Stem cells are characterized by their ability to
self-renew and their capacity to differentiate to specialized cell
types (1). Stem cells are found in
most tissues of the human body and are required to maintain tissue
homeostasis (2). They are also
engaged in wound healing (3). A
recent work by Fuchs et al on hair follicle stem cells
suggests that the more adult stem cells are present in the injured
area the faster the wound is healing (4). This might be explained by an
accelerated recruitment of differentiated cells as generated by a
higher number of stem cells. However, there is evidence that
besides differentiation capacity also paracrine functions of stem
cells are important in wound healing (5).
A stem cell type that, for quite some time, is known
to apply paracrine effects to orchestrate wound healing is the
mesenchymal stem cell (MSC), a multipotent stromal progenitor cell
residing preferentially in bone marrow and adipose tissue (6,7).
MSCs are defined by their ability to differentiate to osteoblasts,
chondroblasts and adipocytes, by plastic adherence and by a
particular expression pattern of certain surface proteins (8,9).
Strongly attracted to wounds, MSCs are mobilized by injuries which
they enter to modulate inflammatory responses and stimulate tissue
regeneration (10). MSCs are a
heterogeneous population and can also emerge from pericytes or
endothelial cells (11), which may
help to accelerate local MSC recruitment. MSCs were originally
reported to contribute to tissue repair by trans-differentiating
into cells, such as epithelial cells or neurons, that are required
to restore the injured tissue (12–15).
However, later it became evident that their paracrine activities
are more important for wound healing than their differentiation
potential (11,16,17).
It is now well accepted that, also in cancer,
stem-like cells, so-called cancer stem cells (CSCs), exist
(18–21). These cells are thought to be
responsible for tumor initiation and metastasis. As wounds that
never heal (22) cancers resemble
wounds in a number of aspects, e.g., in their ability to attract
MSCs (23). CSCs are thought to
contribute to tumor heterogeneity by generating different kind of
differentiated cells. In breast cancer, CSCs can give rise to the
so-called basal and luminal type of breast cancer cells (24). As suggested for adult stem cells,
CSCs may have other functions besides recruitment of differentiated
cells und may use paracrine activities to influence (tumor) tissue
growth and maintenance. In this review, we will summarize the
current knowledge on the importance of normal and cancer stem cells
as producer of paracrine factors. Since there are a number of
excellent reviews that address the paracrine functions of MSCs in
wound healing and cancer (11,25–30),
we focussed here on the paracrine effects of non-MSC stem cells and
describe MSC paracrine activities only for comparative reasons.
There are many ways by which cells can communicate
in a paracrine manner. One way is by proteins, such as growth
factors or cytokines. MSCs secret a plethora of such proteins
(28,29,31)
some of which act as survival factors on neighboring
(differentiated) cells, others stimulate angiogenesis. The cocktail
of proteins that is secreted by cells is called the secretome
(32). Besides the secretome,
additional non-protein factors, such as lipids and RNAs, can be
released from cells into the extracellular space. Some of these
factors, in particular RNAs, may not leave the cell as soluble
substances, but rather as cargos of microvesicles that are
generated by the secreting cell. Microvesicles are circular
fragments which can either be generated from endosomes (called
exosomes; size range, 40–120 nm) or from the plasma membrane
(called shedding vesicles; size range, 100–1,000 nm) (33–35).
They can be distinguished from apoptotic bodies by their lack of
DNA and histones. Both exosomes and shedding vesicles contain
proteins of the lipid raft and lipids, such as cholesterol, as well
as numerous soluble proteins and RNAs (mRNA and microRNA), e.g., in
MSC-derived microvesicles, more than 700 proteins and ∼150 miRNAs
have been identified (36,37). By interacting with microvesicles,
cells can take up the microvesicular contents (37,38)
and use them for biological activities. Microvesicular RNA may be
of particular importance. RNA from microvesicles can be translated
into proteins (39) and RNase
treatment often abrogates the effect of microvesicles on recipient
cells (40,41). Many effects of microvesicles have
been described. Among them are inhibition of apoptosis, stimulation
of stem cell activity or modulation of inflammatory responses
(41–43).
Cardiac stem cells have been shown to improve
recovery of the myocard from ischemia. This has been linked to
their ability to differentiate to cardiomyocytes to replace the
damaged cells. However, a recent report demonstrated that the
differentiation potential of these cells alone was not sufficient
for this repair (44). The
cardioprotective effect of the cardiac stem cells also strictly
depended upon the activation of signal transducer and activator of
transcription 3 (STAT3) in the myocard. STAT3 can be activated by
stromal cell derived factor-1 (SDF-1), a chemokine secreted by
cardiac stem cells and known to support regeneration of the
myocardial tissue (45).
Inhibition of SDF-1 secretion blocked recovery. SDF-1 has a dual
function in myocard repair. It recruits stem cells to the infarcted
heart (45) and improves the
survival of cardiomyocytes (46)
by decreasing caspase 3-dependent apoptosis (44). In the infarcted dog heart,
recruitment of cardiac stem cells could be induced by
administration of insulin growth factor-1 (IGF-1) and hepatocyte
growth factor (HGF), two growth factors that stimulate the
expansion of cardiac stem cells (47).
Besides cardiac stem cells, mesenchymal stem cells
(MSCs) are able to improve post-ischemic recovery of the myocard
(48). It was originally thought
that multipotent MSC differentiate into cardiomyocyte-like cells to
exert this effect, until it was found that the cocktail of proteins
as secreted by MSC was sufficient for MSC-dependent recovery
(5,49,50).
Interestingly, like cardiac stem cells, MSCs induce STAT3
phosphorylation in the myocard (51). Moreover, toll-like receptor 4 (TLR
4)-deficient MSCs that induce much higher STAT3 activation were
more effective in repairing the myocardial tissue than their
wild-type counterpart. In the presence of MSC-conditioned medium
(CM), also SDF-1 levels were higher in the infarcted heart
(52). The SDF-1 level could be
increased when the CM was taken from MSCs that had been forced to
express vascular endothelial growth factor (VEGF). Part of the
SDF-1 protein derived from the MSCs, part from the myocard. Hence,
MSCs and cardiac stem cells may exert their cardioprotective effect
via the same route and by using the same secretory protein(s). In a
porcine model, it could be confirmed that MSCs, in this case
generated from human embryonic stem cells, can improve recovery of
the myocard from ischemia via factors they secrete (53). However, in this study, the
cardioprotective effect was accompanied by decreased
phosphorylation of Smad2, an effector of the transforming growth
factor β pathway, and by reduced expression of caspase 3. In
addition, the component responsible for this effect of the
MSC-derived CM was found to be rather large, a complex of >1,000
kD. Later, a 20S proteasome, that copurifies with MSC-shedded
exosomes, was identified as the likely candidate mediating
MSC-dependent cardioprotection (54). The uptake of this proteosome by
cardiomyocytes decreased the accumulation of misfolded proteins and
may have therefore increased the survival of these cells. This is
in agreement with the observation that MSC-derived CM upregulated
anti-apoptotic protein Bcl2 in cardiomyocytes and protected them
from hypoxia-induced apoptosis (55).
Additionally, MSCs may stimulate angiogenesis in the
infarcted myocard. MSC-derived CM was shown to activate endothelial
cells and to increase capillary density in the infarcted heart
(50,56). Among the pro-angiogenic factors
found in the secretome of MSCs are VEGF and basic fibroblast growth
factor (bFGF) (50,55,57,58).
Blocking VEGF and bFGF by antibody treatment could partly diminish
recovery by MSCs (58). In
addition to VEGF and bFGF, cysteine-rich angiogenic inducer 61
(Cyr61) has been identified as an important MSC-derived soluble
factor that stimulates angiogenesis in the infarcted myocard
(59). The anti-fibrotic activity
of MSCs is also considered to contribute to the beneficial effect
of these cells on the infarcted myocard. MSC-derived CM reduced
cardiac fibrosis by inhibiting the proliferation of cardiac
fibroblast and, thereby, decreasing the deposition of collagen I,
II and III (60,61).
There are at least two more stem/progenitor cell
types, the bone marrow-derived endothelial progenitor cell (EPC)
and the skeletal muscle-derived stem cell (MDSC), which were shown
to be capable of cardioprotection (62,63).
When EPCs were transplanted into the myocard, again, myocardial
expression of SDF-1 was found to be increased (62). In addition, EPS may stimulate
angiogenesis in the myocard by secreting thymosin β4, a protein
known to improve endothelial function (64). MDSCs were barely able to
differentiate to cardiomyocytes, when implanted into the infarcted
heart (65). Again it was their
secretory activity that improved recovery from infarction. The
major component of their secretome responsible for this effect was
determined to be VEGF which stimulated angiogenesis. Blocking VEGF
resulted in reduced neovascularization and adverse remodeling.
Interestingly, mechanical stretching of MDSCs increased VEGF
secretion. This finding, combined with the observation that mice
that exercised after infarction showed higher myocardial VEGF
levels and angiogenesis (66), may
suggest that physical therapy after myocardial infarction improves
recovery by increasing stem cell/VEGF-depending neovascularization
(67).
In a recent study, the cardioprotective activities
of cardiac stem cells, MSCs and EPCs were compared. Most effective
in inducing myocyte differentiation and tube formation were
cardiosphere-derived cells, a population of cells that contained
cardiac stem cells and supporting cells (68). Compared to MSCs or bone
marrow-derived mononuclear cells, these cells produced much higher
levels of SDF-1, HGF and of the proangiogenic proteins VEGF and
bFGF.
In summary, factors secreted by cardioprotective
stem cells seem to have two major functions, i) to improve survival
of cardiomyocytes; and ii) to stimulate neovascularization
(Table I).
Similar to the ischemic myocard, the ischemic brain
requires stem cell-secreted factors for recovery. Here, again, the
pro-angiogenic growth factor VEGF secreted by transplanted human
central nervous system stem cells was found to be critical for stem
cell-dependent repair of stroke-induced lesions (70). Neural stem cells also stimulated
axonal transport and induced increased dendritic branching and
length (71). The effect of neural
stem cell on dendritic plasticity was at least partially dependent
upon thrombospondins 1 and 2, two proteins secreted by the stem
cells. This is in line with the observation that knockout of
thrombospondin 1 and 2 in mice reduced functional recovery after
stroke (72). Neural stem cells
were also reported to improve repair of spinal cord injuries in
rats. Implanted into the lesion area these cells enhanced axonal
outgrowth (73). Neutrotrophic
factors, such as nerve growth factors (NGF) and brain-derived
neurotrophic factor (BDNF), were found to be secreted by the neural
stem cells and were made responsible for this effect. Spinal cord
injured rats also benefitted from CM generated by bone-marrow
derived MSCs (74). Improved motor
recovery in the presence of this medium was the consequence of less
extensive lesions. Though MSCs secrete NGF and BDNF, protect
neurons from apoptosis (74) and
stimulate neurite outgrowth in vitro (75), MSC-CM seem to have no effect on
axonal outgrowth in vivo (74). Rather, MSC-CM appears to exert its
neuroprotective effect in vivo by stimulating angiogenesis.
This was again at least partly dependent on VEGF.
Paracrine effects of stem cells also play a role in
recovery from kidney injury. Tubular adult renal stem/progenitor
cells (tARPC) have been reported to stimulate proliferation and to
inhibit apoptosis of cisplatin-injured proximal tubular epithelial
cells (76). This effect depended
upon the secretion of inhibin A, an inhibitor of the TGFβ
superfamily ligand activin known to inhibit renal tubulogenesis
(77). Evidence was provided that
inhibin A was transported to the tubular epithelial cells as RNA
via microvesicles (76).
Interestingly, inhibin A was only found in microvesicles shedded by
tARPC that had encountered damaged tubular epithelial cells. For
the recognition of apoptotic epithelial cells, toll receptor 2
(TLR2) was required. Also microvesicles shedded from bone
marrow-derived mesenchymal stem cells were found to increase
survival and proliferation of tubular cells after damage (40,41).
As RNase treatment abrogated this effect, again the transfer of
certain RNAs by the epithelial cells was made responsible for this
process. Furthermore, the presence of CD44 and CD29 on the surface
of these microvesicles were found to be crucial for the
communication between the MSC-derived microvesicles and tubular
cells. In addition, soluble factors, namely VEGF, IGF-1 and HGF, as
secreted by MSCs may contribute to the renoprotective effect of
MSCs. These factors may be responsible for the increased survival
of endothelial cells as observed in the presence of MSCs (78,79).
Interestingly, MSCs were found to attach to endothelial cells to
form tubes in a cooperative manner (78).
Stem cell-secreted factors have also been shown to
improve recovery of liver from cirrhosis (81). In this case, Wistar rats poisoned
with dimethylnitrosamine were treated with or without CM from
CD34+ haematopoietic stem cells. The CM from these cells
injected into the tail vein significantly increased liver repair
and animal survival by blocking caspase 3-dependent apoptosis of
liver cells. Among the 32 factors identified in the CM of the
CD34+ stem cells were a number of cytokines, including
members of the CXCL chemokine family, known to be involved in wound
healing. Liver regeneration is closely linked to CXC receptor 2
(82) which recognizes CXCL
chemokines.
In addition to their immunosuppressive effect,
glioma CSCs were found to stimulate angiogenesis. As a
pro-angiogenic factor, CD133+ glioma CSCs secret
substantial amounts of VEGF which leads to enhanced endothelial
migration and tube formation (87). The level of secreted VEGF could be
greatly enhanced by hypoxia. Forced overexpression of VEGF in CSCs
also resulted in increased angiogenesis and tumor formation in
vivo (88) confirming that
CSCs can be a VEGF source to promote angiogenesis in glioma.
Similar data were reported by Folkins et al, who compared
glioma CSC high and low fractions (89). Besides VEGF, the CSC-high fraction
also secreted SDF-1. Both VEGF and SDF-1 were necessary for the
stimulatory effect of the CSC-high fraction on angiogenesis.
Inhibition of either the VEGF receptor VEGFR2 or the SDF-1 receptor
CXCR4 in endothelial cells equally blocked angiogenesis by CM from
CSCs. CXCR4 is also highly expressed in glioma CSCs, where it
stimulates VEGF secretion via the phosphoinositide 3-kinase
(PI3K)/AKT pathway upon binding to SDF-1 (90). This suggests that SDF-1 has two
functions in glioma CSC-driven angiogenesis: i) together with VEGF,
it activates endothelial cells; and ii) it recruits more VEGF by
stimulating its expression in glioma CSCs.
Interestingly, MSCs, which have been shown to
stimulate angiogenesis in prostate cancer (91), suppress angiogenesis in glioma and
hence inhibit glioma growth in vivo (92). Concomitantly, the expression of
pro-angiogenic factors, such as bFGF, platelet-derived growth
factor-BB (PDGF-BB) and IGF-1, were reduced suggesting that MSCs
inhibited the secretion of these factors by the glioma cells.
However, another study using glioma stromal mesenchymal stem-like
cells (GS-MSLCs), which are MSC-like cells residing in glioma,
demonstrated that MSCs are also able to promote angiogenesis
(93). Apparently, the source the
MSCs are isolated from is an important factor that determines the
effect of MSCs in glioma (94).
Pro-angiogenic activities can also be attributed to
CSCs isolated from renal cancer (95). These CD105-expressing CSCs
stimulated angiogenesis by secreting exosome-sized microvesicles
(96). CD105-positive, but not
CD105-negative microvesicles, contained RNAs encoding angiogenic
factors, such as VEGF. The CSC-derived microvesicles induced
invasion of human vascular endothelial cells, protected them from
apoptosis and promoted endothelial/tumor cell adhesion. They also
stimulated angiogenesis in Matrigel plug assays in vivo.
Treatment of lung endothelial cells with these microvesicles
increased their expression of VEGF receptor and of matrix
metalloproteinases 2 and 9. There is also evidence provided that
these CSC-secreted microvesicles promote metastasis formation of
renal cancer cells in the lung.
Recently, ovarian CSCs have been reported to release
CCL5 into the culture medium (102), a chemokine known to play a role
in breast cancer metastasis and whose secretion can be triggered by
co-culturing breast cancer cells with mesenchymal stem cells
(103). CCL5 increased the
migratory and metastatic potential of ovarian CSCs in an autocrine
manner, but had little effect on non-CSC ovarian cancer cells.
However, since the autocrine CCL5 feedback loop fueled expression
of MMP-9 by CSCs, it is possible that secreted MMP-9, a protease
involved in ECM degradation, facilitates invasion also of
neighboring non-CSC tumor cells.
In breast cancer, the vast majority of studies on
paracrine effects of stem cells have been done with MSCs which by
heavily communicating with breast cancer cells via many soluble
factors are able to promote tumor progression (26,104). Interestingly, MSCs may also
affect breast CSCs. Liu et al demonstrated that
IL-6-stimulated MSCs produce the chemokine CXCL7 which further
fuels IL-6 secretion by breast cancer cancer cells (105). In the end, this feedback loop
leads to the release of factors, such as IL-8, that cause the CSC
pool to expand. In a different way, adipose-derived stem cells were
found to increase the breast CSC population. By secreting PDGF-D,
these stem cells induce epithelial-to-mesenchymal transition of
breast cancer cells and, as a consequence, generate additional
stem-like cancer cells (106).
In addition, breast CSCs may themselves be a
provider of bioactive soluble factors. Comparative transcriptome
analyses by serial analysis of gene expression, cDNA microarray and
next generation sequencing of CD44+/CD24−
breast CSCs and bulk tumor cells revealed a highly active TGFβ
pathway in CSCs compared to non-CSC breast cancer cells (107,108). Along with the activation of the
TGFβ pathway, typical TGFβ target genes, such as plasminogen
activator inhibitor-1 (PAI-1), were found to be highly upregulated
in CSCs. PAI-1, a well-established unfavorable prognostic factor in
breast cancer (109), is a
secretory protein able to promote cellular migration and
angiogenesis (110,111). Since PAI-1 secreted by MSCs is
able to enhance migratory activities of cancer cells (112) (Dittmer et al unpublished
data), it is reasonable to assume that CSC-secreted PAI-1 may also
affect cell motility.
Connections between CSCs and endothelial cells have
been demonstrated for squamous tumor of the skin. CSCs in skin
papillomas produce large amounts of VEGF not only to trigger
angiogenesis by stimulating neighboring VEGFR2-expressing
endothelial cells, but also to maintain their stemness in an
autocrine manner via the VEGF co-receptor neuropilin 1 (113). Blocking the function of either
neuropilin 1 in CSCs or of VEGFR2 in endothelial cells reduced both
microvessel density and CSC population. Hence, cutaneous CSCs are
propagated in perivascular niches, which are maintained by the VEGF
produced by the stem cells themselves. Also CD133+
melanoma stem cells have been shown to produce pro-angiogenic
factors, such as VEGF (114). In
pancreatic cancer, CD133+ cancer stem cells have been
found to be the major source of VEGF-C (115).
Though the importance of paracrine effects for the
functions of mesenchymal stem cells in tissue repair and cancer is
well established, we just start to appreciate paracrine activities
of other adult stem cells and cancer stem cells. In the past,
tissue-specific adult stem cells and cancer stem cells were only
viewed as providers of new (differentiated) cells either to fill
the gap that has been caused by cell loss or to fuel tumor growth,
respectively. Now, a new theme is emerging which ascribe to these
stem cells an additional regulatory function in tissue maintenance
or tumor progression. The so-called damage-associated molecular
pattern (DAMP) after acute kidney injury may be a good example that
shows how much stem cell-derived factors are involved in tissue
repair (116). It seems that
adult stem cells orchestrate wound healing by releasing specific
factors that inhibit apoptosis of damaged cells and stimulate
angiogenesis. The secretion of pro-angiogenic factors by stem cells
may be of particular importance, since this activity is shared by
many adult stem cells and cancer stem cells and often found to be
essential for the stem cell-driven tissue regeneration and stem
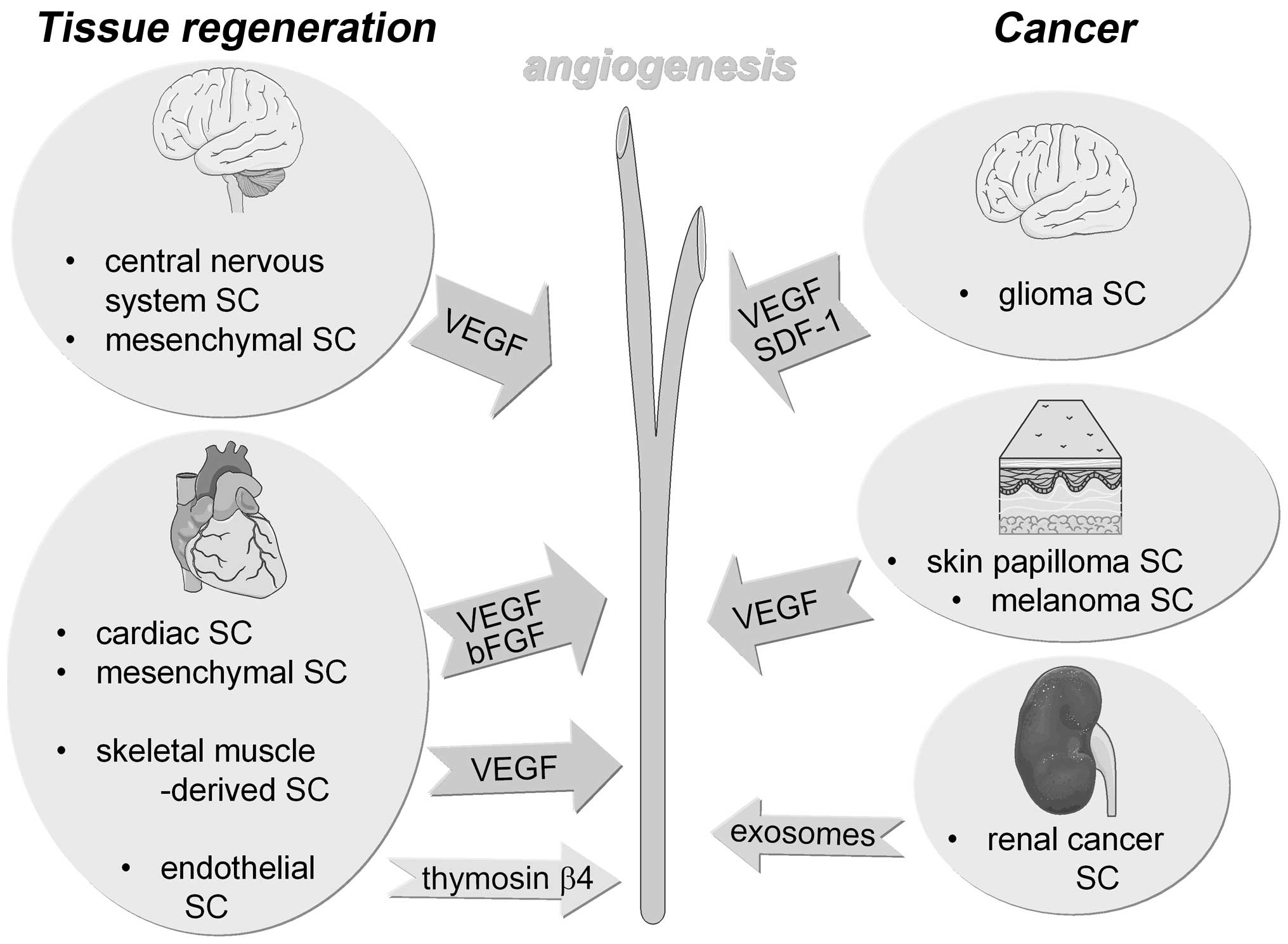
cell-dependent tumor progression, respectively (Fig. 1). Since delivery of oxygen and
nutrients is essential for cell survival timely angiogenesis in
tissue repair and cancer progression is a critical event. Stem
cells may coordinate tissue repair/cancer progression by generating
new cells and, by stimulating angiogenesis, simultaneously
supplying these cells with the substances needed for survival. It
is intriguing that endothelial cells are often in close contact
with stem cells. One example is the haematopoietic stem cells which
are positioned next to endothelial cells when residing in the
endothelial niche in the bone marrow and whose expansion is
dependent on endothelial cells (30). Also glioma stem cells are residing
in endothelial niches (117)
which seem to be of mutual benefit for both cell types (118). Perivascular niches have also been
found to regulate dormancy of breast cancer cells (119) and maintain CSC populations in
skin cancer (113). In addition,
endothelial cells may be strongly involved in cancer metastasis
(120). The link between cancer
stem cells and endothelial cells may theoretically open new evenues
to treat cancer stem cells that are usually resistant to
chemotherapeutics and whose population may even expand in the
presence of these drugs (121).
Anti-angiogenic drugs may dislodge cancer stem cells from their
endothelial feeding layer and stop them from growing and
differentiating. However, anti-angiogenic drugs, such as anti-VEGF,
have been tested for some time in clinical trials to suppress blood
supply to the tumor and showed limited success for several reasons,
e.g., because hypoxia was induced that then fueled cancer
progression (122). More
knowledge is required to understand the role of the cancer
stem/endothelial cell interaction in cancer progression to find
specific drugs that interfere with this kind of cell-cell
communication.
In general, knowing that paracrine effects of stem
cells strongly contribute to tissue repair and cancer may help to
find new ways of therapeutical interventions to facilitate tissue
regeneration and to improve cancer treatment, respectively.
This study was supported by the
Deutsche Krebshilfe, grant no. 109271.
|
1.
|
Fuchs E and Chen T: A matter of life and
death: self-renewal in stem cells. EMBO Rep. 14:39–48. 2013.
View Article : Google Scholar
|
|
2.
|
Hsu YC and Fuchs E: A family business:
stem cell progeny join the niche to regulate homeostasis. Nat Rev
Mol Cell Biol. 13:103–114. 2012. View
Article : Google Scholar : PubMed/NCBI
|
|
3.
|
Chun Q and Liang LS: Stem cell research,
repairing, and regeneration medicine. Int J Low Extrem Wounds.
11:180–183. 2012. View Article : Google Scholar
|
|
4.
|
Fuchs Y, Brown S, Gorenc T, Rodriguez J,
Fuchs E and Steller H: Sept4/ARTS regulates stem cell apoptosis and
skin regeneration. Science. 341:286–289. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
5.
|
Crisostomo PR, Wang M, Markel TA, Lahm T,
Abarbanell AM, Herrmann JL and Meldrum DR: Stem cell mechanisms and
paracrine effects: potential in cardiac surgery. Shock. 28:375–383.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
6.
|
Friedenstein AJ, Piatetzky S II and
Petrakova KV: Osteogenesis in transplants of bone marrow cells. J
Embryol Exp Morphol. 16:381–390. 1966.PubMed/NCBI
|
|
7.
|
Zuk PA, Zhu M, Ashjian P, De Ugarte DA,
Huang JI, Mizuno H, Alfonso ZC, Fraser JK, Benhaim P and Hedrick
MH: Human adipose tissue is a source of multipotent stem cells. Mol
Biol Cell. 13:4279–4295. 2002.
|
|
8.
|
Dominici M, Le Blanc K, Mueller I,
Slaper-Cortenbach I, Marini F, Krause D, Deans R, Keating A,
Prockop D and Horwitz E: Minimal criteria for defining multipotent
mesenchymal stromal cells. The International Society for Cellular
Therapy position statement. Cytotherapy. 8:315–317. 2006.
View Article : Google Scholar
|
|
9.
|
Friedenstein AJ, Chailakhyan RK, Latsinik
NV, Panasyuk AF and Keiliss-Borok IV: Stromal cells responsible for
transferring the microenvironment of the hemopoietic tissues.
Cloning in vitro and retransplantation in vivo. Transplantation.
17:331–340. 1974. View Article : Google Scholar : PubMed/NCBI
|
|
10.
|
Brooke G, Cook M, Blair C, Han R,
Heazlewood C, Jones B, Kambouris M, Kollar K, McTaggart S,
Pelekanos R, Rice A, Rossetti T and Atkinson K: Therapeutic
applications of mesenchymal stromal cells. Semin Cell Dev Biol.
18:846–858. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
11.
|
Bluguermann C, Wu L, Petrigliano F,
McAllister D, Miriuka S and Evseenko DA: Novel aspects of
parenchymal-mesenchymal interactions: from cell types to molecules
and beyond. Cell Biochem Funct. 31:271–280. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
12.
|
Chen J, Li Y, Wang L, Zhang Z, Lu D, Lu M
and Chopp M: Therapeutic benefit of intravenous administration of
bone marrow stromal cells after cerebral ischemia in rats. Stroke.
32:1005–1011. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
13.
|
Ortiz LA, Gambelli F, McBride C, Gaupp D,
Baddoo M, Kaminski N and Phinney DG: Mesenchymal stem cell
engraftment in lung is enhanced in response to bleomycin exposure
and ameliorates its fibrotic effects. Proc Natl Acad Sci USA.
100:8407–8411. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
14.
|
Park KS, Jung KH, Kim SH, Kim KS, Choi MR,
Kim Y and Chai YG: Functional expression of ion channels in
mesenchymal stem cells derived from umbilical cord vein. Stem
Cells. 25:2044–2052. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
15.
|
Rojas M, Xu J, Woods CR, Mora AL, Spears
W, Roman J and Brigham KL: Bone marrow-derived mesenchymal stem
cells in repair of the injured lung. Am J Respir Cell Mol Biol.
33:145–152. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
16.
|
Baraniak PR and McDevitt TC: Stem cell
paracrine actions and tissue regeneration. Regen Med. 5:121–143.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
17.
|
Kassis I, Vaknin-Dembinsky A and Karussis
D: Bone marrow mesenchymal stem cells: agents of immunomodulation
and neuroprotection. Curr Stem Cell Res Ther. 6:63–68. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
18.
|
Clevers H: The cancer stem cell: premises,
promises and challenges. Nat Med. 17:313–319. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
19.
|
Gupta PB, Chaffer CL and Weinberg RA:
Cancer stem cells: mirage or reality? Nat Med. 15:1010–1012. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
20.
|
Visvader JE and Lindeman GJ: Cancer stem
cells in solid tumours: accumulating evidence and unresolved
questions. Nat Rev Cancer. 8:755–768. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
21.
|
Dittmer J and Rody A: Stem cells in breast
cancer. Histol Histopathol. 28:827–838. 2013.
|
|
22.
|
Dvorak HF: Tumors: wounds that do not
heal. Similarities between tumor stroma generation and wound
healing. N Engl J Med. 315:1650–1659. 1986. View Article : Google Scholar
|
|
23.
|
Kidd S, Spaeth E, Klopp A, Andreeff M,
Hall B and Marini FC: The (in) auspicious role of mesenchymal
stromal cells in cancer: be it friend or foe. Cytotherapy.
10:657–667. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
24.
|
Gupta PB, Fillmore CM, Jiang G, Shapira
SD, Tao K, Kuperwasser C and Lander ES: Stochastic state
transitions give rise to phenotypic equilibrium in populations of
cancer cells. Cell. 146:633–644. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
25.
|
Ren G, Chen X, Dong F, Li W, Ren X, Zhang
Y and Shi Y: Concise review: mesenchymal stem cells and
translational medicine: emerging issues. Stem Cells Transl Med.
1:51–58. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
26.
|
Cuiffo BG and Karnoub AE: Mesenchymal stem
cells in tumor development: emerging roles and concepts. Cell Adh
Migr. 6:220–230. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
27.
|
Tetta C, Consiglio AL, Bruno S, Tetta E,
Gatti E, Dobreva M, Cremonesi F and Camussi G: The role of
microvesicles derived from mesenchymal stem cells in tissue
regeneration; a dream for tendon repair? Muscles Ligaments Tendons
J. 2:212–221. 2012.PubMed/NCBI
|
|
28.
|
Zimmerlin L, Park TS, Zambidis ET,
Donnenberg VS and Donnenberg AD: Mesenchymal stem cell secretome
and regenerative therapy after cancer. Biochimie. 95:2235–2245.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
29.
|
Paul G and Anisimov SV: The secretome of
mesenchymal stem cells: Potential implications for
neuroregeneration. Biochimie. 95:2246–2256. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
30.
|
Frenette PS, Pinho S, Lucas D and
Scheiermann C: Mesenchymal stem cell: keystone of the hematopoietic
stem cell niche and a stepping-stone for regenerative medicine.
Annu Rev Immunol. 31:285–316. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
31.
|
Parekkadan B, van Poll D, Suganuma K,
Carter EA, Berthiaume F, Tilles AW and Yarmush ML: Mesenchymal stem
cell-derived molecules reverse fulminant hepatic failure. PLoS One.
2:e9412007. View Article : Google Scholar : PubMed/NCBI
|
|
32.
|
Agrawal GK, Jwa NS, Lebrun MH, Job D and
Rakwal R: Plant secretome: unlocking secrets of the secreted
proteins. Proteomics. 10:799–827. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
33.
|
Camussi G, Deregibus MC, Bruno S,
Cantaluppi V and Biancone L: Exosomes/microvesicles as a mechanism
of cell-to-cell communication. Kidney Int. 78:838–848. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
34.
|
Muralidharan-Chari V, Clancy JW, Sedgwick
A and D'Souza-Schorey C: Microvesicles: mediators of extracellular
communication during cancer progression. J Cell Sci. 123:1603–1611.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
35.
|
Meckes DG Jr and Raab-Traub N:
Microvesicles and viral infection. J Virol. 85:12844–12854. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
36.
|
Kim HS, Choi DY, Yun SJ, Choi SM, Kang JW,
Jung JW, Hwang D, Kim KP and Kim DW: Proteomic analysis of
microvesicles derived from human mesenchymal stem cells. J Proteome
Res. 11:839–849. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
37.
|
Collino F, Deregibus MC, Bruno S, Sterpone
L, Aghemo G, Viltono L, Tetta C and Camussi G: Microvesicles
derived from adult human bone marrow and tissue specific
mesenchymal stem cells shuttle selected pattern of miRNAs. PLoS
One. 5:e118032010. View Article : Google Scholar : PubMed/NCBI
|
|
38.
|
Yuan A, Farber EL, Rapoport AL, Tejada D,
Deniskin R, Akhmedov NB and Farber DB: Transfer of microRNAs by
embryonic stem cell microvesicles. PLoS One. 4:e47222009.
View Article : Google Scholar : PubMed/NCBI
|
|
39.
|
Valadi H, Ekstrom K, Bossios A, Sjostrand
M, Lee JJ and Lotvall JO: Exosome-mediated transfer of mRNAs and
microRNAs is a novel mechanism of genetic exchange between cells.
Nat Cell Biol. 9:654–659. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
40.
|
Bruno S, Grange C, Deregibus MC, Calogero
RA, Saviozzi S, Collino F, Morando L, Busca A, Falda M, Bussolati
B, Tetta C and Camussi G: Mesenchymal stem cell-derived
microvesicles protect against acute tubular injury. J Am Soc
Nephrol. 20:1053–1067. 2009. View Article : Google Scholar
|
|
41.
|
Gatti S, Bruno S, Deregibus MC, Sordi A,
Cantaluppi V, Tetta C and Camussi G: Microvesicles derived from
human adult mesenchymal stem cells protect against
ischaemiareperfusion-induced acute and chronic kidney injury.
Nephrol Dial Transplant. 26:1474–1483. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
42.
|
Ratajczak J, Miekus K, Kucia M, Zhang J,
Reca R, Dvorak P and Ratajczak MZ: Embryonic stem cell-derived
microvesicles reprogram hematopoietic progenitors: evidence for
horizontal transfer of mRNA and protein delivery. Leukemia.
20:847–856. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
43.
|
Ardoin SP, Shanahan JC and Pisetsky DS:
The role of microparticles in inflammation and thrombosis. Scand J
Immunol. 66:159–165. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
44.
|
Huang C, Gu H, Yu Q, Manukyan MC, Poynter
JA and Wang M: Sca-1+ cardiac stem cells mediate acute
cardioprotection via paracrine factor SDF-1 following myocardial
ischemia/reperfusion. PLoS One. 6:e292462011.
|
|
45.
|
Askari AT, Unzek S, Popovic ZB, Goldman
CK, Forudi F, Kiedrowski M, Rovner A, Ellis SG, Thomas JD,
DiCorleto PE, Topol EJ and Penn MS: Effect of stromal-cell-derived
factor 1 on stem-cell homing and tissue regeneration in ischaemic
cardiomyopathy. Lancet. 362:697–703. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
46.
|
Hu X, Dai S, Wu WJ, Tan W, Zhu X, Mu J,
Guo Y, Bolli R and Rokosh G: Stromal cell derived factor-1 alpha
confers protection against myocardial ischemia/reperfusion injury:
role of the cardiac stromal cell derived factor-1 alpha CXCR4 axis.
Circulation. 116:654–663. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
47.
|
Linke A, Muller P, Nurzynska D, Casarsa C,
Torella D, Nascimbene A, Castaldo C, Cascapera S, Bohm M, Quaini F,
Urbanek K, Leri A, Hintze TH, Kajstura J and Anversa P: Stem cells
in the dog heart are self-renewing, clonogenic, and multi-potent
and regenerate infarcted myocardium, improving cardiac function.
Proc Natl Acad Sci USA. 102:8966–8971. 2005. View Article : Google Scholar
|
|
48.
|
Nesselmann C, Ma N, Bieback K, Wagner W,
Ho A, Konttinen YT, Zhang H, Hinescu ME and Steinhoff G:
Mesenchymal stem cells and cardiac repair. J Cell Mol Med.
12:1795–1810. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
49.
|
Noiseux N, Gnecchi M, Lopez-Ilasaca M,
Zhang L, Solomon SD, Deb A, Dzau VJ and Pratt RE: Mesenchymal stem
cells over-expressing Akt dramatically repair infarcted myocardium
and improve cardiac function despite infrequent cellular fusion or
differentiation. Mol Ther. 14:840–850. 2006. View Article : Google Scholar
|
|
50.
|
Uemura R, Xu M, Ahmad N and Ashraf M: Bone
marrow stem cells prevent left ventricular remodeling of ischemic
heart through paracrine signaling. Circ Res. 98:1414–1421. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
51.
|
Wang Y, Abarbanell AM, Herrmann JL, Weil
BR, Manukyan MC, Poynter JA and Meldrum DR: TLR4 inhibits
mesenchymal stem cell (MSC) STAT3 activation and thereby exerts
deleterious effects on MSC-mediated cardioprotection. PLoS One.
5:e142062010. View Article : Google Scholar : PubMed/NCBI
|
|
52.
|
Tang JM, Wang JN, Zhang L, Zheng F, Yang
JY, Kong X, Guo LY, Chen L, Huang YZ, Wan Y and Chen SY: VEGF/SDF-1
promotes cardiac stem cell mobilization and myocardial repair in
the infarcted heart. Cardiovasc Res. 91:402–411. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
53.
|
Timmers L, Lim SK, Arslan F, Armstrong JS,
Hoefer IE, Doevendans PA, Piek JJ, El Oakley RM, Choo A, Lee CN,
Pasterkamp G and de Kleijn DP: Reduction of myocardial infarct size
by human mesenchymal stem cell conditioned medium. Stem Cell Res.
1:129–137. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
54.
|
Lai RC, Tan SS, Teh BJ, Sze SK, Arslan F,
de Kleijn DP, Choo A and Lim SK: Proteolytic potential of the MSC
exosome proteome: Implications for an exosome-mediated delivery of
therapeutic proteasome. Int J Proteomics.
2012:9719072012.PubMed/NCBI
|
|
55.
|
Xu M, Uemura R, Dai Y, Wang Y, Pasha Z and
Ashraf M: In vitro and in vivo effects of bone marrow stem cells on
cardiac structure and function. J Mol Cell Cardiol. 42:441–448.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
56.
|
Timmers L, Lim SK, Hoefer IE, Arslan F,
Lai RC, van Oorschot AA, Goumans MJ, Strijder C, Sze SK, Choo A,
Piek JJ, Doevendans PA, Pasterkamp G and de Kleijn DP: Human
mesenchymal stem cell-conditioned medium improves cardiac function
following myocardial infarction. Stem Cell Res. 6:206–214. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
57.
|
Gnecchi M, He H, Noiseux N, Liang OD,
Zhang L, Morello F, Mu H, Melo LG, Pratt RE, Ingwall JS and Dzau
VJ: Evidence supporting paracrine hypothesis for Akt-modified
mesenchymal stem cell-mediated cardiac protection and functional
improvement. FASEB J. 20:661–669. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
58.
|
Kinnaird T, Stabile E, Burnett MS, Shou M,
Lee CW, Barr S, Fuchs S and Epstein SE: Local delivery of
marrow-derived stromal cells augments collateral perfusion through
paracrine mechanisms. Circulation. 109:1543–1549. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
59.
|
Estrada R, Li N, Sarojini H, An J, Lee MJ
and Wang E: Secretome from mesenchymal stem cells induces
angiogenesis via Cyr61. J Cell Physiol. 219:563–571. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
60.
|
Ohnishi S, Sumiyoshi H, Kitamura S and
Nagaya N: Mesenchymal stem cells attenuate cardiac fibroblast
proliferation and collagen synthesis through paracrine actions.
FEBS Lett. 581:3961–3966. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
61.
|
Xu X, Xu Z, Xu Y and Cui G: Effects of
mesenchymal stem cell transplantation on extracellular matrix after
myocardial infarction in rats. Coron Artery Dis. 16:245–255. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
62.
|
Cho HJ, Lee N, Lee JY, Choi YJ, Ii M,
Wecker A, Jeong JO, Curry C, Qin G and Yoon YS: Role of host
tissues for sustained humoral effects after endothelial progenitor
cell transplantation into the ischemic heart. J Exp Med.
204:3257–3269. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
63.
|
Oshima H, Payne TR, Urish KL, Sakai T,
Ling Y, Gharaibeh B, Tobita K, Keller BB, Cummins JH and Huard J:
Differential myocardial infarct repair with muscle stem cells
compared to myoblasts. Mol Ther. 12:1130–1141. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
64.
|
Kupatt C, Bock-Marquette I and Boekstegers
P: Embryonic endothelial progenitor cell-mediated cardioprotection
requires thymosin beta4. Trends Cardiovasc Med. 18:205–210. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
65.
|
Payne TR, Oshima H, Okada M, Momoi N,
Tobita K, Keller BB, Peng H and Huard J: A relationship between
vascular endothelial growth factor, angiogenesis, and cardiac
repair after muscle stem cell transplantation into ischemic hearts.
J Am Coll Cardiol. 50:1677–1684. 2007. View Article : Google Scholar
|
|
66.
|
Wu G, Rana JS, Wykrzykowska J, Du Z, Ke Q,
Kang P, Li J and Laham RJ: Exercise-induced expression of VEGF and
salvation of myocardium in the early stage of myocardial
infarction. Am J Physiol Heart Circ Physiol. 296:H389–H395. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
67.
|
Ambrosio F, Wolf SL, Delitto A, Fitzgerald
GK, Badylak SF, Boninger ML and Russell AJ: The emerging
relationship between regenerative medicine and physical
therapeutics. Phys Ther. 90:1807–1814. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
68.
|
Li TS, Cheng K, Malliaras K, Smith RR,
Zhang Y, Sun B, Matsushita N, Blusztajn A, Terrovitis J, Kusuoka H,
Marban L and Marban E: Direct comparison of different stem cell
types and subpopulations reveals superior paracrine potency and
myocardial repair efficacy with cardiosphere-derived cells. J Am
Coll Cardiol. 59:942–953. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
69.
|
Duran JM, Makarewich CA, Sharp TE,
Starosta T, Zhu F, Hoffman NE, Chiba Y, Madesh M, Berretta RM, Kubo
H and Houser SR: Bone-derived stem cells repair the heart after
myocardial infarction through transdifferentiation and paracrine
signaling mechanisms. Circ Res. 113:539–552. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
70.
|
Horie N, Pereira MP, Niizuma K, Sun G,
Keren-Gill H, Encarnacion A, Shamloo M, Hamilton SA, Jiang K, Huhn
S, Palmer TD, Bliss TM and Steinberg GK: Transplanted stem
cell-secreted vascular endothelial growth factor effects
post-stroke recovery, inflammation, and vascular repair. Stem
Cells. 29:274–285. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
71.
|
Andres RH, Horie N, Slikker W, Keren-Gill
H, Zhan K, Sun G, Manley NC, Pereira MP, Sheikh LA, McMillan EL,
Schaar BT, Svendsen CN, Bliss TM and Steinberg GK: Human neural
stem cells enhance structural plasticity and axonal transport in
the ischaemic brain. Brain. 134:1777–1789. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
72.
|
Liauw J, Hoang S, Choi M, Eroglu C, Sun
GH, Percy M, Wildman-Tobriner B, Bliss T, Guzman RG, Barres BA and
Steinberg GK: Thrombospondins 1 and 2 are necessary for synaptic
plasticity and functional recovery after stroke. J Cereb Blood Flow
Metab. 28:1722–1732. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
73.
|
Lu P, Jones LL, Snyder EY and Tuszynski
MH: Neural stem cells constitutively secrete neurotrophic factors
and promote extensive host axonal growth after spinal cord injury.
Exp Neurol. 181:115–129. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
74.
|
Cantinieaux D, Quertainmont R, Blacher S,
Rossi L, Wanet T, Noel A, Brook G, Schoenen J and Franzen R:
Conditioned medium from bone marrow-derived mesenchymal stem cells
improves recovery after spinal cord injury in rats: an original
strategy to avoid cell transplantation. PLoS One. 8:e695152013.
View Article : Google Scholar
|
|
75.
|
Crigler L, Robey RC, Asawachaicharn A,
Gaupp D and Phinney DG: Human mesenchymal stem cell subpopulations
express a variety of neuro-regulatory molecules and promote
neuronal cell survival and neuritogenesis. Exp Neurol. 198:54–64.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
76.
|
Sallustio F, Costantino V, Cox SN, Loverre
A, Divella C, Rizzi M and Schena FP: Human renal stem/progenitor
cells repair tubular epithelial cell injury through TLR2-driven
inhibin-A and microvesicle-shuttled decorin. Kidney Int.
83:392–403. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
77.
|
Maeshima A, Zhang YQ, Furukawa M, Naruse T
and Kojima I: Hepatocyte growth factor induces branching
tubulogenesis in MDCK cells by modulating the activin-follistatin
system. Kidney Int. 58:1511–1522. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
78.
|
Togel F, Weiss K, Yang Y, Hu Z, Zhang P
and Westenfelder C: Vasculotropic, paracrine actions of infused
mesenchymal stem cells are important to the recovery from acute
kidney injury. Am J Physiol Renal Physiol. 292:F1626–1635. 2007.
View Article : Google Scholar
|
|
79.
|
Imberti B, Morigi M, Tomasoni S, Rota C,
Corna D, Longaretti L, Rottoli D, Valsecchi F, Benigni A, Wang J,
Abbate M, Zoja C and Remuzzi G: Insulin-like growth factor-1
sustains stem cell mediated renal repair. J Am Soc Nephrol.
18:2921–2928. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
80.
|
Van Koppen A, Joles JA, van Balkom BW, Lim
SK, de Kleijn D, Giles RH and Verhaar MC: Human embryonic
mesenchymal stem cell-derived conditioned medium rescues kidney
function in rats with established chronic kidney disease. PLoS One.
7:e387462012.PubMed/NCBI
|
|
81.
|
Mintz PJ, Huang KW, Reebye V, Nteliopoulos
G, Lai HS, Saetrom P, Kasahara N, Jensen S, Pai M, Gordon MY,
Marley SB, Behan R, Spalding DR, Haoudi A, Emara MM, Nicholls J,
Rossi JJ and Habib NA: Exploiting human CD34 stem cell-conditioned
medium for tissue repair. Mol Ther. 22:149–159. 2014.PubMed/NCBI
|
|
82.
|
Hogaboam CM, Bone-Larson CL, Steinhauser
ML, Lukacs NW, Colletti LM, Simpson KJ, Strieter RM and Kunkel SL:
Novel CXCR2-dependent liver regenerative qualities of
ELR-containing CXC chemokines. FASEB J. 13:1565–1574.
1999.PubMed/NCBI
|
|
83.
|
Wei J, Barr J, Kong LY, Wang Y, Wu A,
Sharma AK, Gumin J, Henry V, Colman H, Priebe W, Sawaya R, Lang FF
and Heimberger AB: Glioblastoma cancer-initiating cells inhibit
T-cell proliferation and effector responses by the signal
transducers and activators of transcription 3 pathway. Mol Cancer
Ther. 9:67–78. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
84.
|
Wei J, Barr J, Kong LY, Wang Y, Wu A,
Sharma AK, Gumin J, Henry V, Colman H, Sawaya R, Lang FF and
Heimberger AB: Glioma-associated cancer-initiating cells induce
immunosuppression. Clin Cancer Res. 16:461–473. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
85.
|
Peng W, Wang HY, Miyahara Y, Peng G and
Wang RF: Tumor-associated galectin-3 modulates the function of
tumor-reactive T cells. Cancer Res. 68:7228–7236. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
86.
|
Kuklinski S, Pesheva P, Heimann C, Urschel
S, Gloor S, Graeber S, Herzog V, Pietsch T, Wiestler OD and
Probstmeier R: Expression pattern of galectin-3 in neural tumor
cell lines. J Neurosci Res. 60:45–57. 2000. View Article : Google Scholar
|
|
87.
|
Bao S, Wu Q, Sathornsumetee S, Hao Y, Li
Z, Hjelmeland AB, Shi Q, McLendon RE, Bigner DD and Rich JN: Stem
cell-like glioma cells promote tumor angiogenesis through vascular
endothelial growth factor. Cancer Res. 66:7843–7848. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
88.
|
Oka N, Soeda A, Inagaki A, Onodera M,
Maruyama H, Hara A, Kunisada T, Mori H and Iwama T: VEGF promotes
tumorigenesis and angiogenesis of human glioblastoma stem cells.
Biochem Biophys Res Commun. 360:553–559. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
89.
|
Folkins C, Shaked Y, Man S, Tang T, Lee
CR, Zhu Z, Hoffman RM and Kerbel RS: Glioma tumor stem-like cells
promote tumor angiogenesis and vasculogenesis via vascular
endothelial growth factor and stromal-derived factor 1. Cancer Res.
69:7243–7551. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
90.
|
Ping YF, Yao XH, Jiang JY, Zhao LT, Yu SC,
Jiang T, Lin MC, Chen JH, Wang B, Zhang R, Cui YH, Qian C, Wang J
and Bian XW: The chemokine CXCL12 and its receptor CXCR4 promote
glioma stem cell-mediated VEGF production and tumour angiogenesis
via PI3K/AKT signalling. J Pathol. 224:344–354. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
91.
|
Lin G, Yang R, Banie L, Wang G, Ning H, Li
LC, Lue TF and Lin CS: Effects of transplantation of adipose
tissue-derived stem cells on prostate tumor. Prostate.
70:1066–1173. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
92.
|
Ho IA, Toh HC, Ng WH, Teo YL, Guo CM, Hui
KM and Lam PY: Human bone marrow-derived mesenchymal stem cells
suppress human glioma growth through inhibition of angiogenesis.
Stem Cells. 31:146–155. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
93.
|
Kong BH, Shin HD, Kim SH, Mok HS, Shim JK,
Lee JH, Shin HJ, Huh YM, Kim EH, Park EK, Chang JH, Kim DS, Hong
YK, Lee SJ and Kang SG: Increased in vivo angiogenic effect
of glioma stromal mesenchymal stem-like cells on glioma cancer stem
cells from patients with glioblastoma. Int J Oncol. 42:1754–1762.
2013.
|
|
94.
|
Akimoto K, Kimura K, Nagano M, Takano S,
To'a Salazar G, Yamashita T and Ohneda O: Umbilical cord
blood-derived mesenchymal stem cells inhibit, but adipose
tissue-derived mesenchymal stem cells promote, glioblastoma
multiforme proliferation. Stem Cells Dev. 22:1370–1386. 2013.
View Article : Google Scholar
|
|
95.
|
Bussolati B, Dekel B, Azzarone B and
Camussi G: Human renal cancer stem cells. Cancer Lett. 338:141–146.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
96.
|
Grange C, Tapparo M, Collino F, Vitillo L,
Damasco C, Deregibus MC, Tetta C, Bussolati B and Camussi G:
Microvesicles released from human renal cancer stem cells stimulate
angiogenesis and formation of lung premetastatic niche. Cancer Res.
71:5346–5356. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
97.
|
Todaro M, Alea MP, Di Stefano AB,
Cammareri P, Vermeulen L, Iovino F, Tripodo C, Russo A, Gulotta G,
Medema JP and Stassi G: Colon cancer stem cells dictate tumor
growth and resist cell death by production of interleukin-4. Cell
Stem Cell. 1:389–402. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
98.
|
Francipane MG, Alea MP, Lombardo Y, Todaro
M, Medema JP and Stassi G: Crucial role of interleukin-4 in the
survival of colon cancer stem cells. Cancer Res. 68:4022–4025.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
99.
|
Conticello C, Pedini F, Zeuner A, Patti M,
Zerilli M, Stassi G, Messina A, Peschle C and De Maria R: IL-4
protects tumor cells from anti-CD95 and chemotherapeutic agents via
up-regulation of antiapoptotic proteins. J Immunol. 172:5467–5477.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
100.
|
Todaro M, Zerilli M, Ricci-Vitiani L, Bini
M, Perez Alea M, Maria Florena A, Miceli L, Condorelli G, Bonventre
S, Di Gesu G, De Maria R and Stassi G: Autocrine production of
interleukin-4 and interleukin-10 is required for survival and
growth of thyroid cancer cells. Cancer Res. 66:1491–1499. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
101.
|
Emmink BL, Verheem A, Van Houdt WJ,
Steller EJ, Govaert KM, Pham TV, Piersma SR, Borel Rinkes IH,
Jimenez CR and Kranenburg O: The secretome of colon cancer stem
cells contains drug-metabolizing enzymes. J Proteomics. 2013.84–96.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
102.
|
Long H, Xie R, Xiang T, Zhao Z, Lin S,
Liang Z, Chen Z and Zhu B: Autocrine CCL5 signaling promotes
invasion and migration of CD133+ ovarian cancer
stem-like cells via NF-kappaB-mediated MMP-9 upregulation. Stem
Cells. 30:2309–2319. 2012.PubMed/NCBI
|
|
103.
|
Karnoub AE, Dash AB, Vo AP, Sullivan A,
Brooks MW, Bell GW, Richardson AL, Polyak K, Tubo R and Weinberg
RA: Mesenchymal stem cells within tumour stroma promote breast
cancer metastasis. Nature. 449:557–563. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
104.
|
Dittmer J, Oerlecke I and Leyh B:
Involvement of mesenchymal stem cells in breast cancer progression.
Breast Cancer-Focusing Tumor Microenvironment, Stem Cells and
Metastasis. Gunduz M and Gunduz E: INTECH Open Access Publisher;
Rijeka: pp. 247–272. 2011
|
|
105.
|
Liu S, Ginestier C, Ou SJ, Clouthier SG,
Patel SH, Monville F, Korkaya H, Heath A, Dutcher J, Kleer CG, Jung
Y, Dontu G, Taichman R and Wicha MS: Breast cancer stem cells are
regulated by mesenchymal stem cells through cytokine networks.
Cancer Res. 71:614–624. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
106.
|
Devarajan E, Song YH, Krishnappa S and Alt
E: Epithelialmesenchymal transition in breast cancer lines is
mediated through PDGF-D released by tissue-resident stem cells. Int
J Cancer. 131:1023–1031. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
107.
|
Shipitsin M, Campbell LL, Argani P,
Weremowicz S, Bloushtain-Qimron N, Yao J, Nikolskaya T,
Serebryiskaya T, Beroukhim R, Hu M, Halushka MK, Sukumar S, Parker
LM, Anderson KS, Harris LN, Garber JE, Richardson AL, Schnitt SJ,
Nikolsky Y, Gelman RS and Polyak K: Molecular definition of breast
tumor heterogeneity. Cancer Cell. 11:259–273. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
108.
|
Hardt O, Wild S, Oerlecke I, Hofmann K,
Luo S, Wiencek Y, Kantelhardt E, Vess C, Smith GP, Schroth GP,
Bosio A and Dittmer J: Highly sensitive profiling of
CD44(+)/CD24(−) breast cancer stem cells by combining global mRNA
amplification and next generation sequencing: Evidence for a
hyperactive PI3K pathway. Cancer Lett. 325:165–174. 2012.
|
|
109.
|
Harbeck N, Schmitt M, Meisner C, Friedel
C, Untch M, Schmidt M, Sweep CG, Lisboa BW, Lux MP, Beck T,
Hasmuller S, Kiechle M, Janicke F and Thomssen C: Ten-year analysis
of the prospective multicentre Chemo-N0 trial validates American
Society of Clinical Oncology (ASCO)-recommended biomarkers uPA and
PAI-1 for therapy decision making in node-negative breast cancer
patients. Eur J Cancer. 49:1825–1835. 2013.
|
|
110.
|
Dellas C and Loskutoff DJ: Historical
analysis of PAI-1 from its discovery to its potential role in cell
motility and disease. Thromb Haemost. 93:631–640. 2005.PubMed/NCBI
|
|
111.
|
Czekay RP, Wilkins-Port CE, Higgins SP,
Freytag J, Overstreet JM, Klein RM, Higgins CE, Samarakoon R and
Higgins PJ: PAI-1: An integrator of cell signaling and migration.
Int J Cell Biol. 2011:5624812011. View Article : Google Scholar : PubMed/NCBI
|
|
112.
|
Hogan NM, Joyce MR, Murphy JM, Barry FP,
O'Brien T, Kerin MJ and Dwyer RM: Impact of mesenchymal stem cell
secreted PAI-1 on colon cancer cell migration and proliferation.
Biochem Biophys Res Commun. 435:574–579. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
113.
|
Beck B, Driessens G, Goossens S, Youssef
KK, Kuchnio A, Caauwe A, Sotiropoulou PA, Loges S, Lapouge G, Candi
A, Mascre G, Drogat B, Dekoninck S, Haigh JJ, Carmeliet P and
Blanpain C: A vascular niche and a VEGF-Nrp1 loop regulate the
initiation and stemness of skin tumours. Nature. 478:399–403. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
114.
|
Monzani E, Facchetti F, Galmozzi E,
Corsini E, Benetti A, Cavazzin C, Gritti A, Piccinini A, Porro D,
Santinami M, Invernici G, Parati E, Alessandri G and La Porta CA:
Melanoma contains CD133 and ABCG2 positive cells with enhanced
tumourigenic potential. Eur J Cancer. 43:935–946. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
115.
|
Maeda S, Shinchi H, Kurahara H, Mataki Y,
Maemura K, Sato M, Natsugoe S, Aikou T and Takao S: CD133
expression is correlated with lymph node metastasis and vascular
endothelial growth factor-C expression in pancreatic cancer. Br J
Cancer. 98:1389–1397. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
116.
|
Romagnani P and Anders HJ: What can
tubular progenitor cultures teach us about kidney regeneration?
Kidney Int. 83:351–353. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
117.
|
Calabrese C, Poppleton H, Kocak M, Hogg
TL, Fuller C, Hamner B, Oh EY, Gaber MW, Finklestein D, Allen M,
Frank A, Bayazitov IT, Zakharenko SS, Gajjar A, Davidoff A and
Gilbertson RJ: A perivascular niche for brain tumor stem cells.
Cancer Cell. 11:69–82. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
118.
|
Eyler CE and Rich JN: Survival of the
fittest: cancer stem cells in therapeutic resistance and
angiogenesis. J Clin Oncol. 26:2839–2845. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
119.
|
Ghajar CM, Peinado H, Mori H, Matei IR,
Evason KJ, Brazier H, Almeida D, Koller A, Hajjar KA, Stainier DY,
Chen EI, Lyden D and Bissell MJ: The perivascular niche regulates
breast tumour dormancy. Nat Cell Biol. 15:807–617. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
120.
|
Descot A and Oskarsson T: The molecular
composition of the metastatic niche. Exp Cell Res. 319:1679–1686.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
121.
|
Gupta PB, Onder TT, Jiang G, Tao K,
Kuperwasser C, Weinberg RA and Lander ES: Identification of
selective inhibitors of cancer stem cells by high-throughput
screening. Cell. 138:645–659. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
122.
|
Bottos A and Bardelli A: Oncogenes and
angiogenesis: a way to personalize anti-angiogenic therapy? Cell
Mol Life Sci. 70:4131–4140. 2013. View Article : Google Scholar : PubMed/NCBI
|