Contents
Introduction
Tumor-induced angiogenesis and lymphangiogenesis
The molecular mechanism of tumor-induced
angiogenesis and lymphangiogenesis
The origin of tumor neogenetic endothelial cells
CSCs and tumor metastasis
CSCs and vasculature of tumor
CSCs and lymphatic vasculature as a significant
thera peutic target
Summary and conclusions
Introduction
Metastasis is defined as the spread of cancer cells
from the site of an original malignant primary tumor to one or more
other places in the body. Over 90% of cancer suffering and death is
associated with metastatic spread. Therefore, a significant aim of
cancer research is to understand the molecular and cellular
mechanisms that underlie the processes of metastasis.
Metastasis is most often associated with solid
tumors. Evidence suggests that an important initial event in the
spread of solid tumors is through the lymphatic system
(lymphanogenous spread), while spread via blood vessels
(hematogenous spread) may be secondary (1). Indeed, metastatic spread to regional
lymph nodes is considered a prognostic indicator and may help to
determine cancer management and therapy (1).
It is widely accepted that angiogenesis is involved
in solid tumor growth and hematogenous spread. However, current
opinion indicates that lymphangiogenesis plays the key role in
promoting the initial spread of malignant tumors. Despite this, the
mechanism that underlies lymphatic spread and the role of
lymphangiogenesis in tumor metastasis is not clear.
Recently, the cancer stem cell (CSC) theory has
emerged as an attractive hypothesis for tumor development and
progression. The theory suggests that tumors consist of subsets of
cells with functional heterogeneity. In the CSC model, one small
subset of cancer cells has the characteristics of stem cells. These
CSCs have the capability of both self-renewal and differentiation
into diverse cancer cells, which play a decisive role in
maintaining capacity for malignant proliferation, invasion,
metastasis, and tumor recurrence (2,3).
Assuming CSCs are relatively refractory to the therapies developed
to eradicate the non-stem cell component of tumors, the CSC model
provides a theoretical basis for developing therapies that target
the minority CSC population and presents a new perspective for the
treatment of cancer (3).
So how do CSCs play a role in tumor metastasis and
especially in lymphatic metastasis? Accumulating evidence suggests
that CSCs closely correlate to tumor metastasis (1–5).
This idea is supported by previous experimental observations
including: a) CSCs can induce cancer metastasis through multiple
pathways; b) angiogenesis and lymphangiogenesis are significant
pathological changes in the process of tumor metastasis; and c)
CSCs participate in angiogenesis and lymphangiogenesis directly and
indirectly.
In this review we investigate the possible
relationship between CSCs, tumor-induced lymphangiogenesis, and
lymphatic metastasis in an attempt to reveal cellular mechanisms
associated with metastatic spread, and to inform research on the
development of approaches to block tumor lymphatic metastasis
(1,4).
Tumor-induced angiogenesis and
lymphangiogenesis
Tumor-induced angiogenesis and lymphangiogenesis
play an important role in promoting tumor growth and metastasis
(5). The continued tumor growth is
often associated with neovascularization. Intratumoral hypoxia
upregulates the expression of the vascular endothelial growth
factor (VEGF) which induces angiogenesis, offering the necessary
routes for cell dissemination, changing vascular integrity and
permeability and even promoting intravasation and extravasation
(6). Moreover, hypoxia selects a
subpopulation of tumor cells with an invasive and metastatic
phenotype that have the capacities of escaping from the primary
tumors (7).
Lymphangiogenesis is also considered as a potential
facilitator of cancer metastasis. Cancer cells move to the regional
lymph nodes draining the primary tumor tissues promote tumor cells
to migrate to local lymph nodes and even to distant organs
(23). A number of studies have
confirmed the association between lymphatic vessel density and
survival rate in patients with different types of cancer.
Schoppmann et al investigated invasive breast cancer by
immunohistochemical staining for the lymphatic endothelial marker
podoplanin and the vascular endothelial marker CD34, and showed
that lymphatic microvessel density and lymphovascular invasion
correlated with lymph node metastasis (8). Beasley et al analyzed samples
from human head and neck cancers by immunohistochemical staining
for the lymphatic endothelial marker LYVE-1, CD34, and the pKi67
proliferation marker, and quantified the lymphangiogenic growth
factor, vascular endothelial growth factor C, by real-time
polymerase chain reaction. Their study provided evidence that
proliferating lymphatics occur in human malignant tumors, and they
reported that a high intratumoral lymph vessel density was
significantly correlated with cervical node metastases and an
infiltrating margin of tumor invasion (9).
The lymphatic system is the primary conduit for
initial metastasis in numerous types of solid tumors, including
breast, colon, and prostate cancers. Metastatic spread is enhanced
by increased lymphangiogenesis in and around the primary tumor
(10,11). Tumor-induced lymphangiogenesis at
the tumor periphery correlates with lymph node metastasis.
Elucidating the interaction between lymphangiogenesis and tumor
metastasis will bring us new insights into mechanisms of lymphatic
metastasis.
The molecular mechanism of tumor-induced
angiogenesis and lymphangiogenesis
Various lymphatic growth factors and vascular growth
factors participate in regulating tumor-induced angiogenesis and
lymphangiogenesis. Most of these factors are shown to have dual
effects and interact with each other during angiogenesis and
lymphangiogenesis making distinguishing difficult. It is believed
that these factors are secreted by tumor cells, stromal cells, and
inflammatory cells in the tumor microenvironment (12–14).
Vascular endothelial growth factors (VEGFs)
stimulate angiogenesis and lymphangiogenesis by activating VEGF
receptor (VEGFR) tyrosine kinases in endothelial cells. The major
signaling pathways for lymphangiogenesis include secreting type
glucoprotein VEGF-C and VEGF-D, which function through VEGFR-3
(Flt4) expressed on the surface of lymphatic endothelial cells
(LECs). At present, the VEGF-C/ VEGF-D/VEGFR-3 pathway is the most
understood pathway regulating lymphangiogenesis (15,16).
However, Tammela et al showed that VEGFR-3 as a regulator of
vascular network formation. Targeting VEGFR-3 may provide
additional efficacy for anti-angiogenic therapies, especially
towards vessels that are resistant to VEGF or VEGFR-2 inhibitors
(17).
VEGF-A, another VEGF family member, initially
identified as an important promoter of angiogenesis, primarily
binds to VEGFR-1 and VEGFR-2 (18). However, VEGF-A can also induce
tumor lymphangiogenesis and promote tumor metastasis to regional
and distant lymph nodes (19).
Tie-1,-2 and their ligand angiopoietins-1, -2 and -4 (Ang-1, -2 and
-4) play a role in tumor angiogenesis and lymphangiogenesis. Ang/
Tie signaling pathway closely relates to the VEGF/VEGFR signaling
pathway. VEGF-A, -C and PDGFB can upregulate Ang-2. Moreover,
hepatocyte growth factor (HGF), fibroblast growth factor-2 (FGF-2),
platelet-derived growth factor-BB (PDGFBB), insulin-like growth
factors 1 and 2 (IGF-1 and -2), and endothelin-1 (ET-1) have been
identified as inducers of angiogenesis and lymphangiogenesis. The
main angiogenic and lymphangiogenic growth factors are summarized
in Fig. 1.
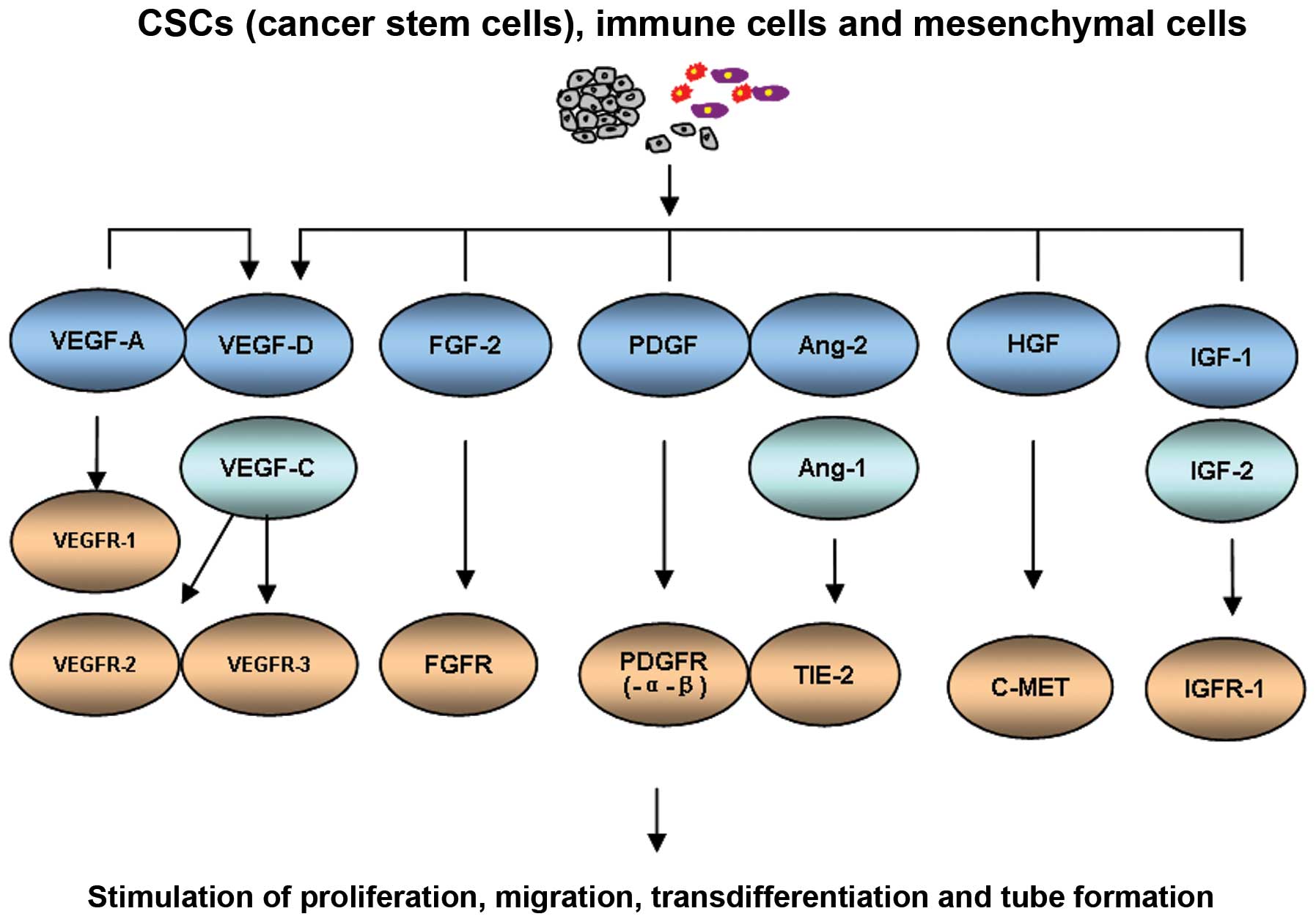 | Figure 1.Lymphangiogenic growth factors and
their receptors. Ang-1 and -2, angiopoietins-1 and -2; ET-1,
endothelin-1; FGF-2, fibroblast growth factor-2; FGFR, fibroblast
growth factor receptor; HGF, hepatocyte growth factor; IGF-1, -2,
insulin-like growth factors-1 and -2; IGFR-1, insulin-like growth
factor receptor-1; PDGF, platelet-derived growth factor; PDGFR,
platelet-derived growth factor receptor; TIE-2, endothelial
cell-specific receptor tyrosine kinase; VEGF-A, -C and -D, vascular
endothelial growth factors-A, -C and -B; VEGFR-1, -2 and -3,
vascular endothelial growth factor receptors-1, -2 and -3. |
CSCs show greater potential for angiogenesis and
lymphangiogenesis than non-stem cell-like tumor cells. Malignant
gliomas are highly lethal cancers dependent on angiogenesis. Bao
et al (20) examined the
potential of stem cell-like glioma cells (SCLGC) to support tumor
angiogenesis. In comparison with matched non-SCLGC populations,
SCLGC consistently secreted markedly elevated levels of vascular
endothelial growth factor (VEGF), which were further induced by
hypoxia. The VEGF expression in CD133+ SCLGC was
10–20-fold upregulated, combined with a dramatically increased
vascular density identified by CD31 staining. In an in vitro
model of angiogenesis, SCLGC-conditioned medium significantly
increased endothelial cell migration and tube formation compared
with non-SCLGC tumor cell-conditioned medium. Furthermore, therapy
with VEGF neutralizing antibody (bevacizumab) can deplete
SCLGC-induced vascular endothelial cell migration and tube
formation. These data indicate that stem cell-like tumor cells can
be a crucial source of key angiogenic factors in cancers and that
targeting proangiogenic factors from stem cell-like tumor
populations may be critical for patient therapy. Other studies
support this finding in CSCs isolated from U87 glioma human cell
lines and GL261 murine glioma cell lines (21,22).
Additionally it was reported that CSCs contribute to tumor vascular
development in glioma. The results revealed that tumor with larger
CSC population recruited a substantially higher amount of
endothelial progenitor cells (EPC), suggesting that CSCs promote
local angiogenesis and EPC mobilization via stimulating
proangiogenic factors such as VEGF and SDF-1 (23).
Related research has explored this phenomenon using
high throughput assays in liver cancer, qRT-PCR assessment and
tissue microarray (TMAs) validation (24). In the high hepatic stem/progenitor
cell (HSC/HPC) profile group (CD133, Nestin CD44 and ABCG2), the
MVD and angiogenic factors (VEGF and PD-ECGF) are significantly
higher than in the low HSC/ HPC profile group and related to a poor
prognosis. Both stemness and angiogenesis associated factors might
be potential biomarkers for clinical prediction (24).
Moreover, recent research showed that there are
signaling pathways associated with CSCs and angiogenesis. One is
bone morphogenic protein (BMP) signaling. The BMP was shown to play
a vital role in CSC tumorigenesis and angiogenesis (25,26).
Another important mechanism is the Notch signaling pathway.
Recently, Hovinga et al showed that the Notch pathway
combines glioblastoma angiogenesis and cancer stem cell
self-renewal (27). These two
pathways are both related to CSCs and angiogenesis, however,
further experiments need to be done to show the fundamental cause
and effect.
The origin of tumor neogenetic endothelial
cells
Identifying the origin of tumor neovascularized
blood endothelial cells (BECs) and lymphatic endothelial cells
(LECs) can help elucidate the cellular mechanisms of angiogenesis
and lymphangiogenesis.
Potential cellular origins of LECs include
pre-existing vasculature as well as bone marrow-derived progenitor
cells (BMDCs). A large body of evidence suggests that newly formed
lymphatic vessels primarily arise from the pre-existing local
vascular/lymphatic network during angiogenesis/lymphangiogenesis
and hematogenous/lymphanogenous spread (28–30).
However, as BM-derived vascular endothelial progenitor cells (EPCs)
support the formation of new blood vessels during tumor
angiogenesis, it is possible that BMDCs also contribute to the
expansion of the lymphatic vasculature during tumor metastasis
(28,31–33).
These findings have recently been challenged by the study of De
Palma et al (34), who
demonstrated BM-derived hemopoietic cells
(CD45+/CD11b+/CD31−/Tie2+)
rather than EPC (CD31+), homed specifically to tumors,
without any evidence of incorporation. The reason for such
diametrically conflicting results remains unclear. Indeed, recent
research indicates that lymphangiogenesis can occur from BM-derived
lymphatic lineage cells and that BM-derived non-endothelial cells
can transdifferentiate into tumor LECs under certain conditions. In
a model of mouse inflammation after corneal transplant, Maruyama
et al (35) demonstrated
that CD11b+ macrophages infiltrated the corneal stroma,
transdifferentiated into LECs, and integrated into existing
lymphatic vessels. In addition, tumor-associated macrophages (TAMs)
express the lymphatic marker VEGFR-3. However, the
transdifferentiation of TAMs into LECs during tumorigenesis
requires further investigation (36).
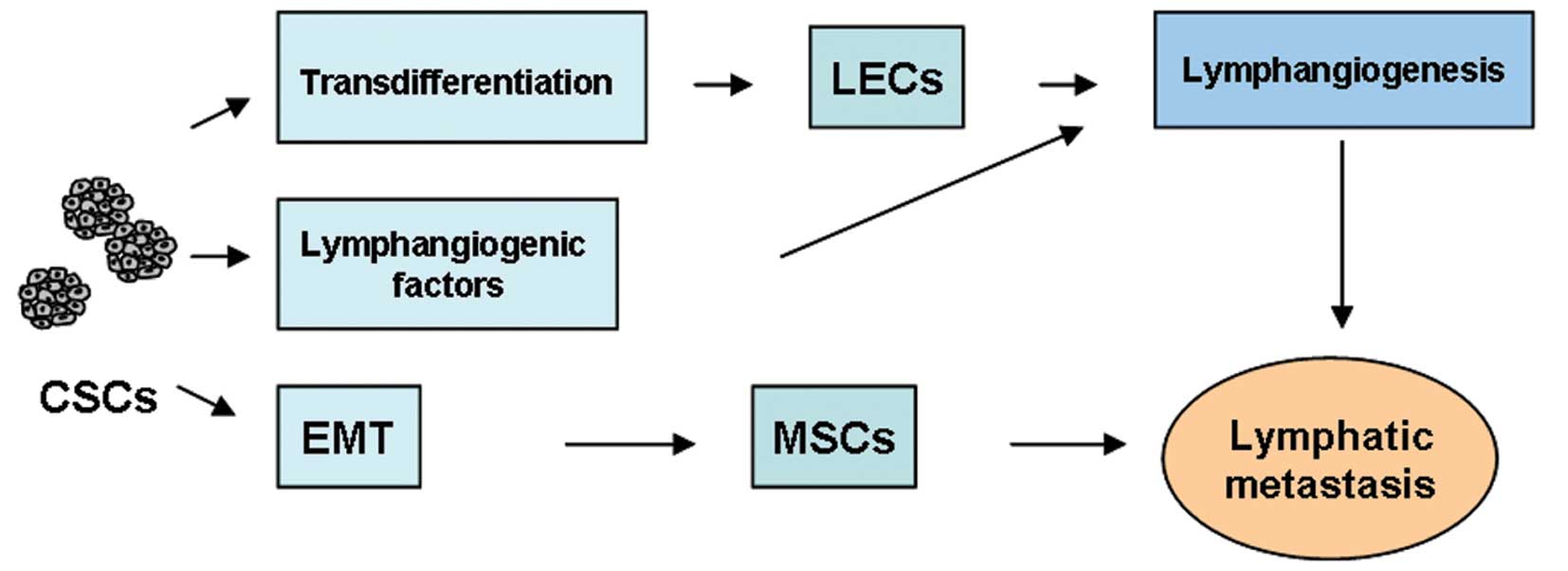
BM-derived mesenchymal stem cells (MSCs) may also
contribute to tumor angiogenesis and lympahgiogenesis. MSCs can
infiltrate tumors and may enhance breast cancer cell metastasis
(37). Furthermore, MSCs have the
ability to differentiate into endothelial cells (ECs) under certain
conditions, and ECs and MSCs are able to transdifferentiate and
interchange their phenotypes (37–40).
Such transdifferentiation may be facilitated by the tumor
microenvironment and could contribute to tumor progression.
Several studies have shown that LECs can arise by
transdifferentiation from blood endothelial cells (BECs). During
embryonic lymphangiogenesis, lymphatic endothelial precursor cells
are derived from venous ECs in the cardinal vein. This population
of venous ECs expresses transcription factors, including COUP-TFII,
PROX-1 and SOX18, which regulate the transdifferentiation of venous
ECs into LECs (41–43). Therefore, reactivation of a
specific set of transcription factors in the adult under certain
pathological conditions may regulate the differentiation of ECs by
turning on the molecular program required for transition from a BEC
to a LEC phenotype. In support of this concept, studies show that
blood vessels express the lymphatic marker VEGFR-3 in some tumors
(44,45). The expression of VEGFR-3 on BECs
can increase angiogenic activation by the VEGF pathway and induce
the LEC phenotype and may be indicative of a phenotypic transition
between blood and lymphatic vessels. The key pathways that trigger
the angiogenesis and lymphangiogenic switch during tumorigenesis
are shown in Table I.
 | Table I.Proposed cellular origin of lymphatic
endothelial cells and blood endothelial cells. |
Table I.
Proposed cellular origin of lymphatic
endothelial cells and blood endothelial cells.
| Authors (Ref.) | Possible cellular
origin | Main findings |
|---|
De Palma et
al (31)
Lyden et al (32) | Bone-marrow-derived
endothelial and hematopoietic precursor cells | Recruited to
angiogenic sites and support the formation of new vessels |
| Maruyama et
al (35) | Tumor-associated
macrophages (TAMS) | Transdifferentiated
into lymphatic endothelial cells that integrate into existing
lymphatic vessels |
| Medici et al
(40) | Bone marrow-derived
mesenchymal stem cells (MSCs) | ECs and MSCs are
able to interchange their phenotypes |
Paavonen et
al (44)
Valtola R et al (45) | Blood endothelial
cells (BECs) | The expression of
VEGFR-3 on BECs in some tumors and chronic wounds |
Shen et al
(55)
Bussolati et al (56) | Cancer stem cells
(CSCs) |
Transdifferentiation to blood vessel
endothelial cells |
CSCs and tumor metastasis
Studies show that CSCs closely correlate with tumor
metastasis (2,46). Pandit et al compared
differentially expressed genes in cell lines of high (468LN) vs.
low (468GFP) lymphatic metastatic ability to identify genes of
potential clinical relevance. This approach revealed that 468LN
cells have a higher proportion of cells with a CSC-like
(CD44+/CD24−) phenotype, have a higher
clonogenic potential, and a greater ability to survive, establish
and grow in a foreign microenvironment, relative to 468GFP cells
(47). Wakamatsu et al
immunohistochemically examined the expression and distribution of
representative CSC markers ALDH1, CD44, and CD133 from the primary
tumor and the lymph node metastasis of gastric cancer. They showed
ALDH1 positivity to be significantly higher in diffuse-type lymph
node metastasis than in the primary tumor. They concluded that this
CSC marker is important for tumor invasion and metastasis, and that
CSCs can promote the heterogeneity and lymphatic metastasis of
cancer (48).
Li et al (49) hypothesized that a single cancer
cell can be considered a CSC as long as it can: a) develop into a
tumor and b) its filial generation can inherit its biological
features. They cultured CD133+ colorectal cancer
monoplast cells in vitro and analyzed the invasive and
metastatic capabilities of CD133+ single cell-derived
progenies (SCPs) in a nude mouse model. They found that
CD133+ SCPs were more likely to produced tumors after
nude mice transplantation compared with CD133− SCPs, and
that CD133+ cells were heterogeneous in invasion and
metastasis in vitro and in vivo. They concluded that
colorectal CSCs constitute a diverse subpopulation.
To study the role of CSCs in the process of tumor
metastasis, Brabletz et al (50) suggested the migrating cancer stem
(MCS)-cell concept. They proposed that CSCs in situ can
transform to MCS cells by epithelial-mesenchymal transition (EMT).
Subsequently, the MCS cells disseminate and form metastatic
colonies. In support of this concept, several studies have shown
that cells possessing both the stem and tumorigenic characteristics
of CSCs can be derived from human mammary epithelial cells
(51,52).
CSCs and vasculature of tumor
It is believed that tumor metastasis is a complex
multistep process, characterized by local invasion followed by
intravasation of cancer cells into blood and lymphatic vessels
(53). The intrinsic properties of
the tumor itself and the tumor microenvironment are likely to be
the main triggers that determine the ability of cancer cells to
metastasize (54).
Several studies have shown that CSCs can promote
angiogenesis and lymphangiogenesis in tumor metastasis. Angiogenic
and lymphangiogenic factors are highly expressed by CSCs under
conditions of hypoxia, which suggests that CSCs can indirectly
promote angiogenesis and lymphangiogenesis during tumorigenesis and
progression. Moreover, CSCs may directly participate in
angiogenesis by transforming into tumor vasculogenic
stem/progenitor cells or constructing a tumor microcirculation by
developing vasculogenic mimicry without an endothelial pattern.
Shen et al (55) isolated
the CSC line 2C4 from the spleen of mice with leukemia. By
subcutaneously implanting enhanced green fluorescent protein
(GFP)-expressing transfected 2C4 cells into SCID CB17 mice, a
xenotransplant tumor was formed. CSC-derived GFP+
endothelial-like cells were identified by green fluorescence in
10-mm diameter tumors. In vitro, the morphology of the CSCs
was altered to elongated endothelial-like cells under conditions of
hypoxia, and the expression of VEGFR2 was unregulated in the
presence of cytokines, such as IL-13 and GM-CSF. The authors
concluded that CSCs transdifferentiated to blood vessel ECs and
were important for tumor vasculogenesis. Bussolati et al
(56) isolated and cloned a
population of breast tumor stem cells, which expressed the
endothelial markers CD31, VEGFR2 and FVIII, when cultured in the
presence of VEGF. The endothelial differentiated breast tumor stem
cells acquired the ability to organize into capillary-like
structures after 6 h in culture on Matrigel.
The ability of CSCs to participate in the
development of endothelium directly contributes to understanding of
the mechanisms of tumorigenesis and development. It challenges
traditional theory on cancer and anti-angiogenesis therapy and
emphasizes the potential of tumor lymphatic metastasis-resistant
therapy.
CSCs and lymphatic vasculature as a
significant therapeutic target
The source of tumor neolymphangiogenesis remains to
be elucidated. We hypothesize that direct transdifferentiation of
CSCs into LECs occurs during tumor lymphatic metastasis.
Establishing the exact relationship of CSCs, lymphangiogenesis, and
lymphatic metastasis will help to reveal the mechanisms of tumor
metastasis at the cellular level, and create new challenges for
future research.
Limitations of anti-angiogenic therapy have been
reported (57,58). Although preclinical and clinical
studies have established that anti-angiogenic therapies have
antitumoral effects and survival benefits, there are studies
showing that tumor cells can develop multiple mechanisms of
resistance, which can increase tumor invasion and distant
metastasis. A more significant therapeutic strategy could target
both blood and lymphatic vessels to maximize anti-tumor and
anti-metastasis effects.
The critical challenge of anti-lymphangiogenic
therapy is to control metastatic disease after surgical removal of
the primary tumor or inhibition by anti-angiogenic agents.
Anti-lymphangiogenic therapies may help prevent both lymph node and
distant organ metastasis by targeting lymphangiogenic growth
factors, lymph node metastasis, and cellular mechanisms of
differentiation and transdifferenatiation to LECs. Therefore, the
simultaneous use of anti-angiogenic and anti-lymphangiogenic agents
may improve current therapy.
It is well accepted that CSCs play a significant
role in tumorigenesis, metastasis, and recurrence. More and more
studies will focus on the role of CSCs in lymphangiogenesis,
possibly revealing new targets for anti-CSCs and
anti-lymphangiogenic therapy.
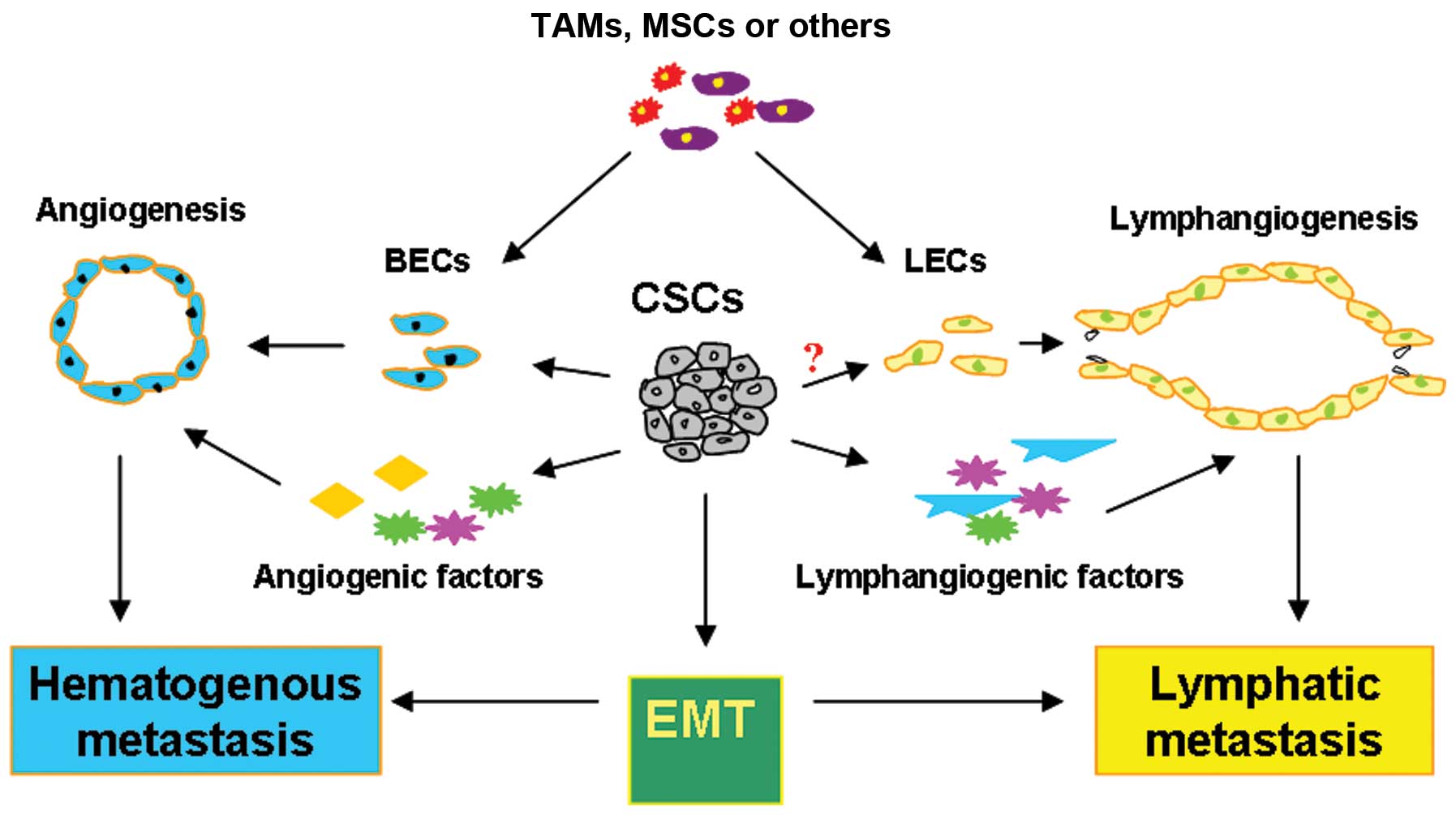
Summary and conclusions
In this review, we propose a relationship between
CSCs and tumor metastasis. Clarification of this relationship may
shed new light on cancer biotherapy. The multiple pathways through
which CSCs can promote tumor metastasis are summarized in Figs. 2 and 3.
Abbreviations:
|
CSC
|
cancer stem cell;
|
|
LEC
|
lymphatic endothelial cell;
|
|
BEC
|
blood endothelial cells
|
Acknowledgements
This study was sponsored by a Grant
from the National Natural Science Foundation of China (no.
81101749).
References
|
1.
|
Stacker SA, Baldwin ME and Achen MG: The
role of tumor lymphangiogenesis in metastatic spread. FASEB J.
16:922–934. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
2.
|
Campbell LL and Polyak K: Breast tumor
heterogeneity: cancer stem cells or clonal evolution? Cell Cycle.
6:2332–2338. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
3.
|
Clarke MF, Dick JE, Dirks PB, Eaves CJ,
Jamieson CH, Jones DL, et al: Cancer stem cells - perspectives on
current status and future directions: AACR Workshop on cancer stem
cells. Cancer Res. 66:9339–9344. 2006. View Article : Google Scholar
|
|
4.
|
Van der Auwera I, Cao Y, Tille JC, Pepper
MS, Jackson DG, Fox SB, et al: First international consensus on the
methodology of lymphangiogenesis quantification in solid human
tumours. Br J Cancer. 95:1611–1625. 2006.PubMed/NCBI
|
|
5.
|
Sundar SS and Ganesan TS: Role of
lymphangiogenesis in cancer. J Clin Oncol. 25:4298–4307. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
6.
|
Gilkes DM and Semenza GL: Role of
hypoxia-inducible factors in breast cancer metastasis. Future
Oncol. 9:1623–1636. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
7.
|
Mimeault M and Batra SK: Hypoxia-inducing
factors as master regulators of stemness properties and altered
metabolism of cancer- and metastasis-initiating cells. J Cell Mol
Med. 17:30–54. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
8.
|
Schoppmann SF, Birner P, Studer P and
Breiteneder-Geleff S: Lymphatic microvessel density and
lymphovascular invasion assessed by anti-podoplanin immunostaining
in human breast cancer. Anticancer Res. 21:2351–2355.
2001.PubMed/NCBI
|
|
9.
|
Beasley NJ, Prevo R, Banerji S, Leek RD,
Moore J, van Trappen P, et al: Intratumoral lymphangiogenesis and
lymph node metastasis in head and neck cancer. Cancer Res.
62:1315–1320. 2002.PubMed/NCBI
|
|
10.
|
Lohela M, Bry M, Tammela T and Alitalo K:
VEGFs and receptors involved in angiogenesis versus
lymphangiogenesis. Curr Opin Cell Biol. 21:154–165. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
11.
|
Raica M and Ribatti D: Targeting tumor
lymphangiogenesis: an update. Curr Med Chem. 17:698–708. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
12.
|
Mandriota SJ, Jussila L, Jeltsch M,
Compagni A, Baetens D, Prevo R, et al: Vascular endothelial growth
factor-C-mediated lymphangiogenesis promotes tumour metastasis.
EMBO J. 20:672–682. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
13.
|
Krishnan J, Kirkin V, Steffen A, Hegen M,
Weih D, Tomarev S, et al: Differential in vivo and in vitro
expression of vascular endothelial growth factor (VEGF)-C and
VEGF-D in tumors and its relationship to lymphatic metastasis in
immunocompetent rats. Cancer Res. 63:713–722. 2003.PubMed/NCBI
|
|
14.
|
Straume O, Jackson DG and Akslen LA:
Independent prognostic impact of lymphatic vessel density and
presence of low grade lymphangiogenesis in cutaneous melanoma. Clin
Cancer Res. 9:250–256. 2003.PubMed/NCBI
|
|
15.
|
Mattila MM, Ruohola JK, Karpanen T,
Jackson DG, Alitalo K and Härkönen PL: VEGF-C induced
lymphangiogenesis is associatedwith lymph node metastasis in
orthotopic MCF-7 tumors. Int J Cancer. 98:946–951. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
16.
|
Baldwin ME, Stacker SA and Achen MG:
Molecular control of lymphangiogenesis. Bioessays. 24:1030–1040.
2002. View Article : Google Scholar
|
|
17.
|
Tammela T, Zarkada G, Wallgard E,
Murtomäki A, Suchting S, Wirzenius M, Waltari M, Hellström M,
Schomber T, Peltonen R, Freitas C, Duarte A, Isoniemi H, Laakkonen
P, Christofori G, Ylä-Herttuala S, Shibuya M, Pytowski B, Eichmann
A, Betsholtz C and Alitalo K: Blocking VEGFR-3 suppresses
angiogenic sprouting and vascular network formation. Nature.
454:656–660. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
18.
|
Ferrara N, Gerber HP and LeCouter J: The
biology of VEGF and its receptors. Nat Med. 9:669–676. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
19.
|
Hirakawa S, Kodama S, Kunstfeld R, Kajiya
K, Brown LF and Detmar M: VEGF-A induces tumor and sentinel lymph
node lymphangiogenesis and promotes lymphatic metastasis. J Exp
Med. 201:1089–1099. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
20.
|
Bao S, Wu Q, Sathornsumetee S, Hao Y, Li
Z, Hjelmeland AB, Shi Q, McLendon RE, Bigner DD and Rich JN: Stem
cell-like glioma cells promote tumor angiogenesis through
vascularendothelial growth factor. Cancer Res. 66:7843–7848. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
21.
|
Pellegatta S, Poliani PL, Corno D, Menghi
F, Ghielmetti F, Suarez-merino B, Caldera V, Nava S, Ravanini M,
Facchetti F, et al: Neurospheres enriched in cancer stem-like cells
are highly effective in eliciting a dendritic cell-mediated immune
response against malignant gliomas. Cancer Res. 66:10247–10252.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
22.
|
Yao XH, Ping YF, Chen JH, Xu CP, Chen DL,
Zhang R, Wang JM and Bian XW: Glioblastoma stem cells produce
vascular endothelial growth factor by activation of a G-protein
coupled formylpeptide receptor FPR. J Pathol. 215:369–376. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
23.
|
Folkins C, Shked Y, Man S, Tang T, Lee CR,
Zhu ZP, Hoffman RM and Kerbel RS: Glioma tumor stem-like cells
promote tumor angiogenesis and vasculogenesis via vascular
endothelial growth factor and stromal-derived factor 1. Cancer Res.
69:7243–7251. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
24.
|
Yang XR, Xu Y, Yu B, Zhou J, Qiu SJ, Shi
GM, Zhang BH, Wu WZ, Shi YH, Wu B, et al: High expression levels of
putative hepatic stem/progenitor cell biomarkers related to tumour
angiogenesis and poor prognosis of hepatocellular carcinoma. Gut.
59:953–962. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
25.
|
Shao ES, Lin L, Yao Y and Bostrom KI:
Expression of vascular endothelial growth factor is coordinately
regulated by the activin-like kinase receptors 1 and 5 in
endothelial cells. Blood. 114:2197–2206. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
26.
|
Piccirillo SG, Reynolds BA, Zanetti N,
Lamorte G, Binda E, Broggi G, Brem H, Olivi A, Dimeco F and Vescovi
AL: Bone morphogenetic proteins inhibit the tumorigenic potential
of human brain tumour-initiating cells. Nature. 444:761–765. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
27.
|
Hovinga KE, Shimizu F, Wang R,
Panagiotakos G, Van Der Heijden M, Moayedpardazi H, Sofia Correia
A, Soulet D, Major T, Menon J, et al: Inhibition of notch signaling
in glioblastoma targets cancer stem cells via an endothelial cell
intermediate. Stem Cells. 28:1019–1029. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
28.
|
Alitalo K, Tammela T and Petrova TV:
Lymphangiogenesis in development and human disease. Nature.
438:946–953. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
29.
|
He Y, Rajantie I, Ilmonen M, Makinen T,
Karkkainen MJ, Haiko P, et al: Preexisting lymphatic endothelium
but not endothelial progenitor cells are essential for tumor
lymphangiogenesis and lymphatic metastasis. Cancer Res.
64:3737–3740. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
30.
|
He Y, Rajantie I, Pajusola K, Jeltsch M,
Holopainen T, Yla-Herttuala S, et al: Vascular endothelial cell
growth factor receptor 3-mediated activation of lymphatic
endothelium is crucial for tumor cell entry and spread via
lymphatic vessels. Cancer Res. 65:4739–4746. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
31.
|
De Palma M, Venneri MA, Galli R, Sergi
Sergi L, Politi LS, Sampaolesi M, et al: Tie2 identifies a
hematopoietic lineage of proangiogenic monocytes required for tumor
vessel formation and a mesenchymal population of pericyte
progenitors. Cancer Cell. 8:211–226. 2005.PubMed/NCBI
|
|
32.
|
Lyden D, Hattori K, Dias S, Costa C,
Blaikie P, Butros L, et al: Impaired recruitment of
bone-marrow-derived endothelial and hematopoietic precursor cells
blocks tumor angiogenesis and growth. Nat Med. 7:1194–1201. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
33.
|
Grunewald M, Avraham I, Dor Y,
Bachar-Lustig E, Itin A, Jung S, et al: VEGF-induced adult
neovascularization: recruitment, retention, and role of accessory
cells. Cell. 124:175–189. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
34.
|
De Palma M, Venneri MA, Roca C and Naldini
L: Targeting exogenous genes to tumor angiogenesis by
transplantation of genetically modified hematopoietic stem cells.
Nat Med. 9:789–795. 2003.PubMed/NCBI
|
|
35.
|
Maruyama K, Ii M, Cursiefen C, Jackson DG,
Keino H, Tomita M, et al: Inflammation induced lymphangiogenesis in
the cornea arises from CD11b-positive macrophages. J Clin Invest.
115:2363–2372. 2005. View
Article : Google Scholar : PubMed/NCBI
|
|
36.
|
Schoppmann SF, Birner P, Stöckl J, Kalt R,
Ullrich R, Caucig C, et al: Tumor-associated macrophages express
lymphatic endothelial growth factors and are related to peritumoral
lymphangiogenesis. Am J Pathol. 161:947–956. 2002. View Article : Google Scholar
|
|
37.
|
Karnoub AE, Dash AB, Vo AP, Sullivan A,
Brooks MW, Bell GW, et al: Mesenchymal stem cells within tumour
stroma promote breast cancer metastasis. Nature. 449:557–563. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
38.
|
Bagley RG, Weber W, Rouleau C, Yao M,
Honma N, Kataoka S, et al: Human mesenchymal stem cells from bone
marrow express tumor endothelial and stromal markers. Int J Oncol.
34:619–627. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
39.
|
Roobrouck VD, Clavel C, Jacobs SA,
Ulloa-Montoya F, Crippa S, Sohni A, et al: Differentiation
potential of human postnatal mesenchymal stem cells,
mesoangioblasts, andmultipotent adult progenitor cells reflected in
their transcriptome and partially influenced by the culture
conditions. Stem Cells. 29:871–882. 2011. View Article : Google Scholar
|
|
40.
|
Medici D, Shore EM, Lounev VY, Kaplan FS,
Kalluri R and Olsen BR: Conversion of vascular endothelial cells
into multi-potent stem-like cells. Nat Med. 16:1400–1406. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
41.
|
François M, Caprini A, Hosking B, Orsenigo
F, Wilhelm D, Browne C, et al: Sox18 induces development of the
lymphatic vasculature in mice. Nature. 456:643–647. 2008.PubMed/NCBI
|
|
42.
|
Wigle JT and Oliver G: Prox1 function is
required for the development of the murine lymphatic system. Cell.
98:769–778. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
43.
|
Srinivasan RS, Geng X, Yang Y, Wang Y,
Mukatira S, Studer M, et al: The nuclear hormone receptor Coup-TFII
is required for the initiation and early maintenance of Prox1
expression in lymphatic endothelial cells. Genes Dev. 24:696–707.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
44.
|
Paavonen K, Puolakkainen P, Jussila L,
Jahkola T and Alitalo K: Vascular endothelial growth factor
receptor-3 in lymphangiogenesis in wound healing. Am J Pathol.
156:1499–1504. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
45.
|
Valtola R, Salven P, Heikkilä P, Taipale
J, Joensuu H, Rehn M, et al: VEGFR-3 and its ligand VEGF-C are
associated with angiogenesis in breast cancer. Am J Pathol.
154:1381–1390. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
46.
|
Song EW: Research progress of solid tumor
stem cells. J SUN Yat-sen Univ. 31:172–178. 2010.
|
|
47.
|
Pandit TS, Kennette W, Mackenzie L, Zhang
G, Al-Katib W, Andrews J, et al: Lymphatic metastasis of breast
cancer cells is associated with differential gene expression
profiles that predict cancer stem cell-like properties and the
ability to survive, establish and grow in a foreign environment.
Int J Oncol. 35:297–308. 2009.
|
|
48.
|
Wakamatsu Y, Sakamoto N, Oo HZ, Naito Y,
Uraoka N, Anami K, et al: Expression of cancer stem cell markers
ALDH1, CD44 and CD133 in primary tumor and lymph node metastasis of
gastric cancer. Pathol Int. 62:112–119. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
49.
|
Li G, Liu C, Yuan J, Xiao X, Tang N, Hao
J, et al: CD133(+) single cell-derived progenies of colorectal
cancer cell line SW480 with different invasive and metastatic
potential. Clin Exp Metastasis. 27:517–527. 2010.
|
|
50.
|
Brabletz T, Jung A, Spaderna S, Hlubek F
and Kirchner T: Opinion: migrating cancer stem cells-an integrated
concept of malignant tumour progression. Nat Rev Cancer. 5:744–749.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
51.
|
Morel AP, Lièvre M, Thomas C, Hinkal G,
Ansieau S and Puisieux A: Generation of breast cancer stem cells
through epithelial-mesenchymal transition. PLoS One. 3:e28882008.
View Article : Google Scholar : PubMed/NCBI
|
|
52.
|
Asiedu MK, Ingle JN, Behrens MD, Radisky
DC and Knutson KL: TGFbeta/TNF(alpha)-mediated
epithelial-mesenchymal transition generates breast cancer stem
cells with a claudin-low phenotype. Cancer Res. 71:4707–4719. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
53.
|
Fidler IJ: The pathogenesis of cancer
metastasis: the ‘seed and soil’ hypothesis revisited. Nat Rev
Cancer. 3:453–458. 2003.
|
|
54.
|
Fidler IJ: Cancer metastasis. Br Med Bull.
47:157–177. 1991.
|
|
55.
|
Shen R, Ye Y, Chen L, Yan Q, Barsky SH and
Gao JX: Precancerous stem cells can serve as tumor vasculogenic
progenitors. PLoS One. 3:e16522008. View Article : Google Scholar : PubMed/NCBI
|
|
56.
|
Bussolati B, Grange C, Sapino A and
Camussi G: Endothelial cell differentiation of human breast tumor
stem/progenitor cells. J Cell Mol Med. 13:309–319. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
57.
|
Sie M, den Dunnen WF, Hoving EW and de
Bont ES: Anti-angiogenic therapy in pediatric brain tumors: an
effective strategy? Crit Rev Oncol Hematol. 2013.10:pii:
S1040-8428(13)00210-2. View Article : Google Scholar
|
|
58.
|
Fujimoto J: Novel strategy of
anti-angiogenic therapy for uterine cervical carcinomas. Anticancer
Res. 29:2665–2669. 2009.PubMed/NCBI
|