Introduction
Ovarian cancer is the seventh leading cause of
female cancer-related death globally (1). Although cisplatin and its platinum
derivative therapies are effective in treating ovarian cancer, most
patients exhibit resistance to this type of chemotherapy (2). It was reported that platinum with
taxane was effective for ovarian cancer, but the side effects were
relatively severe (3). There is
thus an urgent need to develop new chemotherapeutic combinations to
maximize clinical benefit while minimizing toxicity.
Emetine is a natural alkaloid derived from
Psychotria ipecacuanha that strongly inhibits the synthesis
of biomolecules (4). It has been
widely used as an anti-amoebiasis drug since the early 1900s
(5). Evidence of emetine’s effect
against tumor cells first came to light in 1918 (6). Following several preclinical studies
of its anticancer activity, phase I/II clinical trials using
emetine were performed by the NCI in the mid-1970s (7–10).
However, emetine as a single agent showed no clinical benefit with
several side effects, such as cardiac damage. Since then, emetine
has been found to induce apoptosis in various human leukemic cell
lines (11–14) and a lung cancer cell line (15). Recently, emetine has been reported
to regulate the alternative splicing of bcl-x in breast cancer
MCF-7, prostate cancer PC-3, cervical cancer C33 and lung cancer
A549 cell lines, with downregulation of anti-apoptotic bcl-xL mRNA
and upregulation of pro-apoptotic bcl-xS mRNA (16).
To date, emetine has been indicated to enhance
cisplatin-induced apoptosis in leukemia cells (17) and bladder cancer cells (18). However, the precise molecular
mechanisms remain unclear. In the present study, we investigated
the effect of emetine with or without cisplatin on the ovarian
cancer cell line SKOV3. We further clarified that downregulation of
bcl-xL is responsible for the synergistic effects of emetine and
cisplatin.
Materials and methods
Cell culture
Human ovarian cancer cell line SKOV3 was obtained as
a cell line of the NCI-60 from the National Cancer Institute
Developmental Therapeutics Program (NCI DTP, Bethesda, MD, USA).
SKOV3 cells were maintained in DMEM (Nissui Pharmaceutical, Tokyo,
Japan) supplemented with 10% FBS (PAA Laboratories, Pasching,
Austria), 4 mM glutamine (Nacalai Tesque, Kyoto, Japan), 100 U/ml
penicillin (Meiji Seika Pharma, Tokyo, Japan) and 100 mg/ml
streptomycin (Nacalai Tesque). Cell culture was incubated at 37°C
in a humidified atmosphere of 5% CO2.
Reagents
Cisplatin (Wako, Osaka, Japan), emetine (Tokyo
Chemical Industry, Tokyo, Japan) and zVAD-fmk (R&D Systems,
Minneapolis, MN, USA) were dissolved in DMSO (Nacalai Tesque).
Cell growth assay
The number of viable cells was measured by a Cell
Counting Kit-8 assay (Dojindo, Kumamoto, Japan) according to the
manufacturer’s instructions. Cells were seeded at a density of
1,500 cells in each well of 96-well plates (Becton-Dickinson,
Franklin Lakes, NJ, USA). After culturing for 24 h, cells were
treated with DMSO as a control or cisplatin at the indicated
concentrations for 72 h. Then, kit reagent WST-8 was added to the
medium and incubated for 4 h. The absorbance of the samples at 450
nm was measured using a multi-plate reader (DS Pharma Biomedical,
Osaka, Japan).
Analyses of cell cycle and apoptosis
Cells were incubated with various agents for 24, 48
or 72 h, and then harvested by trypsinization. After
centrifugation, the cells were suspended in PBS containing 0.1%
Triton X-100 (Nacalai Tesque), 150 mg/ml RNase A (Sigma, St.
Louis, MO, USA) and 25 mg/ml propidium iodide (Sigma). The
stained cells were analyzed using FACSCalibur (Becton-Dickinson).
The data were analyzed using Modifit LT software and CellQuest
software (Becton-Dickinson).
Protein isolation and western blot
analysis
Cells were lysed with a buffer containing 50 mM
Tris-HCl (Nacalai Tesque), 1% SDS (Nacalai Tesque), 1 mM DTT
(Nacalai Tesque), 0.43 mM 4-(2-aminoethyl) benzenesulfonyl fluoride
hydrochloride (AEBSF) (Wako) and phosphatase inhibitor cocktail
(Nacalai Tesque). The lysate was sonicated and centrifuged at
15,000 g for 20 min at 4°C, and the supernatant was then collected.
Equal amounts of the protein extract were subjected to SDS-PAGE and
transferred to a PVDF membrane (Millipore, Bedford, MA, USA). The
following were used as the primary antibody: rabbit anti-human
polyclonal antibody bcl-x (Santa Cruz Biotechnology, Santa Cruz,
CA, USA), rabbit anti-human monoclonal antibody caspase-3 (Cell
Signaling Technology, Beverly, MA, USA), rabbit anti-human
monoclonal antibody cleaved-caspase-3 (Cell Signaling Technology),
mouse anti-human monoclonal antibody caspase-7 (R&D Systems),
mouse anti-human monoclonal antibody caspase-8 (Medical &
Biological Laboratories, Nagoya, Japan) and anti-β-actin (Sigma).
The signals were detected with a Chemi-lumi One L (Nacalai Tesque)
or an Immobilon Western Chemiluminescent HRP Substrate
(Millipore).
Colony formation assay
SKOV3 cells were seeded at a density of 250 cells
per well in 6-well plates. After incubation for 24 h, cells were
treated with cisplatin at 20 μM with or without emetine at 2.5 μM.
The medium was then replaced with a fresh one, and the cells were
cultured for 10 days. The colonies fixed in 10% formaldehyde
solution (Nacalai Tesque) were stained in crystal violet (Nacalai
Tesque), and the number of viable colonies was counted.
Small interfering (si) RNA
transfection
Knockdown of bcl-xL was achieved by transfection
with small interfering RNA (siRNA) (Invitrogen, Carlsbad, CA, USA),
as previously reported (19),
using Lipofectamine RNAiMAX (Invitrogen).
Statistical analysis
Data are expressed as mean ± SD of three
determinations. Statistical analysis was performed using the
Student’s t-test. Samples were considered significantly different
when P<0.05.
Results
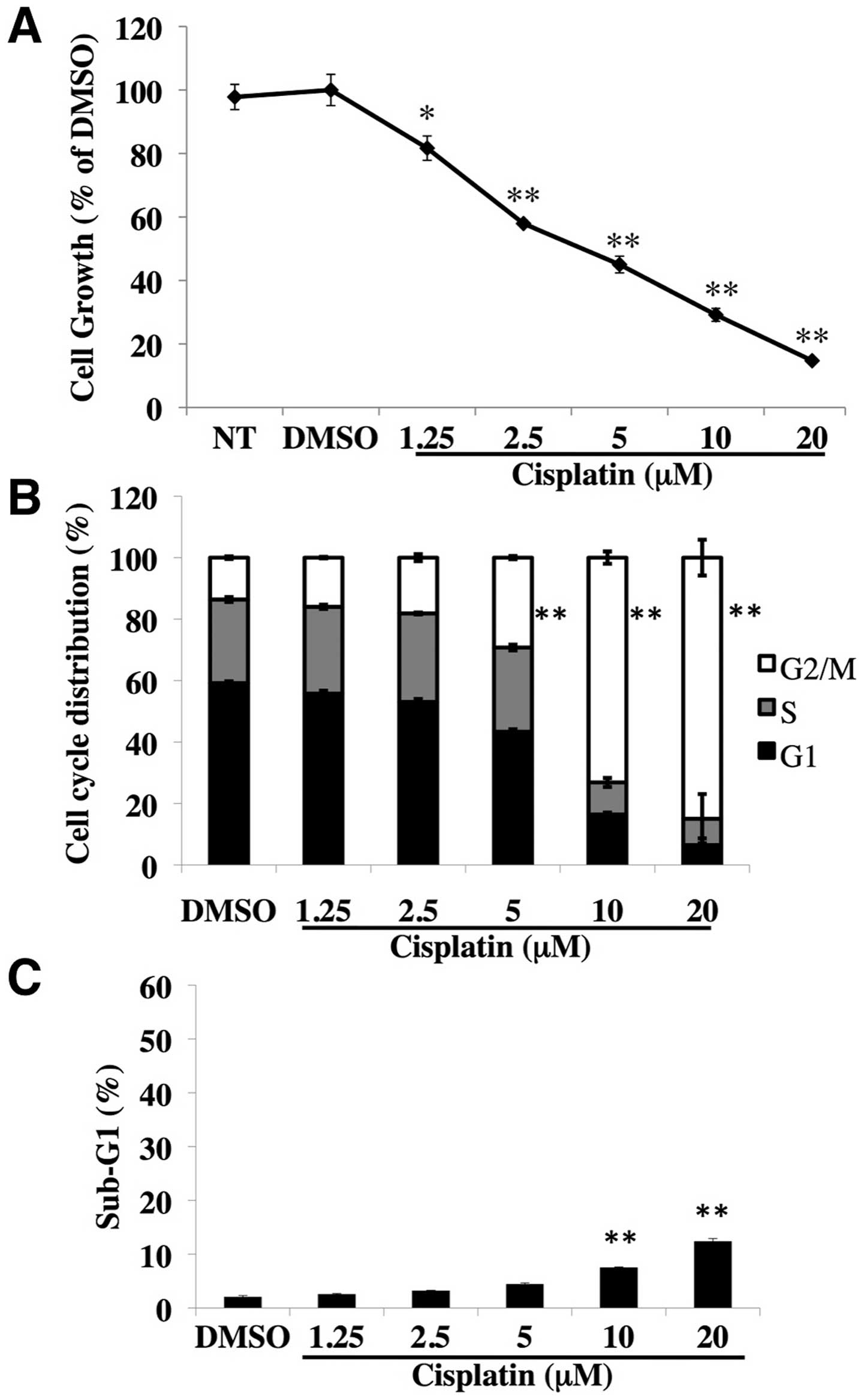
Cisplatin inhibits cell growth with
G2/M-phase arrest in ovarian cancer SKOV3 cells
High resistance to cisplatin in many human ovarian
cancer cell lines has been reported (20). Here, we investigated the effects of
cisplatin on ovarian cancer SKOV3 cells. After treatment with the
indicated concentrations of cisplatin for 72 h, cisplatin
dose-dependently inhibited the cell growth (Fig. 1A). We then performed cell cycle
analysis and measured the sub-G1 population to quantify apoptotic
cells. The treatment with cisplatin at 5 μM or more for 72 h
increased the G2/M phase in a dose-dependent manner (Fig. 1B), but barely induced apoptosis
(Fig. 1C). These results clearly
show that cisplatin inhibited the growth of human ovarian cancer
SKOV3 cells with G2/M-phase arrest.
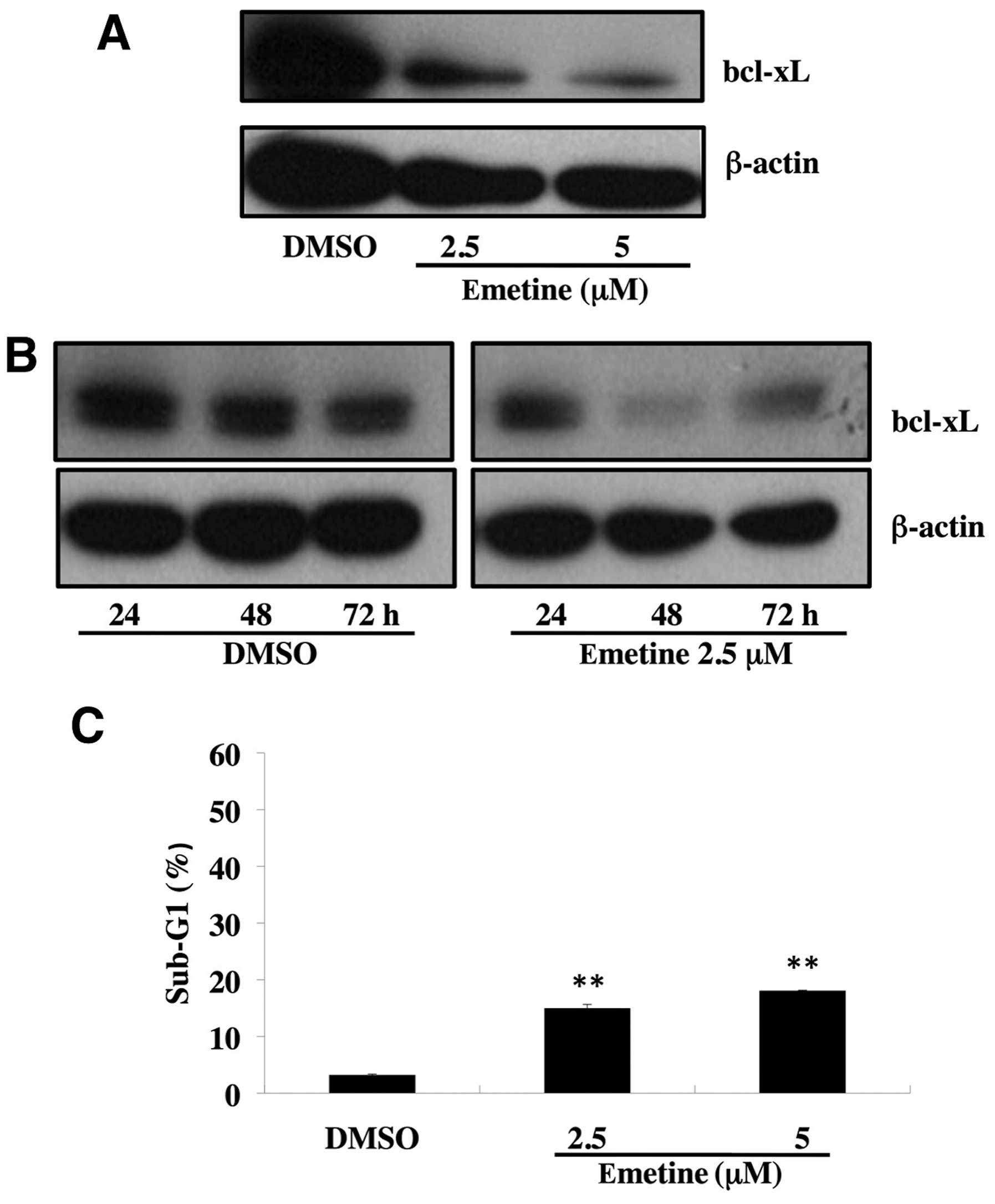
Emetine reduces anti-apoptotic bcl-xL
expression with slight induction of apoptosis
Among anti-apoptotic members, bcl-xL expression is
frequently upregulated during carcinogenesis (21), and is associated with resistance to
chemotherapeutic agents in malignant tumors of various origins
(22–25). In this study, we found that
treatment of emetine at 2.5 or 5 μM for 72 h decreased the protein
expression of bcl-xL (Fig. 2A) in
ovarian cancer SKOV3 cells, whereas treatment of 2.5 μM emetine for
24 h did not reduce the expression of bcl-xL (Fig. 2B). As described, emetine at 2.5 or
5 μM effectively downregulated the anti-apoptotic bcl-xL protein
expression (Fig. 2A), but it could
induce only weak apoptosis (Fig.
2C).
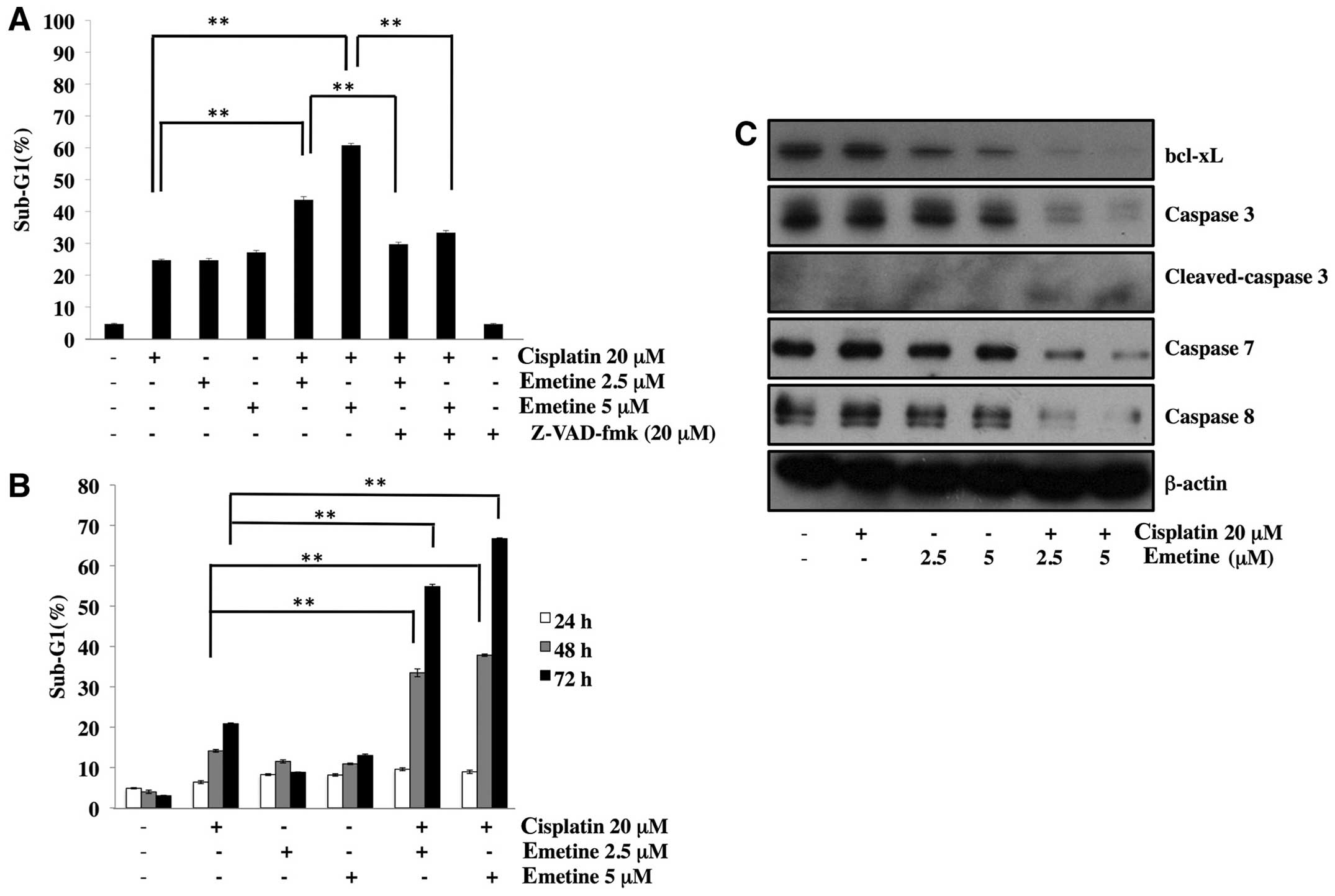
Combination treatment with cisplatin and
emetine time-dependently induce caspase-dependent apoptosis
We next investigated whether emetine sensitized
SKOV3 cells to cisplatin. As shown in Fig. 3A, co-treatment with 20 μM cisplatin
and 2.5 or 5 μM emetine significantly induced apoptosis in SKOV3
cells. The time-dependence of apoptosis induced by the combination
of cisplatin and emetine is shown in Fig. 3B. Combined treatment for 24 h did
not induce increased apoptosis. However, co-treatment with
cisplatin and emetine for 48 and 72 h apparently increased it.
These results are in accordance with the data shown in Fig. 2B that emetine at 2.5 μM for 24 h
did not reduce the expression of bcl-xL, but it was decreased by
the treatment for 48 and 72 h. To investigate whether apoptosis was
caspase-dependent, we analyzed the effect of a pan-caspase
inhibitor, zVAD-fmk. Apoptosis induced by the combination was
partially inhibited by this inhibitor (Fig. 3A). Furthermore, the combination
clearly enhanced the activation of caspases with reduction of
bcl-xL expression (Fig. 3C).
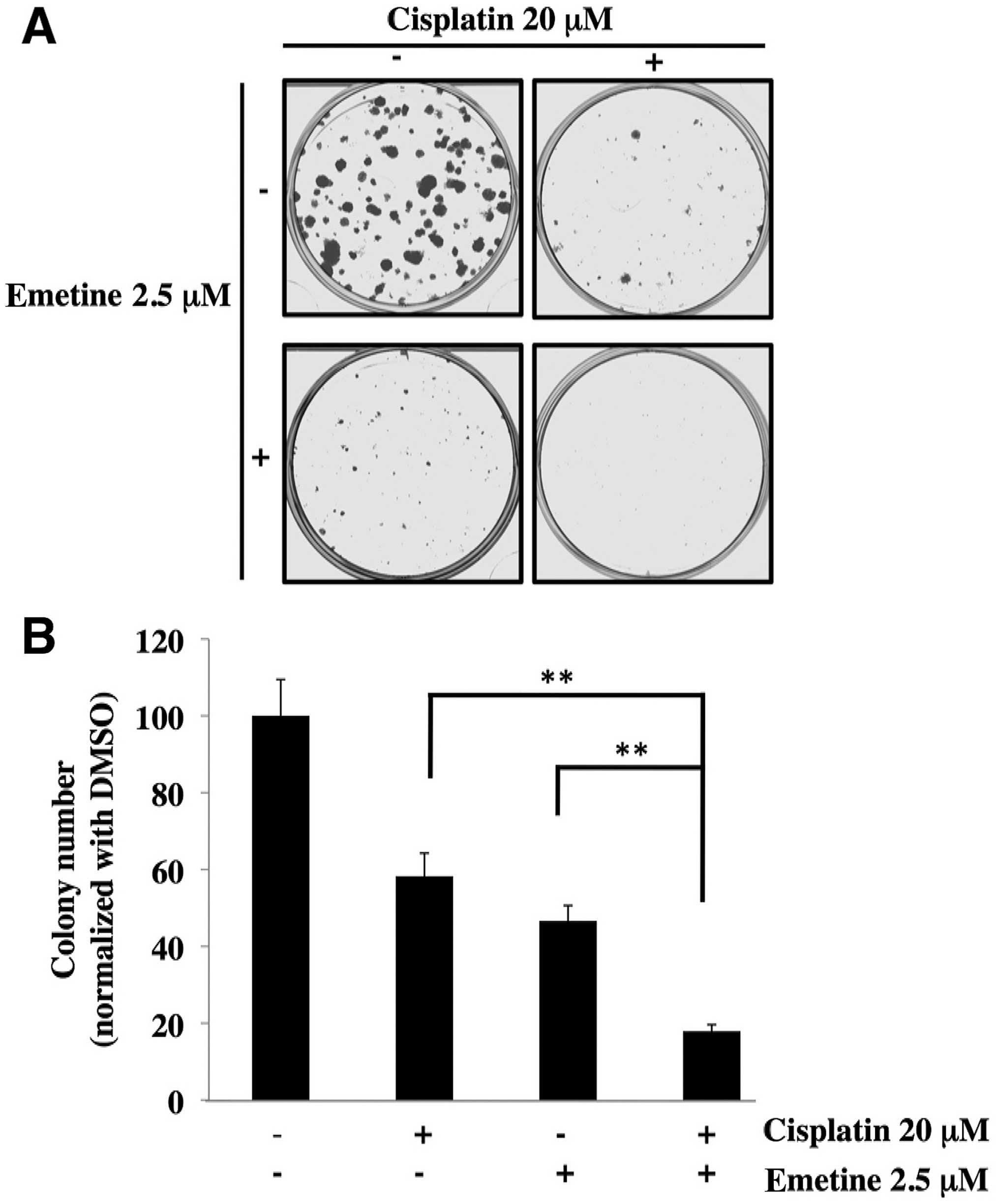
Combination treatment with cisplatin and
emetine decreases colony formation
We then performed a colony formation assay to
investigate the effect of the combined treatment with cisplatin and
emetine. Cisplatin at 20 μM or emetine at 2.5 μM reduced the colony
number to 58 and 47%, respectively, whereas combined treatment with
20 μM cisplatin and 2.5 μM emetine markedly decreased it to 17%
(Fig. 4). These results indicate
that emetine could sensitize SKOV3 cells to cisplatin.
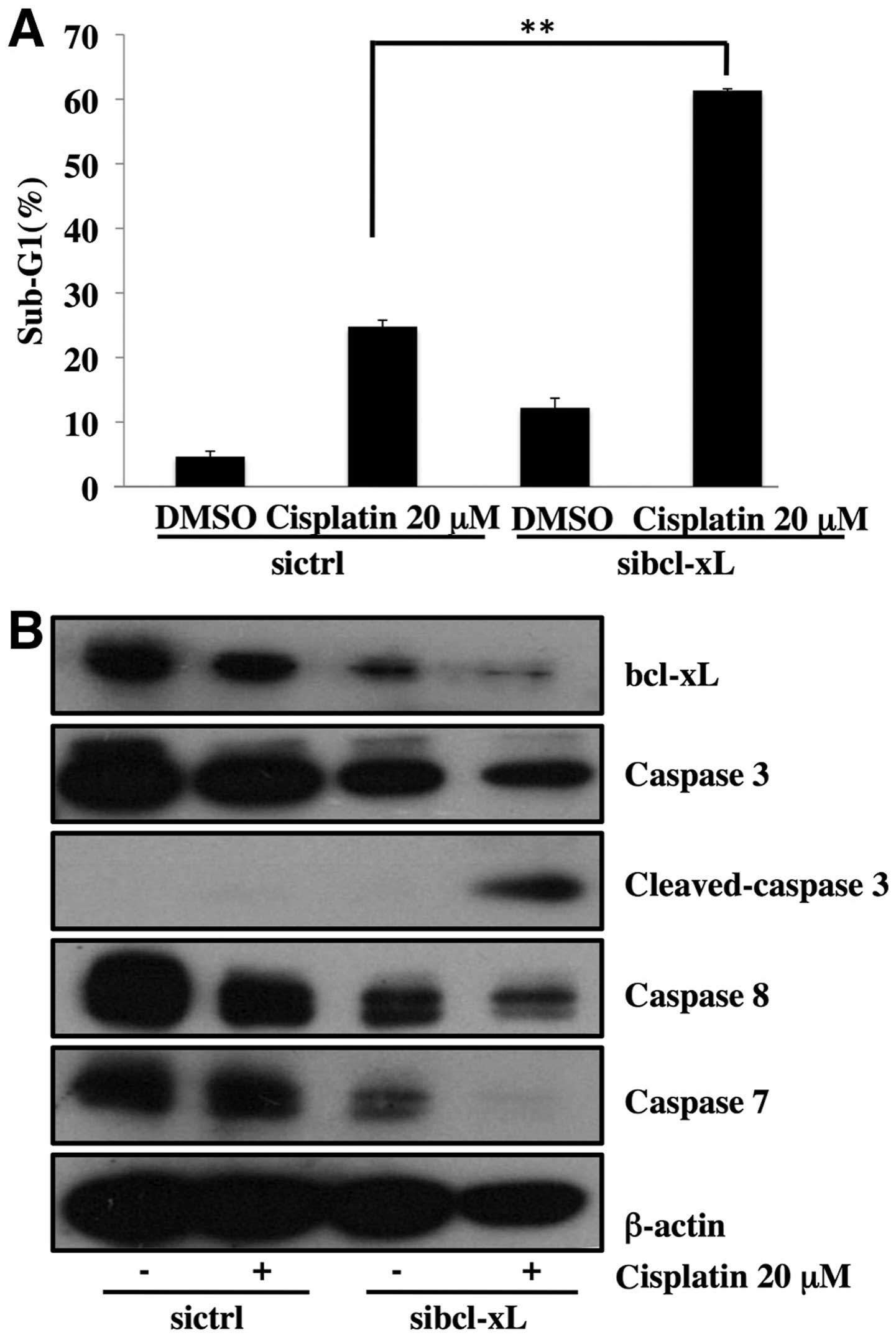
Downregulation of bcl-xL enhances
apoptosis induced by cisplatin
To confirm the contribution of the downregulation of
bcl-xL by emetine to the enhancement of apoptosis induced by
cisplatin, we performed the knockdown of bcl-xL by siRNA. As shown
in Fig. 5A, knockdown of bcl-xL
markedly enhanced the apoptosis by cisplatin in SKOV3 cells.
Moreover, the knockdown of bcl-xL caused the cleavage of caspases
(Fig. 5B). These results suggest
that the downregulation of bcl-xL expression contributed to the
enhancement of apoptosis by the combination of cisplatin and
emetine.
Discussion
One of the major goals of cancer chemotherapy is to
induce apoptosis in tumor cells by exposure to antitumor agents.
Although cisplatin is a potent inducer of apoptosis in ovarian
cancer cells, the resistant tumor cells have been found to fail to
undergo apoptosis upon treatment with cisplatin (26–28).
We demonstrated here that cisplatin inhibited cell growth with
G2/M-phase arrest but slightly increased apoptosis in ovarian
carcinoma SKOV3 cells (Fig. 1). To
enhance the sensitivity, the identification of new agents that can
sensitize SKOV3 cells to apoptosis induced by cisplatin appears as
a major challenge. In the present study, we found that co-treatment
of cisplatin and emetine remarkably induced apoptosis and reduced
colony formation of SKOV3 cells (Figs.
3 and 4). It is interesting
that emetine, which was used as an anti-amoebiasis drug ~100 years
ago, could sensitize SKOV3 cells to apoptosis induced by
cisplatin.
There are several reports describing that bcl-xL
expression contributed to chemotherapy resistance in ovarian
carcinoma by inhibiting chemotherapy-induced apoptosis (29–31).
Therefore, downregulation of bcl-xL is reasonable for sensitizing
ovarian cancer cells to chemotherapy. We found that emetine could
reduce the expression of bcl-xL in SKOV3 cells for the first time
(Fig. 2A and B). However, the
reduction of bcl-xL expression by emetine alone was insufficient to
enhance apoptosis in SKOV3 cells (Fig.
2C). We speculated that emetine might sensitize SKOV3 cells to
apoptosis induced by cisplatin through the downregulation of
bcl-xL. The present experiments showed that single treatment of
cisplatin did not reduce the expression of bcl-xL, but co-treatment
of cisplatin and emetine did, which led to the induction of
apoptosis with activated caspases (Fig. 3). Moreover, the knockdown of bcl-xL
with siRNA enhanced the sensitivity to cisplatin of SKOV3 cells
(Fig. 5), which was consistent
with the results for the combination of cisplatin and emetine
(Figs. 3 and 4). Taken together, these studies indicate
that emetine as a downregulator of bcl-xL might be a promising
agent to sensitize ovarian cancer cells to cisplatin.
Previously it was reported that emetine sensitizes
leukemic cancer cells and bladder cancer cells to chemotherapy
(17,18). However, the precise molecular
mechanisms by which emetine improves the sensitivity of cancer
cells remain unclear. Recent studies reported that emetine enhanced
the sensitivity of pancreatic cancer cells to TRAIL-induced
apoptosis by downregulating mcl-1 protein (32); the authors suggested that emetine
was highly specific to TRAIL since it did not have any effect on
other cell death-inducing agents, such as thapsigargin,
daunorubicin and paclitaxel (32).
However, our study, we found that emetine sensitized ovarian
carcinoma cells to cisplatin through downregulation of bcl-xL.
These studies suggest that the functions of emetine as a sensitizer
for chemotherapeutic agents might vary depending on the cancer cell
type. Therefore, the mechanisms for emetine’s involvement in the
enhancement of the sensitivity of cancer cells require further
investigation.
In conclusion, we found that emetine sensitizes
ovarian carcinoma SKOV3 cells, which were initially resistant to
cisplatin-induced apoptosis. We demonstrated, for the first time,
that the downregulation of bcl-xL by emetine is a key factor in
sensitizing SKOV3 cells to cisplatin. The combination of cisplatin
with emetine might be worth developing as a possible treatment for
ovarian cancer. Furthermore, the use of emetine in combination with
other chemotherapeutic agents for other cancer cells remains an
interesting topic for further studies.
Acknowledgments
Acknowledegements
This study was supported in part by the Ministry of
Education, Culture, Sports, Science and Technology of Japan.
References
|
1
|
Jemal A, Bray F, Center MM, Ferlay J, Ward
E and Forman D: Global cancer statistics. CA Cancer J Clin.
61:69–90. 2011. View Article : Google Scholar
|
|
2
|
Ali AY, Farrand L, Kim JY, Byun S, Suh JY,
Lee HJ and Tsang BK: Molecular determinants of ovarian cancer
chemoresistance: new insights into an old conundrum. Ann NY Acad
Sci. 1271:58–67. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
McGuire WP III and Markman M: Primary
ovarian cancer chemotherapy: current standards of care. Br J
Cancer. 89:S3–S8. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Grollman AP: Inhibitors of protein
biosynthesis. V Effects of emetine on protein and nucleic acid
biosynthesis in HeLa cells. J Biol Chem. 243:4089–4094.
1968.PubMed/NCBI
|
|
5
|
Lambert AC: The treatment of amoebic
dysentery with emetine and bismuth iodide. Br Med J. 1:116–118.
1918. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Lewisohn R: Action of emetine on malignant
tumors. JAMA. 70:9–10. 1918. View Article : Google Scholar
|
|
7
|
Panettiere F and Coltman CA Jr: Experience
with emetine hydrochloride (NSC 33669) as an antitumor agent.
Cancer. 27:835–841. 1971. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Mastrangelo MJ, Grage TB, Bellet RE and
Weiss AJ: A phase I study of emetine hydrochloride (NSC 33669) in
solid tumors. Cancer. 31:1170–1175. 1973. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Siddiqui S, Firat D and Olshin S: Phase II
study of emetine (NSC-33669) in the treatment of solid tumors.
Cancer Chemother Rep. 57:423–428. 1973.PubMed/NCBI
|
|
10
|
Kane RC, Cohen MH, Broder LE, Bull MI,
Creaven PJ and Fossieck BE Jr: Phase I-II evaluation of emetine
(NSC-33669) in the treatment of epidermoid bronchogenic carcinoma.
Cancer Chemother Rep. 59:1171–1172. 1975.PubMed/NCBI
|
|
11
|
Bicknell GR, Snowden RT and Cohen GM:
Formation of high molecular mass DNA fragments is a marker of
apoptosis in the human leukaemic cell line, U937. J Cell Sci.
107:2483–2489. 1994.PubMed/NCBI
|
|
12
|
Möller M, Weiss J and Wink M: Reduction of
cytotoxicity of the alkaloid emetine through P-glycoprotein
(MDR1/ABCB1) in human Caco-2 cells and leukemia cell lines. Planta
Med. 72:1121–1126. 2006.PubMed/NCBI
|
|
13
|
Möller M and Wink M: Characteristics of
apoptosis induction by the alkaloid emetine in human tumour cell
lines. Planta Med. 73:1389–1396. 2007.PubMed/NCBI
|
|
14
|
Rosenkranz V and Wink M: Alkaloids induce
programmed cell death in bloodstream forms of trypanosomes
(Trypanosoma b. brucei). Molecules. 13:2462–2473. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Watanabe N, Iwamoto T, Dickinson DA, Iles
KE and Forman HJ: Activation of the mitochondrial caspase cascade
in the absence of protein synthesis does not require c-Jun
N-terminal kinase. Arch Biochem Biophys. 405:231–240. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Boon-Unge K, Yu Q, Zou T, Zhou A,
Govitrapong P and Zhou J: Emetine regulates the alternative
splicing of Bcl-x through a protein phosphatase 1-dependent
mechanism. Chem Biol. 14:1386–1392. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Möller M, Herzer K, Wenger T, Herr I and
Wink M: The alkaloid emetine as a promising agent for the induction
and enhancement of drug-induced apoptosis in leukemia cells. Oncol
Rep. 18:737–744. 2007.PubMed/NCBI
|
|
18
|
Foreman KE, Jesse JN III, Kuo PC and Gupta
GN: Emetine dihydrochloride: a novel therapy for bladder cancer. J
Urol. 191:502–509. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Qian J, Zou Y, Rahman JS, Lu B and Massion
PP: Synergy between phosphatidylinositol 3-kinase/Akt pathway and
Bcl-xL in the control of apoptosis in adenocarcinoma cells of the
lung. Mol Cancer Ther. 8:101–109. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Godwin AK, Meister A, O’Dwyer PJ, Huang
CS, Hamilton TC and Anderson ME: High resistance to cisplatin in
human ovarian cancer cell lines is associated with marked increase
of glutathione synthesis. Proc Natl Acad Sci USA. 89:3070–3074.
1992. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Kirkin V, Joos S and Zörnig M: The role of
Bcl-2 family members in tumorigenesis. Biochim Biophys Acta.
1644:229–249. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Lebedeva I, Rando R, Ojwang J, Cossum P
and Stein CA: Bcl-xL in prostate cancer cells: effects of
overexpression and down-regulation on chemosensitivity. Cancer Res.
60:6052–6060. 2000.PubMed/NCBI
|
|
23
|
Agarwal R and Kaye SB: Ovarian cancer:
strategies for overcoming resistance to chemotherapy. Nat Rev
Cancer. 3:502–516. 2003. View
Article : Google Scholar : PubMed/NCBI
|
|
24
|
Sasazawa Y, Futamura Y, Tashiro E and
Imoto M: Vacuolar H+-ATPase inhibitors overcome
Bcl-xL-mediated chemoresistance through restoration of a
caspase-independent apoptotic pathway. Cancer Sci. 100:1460–1467.
2009. View Article : Google Scholar
|
|
25
|
Lee SJ, Park HJ, Kim YH, et al: Inhibition
of Bcl-xL by ABT-737 enhances chemotherapy sensitivity in
neurofibromatosis type 1-associated malignant peripheral nerve
sheath tumor cells. Int J Mol Med. 30:443–450. 2012.PubMed/NCBI
|
|
26
|
Fajac A, Da Silva J, Ahomadegbe JC, Rateau
JG, Bernaudin JF, Riou G and Bénard J: Cisplatin-induced apoptosis
and p53 gene status in a cisplatin-resistant human ovarian
carcinoma cell line. Int J Cancer. 68:67–74. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Henkels KM and Turchi JJ: Induction of
apoptosis in cisplatin-sensitive and -resistant human ovarian
cancer cell lines. Cancer Res. 57:4488–4492. 1997.PubMed/NCBI
|
|
28
|
Singh M, Chaudhry P, Fabi F and Asselin E:
Cisplatin-induced caspase activation mediates PTEN cleavage in
ovarian cancer cells: a potential mechanism of chemoresistance. BMC
Cancer. 13:2332013. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Liu JR, Fletcher B, Page C, Hu C, Nunez G
and Baker V: Bcl-xL is expressed in ovarian carcinoma and modulates
chemotherapy-induced apoptosis. Gynecol Oncol. 70:398–403. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Williams J, Lucas PC, Griffith KA, Choi M,
Fogoros S, Hu YY and Liu JR: Expression of Bcl-xL in ovarian
carcinoma is associated with chemoresistance and recurrent disease.
Gynecol Oncol. 96:287–295. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Dodier P and Piché A: Bcl-X(L) is
functionally non-equivalent for the regulation of growth and
survival in human ovarian cancer cells. Gynecol Oncol. 100:254–263.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Han Y, Park S, Kinyua AW, Andera L, Kim KW
and Kim I: Emetine enhances the tumor necrosis factor-related
apoptosis-inducing ligand-induced apoptosis of pancreatic cancer
cells by downregulation of myeloid cell leukemia sequence-1
protein. Oncol Rep. 31:456–462. 2014.
|