Introduction
Cervical cancer contributes to be an important world
health problem for women. It is the third most commonly diagnosed
cancer and the fourth cause of cancer death in females world-wide.
The role of human papillomavirus (HPV) infection has been
extensively investigated and considered as the pathogenesis of both
cervical cancer and its precursor damage (1). Of note, increasing evidence indicates
that additional non-viral molecular pathways are involved in the
initiation and progression of the desease. Thus, to explore the
effects of the signaling pathways on cell growth and apoptosis is
useful to identify the underlying mechanisms of carcinogenesis and
to find the potential therapeutic targets.
Nuclear factor-κB (NF-κB) is a transcription factor
that controls numerous genes regulating various cell functions
(2). NF-κB family is composed five
subunits, including p50 (NF-κB1), p52 (NF-κB2), p65 (RelA), RelB
and c-Rel, each of which may form homo- or heterodimers. Among
them, nuclear translocation of p65 subunit is a key step in the
activation of NF-κB (3). NF-κB
signaling pathway plays important roles in general inflammatory
response and cell death (4–6). We
have recently shown that the inhibition of NF-κB reduces the levels
of inflammatory factors, such as IL-6 and IL-8, as well as COX-2 in
chemical hypoxia-treated HaCat cells (4,5).
Furthermore, it has reported that NF-κB has a critical role in
malignancies related to chronic inflammation, due to the activation
of genes that promote cell proliferation, angiogenesis and survival
(7,8). The activation of NF-κB pathway
triggered by HPV 16E5, E6 and E7 oncoproteins is associated with
cervical carcinogenesis and progression (9). NF-κB is constitutively activated,
which promotes human cervical cancer progression and poor prognosis
(10,11). Notably, NF-κB is also involved in
overexpression of COX-2 by HPV 16E5 (9).
COX is the rate-limiting enzyme in the arachidonic
acid cascade that produces prostaglandins (PG) (12). There are two isoforms of COX. COX-1
is constitutively expressed in almost all tissues and involved in
maintaining homeostasis. In contrast, COX-2 is induced immediately
in response to inflammatory stimuli including mitogens, growth
factors and cytokine (13).
Increasing evidence has indicated that COX-2 levels are elevated in
several malignancies, such as liver, colon, prostate, gall bladder,
lung, skin and gynecologic cancer (14–21).
Accumulating studies have demonstrated that overexpressed COX-2 in
cervical cancer is related to lymph node metastasis (22) and resistance to radiation therapy
and chemotherapy (21,23). In addition, oxyphenbutazone (a
non-selective COX inhibitor) treatment markedly improves 5-year
survival rates in cervical cancer patients receiving radiation
therapy (24). A recent study also
reported that celecoxib, a selective COX-2 inhibitor,
radiosensitizes the human cervical cancer HeLa cells (25). Although NF-κB has been shown to be
a positive regulator of COX-2 expression in response to various
cytokines and growth factors in some cell types (26,27),
such as in human colorectal cancer cells (27), whether COX-2 was implicated in the
regulation of NF-κB expression in cervical cancer cells in
unclear.
Another signal molecule involved in the activation
of NF-κB is caspase-1. Caspase-1, also known as interleukin 1β
(IL-1β) converting enzyme, is an initiator caspase that is
originally the active proinflammatory cytokine IL-1β (28). Caspase-1 has been shown to be an
activator of NF-κB in B cells (29), whereas caspase-1 knockout
macrophages have decreased NF-κB activity (30). Although generally categorized as
cytokine-processing caspase, caspase-1 also plays important roles
in apoptosis in some cells, such as neurons (31), human ovarian cancer cells (32), prostate cancer cells (33) and pancreatic cancer cells (34). However, it is unknown what is the
relationship among caspase-1, NF-κB and COX-2, and what are the
roles of caspase-1 in cell growth and apoptosis in cervical cancer
cells.
Flavonoids are a large class of natural polyphenolic
compounds, existing in a wide variety of fruits and vegetables
regularly consumed by humans. Naringin (NRG, the glycoside of the
flavonone, naringenin), one of the most abundant flavonoids in
grape fruit and other citrus fruits, has been reported to have
multiple pharmacological effects, for example, antioxidant
(35,36), anti-inflammatory (36,37),
antiapoptotic (35,38,39)
and antiviral (40) effects.
Importantly, recent studies have demonstrated that increased
dietary consumption of NRG is related to attenuation of the risk of
certain cancers, such as breast cancer and lung cancers (41,42).
In addition, the findings from in vivo and in vitro
studies indicated that NRG suppresses colon cancer cells (43), urinary blader cancer cells
(44), human breast cancer cells
(45) and human cervical cancer
(SiHa) cells (46). Although many
studies have analyzed the effects of NRG on the tumor growth
inhibition and apoptosis induction, in several cell lines (41,46),
the signaling pathways involved in these effects on cancer cells
remain to be investigated.
The present study soughs to explore: i) the roles of
NF-κB-COX-2/caspase-1 pathway in regulating cell growth and
apoptosis; ii) the roles of inhibition of this pathway in
NRG-induced cell growth inhibition and apoptosis in cervical cancer
(HeLa) cells. This study demonstrated that NRG induces not only
cell growth inhibition, but also apoptosis by inhibiting the
NF-κB-COX-2/caspase-1 pathway in cervical cells.
Materials and methods
Materials
Naringin, Hoechst 33258 and NS-398 were purchased
from Sigma-Aldrich (St. Louis, MO, USA). The cell counter kit-8
(CCK-8) was supplied by Dojindo Laboratories (Kumamoto, Japan).
Fetal bovine serum (FBS) and Dulbecco’s modified Eagle’s medium
(DMEM) were obtained from Gibco-BRL (Grand Island, NY, USA).
Anti-COX-2 antibody, anti-p-NF-κB p65 antibody, anti-total
(t)-NF-κB p65 antibody, anti-caspase-1 antibody and anti-caspase-3
were purchased from Cell Signaling Technology (Boston, MA, USA),
HRP-conjugated secondary antibody and BCA protein assay kit were
obtained from KangChen Bio-tech, Inc (Shanghai, China). Enhanced
chemiluminescence (ECL) solution was purchased from KeyGen Biotech
(Nanjing, China).
Culture and treatments
The HeLa cells, a human cervical cancer HeLa cell
line, were supplied by Sun Yat-sen University Experimental Animal
Center (Guangzhou, China). The cells were cultured in DMEM medium
supplemented with 10% FBS at 37°C under an atmosphere of 5%
CO2 and 95% air.
To explore the anticancer effects of NRG on growth
and apoptosis, HeLa cells were treated with 1,000 μmol/l NRG for
the indicated times. To determine the roles of NF-κB, COX-2 and
caspase-1 in the growth and apoptosis, HeLa cells were treated with
1,000 μmol/l PDTC (an inhibitor of NF-κB) or 10 μmol/l NS-398 (an
inhibitor of COX-2) or 0.1 μg/ml SC-3069 for 24 h.
Cell viability assay
HeLa cells were cultured in 96-well plates at a
concentration of 1×104/ml, the CCK-8 assay was employed
to assess the cell viability of HeLa cells. After the indicated
treatments, 10 μl CCK-8 solution at a 1/10 dilution was added to
each well and then the plate was incubated for 1.5 h in the
incubator. Absorbance at 450 nm was tested with a microplate reader
(Molecular Devices, Sunnyvale, CA, USA). The means of the optical
density (OD) of five wells in the indicated groups were used to
calculate the percentage of cell viability according to the formula
below: cell viability (%) = (OD treatment group/OD control group)
×100%. The experiment was repeated in triplicate.
Hoechst 33258 nuclear staining for
assessment of apoptosis
Apoptotic cell death was measured by the Hoechst
33258 staining followed by photofluorography. Firstly, HeLa cells
were plated in 35-mm dishes at a density of 1×106
cells/well. After the indicated treatments, HeLa cells were fixed
with 4% paraformaldehyde in 0.1 mol/l phosphate-buffered saline
(PBS, pH 7.4) for 15 min. And then the slides were washed three
times with PBS. After staining by 5 mg/ml Hoechst 33258 for 10 min,
HeLa cells were washed three times with PBS. The cells were
visualized under a fluorescence microscope (Bx50-FLA; Olympus,
Tokyo, Japan). Viable HeLa cells displayed a uniform blue
fluorescence throughout the nucleus and normal nuclear size,
however, apoptotic HeLa cells showed condensed, distorted or
fractured nuclei. The experiment was carried out in
triplicates.
Western blot assay for expression of
protein
After the indicated treatments, HeLa cells were
harvested and lysed with cell lysis solution at 4°C for 30 min. The
total proteins were quantified using the BCA protein assay kit.
Loading buffer was added to cytosolic extracts, and boiled for
about 5 min, then the same amount of supernatant from each sample
were fractionated by 10% sodium dodecyl sulphate-polyacrylamide gel
electrophoresis (SDS-PAGE), and the total proteins were transferred
into polyvinylidene difluoride (PVDF) membranes. The membranes were
blocked with 5% fat-free milk for 1 h in fresh blocking buffer
[0.1% Tween-20 in Tris-buffered saline (TBS-T)] at room
temperature, and incubated with either anti-COX-2 antibody (1:1,000
dilution), anti-p-NF-κB p65 antibody (1:1,000 dilution), anti-total
(t)-NF-κB p65 antibody (1:1,000 dilution), anti-caspase-1 antibody
(1:1,000 dilution) or anti-caspase-3 antibody (1:1,000 dilution) in
freshly prepared TBS-T with 3% free-fat milk overnight with gentle
agitation at 4°C. The membranes were washed 3 times with TBST and
then incubated with a secondary antibody for 1.5 h at room
temperature in TBST with 3% fat-free milk [horseradish peroxidase
(HRP)-conjugated goat anti-rabbit secondary antibody, 1:2,500
dilution; Kangchen Biotech, Shanghai, China]. Then membranes were
washed three times with TBST for 5 min. The immunoreactive signals
were visualized by using enhanced chemiluminescence (ECL)
detection. In order to quantify the protein expression, the X-ray
film was scanned and analyzed with ImageJ 1.47 i software. The
experiment was repeated 3 times.
Statistical analysis
All data are presented as the mean ± SEM.
Differences between groups were analyzed by one-way analysis of
variance (ANOVA) by using SPSS 13.0 (SPSS, Chicago, IL, USA)
software, and followed by LSD post hoc comparison test. A
significance of p<0.05 was considered significant.
Results
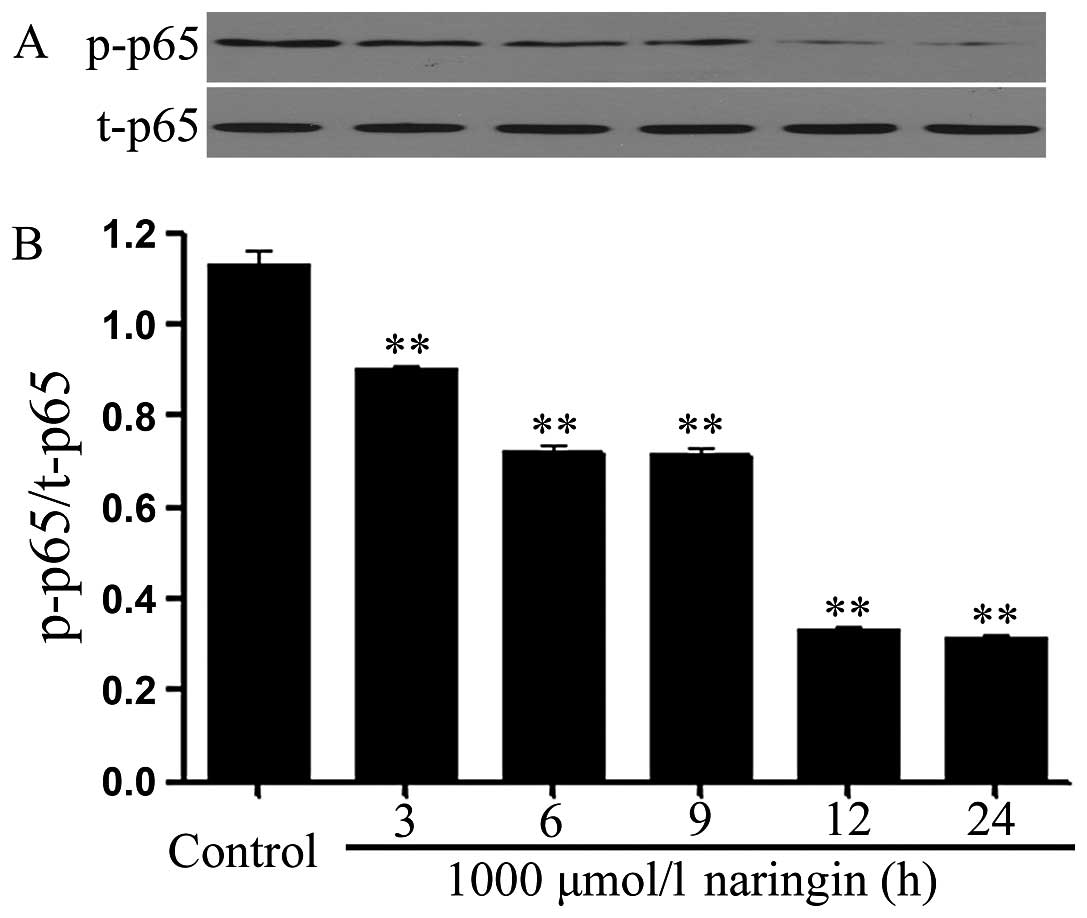
Naringin attenuates the expression of
phosphorylated NF-κB p65 in HeLa cells
In order to observe the effect of NRG on activation
of NF-κB pathway, HeLa cells were treated with 1,000 μmol/l NRG for
3, 6, 9, 12 and 24 h, respectively. As shown in Fig. 1, the expression level of
phosphorylated (p) NF-κB p65 was markedly reduced after exposure of
the cells to NRG for the indicated times. The maximal inhibition of
p-NF-κB p65 expression appeared after exposure to NRG for 12 to 24
h. However, exposure of HeLa cells to 1,000 μmol/l NRG did not
alter the total (t) expression of NF-κB p65 at the indicated
times.
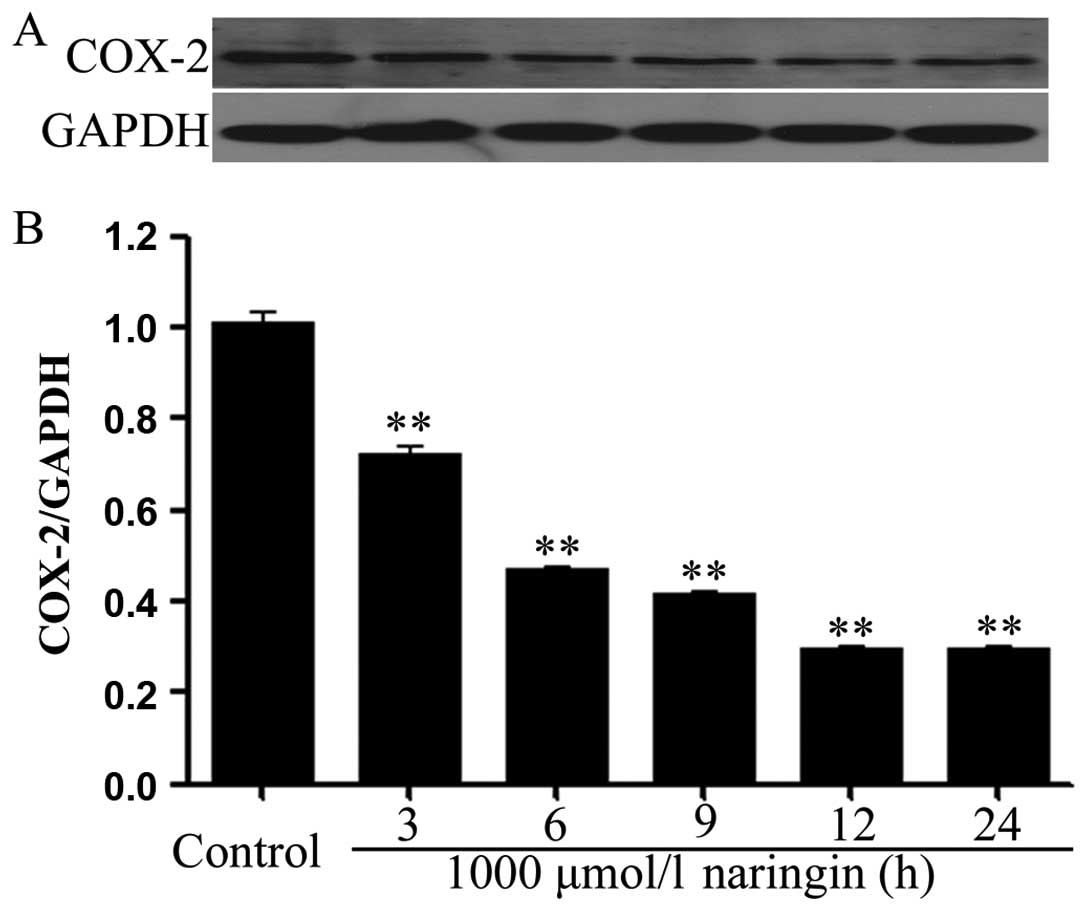
Naringin ameliorates the expression of
COX-2 in HeLa cells
Similarily, the expression of COX-2 was
significantly depressed after exposure of HeLa cells to 1,000
μmol/l NRG for the indicated times (3, 6, 9, 12 and 24 h),
respectively. The maximal inhibition of COX-2 expression by NRG was
recorded at 12 to 24 h (Fig.
2).
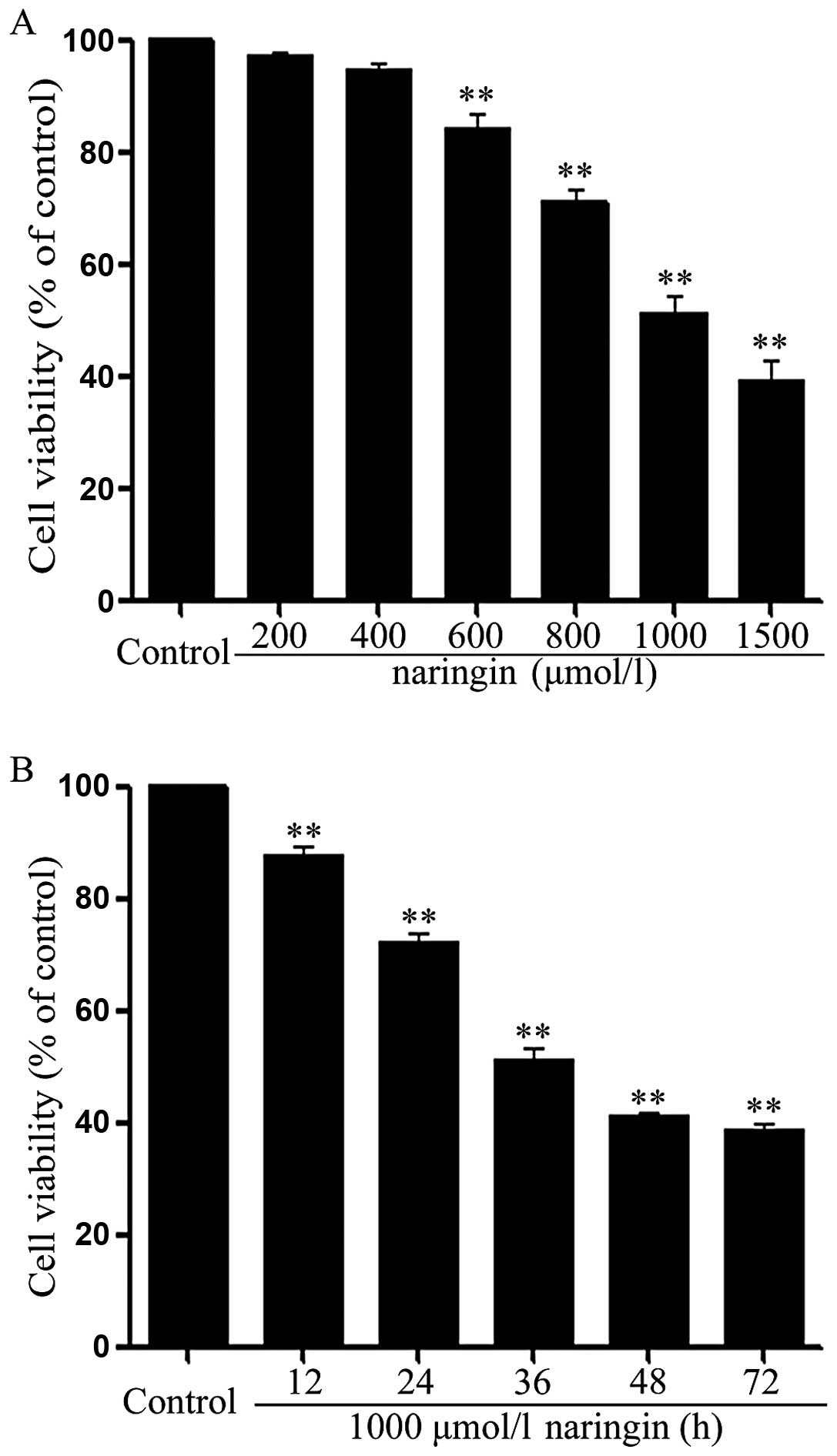
Naringin inhibits growth in HeLa
cells
To explore the effect of NRG on growth of HeLa
cells, we observed the effect of NRG at different concentrations
(200, 400, 600, 800, 1,000 and 1,500 μmol/l). As shown in Fig. 3A, HeLa cells were exposed to the
indicated concentrations of NRG for 24 h. At the range from 400 to
1,500 μmol/l, NRG dose-dependently attenuated the cell viability of
HeLa cells. NRG at 1,000 μmol/l reduced the cell viability to
51.1–3.1% (p<0.01), compared with the control group. Based on
these data, NRG at 1,000 μmol/l was used to perform a time response
experiment on growth of HeLa cells. As presented in Fig. 3B, the cell viability was
time-dependently inhibited after exposure of HeLa cells to 1,000
μmol/l RNG for the indicated times (12, 24, 36 and 48 h).
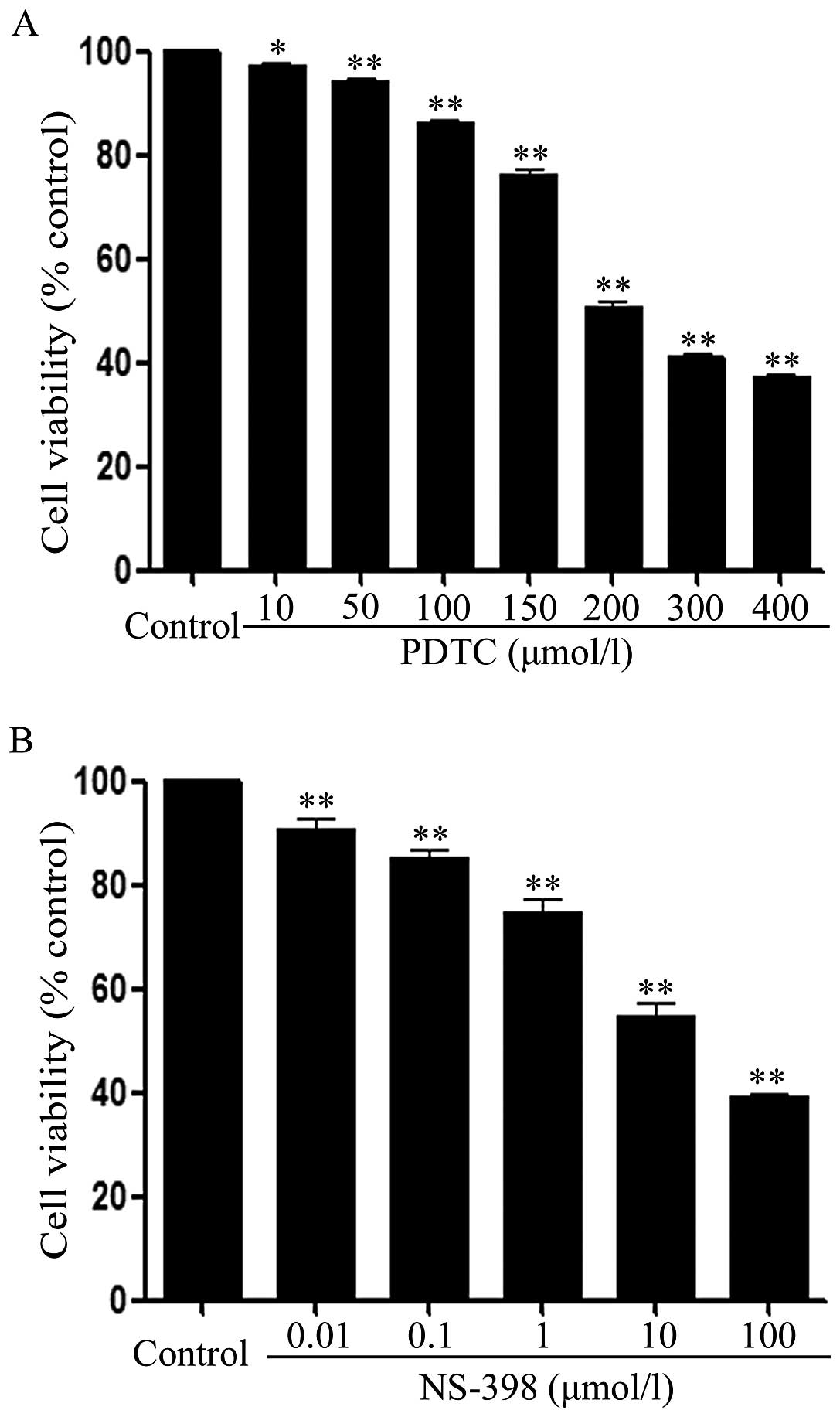
NF-κB inhibitor and COX-2 inhibitor block
growth of HeLa cells
To investigate the roles of both NF-κB and COX-2
pathways in the inhibitory effect of NRG on HeLa cell growth, the
effects of PDTC (an inhibitor of NF-κB) and NS-398 (an inhibitor of
COX-2) on the growth were observed. As shown in Fig. 4A, after HeLa cells were exposed to
different concentrations (10, 50, 100, 150, 200, 300 and 400
μmol/l) of PDTC for 36 h, the cell viability was decreased in a
dose-dependent manner. Similarly, exposure of the cells to the
indicated concentrations (0.01, 0.1, 1, 10 and 100 μmol/l) of
NS-398 for 36 h dose-dependently attenuated the cell viability
(Fig. 4B).
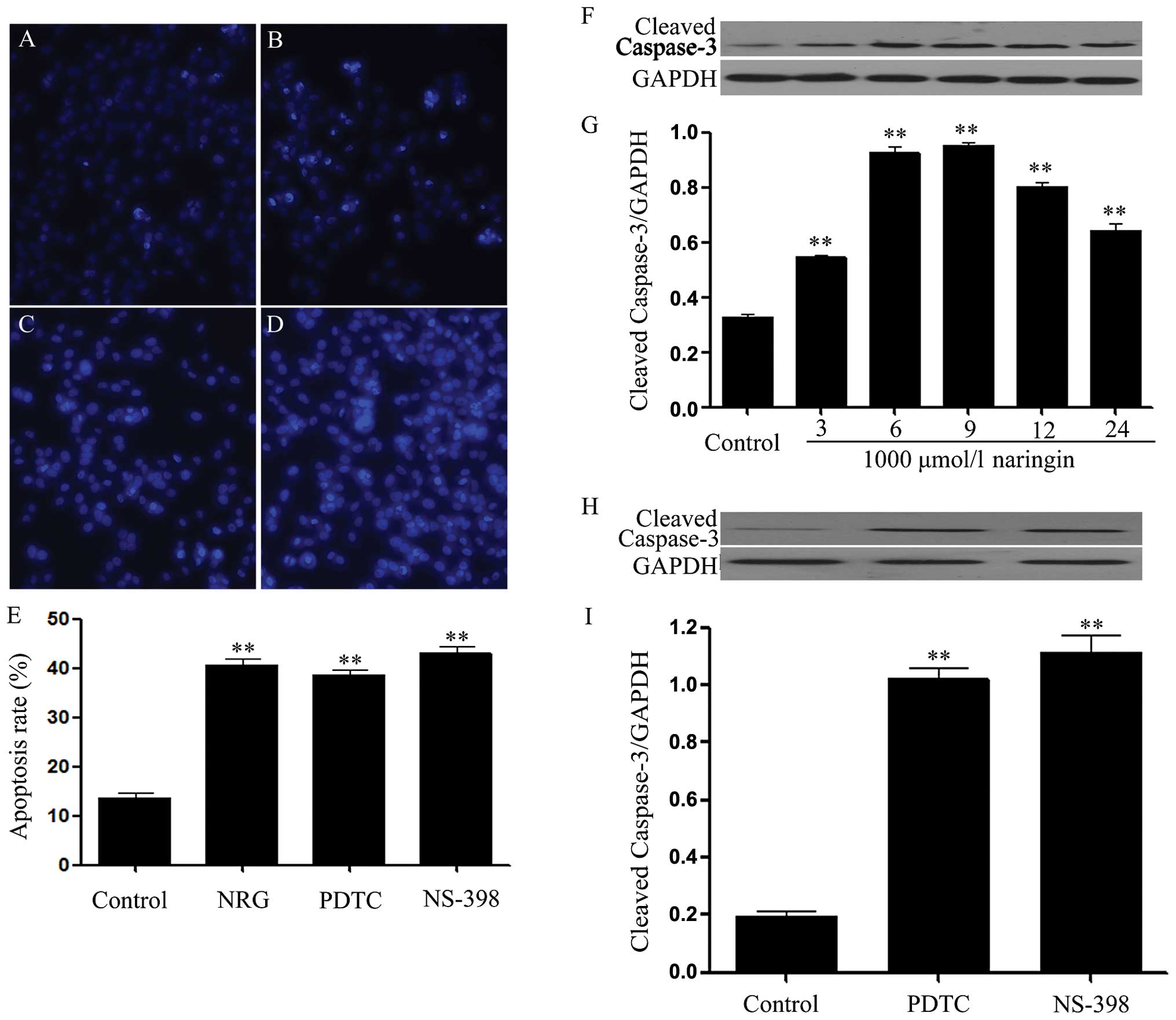
Naringin, PDTC and NS-398 induce
apoptosis in HeLa cells
As shown in Fig.
5B, exposure of HeLa cells to 1,000 μmol/l NRG for 24 h induced
typical characteristics of apoptosis, as evidenced by the
condensation of chromatin, the shrinkage of nuclei and the
formation of apoptotic bodies. Similar to the apoptotic effect of
NRG, exposure of the cells to 200 μmol/l PDTC or 10 μmol/l NS-398
for 24 h also enhanced the number of apoptotic HeLa cells (Fig. 5C and E).
On the other hand, the effect of NRG on the
expression of cleaved caspase-3 (an apoptotic effector) was
observed. As illustrated in Fig. 5F
and G, exposure of HeLa cells to 1,000 μmol/l NRG for the
indicated times (3, 6, 9, 12 and 24 h) markedly upregulated the
expression level of cleaved caspase-3, peaking at 9 h. Similarly,
exposure of the cells to 200 μmol/l PDTC or 10 μmol/l NS-398 for 24
h also increased the expression level of cleaved caspase-3,
respectively (Fig. 5H and I).
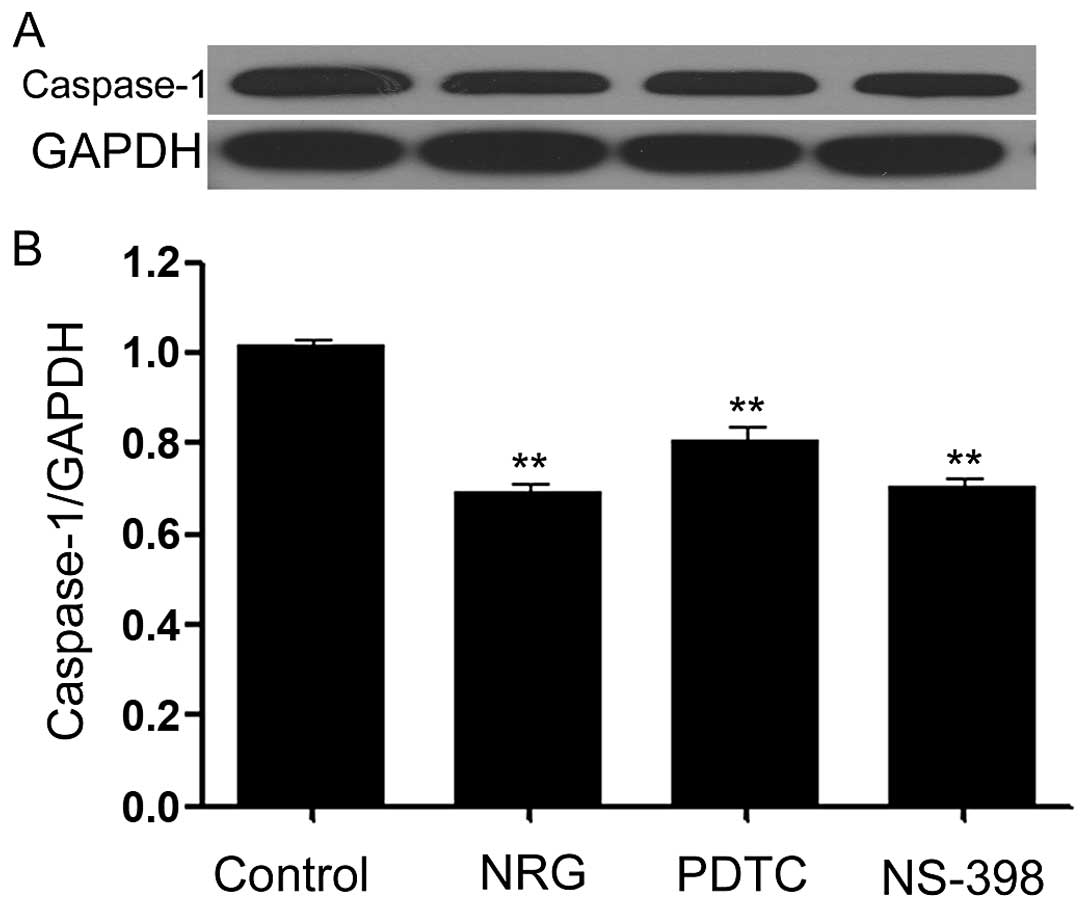
Naringin, PDTC and NS-398 downregulate
the expression of caspase-1 in HeLa cells
Caspase-1 is one member of inflammasome family
(47,48), which has been reported to
contribute to tumor growth. Thus, we explored the influences of
NRG, PDTC and NS-398 on caspase-1 expression in HeLa cells. As
shown in Fig. 6, expression of
caspase-1 was observed in the cells. Of note, exposure of the cells
to 1,000 μmol/l NRG for 24 h obviously reduced the expression level
of caspase-1. In addition, treatment of the cells with 200 μmol/l
PDTC or 10 μmol/l NS-398 for 24 h also attenuated caspase-1
expression.
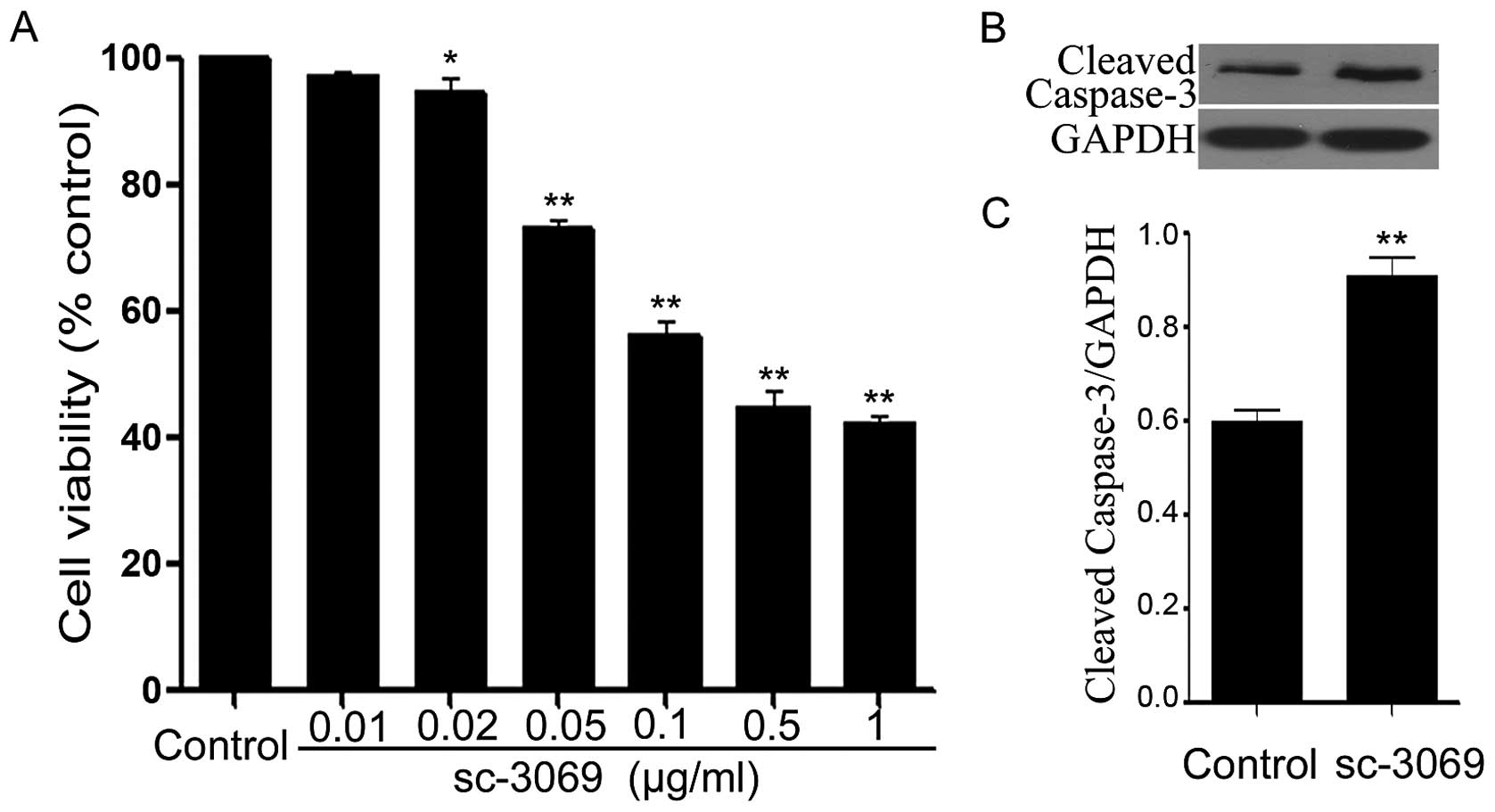
Caspase-1 inhibitor reduces growth and
induces apoptosis in HeLa cells
To investigate the roles of caspase-1 in growth of
the cells, we examined the effects of SC-3069 (an inhibitor of
caspase-1) on the cell viability and apoptosis. As illustrated in
Fig. 7A, exposure of the cells to
SC-3069 at different concentrations (0.01, 0.02, 0.05, 0.1, 0.5 and
1 μg/ml) for 24 h dose-dependently decreased cell viability.
Furthermore, as shown in Fig. 7B, treatment of HeLa cells with 0.1
μg/ml sc-3096 for 12 h significantly increased expression level of
caspase-3, compared with the control group.
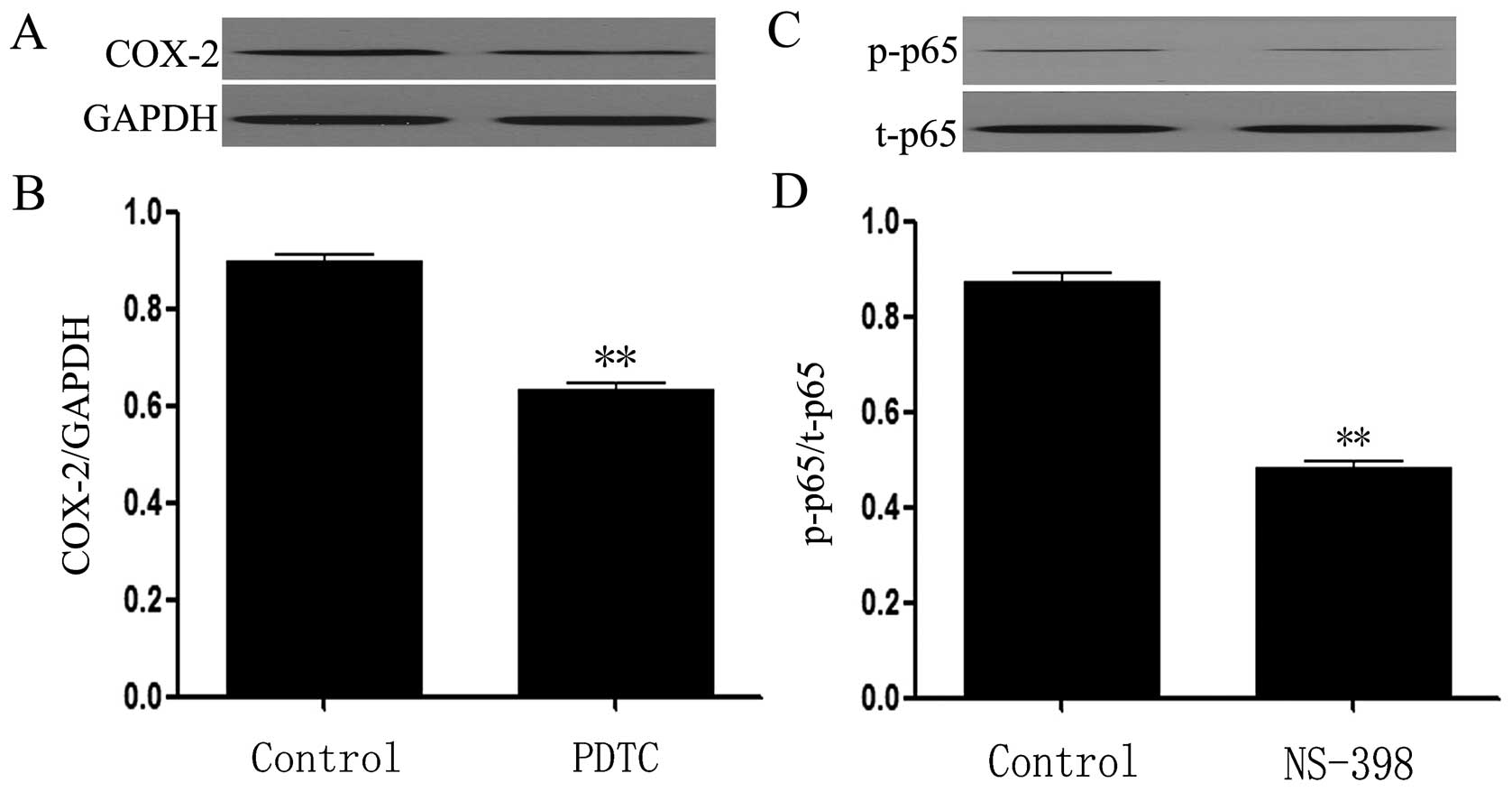
There is a positive interaction between
NF-κB and COX-2 pathway in HeLa cells
The data from western blot assay showed that
exposure of the cells to 200 μmol/l PDTC for 24 dramatically block
the expression of COX-2 (Fig. 8A and
B, p<0.01), indicating contribution of NF-κB to the
activation of COX-2 pathway. Interestingly, treatment of the cells
with 10 μmol/l NS-398 for 24 h considerably antagonized the
expression of p-NF-κB p65 (Fig. 8C and
D, p<0.01), revealing involvement of COX-2 in the activity
of NF-κB pathway.
Discussion
The activation of NF-κB (9,10,12)
or COX-2 pathway (21–25,49)
has been correlated with cervical carcinogenesis and progression.
Furthermore, NF-κB is implicated in overexpression of COX-2 by HPV
16E5 (9). However, the
relationship between NF-κB and COX-2 in cell proliferation and
apoptosis of cervical cancer cells is incomplely understood. In the
present study, we provided novel evidence that there is a positive
interaction between NF-κB and COX-2 pathway, which may be an
important mechanism responsible for cell growth and antiapoptosis
in cervical cancer cells. This mechanism is supported by the
following results: i) treatment with PDTC, an inhibitor of NF-κB,
attenuated the expression level of COX-2; ii) NS-398, an inhibitor
of COX-2, reduced the expression of NF-κB p65 subunit; iii)
exposure of HeLa cells to PDTC or NS-398 induced growth inhibition
and apoptosis, as demonstrated by the decreased cell viability and
increased amount of apoptotic cells and cleaved caspase-3
expression.
Apoptosis is the physiologically relevant model of
programmed cell death that counterbalances cell proliferation.
Activation of the caspase cascade is involved in the execution of
apoptosis in a variety of cellular systems. Caspase-1, a member of
the so-called ‘inflammatory caspases’ group, is an initiator
caspase. Recently, the role of caspase-1 in apoptosis of cancer
cells has been receiving attention, but previous studies concering
the role of caspase-1 are controversial. Jarry et al
(50) have demonstrated obvious
downregulation of caspase-1 expression in human colon cancer.
Overexpression of caspase-1 enhances the rate of apoptosis in
vitro and in vivo in renal cancer cell lines (51). In constrast, Schlosser et al
(34) reported that caspase-1 has
antiapoptotic function in pancreatic carcinoma. Thus, to explore
the functional roles of caspase-1 in cell growth and apoptosis will
enhance the understanding of the molecular basis of cervical
carcinogenesis and progression. The findings of this study showed
that both NF-κB inhibitor (PDTC) and COX-2 inhibitor (NS-398)
significantly reduced the expression level of caspase-1, indicating
the contribution of NF-κB-COX-2 pathway to activation of caspase-1
in HeLa cells. In addition, SC-3069, an inhibitor of caspase-1,
suppressed cell viability and upregulated the expression of cleaved
caspase-3 (an executioner of apoptosis), suggesting involvement of
caspase-1 in cell growth and antiapoptotic effect in cervical
cancer cells. Our results are comparable with those of a previous
study that caspase-1 has antiapoptotic effect in pancreatic cancer
cells (34). However, our data
contrast with previous observations that caspase-1 contributes to
apoptotic induction in human ovarian cancer cells (32), prostate cancer cells (33), human colon cancer cells (50) and renal cancer cell lines (51). The apparent discrepancy may reflect
specific differences in the molecular mechanisms responsible for
the development of various human malignancies. Alternatively, a
potential antiapoptotic function for this caspase may be a result
of alternative splicing of caspase-1, since caspase-1 has four
known isoforms, at least two of which may have antagonistic effect
against apoptosis (52).
Another novel finding of the present study is that
NRG, a citrus flavonone, exerts its anticancer effects by
inhibiting the NF-κB-COX-2/caspase-1 pathway in cervical cancer
cells. Citrus fruits contain various flavonoids, and among these
natural compounds, NRG has been pharmacologically considered as a
potential anticancer agent, due to its anticancer effect on various
cancer cells, such as colon cancer cells (43,53),
urinary blader cancer cells (44),
human breast cancer cells (45)
and human cervical cancer (SiHa) cells (46). However, the molecular mechanisms
responsible for the anticancer effect of NRG have yet to be
complely understood. To the best of our knowledge, no previous
studies have focused on NRG-induced cell growth inhibition and
apoptosis through the inhibition of NF-κB-COX-2/caspase-1 pathway
in human cervical cancer (HeLa) cells.
To investigate this topic, firstly, we observed the
roles of NRG in cell growth inhibition and apoptosis in HeLa cells.
In agreement with the results of previous studies (43–46),
our data showed that NRG had marked anticancer effects, as
evidenced by a decrease in cell viability and increases in number
of apoptotic cells as well as the expression level of cleaved
caspase-3 in HeLa cells. In human cervical cancer (SiHa) cells,
administration of NRG also has anti-proliferative effect and
increases the expression of caspase-3 and -9 (46), which support our results. Based on
the above, it is suggested that NRG may be a potential inducer of
cleaved caspase-3 (an executioner of apoptosis) in human cervical
cancer (including SiHa and HeLa) cells.
As described above, the present study demonstrated
the involvement of NF-κB-COX-2/caspase-1 pathway in cell growth and
anti-apoptosis, thus, we further explored the effects of NRG on the
activation of NF-κB-COX-2/caspase-1 pathway. The findings of the
current study showed that treatment of HeLa cells with NRG markedly
attenuated the expression levels of NF-κB p65 subunit, COX-2 and
caspase-1. These results revealed that NRG induces growth
inhibition and apoptosis, at least in part, through the inhibition
of NF-κB-COX-2/caspase-1 pathway in HeLa cells.
In summary, the present study provides novel
evidence that the activation of NF-κB-COX-2/caspase-1 pathway
contributes to cervical carcinogenesis, including cell
proliferation and antiapoptosis. The understanding of the roles of
such a signaling pathway is important, as it may lead to the
development of novel treatment strategies designed to inhibit this
signal cascade for cervical cancer cells. Additionally, the present
study provides important new insight into the molecular mechanisms
underlying the anticancer effect of NRG in cervical cancer cells.
First, NRG reduces cell viability and induces apoptosis. Second,
the NRG-induced growth inhibition and apoptosis appears to be
linked to the inhibition of activation of NF-κB-COX-2/caspase-1
pathway. The findings of the present study may, in part, explain
the therapeutic effects of NRG in treatment of human cervical
cancer cells.
Acknowledgements
This study was supported by Special Competitive
Allocation Project of Science and Technology Special Financial
Funding of Zhanjiang (no. 2013A304) and Science Research Funding of
the Guangdong Provincial Population and Family Planning Commission
(no. 20110264).
References
|
1
|
Münger K, Baldwin A, Edwards KM, et al:
Mechanisms of human papillomavirus-induced oncogenesis. J Virol.
78:11451–11460. 2004.
|
|
2
|
Karin M and Greten FR: NF-kappaB: linking
inflammation and immunity to cancer development and progression.
Nat Rev Immunol. 5:749–759. 2005. View
Article : Google Scholar : PubMed/NCBI
|
|
3
|
Baldwin AS Jr: The NF-kappa B and I kappa
B proteins: new discoveries and insights. Annu Rev Immunol.
14:649–683. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Yang C, Ling H, Zhang M, et al: Oxidative
stress mediates chemical hypoxia-induced injury and inflammation by
activating NF-κb-COX-2 pathway in HaCaT cells. Mol Cells.
31:531–538. 2011.PubMed/NCBI
|
|
5
|
Yang C, Yang Z, Zhang M, et al: Hydrogen
sulfide protects against chemical hypoxia-induced cytotoxicity and
inflammation in HaCaT cells through inhibition of ROS/NF-κB/COX-2
pathway. PLoS One. 6:e219712011.PubMed/NCBI
|
|
6
|
Guo RM, Xu WM, Lin JC, et al: Activation
of the p38 MAPK/NF-κB pathway contributes to doxorubicin-induced
inflammation and cytotoxicity in H9c2 cardiac cells. Mol Med Rep.
8:603–608. 2013.
|
|
7
|
Lu H, Ouyang W and Huang C: Inflammation,
a key event in cancer development. Mol Cancer Res. 4:221–233. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Pollard JW: Tumour-educated macrophages
promote tumour progression and metastasis. Nat Rev Cancer. 4:71–78.
2004. View
Article : Google Scholar : PubMed/NCBI
|
|
9
|
Kim SH, Oh JM, No JH, Bang YJ, Juhnn YS
and Song YS: Involvement of NF-kappaB and AP-1 in COX-2
upregulation by human papillomavirus 16 E5 oncoprotein.
Carcinogenesis. 30:753–757. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Li J, Jia H, Xie L, et al: Association of
constitutive nuclear factor-kappaB activation with aggressive
aspects and poor prognosis in cervical cancer. Int J Gynecol
Cancer. 19:1421–1426. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Wu Z, Peng X, Li J, Zhang Y and Hu L:
Constitutive activation of nuclear factor κB contributes to cystic
fibrosis transmembrane conductance regulator expression and
promotes human cervical cancer progression and poor prognosis. Int
J Gynecol Cancer. 23:906–915. 2013.
|
|
12
|
Agarwal B, Rao CV, Bhendwal S, Ramey WR,
Shirin H, Reddy BS and Holt PR: Lovastatin augments
sulindac-induced apoptosis in colon cancer cells and potentiates
chemopreventive effects of sulindac. Gastroenterology. 117:838–847.
1999. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Vane JR, Bakhle YS and Botting RM:
Cyclooxygenases 1 and 2. Annu Rev Pharmacol Toxicol. 38:97–120.
1998. View Article : Google Scholar
|
|
14
|
Bae SH, Jung ES, Park YM, Kim BS, Kim BK,
Kim DG and Ryu WS: Expression of cyclooxygenase-2 (COX-2) in
hepato-cellular carcinoma and growth inhibition of hepatoma cell
lines by a COX-2 inhibitor, NS-398. Clin Cancer Res. 7:1410–1418.
2001.PubMed/NCBI
|
|
15
|
Dubois RN: Review article: cyclooxygenase
- a target for colon cancer prevention. Aliment Pharmacol Ther.
14(Suppl 1): 64–67. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Hsu AL, Ching TT, Wang DS, Song X,
Rangnekar VM and Chen CS: The cyclooxygenase-2 inhibitor celecoxib
induces apoptosis by blocking Akt activation in human prostate
cancer cells independently of Bcl-2. J Biol Chem. 275:11397–11403.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Grossman EM, Longo WE, Panesar N, Mazuski
JE and Kaminski DL: The role of cyclooxygenase enzymes in the
growth of human gall bladder cancer cells. Carcinogenesis.
21:1403–1409. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Khuri FR, Wu H, Lee JJ, et al:
Cyclooxygenase-2 overexpression is a marker of poor prognosis in
stage I non-small cell lung cancer. Clin Cancer Res. 7:861–867.
2001.PubMed/NCBI
|
|
19
|
Higashi Y, Kanekura T and Kanzaki T:
Enhanced expression of cyclooxygenase (COX)-2 in human skin
epidermal cancer cells: evidence for growth suppression by
inhibiting COX-2 expression. Int J Cancer. 86:667–671. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Kulkarni S, Rader JS, Zhang F, et al:
Cyclooxygenase-2 is overexpressed in human cervical cancer. Clin
Cancer Res. 7:429–434. 2001.PubMed/NCBI
|
|
21
|
Ferrandina G, Lauriola L, Distefano MG, et
al: Increased cyclooxygenase-2 expression is associated with
chemotherapy resistance and poor survival in cervical cancer
patients. J Clin Oncol. 20:973–981. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Ryu HS, Chang KH, Yang HW, Kim MS, Kwon HC
and Oh KS: High cyclooxygenase-2 expression in stage IB cervical
cancer with lymph node metastasis or parametrial invasion. Gynecol
Oncol. 76:320–325. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Gaffney DK, Holden J, Davis M, Zempolich
K, Murphy KJ and Dodson M: Elevated cyclooxygenase-2 expression
correlates with diminished survival in carcinoma of the cervix
treated with radiotherapy. Int J Radiat Oncol Biol Phys.
49:1213–1217. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Weppelmann B and Mönkemeier D: The
influence of prostaglandin antagonists on radiation therapy of
carcinoma of the cervix. Gynecol Oncol. 17:196–199. 1984.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Wang AH, Tian XY, Yu JJ, Mi JQ, Liu H and
Wang RF: Celecoxib radiosensitizes the human cervical cancer HeLa
cell line via a mechanism dependent on reduced cyclo-oxygenase-2
and vascular endothelial growth factor C expression. J Int Med Res.
40:56–66. 2012. View Article : Google Scholar
|
|
26
|
Kang YJ, Wingerd BA, Arakawa T and Smith
WL: Cyclooxygenase-2 gene transcription in a macrophage model of
inflammation. J Immunol. 177:8111–8122. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Liu W, Reinmuth N, Stoeltzing O, et al:
Cyclooxygenase-2 is up-regulated by interleukin-1 beta in human
colorectal cancer cells via multiple signaling pathways. Cancer
Res. 63:3632–3636. 2003.PubMed/NCBI
|
|
28
|
Thornberry NA, Bull HG, Calaycay JR, et
al: A novel heterodimeric cysteine protease is required for
interleukin-1 beta processing in monocytes. Nature. 356:768–774.
1992. View
Article : Google Scholar : PubMed/NCBI
|
|
29
|
Lamkanfi M, Kalai M, Saelens X, Declercq W
and Vandenabeele P: Caspase-1 activates nuclear factor of the
kappa-enhancer in B cells independently of its enzymatic activity.
J Biol Chem. 279:24785–24793. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Sarkar A, Duncan M, Hart J, Hertlein E,
Guttridge DC and Wewers MD: ASC directs NF-kappaB activation by
regulating receptor interacting protein-2 (RIP2) caspase-1
interactions. J Immunol. 176:4979–4986. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Zhang WH, Wang X, Narayanan M, Zhang Y,
Huo C, Reed JC and Friedlander RM: Fundamental role of the
Rip2/caspase-1 pathway in hypoxia and ischemia-induced neuronal
cell death. Proc Natl Acad Sci USA. 100:16012–16017. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Feng Q, Li P, Salamanca C, Huntsman D,
Leung PC and Auersperg N: Caspase-1alpha is down-regulated in human
ovarian cancer cells and the overexpression of caspase-1alpha
induces apoptosis. Cancer Res. 65:8591–8596. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Winter RN, Rhee JG and Kyprianou N:
Caspase-1 enhances the apoptotic response of prostate cancer cells
to ionizing radiation. Anticancer Res. 24:1377–1386.
2004.PubMed/NCBI
|
|
34
|
Schlosser S, Gansauge F, Ramadani M, Beger
HG and Gansauge S: Inhibition of caspase-1 induces cell death in
pancreatic carcinoma cells and potentially modulates expression
levels of bcl-2 family proteins. FEBS Lett. 491:104–108. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Chen J, Guo R, Yan H, et al: Naringin
inhibits ROS-activated MAPK pathway in high glucose-induced
injuries in H9c2 cardiac cells. Basic Clin Pharmacol Toxicol.
114:293–304. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Mahmoud AM, Ashour MB, Abdel-Moneim A and
Ahmed OM: Hesperidin and naringin attenuate hyper-glycemia-mediated
oxidative stress and proinflammatory cytokine production in high
fat fed/streptozotocin-induced type 2 diabetic rats. J Diabetes
Complications. 26:483–490. 2012. View Article : Google Scholar
|
|
37
|
Nie YC, Wu H, Li PB, et al:
Anti-inflammatory effects of naringin in chronic pulmonary
neutrophilic inflammation in cigarette smoke-exposed rats. J Med
Food. 15:894–900. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Huang H and Wu K, You Q, Huang R, Li S and
Wu K: Naringin inhibits high glucose-induced cardiomyocyte
apoptosis by attenuating mitochondrial dysfunction and modulating
the activation of the p38 signaling pathway. Int J Mol Med.
32:396–402. 2013.
|
|
39
|
Chen J, Mo H, Guo R, You Q, Huang R and Wu
K: Inhibition of the leptin-induced activation of the p38 MAPK
pathway contributes to the protective effects of naringin against
high glucose-induced injury in H9c2 cardiac cells. Int J Mol Med.
33:605–612. 2014.PubMed/NCBI
|
|
40
|
Kaul TN, Middleton E Jr and Ogra PL:
Antiviral effect of flavonoids on human viruses. J Med Virol.
15:71–79. 1985. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
So FV, Guthrie N, Chambers AF, Moussa M
and Carroll KK: Inhibition of human breast cancer cell
proliferation and delay of mammary tumorigenesis by flavonoids and
citrus juices. Nutr Cancer. 26:167–181. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Le Marchand L, Murphy SP, Hankin JH,
Wilkens LR and Kolonel LN: Intake of flavonoids and lung cancer. J
Natl Cancer Inst. 92:154–160. 2000.PubMed/NCBI
|
|
43
|
Vanamala J, Leonardi T, Patil BS, et al:
Suppression of colon carcinogenesis by bioactive compounds in
grapefruit. Carcinogenesis. 27:1257–1265. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Kim DI, Lee SJ, Lee SB, Park K, Kim WJ and
Moon SK: Requirement for Ras/Raf/ERK pathway in naringin-induced
G1-cell-cycle arrest via p21WAF1 expression. Carcinogenesis.
29:1701–1709. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Schindler R and Mentlein R: Flavonoids and
vitamin E reduce the release of the angiogenic peptide vascular
endothelial growth factor from human tumor cells. J Nutr.
136:1477–1482. 2006.PubMed/NCBI
|
|
46
|
Ramesh E and Alshatwi AA: Naringin induces
death receptor and mitochondria-mediated apoptosis in human
cervical cancer (SiHa) cells. Food Chem Toxicol. 51:97–105. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Choi JS and Ryter SW: Inflammasomes:
Molecular Regulation and Implications for Metabolic and Cognitive
Diseases. Mol Cells. May 19–2014.(Epub ahead of print).
|
|
48
|
Man SM, Tourlomousis P, Hopkins L, Monie
TP, Fitzgerald KA and Bryant CE: Salmonella infection
induces recruitment of Caspase-8 to the inflammasome to modulate
IL-1β production. J Immunol. 191:5239–5246. 2013. View Article : Google Scholar
|
|
49
|
Yusup G, Akutsu Y, Mutallip M, et al: A
COX-2 inhibitor enhances the antitumor effects of chemotherapy and
radiotherapy for esophageal squamous cell carcinoma. Int J Oncol.
44:1146–1152. 2014.PubMed/NCBI
|
|
50
|
Jarry A, Vallette G, Cassagnau E, et al:
Interleukin 1 and interleukin 1beta converting enzyme (caspase 1)
expression in the human colonic epithelial barrier. Caspase 1
downregulation in colon cancer. Gut. 45:246–251. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Ueki T, Takeuchi T, Nishimatsu H, et al:
Silencing of the caspase-1 gene occurs in murine and human renal
cancer cells and causes solid tumor growth in vivo. Int J Cancer.
91:673–679. 2001. View Article : Google Scholar
|
|
52
|
Alnemri ES, Fernandes-Alnemri T and
Litwack G: Cloning and expression of four novel isoforms of human
interleukin-1 beta converting enzyme with different apoptotic
activities. J Biol Chem. 270:4312–4317. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Chen YC, Shen SC, Chow JM, Ko CH and Tseng
SW: Flavone inhibition of tumor growth via apoptosis in
vitro and in vivo. Int J Oncol. 25:661–670.
2004.PubMed/NCBI
|