Introduction
Kefir is a well-known fermented milk product
obtained by the fermentation of milk with kefir grains. It is
highly consumed in many countries, mostly in Eastern Europe, but
also in Asia and America (1,2).
Around the world, kefir is manufactured and marketed as a
refreshing, slightly alcoholic beverage, under different names
(Kephir, Kiaphur, Kefer, Kepi and Kippi) (1–3).
Kefir grains are a white, soft, gelatin-like mass
composed of bacteria and yeast existing in a matrix of proteins,
fat, and polysaccharides, with kefiran being the most important
water-soluble polysaccharide (4).
Around 50 types of these microorganisms co-exist in a symbiotic
relationship in the kefir grains: these include lactic acid
bacteria, yeasts, streptococci, lactococci and infrequently acetic
acid bacteria among others (5).
It was always assumed among Bulgarian farmers that
kefir held special healing powers, and they believed that their
longevity was attributed to their ingestion of kefir (6). Kefir has been made in the Caucasus
Mountains for hundreds of years, yet it was kept in secrecy within
tribes who held it as their legacy, and not until recently have
kefir grains been distributed to different regions of the world
(2). Since then, many health
benefits have been attributed to kefir, including its ability to
stimulate the immune system (7),
improve lactose digestion in lactose-intolerant individuals
(8), decrease serum cholesterol
levels (9), provide resistance
against enteric and diarrheal diseases (10), act as an antifungal and
antibacterial agent against pathogenic organisms (12), as well as possessing an
anti-tumoral activity (11).
The first publication to show antitumoral activity
of a water-soluble polysaccharide (KGF-C) separated from kefir
grains was published in 1982 by Shiomi et al. The study
showed that KGF-C prevented the growth of Ehrlich carcinoma or
Sarcoma 180 when administered orally or peritoneally (13,14).
Later studies determined that KGF-C polysaccharide enhances the
immune system by affecting T- and not B-cell action (15). Additionally, studies have also
shown that Lewis lung and Ehrlich ascites carcinoma were inhibited
upon the oral administration of kefir (16,17).
Moreover, kefir produced from soy milk and cow’s milk considerably
repressed tumor growth in mice injected with Sarcoma 180 as
compared to unfermented milk. Microscopic observations suggested
that the tumor size was decreased due to apoptosis (6). Yet the mechanism of action behind
kefir’s anti-tumoral activity remained elusive.
Recently, in vitro studies were performed to
determine whether kefir has any direct effect on cancer cells.
Cell-free kefir fractions exhibited an anti-proliferative effect on
human mammary cancer cells (MCF-7) but did not affect normal
mammary epithelial cells (11).
Similarly, previous studies-done in our laboratory showed that
cell-free kefir fractions have anti-proliferative and pro-apoptotic
effects on human T-lymphotropic virus type I (HTLV-1) positive and
HTLV-1 negative T-lymphocytes but did not exert any effect on
lymphocytes removed from the peripheral blood of healthy
individuals (18,19). Among the few studies that examine
the anti-tumoral activity of kefir, only one study discussed the
anti-metastatic ability of kefir on cancer cells in vivo. A
study in 2000 by Furukawa et al showed that when the
water-soluble polysaccharide fraction from kefir grains is
administered orally either before or after tumor transplantation,
it inhibited the pulmonary metastasis of Lewis lung carcinoma.
Similarly, the water insoluble fraction of kefir grains was able to
prevent metastasis in mice injected with the highly metastatic B16
melanoma (20).
In 2013, the American Cancer Society ranked
colorectal cancer (CRC) as the third leading cause of
cancer-related mortalities in the US in both men and women. It was
estimated that by the end of 2013, >50,000 death cases will
arise due to CRC (21). The
sequence of events leading to the formation of CRC takes place over
a period of 10–15 years allowing sufficient time for chances to
screen, properly interfere, and to potentially save the lives of
more patients (22,23). Significant successes have been
achieved through targeted therapy in the treatment of CRC (24–26).
Yet, by the time people are diagnosed with CRC, 25% of patients
would have already developed metastasis. Among these patients with
metastatic CRC, 50% will survive (27). Whether it initially exists during
diagnosis, or occurs during treatment or relapse, metastasis
remains the primary cause of death in cancer patients (28,29).
Targeting any step in the metastatic process such as motility or
invasion can limit the metastatic ability of cancer cells and
retain them in their primary location.
The current study aims at examining the effect of
cell-free fractions of kefir on the viability, proliferation,
apoptosis and motility of CRC cells in vitro, and
determining the underlying mechanism of action.
Materials and methods
Cell line and cell culture
Human colorectal adenocarcinoma cell lines (Caco-2
and HT-29) and human breast cancer cell lines (MCF-7 and
MDA-MB231), obtained from ATCC (American Type Culture Collection),
were cultured in DMEM medium supplemented with 10% FBS and 100 U
penicillin/streptomycin at 37°C and 5% CO2 in a
humidified chamber.
Antibodies and reagents
Mouse monoclonal IgG anti-β-actin, anti-Bax,
anti-Bcl2, anti-p53, anti MMP-2, and anti-MMP-9 antibodies and
rabbit polyclonal anti-p21 were obtained from Santa Cruz
Biotechnology, Inc. Anti-mouse and anti-rabbit IgG HRP-conjugated
secondary antibodies were obtained from Promega.
Preparation of kefir cell-free fraction
and milk
Pasteurized skimmed milk (150 ml) was inoculated
with kefir grains (50 g). Inoculated milk samples were incubated at
20°C for 24 h in a sealed-glass container. At the end of
fermentation, the milk was strained to remove the kefir grains. The
yeast and bacteria in the filtrate were removed by centrifugation
(35,000 rpm for 10 min at 4°C). The supernatant was stored at −20°C
until needed for treatment of cells. On the day of treatment, the
kefir supernatant was thawed and then passed through a 0.22-μm
filter (Millipore). This cell-free fraction of fermented milk, also
termed kefir, was applied directly to the cells in different
volumes to establish the different concentrations required.
Non-inoculated milk samples were similarly prepared
but passed consecutively through a 0.45-μm then 0.22-μm filter
(Millipore).
Cytotoxicity: trypan blue exclusion
method
HT-29 and Caco-2 cells were grown in 24-well plates
(growth area: 2 cm2) at a density of 2×106
cells/ml. Cells were treated with milk and kefir at the following
concentrations (v/v): 0, 5, 10, 15, and 20%. After 24, 48, and 72
h, the supernatant from each well was collected, cells were washed
with phosphate-buffered saline (PBS), and the washes were added to
the supernatant of each well. Cells were then trypsinised and
collected separately from the well contents and PBS. From each
collection tube 20 μl were mixed with 20 μl of trypan blue
(Sigma-Aldrich). This mixture was placed in a counting chamber
under the microscope and the percentage of viable cells was
reported.
Proliferation: cell proliferation reagent
(WST-1)
HT-29 and Caco-2 cells were seeded in 96-well plates
(growth area: 0.6 cm2) at a concentration of
1×106 cells/ml. After 24 h of seeding, cells were
treated with 0, 5, 10, 15, and 20% milk and kefir (v/v). For every
milk and kefir concentration, a blank well was prepared, containing
only media and the corresponding volume of kefir or milk. After 24,
48, and 72 h, 10 μl of cell proliferation reagent (WST-1; Roche)
was added to each well. The plates were put in a humidified
incubator (37°C) for 1.5 h. Absorbance was then read at 450 nm. The
absorbance of the each blank well was subtracted from the
corresponding sample well. The results were normalized to the
untreated controls, and the percent proliferation was reported.
Cell cycle analysis: flow cytometry
Caco-2 and HT-29 cells were seeded in 6-well plates
(growth area: 9.5 cm2). Treatment was done for 24 h,
with 10% milk and kefir. After treatment, cells were trypsinized
and detached, then centrifuged at 1,200 rpm at 5°C for 5 min. The
pellet was washed in 1 ml of ice-cold PBS, centrifuged, and
resuspended again in 1 ml of ice-cold PBS. Ethanol was then added
to a final concentration of 70%. The fixed cells were left
overnight at 40°C. The following day, cells were centrifuged and
washed with PBS. The pellet was resuspended in 500 μl of binding
buffer, and then 10 μl of propidium iodide (PI) was added to each
sample. The samples were incubated in the dark for 10 min.
Cells were analyzed using an Accuri C6 flow
cytometer (Accuri Cytometers Inc.), which indicated the
distribution of the cells into their respective cell cycle phases
based on their DNA content. Cell DNA content was determined by
CFlow® software. An increase in cells in the pre-G phase
is indicative of an increase in cell death. The percentage of cells
in the sub-G0/G1 phase was compared to that of the control.
Cell death ELISA
HT-29 and Caco-2 cells were grown in 96-well plates
(growth area: 0.6 cm2) at 1×105 cells/ml.
After 24 h, cells were treated with relative concentrations (v/v):
0, 5, 10, and 15%. After 24 or 48 h, cells were lysed with lysis
buffer, and incubated for 30 min at room temperature. The plates
were then centrifuged for 10 min at 200 g. The supernatant (20 μl)
was placed in streptavidin-coated microtiter plates, followed by
the addition of biotin-labeled anti-histone and
peroxidase-conjugated anti-DNA antibodies. The anti-histone
antibody, bound to the plate via biotin-streptavidin, also bound
histones from released nucleosomes. The plate was then incubated at
room temperature for 2 h, after which
2,2′-azino-di[3-ethylbenzthiazolin-sulfonate] (ABTS) was added as a
substrate for peroxidase enzyme. Enrichment factor (EF) was
calculated as the recorded absorbance of each sample, divided by
that of the untreated cells, according to manufacturer’s
instructions (Roche).
Western blotting
Cell lysates were prepared by scraping the cells in
a sample buffer consisting of 4% SDS, 10% β-mercaptoethanol, 20%
glycerol, 0.004% bromophenol blue, and 0.125 M Tris-HCl at pH 6.8.
The resulting lysates were boiled for 5 min. Protein samples were
separated by SDS-PAGE on 10% (for β-actin, p53, Mmp-2, and Mmp-9)
or 12% (for Bax, Bcl-2, and p21) gels and transferred to PVDF
membranes overnight at 30 V. The membranes were then blocked with
5% BSA in PBS containing 0.1% Tween-20 for 1 h at room temperature
and incubated with primary antibody at a concentration of 1:1,000
for 2 h at room temperature. After the incubation with the primary
antibody, the membranes were washed and incubated with secondary
antibody at a concentration of 1:1,000 for 1 h at room temperature.
The membranes were then washed, and the bands visualized by
treating the membranes with western blotting enhanced
chemiluminescent reagent ECL (GE Healthcare). The results were
obtained on X-ray film (Agfa Healthcare). The levels of protein
expression were compared by densitometry using the ImageJ
software.
Reverse-transcription PCR
Cells were grown in 6-well plate at density of
1×106 cells/ml. After 24 h, cells were treated with 0,
5, and 10% cell-free fractions of kefir for 24 h, after which total
RNA was extracted using RNeasy extraction kit (Qiagen) according to
manufacturer’s instruction. Reverse transcriptase-polymerase chain
reaction (RT-PCR) was used to amplify RNA of β-actin (ACTB),
transforming growth factor α (TGF-α) and transforming growth
factor-β1 (TGF-β1). RNA (2 μg) was converted to cDNA using
the OneStep RT-PCR kit (Qiagen) as described by the manufacturer.
All gene-specific primers designed to detect cDNA were obtained
from Sigma-Aldrich. Primer sequences used were: β-actin, forward:
5′-ATGAAGATCCTGACCGAGCGT-3′, and reverse:
5′-AACGCAGCTCAGTAACAGTCCG-3′; TGF-α, forward:
5′-ATGTTGTTCCCTGCAAGTCC-3′, and reverse:
5′-ACTATGGAGAGGGGTCGCTT-3′; TGF-β, forward:
5′-GAAGTCACCCGCGTGCTAATGG-3′, and reverse:
5′-GGATGTAAACCTCGGACCTGTGTG-3′. The RT-PCR reagents and thermal
cycler conditions were used according to manufacturer’s instruction
with an annealing temperature of 52°C for β-actin, 48°C for TGF-α
and 50°C for TGF-β1 for 1 min. From the PCR product, 10 μl
were run on 0.8% agarose gel stained with ethidium bromide at 100 V
for 30 min. The resulting bands were visualized under UV light and
photographed. β-actin was used as a loading control.
Wound healing
Cells were grown to confluence on culture plates and
a wound was made in the monolayer with a sterile pipette tip. After
wounding, the cells were washed twice with PBS to remove debris and
fresh medium was added. Phase-contrast images of the wounded area
were taken at 0 and 21 or 24 h after wounding. Wound widths were
measured at 11 different points for each wound, and the average
rate of wound closure was calculated in μm/h using ImageJ
software.
Motility assay
For motility analysis, images of cells moving
randomly in serum were collected every 60 sec for 2 h using a 20×
objective. During imaging, the temperature was controlled using a
Nikon heating stage which was set at 37°C. The medium was buffered
using HEPES and overlayed with mineral oil. The speed of cell
movement was quantified using the ROI tracker plugin in ImageJ
software, which was used to calculate the total distance travelled
by individual cells. The speed was then calculated by dividing this
distance by the time (120 min) and reported in μm/min. The speed of
at least 10 cells for each condition was calculated.
Invasion assay
Cells were grown in 6-well plates. After 24 h, cells
were treated with 0, 5, and 10% of kefir for another 24 h. Invasion
assay was performed following treatment period using the
collagen-based invasion assay (Millipore) according to
manufacturer’s instructions. Briefly, 24 h prior to assay, cells
were starved with serum free medium. Cells were harvested,
centrifuged and then resuspended in quenching medium (serum free).
Cells were then brought to a concentration of 1×106
cells/ml. Inserts were rehydrated with 300 μl of serum free medium
for 30 min at room temperature, 250 μl of media was then removed
from inserts and 250 μl of cell suspension was added. Inserts were
then placed in a 24-well plate, and 500 μl of complete media was
added to the lower wells. Plates were incubated for 24 h at 37°C in
a CO2 incubator. Later, inserts were stained for 20 min
at room temperature with 400 μl of cell stain provided with the
kit. Stain was then extracted with extraction buffer. The extracted
stain (100 μl) was then used for colorimetric measurement using a
plate reader. Optical density was measured at 560 μm.
Statistical analysis
All reported results represent average values from
three independent experiments. The error estimates are given as
mean ± SEM. The p-values were calculated by t-tests or
χ2 tests depending on the experiment, using the
VassarStats: Website for Statistical Computation (http://vassarstats.net/).
Results
Kefir treatment reduces the viability of
CRC cells
To assess kefir’s cytotoxicity on the two cell
lines, we began by determining the percentage viability after
treating the cells with increasing kefir concentrations. Upon kefir
treatment, the viability of the cells decreased in a time- and
dose-dependent manner.
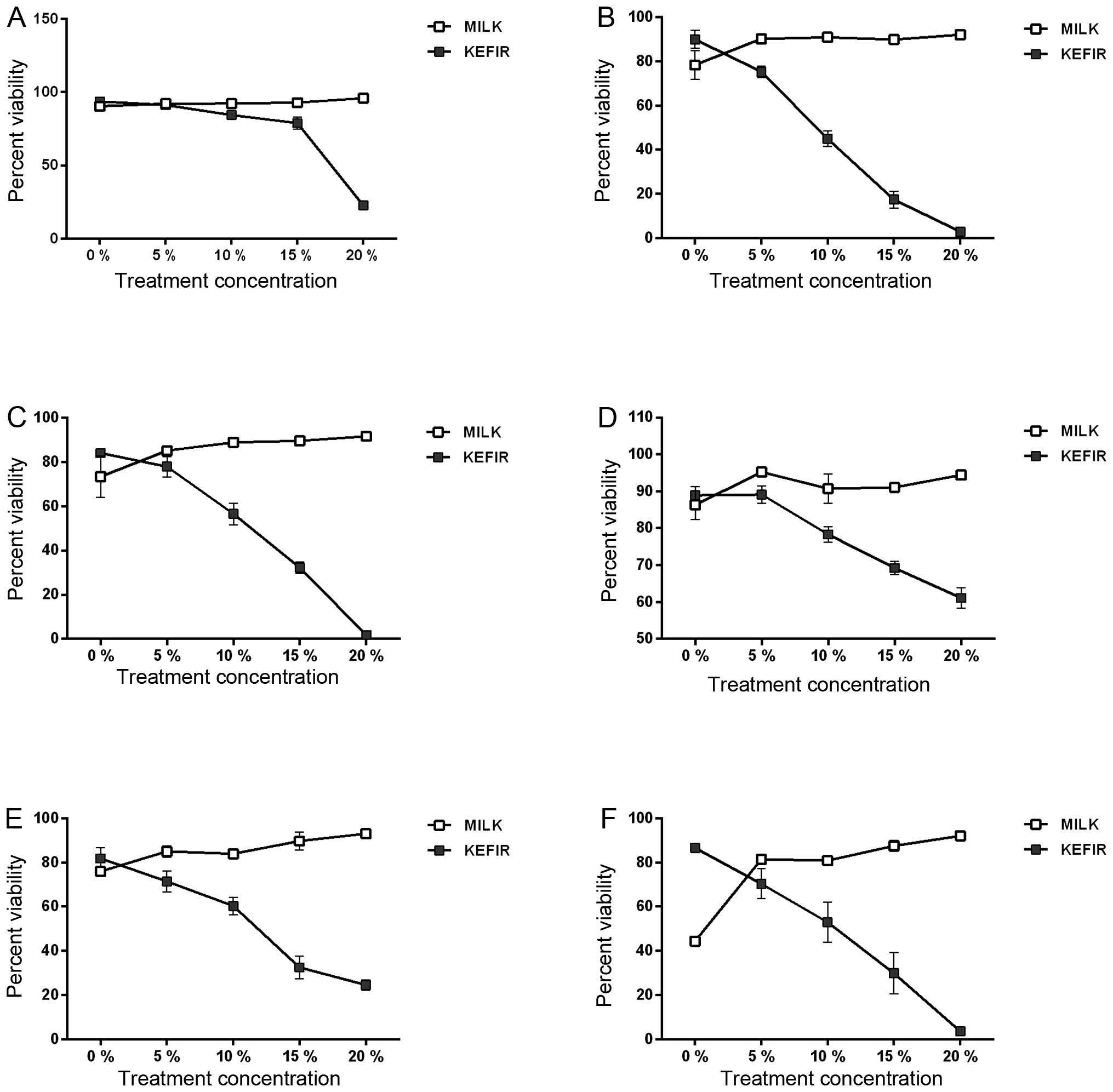
Results demonstrated that kefir’s inhibitory
concentration 50 (IC50) for Caco-2 cells ranges between 10, 12, and
18% (v/v) at 72, 48, and 24 h, respectively (Fig. 1A–C). For HT-29 cells, the IC50 was
only reached at 48 and 72 h of treatment, where it was determined
to be 12 and 10%, respectively (Fig.
1D–F). Past the IC 50, the viability of both cell lines
decreased significantly (p<0.05). Cytotoxicity levels were shown
to be dose- and time-dependent. The viability of both cell lines,
24, 48, and 72 h after treatment with various milk concentrations
was not reduced, but rather, a slight increase in percentage
viability was detected, significant compared to kefir (p<0.05)
(Fig. 1A–F).
The viability of both cell lines was not reduced 6 h
post-treatment (data not shown), suggesting that the cells were
dying through apoptosis rather than necrosis.
Kefir treatment reduces proliferation of
Caco-2 and HT-29 cells
The effect of kefir on proliferation of CRC cells
was determined by the activity of mitochondrial dehydrogenases.
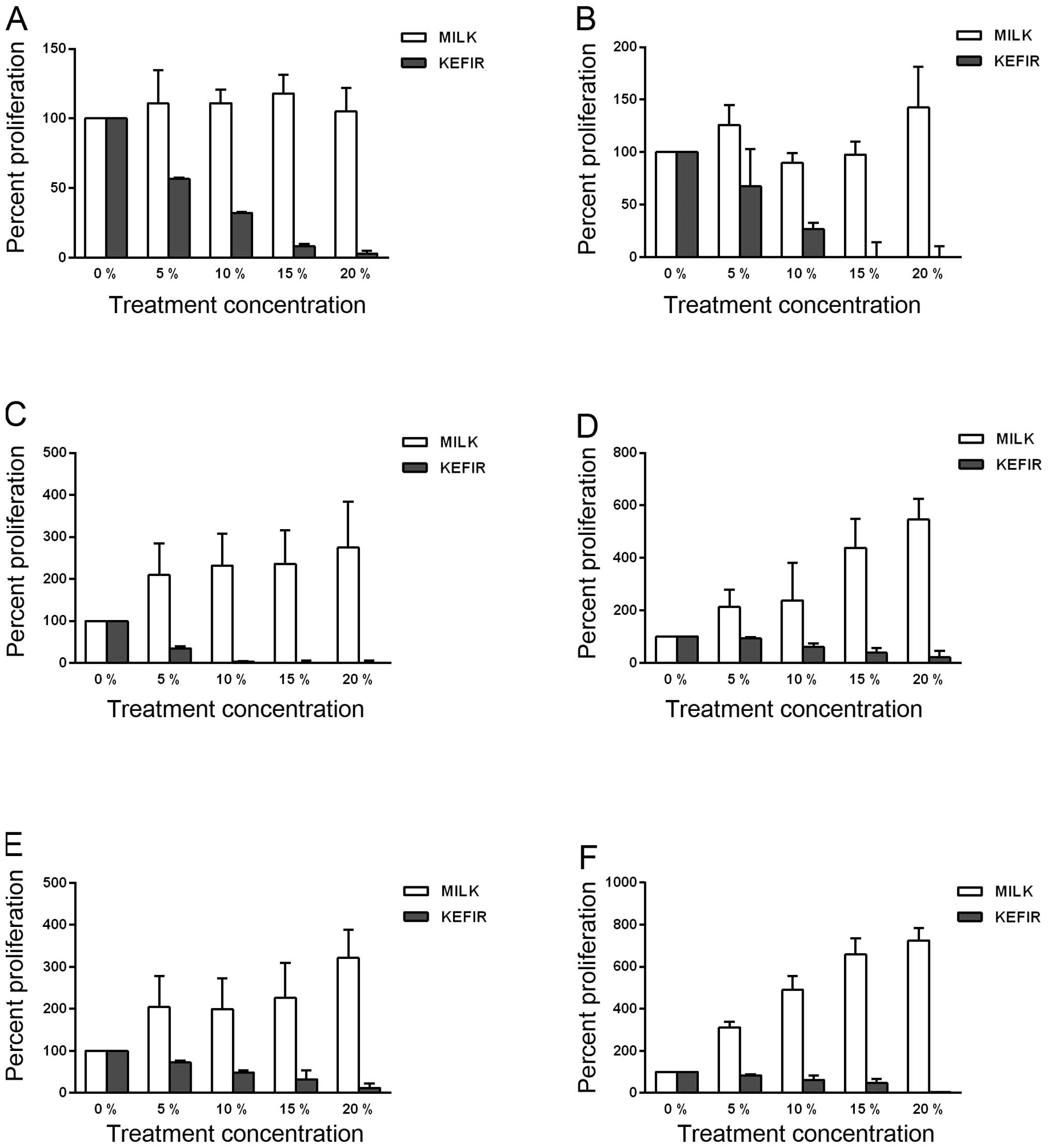
Results have shown that kefir significantly inhibited the
proliferation of HT-29 and Caco-2 cells (p<0.05) (Fig. 2A–F). At the kefir concentrations
where the IC50 was calculated in the trypan blue exclusion method
with Caco-2 cells, the recorded decrease in proliferation was 95%
at 24 h, 94% at 48 h, and 97% at 72 h compared to untreated cells
(Fig. 2A–C).
HT-29 cells as well showed significant inhibition of
proliferation upon kefir treatment, even though the effect was
slightly less than that exhibited by the Caco-2 cells (Fig. 2D–F). At the concentrations
corresponding to the IC50, the percent decrease was calculated to
be 60% at 48 h, and 38% at 72 h (Fig.
2E and F).
All cells treated with milk showed a significant
increase in proliferation, compared to the untreated and
kefir-treated cells (p<0.05). Hence, kefir treatment
significantly reduces proliferation of CRC cell lines in a time-
and dose-dependent manner.
Kefir induces cell cycle arrest at the G1
phase
After verifying that kefir inhibited cell
proliferation in CRC cells, we aimed to evaluate whether this
effect was through an induction of cell cycle arrest, using flow
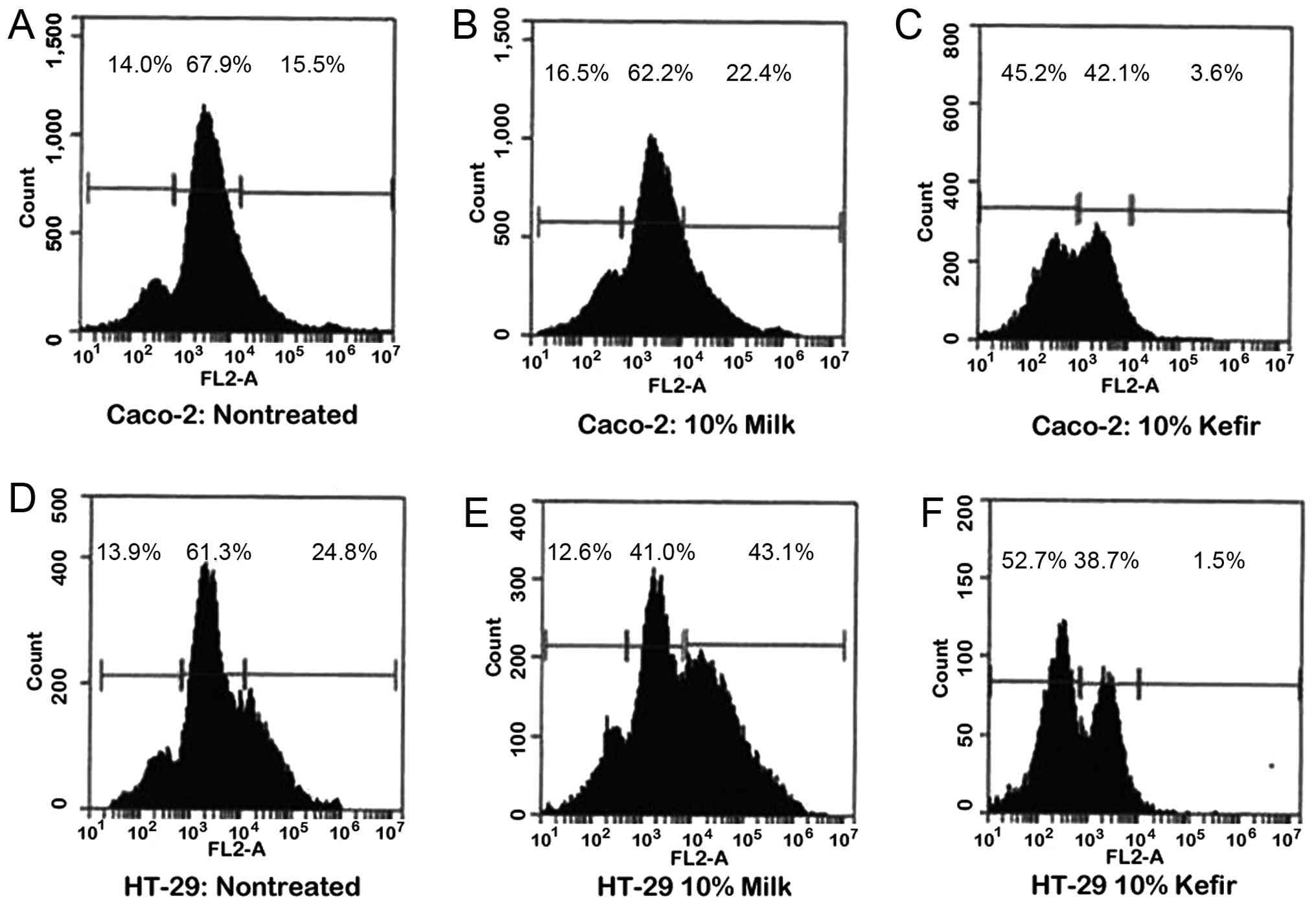
cytometry. After analyzing the cell’s DNA content, cells were
assigned to their respective phases: sub-G 0/G1 cells were <2n,
G0/G1 cells were 2n, and S/M phase cells were >2n.
Consistent with the results of the proliferation
assay, the sub-G0/G1 population of Caco-2 cells increased from 14
to 5.2% as a result of 10% kefir treatment, while the S/M phase
cells decreased from 15.5 to 3.6% (Fig. 3A and C). The same pattern of cell
cycle shift was seen upon treatment of HT-29 cells with 10% kefir.
The sub-G0/G1 population increased from 13.9 to 52.7%, accompanied
by a significant decrease in S/M phase population from 24.8 to 1.5%
(Fig. 3D and F). When these cells
were treated with 10% milk, a slight increase in sub-G0/G1
population and a noticeable increase in S/M were detected in both
Caco-2 and HT-29 cells (Fig. 3A, B, D
and E), significant compared to kefir treatment
(p<0.05).
It is thus implied that kefir causes a cell cycle
arrest at the G1 transition checkpoint which explains its
anti-proliferative effect.
Kefir has a pro-apoptotic effect on
Caco-2 and HT-29 cells
To verify if kefir reduce the viability of the CRC
cells through an induction of apoptosis, we used the cell death
detection ELISA assay, where the absorbance obtained at 405 nm
reflects the quantity of released nucleosomes, a hallmark of
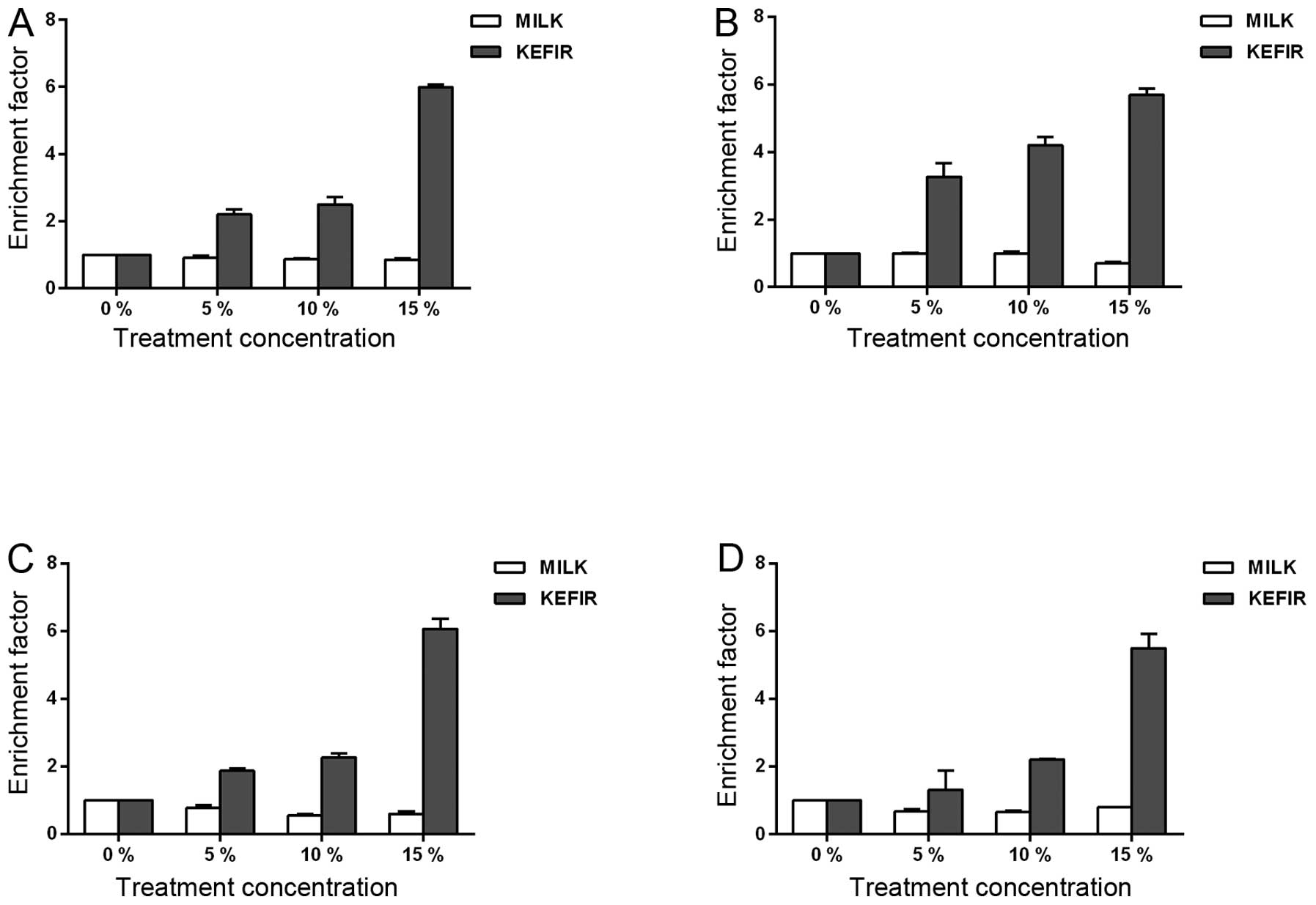
apoptosis. The EF, which is the ratio of the absorbance measured
for each concentration to that of the untreated controls, was
calculated. In Caco-2 kefir-treated cells, the EF increased around
2.3-, 2.6-, and 6-fold, 24 h after treatment with 5, 10, and 15%
kefir, respectively (Fig. 4A).
Upon 48 h of treatment, the calculated EF showed a 3.5-, 4-, and
5.6-fold increase with 5, 10, and 15% kefir, respectively (Fig. 4B). HT-29 cells also showed a
significant increase in apoptosis induction upon kefir treatment.
In the 24-h kefir-treated HT-29 cells, the EFs were ~1.9, 2.4, and
6.3 upon treatment with 5, 10, and 15% kefir, respectively
(Fig. 4C). Similarly, after 48 h
of kefir treatment with 5, 10, and 15% kefir, the EFs were
determined to be 1.3, 2.2, and 5.5, respectively (Fig. 4D). Also, in both cell lines, milk
treatment induced a decrease in apoptosis as compared to the
untreated controls with an EF <1, significantly lower than kefir
(p<0.05) (Fig. 4A–D). The
results obtained confirm that the decreased viability resulting
from kefir treatment involves the induction of apoptosis.
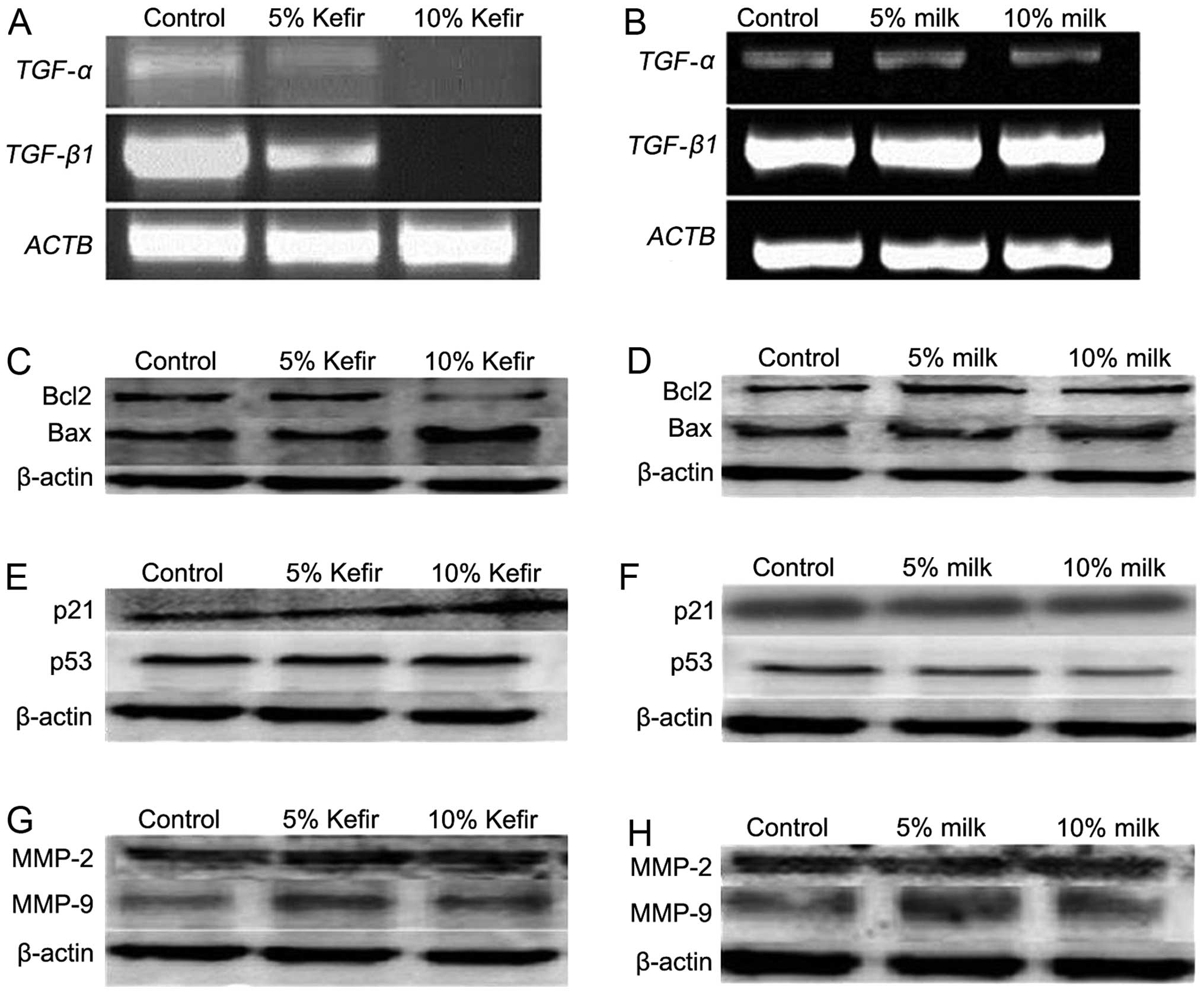
Kefir modulates the expression of genes
involved in proliferation and apoptosis
In order to determine a possible mechanism for the
anti-proliferative effect of kefir observed in the CRC cells with
the WST-1 assay, the expression of TGF-α and TGF-β1
was assessed at the mRNA level using RT-PCR in HT-29 cells. The
results showed a significant decrease in the expression of both
cytokines in a dose-dependent manner upon kefir treatment in HT-29
cells for 24 h (Fig. 5A),
consistent with the observed reduction in proliferation. The
expression of both cytokines did not change in milk-treated cells
(Fig. 5B). To examine the
mechanism behind kefir’s pro-apoptotic effect on CRC cells, the
expression of Bax, Bcl-2, p53, and p21 was assessed at the protein
level using western blotting in HT-29 cells. Consistent with the
induction of apoptosis seen with the cell death assay, we observed
an increase in the Bax:Bcl-2 ratio upon kefir treatment, while a
slight decrease in this ratio was observed upon milk treatment
(Fig. 5C and D). Furthermore,
results showed no significant increase in the expression of p53 in
kefir-treated cells, yet p21 levels showed an increase upon
treatment with 10% kefir (Fig.
5E). For milk-treated cells, the expression of p53 and p21 does
not significantly vary between control and treated cells (Fig. 5F). In addition, we examined the
expression of matrix metalloproteinases MMP-2 and MMP-9 in treated
HT-29 cells and observed that it was unaffected by either kefir or
milk treatment (Fig. 5G and H).
These results suggest that kefir’s anti-proliferative and
pro-apoptotic effects involve a reduction in TGF-α and
TGF-β1, and an upregulation in the Bax:Bcl-2 ratio as well
as p53-independent p21 expression, respectively, with no effect on
the levels of MMPs.
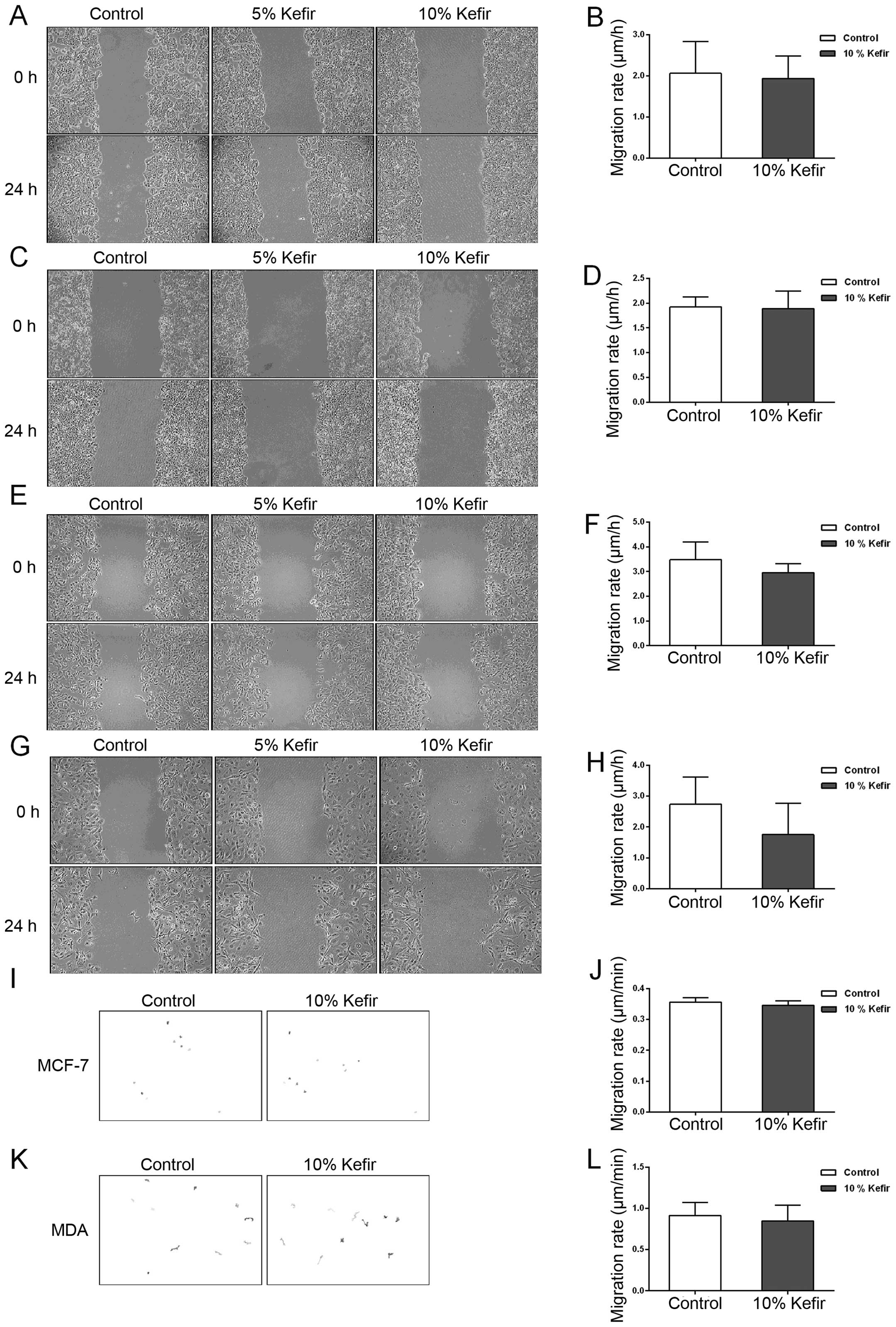
Kefir treatment does not affect the
motility of colon and breast cancer cell lines
The effect of kefir on the metastatic ability of
cancer cells was assessed in a single study, in vivo, by
Furukawa et al (20).
Therefore, we aimed to determine whether there is a direct effect
on the motility of cancer cells in vitro upon kefir
treatment. Using wound-healing analysis, we observed no significant
difference in the migration rate of control and kefir-treated
colorectal cells (Caco-2 and HT-29) after 24 h of treatment
(p>0.05) (Fig. 6A–D). We thus
decided to explore whether kefir might induce an effect on the
motility of other cancer types in vitro. For that purpose,
two breast cancer cell lines, MCF-7, whose proliferation has
previously been shown to be reduced by kefir treatment by Chen
et al (11), and MDA-MB-231
were used. In both of these cell lines, we observed a slight but
non-significant (p>0.05) decrease in the migration ability
between control and treated cells (Fig. 6E–H). Kefir’s effect on the
migration ability of both colorectal and breast cancer cell lines
was also assessed using time-lapse movies. Consistent with the
results of the wound-healing assay, we observed a slight decrease
in the migration ability of both cell lines upon kefir treatment,
yet the decrease was non-significant (Fig. 6I–L).
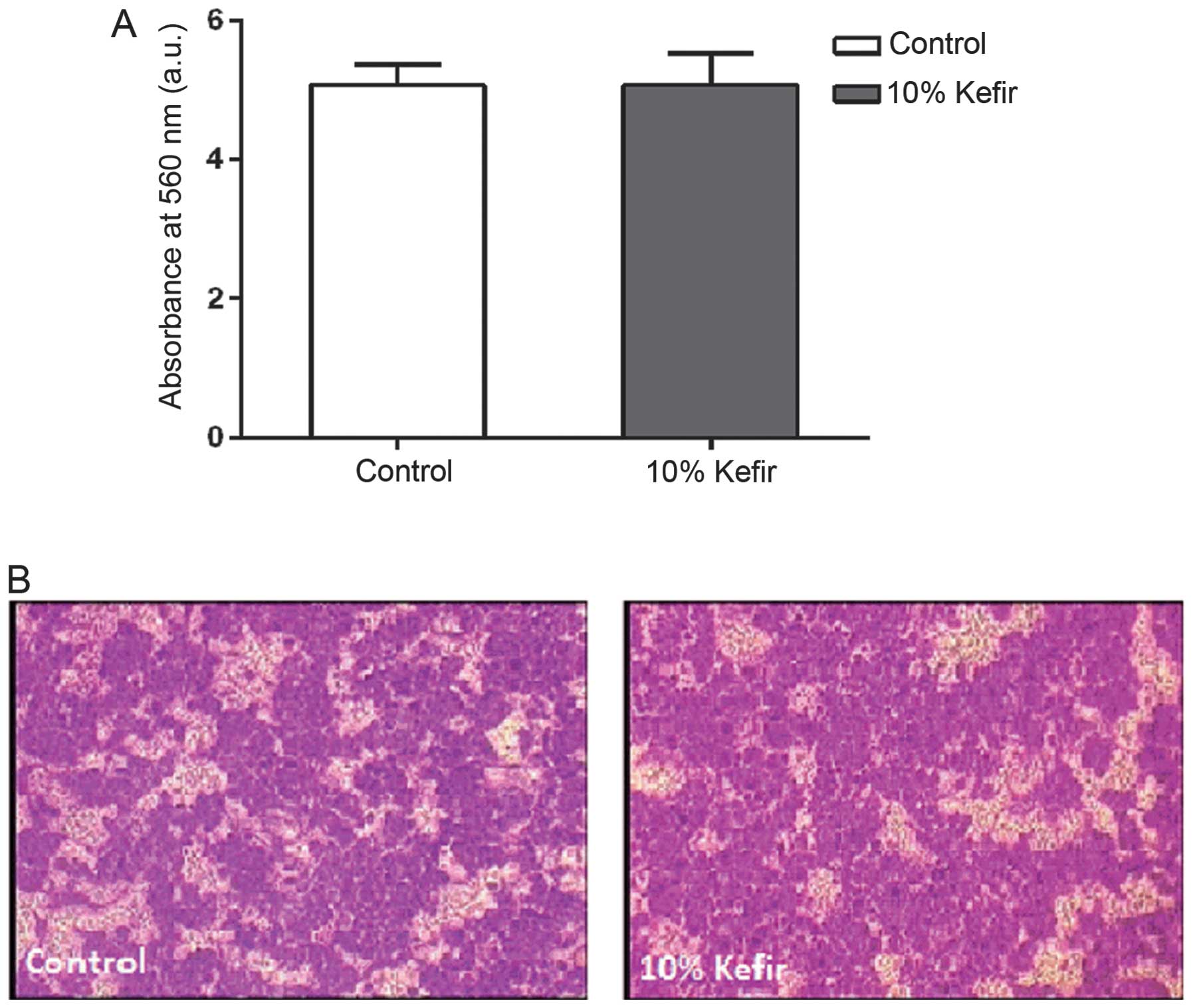
In vitro invasion of HT-29 cells is not
affected by kefir treatment
After looking at the motility of colorectal and
breast cancer cell lines in two-dimensions and observing no effect
upon kefir treatment, we decided to look at whether kefir has any
effect on the invasive ability of the CRC HT-29 cells. Using the
collagen-based invasion assay, we observed no significant
difference in the invasive ability of HT-29 cell lines between
control and 10% kefir-treated cells in vitro (p>0.05)
(Fig. 7).
Discussion
In the present study, we aimed to investigate
whether kefir’s anti-cancerous effect, previously proven on several
types of cancers, both in vivo and in vitro, also
applies to CRC cell lines. The results obtained are in accordance
with previous study on breast and leukemic cancer cell lines,
showing an anti-proliferative and pro-apoptotic effect of kefir on
these cells (11,18,19).
We first determined that the IC50 is reached with
18, 12, and 10% v/v (kefir cell-free fractions) at 24, 48, and 72 h
respectively, for Caco-2 cells, compared to 12 and 10% at 48 and 72
h, respectively, for HT-29 cells. For both cell lines, treatment
with kefir reduced viability in a time- and dose-dependent manner.
Since 6 h post-treatment, the viability of the cell lines was not
reduced (data not shown), it was assumed that the cell numbers were
reduced due to cell death. The observation that the milk-treated
cells showed no decrease in viability suggests that kefir’s effect
is due to products released by the microorganisms during
fermentation.
A notable decrease in proliferation upon kefir
treatment was detected in the cells using the WST-1 reagent. Caco-2
cells exhibited a 91, 74, and 96% decrease in proliferative
activity upon treatment with 18, 12, and 10% at 24, 48, and 72 h,
respectively (IC50 concentrations). For HT-29 cells, treatment with
IC50 concentrations (12 and 10% at 48 and 72 h, respectively),
caused 64 and 40% decrease. As expected, milk-treated cells results
showed an increase in proliferation. Through flow cytometry, it was
verified that kefir, but not milk, causes a shift in the cell cycle
of the treated cells towards the sub-G0/G1 phase, by inducing cell
cycle arrest at the G1 checkpoint. Kefir did not only increase the
percentage of cells in the sub-G0/G1 phase, but also caused a
reduction in the S/M cell population as well. The increase in
sub-G0/G1 implies that kefir induces death in CRC cell lines. To
verify that the cells were dying through apoptosis and not
necrosis, as assumed, the cell death ELISA kit assay was performed.
The results of this assay confirmed that kefir was indeed inducing
apoptosis in Caco-2 and HT-29 cells.
TGF-α is a known mitogen whose expression is
upregulated in many types of tumors, especially CRC, where its
expression exceeds four times that of normal colorectal tissues
(9,31). TGF-α expression was
downregulated in HT-29 cells, dose-dependently, upon treatment with
non-cytotoxic doses of kefir (5 and 10%), but not milk. These
results are consistent with the observed decrease in proliferation
of HT-29 and consistent with a previous study (19). Moreover, given the fact that TGF-α
also plays a significant role in invasion and metastasis in
vivo, this further shows the importance of targeting it, not
only to inhibit cancer growth, but also invasion and metastasis
(30,32–34).
A downregulation in the expression level of
TGF-β1 was also observed in HT-29 cells upon treatment with
kefir but not milk. This observation was at first perplexing, as it
is not in accordance with a previous study (19). TGF-β1 has long been assumed to act
as a tumor suppressor yet many studies have shown data conflicting
with its known role and showed that the response of a cell to
TGF-β1 is context dependent (35).
It has been previously reported that TGF-β1 is able to stimulate
the growth of HT-29 cells (36).
Therefore, the observed decrease in its expression obtained is
consistent with the decrease in proliferation upon kefir treatment.
In CRC, TGF-β1 suppresses immune system cells in the
microenvironment, recruits cells that enhance invasion, and
stimulates angiogenesis (37–39).
Thus, our data might be of great significance given the fact that
drugs targeting the TGF-β signaling pathway, especially by
inhibiting the expression of TGF-β have now reached phase III
clinical trials (40,41).
To determine a possible explanation for the
pro-apoptotic effect of kefir, the expression of Bax and Bcl-2 at
the protein level was assessed. Our findings show upregulation in
Bax:Bcl-2 expression in HT-29 cells, consistent with the observed
increase in apoptosis using the cell death ELISA assay. For the
milk-treated HT-29 cells, a decrease in Bax:Bcl-2 ratio was
observed which is also in accordance with the decrease in apoptosis
and increase in proliferation observed for milk-treated cells.
To further elucidate the mechanism of action of
kefir, the expression levels of p53 and p21 proteins were assessed.
Kefir treatment at 5 and 10% (v/v) caused no difference in p53
levels but a noticeable increase in p21 levels when treated with
10% kefir. This increase in p21 levels might explain the cell cycle
arrest observed at the G1 phase through cell cycle analysis by flow
cytometry. Our data suggest that p21 induction is
p53-independent.
Since metastasis remains the main cause of death in
cancer patients, we assessed the effect of cell-free fractions of
kefir on the motility of CRC cells in vitro (29). Using wound-healing analysis, no
difference in motility between the control and 10% kefir-treated
HT-29 and Caco-2 cells was observed, in contrast to a previous
study showing that kefir inhibits metastasis of Lewis lung
carcinoma and B16 melanoma in vivo (20). The effect of cell-free fractions of
kefir was then assessed on weakly metastatic MCF-7 and highly
metastatic MDA-MB-231 breast cancer cell lines. Using both
time-lapse movies and wound-healing assays, we noted a
non-significant decrease in the motility of these cells. Yet
metastasis does not depend only on the motility of the cells, but
also involves invasion of the microenvironment, intravasation into
vessels, survival in the blood or lymph, extravasion, and
colonization at secondary sites (42). Therefore, we decided to look at the
effect of kefir on invasion using a collagen-based in vitro
assay. A decrease in the invasive ability of HT-29 cells was
expected since downregulation in TGF-α and TGF-β1, both of which
play an important role in enhancing invasion of cancer cells, was
observed upon kefir treatment (34–37).
However, results showed no difference in the invasive ability
between control and 10% treated cells. Also, the expression of the
matrix metalloproteinases MMP-2 and MMP-9, which are required for
the degradation of the extracellular matrix, were not altered
between control and kefir-treated cells at the protein level. Our
data do not rule out the fact that kefir could affect metastasis
since it might exhibit an indirect effect inhibiting metastasis
in vivo through modulating the immune system or the
microenvironment of the cells. Another hypothesis could be that
kefir might be intervening in other processes involved in
metastasis besides motility and invasion. A third possibility is
that components found in kefir might be metabolized in vivo
leading to the formation of active compounds which are able to
inhibit metastasis.
In conclusion, kefir has become globally known as a
complex probiotic, to which many health benefits have been
attributed. These include anti-microbial, anti-inflammatory,
immunomodulatory, and metabolic benefits. This study focused on
assessing kefir’s anti-cancerous potential. Through several
experiments, we have established that kefir exhibits pro-apoptotic
and anti-proliferative properties on colorectal adenocarcinoma
cells, namely Caco-2 and HT-29, in vitro. It was also
demonstrated that kefir causes cell cycle arrest at G1 phase. The
results of our experiments, which were correspondingly performed
with milk treatment, affirm that kefir’s beneficial effects are due
to products produced by the microorganisms during fermentation.
This study is the first to elucidate a potential mechanism of
action for kefir’s effect on CRC in vitro. The
downregulation in the expression of TGF-α and TGF-β1
explain the decrease in the proliferation of HT-29 cells in
vitro. Also the observed overexpression of p21, which was seen
to be p53-independent, could be the reason for the cell cycle
arrest at the G1 phase observed upon kefir treatment. Moreover, we
report that the ratio of Bax:Bcl-2 increases upon kefir treatment
consistent with the increase in apoptosis induced by kefir. On the
other hand, the effect of kefir on the motility and invasion of CRC
cell lines is insignificant, but this does not rule out the fact
that kefir could affect the metastatic ability of cancer cells.
Kefir’s effect on metastasis could be by modulating other steps in
the metastatic process or that its effect can only be detected
in vivo. Future study will focus on confirming the effect of
kefir on the growth of CRC in vivo, and assessing its effect
on the metastasis through the intra-splenic injection of HT-29 that
causes liver metastasis.
Acknowledgements
ROI tracker software was supplied by David Entenberg
and John Condeelis as supported by CA100324 and GM064346. This
study was partially funded by the National Council for Scientific
Research-Lebanon, and the Univeristy Research Council-Lebanese
American University.
Abbreviations:
|
CRC
|
colorectal cancer
|
|
RT-PCR
|
reverse transcriptase-polymerase chain
reaction
|
|
HTLV-1
|
human T-lymphotropic virus type I
|
|
EGFR
|
epidermal growth factor receptor
|
|
TGF-α
|
transforming growth factor-α
|
|
TGF-β1
|
transforming growth factor-β1
|
|
IC50
|
inhibitory concentration 50
|
|
EF
|
enrichment factor
|
References
|
1
|
Adriana P and Socaciu C: Probiotic
activity of mixed cultures of kefir’s lactobacilli and non-lactose
fermenting yeasts. Agric. 65:329–334. 2008.
|
|
2
|
Farnworth ER: Kefir - a complex probiotic.
Food Sci Technol Bull. 2:1–17. 2005.
|
|
3
|
Angulo L, Lopez E and Lema C: Microflora
present in kefir grains of the Galician region (north-west of
Spain). J Dairy Res. 60:263–267. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Lopitz-Otsoa F, Rementeria A, Elguezabal N
and Garaizar J: Kefir: a symbiotic yeasts-bacteria community with
alleged healthy capabilities. Rev Iberoam Micol. 23:67–74.
2006.PubMed/NCBI
|
|
5
|
Simova E, Simov Z, Beshkova D, Frengova G,
Dimitrov Z and Spasov Z: Amino acid profiles of lactic acid
bacteria, isolated from kefir grains and kefir starter made from
them. Int J Food Microbiol. 107:112–123. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Liu JR, Wang SY, Lin YY and Lin CW:
Antitumor activity of milk kefir and soy milk kefir in
tumor-bearing mice. Nutr Cancer. 44:182–187. 2002.PubMed/NCBI
|
|
7
|
Vinderola G, Perdigón G, Duarte J,
Farnworth E and Matar C: Effects of the oral administration of the
exopolysaccharide produced by Lactobacillus kefiranofaciens
on the gut mucosal immunity. Cytokine. 36:254–260. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Hertzler SR and Clancy SM: Kefir improves
lactose digestion and tolerance in adults with lactose
maldigestion. J Am Diet Assoc. 103:582–587. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Liu JR, Wang SY, Chen MJ, Chen HL, Yueh PY
and Lin CW: Hypocholesterolaemic effects of milk-kefir and
soyamilk-kefir in cholesterol-fed hamsters. Br J Nutr. 95:939–946.
2006. View Article : Google Scholar
|
|
10
|
Preidis GA, Hill C, Guerrant RL,
Ramakrishna BS, Tannock GW and Versalovic J: Probiotics, enteric
and diarrheal diseases, and global health. Gastroenterology.
140:8–14. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Chen C, Chan HM and Kubow S: Kefir
extracts suppress in vitro proliferation of estrogen-dependent
human breast cancer cells but not normal mammary epithelial cells.
J Med Food. 10:416–422. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Rodrigues KL, Caputo LR, Carvalho JC,
Evangelista J and Schneedorf JM: Antimicrobial and healing activity
of kefir and kefiran extract. Int J Antimicrob Agents. 25:404–408.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Shiomi M, Sasaki K, Murofushi M and Aibara
K: Antitumor activity in mice of orally administered polysaccharide
from Kefir grain. Jpn J Med Sci Biol. 35:75–80. 1982. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Murofushi M, Shiomi M and Aibara K: Effect
of orally administered polysaccharide from kefir grain on
delayed-type hypersensitivity and tumor growth in mice. Jpn J Med
Sci Biol. 36:49–53. 1983. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Murofushi M, Mizuguchi J, Aibara K and
Matuhasi T: Immunopotentiative effect of a polysaccharide from
kefir grain, KGF-C, administered orally in mice.
Immunopharmacology. 12:29–35. 1986. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Furukawa N, Matsuoka A and Yamanaka Y:
Effects of orally administered yoghurt and kefir on tumor growth in
mice. J Jpn Soc Nutr Food Sci. 43:450–453. 1990. View Article : Google Scholar
|
|
17
|
Kubo M, Odani T, Nakamura S, Tokumaru S
and Matsuda H: Pharmacological study on kefir - a fermented milk
product in Caucasus. I. On antitumor activity (1). Yakugaku Zasshi.
112:489–495. 1992.(In Japanese).
|
|
18
|
Rizk S, Maalouf K and Baydoun E: The
antiproliferative effect of kefir cell-free fraction on HuT-102
malignant T lymphocytes. Clin Lymphoma Myeloma. (Suppl 3):
S198–S203. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Maalouf K, Baydoun E and Rizk S: Kefir
induces cell-cycle arrest and apoptosis in HTLV-1-negative
malignant T-lymphocytes. Cancer Manag Res. 3:39–47. 2011.PubMed/NCBI
|
|
20
|
Furukawa N, Matsuoka A, Takahashi T and
Yamanaka Y: Anti-metastatic effect of kefir grain components on
Lewis lung carcinoma and highly metastatic B16 melanoma in mice. J
Agric Sci Tokyo Nogyo Daigaku. 45:62–70. 2000.
|
|
21
|
Siegel R, Naishadham D and Jemal A: Cancer
statistics, 2013. Cancer J Clin. 2013.63:11–30
|
|
22
|
Hamilton SR: The molecular genetics of
colorectal neoplasia. Gastroenterology. 105:3–7. 1993.PubMed/NCBI
|
|
23
|
Hoff G, Foerster A, Vatn MH, Sauar J and
Larsen S: Epidemiology of polyps in the rectum and colon. Recovery
and evaluation of unresected polyps 2 years after detection. Scand
J Gastroenterol. 21:853–862. 1986.PubMed/NCBI
|
|
24
|
Wolpin BM and Mayer RJ: Systemic treatment
of colorectal cancer. Gastroenterology. 134:1296–1310. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Messersmith WA and Hidalgo M: Panitumumab,
a monoclonal anti-epidermal growth factor receptor antibody in
colorectal cancer: another one or the one? Clin Cancer Res.
13:4664–4666. 2007. View Article : Google Scholar
|
|
26
|
Arteaga C: Targeting HER1/EGFR: a
molecular approach to cancer therapy. Semin Oncol. (3 Supp 7):
S3–S14. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Rodriguez-Bigas MA, Lin EH and Crane CH:
Stage IV colorectal cancer. Holland-Frei Cancer Medicine. Kufe DW,
Pollock RE, Weichselbaum RR, Bast RC, Gansler TS, Holland JF and
Frei E: 2. 6th edition. Hamilton, ON: 2003
|
|
28
|
Hassan MS, Ansari J, Spooner D and Hussain
SA: Chemotherapy for breast cancer (Review). Oncol Rep.
24:1121–1131. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Palmer TD, Ashby WJ, Lewis JD and Zijlstra
A: Targeting tumor cell motility to prevent metastasis. Adv Drug
Deliv Rev. 63:568–581. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Ji H, Zhao X, Yuza Y, Shimamura T, Li D,
Protopopov A, Jung BL, McNamara K, Xia H, Glatt KA, et al:
Epidermal growth factor receptor variant III mutations in lung
tumorigenesis and sensitivity to tyrosine kinase inhibitors. Proc
Natl Acad Sci USA. 103:7817–7822. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Liu C, Woo A and Tsao MS: Expression of
transforming growth factor-alpha in primary human colon and lung
carcinomas. Br J Cancer. 62:425–429. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Sasaki T, Nakamura T, Rebhun RB, Cheng H,
Hale KS, Tsan RZ, Fidler IJ and Langley RR: Modification of the
primary tumor microenvironment by transforming growth factor
alpha-epidermal growth factor receptor signaling promotes
metastasis in an orthotopic colon cancer model. Am J Pathol.
173:205–216. 2008. View Article : Google Scholar
|
|
33
|
Ueda M, Fujii H, Yoshizawa K, Terai Y,
Kumagai K, Ueki K and Ueki M: Effects of EGF and TGF-alpha on
invasion and proteinase expression of uterine cervical
adenocarcinoma OMC-4 cells. Invasion Metastasis. 18:176–183. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Hölting T, Siperstein AE, Clark OH and Duh
QY: Epidermal growth factor (EGF)-and transforming growth factor
alpha-stimulated invasion and growth of follicular thyroid cancer
cells can be blocked by antagonism to the EGF receptor and tyrosine
kinase in vitro. Eur J Endocrinol. 132:229–235. 1995.
|
|
35
|
Massagué J: TGFβ signalling in context.
Nat Rev Mol Cell Biol. 13:616–630. 2012.
|
|
36
|
Halder SK, Beauchamp RD and Datta PK: A
specific inhibitor of TGF-beta receptor kinase, SB-431542, as a
potent antitumor agent for human cancers. Neoplasia. 7:509–521.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Xiong B, Yuan HY, Hu MB, Zhang F, Wei ZZ,
Gong LL and Yang GL: Transforming growth factor-beta1 in invasion
and metastasis in colorectal cancer. World J Gastroenterol.
8:674–678. 2002.PubMed/NCBI
|
|
38
|
Thiery JP, Acloque H, Huang RY and Nieto
MA: Epithelial-mesenchymal transitions in development and disease.
Cell. 139:871–890. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Ramesh S, Wildey GM and Howe PH:
Transforming growth factor beta (TGFbeta)-induced apoptosis: the
rise and fall of Bim. Cell Cycle. 8:11–17. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Akhurst RJ and Hata A: Targeting the TGFβ
signalling pathway in disease. Nat Rev Drug Discov. 11:790–811.
2012.
|
|
41
|
Morris JC, Shapiro GI, Tan AR, Lawrence
DP, Olencki TE, Dezube BJ, Hsu FJ, Reiss M and Berzofsky JA: Phase
I/II study of GC1008: a human anti-transforming growth factor-beta
(TGFβ) monoclonal antibody (MAb) in patients with advanced
malignant melanoma (MM) or renal cell carcinoma (RCC). J Clin
Oncol. 26(Suppl 15): S90282008.
|
|
42
|
Fidler IJ: The pathogenesis of cancer
metastasis: the ‘seed and soil’ hypothesis revisited. Nat Rev
Cancer. 3:453–458. 2003.
|