Introduction
Meisoindigo, a second-generation derivative of
indirubin, has been a routine therapeutic agent in the clinical
treatment of CML in China since 1980s (1,2). In
the phase III clinical trial of meisoindigo involving 402 patients,
it was shown that meisoindigo was equally efficient for both newly
diagnosed and previously treated CML patients after oral
administration. The hematological complete response (CR) and
partial response (PR) rates, respectively, were 45.0 and 39.3% for
newly diagnosed patients and 35.9 and 41.4% for previously treated
patients (2). Meisoindigo was
generally well tolerated. The most frequent side-effects were bone,
joint and/or muscle pain of varying degrees when the dosage was
more than the suitable one (1).
The molecular mechanism of action of meisoindigo is
still not well understood. It appears that growth inhibition and
apoptosis of the treated cancer cells might be the major mechanism
of action of meisoindigo (2).
Previous studies have indicated that meisoindigo strongly inhibits
DNA biosynthesis in cancer cells and inhibits the assembly of
microtubules (3,4). Experimental results on the mouse
leukemia L1210 cell cycle showed that meisoindigo induced
accumulation of S phase cells. The movement of cells in G2+M phase
to G1 phase may also be blocked to some extent (3). The induction of cancer cell
differentiation associated with decreased c-myb oncogene
expression might also account for the anticancer action and low
toxicity of meisoindigo (4).
Another study indicated that the anti-angiogenesis effect of
meisoindigo may contribute to the antileukemic effect of this drug
(5).
As meisoindigo is clinically effective in the
management of CML with high efficacy and low toxicity, it could be
effective in the treatment of other types of myeloid leukemia
including acute promyelocytic leukemia (APL), acute myeloid
leukemia (AML) and myelomonocytic leukemia. However, the
information on the effects of meisoindigo in these myeloid
leukemias is still limited. One previous study reported that
meisoindigo showed promising in vitro and in vivo
activity against AML (6). In the
present study, we tested the antileukemic effects of meisoindigo in
the human NB4 (APL cells, FAB-M3), NB4.007/6 (retinoic
acid-resistant cells derived from NB4), HL60 (AML cells, FAB-M2),
and U937 (myelomonocytic leukemia cells, FAB-M5). Among them, NB4
and HL60 are retinoic acid-sensitive cells (7,8),
whereas NB4.007/6 and U937 are retinoic acid-resistant cells
(9,10).
To improve the understanding of its efficacy and
safety characteristics, detailed investigation of meisoindigo
metabolism is warranted. In our previous studies, the in
vitro metabolic profiles of meisoindigo in rat, pig and human
liver microsomes were explored from both the qualitative and
quantitative aspects (11,12). The in vivo metabolic
profiles of meisoindigo in rat plasma, urine and feces were
examined from the qualitative aspect (13). The major circulatory metabolites of
meisoindigo in rat plasma were identified as 3,3′ double bond
reduction products by LC-MS/MS. However, to our knowledge,
quantitative information relevant to the in vivo metabolic
profile of meisoindigo is lacking. Another objective of this study
was to elucidate the pharmacokinetic properties of meisoindigo and
its major circulatory metabolites in rats following oral
administration. The latter experiments served as an in vivo
model to illustrate the metabolism and disposition of meisoindigo
and its metabolites after oral administration.
Materials and methods
Chemicals and reagents
Cell culture medium and reagents, propidium iodide
(PI), DMSO (molecular biology grade), sodium carboxymethyl
cellulose (CMC-Na) and formic acid were purchased from Sigma
Chemical Co. (St. Louis, MO, USA). Fetal bovine serum (FBS) was
obtained from HyClone (Logan, UT, USA). RNase was bought from Roche
(Basel, Switzerland). Meisoindigo was provided by the Institute of
Materia Medica, Chinese Academy of Medical Sciences and Peking
Union Medical College (Beijing, China). Indirubin (internal
standard) was purchased from the National Institute for the Control
of Pharmaceutical and Biological Products (Beijing, China). HPLC
grade ethyl acetate, methanol and acetonitrile were purchased from
Fisher Scientific Co. (Fair Lawn, NY, USA). Milli-Q water was
obtained from a Millipore water purification system (Billerica, MA,
USA) and used to prepare buffer solutions and other aqueous
solutions.
Cell cultures
NB4 (APL cells, M3 subtype according to FAB)
(7), NB4.007/6 (retinoic acid
resistant cells developed through continuously culturing NB4 in the
medium containing retinoic acid (9), HL60 (AML cells, M2 subtype according
to FAB) (14), and U937
(myelomonocytic leukemia cells, M5 subtype according to FAB)
(10,15), were maintained in RPMI-1640 medium
supplemented with 10% heat-inactive fetal bovine serum, 100 U/ml
penicillin, and 100 μg/ml streptomycin in a humidified 5%
CO2 atmosphere at 37°C. The cells were subjected to
sub-culture by 1:2 dilution with fresh medium every 3 days.
Cultured cells with a passage number of 10–20 were used in the
experiments to reduce variability due to cell culture
conditions.
Inhibitory effects of meisoindigo on the
four human leukemic cells
The inhibitory effects of meisoindigo on the four
human leukemic cells were tested by treating the cells with
meisoindigo at various concentrations (0, 1, 2, 4, 8, 12, 16, 20
μM). The cells were cultured in 24-well plates. The number of the
cells was counted by trypan blue exclusion method. The initial cell
numbers (NB4, NB4.007/6, HL60 and U937) were 3×106
cell/ml. After incubation for 24, 48 and 72 h, the plates were
taken out and the number of viable cells was counted. All
experiments were carried out in eight replicates. The cell activity
was defined as the number of viable cells in the test culture over
the mean number of viable cells in the control. The 50% inhibitory
concentration (IC50) on cell activity was estimated
based on the cell number obtained after incubation for 72 h. The
data were fitted to the Inhibitory Effect Sigmoid Emax
Model with the software, WinNonlin version 1.0 (Lexington, KY,
USA). All regressions were carried out with equal weighting.
Flow cytometry analysis for apoptosis and
cell cycle distribution
Apoptosis and cell cycle distribution of the tested
leukemic cells were examined by flow cytometry analysis. The
leukemic cells were treated with meisoindigo of various
concentrations (0, 4, 8, 12, 16 and 20 μM) for 24, 48 and 72 h in
the respective 6-well plates. At the end of incubation, the cells
were harvested, washed twice with 3 ml PBS (pH 7.4 contain 1% FBS),
and then fixed with 70% ice-cold ethanol and kept at −20°C for at
least 24 h. On the day before flow cytometry assay, the fixed
leukemic cells were spun down, washed with 3 ml PBS with 1% FBS and
spun down again. The cells were then dyed in 500 μl PBS containing
100 μg/ml propidium iodide (PI) and 100 μg/ml RNase and stored at
4°C overnight. On the following day, the samples were first
filtered through a 30 μm pore size nylon mesh prior to the flow
cytometry analysis. The samples were analyzed on a Coulter Epics
Elite ESP flow cytometer (Beckman Coulter, FL, USA) equipped with a
15 mW argon-ion laser source of 488 nm. Red fluorescence of
propidium iodide (PI) was collected with a 610 nm bandpass filter.
Typical flow rates were about 200 to 300 particles per sec. A total
of 10,000 cells were analyzed per sample. The experiment was
carried in triplicate. The data obtained were analyzed using WinMDI
2.8 software (La Jolla, CA, USA). From the DNA histogram, the
percentages of cells in different cell cycle phases were
determined. Cells with DNA concentration less than the G1 phase
were regarded as apoptotic cells.
Statistics
Statistical analysis was performed by using SPSS
10.0 (Chicago, IL, USA) or GraphPad Prism 2.00 (La Jolla, CA, USA).
All experimental data were analyzed with One-Sample
Kolmogorov-Smirnov Test for their distribution. As the parameters
were found to be normally distributed, data were expressed as mean
± standard derivation (SD). EC50 on growth inhibition
was compared at their 95% confidential level. The percentages of
apoptotic cells at different meisoindigo concentrations were
compared with the one-way ANOVA and the posthoc Tukey’s test. A
value of p<0.05 was adopted to indicate statistical
significance.
Synthesis and purification of meisoindigo
reductive metabolites
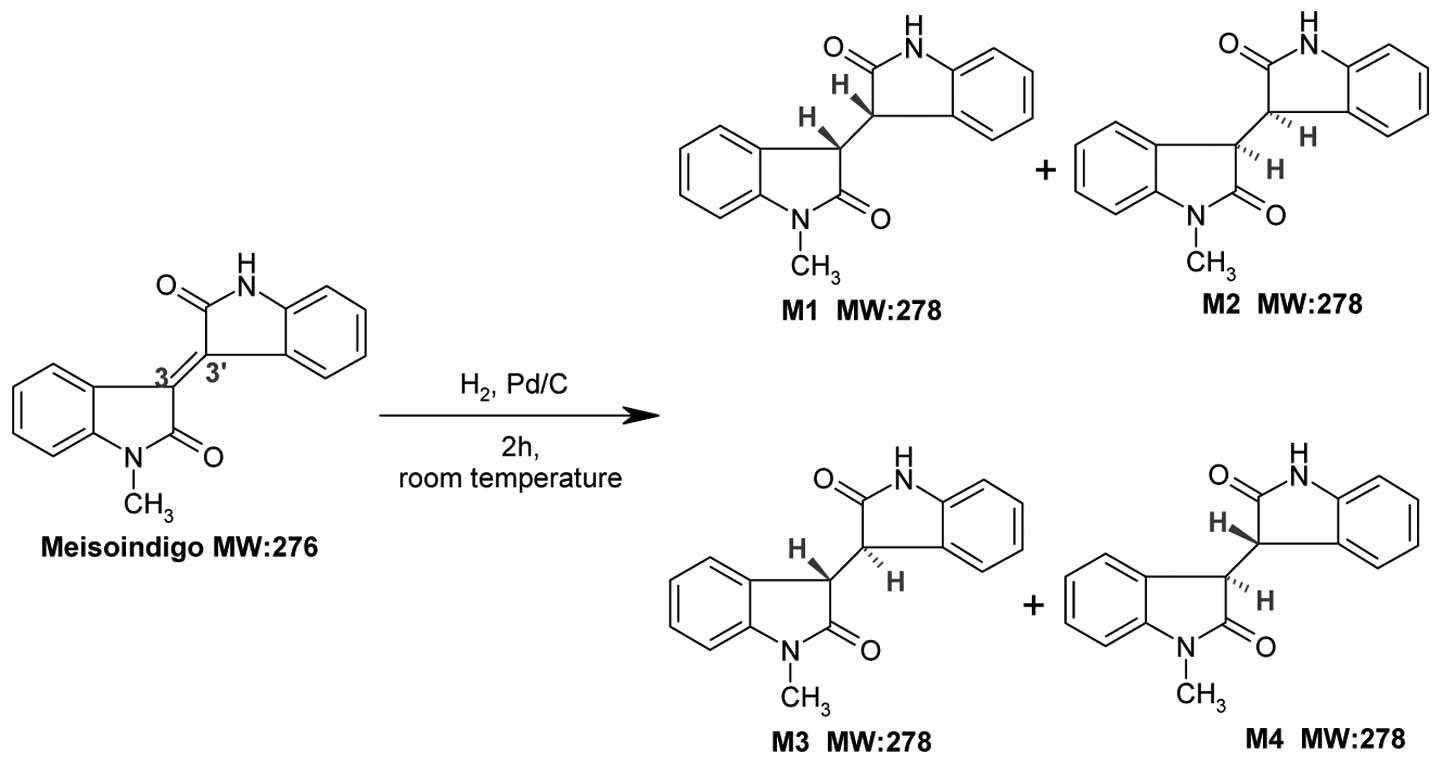
Synthetic routes of reductive metabolite standards
(M1+M2 and M3+M4) are shown in Fig.
1 as previously described (11). The starting material of meisoindigo
(50 mg) was dissolved in methanol (100 ml). After adding 10%
palladium on carbon (10 mg), the mixture was placed inside the
hydrogenator apparatus and kept shaking at 40 psi for 2 h at room
temperature. The reaction mixture was filtered through filter paper
and the filter pad was washed with distilled water. The filtrate
was evaporated under reduced pressure and the precipitate was
purified by Agilent 1100 preparative HPLC system (Palo Alto, CA,
USA) on a Peeke Scientific Combi-A C18 column (50 × 20 mm, 5 μm)
(Redwood City, CA, USA) with a guard cartridge at ambient
temperature. The isocratic mobile phase consisted of 50% water
(solvent A) and 50% methanol (solvent B) and was delivered at a
flow rate of 4 ml/min within 20 min. The UV absorbance was
monitored at 254 nm. The combined metabolites fractions (total
M1+M2 and M3+M4) were evaporated under reduced pressure and stored
at −20°C.
LC-MS/MS conditions for simultaneous
quantification of meisoindigo and its reductive metabolites
The LC-MS/MS system consisted of an Agilent 1200
HPLC (Palo Alto, CA, USA) with a Q TRAP™ 3200 hybrid triple
quadrupole linear ion trap mass spectrometer from Applied
Biosystems/MDS Sciex (Concord, Ontario, Canada). Chromatographic
separations for rat plasma samples were performed on a Phenomenex
Luna C18 column (150 mm × 2.00 mm i.d., 5 μm) (Torrance, CA, USA)
with a guard cartridge. The injection volume and column temperature
were 5 μl and 25°C, respectively. The mobile phases consisted of
0.1% formic acid in water (solvent A) and acetonitrile (solvent B).
The gradient elution was set at a flow rate of 0.3 ml/min. The
optimized linear gradient elution conditions were: 30 to 95% B over
13 min, followed by an isocratic hold at 95% B for another 2 min.
At 15 min, B was returned to 30% in 1 min and the column was
equilibrated for 14 min before the next injection. The total run
time was 30 min. During LC-MS/MS analysis, up to 4 min of the
initial flow was diverted away from the mass spectrometer before
the data acquisition. The mass spectrometer was operated in the
positive ion mode with a TurboIonSpray source. Enhanced product ion
(EPI) scans were used to investigate the fragmentation patterns of
authentic standard compounds. A list of three Multiple Reaction
Monitoring (MRM) transitions in Table
I was selected for the quantification of meisoindigo, its
reductive metabolites and indirubin (internal standard). The Q1,
Q3, declustering potential (DP), and collision energy (CE) values
in Table I were based on the MS/MS
results of those respective standards. The other ionization
parameters were as follows: curtain gas (CUR), 20 (arbitrary
units); ion source gas 1 (GS1), 40 (arbitrary units); ion source
gas 2 (GS2), 50 (arbitrary units); source temperature (TEM), 550°C;
entrance potential (EP), 10 V. The dwell time of each MRM
transition was 150 msec. The HPLC system and the mass spectrometer
were controlled by Analyst™ 1.4.2 software from Applied
Biosystems/MDS Sciex.
 | Table IMRM transition parameters for
meisoindigo, its reductive metabolites and indirubin (internal
standard) in rat plasma. |
Table I
MRM transition parameters for
meisoindigo, its reductive metabolites and indirubin (internal
standard) in rat plasma.
| Q1 (amu) | Q3 (amu) | DP (V) | CE (eV) |
|---|
| Meisoindigo | 277.1 | 234.2 | 66 | 45 |
| Reductive
metabolites | 279.1 | 147.1 | 61 | 30 |
| Indirubin (internal
standard) | 263.1 | 190.1 | 80 | 50 |
Preparation of standards and quality
control samples
Stock solutions of meisoindigo and its reductive
metabolites (M1+M2 and M3+M4) were prepared by dissolving the
accurately weighed compounds in methanol to give a final
concentration of 1 mg/ml for both. Solution of indirubin (internal
standard) was prepared in methanol at the concentration 1 mg/ml and
diluted to 1 μg/ml with methanol. Blank rat plasma (drug free) was
obtained by centrifugation of predose rat blood. Calibration curves
were prepared by spiking appropriate standard solutions of the
parent drug meisoindigo and its reductive metabolites,
respectively, to the blank plasma. Concentrations in plasma samples
were 1, 2.5, 5, 10, 50, 250 and 500 ng/ml for meisoindigo and 2.5,
5, 50, 100, 250, 500 and 1,000 ng/ml for its reductive metabolites.
Quality control (QC) samples were separately prepared in blank
plasma samples at the concentration of 5, 50 and 500 ng/ml for
meisoindigo and its reductive metabolites, respectively.
Rat plasma sample preparation
Each rat plasma sample (45 μl) was added with 5 μl
of internal standard indirubin (100 ng/ml). The samples were
briefly mixed before extraction with 2 × 2-fold volume of cold
ethyl acetate saturated with H2O. The organic phases
from the extracted plasma were combined and dried at 35°C under a
gentle stream of nitrogen. The residue was reconstituted with 50 μl
acetonitrile and H2O (1:1) for LC-MS/MS analysis.
LC-MS/MS method validation
Selectivity
Blank rat plasma were analyzed for interference
using the proposed sample preparation procedure and LC-MS/MS
conditions, compared to blank plasma spiking with meisoindigo and
its reductive metabolites.
Calibration curve
Through the internal standard method, seven non-zero
samples with meisoindigo and its reductive metabolites were used in
establishing two standard calibration curves that covered the
expected range. Calibration curves were generated using linear
least square regression. The lower limit of quantification (LLOQ)
of the assay was also determined.
Accuracy and precision
Intra-day accuracy and precision were determined by
the analysis at the concentrations of 1, 2.5, 5, 10, 50, 250 and
500 ng/ml for meisoindigo and 2.5, 5, 50, 100, 250, 500 and 1,000
ng/ml for its reductive metabolites. Three replicates of each
concentration were analyzed within the same day for this purpose.
Inter-day accuracy and precision were evaluated by the analysis
carried out on three consecutive days. Accuracy was calculated as
the percentage ratio of the mean of the measured concentration to
the spiked concentration. Precision was expressed by the relative
standard deviation.
Extraction recovery
The extraction recoveries of meisoindigo and its
reductive metabolites at three quality control levels (5, 50 and
500 ng/ml) were evaluated in triplicate by comparing peak area
ratios of meisoindigo and its reductive metabolites obtained from
plasma samples with those obtained from the standard solutions at
the same concentration.
Stability
For short-term stability, three aliquots of each of
the low (5 ng/ml) and high (500 ng/ml) concentrations samples were
prepared from the stock solutions and stored at −80°C. They were
thawed at room temperature and kept at this temperature for 4 h on
the same day before analyzing. For long-term stability, three
aliquots of each of the 5 and 500 ng/ml samples were stored at
−80°C for 4 weeks before being thawed and analyzed. For freeze and
thaw stability, three aliquots of 5 and 500 ng/ml samples underwent
three freeze and thaw cycles for three consecutive days before
being analyzed. For stock solution stability, three aliquots of the
5 and 500 ng/ml stock solutions were thawed and kept at room
temperature for 6 h before evaluating. For post-preparative
stability, three aliquots of 5 and 500 ng/ml samples were kept in
the LC-MS/MS auto-sampler chamber for 8 h before being analyzed.
Stability was expressed as the percentage ratio of the mean of the
measured concentration to the spiked concentration.
Pharmacokinetic studies of meisoindigo
and its reductive metabolites
The pharmacokinetic profiles of meisoindigo and its
reductive metabolites after oral administration were studied in
rats. The animal experimental protocols were reviewed and approved
by the Institutional Animal Care and Use Committee of the National
University of Singapore (NUS). For the study, four healthy male
Sprague-Dawley (SD) rats (7–8 weeks of age, average 250 g) were
purchased from Animal Holding Unit, National University of
Singapore. They were provided with a standard diet and water ad
libitum. The room was kept on a 12/12-h light/dark cycle at a
temperature of 23±1°C and relative humidity of 50±10%. At least one
week of acclimatization period was allowed for the rats prior to
drug administration. The rats were fasted 12 h prior to
administration of the dose and were fed 12 h after the dose. The
dose was formulated in a mixture of meisoindigo suspended in 1%
sodium carboxymethyl cellulose (CMC-Na) solution at a target
concentration of 3.75 mg/ml. The rats received meisoindigo solution
by gavage administration with a single dosage of 10 mg/kg body
weight. Blood samples were collected in heparinized tubes via
caudal vein predose and at 1, 2, 3, 4, 5, 6, 8, 10 and 24 h
postdose. Blood samples were immediately centrifuged at 6,000 rpm
for 10 min to obtain the plasma. Plasma samples were transferred to
clean tubes and underwent the sample preparation procedures as
described above before LC-MS/MS analysis. Pharmacokinetic
parameters were calculated by non-compartmental analysis using the
software of WinNonlin standard version 1.0 from Scientific
Consulting Inc. (Apex, NC, USA).
Results
Growth inhibition of meisoindigo on the
four human leukemic cells
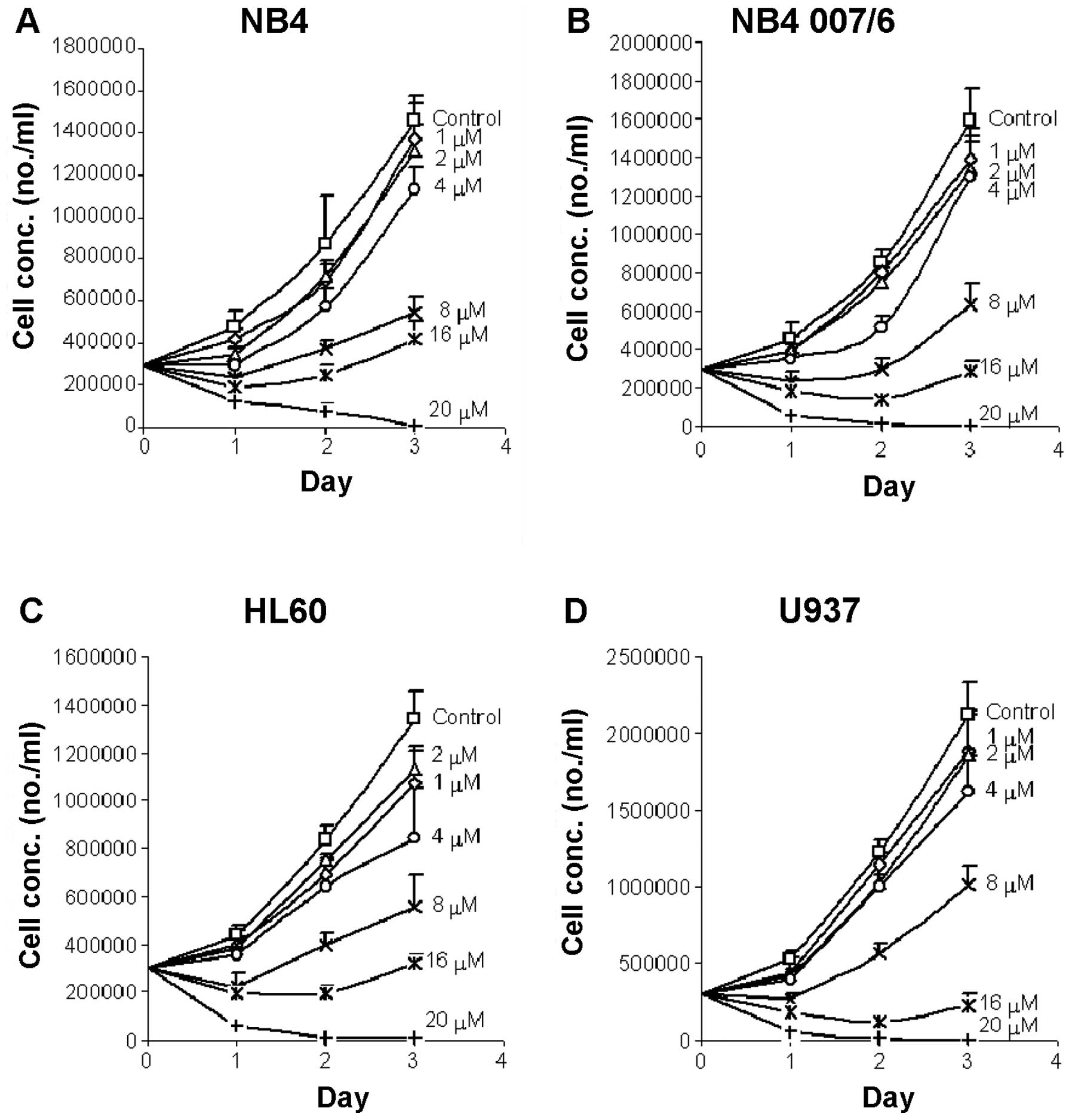
As shown in Fig. 2,
the leukemic cells grew well in the absence of meisoindigo with a
relatively rapid proliferation rate, suggesting appropriate cell
culture conditions were applied. The NB4, NB4.007/6 and HL60 cells
proliferated 4–5 times during a 3-day period, while U937
proliferated about 7 times during the same period. There was always
a delay in growth on the first day, before the growth was
accelerated. In our preliminary study, the growth and/or
proliferation of the test cells were observed to decline on the 4th
day after culture, if no fresh medium was added. In order to avoid
this fluctuation in growth due to change of media, the inhibitory
effects of meisoindigo was studied over three days.
Meisoindigo effectively inhibited the growth and
proliferation of the retinoic acid sensitive cells (NB4 and HL60),
as well as the retinoic acid resistant cells (NB4.007/6 and U937).
The inhibitory effects of meisoindigo on the growth and
proliferation of various leukemic cells exhibited a dose-dependent
characteristic. The 50% inhibitory concentrations (IC50)
were estimated based on the values of growth obtained after 72 h
incubation and the data fitted to an Inhibitory Effect Sigmoid
Emax Model. The correlation of regression of the model
fittings was 0.986, indicating an appropriate pharmacodynamic model
was applied. The IC50 values were 7.9, 7.1, 7.1 and 7.5
μM in NB4, NB4.007/6, HL60 and U937 cells, respectively. No
statistically significant difference among these IC50
values was observed. After exposure to meisoindigo at 8 μM or above
for 72 h, all leukemic cells were clearly inhibited and there was a
greater than 50% decrease in the number of viable cells in the
meisoindigo treated group over the control (Fig. 2). When treated with 20 μM
meisoindigo for 48 to 72 h, most of the cells did not survive. The
antileukemic effects of meisoindigo seemed to be time-dependent.
Prolonged incubation with high concentrations of meisoindgo (12, 16
or 20 μM) led to enhanced inhibitory effects. The calculation of
IC50 with counts on day 1, 2 or 3 resulted in very
similar finding in the four leukemic cells. Interestingly, the
finding that meisoindigo was effective in inhibiting the growth and
proliferation of retinoic acid resistant NB4.007/6 and U937 cells,
suggests a less possibility of cross-resistance with retinoic acid.
Thus meisoindigo could be a potential treatment for leukemia,
either sensitive or resistant to retinoic acid.
Apoptosis induction of meisoindigo in
four human leukemic cell types
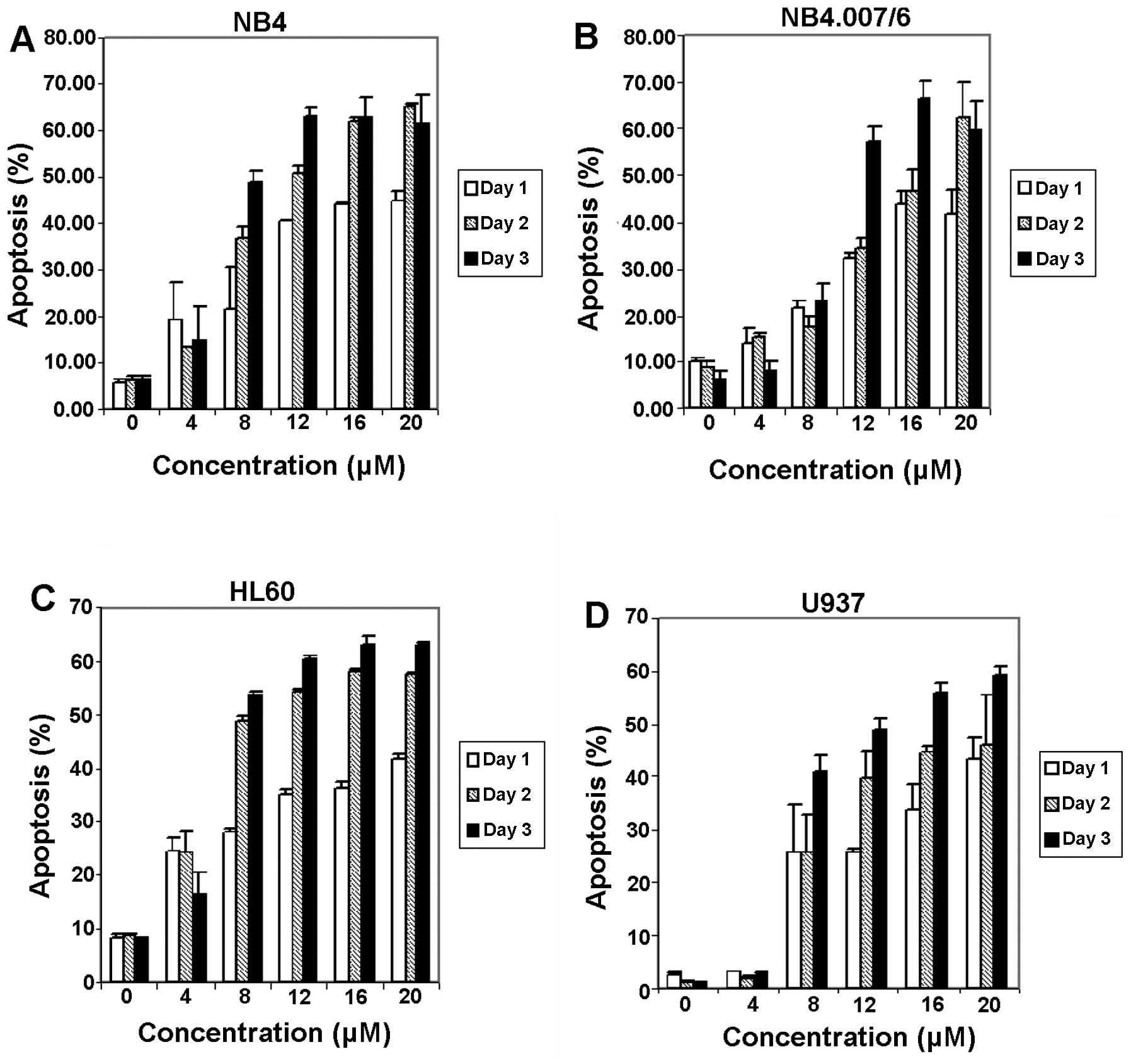
The flow cytometry analysis showed that NB4,
NB4.007/6, HL60 and U937 cells grew well in the absence of
meisoindigo with about 20–30% of the cells distributed in the
respective M/G2 phase and S phase (Table II), suggesting that these cells
have active DNA synthesis and cell division. This phenomenon was in
good accord with the rapid growth and proliferation of these cells.
After treatment with meisoindigo, the percentage of sub-G1 cells
increased substantially over time in all the four cell types
(Table II). The percentage of
sub-G1 cells, which is regarded as the percentage of apoptosis
shown in Fig. 3, indicated that
the induction of apoptosis was both time- and dose-dependent. The
percentages of sub-G1 cells in all the four cell types were quite
similar at high concentrations of meisoindigo (12, 16, 20 μM),
suggesting that the apoptosis-inductive effect of meisoindigo was
saturable at high concentrations. Also, the percentage of G1/G0, S
and M/G2 dropped substantially as the dosages increased (Table II), which suggested that no phase
arrest or cell phase accumulation occurred in these
meisoindigo-treated cells, and thus the antileukemic effects of
meisoindigo in the cells might be independent of cell cycle arrest.
However, the apoptotic effect is not associated with DNA
fragmentation (data not shown).
 | Table IIPercentages in phase distribution of
the leukemic cells after incubation with meisoindigo for 48 h. |
Table II
Percentages in phase distribution of
the leukemic cells after incubation with meisoindigo for 48 h.
| Conc. | Sub-G1 | G1/G0 | S | M/G2 |
|---|
| NB4 cells |
| 0 | 6.2±0.9a | 46.6±0.7 | 29.8±0.5 | 17.8±1.0 |
| 8 | 36.8±2.2 | 34.3±1.4 | 19.8±0.1 | 9.4±0.7 |
| 12 | 50.8±1.8 | 30.1±0.7 | 12.7±0.5 | 6.4±0.6 |
| NB4.007/6
cells |
| 0 | 8.9±1.0 | 38.2±1.0 | 29.8±0.5 | 23.4±1.5 |
| 8 | 17.6±2.4 | 36.6±2.4 | 27.9±0.1 | 18.2±1.9 |
| 12 | 34.3±2.2 | 29.1±2.2 | 24.3±2.0 | 12.9±1.6 |
| HL60 cells |
| 0 | 8.5±0.6 | 45.4±2.1 | 25.3±0.6 | 21.1±1.5 |
| 8 | 48.8±0.8 | 29.0±0.6 | 15.0±0.4 | 7.7±0.4 |
| 12 | 54.6±0.2 | 28.9±0.3 | 11.2±0.3 | 5.8±0.3 |
| U937 cells |
| 0 | 1.1±0.3 | 48.1±0.5 | 24.5±1.3 | 26.6±1.0 |
| 8 | 25.7±7.2 | 37.5±3.0 | 19.0±2.0 | 18.1±2.5 |
| 12 | 39.9±5.1 | 34.9±1.9 | 15.0±1.0 | 10.6±2.1 |
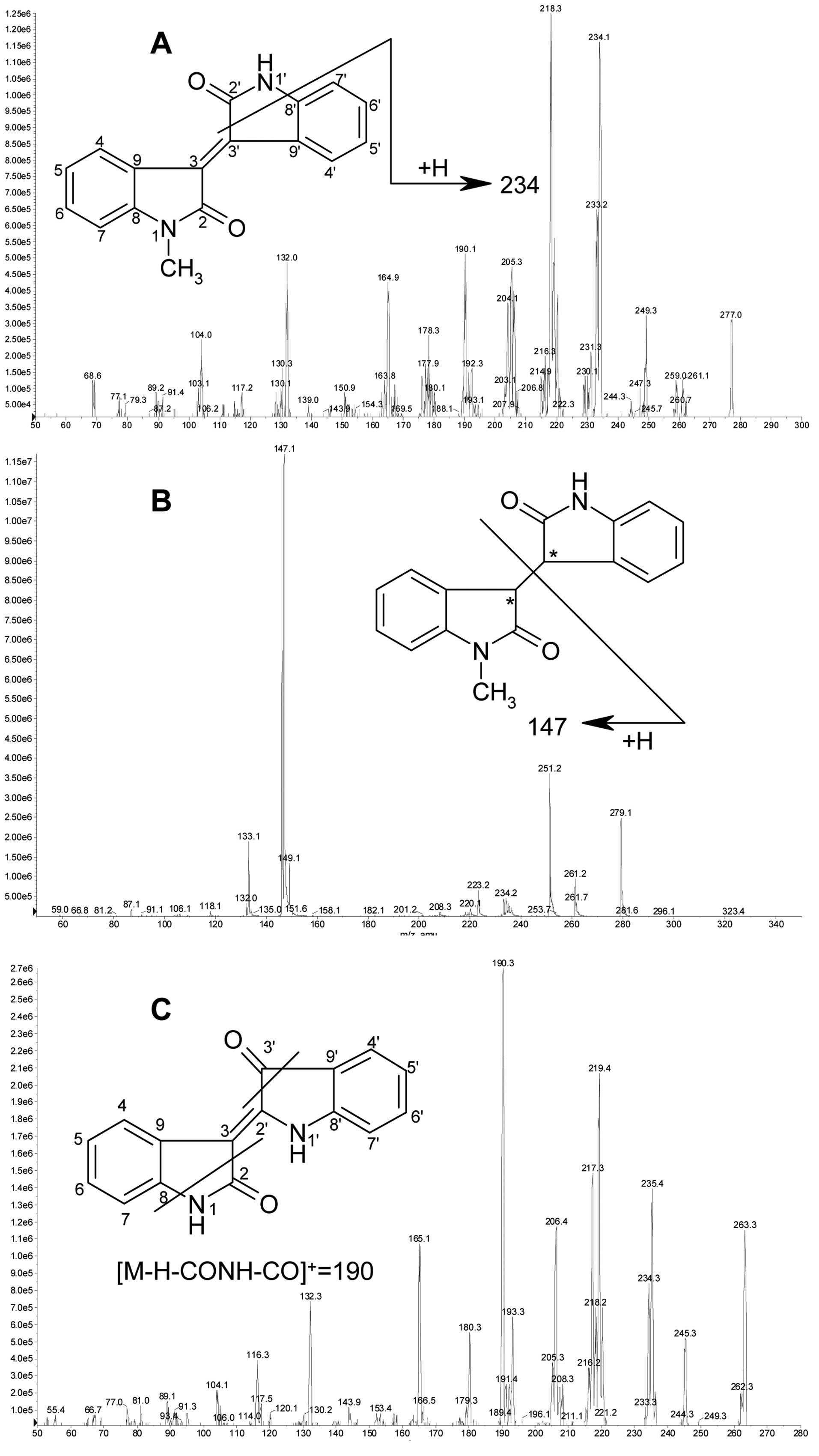
LC-MS/MS method development
The LC conditions were optimized by varying the
mobile phase, gradient elution, flow rate and columns. The MS
conditions were optimized by tuning with authentic standards of
meisoindigo, its reductive metabolites and indirubin. The proposed
fragmentation scheme and MS/MS spectrum of protonated meisoindigo
(m/z 277) are shown in Fig.
4A. Loss of CONH (43 Da) following gain of one H generated a
major product ion at m/z 234. Loss of CONCH3 (57
Da) following loss of one H generated the most abundant product ion
at m/z 218. The product ion at m/z 234 was selected
to form the MRM transition for quantification due to its better
selectivity without compromising the sensitivity. As is shown in
Fig. 1, the reductive metabolites
of meisoindigo M1+M2 were a pair of (3-R, 3′-R) and (3-S, 3′-S)
reduced-meisoindigo enantiomers with two hydrogens located at the
same side of 3,3′-single bond, whereas M3+M4 were another pair of
(3-R, 3′-S) and (3-S, 3′-R) enantiomers with two hydrogens located
at the opposite sides. The MS/MS spectra of M1+M2 and M3+M4 were
identical and are shown in Fig.
4B. The proposed fragmentation scheme of protonated reductive
metabolites (m/z 279) showed that cleavage of the 3,3′ bond
generated the dominant product ion at m/z 147, which was
thus selected to form the MRM transition for quantification. The
proposed fragmentation scheme and MS/MS spectrum of protonated
indirubin (m/z 263) are shown in Fig. 4C. Loss of CONH (43 Da) and CO (28
Da) from indirubin following loss of one H generated the most
abundant product ion at m/z 190. Thus the product ion at
m/z 190 was selected to form the MRM transition for
quantification.
LC-MS/MS method validation
Full validation was conducted for the
pharmacokinetic study of meisoindigo and its reductive metabolites
in rat plasma because this bioanalytical method was developed for
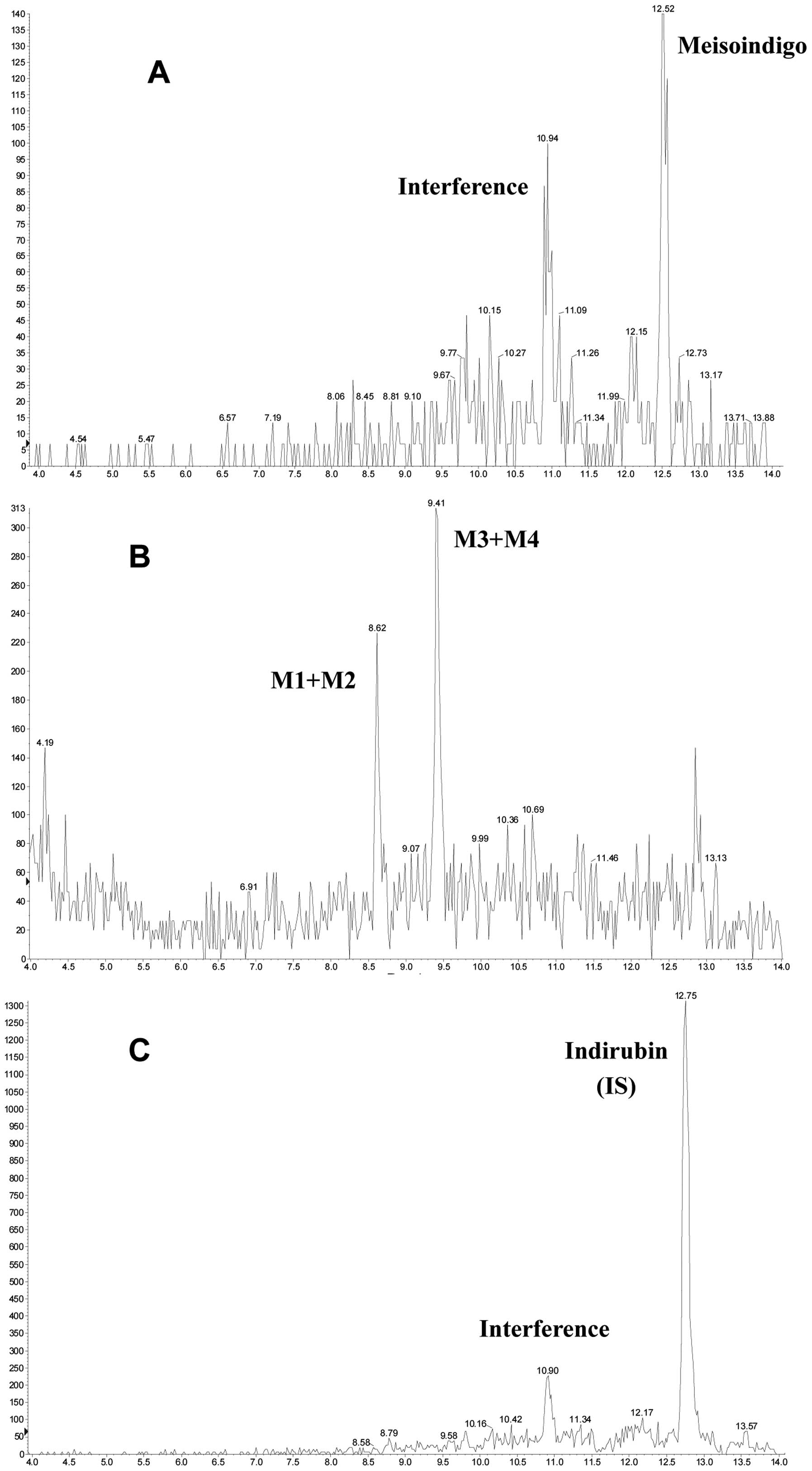
the first time. The assay selectivity was confirmed as no
significant interference was observed at the retention time of
meisoindigo in the blank rat plasma. The matrix effect on the
analyte determination was not taken into account due to the
relative slow chromatographic separation. The linear range of
calibration curve was 1–500 ng/ml with correlation coefficients
greater than 0.9998. LLOQ was 1 ng/ml for meisoindigo (Fig. 5A). The linear range of calibration
curve was 2.5–1,000 ng/ml with correlation coefficients greater
than 0.9928. LLOQ was 0.5 ng/ml for the reductive metabolites of
meisoindigo (Fig. 5B).
Table III shows
both intra- and inter-day accuracy and precision data determined by
the analysis of meisoindigo and its reductive metabolites. These
results fulfilled the criteria of validation with accuracy and
precision not more than ±20%, indicating that this assay is
consistent and reliable with good accuracy and precision. The
extraction recoveries of low, medium, high concentrations were
within ±20% and reproducible. The results of short-term, long-term,
freeze and thaw, stock solution and post-preparative stability
evaluated at a low concentration of 5 ng/ml and a high
concentration of 500 ng/ml of meisoindigo and its reductive
metabolites in rat plasma were summarized in Table IV.
 | Table IIIIntra- and inter-day accuracy and
precision (n=3) for meisoindigo and its reductive metabolites in
male rat plasma. |
Table III
Intra- and inter-day accuracy and
precision (n=3) for meisoindigo and its reductive metabolites in
male rat plasma.
| Meisoindigo |
|---|
| Spiked
concentration (ng/ml) | 1 | 2.5 | 5 | 10 | 50 | 250 | 500 |
|---|
| Intra-day accuracy
(%) | 113.8 | 92.3 | 101.5 | 103.2 | 104.1 | 97.9 | 97.0 |
| Intra-day RSD
(%) | 4.1 | 10.6 | 1.7 | 18.0 | 11.7 | 11.6 | 2.3 |
| Inter-day accuracy
(%) | 97.1 | 95.4 | 106.3 | 88.9 | 95.6 | 100.2 | 102.4 |
| Inter-day RSD
(%) | 15.6 | 17.3 | 2.9 | 18.8 | 11.3 | 10.4 | 7.3 |
|
| Reductive
metabolites |
| Spiked
concentration (ng/ml) | 2.5 | 5 | 50 | 100 | 250 | 500 | 1,000 |
|
| Intra-day accuracy
(%) | 97.9 | 110.6 | 85.2 | 100.5 | 97.3 | 107.3 | 102.5 |
| Intra-day RSD
(%) | 5.4 | 10.8 | 3.3 | 1.9 | 6.6 | 6.9 | 5.3 |
| Inter-day accuracy
(%) | 107.6 | 110.6 | 86.0 | 95.5 | 101.8 | 87.0 | 106.6 |
| Inter-day RSD
(%) | 5.0 | 10.5 | 8.5 | 4.9 | 9.3 | 14.9 | 8.8 |
 | Table IVStability of meisoindigo and its
reductive metabolites in male rat plasma under various
conditions. |
Table IV
Stability of meisoindigo and its
reductive metabolites in male rat plasma under various
conditions.
| Percentage of
initial value (mean ± SD%, n=3) |
|---|
| Meisoindigo | Reductive
metabolites |
|---|
|
|
|
|---|
| Type of
stability | 5 ng/ml | 500 ng/ml | 5 ng/ml | 500 ng/ml |
|---|
| Short-term | 80.5±10.7 | 89.1±6.9 | 101.2±13.7 | 80.1±6.9 |
| Long-term | 75.4±13.9 | 67.4±1.9 | 39.6±6.4 | 64.8±11.1 |
| Freeze and
thaw | 59.2±7.6 | 45.8±7.0 | 94.4±4.7 | 115.7±22.3 |
| Stock solution | 97.2±19.0 | 88.2±1.8 | 65.6±5.8 | 58.6±4.8 |
|
Post-preparative | 95.7±15.7 | 98.0±7.7 | 96.8±8.5 | 86.1±17.0 |
Pharmacokinetic profiles of meisoindigo
and its reductive metabolites
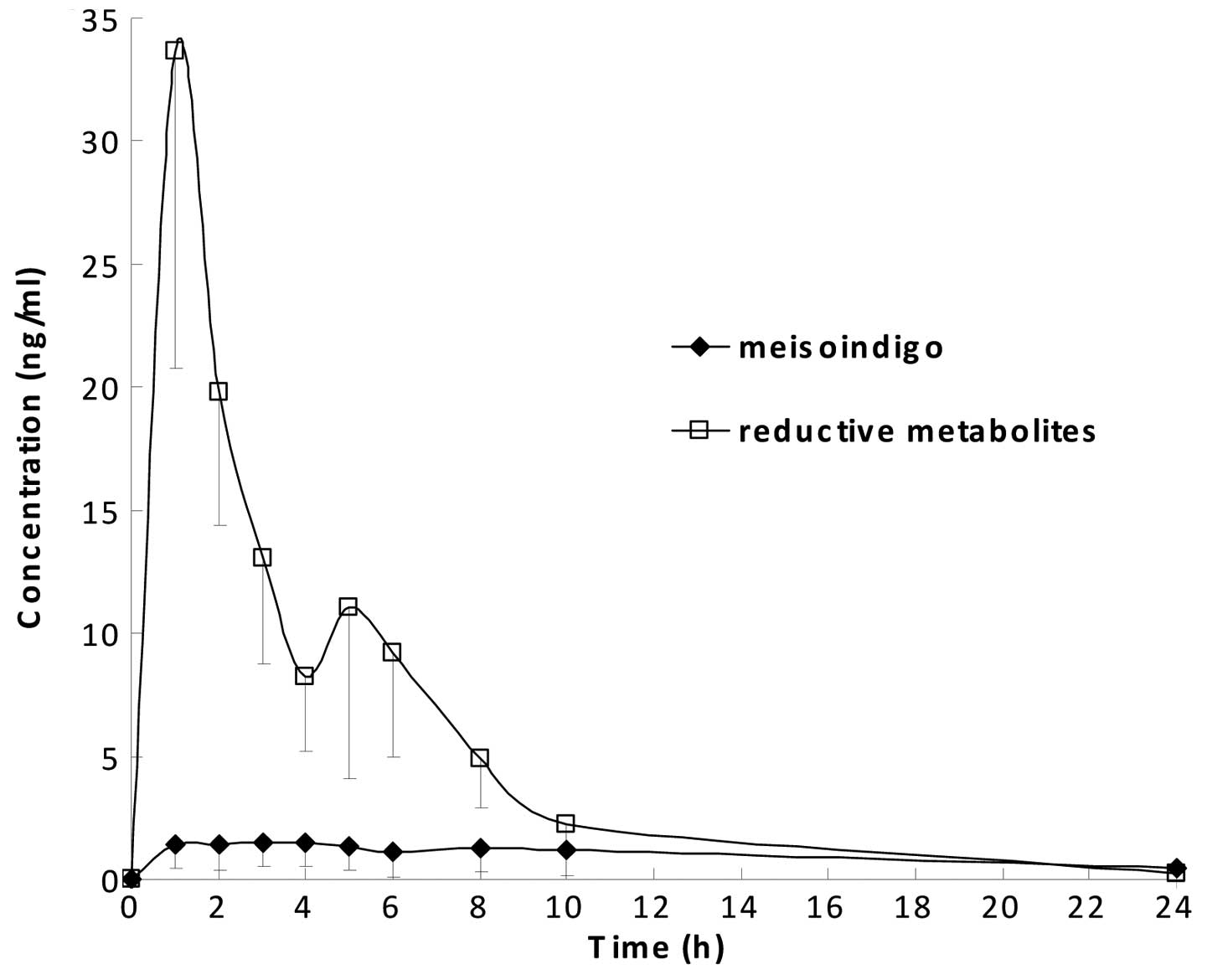
The pharmacokinetic profiles of meisoindigo and its
reductive metabolites in rat plasma are shown in Fig. 6. Data points were the average of
four measurements with standard deviation (SD) as the error bars.
The major pharmacokinetic parameters are listed in Table V. It is shown in Fig. 6 that meisoindigo was converted to
its reductive metabolites rapidly, indicating the 3,3′ double bond
within the molecule of meisoindigo is metabolically unstable.
Plasma level of the parent drug was low, whereas plasma level of
its reductive metabolites was much higher suggesting the occurrence
of extensive first pass effect. The wide variation in plasma levels
of the drug and its reductive metabolites also indicated possible
incomplete absorption due to the poor biopharmaceutical properties
of the drug. In Table V, the low
AUC0→24 h and long t1/2 (a composite
parameter determined by volume of distribution and clearance) could
be partly caused by a large volume of distribution of meisoindigo
due to its lipophilicity.
 | Table VPharmacokinetics parameters (mean ±
SD, n=4) of meisoindigo and its reductive metabolites in rat plasma
after oral administration of dosage 10 mg/kg. |
Table V
Pharmacokinetics parameters (mean ±
SD, n=4) of meisoindigo and its reductive metabolites in rat plasma
after oral administration of dosage 10 mg/kg.
| Parameters | Meisoindigo | Reductive
metabolites |
|---|
| AUC0→24
h (ng·h/ml) | 19.6±15.8 | 148.3±33.1 |
|
Tmax (h) | 2.5±2.1 | 1.4±0.2 |
|
t1/2 (h) | - | 3.6±1.5 |
|
Cmax (ng/ml) | 1.9±0.1 | 38.8±10.7 |
Discussion
It has been reported in previous in vitro
studies that meisoindigo could effectively inhibit the growth
and/or proliferation and induce apoptosis in the leukemic cells
isolated from newly diagnosed CML patients and CML K562 cells
(1,16). In the present study, a similar
effect of meisoindigo was observed in the other four human leukemic
cells, including NB4, NB4.007/6, HL60 and U937. IC50 of
meisoindigo for growth inhibition of the four human leukemic cell
types ranged from 7–8 μM; and at concentration of 12 μM,
meisoindigo was able to effectively induce apoptosis in all the
cells. These concentrations were even lower than the effective
concentration of meisoindigo (20 μM) in K562 cells, a cell line
derived from a CML patient in blast crisis (16). As the clinical efficacy of
meisoindigo for CML has been well documented (1,2),
similar efficacy would be expected in the clinical treatment of
other leukemias, such as APL and AML.
It is interesting to note that meisoindigo is active
in both the retinoic acid-sensitive APL cells (NB4) and retinoic
acid-resistant APL cells (NB4.007/6). This could be explained by
the different antileukemic mechanisms of retinoic acid and
meisoindigo. Retinoic acid exerts its antileukemic effects mainly
through differentiation induction (17), while meisoindigo probably exerts
its effects through induction of apoptosis and/or differentiation,
depending on the concentration. It might be beneficial to apply
meisoindigo in the management of retinoic acid resistant APL.
However, such postulation needs to be established through further
in vitro and in vivo pre-clinical studies.
In this study, flow cytometry and cytomorphology
results showed that the therapeutic effects of meisoindigo in the
four human leukemic cells were related to apoptosis, while the
apoptotic process was not associated with DNA fragmentation. Though
the detection of the DNA fragments by gel electrophoresis, as a DNA
ladder, is currently used as the major biochemical index of
apoptosis, apoptosis can also occur without such phenomenon
(18,19). It is well known that
internucleosomal cleavage of DNA is a later event in the apoptotic
process. Key morphological changes of apoptosis can be dissociated
experimentally from the DNA fragmentation produced by endonuclease
activity if the apoptosis is induced in the presence of an
inhibitor of endonuclease (18,19).
In light of the findings from the previous study (16) and the present study, we postulate
that meisoindigo might be an inhibitor of endonuclease or that it
induced apoptosis in these leukemic cells through an
endonuclease-independent process. The molecular mechanisms for the
apoptosis induction effects of meisoindigo are still unknown.
Several possible mechanisms might be involved, including
downregulation of bcl-2 gene and/or pathogenic oncogene
PML-RARα; phase arrest through inhibition of CDKs; inhibition of
DNA synthesis; inhibition of assembly of microtubule protein; and
partial induction of differentiation (1–4,16,20).
The antileukemic effects of meisoindigo could be the combined
effects of the above-mentioned functions.
As the clinical usage of meisoindigo is mainly
through oral administration, pharmacokinetic parameters were
determined from plasma in rats following oral administration of
meisoindigo in this study. The low and variable level of the parent
drug meisoindigo in rat plasma shown from in vivo
pharmacokinetics studies could be due to extensive first pass
effect and absorption incompleteness and variation. The latter
problem could partially be rectified by formulation modification.
It was found in our previous study that, compared with the
metabolic profile of meisoindigo in rat liver microsomes, the types
of metabolites produced in rat small intestine microsomes are much
less and their quantities are much lower (21). Therefore, it could be deduced that
the in vivo extensive first pass effect of meisoindigo is
mainly due to its metabolism in the liver.
Interestingly, an apparent disconnection between
in vitro and in vivo pharmacological data of
meisoindigo was revealed in this study. It was reported that the
production of vascular endothelial growth factor (VEGF) secreted by
human CML cells was decreased, and time-dependent apoptosis of
human ECV304 cells was induced, after treatment with 10 μM
meisoindigo (5). This required
concentration for the respective effects is much higher than the
in vivo nano-level plasma concentration of meisoindigo
observed in this study (slightly above 1 ng/ml). This in
vivo plasma concentration in rats could reflect the plasma
concentration of meisoindigo in humans, considering no significant
difference in the metabolic stability profiles of meisoindigo
between rat and human (12) as
well as the comparable dosages used in our rat model (10 mg/kg) and
in human application (100–150 mg/day) (2). However, it has been reported that
meisoindigo was equally efficient for both newly diagnosed and
previously treated CML patients (2). Therefore, the contradicting finding
of the observed sub-therapeutic plasma concentration of the parent
drug and clinical efficacy could be a strong indication of the
presence of active metabolites of meisoindigo, which led to a
greater in vivo pharmacological response inconsistent with
in vitro biological data and in vivo pharmacokinetics
profile (22).
Since the reductive metabolites of meisoindigo are
dominant in rat plasma (13), they
were synthesized as authentic standards and thus quantified in this
study. The reductive metabolites of meisoindigo are actually
comprised of two pairs of enantiomers (M1+M2, M3+M4), which can be
eluted as two peaks on conventional HPLC column and four peaks on
Chiral HPLC column (11).
Unfortunately, it was observed that one pair of enantiomers is
unstable in the liquid state and converts gradually into the other
pair of enantiomers (11). Thus,
it is challenging to accurately quantify the two peaks on
conventional HPLC column or four peaks on Chiral HPLC column. In
this study, conventional HPLC column was chosen and the peak area
sum of the two peaks corresponding to two pairs of enantiomers was
used to quantify the total amounts of reductive metabolites of
meisoindigo in rat plasma. A previous study suggested that an
intact exocyclic double bond was essential to maintain planarity
and rigidity of the isoindigo scaffold, a feature essential for its
activity (23). Since the
reductive metabolites of meisoindigo lost the double bond in their
structures, they are anticipated to be inactive.
In our previous studies, the in vivo
metabolic profiles of meisoindigo in rat urine and feces were also
examined qualitatively (13). The
parent drug and its reductive metabolites existed in rat urine with
trace amount which is close to LOD and thus was not analyzed
quantitatively. It is unnecessary to quantify the parent drug and
its reductive metabolites in rat feces, because both of them are
absent in feces samples. Prospective studies involving the
quantitative analysis of the dominant metabolites of meisoindigo at
m/z 295 undergone reduction followed by phenyl
mono-oxidation as a function of time in rat urine and feces could
be considered to provide a more comprehensive in vivo
metabolic profile of this drug.
In conclusion, the antileukemic effects of
meisoindigo were investigated in four human leukemic cells (NB4,
NB4.007/6, HL-60 and U937) including both retinoic acid sensitive
and retinoic acid resistant cells. We found that meisoindigo could
effectively inhibit the growth and/or proliferation of these four
cell types at μM levels. The effects of meisoindigo in these cells
are related to its proliferation inhibition and apoptosis
induction, and are independent of cell cycle arrest. However, the
apoptotic effect is not associated with DNA fragmentation. Clinical
efficacy of meisoindigo could be possible in the treatment of other
leukemias, such as APL and AML. It might be beneficial to apply
meisoindigo in the management of retinoic acid-resistant APL. In
addition, a sensitive and selective LC-MS/MS method was developed
and validated for simultaneous determination of meisoindigo and its
reductive metabolites whose concentration are at different order of
magnitude in rat plasma. The profiles of plasma concentration
versus time were plotted and the relevant pharmacokinetic
parameters were calculated for meisoindigo and its reductive
metabolites. Poor pharmacokinetic characteristics of meisoindigo
could be partly caused by the poor biopharmaceutical properties of
the drug. It warrants exploring whether formulation modification
could improve some of these pharmacokinetic characteristics, such
as the bioavailability of this drug. In particular, the
contradiction of poor pharmacokinetic characteristics and clinical
efficacy could be a strong indication of the presence of active
metabolites of meisoindigo.
Acknowledgements
This study was financially supported by the National
University of Singapore Academic Research Fund
R148-000-104-112.
Abbreviations:
|
AML
|
acute myeloid leukemia
|
|
APL
|
acute promyelocytic leukemia
|
|
CML
|
chronic myelogenous leukemia
|
|
EPI
|
enhanced product ion
|
|
ESI
|
electrospray ionization
|
|
LC-MS/MS
|
liquid chromatography-tandem mass
spectrometry
|
|
MRM
|
multiple reaction monitoring
|
|
QTRAP
|
hybrid triple quadrupole linear ion
trap
|
References
|
1
|
Xiao Z, Qian L, Liu B and Hao Y:
Meisoindigo for the treatment of chronic myelogenous leukaemia. Br
J Haematol. 111:711–712. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Xiao Z, Hao Y, Liu B and Qian L: Indirubin
and meisoindigo in the treatment of chronic myelogenous leukemia in
China. Leuk Lymphoma. 43:1763–1768. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Ji XJ, Liu XM, Li K, Chen RH and Wang LG:
Pharmacological studies of meisoindigo: absorption and mechanism of
action. Biomed Environ Sci. 4:332–337. 1991.PubMed/NCBI
|
|
4
|
Liu XM, Wang LG, Li HY and Ji XJ:
Induction of differentiation and down-regulation of c-myb gene
expression in ML-1 human myeloblastic leukemia cells by the
clinically effective anti-leukemia agent meisoindigo. Biochem
Pharmacol. 51:1545–1551. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Xiao Z, Wang Y, Lu L, et al:
Anti-angiogenesis effects of meisoindigo on chronic myelogenous
leukemia in vitro. Leuk Res. 30:54–59. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Lee CC, Lin CP, Lee YL, Wang GC, Cheng YC
and Liu HE: Meisoindigo is a promising agent with in vitro and in
vivo activity against human acute myeloid leukemia. Leuk Lymphoma.
51:897–905. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Lanotte M, Martin-Thouvenin V, Najman S,
Balerini P, Valensi F and Berger R: NB4, a maturation inducible
cell line with t(15;17) marker isolated from a human acute
promyelocytic leukemia (M3). Blood. 77:1080–1086. 1991.PubMed/NCBI
|
|
8
|
Breitman TR, Selonick SE and Collins SJ:
Induction of differentiation of the human promyelocytic leukemia
cell line (HL-60) by retinoic acid. Proc Natl Acad Sci USA.
77:2936–2940. 1980. View Article : Google Scholar
|
|
9
|
Dermime S, Grignani F, Rogaia D,
Liberatore C, Marchesi E and Gambacorti-Passerini C: Acute
promyelocytic leukaemia cells resistant to retinoic acid show
further perturbation of the RAR alpha signal transduction system.
Leuk Lymphoma. 16:289–295. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Olsson IL and Breitman TR: Induction of
differentiation of the human histiocytic lymphoma cell line U-937
by retinoic acid and cyclic adenosine 3′:5′-monophosphate-inducing
agents. Cancer Res. 42:3924–3927. 1982.
|
|
11
|
Huang M, Goh LT and Ho PC: Identification
of stereoisomeric metabolites of meisoindigo in rat liver
microsomes by achiral and chiral liquid chromatography/tandem mass
spectrometry. Drug Metab Dispos. 36:2171–2184. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Huang M and Ho PC: Identification of
metabolites of meisoindigo in rat, pig and human liver microsomes
by UFLC-MS/MS. Biochem Pharmacol. 77:1418–1428. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Huang M, Lee YS and Ho PC: Identification
of circulatory and excretory metabolites of meisoindigo in rat
plasma, urine and feces by high-performance liquid chromatography
coupled with positive electrospray ionization tandem mass
spectrometry. Rapid Commun Mass Spectrom. 24:729–741. 2010.
View Article : Google Scholar
|
|
14
|
Collins SJ, Gallo RC and Gallagher RE:
Continuous growth and differentiation of human myeloid leukaemic
cells in suspension culture. Nature. 270:347–349. 1977. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Sundstrom C and Nilsson K: Establishment
and characterization of a human histiocytic lymphoma cell line
(U-937). Int J Cancer. 17:565–577. 1976. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Song L and Qian L: Apoptosis inducing
effect of meisoindigo on K562 cells. Zhongguo Zhong Xi Yi Jie He Za
Zhi. 19:353–355. 1999.(In Chinese).
|
|
17
|
Lin RJ, Egan DA and Evans RM: Molecular
genetics of acute promyelocytic leukemia. Trends Genet. 15:179–184.
1999. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Bedner E, Li X, Gorczyca W, Melamed MR and
Darzynkiewicz Z: Analysis of apoptosis by laser scanning cytometry.
Cytometry. 35:181–195. 1999. View Article : Google Scholar
|
|
19
|
Cohen GM, Sun XM, Snowden RT, Dinsdale D
and Skilleter DN: Key morphological features of apoptosis may occur
in the absence of internucleosomal DNA fragmentation. Biochem J.
286:331–334. 1992.PubMed/NCBI
|
|
20
|
Hoessel R, Leclerc S, Endicott JA, et al:
Indirubin, the active constituent of a Chinese antileukaemia
medicine, inhibits cyclin-dependent kinases. Nat Cell Biol.
1:60–67. 1999.PubMed/NCBI
|
|
21
|
Huang M, Choo LW and Ho PC:
Characterization of metabolites of meisoindigo in male and female
rat kidney microsomes by high-performance liquid chromatography
coupled with positive electrospray ionization tandem mass
spectrometry. Rapid Commun Mass Spectrom. 22:3835–3845. 2008.
View Article : Google Scholar
|
|
22
|
Fura A: Role of pharmacologically active
metabolites in drug discovery and development. Drug Discov Today.
11:133–142. 2006. View Article : Google Scholar
|
|
23
|
Wee XK, Yeo WK, Zhang B, et al: Synthesis
and evaluation of functionalized isoindigos as antiproliferative
agents. Bioorg Med Chem. 17:7562–7571. 2009. View Article : Google Scholar : PubMed/NCBI
|