Introduction
Antiangiogenic therapy is an effective method of
reducing tumor growth in animal models (1). Recently, several angiogenesis
inhibitors such as bevacizumab and endostatin have been applied in
clinical therapy. In addition, a growing number of compounds have
in recent years been discovered to inhibit tumor angiogenesis in
laboratory cancer studies (2,3).
Notably, some traditional medicines in clinical applications are
also found to have antiangiogenic properties (4).
Tetrandrine, a bisbenzylisoquinoline alkaloid
originally isolated from the roots of the medicinal plant
Stephaniae tetrandrae S. Moore (Han-Fang-Ji in Chinese), is
found to have a variety of pharmacological properties, including
anti-allergic, anti-inflammatory and anticancer activities
(5–7). For example, tetrandrine is shown to
inhibit proliferation and induce apoptosis in vitro in
hepatocellular, lung carcinoma, bladder cancer and colon carcinoma
(8–13). Tetrandrine is able to increase the
level of ROS and decrease the expression of glutathione, leading to
the inhibition of tumor cell proliferation (13,14).
It is also reported that tetrandrine can induce cell cycle arrest
and apoptosis in Hep G2 cells via the p53 and p21/WAF1 pathway
(15). Previous studies also
indicate that tetrandrine is a promising cancer therapy compound
for its effect on angiogenesis (6,16,17),
however, little is known on the molecular mechanisms for the
effects of tetrandrine in cell cycle and angiogenesis in
endothelial cells and its function in the vessels.
The EOMA cell line is derived from a mixed
hemangioen-dothelioma in an adult mouse and has a characteristic
protein expression profile of endothelial cell phenotype such as
high CD31 and CD45 expression (18–21).
It is known that EOMA cells are widely used in the research field
of angiogenesis inhibitors (22,23).
Endostatin, a clinically used anti-angiogenesis drug, has been
shown to be effective on EOMA cells (24). In the present study, tetrandrine
was able to cause G1/S cell cycle arrest by promoting excessive ROS
accumulation in EOMA cells and downregulating expression of
cyclins. We also show the anti-angiogenic effect in vivo of
tetrandrine in liver tumors in nude mice.
Materials and methods
Chemicals and antibodies
Tetrandrine was purchased from Shanghai Ronghe
Medical (Shanghai, China). 2′-7′-Dichlorodihydrofluorescein
diacetate (DCFH-DA), was purchased from Invitrogen (Carlsbad, CA,
USA). FuGene HD tranfection reagent was from Promega (Madison, WI,
USA) and trypan blue dye was from Sigma (St. Louis, MO, USA). The
ROS Assay kit and LY294002 were purchased from Beyotime (Nantong,
China). Propidium iodide (PI) was purchased from MP Biomedicals
(Solon, OH, USA).
3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT)
was purchased from Amresco LLC (Solon, OH, USA). Antibodies against
β-tubulin, cyclin D1, cyclin D2, cyclin D3, cyclin E1, cyclin E2,
CDK2, CDK4, GSK-3β, p53 and p27 were from Proteintech (Wuhan,
China), Akt and phosphor-Akt were obtained from Cell Signaling
Technology (Boston, MA, USA). Antibody against mouse CD31 was
obtained from Becton-Dickinson (Franklin, NJ, USA).
Cell lines and vectors
EOMA cells were purchased from the American Type
Culture Collection (ATCC, Manassas, VA, USA). Huh7 cells were
purchased from the China Center for Type Culture Collection (CCTCC,
Wuhan, China). All the cells were cultured at 37°C in a humidified
atmosphere of 95% air and 5% CO2 in high-glucose DMEM
supplemented with 10% fetal bovine serum, 1% penicillin and 1%
streptomycin. Cell culture dishes and plates were obtained from the
NEST Biotechnology Co., Ltd., Jiangsu, China.
Empty vector (PUSE) and Akt overexpression vector
(PUSE-CA-Akt) were obtained form Upstate Biotechnology (Lake
Placid, NY, USA). Briefly, EOMA cells were seeded at a
concentration of 5×104 cells/well in a 6-well plate and
grown at 37°C for 22 h before transfection. Then the cells were
transiently transfected using FuGene HD transfection reagent with 4
μg of PUSE or PUSE-CA-Akt, respectively. After transfection for
16–24 h, cells were treated with 30 μM tetrandrine for 48 h.
Cell proliferation assay
EOMA cells were seeded at a concentration of
5×104 cells/well in a 6-well plate and grown at 37°C for
22 h. The effects of tetrandrine on cell proliferation were
characterized by cell counting. In brief, various concentrations of
tetrandrine solution were added to different wells for 48 h. To
assess cellular proliferation, the cells were counted daily using a
hemocytometer.
Cell cycle analysis
EOMA cells were seeded in 60-mm plates at a
concentration of 5×105 cells/plate. After incubation for
22 h, various concentrations of tetrandrine solution were added,
and the cells were incubated for 48 h. EOMA cells were harvested
and supplemented with 70% ice-cold ethanol overnight. The cells
were treated with RNase (50 μg/ml) at 37°C for 1 h, and treated
with PI (20 μg/ml) for 30 min without light at 4°C. The DNA content
was analysed by flow cytometry (Beckman Coulter).
Western blot analysis
EOMA cells were treated with different
concentrations of tetrandrine for 48 h and harvested. Then,
supernatant was collected, and the protein concentration was
determined with a Bicinchoninic acid protein assay kit (Pierce).
Protein samples were separated by sodium dodecyl sulphate
polyacrylamide gel electrophoresis (SDS-PAGE) and transferred to a
PVDF membrane. Following antibody incubation, enhanced
chemiluminescence was used to detect the proteins.
Evaluation of ROS expression using
fluorescence microscopy and flow cytometry
EOMA cells were washed twice with PBS.
2′-7′-Dichlorodihydrofluorescein diacetate (DCFH-DA) was diluted to
10 μM with HG-DMEM and added to the cells. The plates were then
stored in an incubator at 37°C for 1 h. ROS were detected in EOMA
cells, and images were acquired with a fluorescence microscope.
EOMA cells were seeded in 6-well plates for 22 h and
subsequently incubated with various concentrations of tetrandrine,
NAC (20 mM) or pretreated with 20 mM NAC for 1 h followed by 50 μM
tetrandrine. After incubation for 48 h, EOMA cells were harvested,
and cells were centrifuged (150 × g for 10 min), supplemented with
DCFH-DA solution (1 μM) and incubated in the cell incubator at 37°C
for 1 h. The EOMA cells were centrifuged (250 × g for 10 min) and
washed with ice-cold PBS prior to flow cytometry (Beckman
Coulter).
Tumor xenografts
Five-week-old male BALB/c nude mice were obtained
from the Disease Prevention Centre of Hubei Province (Wuhan,
China). The Experimental Animal Centre of Wuhan University approved
the experimental protocols. The Huh7 cells were counted, and
2×107 cells were implanted in the right flank of each
mouse. When the tumor volume reached 150–300 mm3, the
mice were randomly distributed into control and treatment groups
(n=7) and gavaged. The control group received treatment with the
vehicle, which consisted of 0.5% (w/v) methylcellulose and 0.1%
(v/v) Tween-80 in sterile water. The treatment group was given
tetrandrine at 50 mg/kg of body weight for 20 days, and the tumors
were dissected and weighed.
Immunohistochemical analysis
CD31 panendothelial antigen (platelet/endothelial
cell-adhesion molecule) was used for microvessel staining in frozen
sections (25,26). Frozen sections were then blocked
with 5% goat serum and stained with rat anti-mCD31 antibody (1:50
dilution; BD Biosciences-Pharmingen) at room temperature for 1 h.
Sections were then washed with PBS and incubated for 1 h with
biotinylated poly-clonal anti-rat IgG antibody. The sections were
washed three times with PBS, reacted with the ABC peroxidase kit
(Vector Laboratories) at room temperature for 45 min, and washed
twice with PBS prior to mounting for light microscopy and
photography.
The number of vessels was counted in a blinded
manner in 18 sections (2 sections per slide, 3 slides per tissue)
of the tissue using a light microscope at ×200 magnification. Five
fields in each section were randomly selected, and the number of
vessels in each field was averaged (27).
Statistical analysis
The results are expressed as the mean ± SE. The
Tukey-Kramer multiple comparisons test was used to determine
statistical significance. A P-value of <0.05 was considered to
be statistically significant.
Results
Tetrandrine inhibits the proliferation of
EOMA cells
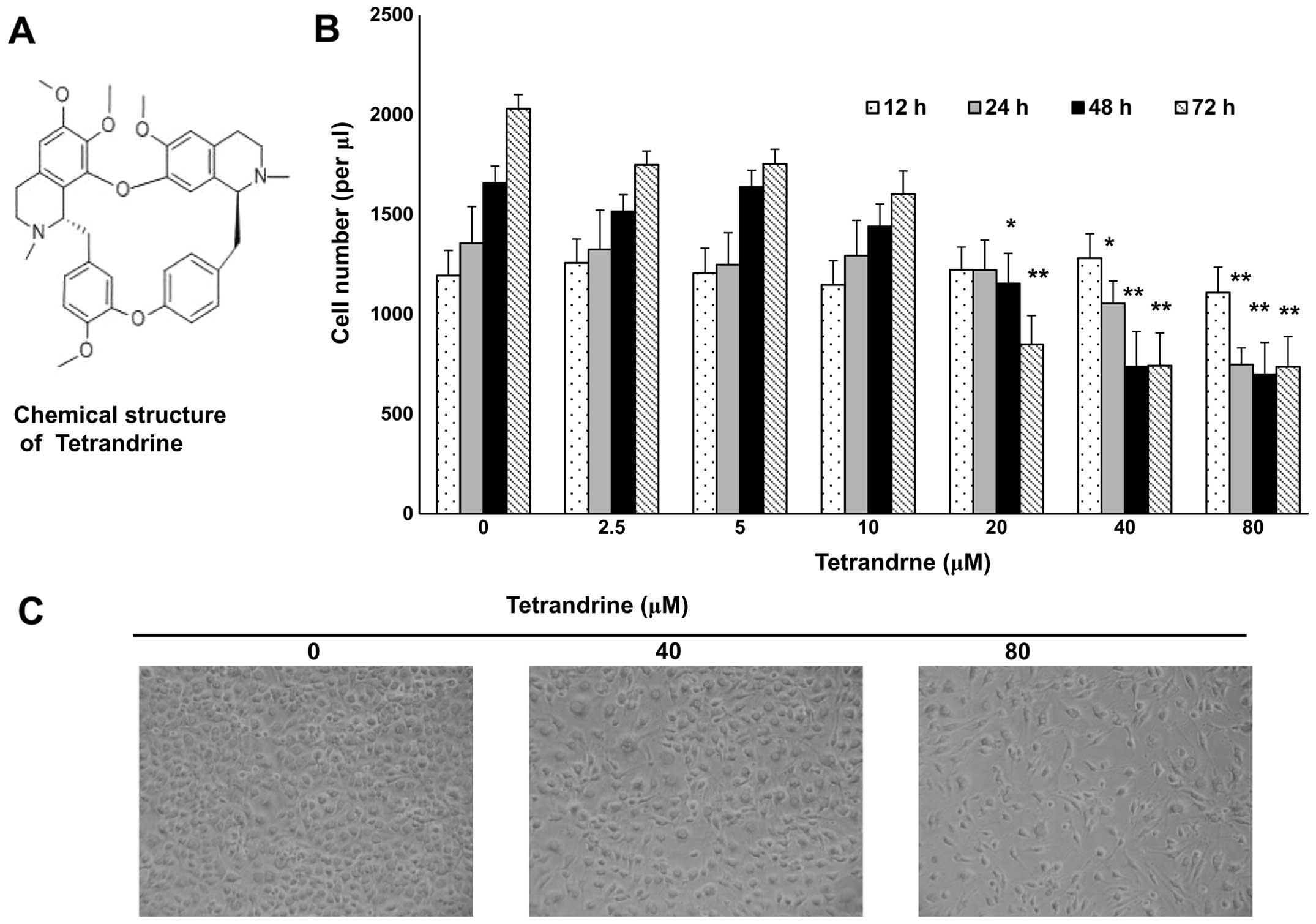
Tetrandrine, a compound isolated from a traditional
Chinese medicine plant (Fig. 1A),
has been found to be an antitumor agent (Fig. 1A) (10,28).
After treatment with tetrandrine at indicated concentrations for
12, 24, 48 and 72 h, EOMA cells were analysed in proliferation
assays. The proliferation of EOMA cells was inhibited, and the
growth of EOMA cells was clearly suppressed after treatment with
tetrandrine from 20 to 80 μM for 48 h (Fig. 1B and C).
Tetrandrine affects the expression of
G1/S cell cycle regulatory proteins in EOMA cells
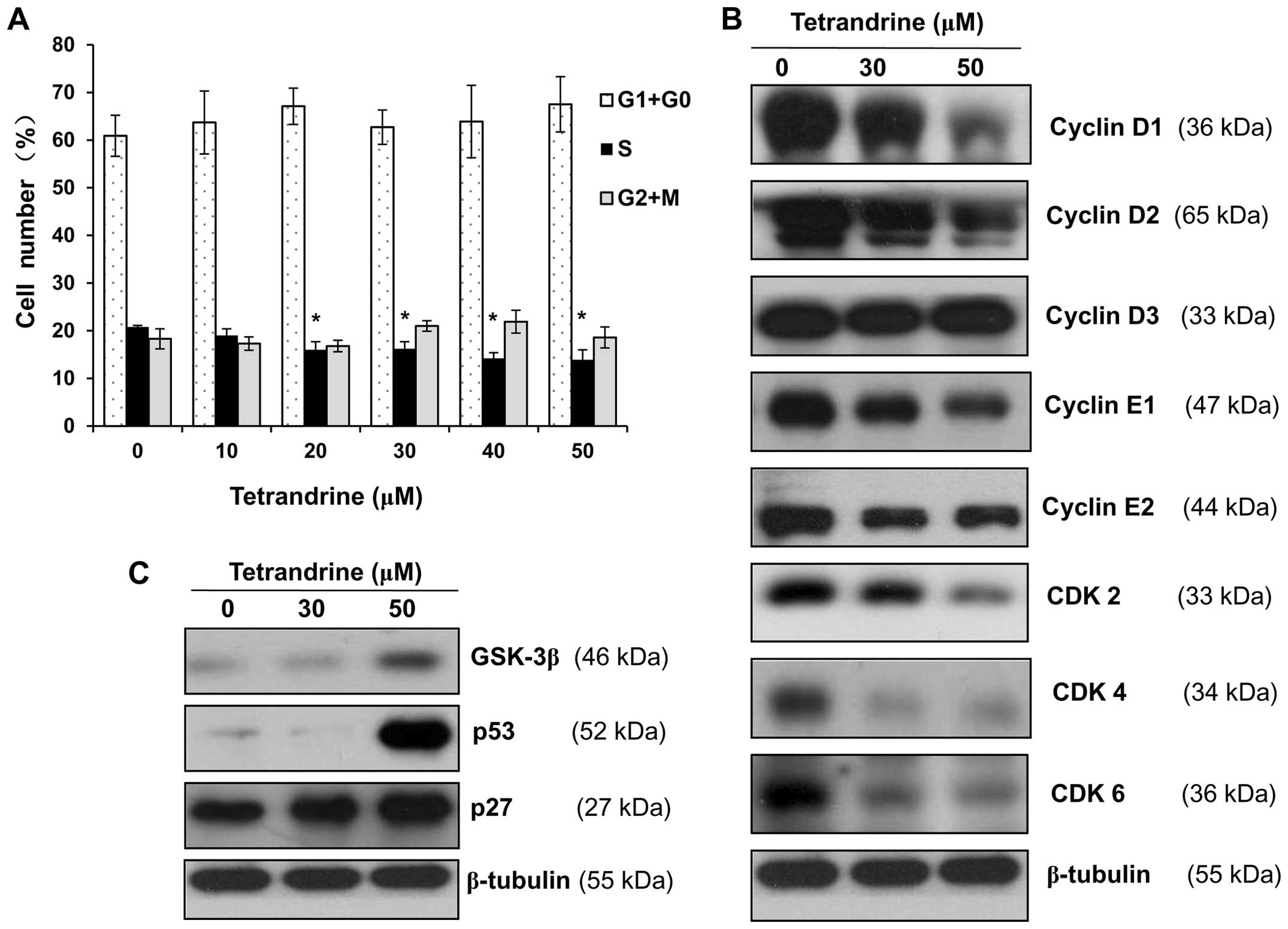
Based on the above results, tetrandrine
concentrations of 10, 20, 30, 40 and 50 μM and an incubation period
of 48 h were selected as conditions for cell cycle analysis by flow
cytometry. As shown in Fig. 2A,
the proportion of EOMA cells in S phase was decreased from 20.8% in
control cells to 19.0, 16.0, 16.2, 14.2 and 13.9% in cells treated
with tetrandrine for 48 h at concentrations of 10, 20, 30, 40 and
50 μM, respectively, while the proportion of cells in G1/G0 was
increased from 60.9 to 63.7, 67.1, 62.7, 63.9 and 67.5% under the
same treatments. Thus, these results indicated that tetrandrine
induced G1/S cell cycle arrest in EOMA cells in a dose-dependent
manner.
To further investigate the observed G1/S cell cycle
arrest, cell cycle regulation proteins, including cyclin D1, cyclin
D2, cyclin D3, cyclin E1, cyclin E2, CDK2, CDK4 and CDK6 were
determined by western blotting. The expression levels of cyclin D1,
cyclin D2, cyclin E1, cyclin E2, CDK2 CDK4 and CDK6 were decreased
in tetrandrine-treated cells compared to the control cells, but the
expression of cyclin D3 was not affected by tetrandrine treatment
(Fig. 2B). In addition, the
expression levels of the proteins, including GSK-3β, p53 and p27,
which were the inhibitors for the cell cycle regulators, were
upregulated with tetrandrine treatment (Fig. 2C).
Tetrandrine upregulates the ROS level in
EOMA cells
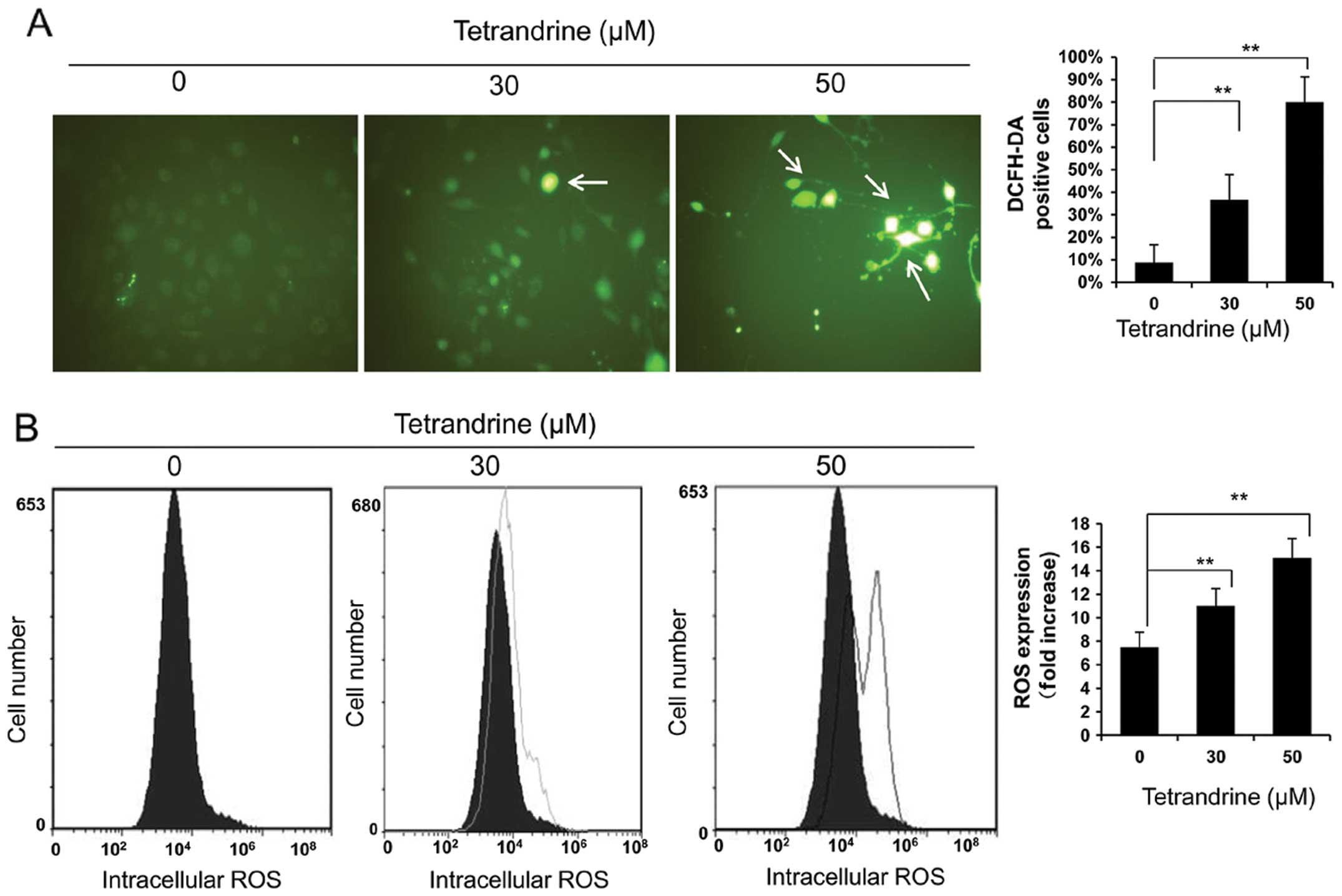
Since tetrandrine has been reported as an anticancer
agent both in vitro and in vivo by inducing apoptosis
through the formation of ROS (29), which can serve as important second
messengers to regulate a variety of downstream signalling pathways
(30–32), we determined the effect of
tetrandrine on intracellular ROS levels in EOMA cells using DCFH-DA
fluorescence. As shown in Fig. 3A,
the number of fluorescent sites, representing the level of ROS, was
higher in tetrandrine-treated cells than in the control cells. Flow
cytometry results also showed that the ROS level was increased by
treatments with 30 and 50 μM of tetrandrine as compared to the
control, up to 2-fold in the 50 μM tetrandrine-treated cells
(Fig. 3B). These results
demonstrate that ROS was produced in EOMA cells in response to
tetrandrine.
NAC inhibits G1/S cell cycle arrest in
tetrandrine-treated EOMA cells
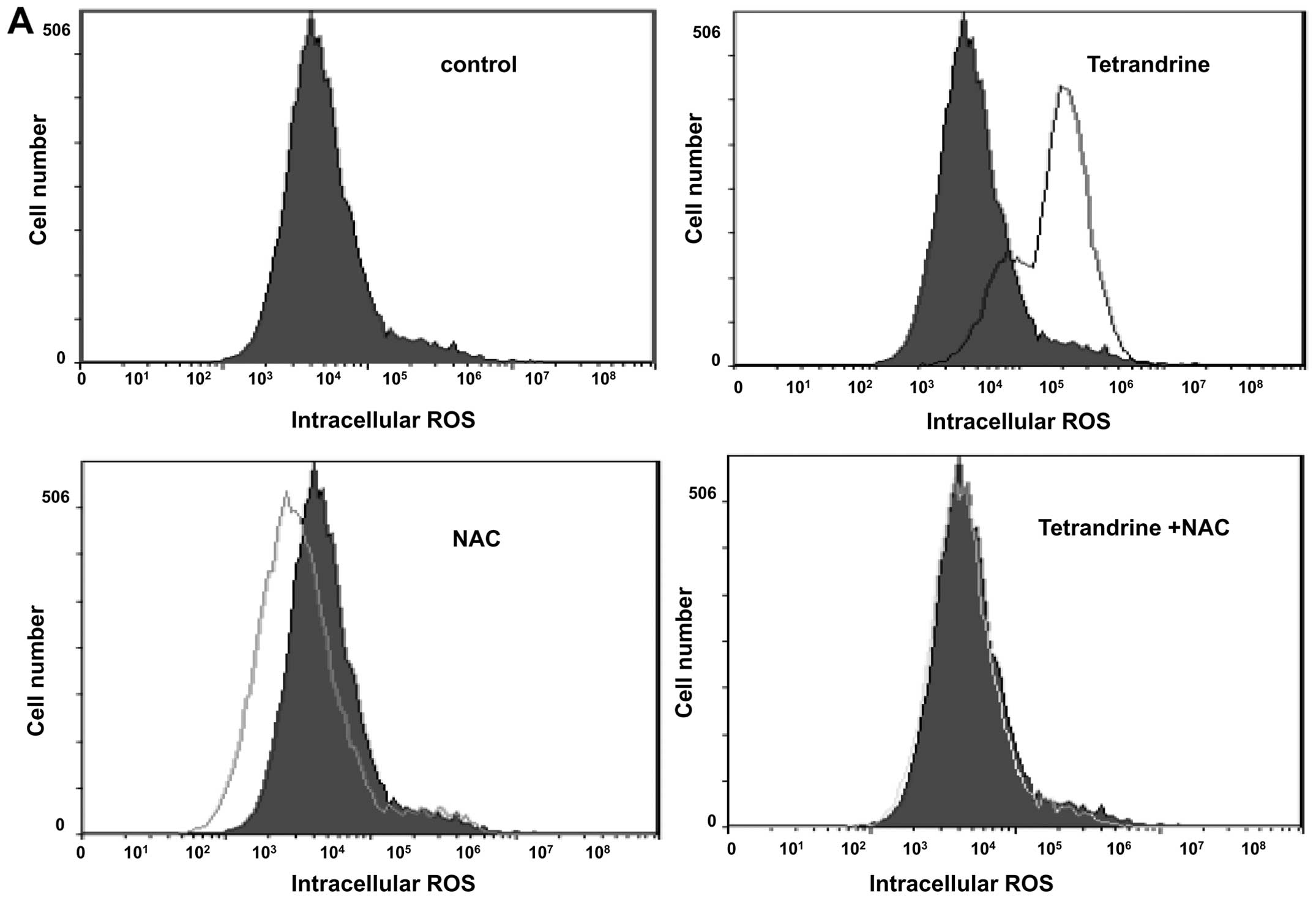
To further investigate the role of ROS in the
upregulation of tetrandrine-treated EOMA cells, the ROS inhibitor
was used. ROS accumulation was observed in EOMA cells after 48 h of
tetrandrine treatment, but was markedly abrogated when cells were
pretreated with the ROS scavenger NAC, demonstrating an effective
elimination of tetrandrine-induced ROS production by NAC (Fig. 4A). Also in the cell cycle analysis,
pretreatment with NAC inhibited the G1/S cell cycle arrest in
tetrandrine-treated EOMA cells (Fig.
4B and C).
To determine the association between cell cycle
arrest and the increased ROS in EOMA cells, the expression levels
of cyclins and their upstream proteins were analysed by western
blotting. The downregulation of cyclin D1, cyclin E1, CDK2, CDK4
and CDK6 in EOMA cells was inhibited by pretreatment with 20 mM of
NAC for 1 h prior to treatment with 50 μM of tetrandrine for 48 h
(Fig. 4D).
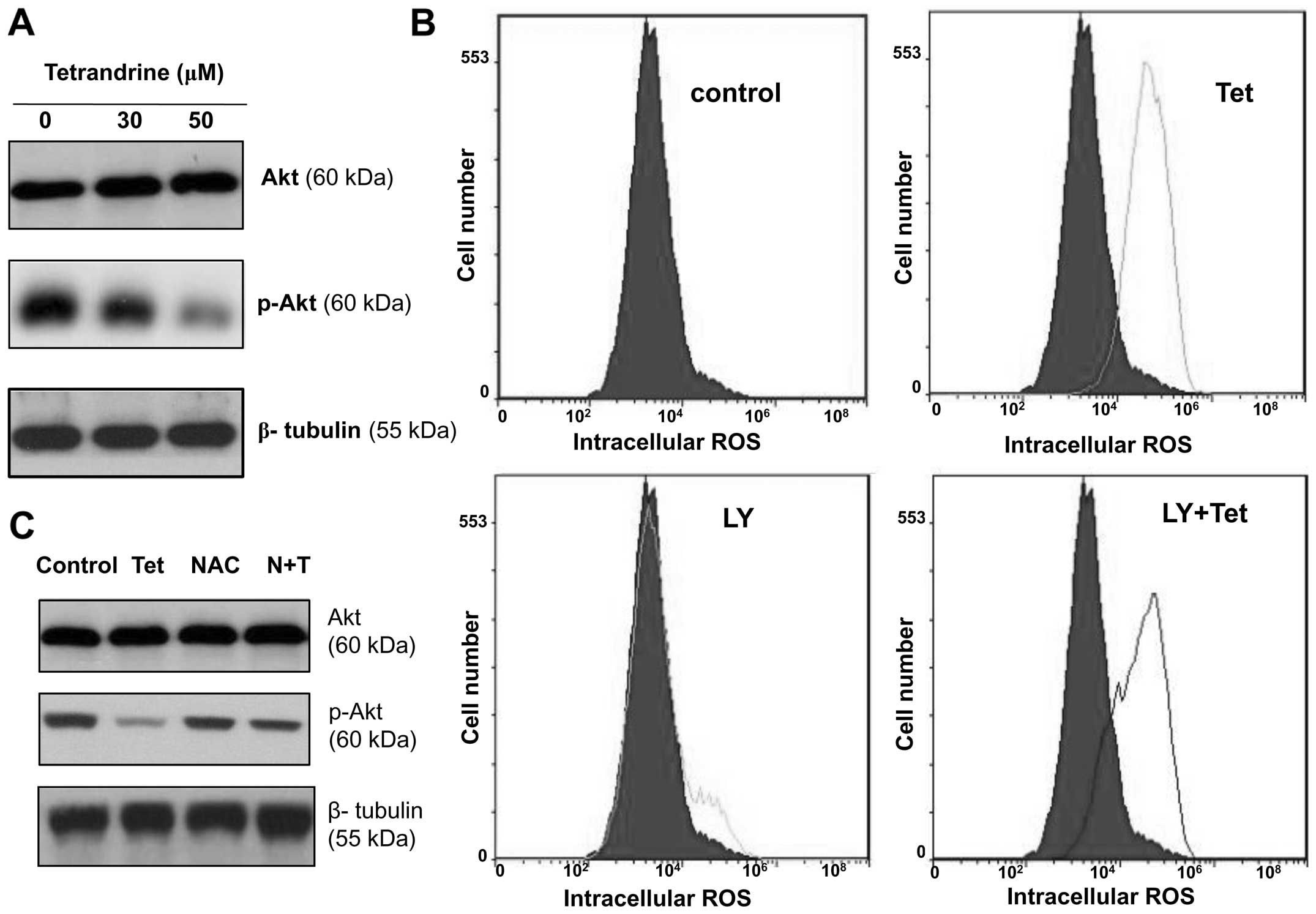
A decreased phospho-Akt protein level
after tetrandrine treatment was reversible with the removal of the
intracellular ROS by NAC
It is known that ROS generation is related with the
PI3K-Akt signalling pathway. Akt is a critical kinase that
regulates a variety of biological processes, including cell
survival, proliferation, autophagy and apoptosis (33–36).
To identify if Akt was involved in tetrandrine treated EOMA cells,
western blots were performed as previously described. As shown in
Fig. 5A, the level of
phosphorylated Akt was decreased after tetrandrine treatment
although the total Akt protein level remained unchanged. Then, to
determine whether tetrandrine induced ROS upregulation by
decreasing Akt activity, the EOMA cells were treated with
tetrandrine in combination with LY294002 (a PI3K/Akt inhibitor) for
48 h and the ROS level was determined by flow cytometry. The
results indicate that LY294002 did not inhibit tetrandrine-induced
increase of ROS in EOMA cells (Fig.
5B).
Next, to determine whether the phosphorylation of
Akt was regulated by the increased levels of ROS, the EOMA cells
were pretreated with 20 mM of NAC for 1 h followed by treatment
with 50 μM of tetrandrine for 48 h, and both total Akt and
phosphorylated Akt were analysed by western blotting. As shown in
Fig. 5C, the tetrandrine-induced
decrease of Akt phosphorylation was blocked by NAC pretreatment.
Taken together, these data suggest that ROS acted upstream of the
Akt pathway to regulate the cell cycle.
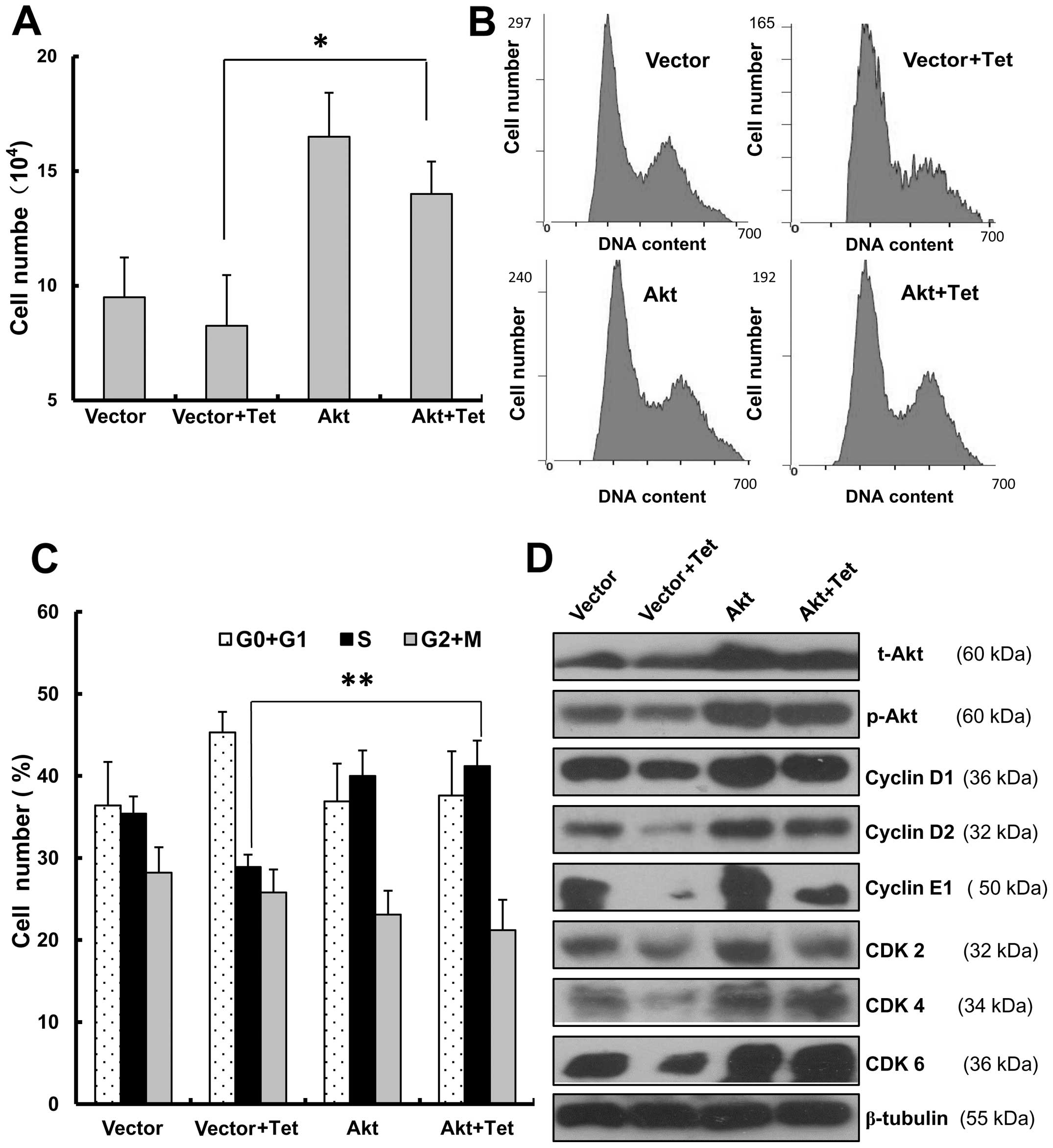
Overexpression of Akt decreased
tetrandrine-induced G1/S arrest
To further establish that Akt was involved in
tetrandrine-induced G1/S arrest, empty vector (PUSE) or PUSE-CA-Akt
was transfected into EOMA cells. After tetrandrine treatment, the
cell number of EOMA cells with Akt overexpression was nearly twice
that of the control cells (Fig.
6A). It indicated that Akt overexpression could promote cell
cycle transition and then had an effect on cell proliferation.
Next, even with tetrandrine treatment, it showed that the
proportion in S phase of EOMA cells with Akt overexpression had a
significantly increase to 41.2%, compared with the control cells
with 28.9% in S phase (Fig. 6B and
C). Finally, the western blot analysis also checked the
expression levels of cyclin D1, cyclin E1, CDK2, CDK4 and CDK6,
which were important for G1/S arrest. As shown in Fig. 6D, when EOMA cells were transfected
with the control vector, and the cells were treated with
tetrandrine, cyclin D1, cyclin E1, CDK2, CDK4 and CDK6 were
significantly downregulated. However, when the EOMA cells were
transfected with Akt overexpressing vector, and treated with
tetrandrine, the protein levels did not change much comparing with
the control cells. This also suggested that tetrandrine-induced
G1/S cell cycle arrest was rescued because of the overexpression of
Akt. Collectively, considering the above, we concluded that the
overexpression of Akt decreases tetrandrine-induced G1/S
arrest.
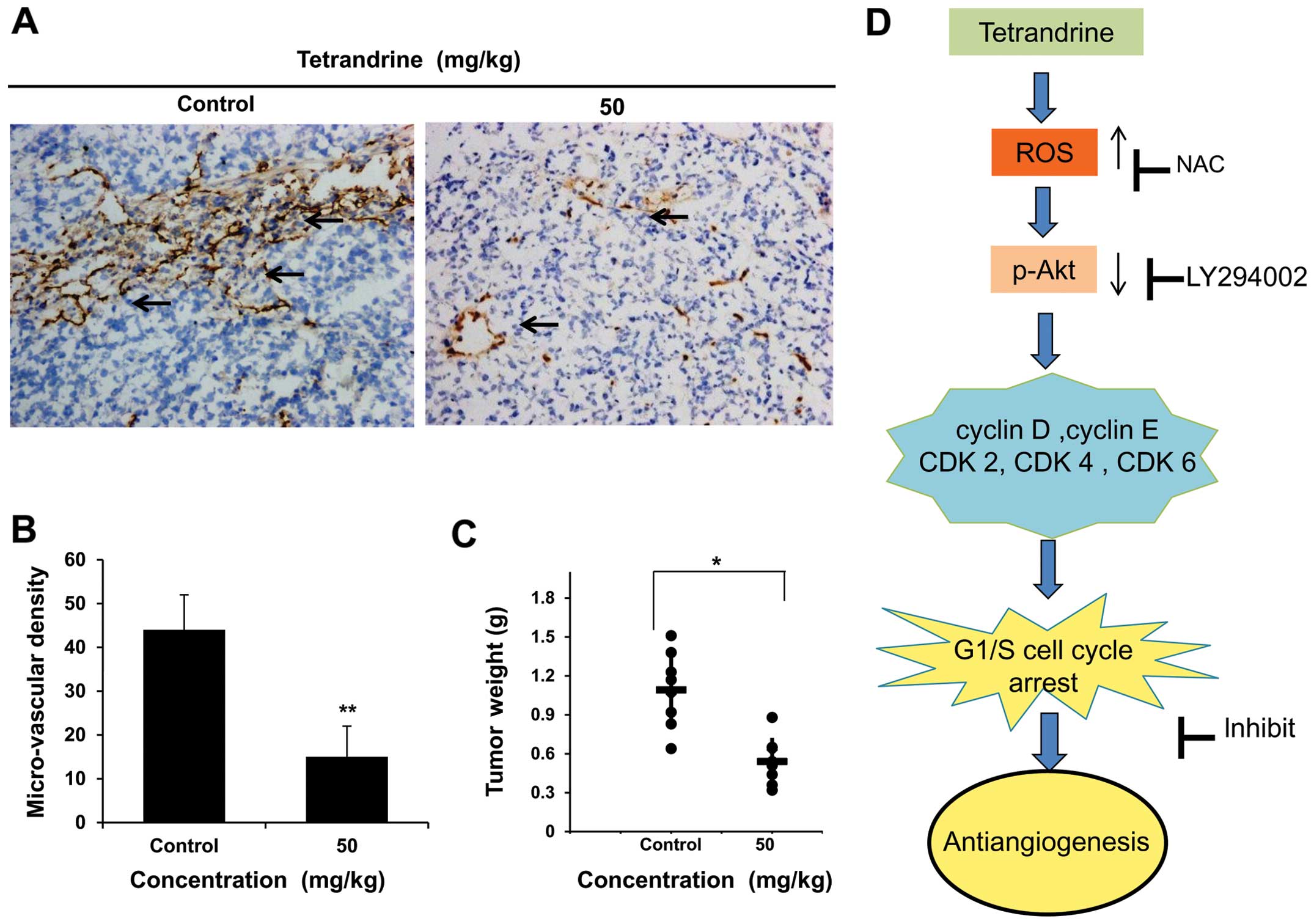
Tetrandrine inhibits angiogenesis in
liver cancer in vivo
Since the regulation of the endothelial cell cycle
is critical to the function of the vascular system (14), it suggests that tetrandrine may be
an effective angiogenesis inhibitor in cancer therapy. To evaluate
the antitumor effect of tetrandrine in vivo, we investigated
whether tetrandrine could inhibit tumor vessel growth in nude mice.
As previously described, the mice bearing Huh7 tumor xenografts
were gavaged with tetrandrine (50 mg/kg of body weight) or vehicle
every other day, and the amount of vessels inside the tumor was
observed in tumor sections after 20 days of treatment (9). Then we found the tumor vascular
growth was visually inhibited by tetrandrine, and the data
confirmed its anti-angiogenic activity (Fig. 7A). Vascular density was also
measured in anti-CD31 stained vasculatures from both control tumor
mice and tetrandrine-treated tumor mice. The results of
immunohistochemical analysis showed a significant decrease of
microvessel density in tetrandrine-treated group (Fig. 7B), further demonstrating the
inhibition of liver cancer vascular growth in nude mice bearing
liver cancer xenografts. Consistent with the reduction of tumor
vascular growth by tetrandrine treatment in vivo, the
average tumor weight in the animals treated with vehicle,
tetrandrine therapy was 1.11 and 0.53 g (P<0.01), respectively
(Fig. 7C). Therefore, tetrandrine
also has antitumor effect and one of the important reasons is its
anti-angiogenic function.
Discussion
In the present study, tetrandrine decreased cyclin
D, cyclin E and CDKs expression, and increased the expression
levels of the inhibitors of G1/S cell cycle arrest in endothelial
cells, resulting in G1/S arrest and anti-angiogenesis. A previous
study also reported that tetrandrine combined with cisplatin
enhances cytotoxicity and could induce G1 arrest and apoptosis in
ovarian cancer cells (37). In
human colon carcinoma HT-29 cells, tetrandrine-induced Akt
dephosphorylation was observed, and GSK-3β was able to induce
subsequent degradation of cyclin D1 (38).
Usually physiological stimuli are capable of
inducing Akt kinase activity through PI3 kinase, and ROS generation
is dependent on the PI3K-Akt signalling pathway (33,39,40).
To determine the relationship between ROS generation and
phosphorylated Akt inhibition in tetrandrine-induced endothelial
cell cycle arrest, NAC (a ROS inhibitor) and LY294002 (a PI3K/Akt
inhibitor) were used to treat EOMA cells. Phosphorylated Akt levels
were decreased after tetrandrine treatment, and this decrease was
prevented by NAC. Then it showed that tetrandrine-induced ROS
generation was not inhibited by LY294002 in EOMA cells. This result
suggested that ROS acts upstream of the PI3K/Akt signalling
pathway, while Akt did not have an effect on the ROS
accumulation.
Further experiments demonstrated that when EOMA
cells were overexpressed with Akt, tetrandrine-induced G1/S arrest
was inhibited indicating that tetrandrine can inhibit cell
proliferation through PI3K/Akt pathway. The PI3K/Akt/mTOR pathway
has an important role in cell metabolism, growth, migration,
survival and angiogenesis. Drug development aimed at this pathway
has been performed and is a frequent occurrence in human cancer
(40–42).
The data revealed that tetrandrine inhibited tumor
angiogenesis in vivo in Huh7 tumor-implanted nude mice.
Because CD31 is an endothelial marker, anti-CD31 staining was used
to assess the vascular density (27,43).
The result confirmed that the vascular density in the tumors in
control mice was higher than that in the tumors treated with
tetrandrine. Additionally, the tumors weight in the mice that were
treated with tetrandrine was lower than the mice that received
vehicle only.
In summary, tetrandrine induces G1/S cell cycle
arrest in EOMA cells by activating ROS and repressing Akt
phosphorylation, and ROS appears to be an upstream regulator of
Akt. Thus, tetrandrine possesses anti-angiogenic activity by
inhibiting EOMA cells proliferation via ROS/Akt pathway (Fig. 7D). In vivo experiments also
demonstrated that tetrandrine reduces the vascular density and
inhibits tumor growth. Therefore, our data suggest that tetrandrine
is a candidate for development as an anti-angiogenic agent.
Acknowledgements
Authors wish to thank Center for Medical Research in
Wuhan University for the help with the flow cytometric analysis.
The present study was supported by the National Program on Key
Basic Research Project (973 Program, no. 2010CB529804), the
National Natural Science Foundation of China (grant nos. 30971456,
31400155, 81472550, 81274048 and 81273540), and by the Innovation
Seed Fund of Wuhan University School of Medicine.
Abbreviations:
|
Tet
|
tetrandrine
|
|
ROS
|
reactive oxygen species
|
|
NAC
|
L-N-acetylcysteine
|
|
MTT
|
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide
|
|
DCFH-DA
|
2′-7′-dichlorodihydrofluorescein
diacetate
|
|
PI3K
|
phosphatidylinositol 3-kinase
|
References
|
1
|
Gerald D, Chintharlapalli S, Augustin HG
and Benjamin LE: Angiopoietin-2: an attractive target for improved
antiangiogenic tumor therapy. Cancer Res. 73:1649–1657. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Turley RS, Fontanella AN, Padussis JC, et
al: Bevacizumab-induced alterations in vascular permeability and
drug delivery: a novel approach to augment regional chemotherapy
for in-transit melanoma. Clin Cancer Res. 18:3328–3339. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Peng F, Xu Z, Wang J, et al: Recombinant
human endostatin normalizes tumor vasculature and enhances
radiation response in xenografted human nasopharyngeal carcinoma
models. PLoS One. 7:e346462012. View Article : Google Scholar
|
|
4
|
Sagar SM, Yance D and Wong RK: Natural
health products that inhibit angiogenesis: a potential source for
investigational new agents to treat cancer - Part 2. Curr Oncol.
13:99–107. 2006.
|
|
5
|
Li SY, Ling LH, Teh BS, Seow WK and Thong
YH: Anti-inflammatory and immunosuppressive properties of the
bis-benzylisoquinolines: in vitro comparisons of tetrandrine and
berbamine. Int J Immunopharmacol. 11:395–401. 1989. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Chen Y, Chen JC and Tseng SH: Tetrandrine
suppresses tumor growth and angiogenesis of gliomas in rats. Int J
Cancer. 124:2260–2269. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Lai JH: Immunomodulatory effects and
mechanisms of plant alkaloid tetrandrine in autoimmune diseases.
Acta Pharmacol Sin. 23:1093–1101. 2002.PubMed/NCBI
|
|
8
|
Wang G, Lemos JR and Iadecola C: Herbal
alkaloid tetrandrine: fron an ion channel blocker to inhibitor of
tumor proliferation. Trends Pharmacol Sci. 25:120–123. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Liu C, Gong K, Mao X and Li W: Tetrandrine
induces apoptosis by activating reactive oxygen species and
repressing Akt activity in human hepatocellular carcinoma. Int J
Cancer. 129:1519–1531. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Lee JH, Kang GH, Kim KC, et al:
Tetrandrine-induced cell cycle arrest and apoptosis in A549 human
lung carcinoma cells. Int J Oncol. 21:1239–1244. 2002.PubMed/NCBI
|
|
11
|
Li X, Su B, Liu R, Wu D and He D:
Tetrandrine induces apoptosis and triggers caspase cascade in human
bladder cancer cells. J Surg Res. 166:e45–51. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Wu JM, Chen Y, Chen JC, Lin TY and Tseng
SH: Tetrandrine induces apoptosis and growth suppression of colon
cancer cells in mice. Cancer Lett. 287:187–195. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Gong K, Chen C, Zhan Y, Chen Y, Huang Z
and Li W: Autophagy-related gene 7 (ATG7) and reactive oxygen
species/extracellular signal-regulated kinase regulate
tetrandrine-induced autophagy in human hepatocellular carcinoma. J
Biol Chem. 287:35576–35588. 2012. View Article : Google Scholar
|
|
14
|
Yu J, Tian S, Metheny-Barlow L, et al:
Modulation of endothelial cell growth arrest and apoptosis by
vascular endothelial growth inhibitor. Circ Res. 89:1161–1167.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Kuo PL and Lin CC: Tetrandrine-induced
cell cycle arrest and apoptosis in Hep G2 cells. Life Sci.
73:243–252. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Kobayashi S, Inaba K, Kimura I and Kimura
M: Inhibitory effects of tetrandrine on angiogenesis in
adjuvant-induced chronic inflammation and tube formation of
vascular endothelial cells. Biol Pharm Bull. 21:346–349. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Gao JL, Ji X, He TC, et al: Tetrandrine
suppresses cancer angio-genesis and metastasis in 4T1 tumor bearing
mice. Evid Based Complement Alternat Med.
2013:2650612013.PubMed/NCBI
|
|
18
|
Felbor U, Dreier L, Bryant RA, Ploegh HL,
Olsen BR and Mothes W: Secreted cathepsin L generates endostatin
from collagen XVIII. EMBO J. 19:1187–1194. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Wen W, Moses MA, Wiederschain D, Arbiser
JL and Folkman J: The generation of endostatin is mediated by
elastase. Cancer Res. 59:6052–6056. 1999.PubMed/NCBI
|
|
20
|
Gordillo GM, Onat D, Stockinger M, et al:
A key angiogenic role of monocyte chemoattractant protein-1 in
hemangioendothelioma proliferation. Am J Physiol Cell Physiol.
287:C866–C873. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Obeso J, Weber J and Auerbach R: A
hemangioendothelioma-derived cell line: its use as a model for the
study of endothelial cell biology. Lab Invest. 63:259–269.
1990.PubMed/NCBI
|
|
22
|
Lannutti BJ, Gately ST, Quevedo ME, Soff
GA and Paller AS: Human angiostatin inhibits murine
hemangioendothelioma tumor growth in vivo. Cancer Res.
57:5277–5280. 1997.PubMed/NCBI
|
|
23
|
O’Reilly MS, Brem H and Folkman J:
Treatment of murine hemangioendotheliomas with the angiogenesis
inhibitor AGM-1470. J Pediatr Surg. 30:325–330. 1995.PubMed/NCBI
|
|
24
|
O’Reilly MS, Boehm T, Shing Y, et al:
Endostatin: an endogenous inhibitor of angiogenesis and tumor
growth. Cell. 88:277–285. 1997.
|
|
25
|
Koukourakis MI, Giatromanolaki A, Thorpe
PE, et al: Vascular endothelial growth factor/KDR activated
microvessel density versus CD31 standard microvessel density in
non-small cell lung cancer. Cancer Res. 60:3088–3095.
2000.PubMed/NCBI
|
|
26
|
Qin L, Zhao D, Liu X, et al: Down syndrome
candidate region 1 isoform 1 mediates angiogenesis through the
calcineurin-NFAT pathway. Mol Cancer Res. 4:811–820. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Davani S, Marandin A, Mersin N, et al:
Mesenchymal progenitor cells differentiate into an endothelial
phenotype, enhance vascular density, and improve heart function in
a rat cellular cardiomyoplasty model. Circulation. 108(Suppl 1):
II253–II258. 2003. View Article : Google Scholar
|
|
28
|
Li X, Lu X, Xu H, et al:
Paclitaxel/tetrandrine coloaded nanoparticles effectively promote
the apoptosis of gastric cancer cells based on ‘oxidation therapy’.
Mol Pharm. 9:222–229. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Heinzelmann S and Bauer G: Multiple
protective functions of catalase against intercellular
apoptosis-inducing ROS signaling of human tumor cells. Biol Chem.
391:675–693. 2010. View Article : Google Scholar
|
|
30
|
Fatehi-Hassanabad Z, Chan CB and Furman
BL: Reactive oxygen species and endothelial function in diabetes.
Eur J Pharmacol. 636:8–17. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Ferreira AK, de-Sa-Junior PL, Pasqualoto
KF, et al: Cytotoxic effects of dillapiole on MDA-MB-231 cells
involve the induction of apoptosis through the mitochondrial
pathway by inducing an oxidative stress while altering the
cytoskeleton network. Biochimie. 99:195–207. 2013. View Article : Google Scholar
|
|
32
|
Gong K, Xie J, Yi H and Li W: CS055
(Chidamide/HBI-8000), a novel histone deacetylase inhibitor,
induces G1 arrest, ROS-dependent apoptosis and differentiation in
human leukaemia cells. Biochem J. 443:735–746. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Sheppard K, Kinross KM, Solomon B, Pearson
RB and Phillips WA: Targeting PI3 kinase/AKT/mTOR signaling in
cancer. Crit Rev Oncog. 17:69–95. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Janku F, McConkey DJ, Hong DS and Kurzrock
R: Autophagy as a target for anticancer therapy. Nat Rev Clin
Oncol. 8:528–539. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Song MS, Salmena L and Pandolfi PP: The
functions and regulation of the PTEN tumour suppressor. Nat Rev Mol
Cell Biol. 13:283–296. 2012.PubMed/NCBI
|
|
36
|
Liu P, Begley M, Michowski W, et al:
Cell-cycle-regulated activation of Akt kinase by phosphorylation at
its carboxyl terminus. Nature. 508:541–545. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Zhang Y, Wang C, Wang H, Wang K, Du Y and
Zhang J: Combination of Tetrandrine with cisplatin enhances
cytotoxicity through growth suppression and apoptosis in ovarian
cancer in vitro and in vivo. Cancer Lett. 304:21–32. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
McCubrey JA, Basecke J, Cervello M,
Martelli AM and Franklin RA: GSK-3beta is a critical mediator of
tetrandrine induced cell cycle arrest and cytotoxicity. Cancer Biol
Ther. 7:10792008. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Lee HG, Lee YJ and Yang JH:
Perfluorooctane sulfonate induces apoptosis of cerebellar granule
cells via a ROS-dependent protein kinase C signaling pathway.
Neurotoxicology. 33:314–320. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Lu CY, Yang YC, Li CC, Liu KL, Lii CK and
Chen HW: Andrographolide inhibits TNFalpha-induced ICAM-1
expression via suppression of NADPH oxidase activation and
induction of HO-1 and GCLM expression through the PI3K/Akt/Nrf2 and
PI3K/Akt/AP-1 pathways in human endothelial cells. Biochem
Pharmacol. 91:40–50. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Muniz-Feliciano L, Van Grol J, Portillo
JA, et al: Toxoplasma gondii-induced activation of EGFR prevents
autophagy protein-mediated killing of the parasite. PLoS Pathog.
9:e10038092013. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Rodon J, Dienstmann R, Serra V and
Tabernero J: Development of PI3K inhibitors: lessons learned from
early clinical trials. Nat Rev Clin Oncol. 10:143–153. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Qin L, Zeng H and Zhao D: Requirement of
protein kinase D tyrosine phosphorylation for VEGF-A165-induced
angiogenesis through its interaction and regulation of
phospholipase Cgamma phosphorylation. J Biol Chem. 281:32550–32558.
2006. View Article : Google Scholar : PubMed/NCBI
|