Introduction
Lung cancer is one of the most common malignancies
worldwide. The high mortality rate is attributed to its early
metastasis, especially in cases of non-small cell lung carcinoma
(NSCLC) (1–3). Metastasis involves a series of
complex processes in which tumor cells invade other organs,
requiring the coordination of several signaling pathways that allow
the detachment of tumor cells, their mobility, degradation of the
extracellular matrix (ECM), invasion, migration, adhesion to
endothelial cells, and reestablishment of growth at a distant site
(4,5). ECM and matrix metalloproteinases
(MMPs) in humans have been identified as key factors involved in
these processes. The degradation of basement membrane and stromal
ECM is a crucial step for tumor invasion and metastasis (6). The MMP family of human zinc-dependent
endopeptidases is responsible for degradation of the ECM components
such as collagen, proteoglycan, fibronectin, elastin, and laminin
(7). Of those, gelatinases, MMP-2,
and MMP-9 are known to play a critical role in the degradation of
native collagen types IV and V (8). MMP-2 is activated on the cell surface
by a multimeric complex consisting of MMP-2, membrane type-1 MMP,
and tissue inhibitor of metalloproteinase-2 (TIMP-2), whereas
TIMP-1 is known as a specific inhibitor of MMP-9 (9). The expression of MMP genes is
primarily regulated through transcriptional factors such as
activator protein-1 (AP-1) and/or nuclear factor κB (NF-κB) via
mitogen-activated protein kinases (MAPKs) or phosphoinositide
3-kinase (PI3K)/protein kinase B (AKT) pathways (10–12).
Several studies on cyclooxygenase (COX)-2 in cancers indicated that
this enzyme stimulated tumor growth, invasion and metastasis in
association with MMPs (13). COX-2
is an inducible isoform of cyclooxygenase that participates in
pro-inflammatory responses to certain stimuli such as mitogens,
cytokines and growth factors (14,15).
These studies revealed that MMPs and their regulatory pathways
might be promising targets for anti-metastatic and chemotherapeutic
therapy.
Mushrooms have been used to treat various diseases
including tumors. Inonotus obliquus, a traditional medicinal
mushroom, has been widely used to promote health and longevity in
humans. Many studies have suggested that polysaccharides from
basidiomycetes mushrooms exhibited highly beneficial therapeutic
effects including: i) direct antitumor activity against various
tumors, ii) synergistic antitumor activity in combination with
chemotherapy, and iii) preventive effects on tumor metastasis
(16–20). However, the anti-metastatic effect
and its underlying mechanistic signaling pathways of
polysaccharides from fruit body of Inonotus obliquus (PFIO)
in human NSCLC remain unknown. Therefore, the present study aimed
to investigate the anti-metastatic effects and potential signaling
pathways of PFIO in the highly metastatic human NSCLC A549 cells
in vitro.
Materials and methods
Preparation of polysaccharides from
Inonotus obliquus
Dried fruiting bodies of Inonotus obliquus
were purchased from a local market and ground in a blender. Milled
mushroom (20 g) was extracted with distilled water (600 ml) at
121°C for 2 h. Extracts were centrifuged at 5,000 rpm for 20 min,
filtered through 0.45-μm Whatman #4 filter paper to remove
insoluble matter and freeze-dried. Polysaccharides were
precipitated from resuspended extracts by ethanol precipitation via
the addition of 75% (v/v) aqueous ethanol, collected by filtration
through 0.45-μm Whatman filter paper, resuspended and dialyzed
against distilled water for 5 days to remove low-molecular weight
compounds (18,19).
Materials
Fetal bovine serum (FBS), penicillin G, streptomycin
and RPMI-1640 media were obtained from Gibco (Grand Island, NY,
USA). 3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide
(MTT), and isopropyl alcohol were purchased from Sigma Chemical Co.
(St. Louis, MO, USA). β-actin monoclonal antibody (mAb),
extracellular signal-regulated kinase (ERK) Ab, phospho-ERK Ab,
stress-activated protein kinase/c-Jun N-terminal kinase (SAPK/JNK)
Ab, phospho-SAPK/JNK Ab, p38 MAPK Ab, phospho-p38 MAPK Ab, AKT Ab,
phospho-AKT Ab, COX-2 Ab, MMP-2 Ab, MMP-9 Ab, TIMP-2 Ab, and NF-κB
p50 Ab were purchased from Cell Signaling Technology (Boston, MA,
USA), Santa Cruz Biotechnology (Santa Cruz, CA, USA), or BD
Bioscience (San Diego, CA, USA). All other chemicals used were of
analytical grade.
Cell culture
Human A549 NSCLC cell line was obtained from the
Korea Cell Line Bank (Seoul, Korea). A549 cells were cultured in
RPMI-1640 supplemented with 10% heat-inactivated FBS, 100 U/ml
penicillin and 100 μg/ml streptomycin. Cells were maintained at
37°C in a humidified 5% CO2 incubator.
Cell viability
A549 cells were seeded into 12-well cell culture
plates and incubated for 24 h. After various treatments, cell
viability was evaluated using the MTT assay, which is based on the
reduction of a tetrazolium salt by mitochondrial dehydrogenase in
viable cells. After treatments, 500 μg/ml MTT solution was added to
each well and incubated for 3 h at 37°C. The formazan crystals in
each well were dissolved in isopropyl alcohol and absorbance was
measured at 595 nm using a microplate reader (Bio-Rad Laboratories,
Hercules, CA, USA).
Flow cytometry
Apoptotic index was determined using a fluorescein
isothiocyanate (FITC)-labeled Annexin V/propidium iodide (PI)
apoptosis detection kit (Molecular Probes, Eugene, OR, USA)
according to the manufacturer’s instructions. Briefly, cells were
harvested, washed with phosphate-buffered saline (PBS) and
centrifuged to collect the cell pellet. The number of cells was
adjusted to 1×106 cells/ml. Then, the cells were
resuspended in binding buffer [10 mM HEPES, 140 mM NaCl, and 2.5 mM
CaCl2 (pH 7.4)] and stained with FITC-labeled Annexin
V/PI at room temperature for 15 min in light-protected conditions.
Flow cytometric analysis was performed using a FACSCalibur flow
cytometer (Becton-Dickinson, Mountain View, CA, USA). The
percentage of apoptotic cells was calculated using CellQuest
software (Becton-Dickinson). Cells in the early phase of apoptosis
were Annexin V-positive and PI-negative, whereas those in the late
phase of apoptosis were positive for both Annexin V and PI. The
apoptotic index (%) was calculated as the sum of cells in the early
and late phases of apoptosis divided by the total number of
events.
Wound healing assay
Wound healing assay was performed as previously
described with some modifications (21). Briefly, A549 cells were cultured to
confluence in 6-well cell culture plates for 24 h in serum-free
medium. The medium was replaced with serum-containing medium
followed by the addition of PFIO at various concentrations (25, 50
and 100 μg/ml) and the cell monolayers were disrupted by scraping
them with a 100-μl micropipette tip. At the indicated time points
(0, 24 and 48 h) after scraping, the cells were washed twice with
PBS (pH 7.4) and photographed using an optical microscope at ×40
magnification.
In vitro migration and invasion
assay
The migration of A549 cells was also measured by
chemotactic directional migration using a 6-well transwell insert.
The 8-μm pore filters (Corning, NY, USA) were coated with gelatin
(Sigma). A549 cells (1×106 cells/ml) were seeded in the
upper chambers with or without PFIO (50 and 100 μg/ml) and allowed
to undergo migration for 24 h. Non-migrated cells in the upper
chambers were then removed with a cotton swab. The filters were
stained with 2% crystal violet. Migrated cells adhered to the
underside of the filters were counted and photographed using an
optical microscope at ×40 magnification. The invasion of A549 cells
was measured using Matrigel-coated transwell cell culture chambers
(8-μm pore size) as previously described. After the cells were
cultured for 24 h in serum-free DMEM, they were collected,
resuspended in serum-free medium, seeded in the upper chambers of
the transwell inserts (1×106 cells/ml), and incubated
with or without PFIO (50 or 100 μg/ml). DMEM containing 10% FBS was
placed in the lower chamber. All the cells in each treatment group
were incubated for 24 h at 37°C in a humidified atmosphere with 95%
air and 5% CO2. The non-invasive cells that remained in
the upper chambers were removed by wiping with a cotton swab, and
the invasive cells were fixed with 4% formaldehyde in PBS and
stained with 2% crystal violet in 2% ethanol. The invasive cells
that penetrated through the Matrigel coating and present on the
lower surface of the filters were counted and photographed using an
optical microscope at ×40 magnification.
Zymography analysis
Sodium dodecyl sulfate-polyacrylamide gel
electrophoresis (SDS-PAGE) substrate-embedded enzymography
(zymography) analysis was used to identify enzyme activities with
collagenase and gelatinase (22).
Briefly, the supernatant collected from cell culture was resolved
in 10% SDS-PAGE gels, which were prepared by the incorporation of
gelatin (1 mg/ml) before casting. After electrophoresis, the gels
were washed twice for 30 min in 2.5% Triton X-100 with shaking.
They were then incubated at 37°C for 24–72 h in reaction buffer
containing 50 mM Tris-HCl (pH 7.6), 10 mM CaCl2, 150 mM
NaCl, and 20% sodium azide, followed by staining with 0.25%
Coomassie brilliant blue G-250 in 50% methanol and 10% acetic acid
for 1–2 h. The completely stained gels were appropriately destained
with 40% methanol and 10% acetic acid. The enzyme activity was
evident as clear (unstained) regions against the dark
background.
Western blot analysis
Treated cells were washed in 1X PBS and lysed in
lysis buffer [10 mM Tris-HCl (pH 7.5), 10 mM
NaH2PO4/NaHPO4 (pH 7.5), 130 mM
NaCl, 1% Triton X-100, 10 mM NaPPi, 1 mM phenylmethylsulphonyl
fluoride and 2 μg/ml pepstatin A] for 30 min on ice. The lysates
were centrifuged at 15,600 rpm for 30 min at 4°C. The supernatant
was collected and its protein content was measured using a Bio-Rad
protein assay kit before analysis. The total or fractionated
protein samples were loaded and separated using SDS-PAGE and
transferred to nitrocellulose membranes (Immun-Blot NC membrane,
0.2 μm; Bio-Rad). Membranes were blocked with 1.5% skim milk in 1X
Tris-buffered saline (TBS) containing 0.1% Tween-20 for 1 h and
incubated with primary antibodies at 4°C overnight. Finally, the
membranes were treated with horseradish peroxidase -linked
secondary antibodies for 1 h at room temperature. TBS washing was
performed after each antibody binding reaction. The expression of
each protein was detected using an enhanced chemiluminescence kit
(Millipore Co., Billerica, MA, USA).
Nuclear protein extraction
Nuclear extracts were prepared by lysing nuclei in
high-salt buffer supplemented with protease and phosphatase
inhibitors using a nuclear extraction kit (Panomics Inc., Fremont,
CA, USA) according to the manufacturer’s protocol. Protein
concentrations were quantified using the Bio-Rad protein assay.
Statistical analysis
Data are expressed as mean ± standard error values,
and results were obtained from at least three independent
experiments performed in triplicates. All data were analyzed using
Student’s t-test to evaluate significant differences. A p-value of
<0.05 was considered statistically significant.
Results
Effect of PFIO on A549 cell
viability
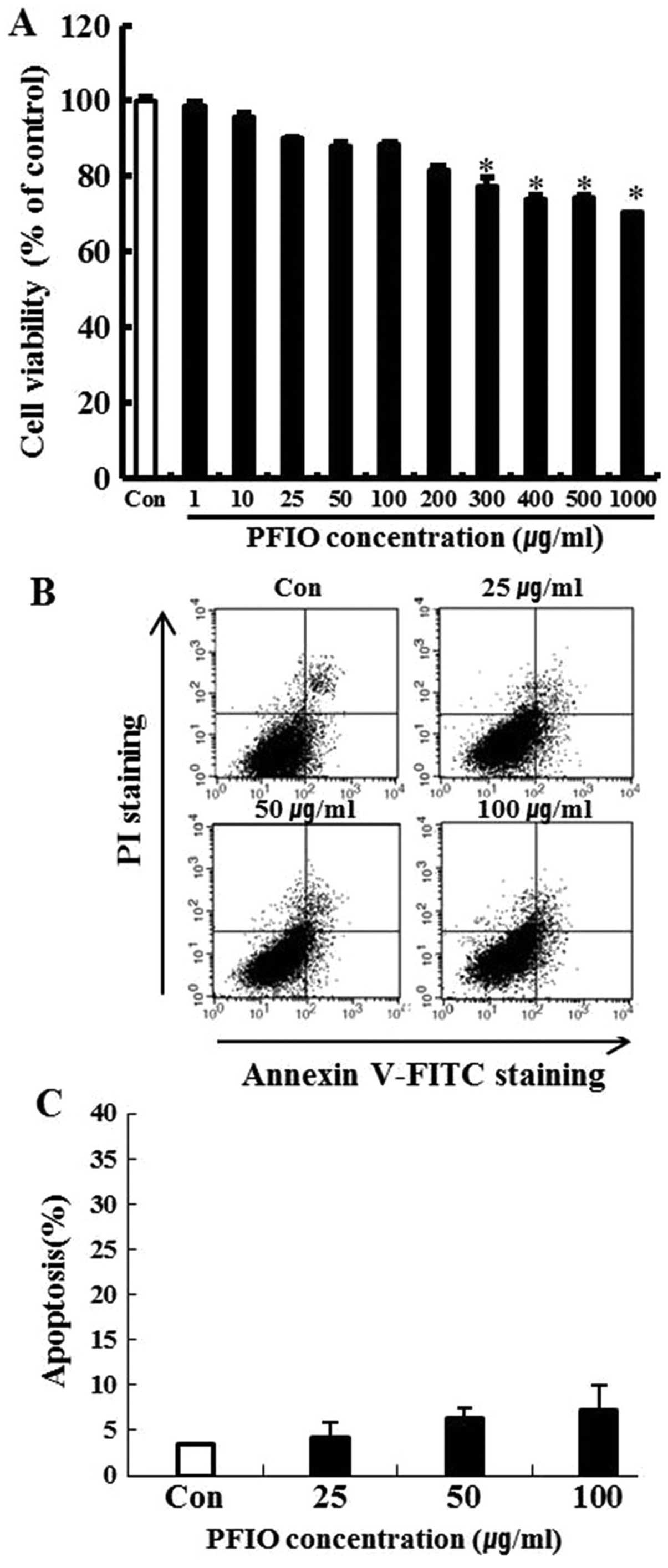
To investigate the cytotoxicity of PFIO, A549 cells
were treated with PFIO at various concentrations ranging from 0 to
1,000 μg/ml for 24 h and cell viability was determined by the MTT
assay. We found that 0–200 μg/ml of PFIO did not significantly
affect A549 cell growth (Fig. 1A).
In these experiments, cell viability was 82% at 200 μg/ml. Results
of the FITC-labeled Annexin V and PI double-staining experiments
revealed that PFIO at 100 μg/ml induced apoptosis in 7.1% of the
cells (Fig. 1B and C), whereas
concentrations ranging from 0 to 100 μg/ml of PFIO did not induce
cell death and apoptosis in the highly metastatic A549 cell line.
This concentration range was then applied in all subsequent
experiments.
Effects of PFIO on the motility of A549
cells
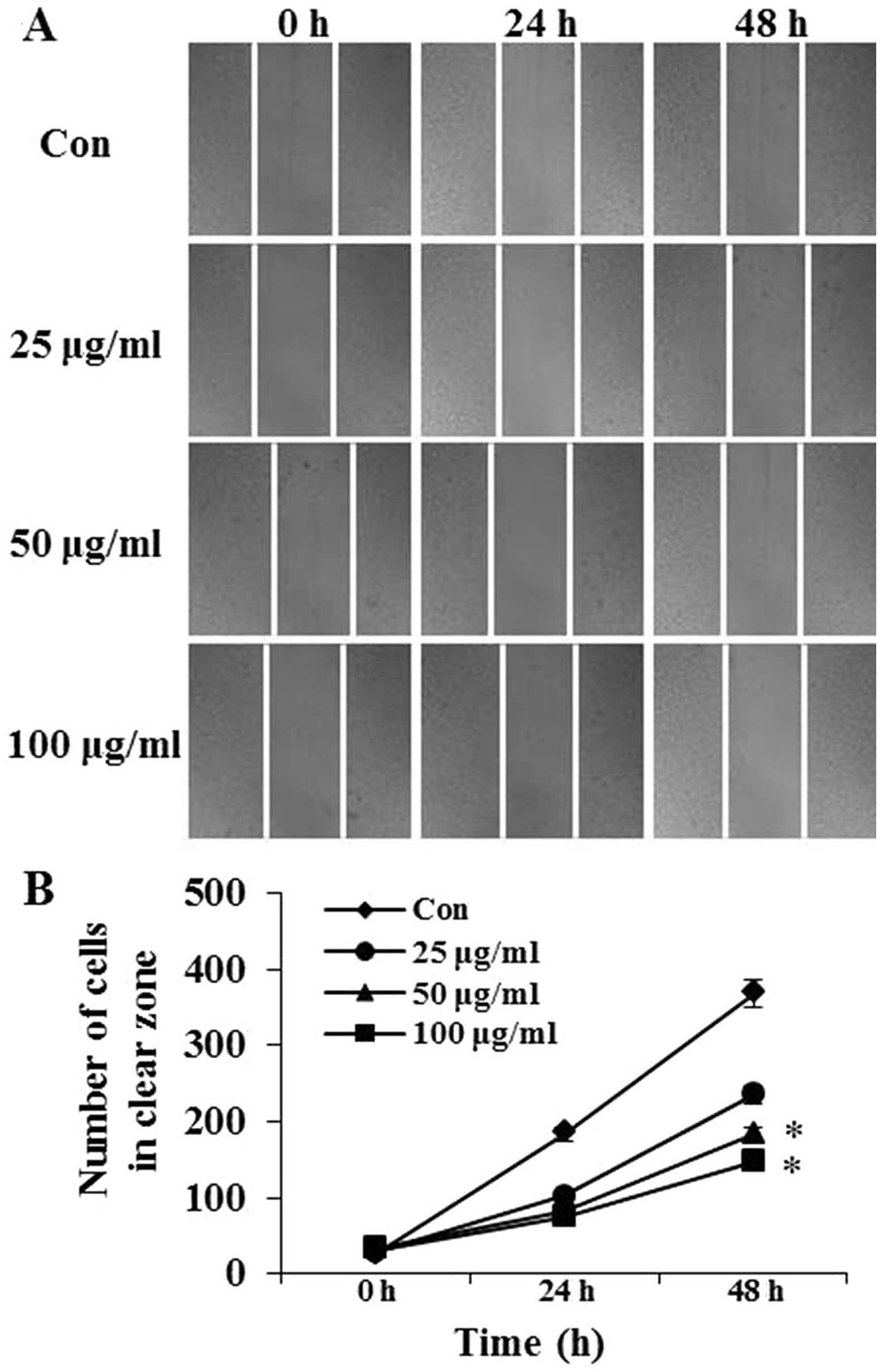
The effects of PFIO on A549 cell migration was
determined using the wound-healing assay in which cells were
stimulated to migrate by physical wounding. As shown in Fig. 2A, when cells were treated with PBS
for 24 and 48 h, an apparent and gradual increase of cells in the
denuded zone was observed under light microscopy. A549 cells
treated with 25, 50 or 100 μg/ml of PFIO displayed a reduced
ability to migrate and fill the wounded area compared with
untreated cells. The quantitative data in Fig. 2B revealed that PFIO could
significantly inhibit the migration of A549 cells.
Effect of PFIO on A549 cell migration and
invasion
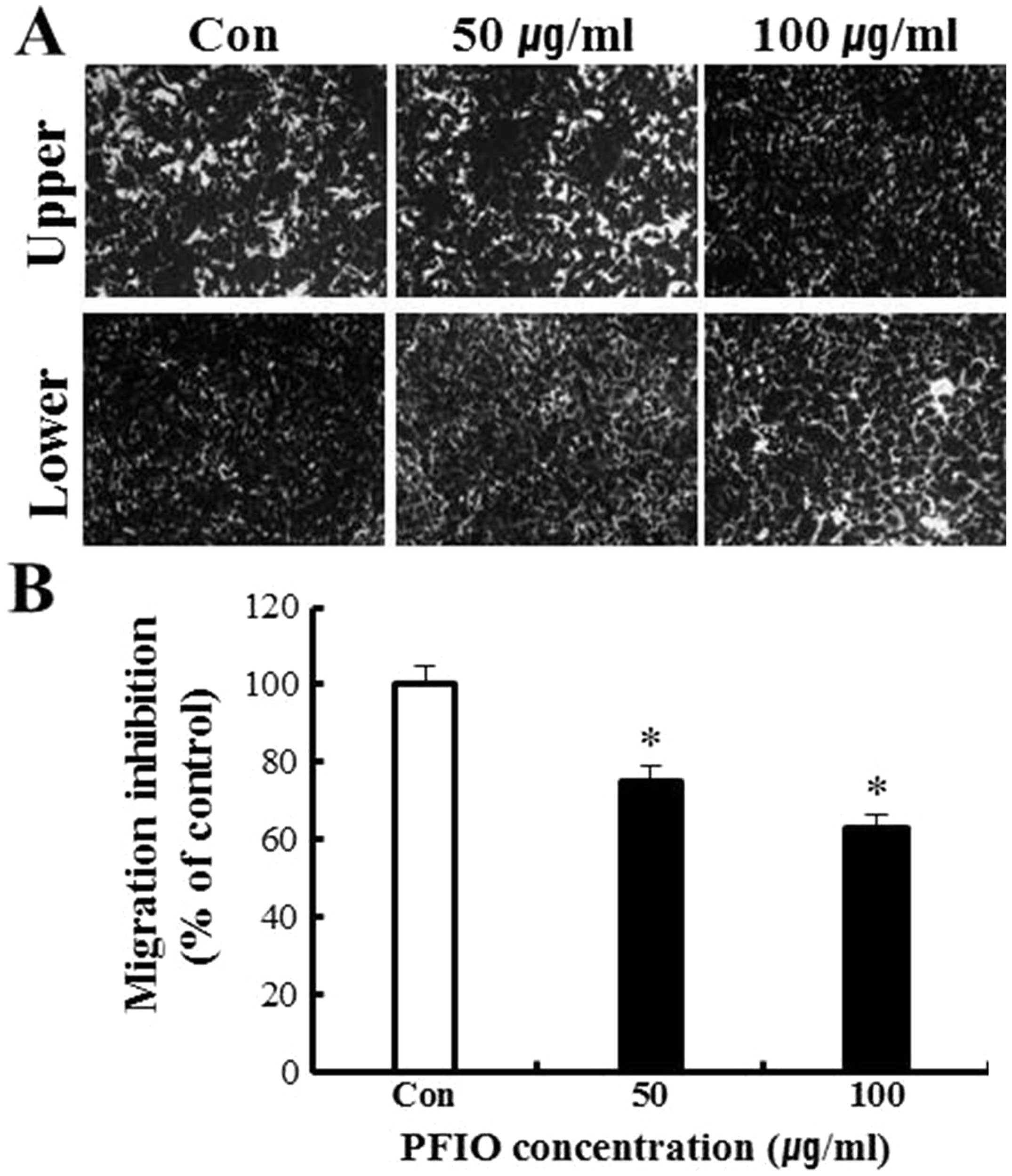
To further evaluate the anti-metastatic activity of
PFIO, we assessed the inhibition of A549 migration and invasion by
PFIO using transwell assay with polycarbonate filters (pore size, 8
μm) pre-coated with gelatin or Matrigel. Our results indicated that
A549 cells were observed moving from the upper to the lower chamber
in the absence of PFIO (control group), suggesting that A549 cells
could migrate across a transwell insert precoated with gelatin.
PFIO at 50 and 100 μg/ml significantly inhibited cell migration by
25 and 37%, respectively (Fig. 3).
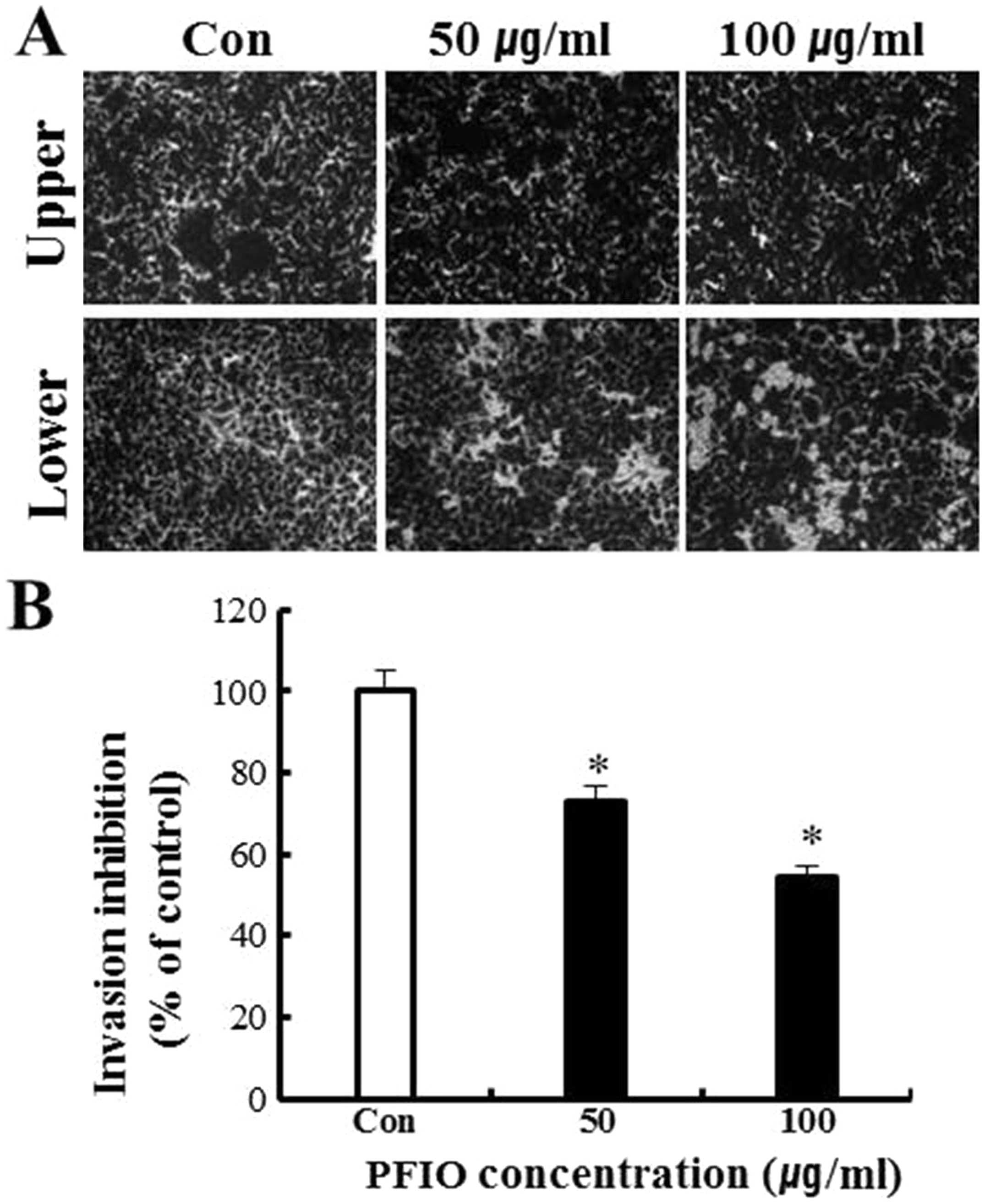
As shown in Fig. 4, results of the
invasion assay illustrated that untreated A549 cells moved from the
upper to the lower chamber, indicating the ability of A549 cells to
invade through Matrigel-coated transwell cell culture chambers.
However, the addition of PFIO to A549 cells resulted in an
inhibitory effect on cellular invasion in a concentration-dependent
manner. Data in Fig. 4B indicated
that 50 and 100 μg/ml of PFIO significantly inhibited A549 cell
invasion by 27 and 46%, respectively. Thus, these results suggested
that PFIO effectively reduced cell migration and invasion.
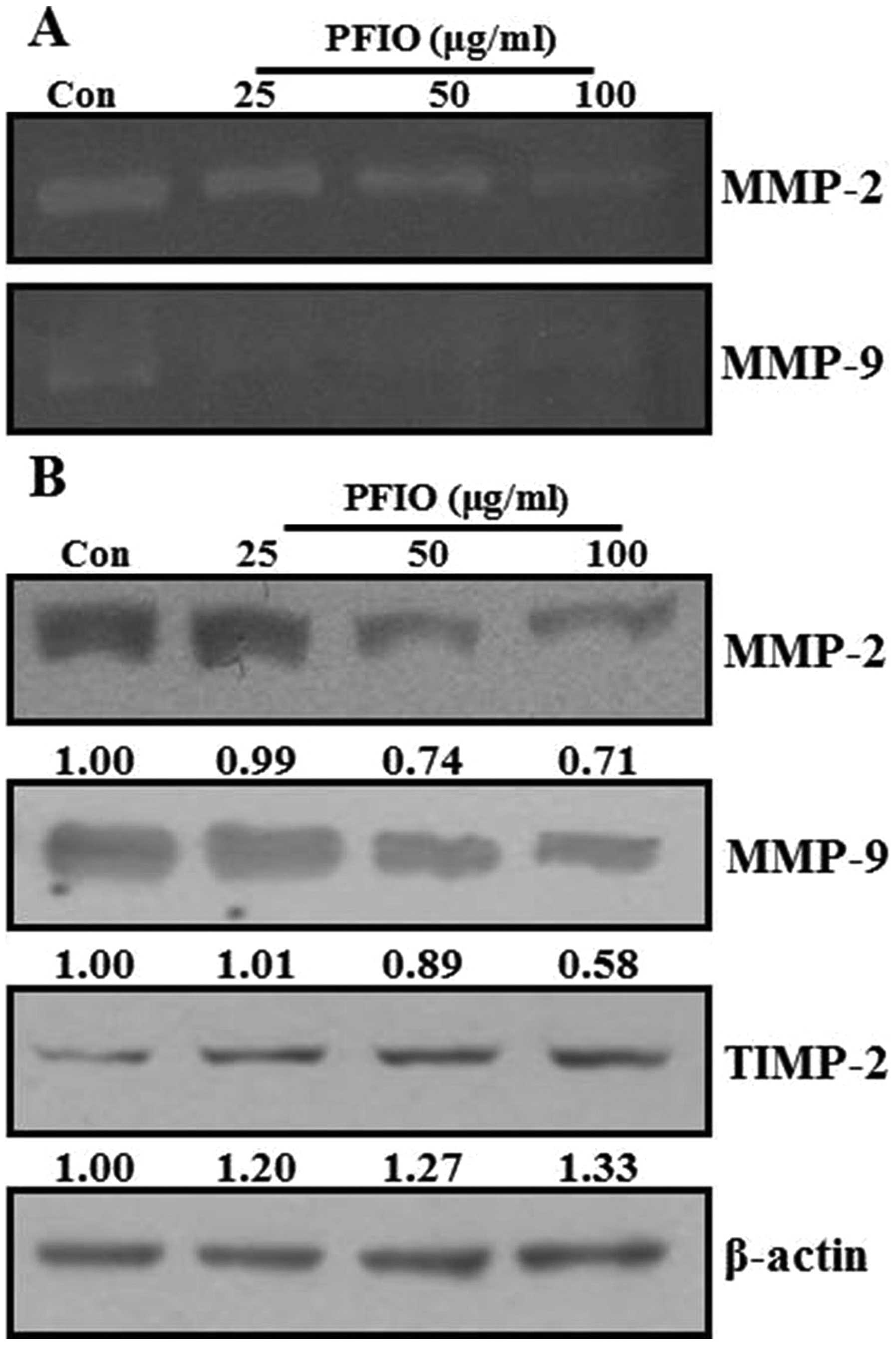
Effect of PFIO on the activities and
expression of MMPs in A549 cells
ECM degradation is crucial to cellular invasion,
indicating the inevitable involvement of matrix-degrading
proteinases (23). Matrix
degradation could, however, be suppressed by altering the balance
between MMP-2 and TIMP-2 (24).
Therefore, the effects of PFIO on protein expression and
gelatinolytic activity of MMPs were investigated by
gelatin-zymography and western blot analysis under serum starvation
condition. As shown in Fig. 5A,
MMP-2 and MMP-9 activity was remarkably decreased in A549 cells
after PFIO treatment at 25, 50 and 100 μg/ml for 24 h compared to
control. When MMP-2, MMP-9, and TIMP-2 protein expression was
determined using western blot analysis, PFIO was found to reduce
the expression levels of MMP-2 and MMP-9 but increase that of
TIMP-2 in A549 cells (Fig. 5B).
Therefore, our results indicated that PFIO could regulate the
expression and activity of MMP-2 and MMP-9.
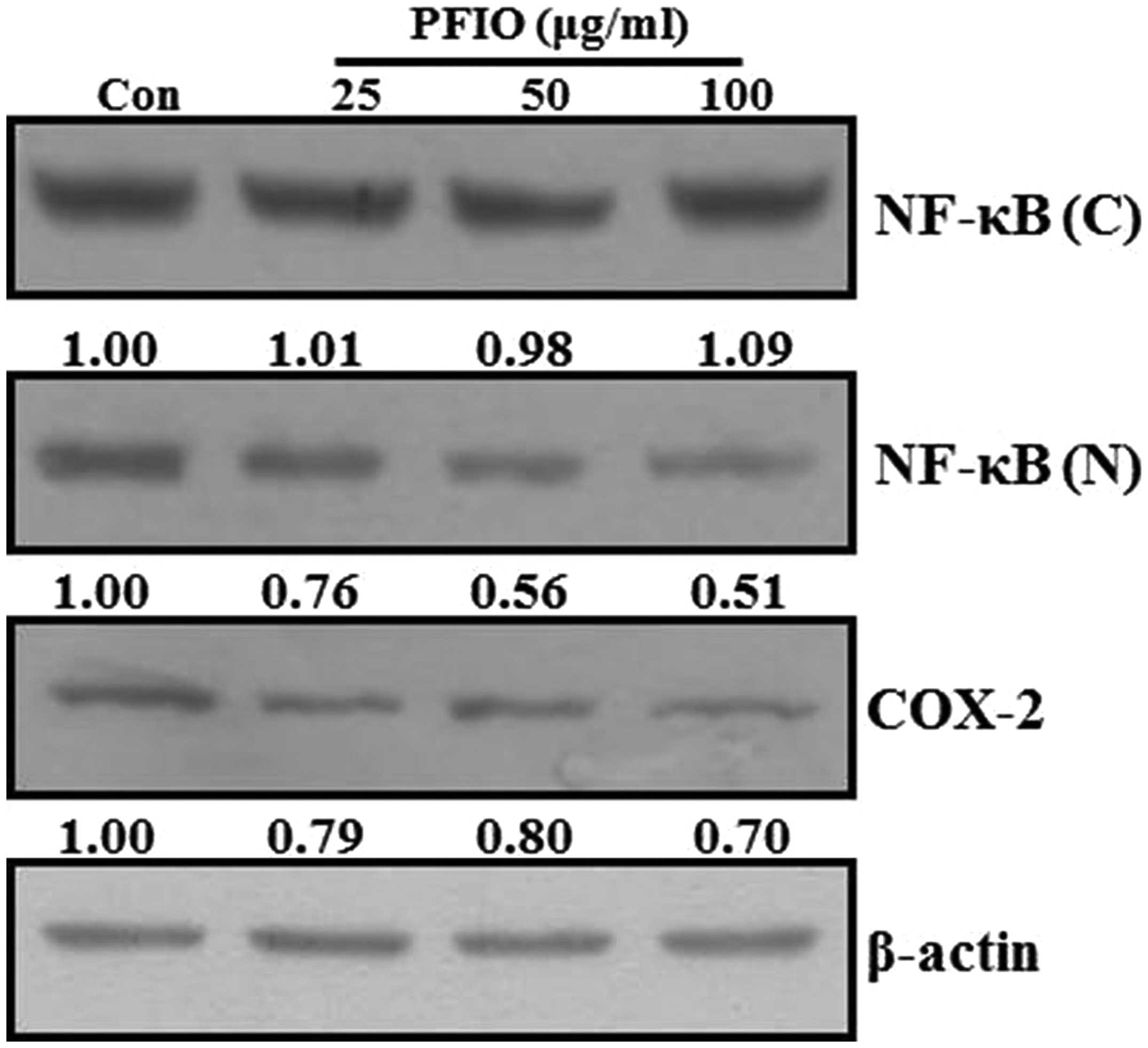
Effect of PFIO on NF-κB nuclear
translocation and COX-2 expression levels in A549 cells
Previous reports have demonstrated that the MMP
promoter contains several transcription factor-binding elements,
including binding sites for AP-1 and NF-κB. Additionally, nuclear
translocation of AP-1 and NF-κB in metastatic cancer cells involves
the expression of MMPs (25,26).
Therefore, NF-κB signal transduction pathway may play an important
role in the regulation of MMP-2 and MMP-9 expression. Moreover,
COX-2, which is regulated by NF-κB activation, also affects the
expression of MMP-9 in highly metastatic cancer cells (27). To investigate whether PFIO could
regulate the NF-κB signaling pathway, A549 cells were treated with
the indicated concentrations of PFIO. The translocation of NF-κB
and COX-2 expression levels were determined by western blot
analysis. As shown in Fig. 6, PFIO
treatment increased the total cytosolic NF-κB protein levels in
A549 cells was compared to untreated control. In contrast, nuclear
levels of NF-κB protein in A549 cells remarkably decreased after
PFIO treatment compared to control. Moreover, PFIO suppressed the
expression levels of COX-2. These results indicated that PFIO could
regulate NF-κB nuclear translocation and COX-2 expression in A549
cancer cells.
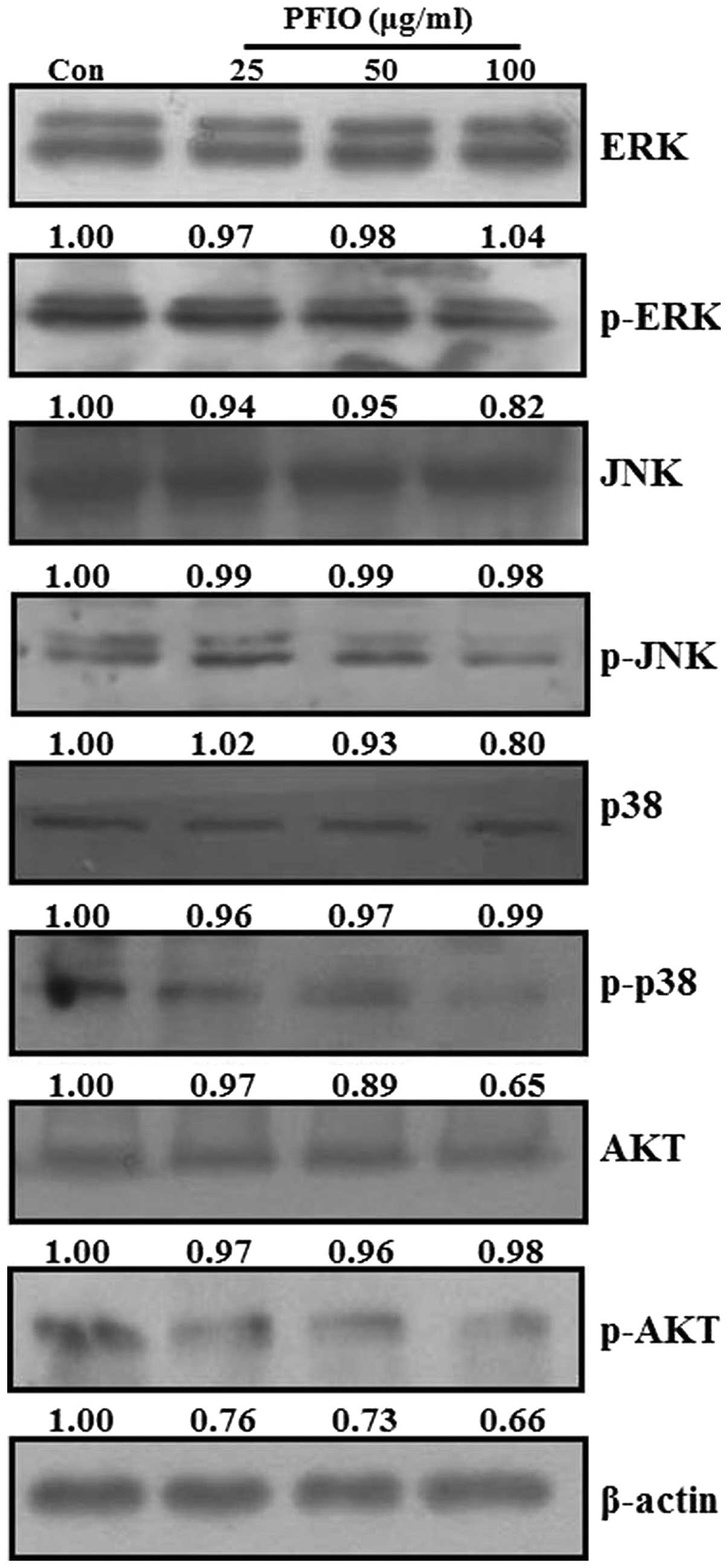
Effect of PFIO on MAPKs and AKT signaling
pathways in A549 cells
Recent studies reported that MAPKs and PI3K/AKT
signaling pathways are involved in cancer cell migration and
invasion (25,28,29).
MAPKs and AKT have been demonstrated to be involved in MMP
induction in various tumor types (30–33).
To examine whether PFIO could regulate MAPKs and AKT signaling
pathways in A549 cells, we analyzed the phosphorylation levels of
all three MAPKs (ERK, JNK and p38 MAPK) and AKT protein in A549
cells by western blot analysis after PFIO treatment (25, 50 and 100
μg/ml) for 24 h. As shown in Fig.
7, PFIO did not affect the expression levels of the three MAPKs
and AKT, but it suppressed the phosphorylation of ERK, JNK, p38
MAPK, and AKT compared to controls. In particular, the
phosphorylation levels of JNK, p38 MAPK, and AKT were inhibited by
the addition of PFIO at 50 and 100 μg/ml. These data indicated that
PFIO could inhibit the phosphorylation of MAPKs and AKT in A549
cells.
Discussion
Cancer is a major health problem worldwide. In
recent years, much attention has been focused on the antitumor
properties of natural components for chemotherapeutic applications.
Polysaccharide is often implicated with meaningful pharmacological
activities (34–39). For instance, polysaccharides
extracted from mushrooms such as L. edodes, C.
militaris, H. erinaceus, and I. obliquus are
well-known to possess important pharmacological properties.
However, the effects of I. obliquus-derived polysaccharides
on cancer metastasis in human NSCLC remain unknown. Cancer cell
invasion and migration are the important prerequisites of tumor
metastasis with invasion of the ECM being a critical step involving
the attachment of tumor cells to the ECM. Numerous reports have
revealed that the inhibition of MMP expression and/or enzymatic
activities could prevent cancer metastasis (26). MMPs have been demonstrated to be
major components of the enzyme cascade responsible for the
degradation of the ECM and basement membrane proteins (40). Proteolysis of these proteins is
part of cell migration, proliferation, differentiation, and tissue
remodeling processes related to airway injury. Lung epithelial
cells are one of the most important sources of MMPs, such as MMP-1
(55-kDa collagenase), MMP-2 (72-kDa gelatinase A), MMP-7 (28-kDa)
and MMP-9 (92-kDa gelatinase B) (8,25).
MMP-2 and MMP-9 are involved in the invasive metastatic potential
of tumor cells. Furthermore, the expression and activity of COX-2
might modulate those of MMPs (27). MMP-2 and MMP-9 are activated on the
cell surface by a multimeric complex with TIMP-1 and TIMP-2, the
activation mechanism of which might help elucidate the process of
cellular motility, invasion, and migration in cancer metastasis
(9). In the present study, we
investigated the effects of PFIO on the migration and invasion of
the highly metastatic A549 cell line in vitro. Our results
from wound healing, migration, and invasion assays demonstrated
that PFIO inhibited the migration and invasion of A549 cells
(Figs. 2–4). In addition, our findings suggested
that PFIO could regulate the expression and activities of MMP-2,
MMP-9, and TIMP-2, which facilitated ECM degradation and played
important roles in cancer cell migration and invasion (Fig. 5). These results indicated that the
anti-metastatic effects of PFIO were associated with the inhibition
of enzymatic degradation processes in metastatic A549 cells.
Previous reports demonstrated that MMP-2 and MMP-9 promoters
consisted of several transcription factor-binding motifs including
those for NF-κB. Multiple pathways leading to the activation of
NF-κB binding factors in tumor cells may contribute to MMP-2 and
MMP-9 transcription and enhanced invasiveness. In this study, we
found that PFIO regulated NF-κB translocation from the cytosol to
the nucleus and COX-2 expression in A549 cells (Fig. 6). Our findings thus implied that
PFIO inhibited A549 metastasis by inhibiting NF-κB translocation
and that it suppressed MMP-2 and MMP-9 expression and activities by
inhibiting the NF-κB signaling pathway. Furthermore, PFIO might
also regulate other signaling pathways related to the migration and
invasion of highly metastatic cancer cells through different
mechanisms such as phosphorylation of MAPKs and AKT (Fig. 7). The MAPK signaling pathway was
found to promote tumor invasion and metastasis in A549 cells. Our
results showed that PFIO inhibited the phosphorylation of AKT in
A549 cells, suggesting that it might inhibit the AKT signaling
pathway. Additionally, PI3K activation reportedly stimulates the
downstream target AKT, which is associated with cell invasion
(25). The PI3K-AKT pathway is
known to play important roles in the cancer cell invasiveness.
In conclusion, as MMPs are very important in tumor
metastasis, their gene expression and enzymatic activity are early
targets for preventing cancer metastasis. Our study suggested that
PFIO inhibited the migration and invasion of highly metastatic A549
cells by inhibition of MMP-2 and MMP-9 activity and expression via
downregulation of NF-κB, AKT, and/or MAPKs signaling pathways.
Acknowledgements
This study was supported by the Basic Science
Research Program of the National Research Foundation of Korea (NRF)
funded by the Korean Ministry of Education (NRF-2011-0011522).
Abbreviations:
|
ECM
|
extracellular matrix
|
|
ERK
|
extracellular signal-regulated protein
kinase
|
|
JNK
|
c-Jun N-terminal kinase
|
|
MAPK
|
mitogen-activated protein kinase
|
|
MMP
|
matrix metalloproteinase
|
|
TIMP
|
tissue inhibitor of
metalloproteinase
|
|
NF-κB
|
nuclear factor κB
|
|
COX
|
cyclooxygenase
|
|
AKT
|
protein kinase B
|
|
PI3K
|
phosphoinositide 3-kinase
|
References
|
1
|
Kanzaki R, Higashiyama M, Fujiwara A, et
al: Occult mediastinal lymph node metastasis in NSCLC patients
diagnosed as clinical N0-1 by preoperative integrated FDG-PET/CT
and CT: risk factors, pattern, and histopathological study. Lung
Cancer. 71:333–337. 2011. View Article : Google Scholar
|
|
2
|
Olmez I, Donahue BR, Butler JS, Huang Y,
Rubin P and Xu Y: Clinical outcomes in extracranial tumor sites and
unusual toxicities with concurrent whole brain radiation (WBRT) and
Erlotinib treatment in patients with non-small cell lung cancer
(NSCLC) with brain metastasis. Lung Cancer. 70:174–179. 2010.
View Article : Google Scholar
|
|
3
|
Spizzo G, Seeber A and Mitterer M: Routine
use of pamidronate in NSCLC patients with bone metastasis: results
from a retrospective analysis. Anticancer Res. 29:5245–5249.
2009.PubMed/NCBI
|
|
4
|
Fidler IJ, Kim SJ and Langley RR: The role
of the organ microenvironment in the biology and therapy of cancer
metastasis. J Cell Biochem. 101:927–936. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Shiraga M, Yano S, Yamamoto A, et al:
Organ heterogeneity of host-derived matrix metalloproteinase
expression and its involvement in multiple-organ metastasis by lung
cancer cell lines. Cancer Res. 62:5967–5973. 2002.PubMed/NCBI
|
|
6
|
Ciborowski P and Finn OJ: Non-glycosylated
tandem repeats of MUC1 facilitate attachment of breast tumor cells
to normal human lung tissue and immobilized extracellular matrix
proteins (ECM) in vitro: potential role in metastasis. Clin Exp
Metastasis. 19:339–345. 2002. View Article : Google Scholar
|
|
7
|
Santos MC, de Souza AP, Gerlach RF,
Trevilatto PC, Scarel-Caminaga RM and Line SR: Inhibition of human
pulpal gelatinases (MMP-2 and MMP-9) by zinc oxide cements. J Oral
Rehabil. 31:660–664. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Corbel M, Boichot E and Lagente V: Role of
gelatinases MMP-2 and MMP-9 in tissue remodeling following acute
lung injury. Brazilian J Med Biol Res. 33:749–754. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Cheng Y, Dong Q, Sun LR, Yang CM and Jiang
BX: Correlation between expression of MMP-2, MMP-9, TIMP-2, TIMP-1
and metastasis of neuroblastoma. Zhonghua Zhong Liu Za Zhi.
27:164–166. 2005.(In Chinese).
|
|
10
|
Chaudhry K, Rogers R, Guo M, et al: Matrix
metalloproteinase-9 (MMP-9) expression and extracellular
signal-regulated kinase 1 and 2 (ERK1/2) activation in
exercise-reduced neuronal apoptosis after stroke. Neurosci Lett.
474:109–114. 2010. View Article : Google Scholar
|
|
11
|
Hu YB, Zong YR, Feng DY, Jin ZY, Jiang HY
and Peng JW: p38/ERK signal pathways regulating the expression of
type I collagen and activity of MMP-2 in TGF-beta1-stimulated
HLF-02 cells. Zhonghua Lao Dong Wei Sheng Zhi Ye Bing Za Zhi.
24:77–80. 2006.(In Chinese).
|
|
12
|
Cheng JC, Chou CH, Kuo ML and Hsieh CY:
Radiation-enhanced hepatocellular carcinoma cell invasion with
MMP-9 expression through PI3K/Akt/NF-kappaB signal transduction
pathway. Oncogene. 25:7009–7018. 2006. View Article : Google Scholar
|
|
13
|
Cui D, Zhang X and Fu Y: Expressions of
COX-2 and MMP-2 in nasopharyngeal carcinoma and the their
relationship with lymph node metastasis]. Lin Chung Er Bi Yan Hou
Tou Jing Wai Ke Za Zhi. 22:692–694. 2008.(In Chinese).
|
|
14
|
Dixon DA, Tolley ND, Bemis-Standoli K, et
al: Expression of COX-2 in platelet-monocyte interactions occurs
via combinatorial regulation involving adhesion and cytokine
signaling. J Clin Invest. 116:2727–2738. 2006. View Article : Google Scholar PubMed/NCBI
|
|
15
|
Shifflett DE, Jones SL, Moeser AJ and
Blikslager AT: Mitogen-activated protein kinases regulate COX-2 and
mucosal recovery in ischemic-injured porcine ileum. Am J Physiol
Gastrointest Liver Physiol. 286:G906–G913. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Lee KR, Lee JS, Kim YR, Song IG and Hong
EK: Polysaccharide from Inonotus obliquus inhibits migration
and invasion in B16-F10 cells by suppressing MMP-2 and MMP-9 via
downregulation of NF-kappaB signaling pathway. Oncol Rep.
31:2447–2453. 2014.
|
|
17
|
Yun JS, Pahk JW, Lee JS, Shin WC, Lee SY
and Hong EK: Inonotus obliquus protects against oxidative
stress-induced apoptosis and premature senescence. Mol Cells.
31:423–429. 2011. View Article : Google Scholar
|
|
18
|
Won DP, Lee JS, Kwon DS, Lee KE, Shin WC
and Hong EK: Immunostimulating activity by polysaccharides isolated
from fruiting body of Inonotus obliquus. Mol Cells.
31:165–173. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Lee JS, Kwon JS, Won DP, et al: Study of
macrophage activation and structural characteristics of purified
polysaccharide from the fruiting body of Cordyceps
militaris. J Microbiol Biotechnol. 20:1053–1060. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Lee JS and Hong EK: Hericium
erinaceus enhances doxorubicin-induced apoptosis in human
hepatocellular carcinoma cells. Cancer Lett. 297:144–154. 2010.
View Article : Google Scholar
|
|
21
|
Rodriguez LG, Wu X and Guan JL:
Wound-healing assay. Methods Mol Biol. 294:23–29. 2005.
|
|
22
|
Toth M, Sohail A and Fridman R: Assessment
of gelatinases (MMP-2 and MMP-9) by gelatin zymography. Methods Mol
Biol. 878:121–135. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Artym VV, Yamada KM and Mueller SC: ECM
degradation assays for analyzing local cell invasion. Methods Mol
Biol. 522:211–219. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Powell K: ECM signals ECM degradation. J
Cell Biol. 172:6422006. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Lee YC, Lin HH, Hsu CH, Wang CJ, Chiang TA
and Chen JH: Inhibitory effects of andrographolide on migration and
invasion in human non-small cell lung cancer A549 cells via
downregulation of PI3K/Akt signaling pathway. Eur J Pharmacol.
632:23–32. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Philip S, Bulbule A and Kundu GC: Matrix
metalloproteinase-2: mechanism and regulation of NF-kappaB-mediated
activation and its role in cell motility and ECM-invasion.
Glycoconj J. 21:429–441. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Callejas NA, Casado M, Diaz-Guerra MJ,
Bosca L and Martin-Sanz P: Expression of cyclooxygenase-2 promotes
the release of matrix metalloproteinase-2 and -9 in fetal rat
hepatocytes. Hepatology. 33:860–867. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Wu KC, Yang ST, Hsia TC, et al:
Suppression of cell invasion and migration by propofol are involved
in down-regulating matrix metalloproteinase-2 and p38 MAPK
signaling in A549 human lung adenocarcinoma epithelial cells.
Anticancer Res. 32:4833–4842. 2012.PubMed/NCBI
|
|
29
|
Ni L, Feng Y, Wan H, et al:
Angiotensin-(1-7) inhibits the migration and invasion of A549 human
lung adenocarcinoma cells through inactivation of the PI3K/Akt and
MAPK signaling pathways. Oncol Rep. 27:783–790. 2012.PubMed/NCBI
|
|
30
|
Lu CC, Yang JS, Chiang JH, et al:
Inhibition of invasion and migration by newly synthesized
quinazolinone MJ-29 in human oral cancer CAL 27 cells through
suppression of MMP-2/9 expression and combined down-regulation of
MAPK and AKT signaling. Anticancer Res. 32:2895–2903.
2012.PubMed/NCBI
|
|
31
|
Jung JS, Jung K, Kim DH and Kim HS:
Selective inhibition of MMP-9 gene expression by mangiferin in
PMA-stimulated human astroglioma cells: involvement of PI3K/Akt and
MAPK signaling pathways. Pharmacol Res. 66:95–103. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Kang MH, Oh SC, Lee HJ, et al: Metastatic
function of BMP-2 in gastric cancer cells: the role of PI3K/AKT,
MAPK, the NF-kappaB pathway, and MMP-9 expression. Exp Cell Res.
317:1746–1762. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Cho SJ, Chae MJ, Shin BK, Kim HK and Kim
A: Akt- and MAPK-mediated activation and secretion of MMP-9 into
stroma in breast cancer cells upon heregulin treatment. Mol Med
Rep. 1:83–88. 2008.PubMed/NCBI
|
|
34
|
Zhang S, Nie S, Huang D, Huang J, Wang Y
and Xie M: Polysaccharide from Ganoderma atrum evokes antitumor
activity via Toll-like receptor 4-mediated NF-kappaB and
mitogen-activated protein kinase signaling pathways. J Agric Food
Chem. 61:3676–3682. 2013. View Article : Google Scholar
|
|
35
|
Cardozo FT, Larsen IV, Carballo EV, et al:
In vivo anti-herpes simplex virus activity of a sulfated derivative
of Agaricus brasiliensis mycelial polysaccharide. Antimicrob Agents
Chemother. 57:2541–2549. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Liu Y, Li Y, Yang W, Zhang L and Cao G:
Anti-hepatoma activity in mice of a polysaccharide from the rhizome
of Anemone raddeana. Int J Biol Macromol. 50:632–636. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Lee JS and Hong EK: Agaricus blazei
Murill enhances doxorubicin-induced apoptosis in human
hepatocellular carcinoma cells by NFkappaB-mediated increase of
intracellular doxorubicin accumulation. Int J Oncol. 38:401–408.
2011.
|
|
38
|
Sinha S, Nosalóva G, Bandyopadhyay SS,
Fleskova D and Ray B: In vivo anti-tussive activity and structural
features of a polysaccharide fraction from water extracted
Withania somnifera. J Ethnopharmacol. 134:510–513. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Li H, Lu X, Zhang S, Lu M and Liu H:
Anti-inflammatory activity of polysaccharide from Pholiota nameko.
Biochemistry Biokhimiia. 73:669–675. 2008. View Article : Google Scholar
|
|
40
|
Baricos WH, Cortez SL, el-Dahr SS and
Schnaper HW: ECM degradation by cultured human mesangial cells is
mediated by a PA/plasmin/MMP-2 cascade. Kidney Int. 47:1039–1047.
1995. View Article : Google Scholar : PubMed/NCBI
|