The protein kinase C (PKC) family is a family of
serine/threonine kinases that play diverse roles in fundamental
cellular processes including cell proliferation, cell death and
differentiation (1,2). PKCs respond to extracellular signals
that promote phospholipid hydrolysis and facilitate the generation
of diacylglycerol (DAG) and release of Ca2+ from
intracellular stores. These two second messengers activate PKCs in
the presence of acidic phospholipids, such as phosphatidylserine
(2). The PKC family garnered
considerable attention by the discovery that PKCs could serve as
receptors for tumor-promoting phorbol esters which was the first
evidence to establish a link between PKCs and cancer (3,4).
These phorbol esters are potent activators of PKCs and can
substitute for the physiological stimulator DAG. Based on the
structural features and cofactor requirements, the PKC family
consists of 10 isozymes categorized as the conventional or the
classical (c) PKCs (α, βI, βII, γ), the novel (n) PKCs (δ, ɛ, η, θ)
and the atypical (a) PKCs (ζ, λ/ι) (1). While the conventional PKCs are
sensitive to Ca2+ and DAG/phorbol ester, novel PKCs are
insensitive to Ca2+ but respond to DAG/phorbol ester and
atypical PKCs are insensitive to both Ca2+ and
DAG/phorbol esters. The distinct structural and biochemical
features of the PKC isozymes pave the way for the distinctive
cellular responses attributed to the PKC family. Owing to the
central role of PKCs in cellular regulation and signal
transduction, significant research efforts have been devoted to the
PKC family. However, much less is known about PKCη, which is a
unique member of the novel PKC family. This review focuses on the
biology of PKCη and its implications in breast cancer.
Protein kinase Cη (PKCη) is a novel member of the
PKC family. It is classified as a calcium-independent but
DAG/phorbol ester-dependent PKC (5). It was first isolated from a cDNA
library of mouse epidermis (5).
PKCη is assigned to human chromosome 14 (14q22–23) and mouse
chromosome 12 (12C3–D2) (6,7) and
contains an open reading frame encoding 683 amino acid residues
(8). Contrary to other PKCs which
are primarily enriched in the brain tissue, PKCη is mainly
expressed in lung, skin and heart tissues (9). PKCη participates in various cellular
processes including proliferation, differentiation, secretion and
apoptosis (10–16). Recent reports have revealed the
role of PKCη in immune function (17,18).
PKCη was shown to be important for T-cell proliferation and
homeostasis (19), and was also
implicated in the regulation of toll-like receptor-2 (TLR-2)
responses in macrophages (20).
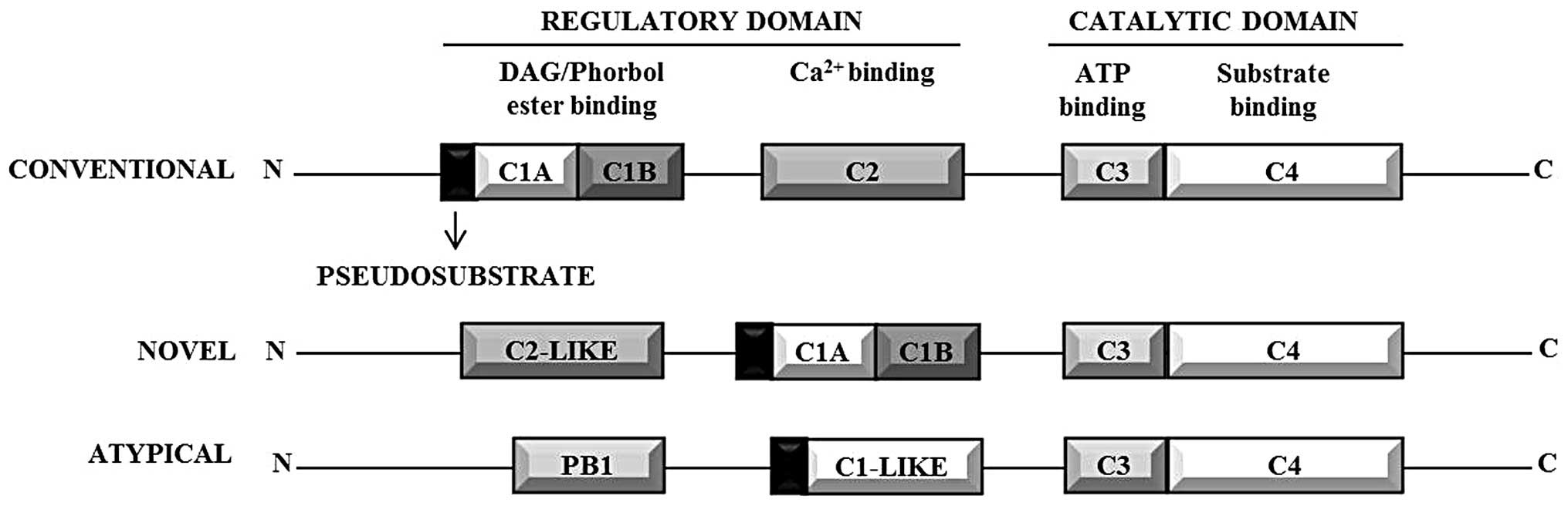
All PKC isozymes contain a common structural
backbone comprised of a highly conserved catalytic domain at the
C-terminal and a regulatory domain at the N-terminal (Fig. 1). PKCs possess 4 conserved modules
(C1–4): C1 and C2 are the membrane targeting modules that along
with the pseudosubstrate region form the regulatory domain; C3 and
C4 comprise the catalytic domain (21). A proteolytically labile hinge
region connects the regulatory domain to the catalytic domain
(22). The catalytic domain
consists of motifs that are required for ATP/substrate binding and
catalysis. The N-terminal contains the autoinhibitory
pseudosubstrate sequence that contains an alanine in place of the
serine/threonine phosphoacceptor site, but otherwise resembles a
PKC substrate. The pseudosubstrate thus holds the enzyme in an
inactive conformation by occupying the catalytic site (21). The pseudosubstrate sequence of PKCη
is the most divergent amongst the PKC isozymes (9).
The structure of PKCη comprises of a highly
conserved catalytic domain at the C-terminal and the regulatory
domain at the N-terminal similar to other PKCs (21). A characteristic cysteine-rich
region is present in the C1 domain of PKCη which allows binding to
physiological stimulator DAG and pharmacological activators such as
tumor-promoting phorbol esters (23). In addition, C1 domain confers
selectivity for phosphatidylserine that acts as the activator for
PKCs (24). The C2 domain of PKCη
lacks the key aspartic acid residues to bind Ca2+ and
consequently renders PKCη insensitive to Ca2+ (23). PKCη shares greatest homology with
PKCɛ, another novel PKC (9).
Similar to other PKC isozymes, PKCη has three
conserved phosphorylation sites - activation loop (Thr-513), turn
motif (Thr-655) and hydrophobic domain (Ser-674) (21). Although the order of priming
phosphorylations of PKCη is not well established,
phosphoinositide-dependent kinase 1 (PDK1) is believed to
phosphorylate PKCη at the activation loop in vitro (25). In mouse A9 fibroblasts infected
with parovirus, Lachmann et al demonstrated that PKCλ
phosphorylates PKCη at the hydrophobic site thus allowing PDK1
access to the activation loop (26). The C2 domain of PKCη was found to
be similar to PKCɛ with significant differences at the putative
lipid binding site. Mass spectrometric analysis of the C2 domain of
PKCη revealed two autophosphorylation sites at Ser-28 and Ser-32
(27). The autophosphorylation
site at Ser-28 but not Ser-32 is conserved in PKCɛ (27). It has been speculated that
autophosphorylation at these sites could affect the lipid-binding
of PKCη (27).
The PKC isozymes are under tight structural and
spatial regulation that underlies their biochemical functions,
intracellular localization and tissue distribution (21). PKCs can be regulated by
phosphorylation, cofactor binding and membrane targeting through
interaction with scaffold proteins (28).
Anionic phospholipids such as phosphatidylserine and
DAG/phorbol esters regulate PKCη (5,9).
However, in contrast to other phorbol-ester sensitive PKC isozymes,
PKCη resists downregulation by prolonged treatment with phorbol
esters, suggesting its unique regulation (11,29,30).
We have shown that PKCη not only resists downregulation by phorbol
esters but is in fact upregulated by several structurally and
functionally distinct PKC activators (31). We further reported that
transphosphorylation by novel PKCɛ is responsible for
activator-induced upregulation of PKCη (31).
PKCη is subject to translational regulation under
both normal and stressed conditions caused by amino acid starvation
(40). Raveh-Amit and colleagues
reported that the 5′-UTR of PKCη is unusually long (659
nucleotides) and rich in GC content and identified two upstream
open reading frames (uORF) in the 5′-UTR which function as
repressive elements under normal growth conditions. However, under
amino acid starvation, the repression is removed by leaky scanning
leading to the translational upregulation of PKCη (40). PKCɛ is the only other PKC isozyme
for which the presence of a regulatory uORF has been reported
(41).
Termination of PKC signaling can occur via different
mechanisms such as release of PKC isozymes from the membrane,
metabolism of DAG by DAG kinases (DGKs) (42,43),
agonist-induced degradation or the removal of priming
phosphorylation which leads to downregulation and rapid degradation
(42,44,45).
Several mechanisms of degradation have been proposed for the PKC
isozymes. Conventional PKCs are believed to be downregulated by
calcium-activated proteases, such as calpains (46,47)
whereas PKCα, −δ and −ɛ were shown to be degraded via
proteasome-mediated pathway (48–50).
Our studies have demonstrated that PKCη can be downregulated by
both proteasomal-dependent and -independent pathways. While
inhibition/knockdown of PDK1 caused PKCη downregulation via the
proteasomal pathway, the downregulation of PKCη caused by the
depletion of PKCɛ or by PKC inhibitors was independent of the
proteasome-mediated pathway (51).
Another study reported that dephosphorylation of PKCη was mediated
by integrin-associated serine threonine phosphatase PP1γ in human
platelets which was shown to be independent of the
ubiquitin-mediated degradation (52). In addition, differential expression
analysis in the neoplastic cell line 8701-BC demonstrated that PKCη
downregulation can be induced by type V collagen (53).
PKCη is localized in the Golgi, endoplasmic
reticulum (ER) and the nuclear envelope (54). Although the C1A domain of PKCη
lacks a Golgi localization signal similar to the other members of
the novel PKC family, the C1B domain of PKCη facilitates its
translocation to the Golgi complex (54). The localization of PKCη in the
Golgi could account for the involvement of PKCη in Golgi vesicular
transport. It has been previously reported that Golgi-cell surface
transport requires protein kinase D (PKD) which is specifically
activated by G protein subunits β1γ2 and β3γ2 via the
Golgi-associated PKCη (55). In
response to serum starvation and PMA, PKCη translocates to the
nuclear envelope. While C1B domain is sufficient to drive Golgi
translocation of PKCη, both the C1 and the pseudosubstrate region
are required for the localization at the nuclear envelope and ER
(54). PKCη is localized in the
plasma membrane and the nuclear envelope upon stimulation with
phorbol esters, while serum starvation leads to distribution only
in the nuclear envelope (54).
Furthermore, a recent study reported that in hepatocellular
carcinoma cells, PKCη is targeted to lipid droplets where it
limited the formation of larger lipid droplets (56).
PKC isozymes have been extensively researched as
potent targets for cancer therapeutics since their discovery as
receptors for tumor promoters (3,57).
The role of PKCη in cancer is controversial owing to its divergent
responses in different cancers. Although, PKCη-deficient mice were
more susceptible to tumor promotion in two-stage skin
carcinogenesis model (58), PKCη
mediates chemotherapeutic resistance in breast cancer (10,59),
glioblastoma (60), lung cancer
(61) and several other cancers
(62,63). It has been reported that PKCη is
downregulated in hepatocellular carcinoma (64) but is associated with the
progression of renal cell carcinoma (65). Thus, PKCη may promote or inhibit
malignant growth depending on the cellular context.
PKCη is a unique member of the PKC family. Its
distinct regulation in response to tumor promoters compared to the
other PKCs has potential implications in cancer. Although several
studies have established the role of PKCη in cell growth,
proliferation and chemoresistance, conflicting reports have added
ambiguity to the functional role of PKCη. Moreover, PKCη interacts
with several signaling pathways, such as the PI3K/Akt, NF-κB and
ERK/Elk-1 (15,76,78).
In addition, most cells express multiple PKC isozymes which display
redundant as well as opposing functions. The distinct biochemical
properties, tissue distribution and subcellular localization of
different PKC isozymes have been reported to result in divergent
responses in cancer (79–81). Thus, the crosstalk between these
proteins will eventually influence the final outcome.
While the published reports have helped discern the
regulation and function of PKCη, many questions remain regarding
the paradoxical actions of PKCη. It would be worthwhile to
understand the specific interactions of PKCη with other signaling
pathways and the subsequent consequences on cellular regulation.
Studies focused on the interaction of transcription factors such as
AP-1 or Ets1 with PKCη in breast cancer could also yield
interesting results. Thus, future studies should help determine the
molecular cues which govern the dynamic role of PKCη.
This work was supported by the Doctoral Bridge
Funding Award (DP) from the University of North Texas Health
Science Center.
|
1
|
Nishizuka Y: Intracellular signaling by
hydrolysis of phospholipids and activation of protein kinase C.
Science. 258:607–614. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Griner EM and Kazanietz MG: Protein kinase
C and other diacylglycerol effectors in cancer. Nat Rev Cancer.
7:281–294. 2007. View
Article : Google Scholar : PubMed/NCBI
|
|
3
|
Blumberg PM: Protein kinase C as the
receptor for the phorbol ester tumor promoters: sixth Rhoads
memorial award lecture. Cancer Res. 48:1–8. 1988.PubMed/NCBI
|
|
4
|
Castagna M, Takai Y, Kaibuchi K, Sano K,
Kikkawa U and Nishizuka Y: Direct activation of calcium-activated,
phospholipid-dependent protein kinase by tumor-promoting phorbol
esters. J Biol Chem. 257:7847–7851. 1982.PubMed/NCBI
|
|
5
|
Osada S, Mizuno K, Saido TC, et al: A
phorbol ester receptor/protein kinase, nPKC eta, a new member of
the protein kinase C family predominantly expressed in lung and
skin. J Biol Chem. 265:22434–22440. 1990.PubMed/NCBI
|
|
6
|
Quan T and Fisher GJ: Cloning and
characterization of the human protein kinase C-eta promoter. J Biol
Chem. 274:28566–28574. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Chida K, Nakada T, Otsuka H, Kuroki T and
Satoh H: Assignment of protein kinase C eta (Pkch) to mouse
chromosome band 12C3–D2 by in situ hybridization. Cytogenet Cell
Genet. 82:30–31. 1998.PubMed/NCBI
|
|
8
|
Kashiwagi M, Ohba M, Chida K and Kuroki T:
Protein kinase C eta (PKC eta): its involvement in keratinocyte
differentiation. J Biochem. 132:853–857. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Bacher N, Zisman Y, Berent E and Livneh E:
Isolation and characterization of PKC-L, a new member of the
protein kinase C-related gene family specifically expressed in
lung, skin, and heart. Mol Cell Biol. 11:126–133. 1991.
|
|
10
|
Akkaraju GR and Basu A: Overexpression of
protein kinase C-eta attenuates caspase activation and tumor
necrosis factor-alpha-induced cell death. Biochem Biophys Res
Commun. 279:103–107. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Basu A: The involvement of novel protein
kinase C isozymes in influencing sensitivity of breast cancer MCF-7
cells to tumor necrosis factor-alpha. Mol Pharmacol. 53:105–111.
1998.PubMed/NCBI
|
|
12
|
Fima E, Shtutman M, Libros P, et al:
PKCeta enhances cell cycle progression, the expression of G1
cyclins and p21 in MCF-7 cells. Oncogene. 20:6794–6804. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Fima E, Shahaf G, Hershko T, Apte RN and
Livneh E: Expression of PKCeta in NIH-3T3 cells promotes production
of the pro-inflammatory cytokine interleukin-6. Eur Cytokine Netw.
10:491–500. 1999.
|
|
14
|
Hussaini IM, Karns LR, Vinton G, et al:
Phorbol 12-myristate 13-acetate induces protein kinase
ceta-specific proliferative response in astrocytic tumor cells. J
Biol Chem. 275:22348–22354. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Raveh-Amit H, Hai N, Rotem-Dai N, Shahaf
G, Gopas J and Livneh E: Protein kinase Ceta activates NF-kappaB in
response to camptothecin-induced DNA damage. Biochem Biophys Res
Commun. 412:313–317. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Ohba M, Ishino K, Kashiwagi M, et al:
Induction of differentiation in normal human keratinocytes by
adenovirus-mediated introduction of the eta and delta isoforms of
protein kinase C. Mol Cell Biol. 18:5199–5207. 1998.PubMed/NCBI
|
|
17
|
Fu G and Gascoigne NR: Protein kinase
Ceta, an emerging player in T-cell biology. Cell Cycle. 11:837–838.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Quann EJ, Liu X, Altan-Bonnet G and Huse
M: A cascade of protein kinase C isozymes promotes cytoskeletal
polarization in T cells. Nat Immunol. 12:647–654. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Fu G, Hu J, Niederberger-Magnenat N, et
al: Protein kinase C eta is required for T cell activation and
homeostatic proliferation. Sci Signal. 4:ra842011.PubMed/NCBI
|
|
20
|
Park DW, Lee HK, Lyu JH, et al: TLR2
stimulates ABCA1 expression via PKC-eta and PLD2 pathway. Biochem
Biophys Res Commun. 430:933–937. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Steinberg SF: Structural basis of protein
kinase C isoform function. Physiol Rev. 88:1341–1378. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Inoue M, Kishimoto A, Takai Y and
Nishizuka Y: Studies on a cyclic nucleotide-independent protein
kinase and its proenzyme in mammalian tissues. II Proenzyme and its
activation by calcium-dependent protease from rat brain. J Biol
Chem. 252:7610–7616. 1977.PubMed/NCBI
|
|
23
|
Hashimoto Y, Osada S, Ohno S and Kuroki T:
A Ca(2+)-independent protein kinase C, nPKC eta: its structure,
distribution and possible function. Tohoku J Exp Med. 168:275–278.
1992. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Johnson JE, Giorgione J and Newton AC: The
C1 and C2 domains of protein kinase C are independent membrane
targeting modules, with specificity for phosphatidylserine
conferred by the C1 domain. Biochemistry. 39:11360–11369. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Balendran A, Hare GR, Kieloch A, Williams
MR and Alessi DR: Further evidence that
3-phosphoinositide-dependent protein kinase-1 (PDK1) is required
for the stability and phosphorylation of protein kinase C (PKC)
isoforms. FEBS Lett. 484:217–223. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Lachmann S, Bar S, Rommelaere J and Nuesch
JP: Parvovirus interference with intracellular signalling:
mechanism of PKCeta activation in MVM-infected A9 fibroblasts. Cell
Microbiol. 10:755–769. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Littler DR, Walker JR, She YM, Finerty PJ
Jr, Newman EM and Dhe-Paganon S: Structure of human protein kinase
C eta (PKCeta) C2 domain and identification of phosphorylation
sites. Biochem Biophys Res Commun. 349:1182–1189. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Gould CM and Newton AC: The life and death
of protein kinase C. Curr Drug Targets. 9:614–625. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Chen CC, Wang JK and Chen WC: TPA induces
translocation but not down-regulation of new PKC isoform eta in
macrophages, MDCK cells and astrocytes. FEBS Lett. 412:30–34. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Resnick MS, Luo X, Vinton EG and Sando JJ:
Selective up-regulation of protein kinase C eta in phorbol
ester-sensitive versus-resistant EL4 mouse thymoma cells. Cancer
Res. 57:2209–2215. 1997.PubMed/NCBI
|
|
31
|
Pal D, Outram SP and Basu A: Novel
regulation of protein kinase C-eta. Biochem Biophys Res Commun.
425:836–841. 2012. View Article : Google Scholar
|
|
32
|
Chida K, Murakami A, Tagawa T, Ikuta T and
Kuroki T: Cholesterol sulfate, a second messenger for the eta
isoform of protein kinase C, inhibits promotional phase in mouse
skin carcinogenesis. Cancer Res. 55:4865–4869. 1995.PubMed/NCBI
|
|
33
|
Okano M, Yokoyama T, Miyanaga T and
Ohtsuki K: Activation of C-kinase eta through its
cholesterol-3-sulfate-dependent phosphorylation by casein kinase I
in vitro. Biol Pharm Bull. 27:109–112. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Redig AJ, Sassano A, Majchrzak-Kita B, et
al: Activation of protein kinase C{eta} by type I interferons. J
Biol Chem. 284:10301–10314. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Uddin S, Sassano A, Deb DK, et al: Protein
kinase C-delta (PKC-delta) is activated by type I interferons and
mediates phosphorylation of Stat1 on serine 727. J Biol Chem.
277:14408–14416. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Srivastava KK, Batra S, Sassano A, et al:
Engagement of protein kinase C-theta in interferon signaling in
T-cells. J Biol Chem. 279:29911–29920. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Ivaska J, Bosca L and Parker PJ:
PKCepsilon is a permissive link in integrin-dependent IFN-gamma
signalling that facilitates JAK phosphorylation of STAT1. Nat Cell
Biol. 5:363–369. 2003. View
Article : Google Scholar : PubMed/NCBI
|
|
38
|
Venkatesan BA, Mahimainathan L,
Ghosh-Choudhury N, et al: PI 3 kinase-dependent Akt kinase and
PKCepsilon independently regulate interferon-gamma-induced
STAT1alpha serine phosphorylation to induce monocyte chemotactic
protein-1 expression. Cell Signal. 18:508–518. 2006. View Article : Google Scholar
|
|
39
|
Karp G, Maissel A and Livneh E: Hormonal
regulation of PKC: estrogen up-regulates PKCeta expression in
estrogen-responsive breast cancer cells. Cancer Lett. 246:173–181.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Raveh-Amit H, Maissel A, Poller J, et al:
Translational control of protein kinase Ceta by two upstream open
reading frames. Mol Cell Biol. 29:6140–6148. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Morrish BC and Rumsby MG: The 5′ UTR of
protein kinase C epsilon confers translational regulation in vitro
and in vivo. Biochem Biophys Res Commun. 283:1091–1098. 2001.
|
|
42
|
Feng X, Zhang J, Barak LS, Meyer T, Caron
MG and Hannun YA: Visualization of dynamic trafficking of a protein
kinase C betaII/green fluorescent protein conjugate reveals
differences in G protein-coupled receptor activation and
desensitization. J Biol Chem. 273:10755–10762. 1998. View Article : Google Scholar
|
|
43
|
Crotty T, Cai J, Sakane F, Taketomi A,
Prescott SM and Topham MK: Diacylglycerol kinase delta regulates
protein kinase C and epidermal growth factor receptor signaling.
Proc Natl Acad Sci USA. 103:15485–15490. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Leontieva OV and Black JD: Identification
of two distinct pathways of protein kinase Calpha down-regulation
in intestinal epithelial cells. J Biol Chem. 279:5788–5801. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Newton AC: Protein kinase C: poised to
signal. Am J Physiol Endocrinol Metab. 298:E395–E402. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Pontremoli S, Melloni E, Damiani G, et al:
Effects of a monoclonal anti-calpain antibody on responses of
stimulated human neutrophils. Evidence for a role for
proteolytically modified protein kinase C. J Biol Chem.
263:1915–1919. 1988.PubMed/NCBI
|
|
47
|
Savart M, Letard P, Bultel S and
Ducastaing A: Induction of protein kinase C down-regulation by the
phorbol ester TPA in a calpain/protein kinase C complex. Int J
Cancer. 52:399–403. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Lee HW, Smith L, Pettit GR and Smith JB:
Bryostatin 1 and phorbol ester down-modulate protein kinase C-alpha
and -epsilon via the ubiquitin/proteasome pathway in human
fibroblasts. Mol Pharmacol. 51:439–447. 1997.PubMed/NCBI
|
|
49
|
Lee HW, Smith L, Pettit GR, Vinitsky A and
Smith JB: Ubiquitination of protein kinase C-alpha and degradation
by the proteasome. J Biol Chem. 271:20973–20976. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Lu Z, Liu D, Hornia A, Devonish W, Pagano
M and Foster DA: Activation of protein kinase C triggers its
ubiquitination and degradation. Mol Cell Biol. 18:839–845.
1998.PubMed/NCBI
|
|
51
|
Pal D, Outram SP and Basu A: Upregulation
of PKCeta by PKCepsilon and PDK1 involves two distinct mechanisms
and promotes breast cancer cell survival. Biochim Biophys Acta.
1830:4040–4045. 2013. View Article : Google Scholar
|
|
52
|
Bynagari YS, Nagy B Jr, Tuluc F, et al:
Mechanism of activation and functional role of protein kinase Ceta
in human platelets. J Biol Chem. 284:13413–13421. 2009. View Article : Google Scholar
|
|
53
|
Luparello C, Sirchia R and Longo A: Type V
collagen and protein kinase C eta down-regulation in 8701-BC breast
cancer cells. Mol Carcinog. 52:348–358. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Maissel A, Marom M, Shtutman M, Shahaf G
and Livneh E: PKCeta is localized in the Golgi, ER and nuclear
envelope and translocates to the nuclear envelope upon PMA
activation and serum-starvation: C1b domain and the pseudosubstrate
containing fragment target PKCeta to the Golgi and the nuclear
envelope. Cell Signal. 18:1127–1139. 2006. View Article : Google Scholar
|
|
55
|
Diaz Anel AM and Malhotra V: PKCeta is
required for beta1gamma2/beta3gamma2- and PKD-mediated transport to
the cell surface and the organization of the Golgi apparatus. J
Cell Biol. 169:83–91. 2005.PubMed/NCBI
|
|
56
|
Suzuki M, Iio Y, Saito N and Fujimoto T:
Protein kinase Ceta is targeted to lipid droplets. Histochem Cell
Biol. 139:505–511. 2013. View Article : Google Scholar
|
|
57
|
Mackay HJ and Twelves CJ: Targeting the
protein kinase C family: are we there yet? Nat Rev Cancer.
7:554–562. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Chida K, Hara T, Hirai T, et al:
Disruption of protein kinase Ceta results in impairment of wound
healing and enhancement of tumor formation in mouse skin
carcinogenesis. Cancer Res. 63:2404–2408. 2003.PubMed/NCBI
|
|
59
|
Rotem-Dai N, Oberkovitz G, Abu-Ghanem S
and Livneh E: PKCeta confers protection against apoptosis by
inhibiting the pro-apoptotic JNK activity in MCF-7 cells. Exp Cell
Res. 315:2616–2623. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Hussaini IM, Carpenter JE, Redpath GT,
Sando JJ, Shaffrey ME and Vandenberg SR: Protein kinase C-eta
regulates resistance to UV- and gamma-irradiation-induced apoptosis
in glioblastoma cells by preventing caspase-9 activation. Neuro
Oncol. 4:9–21. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Sonnemann J, Gekeler V, Ahlbrecht K, et
al: Down-regulation of protein kinase Ceta by antisense
oligonucleotides sensitises A549 lung cancer cells to vincristine
and paclitaxel. Cancer Lett. 209:177–185. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Sonnemann J, Gekeler V, Sagrauske A,
Muller C, Hofmann HP and Beck JF: Down-regulation of protein kinase
Ceta potentiates the cytotoxic effects of exogenous tumor necrosis
factor-related apoptosis-inducing ligand in PC-3 prostate cancer
cells. Mol Cancer Ther. 3:773–781. 2004.
|
|
63
|
Abu-Ghanem S, Oberkovitz G, Benharroch D,
Gopas J and Livneh E: PKCeta expression contributes to the
resistance of Hodgkin’s lymphoma cell lines to apoptosis. Cancer
Biol Ther. 6:1375–1380. 2007.PubMed/NCBI
|
|
64
|
Lu HC, Chou FP, Yeh KT, Chang YS, Hsu NC
and Chang JG: Analysing the expression of protein kinase C eta in
human hepatocellular carcinoma. Pathology. 41:626–629. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Brenner W, Farber G, Herget T, Wiesner C,
Hengstler JG and Thuroff JW: Protein kinase C eta is associated
with progression of renal cell carcinoma (RCC). Anticancer Res.
23:4001–4006. 2003.PubMed/NCBI
|
|
66
|
Masso-Welch PA, Verstovsek G, Darcy K,
Tagliarino C and Ip MM: Protein kinase C eta upregulation and
secretion during postnatal rat mammary gland differentiation. Eur J
Cell Biol. 77:48–59. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Masso-Welch PA, Winston JS, Edge S, et al:
Altered expression and localization of PKC eta in human breast
tumors. Breast Cancer Res Treat. 68:211–223. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Suzuki T, Elias BC, Seth A, et al: PKC eta
regulates occludin phosphorylation and epithelial tight junction
integrity. Proc Natl Acad Sci USA. 106:61–66. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Martin TA, Mason MD and Jiang WG: Tight
junctions in cancer metastasis. Front Biosci (Landmark Ed).
16:898–936. 2011. View
Article : Google Scholar : PubMed/NCBI
|
|
70
|
Lincoln DW II and Bove K: The
transcription factor Ets-1 in breast cancer. Front Biosci.
10:506–511. 2005. View
Article : Google Scholar : PubMed/NCBI
|
|
71
|
Shen Q, Uray IP, Li Y, et al: The AP-1
transcription factor regulates breast cancer cell growth via
cyclins and E2F factors. Oncogene. 27:366–377. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
72
|
Beck J, Bohnet B, Brugger D, et al:
Multiple gene expression analysis reveals distinct differences
between G2 and G3 stage breast cancers, and correlations of PKC eta
with MDR1, MRP and LRP gene expression. Br J Cancer. 77:87–91.
1998. View Article : Google Scholar : PubMed/NCBI
|
|
73
|
Tamarkin A, Zurgil U, Braiman A, et al:
DNA damage targets PKCeta to the nuclear membrane via its C1b
domain. Exp Cell Res. 317:1465–1475. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
74
|
Cabodi S, Calautti E, Talora C, Kuroki T,
Stein PL and Dotto GP: A PKC-eta/Fyn-dependent pathway leading to
keratinocyte growth arrest and differentiation. Mol Cell.
6:1121–1129. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
75
|
Livneh E, Shimon T, Bechor E, Doki Y,
Schieren I and Weinstein IB: Linking protein kinase C to the cell
cycle: ectopic expression of PKC eta in NIH3T3 cells alters the
expression of cyclins and Cdk inhibitors and induces adipogenesis.
Oncogene. 12:1545–1555. 1996.PubMed/NCBI
|
|
76
|
Uht RM, Amos S, Martin PM, Riggan AE and
Hussaini IM: The protein kinase C-eta isoform induces proliferation
in glioblastoma cell lines through an ERK/Elk-1 pathway. Oncogene.
26:2885–2893. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
77
|
Shahaf G, Rotem-Dai N, Koifman G,
Raveh-Amit H, Frost SA and Livneh E: PKCeta is a negative regulator
of AKT inhibiting the IGF-I induced proliferation. Exp Cell Res.
318:789–799. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
78
|
Aeder SE, Martin PM, Soh JW and Hussaini
IM: PKC-eta mediates glioblastoma cell proliferation through the
Akt and mTOR signaling pathways. Oncogene. 23:9062–9069. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
79
|
Koivunen J, Aaltonen V and Peltonen J:
Protein kinase C (PKC) family in cancer progression. Cancer Lett.
235:1–10. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
80
|
Urtreger AJ, Kazanietz MG and Bal de Kier
Joffe ED: Contribution of individual PKC isoforms to breast cancer
progression. IUBMB Life. 64:18–26. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
81
|
Basu A and Pal D: Two faces of protein
kinase Cdelta: the contrasting roles of PKCdelta in cell survival
and cell death. Sci World J. 10:2272–2284. 2010. View Article : Google Scholar : PubMed/NCBI
|