Introduction
Alternative splicing is a process in which either
different combinations of exons or parts of exons are employed to
generate new transcripts through the use of alternative-splicing
sites. More than 90% of human intron-containing genes undergo
alternative splicing, which allows a single transcript to encode a
number of RNAs that produce various protein isoforms with different
functions (1). A complex array of
cis elements (the splicing code) embedded in pre-mRNAs,
together with trans-acting factors that bind to these elements, are
required for the precise process of pre-mRNA splicing and its
regulation. The splicing code for the majority of introns comprises
short sequences that include the 5′ and 3′ splice sites located at
both intron-exon junctions and at the branch point. These sequences
are recognized by the spliceosome, which is composed of five small
nuclear ribonucleoproteins and ~150 proteins. In addition to the
core cis elements in pre-mRNAs, other sequences in introns
and exons, which are recognized by cellular trans-acting
factors, are also critical to the determination of splicing
outcomes (2).
In the last few years, a link has emerged between
cancer development and deregulation of alternative splicing
(3). Approximately 10% of
inherited or somatic mutations are localized within sequences
coding canonical splice sites (4),
and these mutations have been implicated in cancer susceptibility
and tumor progression. One such example is the tumor suppressor
gene p53, the most commonly mutated gene in human cancers. Isoforms
of the p53 gene are created through alternative splicing, and their
tumor suppressor functions differ (5). ‘Silent’ mutations in the p53 gene
have been predicted to affect splicing by creating splice sites in
the middle of an exon (6).
Recently, it has been reported that an attenuated form of familiar
adenomatous polyposis is caused by the insertion of a single T
between the second and third nucleotide of intron 4 of the APC
gene, which leads to the skipping of exon 4, and a predicted
expression of a truncated protein (7). Another good example of altered
splicing in tumorigenesis occurs in the tumor suppressor gene
BRCA1. Mutations in the BRCA1 gene are well-known markers of
susceptibility to ovarian and breast cancer, the latter being the
most common malignancy in women. One such mutation in the BRCA1
gene is an inherited nonsense mutation within exon 18 that disrupts
the exonic splicing enhancer of the gene, provoking exon skipping
(8).
Alterations in signaling pathways cause changes in
the abundance, localization and activity of splicing regulators,
which in turn can cause a general deregulation of splicing, and
result in the occurrence of cancer-specific transcripts that are
associated with tumorigenesis. Recent progress in molecular and
cell biology has indicated that altered splicing profiles of
critical genes may impact all aspects of cancer cell biology
(9), resulting in the inactivation
of tumor suppressor genes (6), or
the gain of function of proteins implicated in cancer
susceptibility and tumor progression (9,10).
Human papillomavirus (HPV) infection is a necessary,
but not sufficient factor in the development of cervical neoplasia,
and persistent infection with high-risk HPV types, especially
HPV16, is a significant risk factor in the development of
precancerous lesions and squamous cell carcinoma (11–14).
HPV-16, -18, -45, -31 and -33 are the most frequently identified
high-risk HPV types in high-grade squamous intraepithelial lesions
and squamous cell carcinoma (15),
though HPV16 predominates (16).
An association has also been suggested between high viral load and
the persistence of HPV infection (17). Promising HPV-related markers of the
risk of progression to cervical cancer include the integration
status of high-risk HPV DNA in precancerous cervical lesions
(18). HPV DNA integration into
the host cell genome usually disrupts the E1 and E2 open reading
frames, but leaves those of E6 and E7 intact (19,20).
The deletion of the E2 open reading frame due to HPV DNA
integration leads to the disruption of E2 protein expression, and
the upregulation of E6 and E7 protein transcription (21); continuous production of the
oncogenic E6 and E7 proteins contribute to malignant
progression.
It was recently demonstrated that level of the
splicing activator ASF/SF2 (also known as arginine/serine-rich
splicing factor 1) is modulated in the presence of HPV infection
(22), as is the splicing silencer
heterogeneous nuclear ribonucleoprotein A1 (hnRNP A1). The latter
is a trans-acting factor that can modulate splice site
selection (23), which may result
in a change in the splicing pattern of a number of mRNAs expressed
in HPV-infected cervical tissue. A previous proteomic analysis we
preformed of vaginal and cervical cancer identified a number of
tumor-specific markers (24). The
biggest increase in cancer-related expression was observed for the
prdx2 protein, which was found to be upregulated in cervical
cancer, and the Rab1b protein (a member of the RAS oncogene
family), which was found to be upregulated in both vaginal and
cervical cancers. Based on these results, the present report
further investigates the splicing patterns of the PRDX2 and RAB1B
genes in normal cervical tissue, preinvasive cervical lesions and
invasive cervical tumors. We also studied the expression patterns
of other RAB gene members of the RAS oncogene family - RAB1A, RAB5A
and RAB25, which are associated with different cancers.
Materials and methods
Between 2012 and 2013, 28 women were enrolled in the
present study: 10 healthy female volunteers (c1–c10), eight women
with preinvasive cervical lesions (p1–p8), and 10 women with
invasive cervical tumors (i1–i10). Each woman underwent a pelvic
examination followed by biopsy collection at one of two departments
in Stockholm, Sweden: the Division of Obstetrics and Gynecology,
Karolinska University Hospital, or the Department of Gynecological
Oncology, Radiumhemmet, Karolinska University Hospital. All
biopsies were immediately flash frozen in liquid nitrogen and then
stored at −80°C. Biopsies were grouped according to morphological
diagnosis. Ethical approval was obtained from the Regional Ethics
Review Board in Stockholm, and an informed consent form was signed
by all participants prior to inclusion.
RNA preparations, cDNA synthesis
Approximately 30 mg of biopsy tissue was homogenized
in microtubes with disposable pestles driven by cordless motor
(VWR, Radnor, PA, USA). Total RNA from homogenized biopsies was
isolated using RNeasy kits (Qiagen, Hilden, Germany) according to
the manufacturer’s instructions. Total RNA (200 ng) was reverse
transcribed with SuperScript III, and amplified with Platinum Taq
DNA polymerase in 30 μl of a one-step RT-PCR system (Invitrogen,
Carlsbad, CA, USA) according to the manufacturer’s instructions.
The reverse transcription reaction was performed at 50°C to destroy
mRNA secondary structures.
3′ RACE RT-PCR
To detect multiple transcripts of the PRDX2, RAB1A,
RAB1B, RAB5A and RAB25 genes, we applied the rapid amplification of
cDNA 3′ ends (3′ RACE) RT-PCR approach, which uses the natural
polyA tail that exists at the 3′ end of most eukaryotic mRNAs for
priming during reverse transcription. cDNAs were generated using an
oligo-dT-adaptor GACTCGAGTCGACATCGATAG(T)19 primer that complements
the polyA stretch and adds a special adaptor sequence to the 5′ end
of each cDNA. Further amplification of cDNAs in the one-step RT-PCR
reaction was performed using the reverse TAILR,
GACTCGAGTCGACATCGATAG primer targeting the adaptor sequence, and
forward PRDX164F, CAGTCATGGCCTCCGGTAA; RAB1A397F,
CTGCAGTGACATGTCCAGCAT; RAB1B16F, CCATGAACCCCGAATATGACTA; RAB5A508F,
CTGGAAGTTCATTGAAGAGTTGA; and 25RAB210F, CTCCATGCGGAGCCAAGAT
primers, corresponding to sequences located in the 5′ regions of
the untranslated parts of the PRDX, RAB1A, RAB1B, RAB5A and RAB25
transcripts, respectively. As these forward primers are located in
front of the initiation ATG codons, PCR products corresponding to
both the PRDX or RAB transcripts included the entire translated
region in each case. The resulting PCR products were gel-purified
using QIAquick gel extraction kits (Qiagen), and then further
amplified and sequenced by BigDye Terminator kits (Applied
Biosystems, Foster City, CA, USA) using the internal reverse
primers PRDX864R, GGTCCCATACTGTGGAGTT; RAB1A2522R,
CCATGTATTTCAATTGCCTGTT (or RAB1A1957R, CTACCTCTACCACAGATGCATT);
RAB1B1701R, GACTTGCTTTCTTGCAGGAAGCA (or RAB1B1551R,
TCCCTGGTGGGCTCCAGAGA); RAB5A2493R, CCAACCTGAGCACCTCAATATA (or
RAB5A2057R, CCTATTTACAGTACAGCTGAAGAT); and 25RAB1077R,
GGACAGATAAAAGAGGTATTTGTG which correspond to the sequences located
within untranslated 3′ regions of the PRDX, RAB1A, RAB1B, RAB5A and
RAB25 genes, respectively. RAB1A, RAB1B, RAB5A and RAB25 internal
reverse primers were also used in 40 cycles of nested PCR for
further analyses of non-specific (NS) DNA generated from the first
round of PCR and separated on agarose gels.
Results
Total RNAs from normal cervical tissue, preinvasive
cervical lesions and invasive cervical tumors, were screened for
the presence of PRDX, RAB1A, RAB1B, RAB5A and RAB25 transcripts by
a sensitive RT-PCR approach. The primers used in the PCR reaction
spanned the entire coding region of all five genes, and each case
included the initiation ATG codon and the polyA tail. All PRDX
amplification products from normal cervical tissue, preinvasive
cervical lesions and invasive cervical tumors consisted of a single
PCR product generated from the full-length transcripts (data not
shown).
All RAB1A amplification products from all samples of
normal cervical tissue, preinvasive cervical lesions and invasive
cervical tumors consisted of a long PCR product and two shorter 400
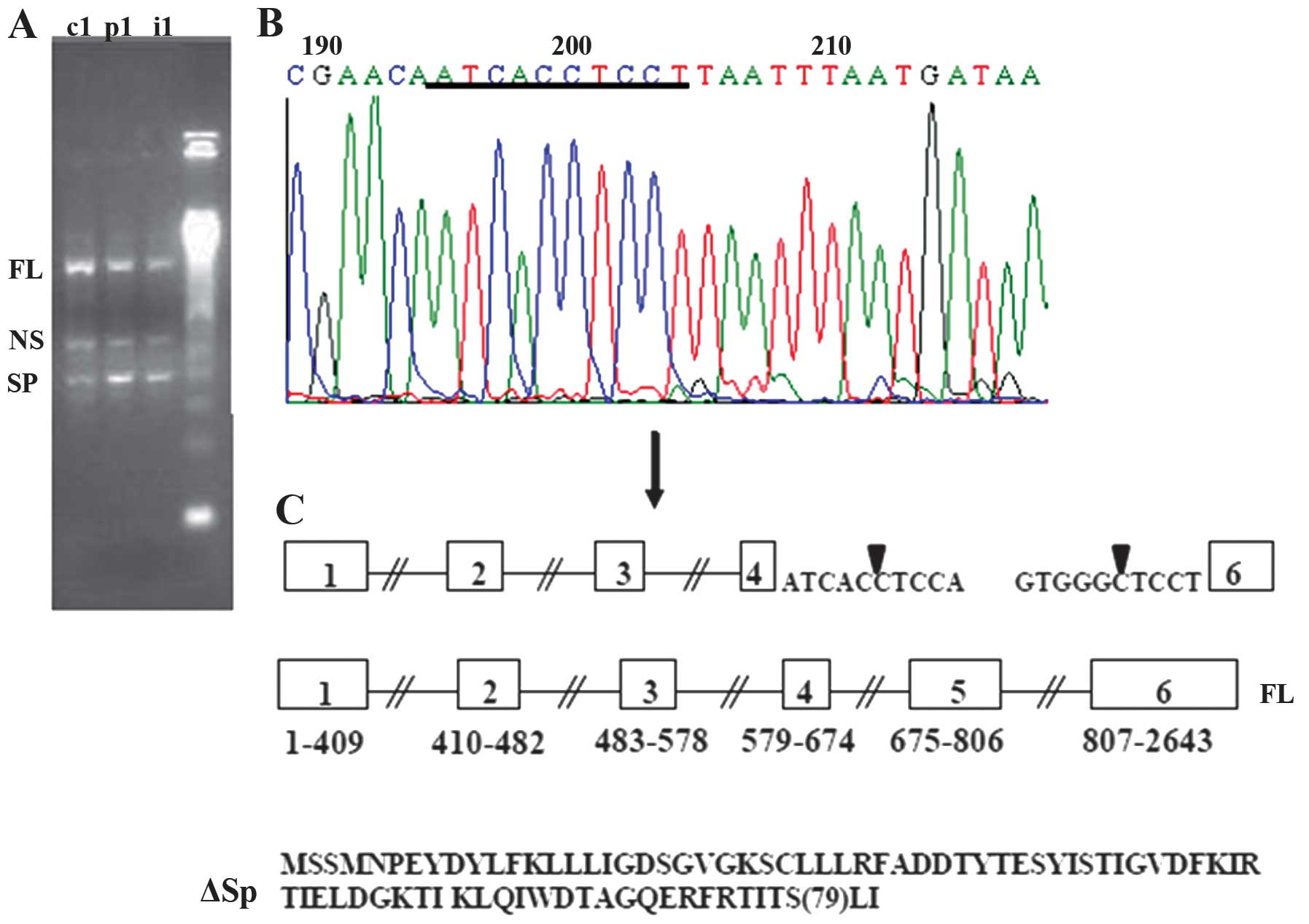
and 550 bp bands (Fig. 1A). All
these products, were cut from the gel, and the DNA was extracted
and amplified by nested PCR employing the RAB116F primer (the same
forward primer used in the first round of PCR) and the RAB1A2522R
primer, which targets the sequence located within the 3′
untranslated region of the transcript. This round of amplification
generated only one shorter (~400 bp) RAB1A-specific band, which was
also extracted from the gel and sequenced using the RAB1A397F
forward and RAB1A2522R or RABA1957R reverse primers. Sequence
analysis revealed that a long PCR product (FL) was generated from
the full-length transcripts while 400 bp band correspond to a new
spliced transcripts (SP) where non-classical splicing sites were
used to excise the RAB1A sequence between exons 4 and 6 (Fig. 1B and C).
To eliminate possibility of missing RAB1A
transcripts which did not amplify sufficiently due to some cDNA/PCR
reaction bias, and were thus undetectable in gel analyses of
first-round PCR product, we also analyzed by nested PCR DNA
extracted from all samples in the areas between FL and NS bands, NS
and SP bands and SP band and 250 bp but did not find any new
RAB1A-specific PCR fragments.
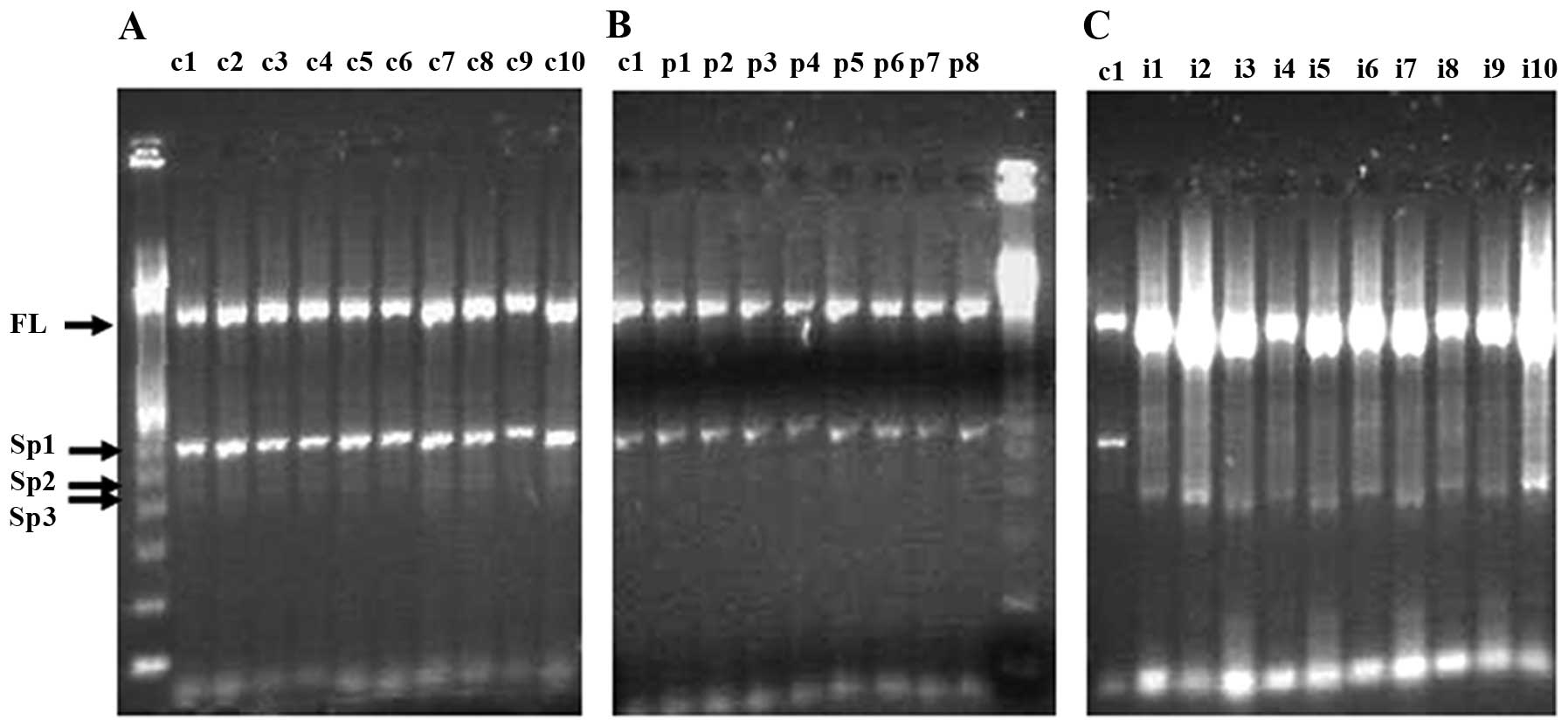
RAB1B amplification products from invasive cervical
tumors consisted of a single PCR product generated from the
full-length transcripts of this gene (Fig. 2C), while amplification products
from all normal cervical tissue and preinvasive cervical lesions
consisted of a shorter PCR product of ~600 bp, together with some
visible less abundant amplification products in the range of
400–500 bp (Fig. 2A and B). To
further analyze full-length and shorter products, they were cut
from the gel, and the DNA was extracted and amplified by a nested
PCR reaction employing the RAB116F primer (the same forward primer
as used in the first round of PCR) and the RAB1B1701R reverse
primer targeting the sequence located within the 3′ untranslated
region of the transcript. This round of amplification generated
three new RAB1B-specific PCR products which were sequenced using
the RAB116F forward and RAB1701R and RAB1551R reverse primers. We
also further amplified first round PCR material from normal and
preinvasive tissues in the range 250–400 and 500–1400 bp (i.e.,
excluding full-length and 400–500 bp product) but did not detect
any shorter RAB1B-specific PCR products.
RAB1B amplification products from invasive cervical
tumors consisted of a single PCR product generated from the
full-length transcripts of this gene. As no visible bands
corresponding to the shorter RAB1B-specific products (found in
normal tissues or preinvasive lesions) were detected in invasive
tumors after analyzing of 2 μl (equal for all samples in all
groups) of standard RT-PCR reactions (data not shown), we analyzed
larger (8 μl) volumes of the RT-PCR reactions from invasive tumors
(Fig. 2C). However, no visible
bands corresponding to new shorter Rab1b-specific products were
observed. DNA from the regions of the gel corresponding
approximately to the range 250–1400 bp (i.e., including the visible
product at ~270 bp (Fig. 2C), but
excluding the prominent full-length product) and also from the
region containing the product corresponding to full-length cDNA,
were extracted from the gel and subjected to nested PCR. However,
this yielded no RAB1B-specific products, suggesting that all this
material represents NS amplification products from the first round
of PCR reactions, in which cDNAs of invasive tumor material were
used as templates.
An interesting feature of the RAB1B alternative
splicing pattern was that the pattern of expression was similar for
all individuals of the same group, reflecting the existence of
common mechanisms for the generation of new transcripts.
The RT-PCR experiments were designed so that the
RNAs of the same amount were treated in the same way from the same
master mix in three independent experiments. Thus, these results
serve as good controls for RNA integrity and confirm that the new
RAB1B forms detected in the study are not artifacts. Sequence
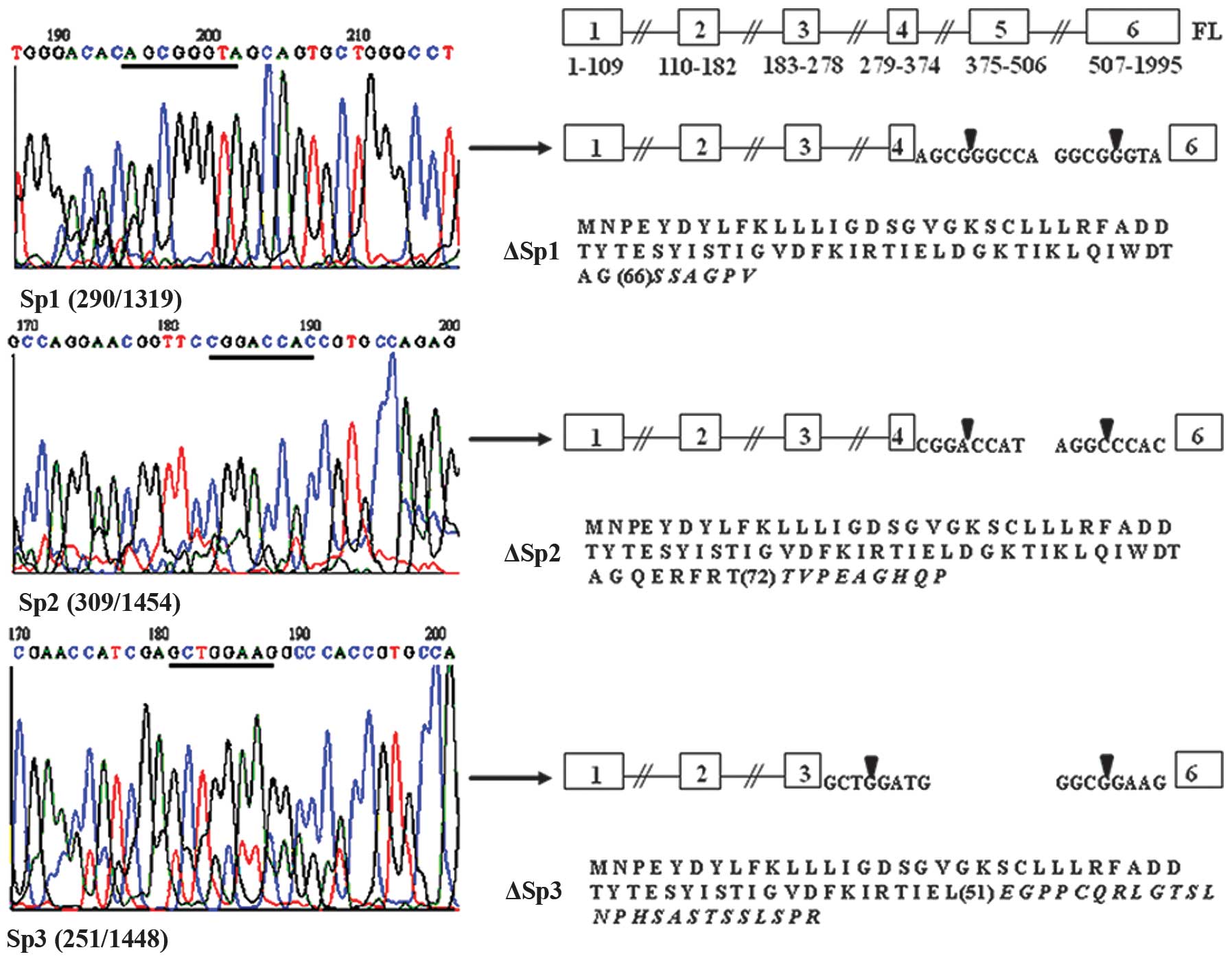
analysis revealed that all of the shorter RAB1B-specific PCR
products Sp1, Sp2 and Sp3 (Fig. 3)
correspond to three new transcripts with sizes of 600, 484 and 422
bp, respectively. In the Sp1 and Sp3 transcripts, classical
splicing sites are used to excise the RAB1B sequence between exons
4 (Sp1) or 3 (Sp3), and exon 6, while the Sp2 transcript is
generated due to splice sites comprised of non-classical splicing
signals (Fig. 3).
All RAB5A and RAB25 amplification products from
normal cervical tissue, preinvasive cervical lesions and invasive
cervical tumors consisted of a single PCR product corresponding to
the full-length transcripts of RAB5A and RAB25 (data not shown). We
extracted all DNA in the area between 250 bp and the full-length
band from all samples and amplified DNA extracts by nested PCR
employing the same forward primers used in the first round of PCR,
and reverse RAB5A- and RAB25-specific primers targeting the
sequence located within the 3′ untranslated regions of the
transcripts. We did not detect any new RAB5A- or RAB25-specific PCR
products.
Discussion
In this study we investigated transcription of
PRDX2, RAB1A, RAB1B, RAB5A and RAB25 genes in normal cervical
tissue, preinvasive cervical lesions and invasive cervical tumors.
PRDX2 and RAB1B gene expression was found to be associated with
cervical cancer in our proteomics study (24). The RAB1A gene is structurally and
functionally very similar to the RAB1B gene, and is associated with
the occurrence of tongue and prostate cancers (25,26).
The RAB5A gene is also similar to the RAB1B gene; it is upregulated
in cervical cancer and expresses a protein that is a key regulator
of intracellular vesicle traffic from the plasma membrane to early
endosomes. Silencing of RAB5A expression in human cervical
carcinoma in HeLa and SiHa cell lines decreased cell motility and
invasiveness (27). RAB25 gene
expression is upregulated in ovarian and breast cancers, and the
level of Rab25 protein overexpression is associated with more
aggressive cancer forms (28).
We found that only the closely related RAB1A and
RAB1B genes could express alternatively spliced RNAs in cervical
tissue. No spliced forms of the RAB1A or RAB1B genes have been
described thus far, making the findings described in this study
novel. We also demonstrate that new RAB1B mRNA transcripts, which
are expressed in normal cervical tissue and preinvasive cervical
lesions, are downregulated in invasive cervical tumors.
Both RAB1A and RAB1B genes consist of five introns
and six exons (Figs. 1 and
2), and belong to a group of small
Ras-related GTP-binding proteins encoded by RAB genes, and
participate in the regulation of vesicular transport from the
endoplasmic reticulum to the Golgi in mammalian cells. Many of the
>60 different human Rab proteins that have been identified have
been localized to discrete subcellular compartments (29).
All new RAB1A and RAB1B transcripts found in this
study contain an initiation translation codon ATG, and therefore
should be capable of being translated into proteins. An interesting
feature of such proteins (ΔSp, ΔSp1, ΔSp2 and ΔSp3) is that all of
them are the products of short reading frames (1–79 aa for ΔSp,
1–66 aa for ΔSp1, 1–72 aa for ΔSp2 and 1–51 aa for ΔSp3) in which
the N-terminus of the RAB1B gene is followed by very short,
unspecific amino acid sequences (Figs.
1 and 2). The Rab1a and Rab1b
protein sequences are very similar and contain several functional
domains. A detailed domain/function description for the Rab1a/b
proteins is not yet available, but it is still possible to predict
that the new proteins contain one or two GTP-binding sites, for
Rab1b protein at positions 15–22 and 63–67, respectively
(http://www.uniprot.org/uniprot/Q9H0U4). All of the
other known domains of the Rab1b protein: the Swich 2, a
determinant of nucleotide-dependent functions aa 64–84, the CAAX
box which directs post-translational modifications, and the
hypervariable region targeting Rab1b to specific membranes (both
located near the -COOH terminus of the protein), are missing from
the new Rab1b (and most probably from Rab1a) proteins. Both Ras and
several Rab proteins are known to be implicated in the development
of different cancers (25–28,30).
Variations in Ras protein expression have tumorigenic potential,
since membrane traffic is of key importance in cancer biology due
to its influence on cell polarity in the metastatic transformation
of tumor cells (29). Ras/Rab
proteins are involved in malignant progression, as they are key
components of the signal transduction pathways triggered by
different mitogens (30). Because
Rab proteins can interact with their molecular targets in the GTP
bound conformation, it could be hypothesized that tissue-specific
alternative splicing and translation of both Rab1a/b and likely
other Rab proteins as well, could generate peptides that only
retain GTP-binding sites; thus the multitude of such peptides
effectively translated from the short reading frames could
influence the intracellular concentrations of GTP, which regulates
tumorigenesis and other cellular functions. The disappearance of
the alternative transcripts of the RAB1B gene in invasive cervical
cancer could be related to known molecular mechanisms of HPV
tumorigenesis. In cervical cancer, one of the key events of
malignant transformation is HPV DNA integration into the host
chromosome. This is followed by the sustained expression of the
viral late genes E6 and E7, whilst other portions of the viral DNA
are deleted or undergo transcription disturbance (31). Such events could reduce cellular
levels of the splicing activator ASF/SF2 (activated by the HPV E2
protein) in tumors, while the splicing silencer hnRNP A1 is
upregulated after HPV infection (23). Thus the splicing of a number of
genes, including RAB1B, which may be dependent on the ASF/SF2:
hnRNP A1 ratio, could be inhibited.
We used nested PCR to amplify all RAB1A, RAB1B,
RAB5A and RAB25 first-round PCR DNA products extracted from all
agarose gel areas which did not contain visible bands, but did not
find any gene-specific products. This eliminated possibility of
missing potential transcripts which did not amplify sufficiently
due to some cDNA/PCR reaction bias, and were thus undetectable in
gel analyses of first-round PCR products.
In conclusion, these results suggest that multiple,
alternatively spliced RAB1A/B transcripts are generated in human
cervical tissue and may be translated into short proteins that are
capable of GTP binding. The expression of RAB1B transcripts is
inhibited in invasive cervical tumors, probably due to a shift in
the HPV-regulated ratio between splicing activators and splicing
inhibitors. A study of other members of the Ras/Rab family in the
same types of tissue as those in this study would be of great
interest to determine whether the silencing of alternative splicing
identified in this study has a more general impact on cervical
cancer development. Analysis of RAB1B may also help to identify
women who are at risk of developing cervical cancer. Indeed, the
definition of an objective molecular marker to identify these women
is of extreme importance, as cytomorphological identification has
proven difficult, and the percentage of HPV positivity in young
women is high. Therefore, we plan to conduct both retrospective
studies using archival liquid-based cytology samples, and
prospective randomized studies using routinely collected
slides.
Acknowledgements
We wish to thank Trudy Perdrix-Thoma for editing
assistance and English language review.
References
|
1
|
Black DL: Mechanisms of alternative
pre-messenger RNA splicing. Annu Rev Biochem. 72:291–336. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Singh RK and Cooper TA: Pre-mRNA splicing
in disease and therapeutics. Trends Mol Med. 18:472–482. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Pal S, Gupta R and Davuluri RV:
Alternative transcription and alternative splicing in cancer.
Pharmacol Ther. 136:283–294. 2012. View Article : Google Scholar
|
|
4
|
Garcia-Blanco MA, Baraniak AP and Lasda
EL: Alternative splicing in disease and therapy. Nat Biotechnol.
22:535–546. 2004. View
Article : Google Scholar : PubMed/NCBI
|
|
5
|
Mills AA: p53: link to the past, bridge to
the future. Genes Dev. 19:2091–2099. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Lamolle G, Marin M and Alvarez-Valin F:
Silent mutations in the gene encoding the p53 protein are
preferentially located in conserved amino acid positions and
splicing enhancers. Mutat Res. 600:102–112. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Neklason DW, Solomon CH, Dalton AL, Kuwada
SK and Burt RW: Intron 4 mutation in APC gene results in splice
defect and attenuated FAP phenotype. Fam Cancer. 3:35–40. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Mazoyer S, Puget N, Perrin-Vidoz L, Lynch
HT, Serova-Sinilnikova OM and Lenoir GM: A BRCA1 nonsense mutation
causes exon skipping. Am J Hum Genet. 62:713–715. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Ghigna C, Valacca C and Biamonti G:
Alternative splicing and tumor progression. Curr Genomics.
9:556–570. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
David CJ and Manley JL: Alternative
pre-mRNA splicing regulation in cancer: pathways and programs
unhinged. Genes Dev. 24:2343–2364. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Bosch FX and Muñoz N: The viral etiology
of cervical cancer. Virus Res. 89:183–190. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Walboomers JM, Jacobs MV, Manos MM, et al:
Human papillomavirus is a necessary cause of invasive cervical
cancer worldwide. J Pathol. 189:12–19. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
zur Hausen H: Papillomaviruses and cancer:
from basic studies to clinical application. Nat Rev Cancer.
2:342–350. 2002.PubMed/NCBI
|
|
14
|
Muñoz N, Bosch FX, de Sanjosé S, et al:
Epidemiologic classification of human papillomavirus types
associated with cervical cancer. N Engl J Med. 348:518–527.
2003.
|
|
15
|
Muñoz N: Human papillomavirus and cancer:
the epidemiological evidence. J Clin Virol. 19:1–5. 2000.
|
|
16
|
Clifford GM, Smith JS, Plummer M, Muñoz N
and Franceschi S: Human papillomavirus types in invasive cervical
cancer worldwide: a meta-analysis. Br J Cancer. 88:63–73. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Sundström K, Ploner A, Dahlström LA, et
al: Prospective study of HPV16 viral load and risk of in situ and
invasive squamous cervical cancer. Cancer Epidemiol Biomarkers
Prev. 22:150–158. 2013.PubMed/NCBI
|
|
18
|
Lazo PA: Papillomavirus integration:
prognostic marker in cervical cancer? Am J Obstet Gynecol.
176:1121–1122. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Kalantari M, Karlsen F, Kristensen G, Holm
R, Hagmar B and Johansson B: Disruption of the E1 and E2 reading
frames of HPV 16 in cervical carcinoma is associated with poor
prognosis. Int J Gynecol Pathol. 17:146–153. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Kalantari M, Blennow E, Hagmar B and
Johansson B: Physical state of HPV16 and chromosomal mapping of the
integrated form in cervical carcinomas. Diagn Mol Pathol. 10:46–54.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Romanczuk H and Howley PM: Disruption of
either the E1 or the E2 regulatory gene of human papillomavirus
type 16 increases viral immortalization capacity. Proc Natl Acad
Sci USA. 89:3159–3163. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
McPhillips MG, Veerapraditsin T, Cumming
SA, et al: SF2/ASF binds the human papillomavirus type 16 late RNA
control element and is regulated during differentiation of
virus-infected epithelial cells. J Virol. 78:10598–10605. 2004.
View Article : Google Scholar
|
|
23
|
Cheunim T, Zhang J, Milligan SG,
McPhillips MG and Graham SV: The alternative splicing factor hnRNP
A1 is up-regulated during virus-infected epithelial cell
differentiation and binds the human papillomavirus type 16 late
regulatory element. Virus Res. 131:189–198. 2008. View Article : Google Scholar
|
|
24
|
Hellman K, Alaiya AA, Becker S, et al:
Differential tissue-specific protein markers of vaginal carcinoma.
Br J Cancer. 100:1303–1314. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Shimada K, Uzawa K, Kato M, et al:
Aberrant expression of RAB1A in human tongue cancer. Br J Cancer.
92:1915–1921. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Sun T, Wang X, He HH, et al: MiR-221
promotes the development of androgen independence in prostate
cancer cells via downregulation of HECTD2 and RAB1A. Oncogene.
33:2790–2800. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Liu SS, Chen XM, Zheng HX, Shi SL and Li
Y: Knockdown of Rab5a expression decreases cancer cell motility and
invasion through integrin-mediated signaling pathway. J Biomed Sci.
18:582011. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Cheng KW, Lahad JP, Kuo WL, et al: The
RAB25 small GTPase determines aggressiveness of ovarian and breast
cancers. Nat Med. 10:1251–1256. 2004. View
Article : Google Scholar : PubMed/NCBI
|
|
29
|
Hutagalung AH and Novick PJ: Role of Rab
GTPases in membrane traffic and cell physiology. Physiol Rev.
91:119–149. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Hernández-Alcoceba R, del Peso L and Lacal
JC: The Ras family of GTPases in cancer cell invasion. Cell Mol
Life Sci. 57:65–76. 2000.PubMed/NCBI
|
|
31
|
Münger K, Baldwin A, Edwards KM, et al:
Mechanisms of human papillomavirus-induced oncogenesis. J Virol.
78:11451–11460. 2004.
|