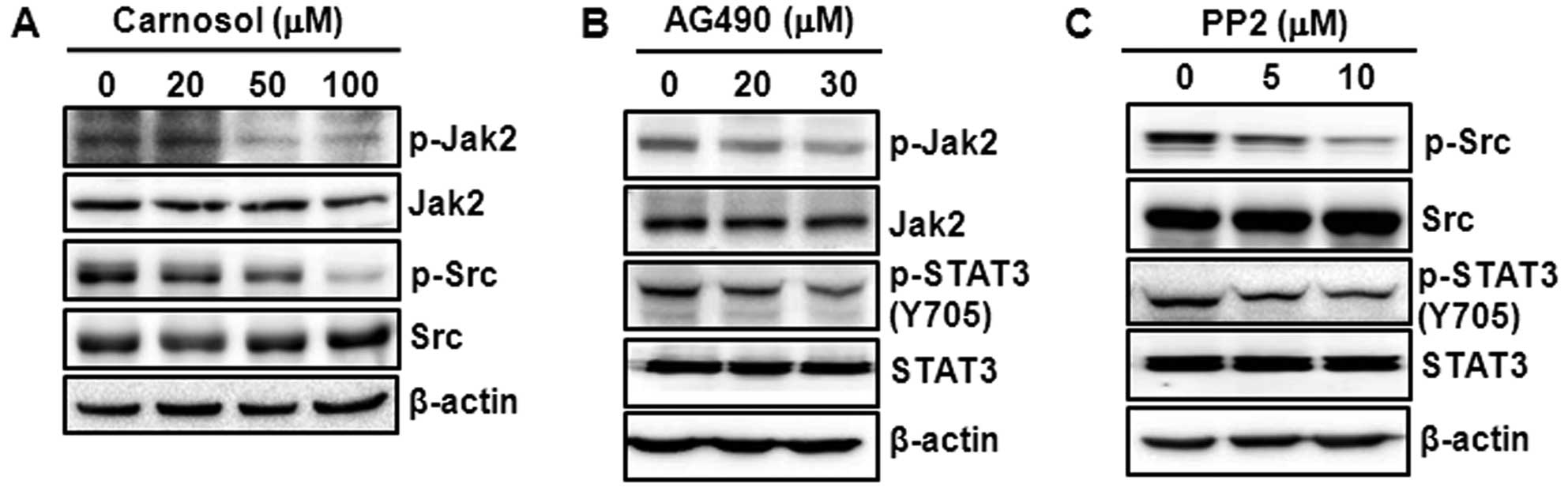
Carnosol-induced STAT3 inactivation is
mediated through inhibition of Jak2 and Src phosphorylation
Several upstream kinases, such as Jak2 and Src, are
known to phosphorylate STAT3 (16,17).
We examined the effect of carnosol on the phosphorylation of Jak2
and Src kinase. As shown in Fig.
5A, treatment with carnosol markedly diminished the
phosphorylation of Jak2 and Src. To validate our findings that the
inhibition of STAT3 activation by carnosol is mediated through its
inhibitory effects on the constitutive phosphorylation of Jak2 and
Src kinase, we incubated cells with AG490 and PP2, the
pharmacological inhibitors of Jak2 and Src kinase, respectively.
Treatment of cells with AG490 (Fig.
5B) or PP2 (Fig. 5C)
diminished the constitutive phosphorylation of STAT3.
Discussion
Carnosol, a diterpene present in the culinary herb
rosemary, has been reported to exert anticancer activities
(20). However, the biochemical
basis of anticancer effects of carnosol remains elusive. In the
present study, we found that carnosol induced apoptosis in HCT116
cells in a time- and concentration-dependent manner. This effect of
carnosol is in good agreement with the apoptosis induction by this
compound in other cancer cells (21,22),
including that in COLO 205 colon cancer cells (6). Then we attempted to delineate the
molecular mechanisms of anticancer effects of carnosol. The
cleavage of pro-caspase-9 by carnosol resulted in the activation of
effector caspase-3 and the cleavage of PARP. Since caspase-9 is an
initiator caspase in mitochondria-mediated death signaling
(9), these findings suggest that
carnosol may induce apoptosis through the intrinsic pathway.
Cells are equipped with a series of anti-apoptotic
(e.g., Bcl-2, Bcl-xl) and apoptosis-inducing proteins (e.g., Bax,
p53). Overexpression of Bcl-2 or Bcl-xl inhibits apoptosis and
promotes survival of cells (23).
According to Zhu et al, the induction of Bax is essential
for death receptor-mediated apoptosis in human colon cancer cells
(24). On the other hand, the
pro-survival protein Bcl-2 is overexpressed in colon cancer
(25) and the Bcl-2-mediated
inhibition of apoptosis restores the tumorigenecity of
spontaneously regressed colon tumors in vivo (26). The inhibition of Bcl-2 and Bcl-xl
expression and the increase in Bax protein level, thus, provide a
mechanistic basis of apoptosis induction by carnosol.
p53, an apoptosis-inducing protein (27), undergoes proteasomal degradation by
its cytosolic repressor Mdm2 (28). Pharmacological inhibition of Mdm2
activates p53 and induces growth arrest in mouse colon tumor and
human colon cancer cells (29).
The inhibition of Mdm2 expression and concomitant increase in p53
expression by carnosol, thus, suggest that carnosol may induce
apoptosis by inducing p53 expression. While the pro-apoptotic Bax
gene is a direct target of p53 (30), the overexpression of p53 in
p53-null cells diminish Bcl-2 expression (31). Thus, carnosol-induced p53
activation may result in cell death through downregulation of Bcl-2
and upregulation of Bax. Since the induction of p53 occurs in
response to oxidative stress (32), we examined if carnosol generates
ROS in HCT116 cells. We found that carnosol induced ROS generation,
which was diminished by ROS scavenger NAC. Moreover, pretreatment
of cells with NAC attenuated carnosol-induced cleavage of caspase-3
and PARP. Thus, carnosol-induced ROS generation may activate p53,
resulting in the downregulation of Bcl-2 and induction of Bax
expression, thereby leading to the activation of caspases and
induction of apoptosis.
Apart from the regulation by p53, the expression of
anti-apoptotic proteins Bcl-2 and Bcl-xl is also regulated by STAT3
(13), which is overexpressed in
colon cancer (12). Persistent
STAT3 activation increases the proliferation of colon cancer cells
in culture and enhances the growth of colon cancer cell xenograft
tumors in nude mice (33).
Moreover, the inhibition of STAT3 signaling induces apoptosis and
cell cycle arrest in colon carcinoma cells (34). Therefore, we examined the effect of
carnosol on STAT3 signaling in HCT116 cells. STAT3 activation
mechanism involves the phosphorylation of its tyrosine 705 residue
followed by the formation of STAT3 dimer, which translocates to the
nucleus and interacts with the promoter region of target genes
(12). Several upstream kinases
including Jak2 (16) and Src
(17) have been reported to
phosphorylate STAT3 at tyrosine705 residue. The decreased
phosphorylation of STAT3, Jak2 and Src kinase by carnosol suggests
that the apoptosis induction by this compound is mediated through
inhibition of STAT3 signaling. This was further supported by the
findings that carnosol diminished STAT3 DNA binding and
STAT3-reporter gene activity, thereby reducing the expression of
D-series cyclins and survivin, which are STAT3 target gene products
(13,35). While, survivin inhibits
Fas-mediated apoptosis in cancer cells (36), cyclins promote cell proliferation
(15). Thus, the reduced
expression of survivin and D-series cyclins by carnosol is
associated with the induction of apoptosis by this compound.
In conclusion, our study demonstrates for the first
time that carnosol-induced generation of ROS, activation of
caspases, induction of p53 and the inhibition of STAT3 signaling
lead to the induction of apoptosis in HCT116 cells, which may
provide a mechanistic basis of the anticancer activity of the
compound.
Acknowledgements
This study was supported by the
College of Pharmacy-specialized Research Fund (the Institute for
New Drug Development) of Keimyung University in 2013.
References
|
1.
|
Jemal A, Center MM, DeSantis C and Ward
EM: Global patterns of cancer incidence and mortality rates and
trends. Cancer Epidemiol Biomarkers Prev. 19:1893–1907. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
2.
|
Jemal A, Siegel R, Xu J and Ward E: Cancer
statistics, 2010. CA Cancer J Clin. 60:277–300. 2010. View Article : Google Scholar
|
|
3.
|
Merika E, Saif MW, Katz A, Syrigos K and
Morse M: Review. Colon cancer vaccines: an update. In Vivo.
24:607–628. 2010.PubMed/NCBI
|
|
4.
|
Chung MY, Lim TG and Lee KW: Molecular
mechanisms of chemopreventive phytochemicals against
gastroenterological cancer development. World J Gastroenterol.
19:984–993. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
5.
|
Moran AE, Carothers AM, Weyant MJ, Redston
M and Bertagnolli MM: Carnosol inhibits beta-catenin tyrosine
phosphorylation and prevents adenoma formation in the C57BL/6J/
Min/+ (Min/+) mouse. Cancer Res. 65:1097–1104. 2005.PubMed/NCBI
|
|
6.
|
Cheng AC, Lee MF, Tsai ML, et al: Rosmanol
potently induces apoptosis through both the mitochondrial apoptotic
pathway and death receptor pathway in human colon adenocarcinoma
COLO 205 cells. Food Chem Toxicol. 49:485–493. 2011. View Article : Google Scholar
|
|
7.
|
Hanahan D and Weinberg RA: Hallmarks of
cancer: the next generation. Cell. 144:646–674. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
8.
|
Neergheen VS, Bahorun T, Taylor EW, Jen LS
and Aruoma OI: Targeting specific cell signaling transduction
pathways by dietary and medicinal phytochemicals in cancer
chemoprevention. Toxicology. 278:229–241. 2010. View Article : Google Scholar
|
|
9.
|
Burz C, Berindan-Neagoe I, Balacescu O and
Irimie A: Apoptosis in cancer: key molecular signaling pathways and
therapy targets. Acta Oncol. 48:811–821. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
10.
|
Martin SJ and Green DR: Protease
activation during apoptosis: death by a thousand cuts? Cell.
82:349–352. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
11.
|
Aggarwal BB, Takada Y and Oommen OV: From
chemoprevention to chemotherapy: common targets and common goals.
Expert Opin Investig Drugs. 13:1327–1338. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
12.
|
Johnston PA and Grandis JR: STAT3
signaling: anticancer strategies and challenges. Mol Interv.
11:18–26. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
13.
|
Masuda M, Suzui M, Yasumatu R, et al:
Constitutive activation of signal transducers and activators of
transcription 3 correlates with cyclin D1 overexpression and may
provide a novel prognostic marker in head and neck squamous cell
carcinoma. Cancer Res. 62:3351–3355. 2002.
|
|
14.
|
Lau GK and Ye D: STAT3 implicated in the
development of colon cancer: a step closer for targeted therapy?
Gastroenterology. 139:353–355. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
15.
|
Lin L, Liu A, Peng Z, et al: STAT3 is
necessary for proliferation and survival in colon cancer-initiating
cells. Cancer Res. 71:7226–7237. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
16.
|
Slattery ML, Lundgreen A, Kadlubar SA,
Bondurant KL and Wolff RK: JAK/STAT/SOCS-signaling pathway and
colon and rectal cancer. Mol Carcinog. 52:155–166. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
17.
|
Laird AD, Li G, Moss KG, et al: Src family
kinase activity is required for signal tranducer and activator of
transcription 3 and focal adhesion kinase phosphorylation and
vascular endothelial growth factor signaling in vivo and for
anchorage-dependent and -independent growth of human tumor cells.
Mol Cancer Ther. 2:461–469. 2003.
|
|
18.
|
Kundu J, Wahab SM, Kundu JK, et al: Tob1
induces apoptosis and inhibits proliferation, migration and
invasion of gastric cancer cells by activating Smad4 and inhibiting
beta-catenin signaling. Int J Oncol. 41:839–848. 2012.PubMed/NCBI
|
|
19.
|
Kundu JK, Shin YK, Kim SH and Surh YJ:
Resveratrol inhibits phorbol ester-induced expression of COX-2 and
activation of NF-kappaB in mouse skin by blocking IkappaB kinase
activity. Carcinogenesis. 27:1465–1474. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
20.
|
Johnson JJ: Carnosol: a promising
anti-cancer and anti-inflammatory agent. Cancer Lett. 305:1–7.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
21.
|
Dorrie J, Sapala K and Zunino SJ:
Carnosol-induced apoptosis and downregulation of Bcl-2 in B-lineage
leukemia cells. Cancer Lett. 170:33–39. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
22.
|
Johnson JJ, Syed DN, Heren CR, Suh Y,
Adhami VM and Mukhtar H: Carnosol, a dietary diterpene, displays
growth inhibitory effects in human prostate cancer PC3 cells
leading to G2-phase cell cycle arrest and targets the
5′-AMP-activated protein kinase (AMPK) pathway. Pharm Res.
25:2125–2134. 2008.PubMed/NCBI
|
|
23.
|
Cherbonnel-Lasserre C and Dosanjh MK:
Suppression of apoptosis by overexpression of Bcl-2 or Bcl-xL
promotes survival and mutagenesis after oxidative damage.
Biochimie. 79:613–617. 1997. View Article : Google Scholar
|
|
24.
|
Zhu S, Li T, Tan J, et al: Bax is
essential for death receptor-mediated apoptosis in human colon
cancer cells. Cancer Biother Radiopharm. 27:577–581. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
25.
|
Valassiadou KE, Stefanaki K, Tzardi M, et
al: Immunohistochemical expression of p53, bcl-2, mdm2 and waf1/p21
proteins in colorectal adenocarcinomas. Anticancer Res.
17:2571–2576. 1997.PubMed/NCBI
|
|
26.
|
Bonnotte B, Favre N, Moutet M, et al:
Bcl-2-mediated inhibition of apoptosis prevents immunogenicity and
restores tumorigenicity of spontaneously regressive tumors. J
Immunol. 161:1433–1438. 1998.PubMed/NCBI
|
|
27.
|
Shaw P, Bovey R, Tardy S, Sahli R, Sordat
B and Costa J: Induction of apoptosis by wild-type p53 in a human
colon tumor-derived cell line. Proc Natl Acad Sci USA.
89:4495–4499. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
28.
|
Kim DH, Kundu JK and Surh YJ: Redox
modulation of p53: mechanisms and functional significance. Mol
Carcinog. 50:222–234. 2011. View
Article : Google Scholar : PubMed/NCBI
|
|
29.
|
Rigatti MJ, Verma R, Belinsky GS,
Rosenberg DW and Giardina C: Pharmacological inhibition of Mdm2
triggers growth arrest and promotes DNA breakage in mouse colon
tumors and human colon cancer cells. Mol Carcinog. 51:363–378.
2012. View
Article : Google Scholar : PubMed/NCBI
|
|
30.
|
Miyashita T and Reed JC: Tumor suppressor
p53 is a direct transcriptional activator of the human bax gene.
Cell. 80:293–299. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
31.
|
Miyashita T, Krajewski S, Krajewska M, et
al: Tumor suppressor p53 is a regulator of bcl-2 and bax gene
expression in vitro and in vivo. Oncogene. 9:1799–1805.
1994.PubMed/NCBI
|
|
32.
|
Lotem J, Peled-Kamar M, Groner Y and Sachs
L: Cellular oxidative stress and the control of apoptosis by
wild-type p53, cytotoxic compounds, and cytokines. Proc Natl Acad
Sci USA. 93:9166–9171. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
33.
|
Corvinus FM, Orth C, Moriggl R, et al:
Persistent STAT3 activation in colon cancer is associated with
enhanced cell proliferation and tumor growth. Neoplasia. 7:545–555.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
34.
|
Lin Q, Lai R, Chirieac LR, et al:
Constitutive activation of JAK3/ STAT3 in colon carcinoma tumors
and cell lines: inhibition of JAK3/STAT3 signaling induces
apoptosis and cell cycle arrest of colon carcinoma cells. Am J
Pathol. 167:969–980. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
35.
|
Kanda N, Seno H, Konda Y, et al: STAT3 is
constitutively activated and supports cell survival in association
with survivin expression in gastric cancer cells. Oncogene.
23:4921–4929. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
36.
|
Asanuma K, Tsuji N, Endoh T, Yagihashi A
and Watanabe N: Survivin enhances Fas ligand expression via
up-regulation of specificity protein 1-mediated gene transcription
in colon cancer cells. J Immunol. 172:3922–3929. 2004. View Article : Google Scholar
|