Introduction
Distant metastasis is a strong prognostic factor in
patients with solid tumors (1–3), and
the presence of circulating tumor cells (CTCs) in peripheral blood
indicates a systemic disease stage (4). The detection of CTCs in peripheral
blood is useful for estimation of prognosis and monitoring of
disease progression in breast, prostate, skin, colon and
gastrointestinal malignancies. Although various methods have been
developed to detect CTCs, the common techniques for the enrichment
and detection of CTCs are density gradient separation (5,6),
direct enrichment by filtration (7), immunomagnetic separation (8), flow cytometry (9), real-time reverse transcriptase
polymerase chain reaction (RT-PCR) (10,11),
and microchip technology (12).
The CellSearch System (Veridex, LLC, Raritan, NJ, USA) (13) is based on immunomagnetic cell
enrichment and is one of the most widely used automated techniques
to enrich and detect CTCs (14–16).
The advantage of immunomagnetic cell separation is that CTCs can be
visualized with a fluorescence microscope. Cells detected with
antibodies against epithelial markers [epithelial cell adhesion
molecules (EpCAMs)] are determined to be CTCs. During
epithelial-mesenchymal transition (EMT), an important developmental
process in CTCs (17), epithelial
surface markers are suggested to decrease (18). Thus, CTCs undergoing EMT may escape
detection by systems using epithelial markers.
Increased telomerase activity is a common
characteristic of malignant tumors, and telomerase plays important
roles in carcinogenesis and disease progression (19,20).
Therefore, we have developed a novel detection system to enrich
cells with high telomerase activity in peripheral blood samples
from cancer patients. We used OBP-401 (TelomeScan, Oncolys
BioPharma, Tokyo, Japan), which is a telomerase-specific,
replication-selective modified viral agent in which the human
telomerase reverse transcriptase (TERT) gene promoter is inserted
into the E1 region, and the green fluorescent protein (GFP) gene is
placed under the control of the cytomegalovirus promoter in the E3
region as a marker of viral replication (21). We obtained 7.5-ml blood samples
from 65 treatment-negative gastric cancer patients before surgery
and 10 healthy volunteers (22).
We detected viable CTCs in the blood samples after incubation with
OBP-401. GFP-positive (GFP+) cells were detected in all
blood samples. Since it has been reported that CTCs are larger than
normal blood cells (23,24), we counted GFP+ cells
having a diameter of at least 7.735 μm (L-GFP+
cells); this threshold was determined by receiver operating
characteristic curve (ROC) analysis. As a result, there was a
significant difference in overall survival between patients with
0–4, and those with ≥5 L-GFP+ cells in both the stage
I–IV disease and stage II–IV advanced disease groups. On the other
hand, the number of L-GFP+ cells showed no significant
correlation to cancer stage. A pathological finding showed that the
number of GFP+ cells was only significantly related to
venous invasion, although there was a trend of higher number of
L-GFP+ cells with disease progression (22).
Our results (22)
suggest that patients with L-GFP+ cells showed
significant survival; however, other studies have shown that tumor
cells undergoing EMT are smaller in size than cells without EMT
features, because of changes in cell shape (25,26).
Thus, CTCs undergoing EMT possibly escape detection using our
technique. Therefore, we analyzed the relationship between the
number of GFP+ cells of any size and patient outcome at
a median-follow up of three years.
Materials and methods
Patients and healthy volunteers
This study is an interim analysis of our prospective
preliminary study on CTCs from 65 patients with treatment-negative
gastric adenocarcinoma, who underwent surgery at the Digestive
Disease Center of the Showa University Northern Yokohama Hospital
between April 2010 and May 2011, and from whom we extracted
peripheral blood samples before treatment. The inclusion criteria
were: i) histologically proven adenocarcinoma of the stomach by
endoscopic biopsy; ii) clinical solitary tumor; iii) no prior
endoscopic resection, chemotherapy, or radiotherapy; iv) ages,
20–80 years; v) Eastern Cooperative Oncology Group performance
status (27) of 0 or 1; vi)
sufficient organ function; and vii) written informed consent. The
exclusion criteria were: i) synchronous or metachronous malignancy;
ii) pregnant or breast-feeding women; iii) active or chronic viral
hepatitis; iv) active bacterial or fungal infection; v) diabetes
mellitus; vi) systemic administration of corticosteroids; and vii)
unstable hypertension. The pathologic stage of the disease was
determined according to the seventh edition American Joint
Committee on Cancer (AJCC)/International Union Against Cancer
(UICC) TNM classification system (28). The depth of the tumor invasion in
four patients without gastrectomy and the regional lymph node
status of seven patients without sufficient lymphadenectomy were
surgically diagnosed.
All the patients were checked regularly every three
months in our hospital after surgery. The patients also underwent
endoscopy and computed tomography at least once a year, according
to their disease stage and course. Healthy volunteers were also
recruited to act as controls. All healthy volunteers were employees
of Sysmex Corporation, which included seven men (mean age, 31.4
years; range, 24–39 years) and three women (mean age, 33.7 years;
range, 26–48 years). All volunteers underwent medical check-ups
upon employment and annually; check-ups included medical
interviews, auscultation, chest radiography, and blood and urine
analyses. In addition, individual interviews were done before
sample collection; any volunteer who was currently receiving
medical treatment, pregnant, or breast-feeding or who had donated
blood within the past month was excluded.
The study was approved by the Institutional Review
Board of the Showa University, Northern Yokohama Hospital (no.
0903-03). The study protocol was explained to the patients and
volunteers before written informed consent was obtained. This study
was registered with the University Hospital Medical Information
Network in Japan (no. 000004026).
Virus
OBP-401, a telomerase-specific,
replication-selective adenoviral agent in which the TERT promoter
element drives the expression of the EIA and EIB genes and into
which the GFP gene is integrated, was used. The sensitivity and
specificity of the assay using OBP-401 have been reported
previously by Kim et al (29). The test was repeated five times. In
the sample containing one MDA-MB-468 (breast carcinoma) cell and
7.5-ml blood, the numbers of GFP+ cells were one, one,
one, two, and three; in the sample containing 20 MDA-MB-468 (breast
carcinoma) cells, the numbers of GFP+ cells were 15, 17,
19, 22, and 24. Viral samples were stored at −80°C.
Sample preparation and
immunostaining
Details of sample preparation and assay have been
described in our previous study (22). A 7.5-ml peripheral vein blood
sample was obtained from each patient before surgery and from each
volunteer. The samples were drawn into tubes containing citric
acid, phosphoric acid, and dextrose and stored at 4°C. The assay
was started within 48 h of sample collection. The samples were
centrifuged for 5 min at 540 x g, and the plasma phase was removed.
The cells were then washed four times with phosphate-buffered
saline (PBS) and twice with Roswell Park Memorial Institute medium.
The samples were infected with 4×108 plaque-forming
units (PFU) of OBP-401 virus by incubation in the medium for 24 h
at 37°C. Dead cells were stained with the red-fluorescent reactive
dye L23102 (Life Technologies, Carlsbad, CA, USA), OBP-401 was
inactivated, and cells were fixed with 2% paraformaldehyde for 20
min at room temperature. The samples were treated with a
surface-active agent (Emalgen 2025G; Kao Chemicals, Tokyo, Japan)
for 10 min at 40°C to degrade red blood cells.
Phycoerythrin-labeled anti-human CD45 antibody (BioLegend, San
Diego, CA, USA) was diluted 1:5, and Pacific Blue-labeled
anti-human CD326 (EpCAM) antibody (BioLegend) was diluted 1:10 in
PBS containing 2% fetal bovine serum. Cells were incubated with the
diluted antibodies for 30 min at 25°C. After being washed with PBS
containing 2% fetal bovine serum, the cells were mounted on two
glass slides for microscopic analysis.
Determination of GFP fluorescence
intensity threshold
The threshold for GFP fluorescence intensity was
determined as previously reported (22). Briefly, ∼30,000 cultured cells were
added into 7.5-ml blood samples from healthy volunteers, which were
mixed with various cancer cell lines: A549 (lung carcinoma), HepG2
(hepatocellular carcinoma), HEC-1 (endometrial carcinoma), KATO-III
(gastric carcinoma), SBC-3 (small cell lung carcinoma), LNCaP
(prostate adenocarcinoma), MDA-MB-MB468 (breast carcinoma), and
OVCAR-3 (ovarian carcinoma); the cell lines were cultured according
to the vendor’s specifications. The blood samples were assayed
using CTC detection assay, and the detectable cells were counted by
fluorescence microscopy. More than 100 cells were analyzed in each
sample. The GFP signal intensity threshold was determined to be
2.85×107 mean equivalent fluorochrome on the basis of
the minimal GFP intensity level observed in the blood samples mixed
with the cell lines. In addition, there was no significant
difference of cell size between the cell before and after OBP-401
infection.
Determination of cell size threshold
In our previous study (22), various sizes of GFP+
cells were observed in each sample, making it difficult to identify
representative GFP+ cells for comparison between
patients and healthy volunteers. Therefore, to establish a constant
value, we used the optimum threshold derived from the ROC analysis
based on cell size, that is, 7.735 μm, as the threshold to
define GFP-positive CTCs. In this study, we categorized
GFP+ cells into two groups: smaller (S-GFP+
cells) or larger (L-GFP+ cells) than 7.735 μm in
diameter (Fig. 1).
Cell counting and analysis
All GFP+ cells on the two slides were
analyzed using a computer-controlled fluorescence microscope (IX71,
Olympus, Tokyo, Japan); the observer was blinded to the sample
detail. S-GFP+ cells with fluorescent emissions
≥2.85×107 mean equivalent fluorochrome were counted as
GFP+ cells. GFP+ cells included epithelial
marker-positive and epithelial marker-negative cells because tumor
cells undergoing EMT have been reported to be epithelial marker,
such as EpCAM and cytokeratin, negative (18). CD45+ cells were excluded
from the analysis.
Statistical analysis
All statistical analysis was performed using JMP Pro
10.0.0.2 (SAS Institute, Cary, NC, USA). Parametric comparisons
were done using analysis of variance, and nonparametric comparisons
were done using the Wilcoxon and Kruskal-Wallis tests. ROC curve
analysis was performed to examine the relationship between patient
outcome and the number of GFP+ cells. The log-rank test
was also used to calculate overall and relapse-free survival rates.
Cox proportional hazards analysis was used to investigate risk
factor for survival; P≤0.05 was considered statistically
significant.
Results
Participant characteristics
The clinicopathological characteristics of 65
patients (46 men and 19 women; mean age 60.7 years; range 33–76
years) are summarized in Table I.
The median follow-up period of surviving patients was 36 months.
Fifty-seven of the 65 patients underwent pathological curative
surgery, and of these patients, nine experienced disease
recurrence. Fourteen patients died. Twenty-nine patients had distal
gastrectomy, 32 had total gastrectomy, and four had exploratory
laparotomy. Twenty-eight of the 65 patients received chemotherapy
after surgery, 19 patients received oral chemotherapy (S-1), and 9
received oral chemotherapy combined with infusion (S-1/cisplatin
and S-1/docetaxel).
 | Table I.Patient characteristics and
pathological findings. |
Table I.
Patient characteristics and
pathological findings.
| Variable | No. of
patients |
|---|
| Gender | |
| Male | 46 |
| Female | 19 |
| Age (years; mean,
range) | 58.8 (33–76) |
| Gastrectomy | |
| Distal | 29 |
| Total | 32 |
| None | 4 |
| Curability | |
| R0 | 57 |
| R1 | 0 |
| R2 | 8 |
| TNM stage | |
| I | 40 |
| II | 6 |
| III | 10 |
| IV | 9 |
| Depth of tumor
invasion | |
| T1 | 36 |
| T2 | 8 |
| T3 | 9 |
| T4 | 12 |
| Lymph node
metastasis | |
| N0 | 39 |
| N1 | 5 |
| N2 | 6 |
| N3 | 15 |
| Distant
metastasis | |
| M0 | 56 |
| M1 | 9 |
| Main histological
typea | |
|
Differentiated | 25 |
|
Undifferentiated | 40 |
| Lymphatic
invasion | |
| L0 | 35 |
| L1 | 26 |
| LX | 4 |
| Venous
invasion | |
| V0 | 35 |
| V1-2 | 26 |
| VX | 4 |
| Postoperative
chemotherapy | |
| Yes (oral) | 19 |
| Yes (oral and
infusion) | 9 |
| No | 37 |
Association of GFP-positive cells with
pathological indices
Comparison of GFP+ cells between healthy
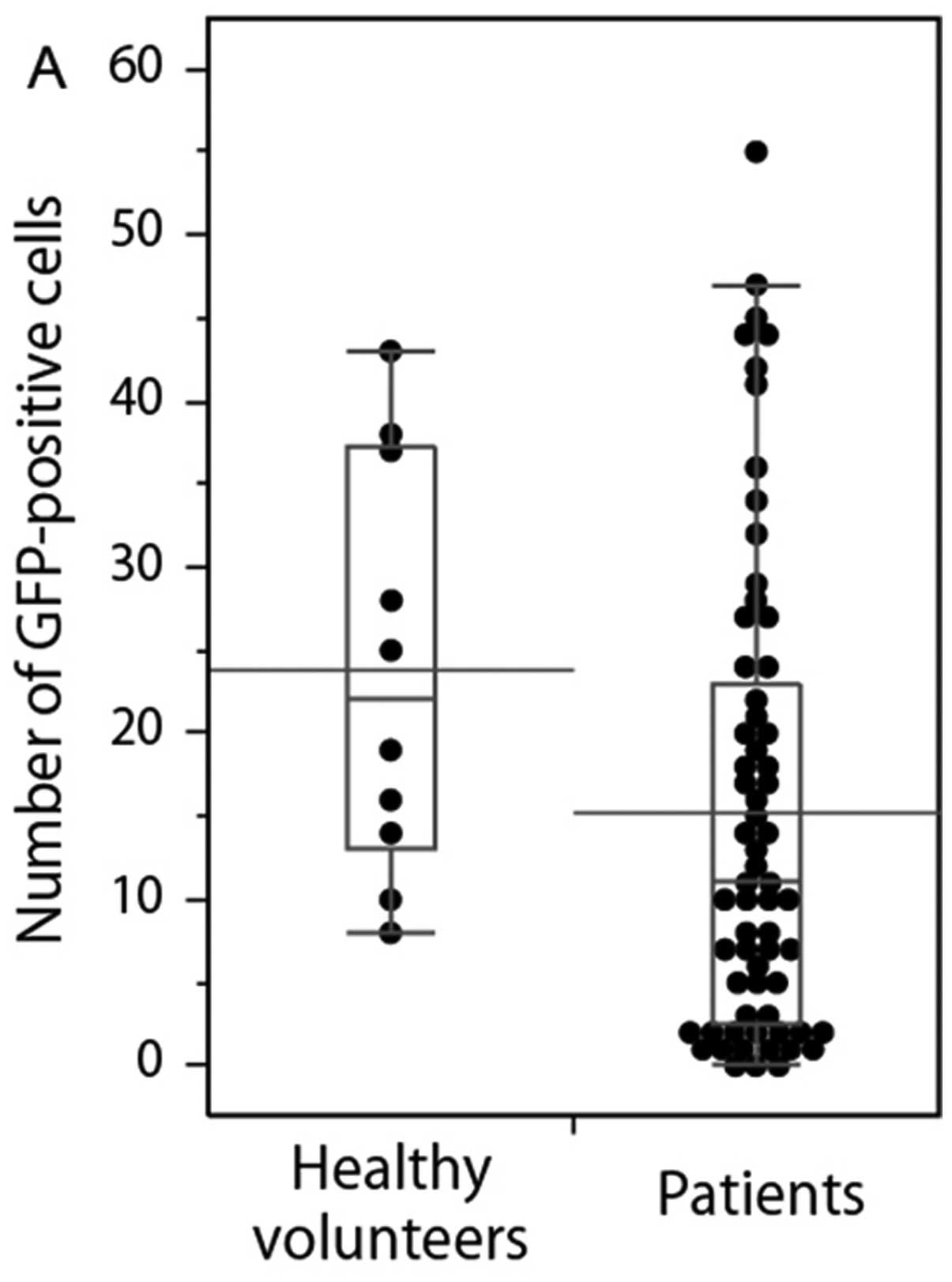
volunteers and patients are shown in Fig. 2. The numbers of GFP+
cells (any size) and S-GFP+ cells in the samples from
the health volunteers were significantly higher than the ones of
the patients (P=0.038 and 0.006). There was no significant
difference in L-GFP+ cells between the samples from
healthy volunteers and the ones from the patients (P=0.760).
There was no significant relationship between the
number of GFP+ cells (any size, P=0.329),
S-GFP+ cells (P=0.424) and L-GFP+ cells
(P=0.213), and cancer stage (Fig.
3A). Although no statistical significance was observed, the
number of GFP+ cells (any size) and S-GFP+
cells tended to increase with the progression of the primary tumor
(Fig. 3B). However, the number of
GFP+ cells in the samples from the node-positive
patients was greater than that in the node-negative patients, there
was no significant difference (Fig.
3C). Compared with the patients without distant metastases,
those with distant metastases had relatively higher numbers of
GFP+ cells (Fig. 3D).
The numbers of GFP+ cells were similar in the samples
from patients with and without lymphatic invasion (Fig. 3E). For venous invasion, the number
of L-GFP+ cells in the samples from the patients with
invasion was significantly higher than that in patients without
invasion (P=0.031) (Fig. 3F).
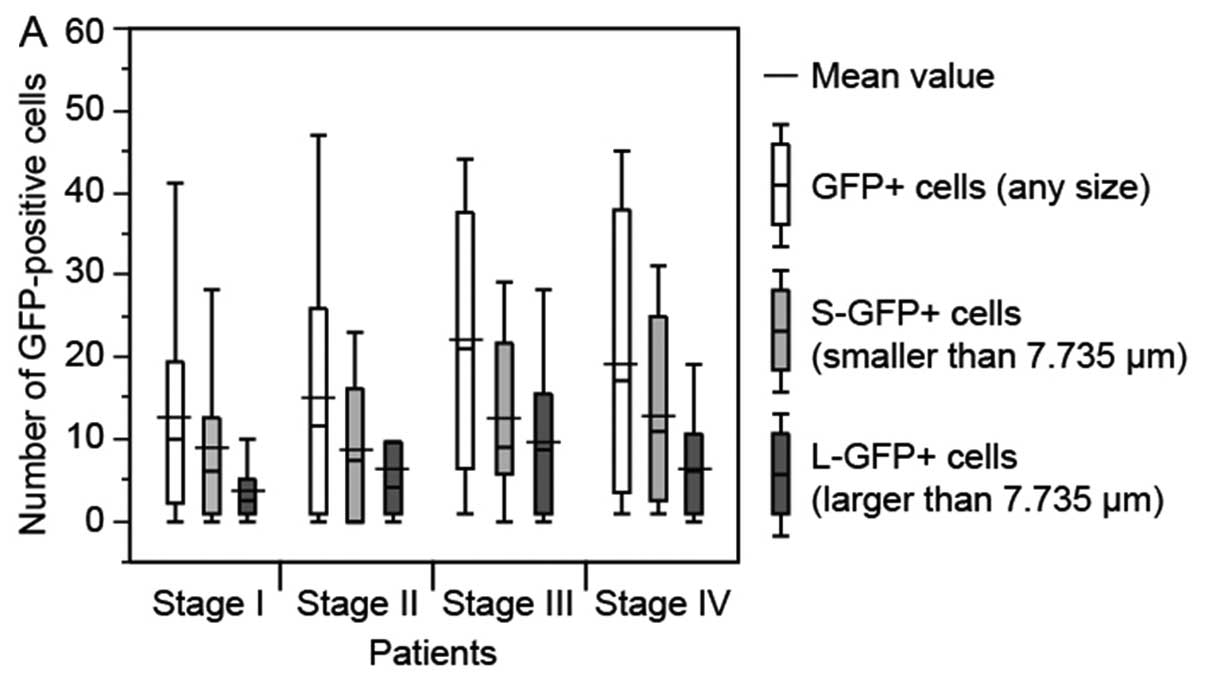 | Figure 3.Relationship between number of
GFP-positive (GFP+) cells in a 7.5-ml blood sample from
gastric cancer patients and pathological findings in the patients.
The bottom and top of the box represent the lower and upper
quartiles, and the band across the box shows the median. The lower
and upper bars at the ends of the whiskers show the lowest data
point within 1.5 interquartile ranges of the lower quartile, and
the highest data point within 1.5 interquartile ranges of the upper
quartile, respectively. (A) TNM stage. (B) Depth of tumor invasion
(T1-T4 indicate increasing depth). (C) Lymph node metastasis (N0,
negative; N1-3, positive). (D) Distant metastasis (M0, negative;
M1, positive). (E) Lymphatic invasion (L0, negative; L1, positive).
(F) Venous invasion (V0, negative; V1-2, positive),
*P<0.05. Although depth of tumor invasion and venous
invasion displayed statistically significant differences, there
were some trends towards an increase in number of GFP+
cells with increasing disease progression. |
Relationship between the patient outcome
and the number and size of GFP-positive cells
The numbers of the detected GFP+ cells in
the peripheral blood samples are shown in Fig. 4. The mean value of GFP+
cells with any size, <7.735 μm and >7.735 μm
were 23.8, 19.0 and 4.8 in the samples from healthy volunteers, and
24, 19 and 5 were prescribed cutoff values of GFP+ cells
with any size, <7.735 μm and >7.735 μm. The
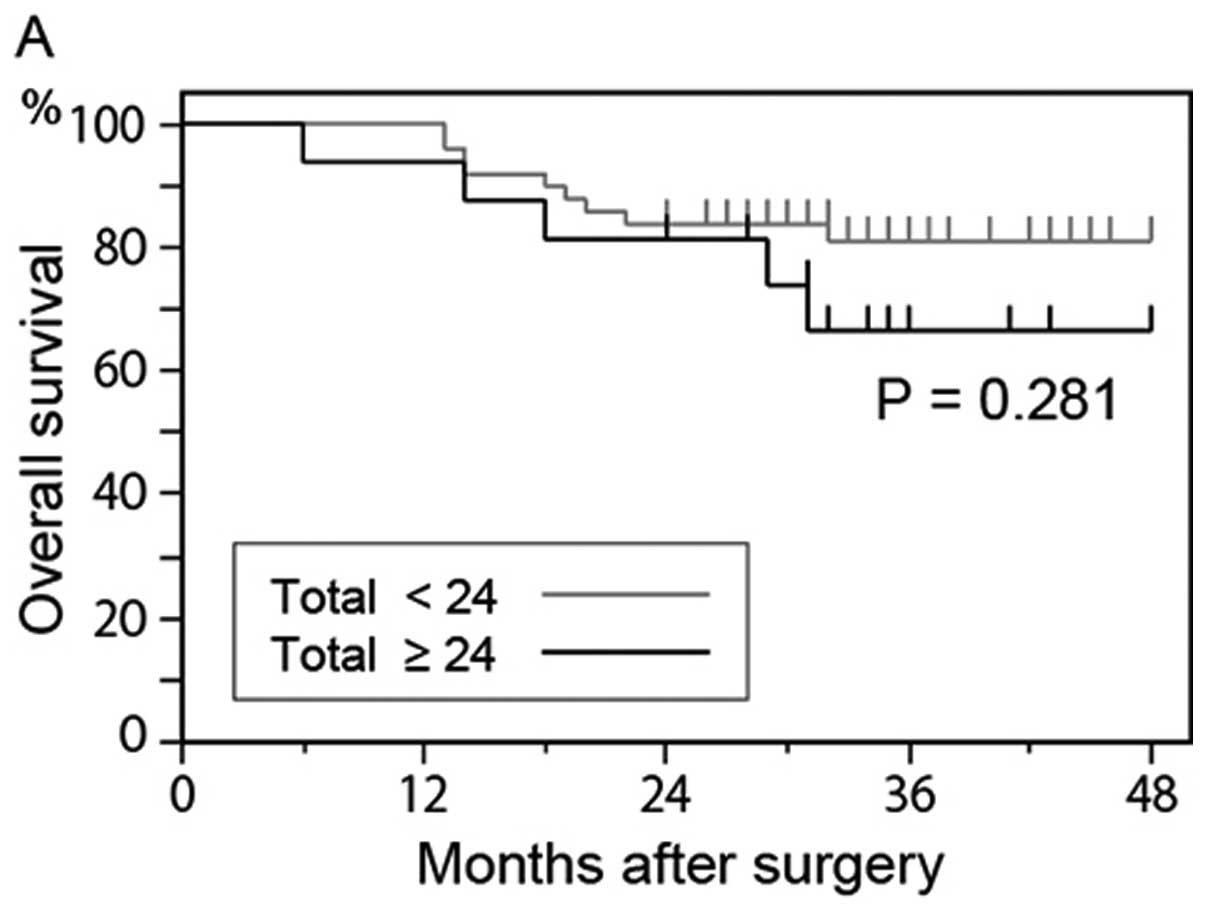
overall survival rate of patients who had 24 or more
GFP+ cells was lower than that of patients who had
<24 GFP+ cells (P= 0.281) (Fig. 4A); however, the difference was not
significant. The overall survival rate of patients who had 20 or
more GFP-positive S-GFP+ cells also tended to be lower
than that of patients who had <20 GFP-positive S-GFP+
cells (P=0.327) (Fig. 4B).
Although there was no significant difference, the overall survival
rate of patients who had 5 or more L-GFP+ cells was
lower than that of patients who had <5 L-GFP+ cells
(P=0.148) (Fig. 4C).
We performed ROC analysis to determine another
cutoff values. The ROC analysis showed that the numbers of
GFP+ cells (P= 0.241, AUC 0.546, cutoff 17, sensitivity
55.6%, and specificity 68.8%) and L-GFP+ cells (P=0.770,
AUC 0.548, cutoff 6, sensitivity 44.4%, and specificity 81.3%) in
the samples from the deceased patients were higher than those in
the samples from the surviving patients (Fig. 5A and B), although the difference
was not significant. No particular tendency was observed in
GFP-positive S-GFP+ cells (P=0.159, AUC 0.557, cutoff
29, sensitivity 22.2%, and specificity 100%) (Fig. 5C). Based on these results, 17 and 6
were prescribed second cutoff values of GFP+ cells with
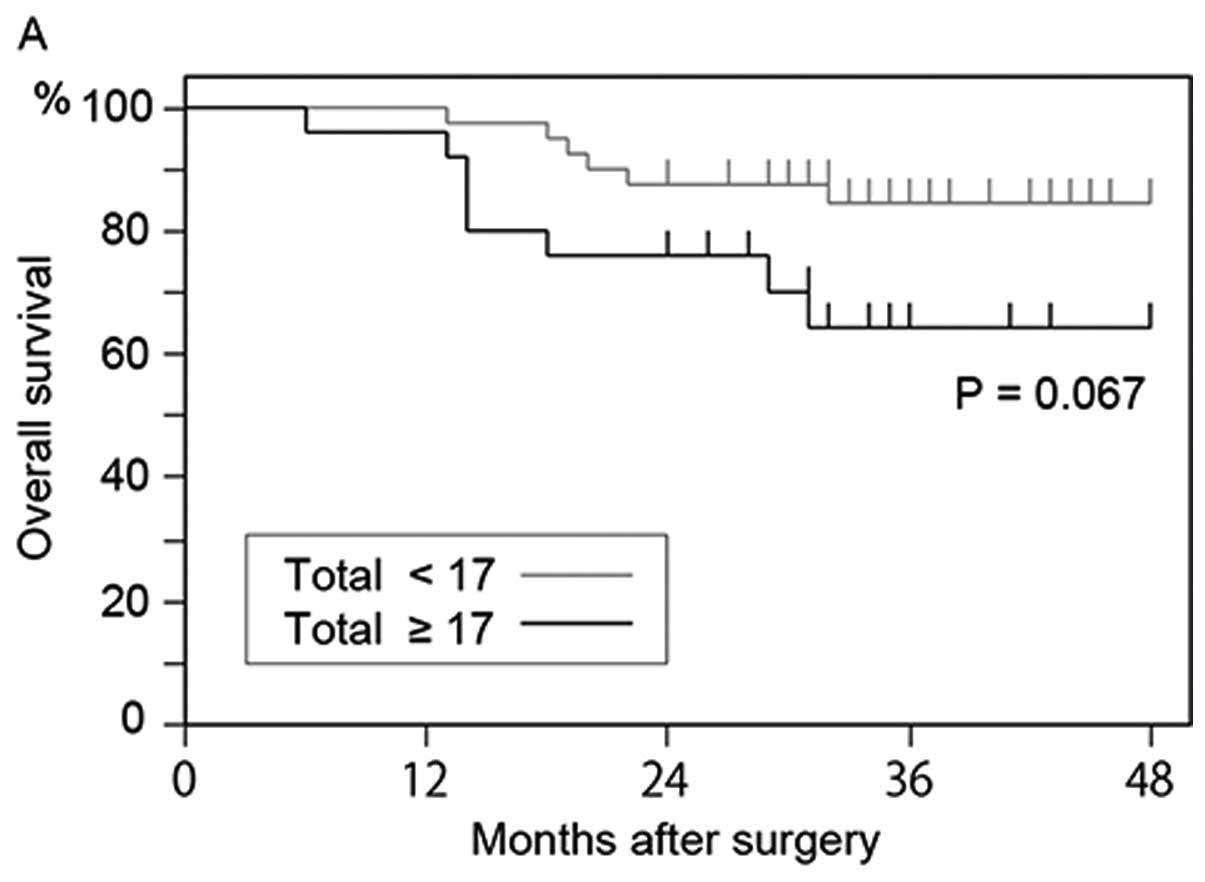
any size and >7.735 μm. The overall survival rate of
patients who had 17 or more GFP+ cells was lower than
that of patients who had <17 GFP+ cells (P=0.067)
(Fig. 6A); however, the difference
was not significant. The overall survival rate of patients who had
6 or more L-GFP+ cells was significantly lower than that
of patients who had <6 L-GFP+ cells (P=0.037)
(Fig. 6B). Moreover, the overall
survival rate of patients who had both 17 or more GFP+
cells and 6 or more L-GFP+ cells was significantly lower
than that of patients who had <17 GFP+ cells or <6
L-GFP+ cells (P=0.004) (Fig. 6C). Seven of 16 (43.8%) patients who
had both 17 or more GFP+ cells and 6 or more
L-GFP+ cells, and 7 of 49 (14.3%) patients who had
<17 GFP+ cells or <6 L-GFP+ cells
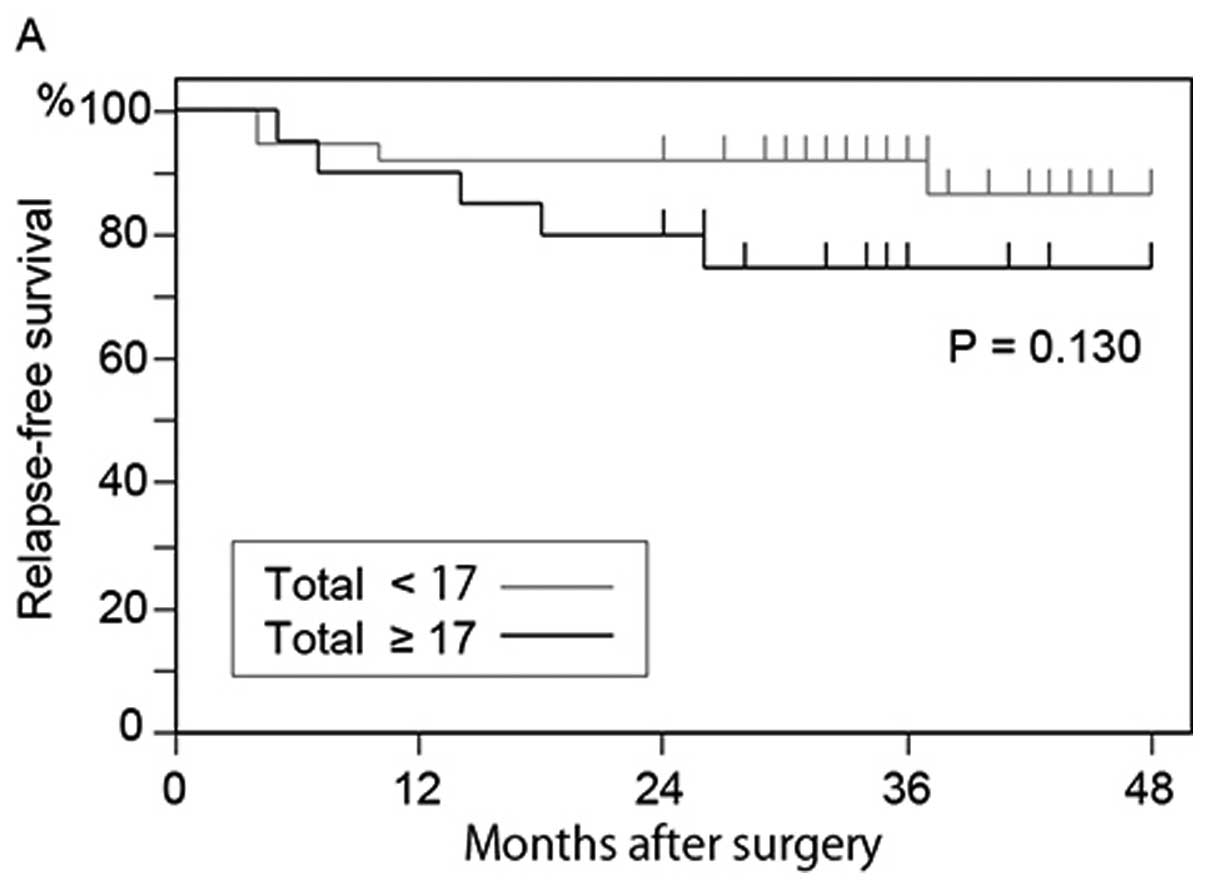
deceased. In the 57 patients who underwent curative surgery, the
relapse-free survival rate of the patients who had 17 or more
GFP+ cells was lower than that of patients who had
<17 GFP+ cells (P=0.130) (Fig. 7A); however, the difference was not
significant. Although there was no significant difference, the
relapse-free survival rate of patients who had 6 or more
L-GFP+ cells was also lower than that of patients who
had <6 L-GFP+ cells (P= 0.124) (Fig. 7B). The relapse-free survival rate
of the patients who had both 17 or more GFP+ cells and 6
or more L-GFP+ cells was significantly lower than that
of the patients who had <17 GFP+ cells or <6
L-GFP+ cells (P=0.015) (Fig. 7C).
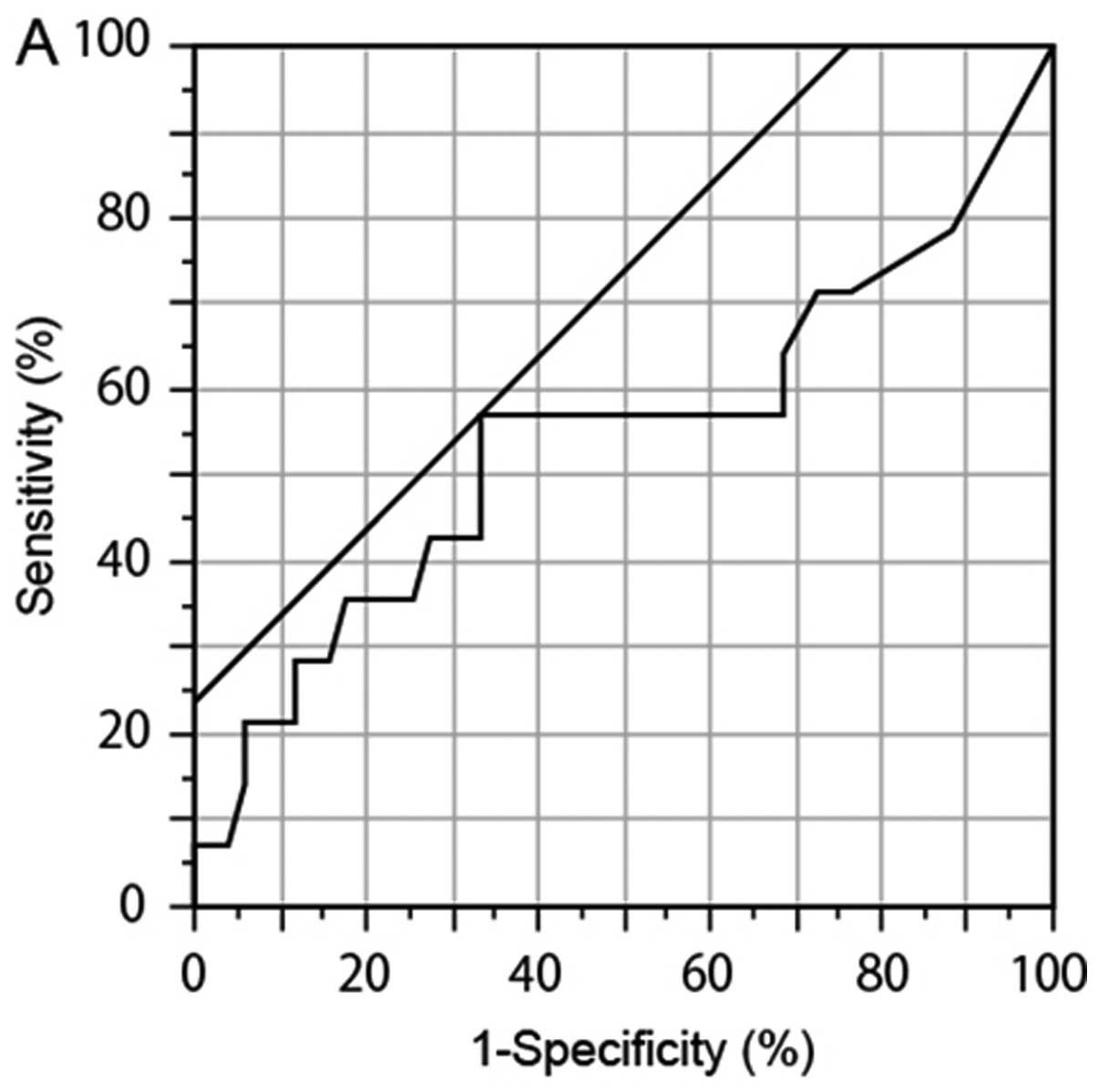 | Figure 5.Comparison of GFP-positive
(GFP+) cell number between surviving patients and
deceased patients. To determine novel threshold, we compared the
numbers of GFP+ cells from surviving patients and
deceased patients with gastric cancer by ROC analysis. (A)
GFP+ cells (any size). (B) Small GFP+ cells
(<7.735 μm). (C) Large GFP+ large cells. The
number of GFP+ cells (P=0.241, AUC 0.546, cutoff 17,
sensitivity 55.6%, and specificity 68.8%) and the number of large
GFP+ cells (P=0.770, AUC 0.548, cutoff 6, sensitivity
44.4%, and specificity 81.3%) in the samples from the deceased
patients were higher than those in the samples from the surviving
patients. A prejudiced value was observed in small GFP+
cells (P= 0.159, AUC 0.557, cutoff 29, sensitivity 22.2%, and
specificity 100%). |
Discussion
In this study, we analyzed the correlation between
CTCs and prognosis in gastric cancer, which is the second leading
cause of cancer-related death worldwide. The usefulness of the
detection of CTCs in the diagnosis and estimation of prognosis has
already been reported for breast (14,30),
prostate (31), lung (32), and digestive tract (11,33)
cancers. The results of the present study indicate that detection
of CTCs may also be useful in the prognosis of gastric cancer.
This study showed two major findings. One was that
the number of L-GFP+ cells is significantly associated
with patient prognosis. In our previous study (22), the prognosis of the patients who
had 5 or more GFP+ cells was significantly lower than
that of the patients who had <5 L-GFP+ cells. In this
study, we obtained a similar result showing that the prognosis of
patients who had 6 or more L-GFP+ cells was
significantly lower than that of patients who had <6
L-GFP+ cells.
Further, we determined whether the number of
GFP+ cells of any diameter may be related to patient
prognosis. Patients who had 17 or more GFP+ cells showed
lower survival rate than those who had <17 GFP+
cells, although the difference was not significant. Since the
combination of the number of total GFP+ cells and
L-GFP+ cells showed a significant correlation with
patient prognosis whereas the number of only L-GFP+
cells did not, we deemed the number of all GFP+ cells to
be related to patient prognosis. On the other hand, the
relationship between the number of S-GFP+ cells and
prognosis was unclear. Although there was a significant difference
in the prognosis between patients who had 29 or more
S-GFP+ cells (n=2) and those who had <29
S-GFP+ cells (n=63), unequal numbers of patients were
enrolled in the two groups. In our previous study (22), S-GFP+ cells were
observed in the blood samples from healthy volunteers. Therefore,
S-GFP+ cells may be detected as false-positive CTCs.
There is possibility that OBP-401 infection caused increased
telomerase activity in non-cancer cells.
One limitation of our study was that the metastatic
potential of the detected CTCs was not determined. Our results
suggested L-GFP+ cells to be a predictive and prognostic
marker; however, further study is needed to determine the
metastatic potential of L-GFP+ cells. On the other hand,
S-GFP+ cells may contain a small population of CTCs with
metastatic potential including tumor cells with EMT. It was
suggested that the CTCs with EMT were included in both of
S-GFP+ cells and L-GFP+ cells in this study.
Clearly, more studies in a larger population of patients, and with
different cancer types, are needed to clarify the clinical
applicability of CTC detection. Thus, further studies should
analyze the functions of viable CTCs after cell sorting, and
identify CTCs with metastatic potential using additional tools such
as DNA ploidy analysis (34,35).
Furthermore, gene expression profiling of viable CTCs, dead cells,
primary tumors, and metastatic tumors will also provide important
insight into the mechanisms of cancer metastasis. Finally, the
results of the present study indicate that CTCs are useful as
predictors of disease progression in gastric cancer patients, but
they do not constitute an independent prognostic factor.
The number of detected L-GFP+ cells
showed a significant relationship with prognosis in gastric cancer.
However, the study used a short follow-up period and only a small
number of participants. In addition, whether all GFP+
cells have true metastatic potential was unclear. Further studies
are warranted to confirm the findings of this study.
Acknowledgements
This study was supported in part by a
Grant-in-Aid for Challenging Exploratory Research (23659308) from
the Ministry of Education, Culture, Sports, Science, and Technology
(MEXT). We are grateful to all the patients and volunteers who
donated blood for this study. We would like to thank Professor
Toshiyoshi Fujiwara (Okayama University Graduate School of
Medicine, Okayama, Japan) for helpful comments and suggestions; Mr.
Yasuo Urata (Oncolys BioPharma, Tokyo, Japan) for supplying the
OBP-401; Dr Yukio Tsujino, Dr Toshiyuki Ozawa, and Dr Akinori
Masago (Sysmex Corporation, Kobe, Japan) for their valuable
support; and the clinical staff.
References
|
1.
|
Kowalski LP: Lymph node metastasis as a
prognostic factor in laryngeal cancer. Rev Paul Med. 111:42–45.
1993.PubMed/NCBI
|
|
2.
|
Nakane Y, Okamura S, Masuya Y, Okumura S,
Akehira K and Hioki K: Incidence and prognosis of para-aortic lymph
node metastasis in gastric cancer. Hepatogastroenterology.
45:1901–1906. 1998.PubMed/NCBI
|
|
3.
|
Arai Y, Kanamaru H, Yoshimura K, Okubo K,
Kamoto T and Yoshida O: Incidence of lymph node metastasis and its
impact on long-term prognosis in clinically localized prostate
cancer. Int J Urol. 5:459–465. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
4.
|
Liotta LA, Kleinerman J and Saidel GM:
Quantitative relationships of intravascular tumor cells, tumor
vessels, and pulmonary metastases following tumor implantation.
Cancer Res. 34:997–1004. 1974.PubMed/NCBI
|
|
5.
|
Hanahan D and Weinberg RA: The hallmarks
of cancer. Cell. 100:57–70. 2000. View Article : Google Scholar
|
|
6.
|
Gertler R, Rosenberg R, Fuehrer K, Dahm M,
Nekarda H and Siewert JR: Detection of circulating tumor cells in
blood using an optimized density gradient centrifugation. Recent
Results Cancer Res. 162:149–155. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
7.
|
Vona G, Sabile A, Louha M, et al:
Isolation by size of epithelial tumor cells: a new method for the
immunomorphological and molecular characterization of circulating
tumor cells. Am J Pathol. 156:57–63. 2000. View Article : Google Scholar
|
|
8.
|
Talasaz AH, Powell AA, Huber DE, et al:
Isolating highly enriched populations of circulating epithelial
cells and other rare cells from blood using a magnetic sweeper
device. Proc Natl Acad Sci USA. 106:3970–3975. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
9.
|
He W, Wang H, Hartmann LC, Cheng JX and
Low PS: In vivo quantitation of rare circulating tumor cells by
multiphoton intravital flow cytometry. Proc Natl Acad Sci USA.
104:11760–11765. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
10.
|
Ito H, Kanda T, Nishimaki T, Sato H,
Nakagawa S and Hatakeyama K: Detection and quantification of
circulating tumor cells in patients with esophageal cancer by
real-time polymerase chain reaction. J Exp Clin Cancer Res.
23:455–464. 2004.PubMed/NCBI
|
|
11.
|
Honma H, Kanda T, Ito H, et al: Squamous
cell carcinoma-antigen messenger RNA level in peripheral blood
predicts recurrence after resection in patients with esophageal
squamous cell carcinoma. Surgery. 139:678–685. 2006. View Article : Google Scholar
|
|
12.
|
Nagrath S, Sequist LV, Maheswaran S, et
al: Isolation of rare circulating tumour cells in cancer patients
by microchip technology. Nature. 450:1235–1239. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
13.
|
Cohen SJ, Punt CJ, Iannotti N, et al:
Relationship of circulating tumor cells to tumor response,
progression-free survival, and overall survival in patients with
metastatic colorectal cancer. J Clin Oncol. 26:3213–3221. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
14.
|
Riethdorf S, Fritsche H, Muller V, et al:
Detection of circulating tumor cells in peripheral blood of
patients with metastatic breast cancer: a validation study of the
CellSearch system. Clin Cancer Res. 13:920–928. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
15.
|
Davis JW, Nakanishi H, Kumar VS, et al:
Circulating tumor cells in peripheral blood samples from patients
with increased serum prostate specific antigen: initial results in
early prostate cancer. J Urol. 179:2187–2191. 2008. View Article : Google Scholar
|
|
16.
|
Hou JM, Greystoke A, Lancashire L, et al:
Evaluation of circulating tumor cells and serological cell death
biomarkers in small cell lung cancer patients undergoing
chemotherapy. Am J Pathol. 175:808–816. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
17.
|
Ksiazkiewicz M, Markiewicz A and Zaczek
AJ: Epithelial-mesenchymal transition: a hallmark in metastasis
formation linking circulating tumor cells and cancer stem cells.
Pathobiology. 79:195–208. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
18.
|
Gorges TM, Tinhofer I, Drosch M, Roese L,
Zollner TM, Krahn T and von Ahsen O: Circulating tumour cells
escape from EpCAM-based detection due to epithelial-to-mesenchymal
transition. BMC Cancer. 12:1782012. View Article : Google Scholar : PubMed/NCBI
|
|
19.
|
Kim NW, Piatyszek MA, Prowse KR, et al:
Specific association of human telomerase activity with immortal
cells and cancer. Science. 266:2011–2015. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
20.
|
Blackburn EH: Telomere states and cell
fates. Nature. 408:53–56. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
21.
|
Fujiwara T, Kagawa S, Kishimoto H, et al:
Enhanced antitumor efficacy of telomerase-selective oncolytic
adenoviral agent OBP-401 with docetaxel: preclinical evaluation of
chemovirotherapy. Int J Cancer. 119:432–440. 2006. View Article : Google Scholar
|
|
22.
|
Ito H, Inoue H, Sando N, et al: Prognostic
impact of detecting viable circulating tumour cells in gastric
cancer patients using a telomerase-specific viral agent: a
prospective study. BMC Cancer. 12:3462012. View Article : Google Scholar
|
|
23.
|
Lin HK, Zheng S, Williams AJ, et al:
Portable filter-based microdevice for detection and
characterization of circulating tumor cells. Clin Cancer Res.
16:5011–5018. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
24.
|
Zheng S, Lin HK, Lu B, Williams A, Datar
R, Cote RJ and Tai YC: 3D microfilter device for viable circulating
tumor cell (CTC) enrichment from blood. Biomed Microdevices.
13:203–213. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
25.
|
Thiery JP: Epithelial-mesenchymal
transitions in tumour progression. Nat Rev Cancer. 2:442–454. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
26.
|
Brabletz T, Hlubek F, Spaderna S,
Schmalhofer O, Hiendlmeyer E, Jung A and Kirchner T: Invasion and
metastasis in colorectal cancer: epithelial-mesenchymal transition,
mesenchymal-epithelial transition, stem cells and beta-catenin.
Cells Tissues Organs. 179:56–65. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
27.
|
Oken MM, Creech RH, Tormey DC, Horton J,
Davis TE, McFadden ET and Carbone PP: Toxicity and response
criteria of the Eastern Cooperative Oncology Group. Am J Clin
Oncol. 5:649–655. 1982. View Article : Google Scholar : PubMed/NCBI
|
|
28.
|
Sobin LH, Gospodarowicz MK and Wittekind
C: International Union Against Cancer: TNM Classification of
Malignant Tumours. 7th edition. Chichester, West Sussex, UK;
Hoboken, NJ: Wiley-Blackwell; 2010
|
|
29.
|
Kim SJ, Masago A, Tamaki Y, et al: A novel
approach using telomerase-specific replication-selective adenovirus
for detection of circulating tumor cells in breast cancer patients.
Breast Cancer Res Treat. 128:765–773. 2011. View Article : Google Scholar
|
|
30.
|
Cristofanilli M, Budd GT, Ellis MJ, et al:
Circulating tumor cells, disease progression, and survival in
metastatic breast cancer. N Engl J Med. 351:781–791. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
31.
|
Moreno JG, Miller MC, Gross S, Allard WJ,
Gomella LG and Terstappen LW: Circulating tumor cells predict
survival in patients with metastatic prostate cancer. Urology.
65:713–718. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
32.
|
Krebs MG, Sloane R, Priest L, et al:
Evaluation and prognostic significance of circulating tumor cells
in patients with non-small-cell lung cancer. J Clin Oncol.
29:1556–1563. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
33.
|
Katsumata K, Sumi T, Mori Y, Hisada M,
Tsuchida A and Aoki T: Detection and evaluation of epithelial cells
in the blood of colon cancer patients using RT-PCR. Int J Clin
Oncol. 11:385–389. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
34.
|
Bonsing BA, Beerman H, Kuipers-Dijkshoorn
N, Fleuren GJ and Cornelisse CJ: High levels of DNA index
heterogeneity in advanced breast carcinomas. Evidence for DNA
ploidy differences between lymphatic and hematogenous metastases
Cancer. 71:382–391. 1993.PubMed/NCBI
|
|
35.
|
Klijanienko J, el-Naggar AK, de Braud F,
et al: Tumor vascularization, mitotic index, histopathologic grade,
and DNA ploidy in the assessment of 114 head and neck squamous cell
carcinomas. Cancer. 75:1649–1656. 1995. View Article : Google Scholar : PubMed/NCBI
|