Introduction
Pancreatic cancer is a common type of tumor, and its
incidence has increased in recent years (1–3). The
effectiveness of pancreatic cancer therapy is unclear, and <3%
of patients live for more than five years. As a result, the
morbidity and mortality rates for pancreatic cancer are very
similar. The main factors that affect the prognosis of pancreatic
cancer are the difficulty of early diagnosis, the low rate of
radical surgical resection, and the insensitivity to chemotherapy
and radiotherapy. Pancreatic carcinogenesis and metastasis are
believed to be correlated with oncogene activation, the
inactivation of tumor suppressor genes, and the aberrant regulation
of molecular signaling pathways, such as those used by the ERM
family (4).
Ezrin and merlin display 45% nucleotide sequence
identity and play important roles in the linkage between
transmembrane proteins and the cytoskeleton. Both proteins belong
to the band 4.1 protein superfamily (5). The members of the
ezrin/radixin/moesin (ERM) family display 75–80% sequence identity
in humans (6). Ezrin proteins are
localized at regions related to cytoskeletal remodeling, such as
ruffling membranes, dynamic actin filaments, and the cleavage
furrows of dividing cells (7).
Active ezrin protein plays critical roles in connecting actin
filaments to the membrane. Ezrin protein expression is closely
associated with cell differentiation, adhesion, metastasis,
invasion, and the extent of cancer malignancy (8–10).
Ezrin mutations and abnormal protein expression have been observed
in many types of cancer. Merlin is encoded by the tumor-suppressing
gene NF2. Merlin proteins are found in the same intracellular
locations as ezrins. Merlin proteins can directly or indirectly
interact with many other types of proteins. The merlin protein
plays a key role in the contact inhibition of cell growth and in
signal transduction. When the NF2 gene is mutated, the merlin
protein becomes inactive and loses its tumor-suppressing function,
resulting in tumorigenesis (11).
Ezrin and merlin protein molecules consist of three
basic functional regions: a spherical membrane-bound N-terminal
domain, an α-helical domain, and a positively charged C-terminal
domain (12). The binding sites
between the plasma membrane and actin are hidden because the N- and
C-termini of the ERM proteins are molecularly bound under normal
conditions. In response to certain types of stimulation, ERM
proteins are phosphorylated by a protein kinase, thereby causing
the ERM protein to be uncovered. The phosphorylated N-terminus of
the ERM protein can then interact with CD44, CD43 and L-selectin,
enabling the transfer of information related to the cell membrane
protein status. The C-terminus can connect to the F-actin protein,
which mediates the reorganization of muscle actin and cytoskeletal
activities within the cell. Such mediation may be related to
tumorigenesis and metastasis.
Merlin has two subtypes, merlin-1 and merlin-2.
Merlin-1 exists in both closed and open conformations, and it is
these versions that undergo phosphorylation (13). Closed merlin-1 inhibits tumor
growth, but open merlin-1 does not. Merlin-2, which lacks exon 17,
has only an open form and does not inhibit tumor growth (14). The present study demonstrates that
merlin is phosphorylated at Ser518. The p21 activation kinase
(PAKs) and cAMP-dependent protein kinase A regulate the
phosphorylation of merlin (15,16).
Phosphorylated merlin can easily form heterodimers with ezrin
proteins, which changes the status of the merlin protein from a
growth-inhibitory state to an open state. Jin et al
described two proteins that interact with merlin (17), the myosin phosphatase MYPT1 PP1-δ
and CPI-17 (protein kinase C-potentiated phosphatase inhibitor of
17 kDa). MYPT1 PP1-δ is involved in the dephosphorylation of merlin
protein, thereby activating the protein and suppressing tumor
growth. CPI-17 is a MYPT1 PP1-δ inhibitor that inactivates merlin
and promotes tumor growth. However, the specific role(s) of
phosphorylated merlin are unclear.
A previous analysis of more than 5,000 samples of
tumor and normal tissues (18)
revealed that nearly all tumor and normal tissues exhibit different
degrees of ezrin expression. Another study indicated high levels of
ezrin expression and activation in many metastatic tumor cells
(19). Yu et al observed
that ezrin is involved in the metastasis of malignant tumors from
different tissues (20). Ohtani
et al showed that the ability of endometrial carcinoma to
invade and metastasize declines after ezrin levels decrease
(21). However, another study
showed that invasion and metastasis can increase significantly in
colorectal cancer cells when ezrin expression is inhibited
(22). In 2009, Ren et al
observed changes in the dynamic regulation of phosphorylated ERM
proteins during different periods of the formation and metastasis
of osteosarcomas (23).
The purpose of the present study was to evaluate the
expression of ERM and merlin in human pancreatic cancer tissues and
cell lines and to determine the relationships of these proteins
with tumorigenesis and metastasis.
Materials and methods
Cell culture and tumor samples
The human pancreatic cancer cell line SW1990 was
cultured in vitro in RPMI-(Gibco BRL, China) with 10%
inactivated fetal bovine serum, 100 U/ml penicillin, and 100 mg/ml
streptomycin (Gibco BRL) under a 5% CO2 atmosphere at
37°C. The SW1990 cells were obtained from the cell bank at the
Chinese Academy of Sciences. A total of 19 paraffin-embedded tissue
specimens of pancreatic cancer were obtained from patients
undergoing surgical resection from June 2006 to September 2008 at
Southeast University Affiliated Zhongda Hospital. Pancreatic cancer
was histologically confirmed in patients who did not undergo
anti-tumor therapy before their operations. All patients completed
postoperative follow-up. The survival time spanned the period from
the date of surgery to the date of death due to recurrence or
metastasis. The 2010 version of the International Union Against
Cancer (UICC) standards was used for the Classification of
Malignant Tumors (TNM) staging (24). The ethics committee of Southeast
University Affiliated Zhongda Hospital reviewed and approved this
study. The participants provided their written consent to
participate in this study.
Immunohistochemistry
Paraffin-embedded sections from surgical specimens
were deparaffinized, rehydrated and immersed in 3% hydrogen
peroxide, incubated for 30 min at room temperature, and washed 3
times with PBS. Antigen retrieval was performed by heating the
slides in 0.01 mol/l sodium citrate buffer (pH 6.0) for 30 min at
95°C. The cooled slides were then incubated in 10% normal blocking
goat serum for 30 min, after which they were then incubated with an
antibody concentration of 1:200 overnight at 4°C. The next day, the
slides were warmed to room temperature and processed using the
labeled horseradish-peroxidase method at 37°C for 15 min. The
slides were washed three times for 5 min each in PBS and stained
with 3,3’-diaminobenzidine (DAB). A total of 10 fields of view on
each slide were analyzed under high magnification (×400, Leitz
microscope, Germany) by two pathologists who were double-blinded to
the goals of the experiment. According to the intensity of
immunostaining, the protein expression was graded as 0 (no
staining), 1 (weak staining), 2 (moderate staining), or 3 (strong
staining). Cell staining was graded as 0 (no cells stained), 1
(<33% of the cells stained), 2 (34–67% of the cells stained), or
3 (>67% of the cells stained) based on the proportion of stained
tumor cells to all tumor cells in tissue sections. The mathematical
products of the staining intensity scores and the staining
proportion scores served as total assessment scores. The
immunohistochemical analysis was classified as positive (a score of
≥3) or negative (a score of <3 score) (25).
Plasmid transfection and target gene
expression
The recombinant plasmids pcDNA3.1+HA,
pcDNA3.1+HA-ezrin, pcDNA3.1+HA-ezrin Thr567 (T567D ezrin, a mutant
which mimics permanent phosphorylation), pcDNA3.1+HA-merlin, and
pcDNA3.1+HA-merlin Ser518 (S518D merlin) were transfected into
SW1990 cells using Lipofectamine 2000. At 24 h after transfection,
the cells were transferred to new six-well plates at a 1:2 ratio.
The transfected cells were cultured in RPMI-1640 containing G418
(500 μg/ml) for another 48 h until approximately day 12,
when all cells in the control group died. Some of the cells
containing the target genome were still visibly alive and exhibited
a nested distribution. Monoclonal cells were selected and cultured
with G418 at 100 μg/ml.
Western blot analysis
The cancerous human pancreatic cell lines were
either left untreated or treated and washed with cold
phosphate-buffered saline (PBS). The four groups of cells were
lysed in Laemmli lysis buffer. Equal amounts of lysate were
separated by electrophoresis on a 12% SDS-PAGE gel, and the
proteins were transferred to nitrocellulose membranes. The
membranes were then blocked with TBS containing 5% low-fat milk and
0.05% Tween for 1 h, washed three times with TBS containing 0.05%
Tween, and incubated with specific primary antibodies for 2 h at
room temperature. The membranes were washed with TBS containing
0.05% Tween and then incubated with peroxidase-conjugated secondary
antibodies labeled with horseradish peroxidase (HRP). The images
were then developed on X-ray film. The optical density of the bands
was calculated using Quantity One software (Bio-Rad Laboratories,
Inc.). Mouse anti-human monoclonal anti-ezrin and anti-β-actin and
mouse anti-human polyclonal anti-p-ezrin Thr567 were purchased from
Santa Cruz Biotechnology, Inc. (USA) at a dilution of 1:500. Mouse
anti-human monoclonal anti-merlin and anti-HA and mouse anti-human
polyclonal anti-p-merlin Ser518 were purchased from Cell Signaling
Technology (USA) and used at a dilution of 1:1000.
Cell proliferation assay
Cell viability was assessed using the MTT assay.
Human pancreatic cancer cells (SW1990, SW1990-HA, SW1990-ezrin,
SW1990-p-ezrin, SW1990-merlin, and SW1990-p-merlin) were cultured
in 96-well plates at a density of 1×104 cells/ml A
20-μl aliquot of MTT (Amresco) labeling reagent was added to
each well containing cells in 150 μl medium, and the cells
were incubated in a humidified incubator at 37°C to permit MTT
metabolism for 4 h. The medium was removed, and the cells were
re-suspended in formazan in 150 μl DMSO. The absorbance of
the samples was measured at 490 nm using a spectrophotometer and
microplate reader, and the viability values were expressed as
percentages of the controls.
Cell cycle and cell apoptosis
Cells in logarithmic growth phase (SW1990,
SW1990-HA, SW1990-p-ezrin, and SW1990-p-merlin) were harvested at
an adjusted cell concentration of 1×106/ml. The prepared
single-cell suspension was fixed in pre-cooled 70% ethanol. The
fixative was then washed away with PBS, the samples were stained
with propidium iodide (Sigma), and RNase A was added. The cells
were then used to detect the cell cycle and apoptosis using flow
cytometry.
Transwell migration assay
Logarithmic growth-phase cells (SW1990, SW1990-HA,
SW1990-p-ezrin and SW1990-p-merlin) were harvested, digested, and
counted. The cells were placed in the upper chamber of a Transwell
containing 200 μl serum-free medium containing 0.1% BSA at a
density of 1×105/ml. The lower chamber of the Transwell
contained 500 μl medium containing 10% fetal bovine serum in
24-well plates at room temperature. After an incubation period of
12 h, the cells that had migrated through the membrane were stained
with H&E and counted.
Cell-matrix adhesion assay
The 96-well plates were coated with Matrigel and
incubated overnight at 4°C. To each well was added 50 μl
serum-free medium containing 0.1% BSA, and the plates were
incubated at 37°C for 30 min. The cells in the 200-μl cell
suspension were seeded at a final concentration of
1×105/ml and incubated at 37°C for 1 or 2 h so that each
group consisted of 4 parallel samples. The cell absorbance of each
well was determined using the MTT assay with a reference to the
control (the basement membrane of the BSA group).
Statistical analysis
The results are expressed as the means ± standard
deviations (SD). Data analyses were performed using SPSS 17.0.
Fisher exact tests were used for comparisons between 2 sample
proportions, and the Pearson Chi-squared test was used for
comparisons of >2 sample proportions. The Kaplan-Meier analysis
and log-rank test were employed for survival analysis. P-values
<0.05 and <0.01 were considered statistically
significant.
Results
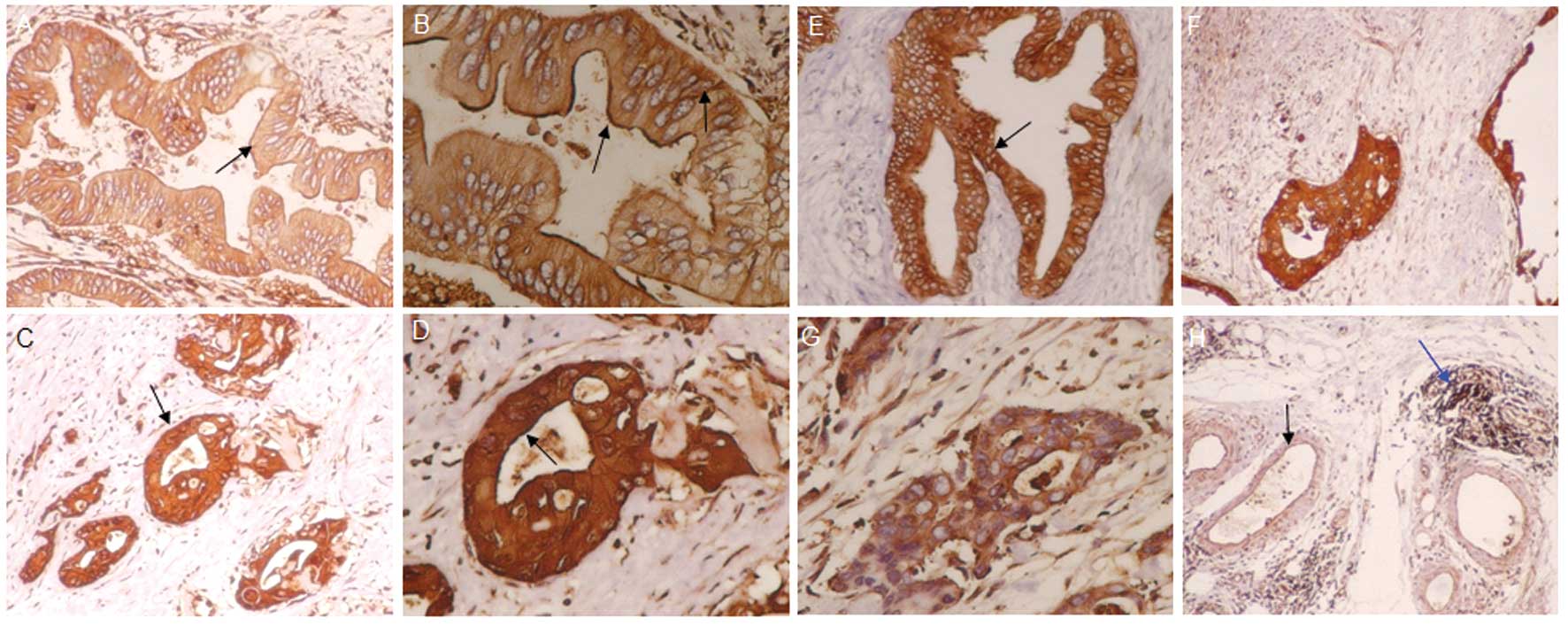
Protein expression of ezrin and merlin in
normal human pancreas and pancreatic carcinoma tissues
Ezrin Thr567, ezrin Tyr353, merlin Ser518, p-ezrin
Thr567, p-ezrin Tyr353 and p-merlin Ser518 showed different levels
of protein expression in tissue samples from normal human
pancreases and pancreatic carcinoma (Fig. 1). The expression of the 6 target
proteins did not significantly differ among these samples. These
proteins were seldom present in the cell membranes of normal
pancreatic cells. The expression of 4 proteins (ezrin Thr567,
p-ezrin Thr567, ezrin Tyr353, p-ezrin Tyr353) was 94.7, 63.2, 94.7,
and 73.7% higher, respectively, in the membranes of pancreatic
cancer cells than in normal cell membranes (P<0.05). The
expression of merlin Ser518 and p-merlin Ser518 protein was lower
in the cell membranes of pancreatic cancer samples than in normal
cell membranes.
The relationship between
clinicopathological factors and protein expression of ezrin and
merlin in pancreatic cancer
The level of expression of p-ezrin 353 and p-ezrin
567 in the cytoplasm of pancreatic cancer cells increased with TNM
stage (P<0.05). The levels of expression were not associated
with gender, age, tumor location or level of differentiation, and
were also independent of lymph node metastasis or neural invasion.
In addition, the clinicopathological factors did not correlate with
the expression of any of the 4 other proteins, ezrin Thr567, ezrin
Tyr353, merlin Ser518, or p-merlin Ser518 (Table I).
 | Table I.Relationship between TNM stage and
protein expression of p-ezrin 353 and p-ezrin 567 in pancreatic
cancers. |
Table I.
Relationship between TNM stage and
protein expression of p-ezrin 353 and p-ezrin 567 in pancreatic
cancers.
| TNM stage | p-ezrin 353
| p-ezrin 567
|
|---|
| Expression | No expression | Expression | No expression |
|---|
| I | 2 | 2 | 1 | 3 |
| II | 2 | 2 | 2 | 2 |
| III | 6 | 0 | 6 | 0 |
| IV | 5 | 0a | 5 | 0b |
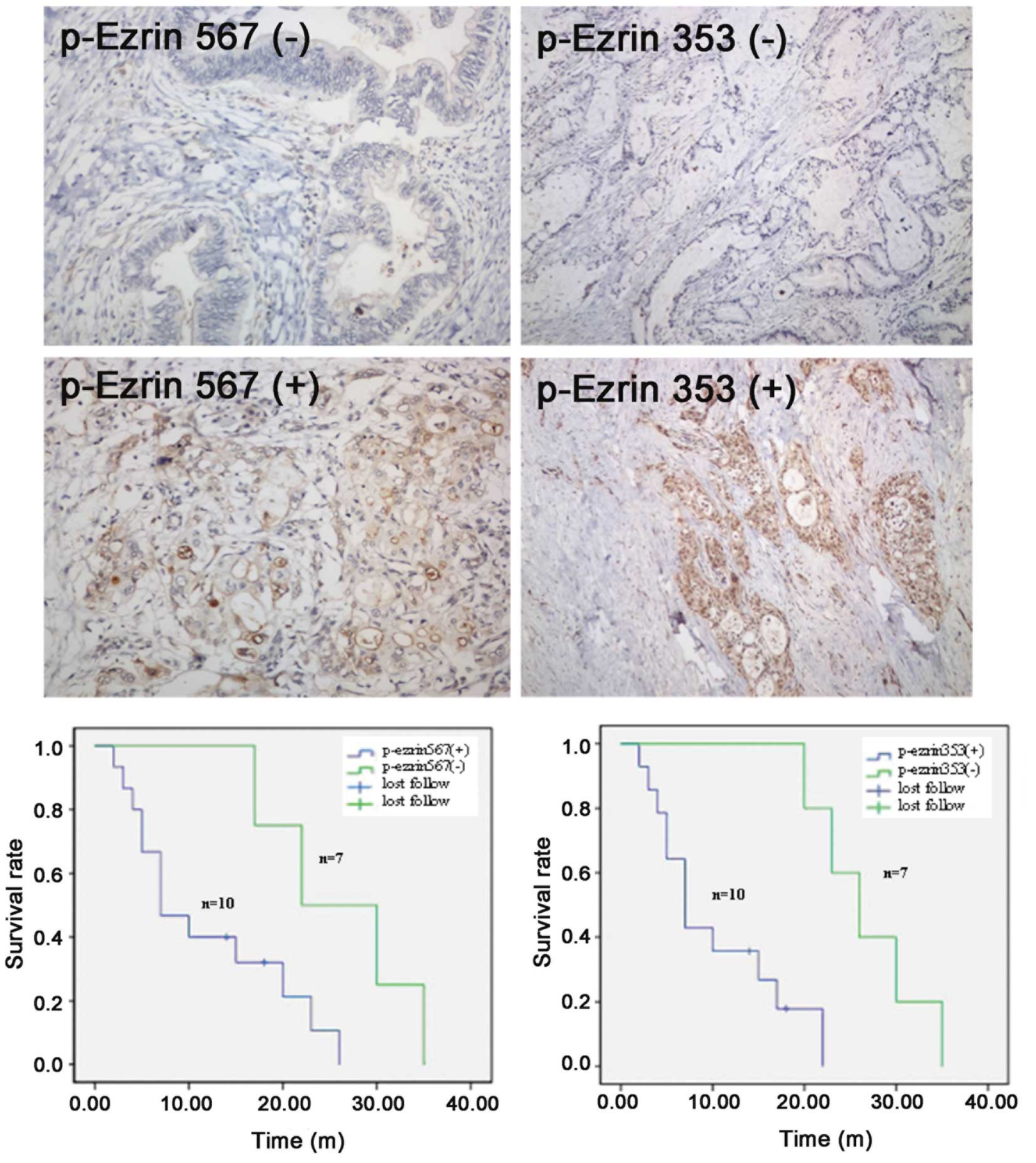
The relationship between p-ezrin
expression and the survival period after pancreatic cancer
treatment
Of a total of 19 cases, 17 were followed up, and 2
were lost to follow-up. The follow-up time ranged from 2 to 35
months. The survival time for the group positive for p-ezrin 567
expression in the cytoplasm of pancreatic cancer cells was 2-26
months, and the average survival time was 11.9 months. For patients
negative for p-ezrin 567 expression, the survival time was 17-35
months, and the median survival time was 26.0 months. Using a
log-rank test, the difference between these two groups was found to
be statistically significant (χ2=4.211, P=0.040). The
survival of the group of patients positive for p-ezrin 353
expression was similar to those positive for p-ezrin 567; the
survival time was 2–22 months, and the median survival time was
10.4 months. Individuals negative for p-exrin 353 expression
exhibited a survival time of 20–35 months and a median survival
time of 26.8 months. Using Kaplan-Meier analysis and the log-rank
test, this difference was found to be statistically significant
(χ2=8.813, P=0.003) (Fig.
2).
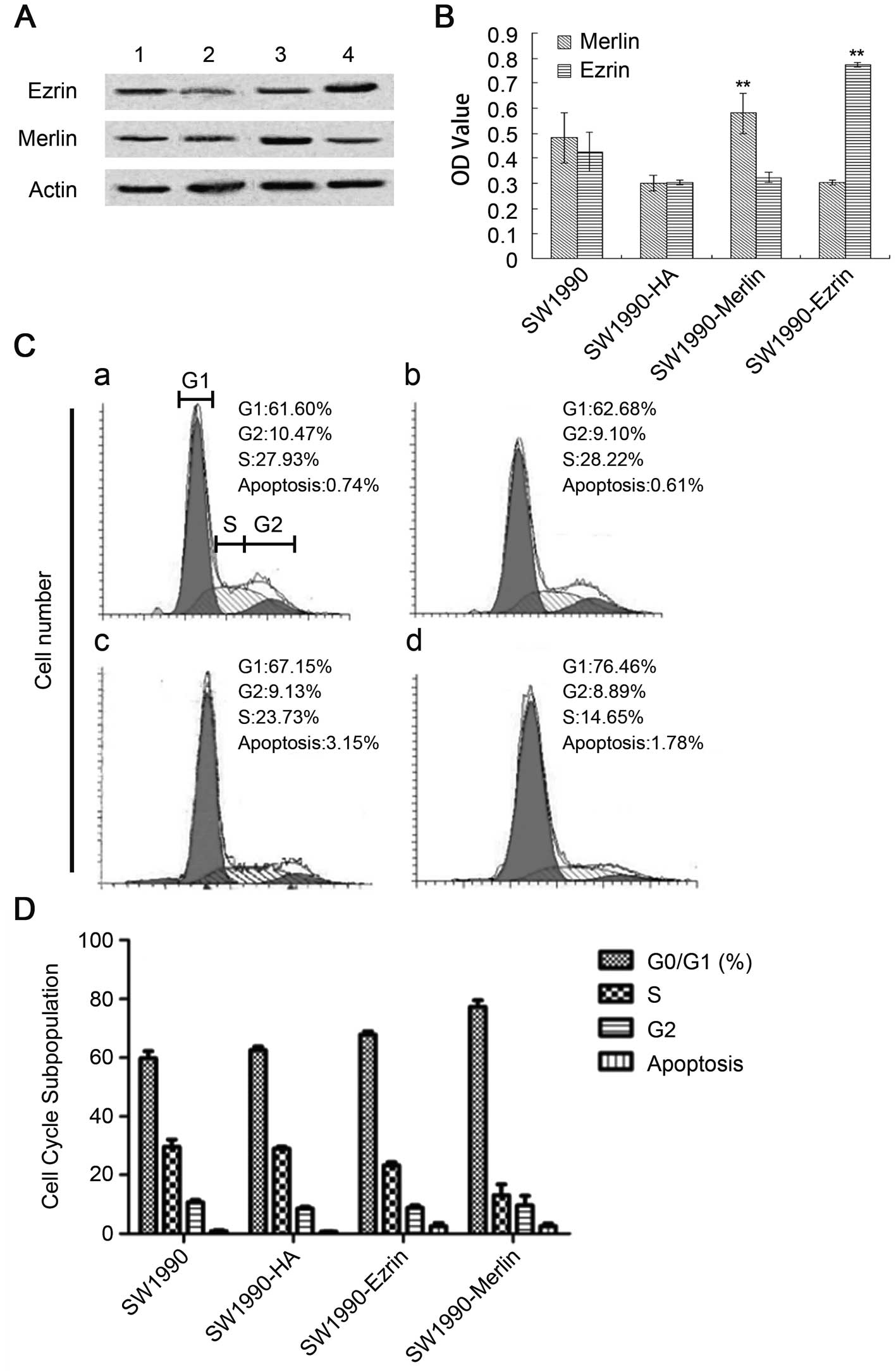
Effect of the overexpression of wild-type
ezrin and merlin on SW1990
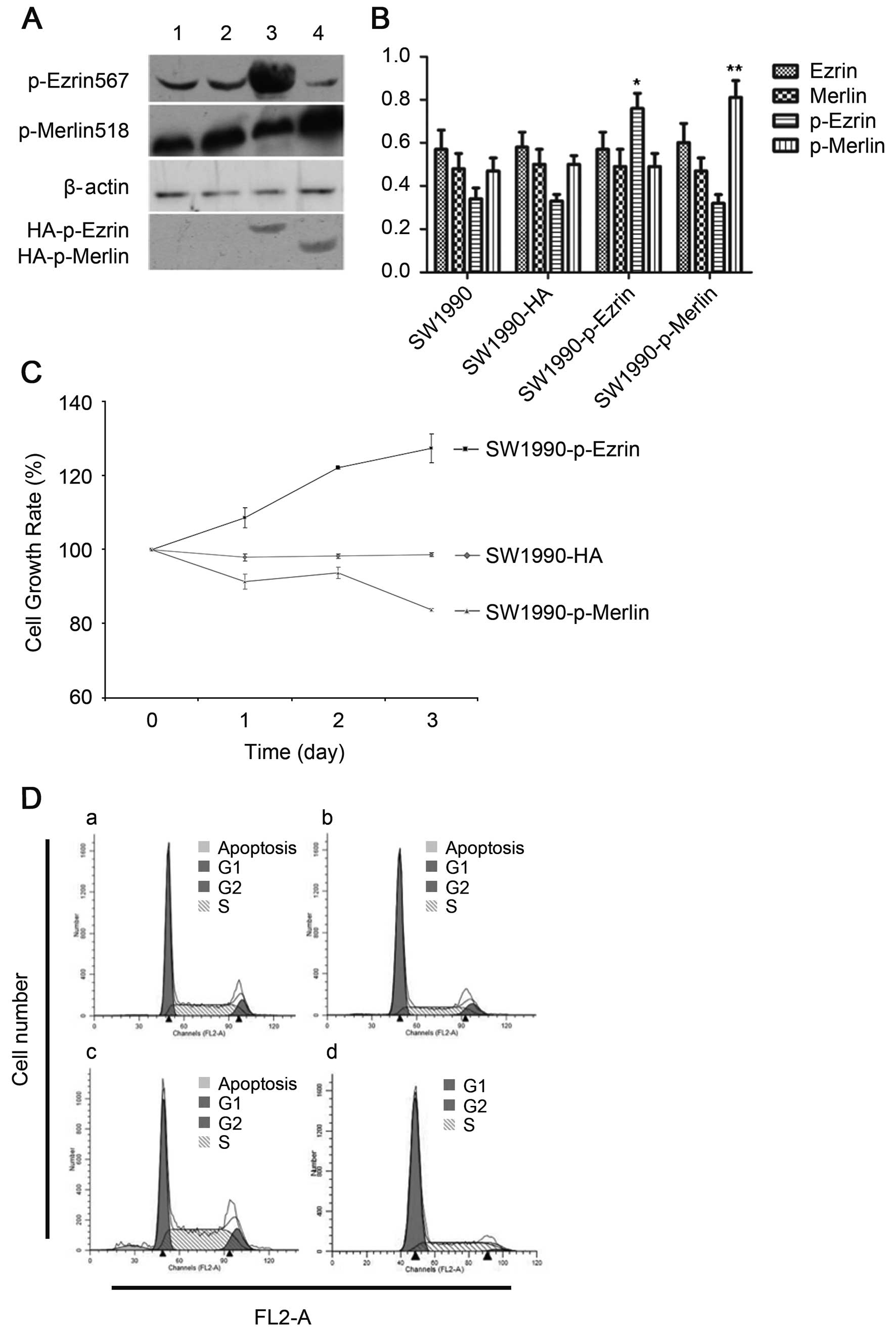
Our results demonstrated that the plasmid was
successfully transfected into cells and was stably expressed. The
proteins ezrin and merlin were highly expressed (Fig. 3A and B). Overexpression of
wild-type ezrin and merlin inhibited the proliferation and growth
of pancreatic cancer cells. The MTT results demonstrated that the
cellular growth rate and proliferative capacity were significantly
lower in the plasmidtransfection group (SW1990-ezrin and
SW1990-merlin) than in untransfected cells (SW1990 cell line) or in
cells transfected with a blank plasmid (SW1990-HA) (P<0.01).
Flow cytometric analysis revealed a significant
increase in the fraction of cells in G0/G1 phase and a significant
decrease in the fraction of cells in S phase (P<0.05) for
SW1990-ezrin and SW1990-merlin cells compared to SW1990 and
SW1990-HA cells. More SW199-merlin cells than SW1990-ezrin cells
were in G0/G1 phase (77.29±2.27%); conversely, more SW1990-ezrin
cells than SW1990-merlin cells were in S phase (P<0.01). The
difference in the rates of apoptosis was not statistically
significant between the two transfected cell types (P>0.05). The
results demonstrated that the effect of merlin on the cell cycle
was more pronounced than that of ezrin. Furthermore, the
overexpression of ezrin and merlin did not significantly affect the
apoptosis of SW1990 (Fig. 3C and
D).
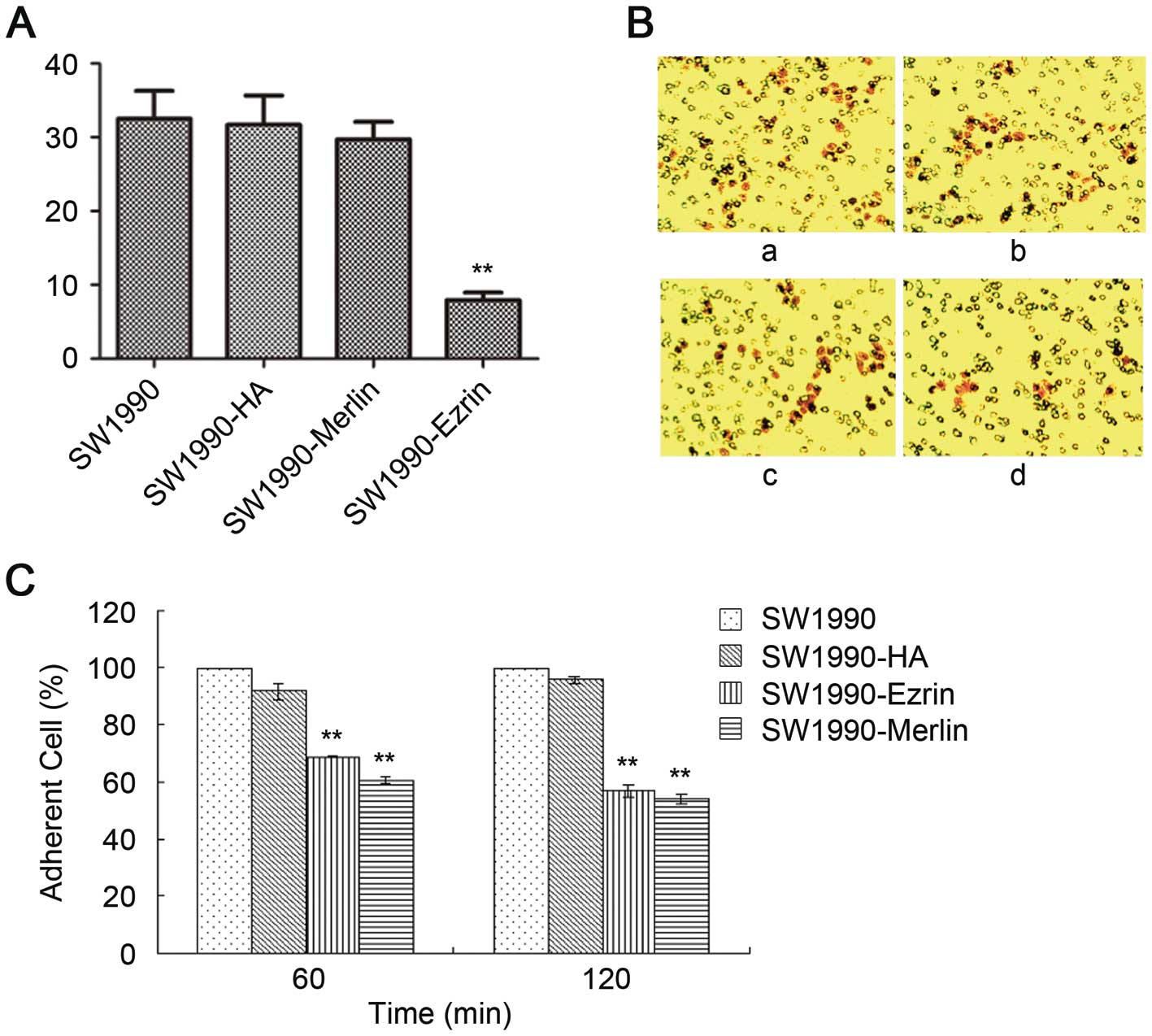
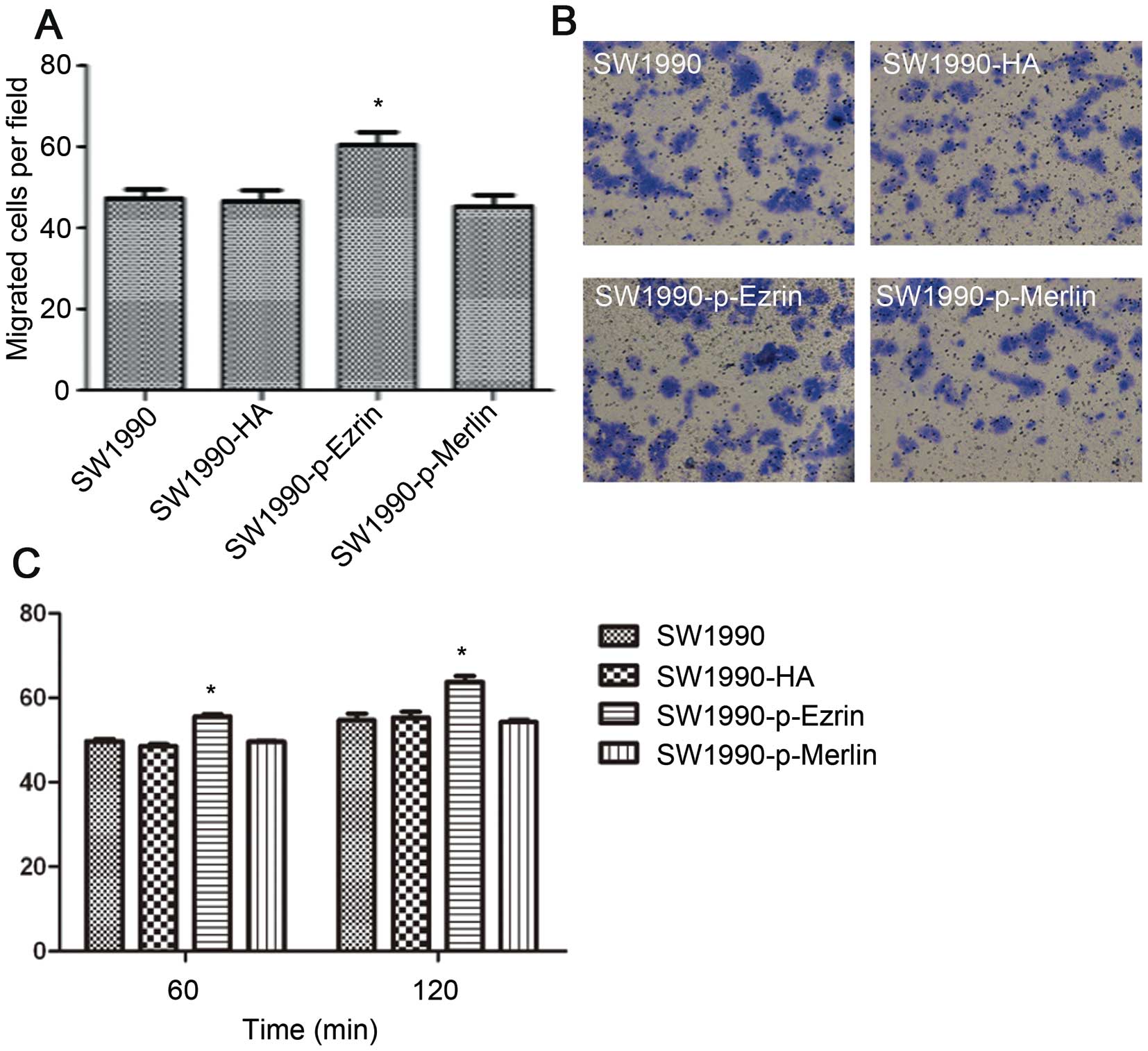
The number of SW1990-HA cells, SW1990-ezrin cells,
and untransfected SW1990 cells that traversed the basement membrane
did not significantly differ as assessed by a Transwell test
(P>0.05). The number of SW1990-merlin cells that passed through
the basement membrane was significantly lower than the number of
SW1990 cells (P<0.05). However, the matrix adhesion ability of
SW1990-ezrin and SW1990-merlin cells was significantly decreased at
60 min and 120 min compared with untransfected cells and SW1990-HA
cells (P<0.01) (Fig. 4).
Effect of the overexpression of
phosphorylated ezrin and merlin on SW1990
Our results demonstrated that the plasmid (a mutant
which mimics permanent phosphorylation) was successfully
transfected into cells and was stably expressed. The phosphorylated
ezrin and merlin proteins were highly expressed (Fig. 5A and B).
Overexpression of T567D ezrin (p-ezrin), a mutant
that mimics permanent phosphorylation, in SW1990-p-ezrin cells
promoted proliferation, and both the proliferative capacity and
growth rate of SW1990-p-merlin cells decreased significantly upon
overexpression of S518D merlin (p-merlin) (P<0.05) (Fig. 5C).
Fewer cells were in G0/G1 phase in SW1990-p-ezrin
cells than in SW1990 cells, and more cells were in S phase and G2
phase. Both proliferation and the rate of apoptosis were increased
in SW1990-p-ezrin cells compared to SW1990 cells (P<0.05). The
SW1990-p-merlin cells ceased in G1 phase, and the rate of apoptosis
also decreased (P<0.05) (Fig. 5D
and E).
More SW1990-p-ezrin cells passed through the
basement membrane than SW1990-HA and SW1990 cells (P<0.05). This
difference suggests that ezrin protein phosphorylation promotes the
migration of SW1990 cells. There were no significant differences in
migration among SW1990 cells, SW1990-HA cells, and SW1990-p-merlin
cells (P>0.05). (Fig. 6A and B)
Although overexpressing wild-type merlin has been shown to inhibit
the migration of SW1990 cells, over-expressing phosphorylated
merlin does not.
The ability of SW1990-p-ezrin cells to adhere to the
matrix significantly increased at 60 min and 120 min (P<0.05)
(Fig. 6C). The matrix adhesion
ability of SW1990-p-merlin was not significantly different from
that of SW1990 and SW1990-HA cells (P>0.05). These results
demonstrate that ezrin phosphorylation enhances cell adhesion and
that the wild-type merlin protein does not inhibit the adhesion of
SW1990 cells. Adhesion appears to be lost after
phosphorylation.
Discussion
Our results showed that the levels of p-ezrin
567/p-ezrin 353 protein expression in the cytoplasm increased with
TNM stage of human pancreatic cancers. The survival time of the
group positive for p-ezrin 567/p-ezrin 353 protein expression was
shorter than that of the negative group. Moreover, we demonstrated
that overexpression of T567D ezrin, a mutant that mimics permanent
phosphorylation, promotes the proliferation, adhesion, and
migration of pancreatic adenocarcinoma in vitro.
Cui et al performed immunohistochemical
analysis of the postoperative specimens of 66 patients with
pancreatic cancer (25), which
revealed that positive expression of p-ezrin Thr353 was associated
with poor cell differentiation and an increased likelihood of lymph
node metastasis in pancreatic cancer. The survival time of
pancreatic cancer patients positive for p-ezrin 353 expression
differed from those negative for p-ezrin353 expression. The
difference in the survival time between the groups positive for
p-ezrin 567/p-ezrin 353 protein expression and those negative for
expression was statistically significant. Coupled with our
observation that the rates of p-ezrin 567/p-ezrin 353 protein
expression in the cytoplasm increase with TNM stage of pancreatic
cancers, these results suggest a close relationship between the
phosphorylation status of ezrin and the adverse biological
behaviors of human pancreatic cancer, such as the capacity for cell
growth, invasion and metastasis.
Ren et al (23) reported that the phosphorylation of
the ERM protein caused dynamic changes in the regulation of
different periods during the onset of metastasis in osteosarcomas.
They also demonstrated that the ezrin protein was widely
distributed in tumor tissues and that the concentration of the
phosphorylated ERM protein was higher at the margins of the
invading tumor. We did not observe this phenomenon.
Hunter (19)
observed high levels of ezrin expression and ezrin protein
activation in many metastatic tumor cell types, such as
osteosarcomas and rhabdomyosarcomas. The ezrin protein is known to
be involved in the metastasis of malignant tumors from various
tissues (20). By contrast, Hiscox
and Jiang (22) demonstrated that
tumor cell invasion and metastasis increased significantly in
colorectal cancer cells when the level of ezrin expression was
suppressed. Similarly, poor prognosis in ovarian cancer is closely
related to low ezrin expression and ezrin deletions (26,27).
In the present study, we did not observe an obvious change in the
migration of SW1990 cells after transfection with ezrin. However,
growth, adhesion, migration and invasion increased markedly after
transfection with phosphorylated ezrin. This finding suggests that
the ezrin protein affects pancreatic cancer via
phosphorylation.
The capacity for growth, proliferation, migration
and cell matrix adhesion was significantly lower in SW1990-merlin
cells than in the control cells, which indicates that merlin
inhibits tumor cell metastasis as well as tumor growth.
Transfection of SW1990 cells with p-merlin resulted in a decrease
in cell proliferation compared to control cells, but cell adhesion,
migration, and invasion did not change.
Our study demonstrates that the expression of
phosphorylated ezrin proteins is related to the clinical and
pathological features of pancreatic cancer. P-ezrin plays a
positive regulatory role in the growth, adhesion, and invasion of
SW1990 cells, while p-merlin serves a negative regulatory role.
In conclusion, the phosphorylation of ezrin may
contribute to the progression of pancreatic carcinoma, and the
level of phosphorylated ezrin may serve as an adverse prognostic
factor for pancreatic carcinoma.
Abbreviations:
|
UICC
|
the International Union Against
Cancer
|
|
TNM
|
Classification of Malignant Tumors
|
|
DAB
|
3,3’-diaminobenzidine
|
|
MTT
|
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide
|
Acknowledgements
This study was supported by the China
National Natural Science Foundation (no. 81071967 and
30872500).
References
|
1.
|
Jemal A, Bray F, Center MM, et al: Global
cancer statistics. CA Cancer J Clin. 61:69–90. 2011. View Article : Google Scholar
|
|
2.
|
Chen WQ, Wang QS, Zhang SW, et al: An
analysis of incidence and mortality of pancreas cancer in China
2003–2007. China Cancer. 21:248–253. 2012.
|
|
3.
|
Siegel R, Naishadham D and Jemal A: Cancer
Statistics, 2012. CA Cancer J Clin. 62:10–29. 2012. View Article : Google Scholar
|
|
4.
|
Cartwright T, Richards DA and Boehm KA:
Cancer of the pancreas: are we making progress? A review of studies
in the US Oncology Research Network. Cancer Control. 15:308–313.
2008.PubMed/NCBI
|
|
5.
|
Wan X, Mendoza A, Khanna C and Helman LJ:
Rapamycin inhibits ezrin-mediated metastatic behavior in a murine
model of osteosarcoma. Cancer Res. 65:2406–2411. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
6.
|
Diakowski W, Grzybek M and Sikorski AF:
Protein 4.1, a component of the erythrocyte membrane skeleton and
its related homologue proteins forming the protein 4.1/FERM
superfamily. Folia Histochem Cytobiol. 44:231–248. 2006.PubMed/NCBI
|
|
7.
|
Wakayama T, Nakata H, Kurobo M, et al:
Expression, localization, and binding activity of the
ezrin/radixin/moesin proteins in the mouse testis. J Histochem
Cytochem. 57:351–362. 2009. View Article : Google Scholar
|
|
8.
|
Arpin M, Chirivino D, Naba A and
Zwaenepoel I: Emerging role for ERM proteins in cell adhesion and
migration. Cell Adh Migr. 5:199–206. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
9.
|
Jörgren F, Nilbert M, Rambech E, et al:
Ezrin expression in rectal cancer predicts time to development of
local recurrence. Int J Colorectal Dis. 27:893–899. 2012.PubMed/NCBI
|
|
10.
|
Arslan AA, Silvera D, Arju R, et al:
Atypical ezrin localization as a marker of locally advanced breast
cancer. Breast Cancer Res Treat. 134:981–988. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
11.
|
Evans DG, Ramsden RT, Shenton A, et al:
What are the implications in individuals with unilateral vestibular
schwannoma and other neurogenic tumors? J Neurosurg. 108:92–96.
2008. View Article : Google Scholar
|
|
12.
|
Tsukita S, Yonemura S and Tsukita S: ERM
proteins: head-to-tail regulation of actin-plasma membrane
interaction. Trends Biochem Sci. 22:53–58. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
13.
|
Ye K: Phosphorylation of merlin regulates
its stability and tumor suppressive activity. Cell Adh Migr.
1:196–198. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
14.
|
Muranen T, Grönholm M, Lampin A, et al:
The tumor suppressor merlin interacts with microtubules and
modulates Schwann cell microtubule cytoskeleton. Hum Mol Genet.
16:1742–1751. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
15.
|
Kissil JL, Wilker EW, Johnson KC, et al:
Merlin, the product of the Nf2 tumor suppressor gene, is an
inhibitor of the p21-activated kinase, Pak1. Mol Cell. 12:841–849.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
16.
|
Alfthan K, Heiska L, Grönholm M, et al:
Cyclic AMP-dependent protein kinase phosphorylates merlin at serine
518 independently of p21-activated kinase and promotes merlin-ezrin
heterodimerization. J Biol Chem. 279:18559–18566. 2004. View Article : Google Scholar
|
|
17.
|
Jin H, Sperka T, Herrlich P and Morrison
H: Tumorigenic transformation by CPI-17 through inhibition of a
merlin phosphatase. Nature. 442:576–579. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
18.
|
Bruce B, Khanna G, Ren L, et al:
Expression of the cytoskeleton linker protein ezrin in human
cancers. Clin Exp Metastasis. 24:69–78. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
19.
|
Hunter KW: Ezrin, a key component in tumor
metastasis. Trends Mol Med. 10:201–204. 2004. View Article : Google Scholar
|
|
20.
|
Yu Y, Khan J, Khanna C, et al: Expression
profiling identifies the cytoskeletal organizer ezrin and the
developmental homeoprotein Six 1 as keymetastatic regulators. Nat
Med. 10:175–181. 2004. View
Article : Google Scholar
|
|
21.
|
Ohtani K, Sakamoto H, Rutherford T, et al:
Ezrin, a membrane-cytoskeletal linking protein, is involved in the
process of invasion of endometrial cancer cells. Cancer Lett.
147:31–38. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
22.
|
Hiscox S and Jiang WG: Ezrin regulates
cell-cell and cell-matrix adhesion, a possible role with
E-cadherin/beta-catenin. Cell Sci. 112:3081–3090. 1999.PubMed/NCBI
|
|
23.
|
Ren L, Hong SH, Cassavaugh J, et al: The
actin-cytoskeleton linker protein ezrin is regulated during
osteosarcoma metastasis by PKC. Oncogene. 28:792–802. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
24.
|
Wittekind C and Oberschmid B: TNM
classification of malignant tumors 2010: General aspects and
amendments in the general section. Pathologe. 31:333–338. 2010.(In
German).
|
|
25.
|
Cui Y, Li T, Zhang D and Han J: Expression
of Ezrin and phosphorylated Ezrin (pEzrin) in pancreatic ductal
adenocarcinoma. Cancer Invest. 28:242–247. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
26.
|
Mathew J, Hines JE, Obafunwa JO, et al:
CD44 is expressed in hepatocellular carcinomas showing vascular
invasion. J Pathol. 179:74–79. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
27.
|
Moilanen J, Lassus H, Leminen A, et al:
Ezrin immumoreactivity in relation to survival in serous ovarian
carcinoma patients. Gynecol Oncol. 90:273–281. 2003. View Article : Google Scholar : PubMed/NCBI
|