Introduction
Bladder cancer is the most common cancer of the
urinary system and is showing each year increases in morbidity and
mortality (1,2). During tumorigenesis and the cancer
development, bladder cancer cells and the host go through three
stages: immune surveillance, equilibrium and immune evasion
(3). Clinical treatment of bladder
cancer is often impeded by immune evasion (4).
Previous studies have shown that impaired apoptotic
signal transduction is one of the important factors promoting
immune evasion by cancer cells (5). Accumulating evidence shows the
apoptotic signal transduction from immune cells to bladder cancer
cells is impaired, resulting in the immune evasion of bladder
cancer cells. For instance, antigen B7-H1 binding to PD-1 results
in functional inhibition of T cells and B cells, inhibition of
body-peculiar cellular and humoral immunity, and induction of
apoptosis in specific cytotoxic T lymphocytes (CTL), all of which
enable immune evasion of bladder cancer cells and promote the
growth of bladder tumors (6).
Changes in the apoptosis receptor Fas/FasL also enable evasion of
the apoptotic effect from CTL cancer cells (7). Increased expression of TGF-β-1 leads
to the escape of bladder cancer cells from host immune surveillance
(8), while deletion or mutation of
the MHC-1 gene blocks the apoptotic response from T cells and NK
cells (9).
However, immune evasion of cancer cells cannot be
completely attributed to the blockage of apoptosis signal
transduction from immune cells to cancer cells. It also involves
the impaired apoptosis signaling transduction in the cancer cells
themselves (9–11). In 2002, microRNA was found to
regulate the proliferation and apoptosis of tumor cells (11). Aberrant expression of microRNA in
bladder cancer cells was first reported in 2007 (12). Recent studies indicate that there
are no binding sites for microRNAs in cell membrane receptor genes
or their ligands in cancer cells, many microRNA binding sites are
present the downstream signaling pathway effector genes. For
example, upregulated miR-221 in malignant bladder tumor inhibits
p27kip1 activity, promote proliferation of bladder
cancer cells, and inhibits TRAIL-mediated apoptosis signaling
(13,14). Given that a microRNA can function
on multiple target mRNAs, we hypothesize that miR-221 may silence
target mRNAs of other genes and suppress the apoptosis of bladder
cancer cells as a result, which would further enable immune evasion
by bladder cancer cells. From www.targetscan.org and www.mirbase.org, we
found that PUMA, a pro-apoptotic protein that promotes apoptosis
might be another target of miR-221. miR-221 and miR-222 have also
been found to specifically target PUMA and promote the survival of
malignant glioma cells (15).
Thus, we hypothesize that increased expression of miR-221 in
bladder cancer may inhibit the apoptosis of bladder cancer cells
through silencing PUMA and maintain the continuous proliferation of
bladder cancer cells, leading to their immune evasion.
The major consequences of immune evasion of tumor
cells are invasive growth and/or metastasis of tumor cells
(16). Previous studies have shown
that the degradation and destruction of the extracellular matrix
and basement membrane of the tumor surface is the basis for tumor
invasion and metastasis (17,18).
Matrix metalloproteinases (MMPs) constitute a family of enzymes
that are responsible for depredating the extracellular matrix and
are closely related with tumor invasion and metastasis. MMP-2 and
MMP-9 are two of the most important MMP proteins. MMP-9 degrades
and destroys extracellular matrix and basement membrane near the
surface of tumor cells, promotes generation of new blood
capillaries, tumor growth and migration. MMP-2 degrades types IV,
IV, VI and X collagen (19–22).
Vascular endothelial growth factor (VEGF) is the most potent known
angiogenic factor and plays an important role in the invasive
growth and metastasis of many human malignancies (23–25).
VEGF can be classified into seven subtypes, namely VEGF-A, VEGF-B,
VEGF-C, VEGF-D, VEGF-E, VEGF-F and PIGF, among which VEGF-C is
closely related to tumor invasion and metastasis. VEGF-C
specifically binds to its cognate receptor on endothelial cells and
facilitates mitotic proliferation of endothelial cells, enhances
the permeability of blood vessels, changes gene expression in
endothelial cells, and thus promotes the synthesis of MMPs
(26–28). Therefore in this study, we directly
measured the expression of VEGF-C, MMP-2 and MMP-9 in tumor cells
to determine the invasion and metastasis state of tumor cells, to
indirectly probe if tumor cells are capable of immune evasion.
Materials and methods
Cell culture
Human bladder cancer cells (T24, 5637 and J82) were
obtained from the China Academia Sinica Cell Repository, Shanghai,
China. T24 and 5637 cell lines were maintained in RPMI-1640, J82
was maintained in MEM. Both media were supplemented with 10%
heat-inactivated fetal bovine serum without antibiotics at 37°C in
a humidified incubator with 5% CO2. All in vitro
experiments were performed in triplicate.
Transient transfection
The FAM-modified 2′-OMe-oligo-nucleotides were
chemically synthesized and purified by high-performance liquid
chromatography by Invitrogen. The sequence of the 2′-O-me-miR-221
inhibitor is: 5′-GAA ACC CAG CAG ACA AUG UAG CU-3′. The
FAM-modified scrambled oligonucleotides are RNA duplexes with the
following sequences: 5′-UUC UCC GAA CGU GUC ACG UTT/ACG UGA CAC GUU
CGG AGA ATT-3′. FAM was attached to the 5′ end of each
oligonucleotide. When cells were grown to 50–60% confluence,
oligonucleotides transfection was performed using the
Lipofectamine™ 2000 transfection reagent (Invitrogen, USA)
according to the manufacturer’s instructions. At 6 h after
transfection, the medium was replaced with fresh medium containing
10% fetal bovine serum. The experiments consisted of three groups:
i) blank (without treatment), ii) negative control (transiently
transfected with scrambled oligonucleotide), and iii) transfection
of miR-221 inhibitor (transiently transfected with miR-221
inhibitor).
Cell viability assay
Cell viability was tested by 3-(4,
5-dimethyl-thiazol-2-yl)-2, 5-diphenyltetrazolium bromide (MTT)
assay. Briefly, cells were seeded into 96-well plates at a density
of 10,000 cells/well in 100 μl culture medium and cultured
overnight before transfection. Cells received fresh medium at 6 h.
After 24 h, 20 μl MTT (5 mg/ml dimethyl thiazolyl diphenyl
tetrazolium, Sigma) was added into each test well and incubated for
4 h in the humidified incubator. Formazan crystals formed by viable
cells were dissolved in 150 ml dimethyl sulf-oxide (Solarbio) and
their absorbance values were measured at 490 nm. Wells without
cells (DMSO alone) were used as a background control. The final
optical density (OD) was calculated according to the formula:
(final optical density = optical density of each group - optical
density of DMSO group). Each test was performed daily for three
consecutive days and repeated in five wells.
Quantitative real-time PCR
T24, 5637 and J82 cells were transfected for 24 h as
described above. Total RNA was extracted using TRIzol (Invitrogen
Life Technologies, Shanghai, China) according to the manufacturer’s
protocol. RNA quality was determined by running a sample with RNA
loading dye (Sigma-Aldrich) on a 1% agarose gel and inspecting for
distinct 18S, 28S and total RNA bands which indicate a lack of
degradation. The quantity of RNA was determined by A260
measurement.
To evaluate miR-221 expression levels,
quantification using the SYBR Green microRNA assay was performed
using two-step RT-PCR according to the manufacturer’s instructions.
In the reverse transcription (RT) step, cDNA was reverse
transcribed from the total RNA sample using specific miR-221
primers from the Bulge-Loop™ hsa-miR-221-5p qRT-PCR Primer Set
(RiboBio, Guang Zhou, China) and the Reverse Transcription System
(Takara, Dalian, China). In the poly-merase chain reaction (PCR)
step, PCR products were amplified from cDNA samples using the
Bulge-Loop™ hsa-miR-221-5p qRT-PCR primer set and using
SYBR® Premix Ex Taq™ II (TliRNase H Plus, Takara) in the
ABI PRISM® 7500 real-time PCR system (Applied
Biosystems, Foster City, CA, USA). The real-time PCR results were
normalized against an internal control U6 and relative expression
levels were evaluated using the 2-ΔΔCt method and then expressed as
fold changes.
For qRT-PCR, 1 μg total RNA was used in the Reverse
Transcription System (Takara) according to the manufacturer’s
instructions, and PCR was performed in the ABI PRISM®
7500 real-time PCR system (Applied Biosystems). The sequences of
gene-specific primers are shown in Table I. The expression level of β-actin
was used as internal control. All reactions were performed at least
in triplicate.
 | Table IOligonucleotide sequences for PCR
amplification. |
Table I
Oligonucleotide sequences for PCR
amplification.
| Gene | PubMed no. | Sequence
(5′-3′) | Product size
(bp) |
|---|
| Bax | NM_138764.4 | F:
ACCAGGGTGGTTGGGTGAGACT | R:
CACCACTGTGACCTGCTCCAGA | 136 |
| Bcl-2 | NM_000633.2 | F:
CCAGCATGCGGCCTCTGTTTGA | R:
TGGGGCAGGCATGTTGACTTCAC | 129 |
| PUMA | NM_001127241.1 | F:
GCGGGGAGGAGGAACAGT | R:
TGTGGCCCCTGGGTAAGG | 177 |
| MMP-2 | NM_004994.2 | F:
CCTCTCCACTGCCTTCGATA | R:
TGGGAGGAGTACAGTCAGCA | 129 |
| MMP-9 | NM_001127891.1 | F:
CTGCAGTGCCCTGAGGACTA | R:
ACTCCTCCCTTTCCTCCAGA | 135 |
| VEGF-C | NM_005429.2 | F:
GGCTGGCAACATAACAGAGAA | R:
CCCCACATCTATACACACCTCC | 159 |
| β-actin | NM_001101.3 | F:
CATGTACGTTGCTATCCAGGC | R:
CTCCTTAATGTCACGCACGAT | 250 |
Invasion assays
The invasion assay was performed in 24-well
transwell plates. Type I collagen was coated on the upper chamber
to reconstitute the basement membrane. Three groups (transfection
of miR-221 inhibitor, negative control and control) of cells
(1×105 per well) were seeded on the upper chamber, and
the lower compartment was filled with RPMI-1640 (T24 and 5637
cells) or MEM (J82 cells) containing 10% fetal calf serum. Cells
were cultured for 24 h, fixed in 10% formaldehyde and stained with
crystal violet staining, while the upper chamber cells were gently
removed using cotton-tipped swabs. Five microscopic fields (×200)
were randomly selected for cell counting. The experiment was
repeated three times.
Flow cytometry
To evaluate cell apoptosis, singe cell suspensions
were prepared for each experimental group, incubated with 5 μl
FITC-conjugated Annexin V and 10 μl propidium iodide (PI) for 20
min at room temperature in the dark and were immediately analysed
with a FACSCalibur flow cytometer (Becton-Dickinson, Franklin
Lakes, NJ, USA). A minimum of 10,000 events were acquired for each
sample.
Acridine orange/ethidium bromide (AO/EB)
staining
Morphological signs of apoptosis were detected by
using acridine orange-ethidium bromide (AO/EB) staining. The cells
were treated as described above. AO/EB (Sigma) was freshly mixed at
0.01/100 (v/v) in dark, and one drop of the mixed solution was
added to each well for 5 min. The apoptotic cells were then counted
under an inverted fluorescence microscope (Eclipse TE300, Nikon,
Japan). The death rate (%) was calculated as the percentage of
positively stained cells, number of cells undergoing programmed
cell death (PCD × 100/total number of cells. The experiments were
repeated twice.
Statistical analysis
All data are presented as the average ± standard
deviation (mean ± SD). All experiments were repeated three times
independently, unless otherwise indicated. Statistical analysis was
performed to determine the significance of the difference between
groups using ANOVA (with post hoc Turkey’s honestly
significant difference test). All statistical analyses were
performed using SPSS 21.0 software for Windows, P<0.05 is
considered as statistically significant.
Results
miR-221 inhibitor impedes the
proliferation of bladder cancer cells
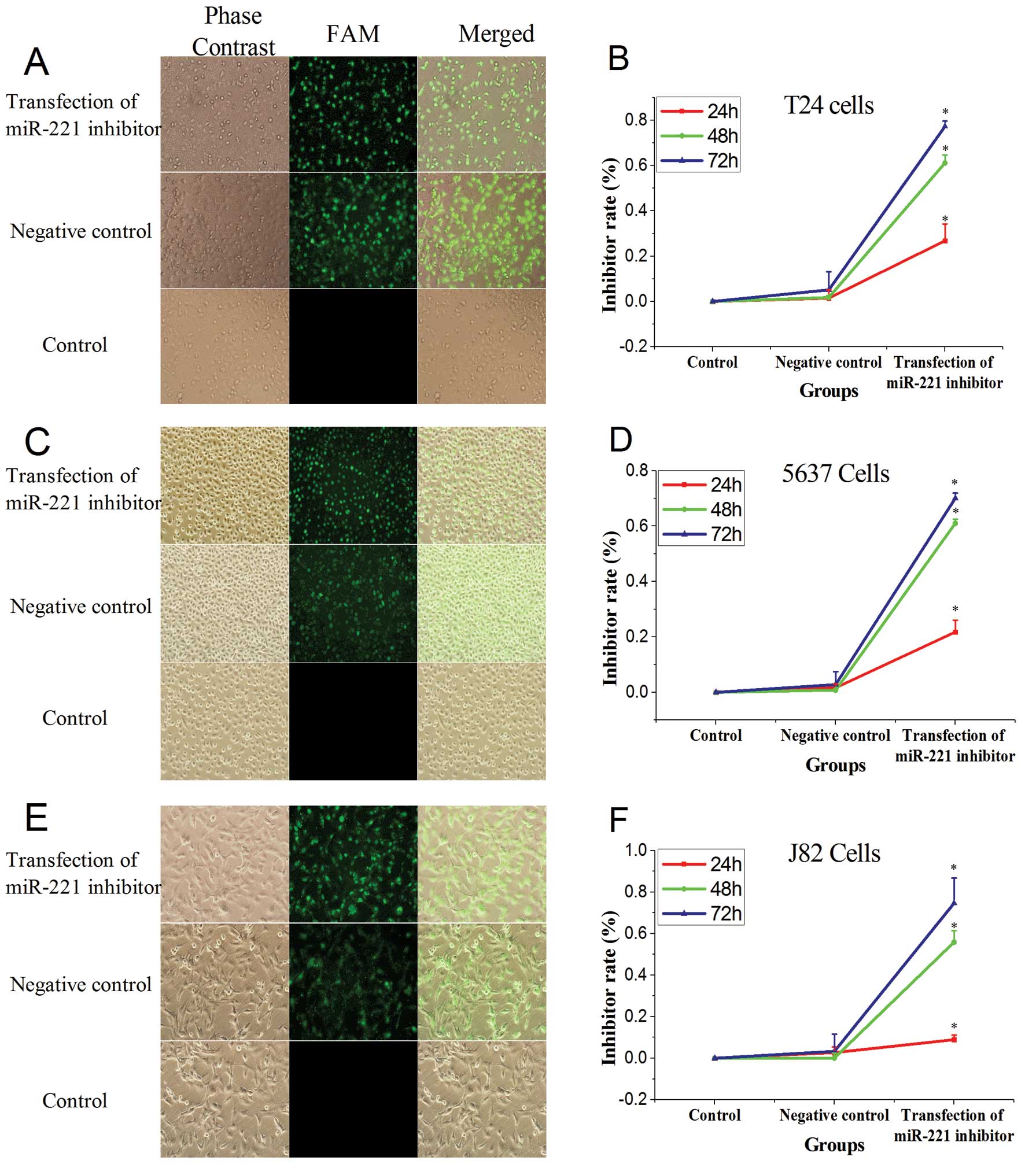
Observation by fluorescence microscopy performed 5 h
after transfection showed that the transfection efficiency for T24,
5637, and J82 cells were 80, 8 and 90%, respectively (Fig. 1). The miR-221 inhibitor was
transfected into bladder cancer T24, 5637 and J82 cells. The cell
viability was determined at 24 h, 48 and 72 h. Cell proliferation
was inhibited in a time-dependent maner, but to varying degrees in
the three tested lines. In addition, the rate of inhibition of cell
proliferation increased with increasing time (P<0.05, vs.
control group, Fig. 1D).
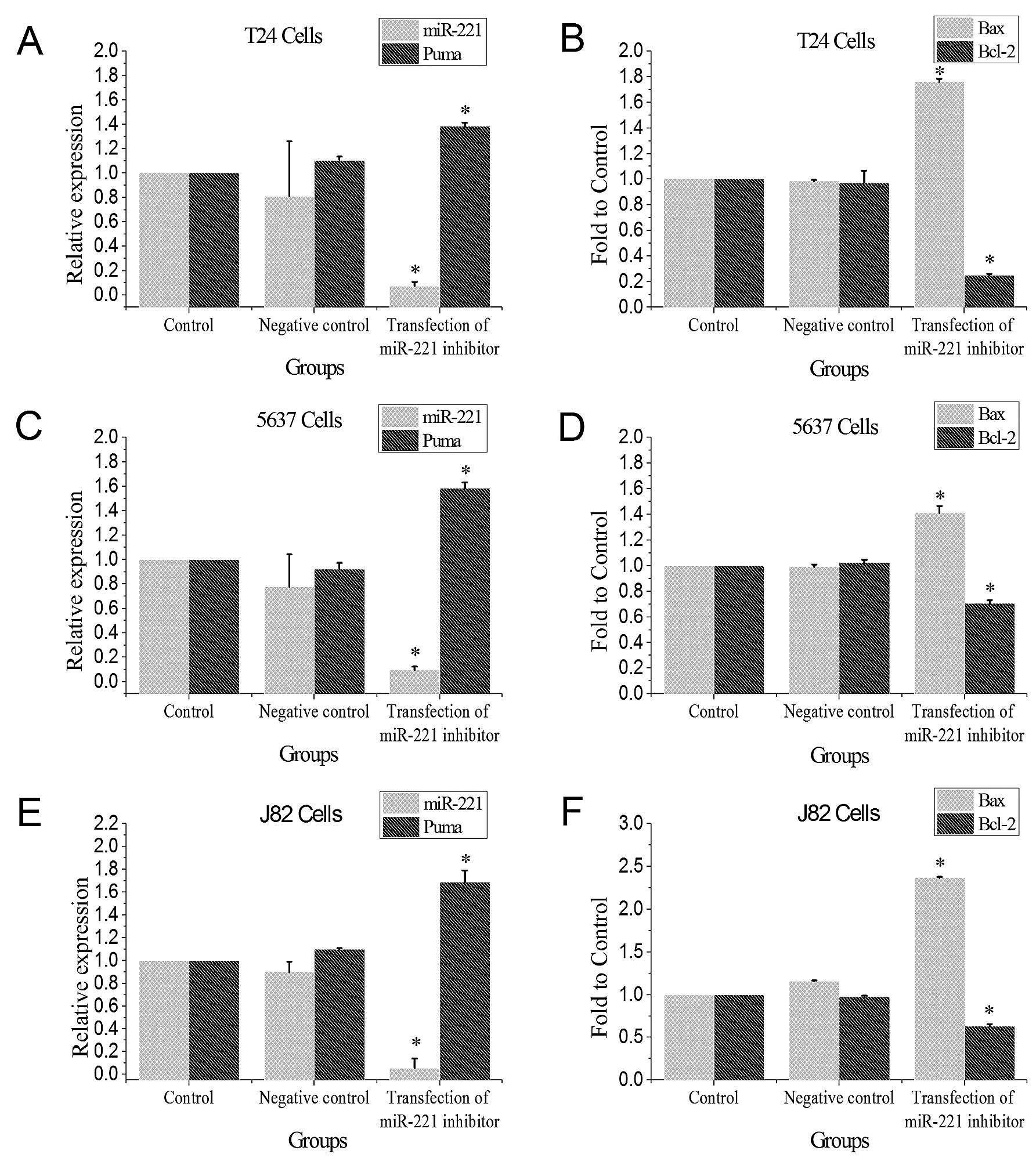
Transfection of miR-221 inhibitor reduces
miR-221 expression
The T24, 5637 and J82 bladder cancer cells were
transfected with miR-221 inhibitor for 48 h, miR-221 expression
levels were determined by qRT-PCR. Transfection of miR-221
significantly reduced miR-221 expression when compared to the
control group and the negative control group (P<0.05, Fig. 2).
miR-221 inhibitor induces mRNAs of PUMA
and Bax, and reduces Bcl-2 mRNA
Results from qRT-PCR showed that after miR-221
expression was inhibited in the three bladder cancer cell lines,
PUMA and Bax mRNA levels were significantly induced, while mRNA of
the anti-apoptotic Bcl-2 was significantly reduced when compared to
the blank group (P<0.05, Fig.
2).
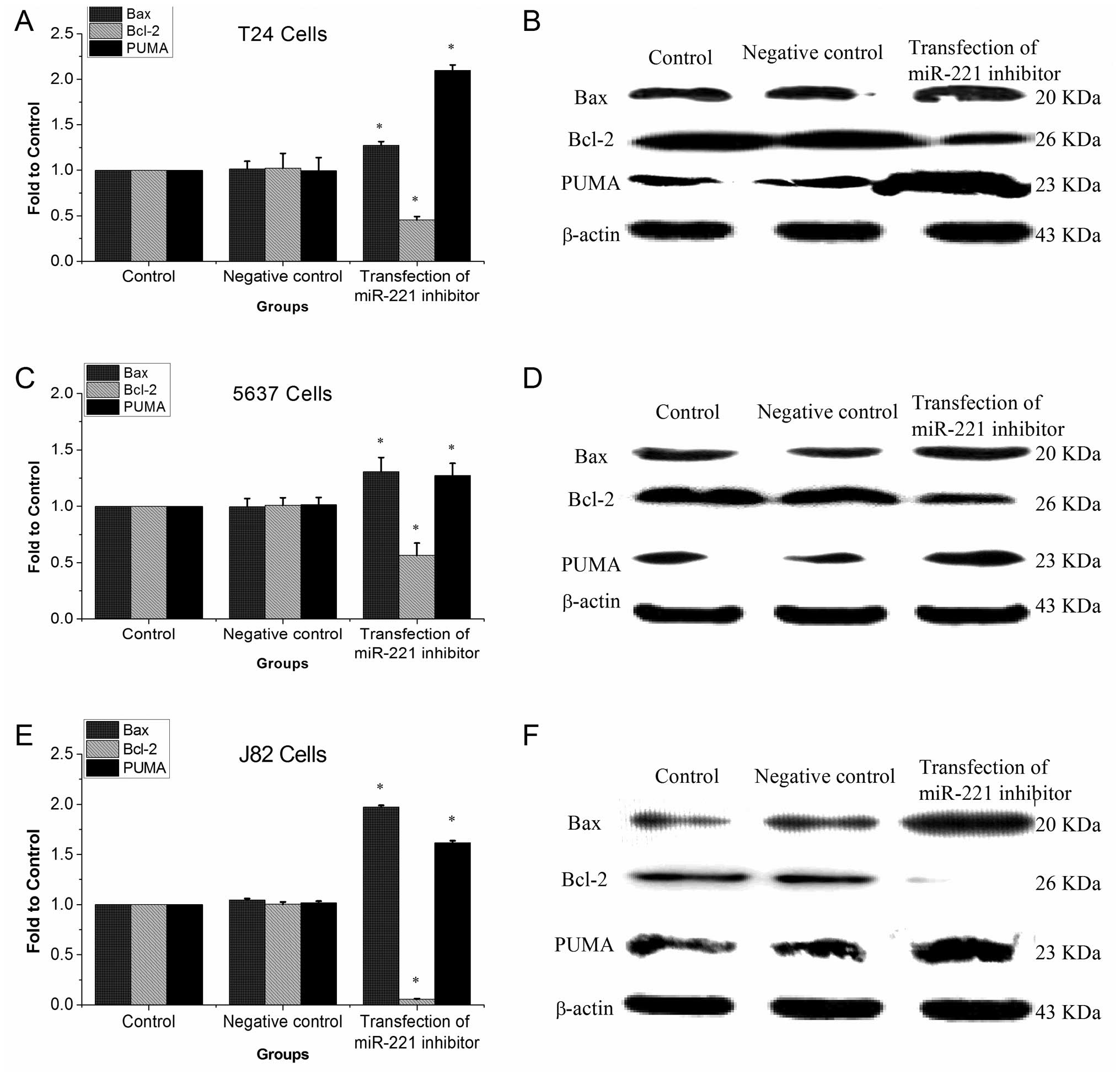
miR-221 inhibitor induces PUMA and Bax
protein expression and reduces Bcl-2 protein expression
After inhibiting the expression of miR-221 in three
bladder cancer cell lines, proteins of PUMA and Bax were
significantly devated, while Bcl-2 protein was significantly
reduced when compared with control group (P<0.05, Fig. 3).
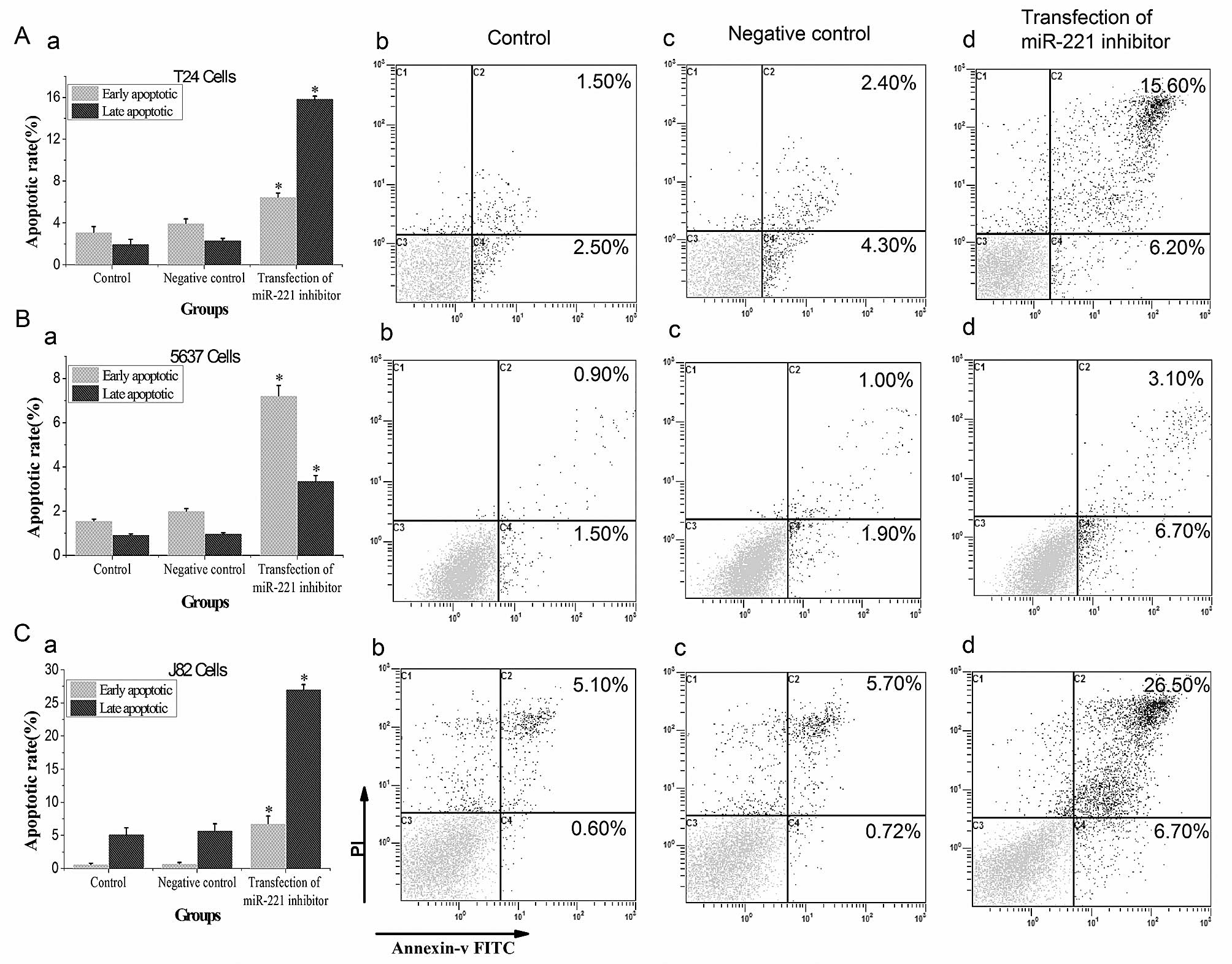
miR-221 inhibitor promotes apoptosis of
bladder cancer cells
Flow cytometry results showed that both the early
and late apoptosis rates in miR-221 inhibitor transfected groups
were significantly higher than those in control groups (P<0.05,
Fig. 4) in T24, 5637 and J82
cells. AO-EB also indicates that DNA damage in miR-221 inhibitor
transfected groups was
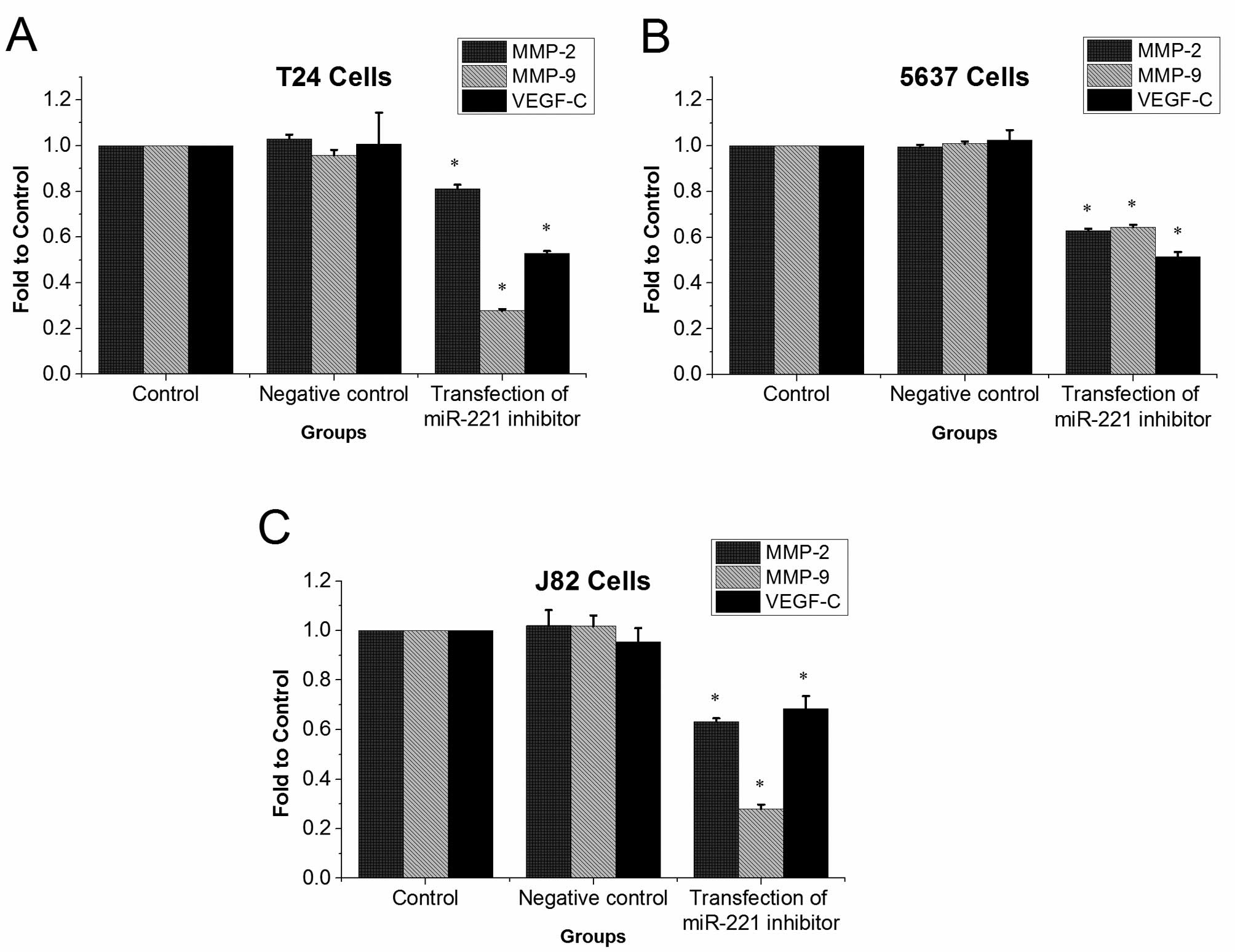
miR-221 inhibitor reduces mRNA levels of
MMP-9, MMP-2, and VEGF-C
Results from qRT-PCR showed that in T24, 5637 and
J82 cells MMP-9, MMP-2, and VEGF-C mRNA levels were significantly
lower in miR-221 inhibitor transfected groups than in control
groups (P<0.05, Fig. 6).
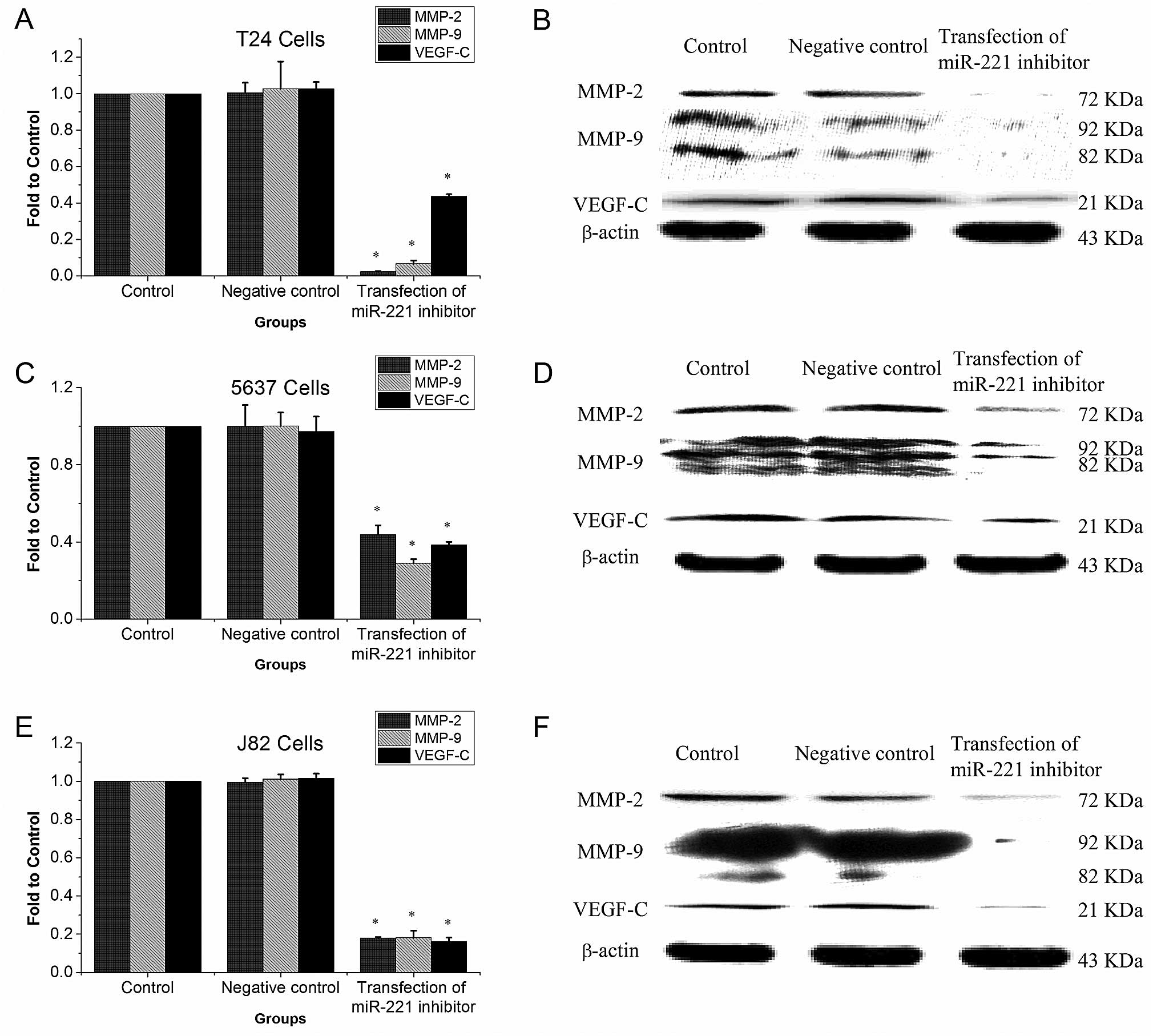
miR-221 inhibitor reduces MMP-9, MMP-2,
and VEGF-C protein expression
Western blotting showed that in T24, 5637, and J82
cells, MMP-9, MMP-2, and VEGF-C protein levels were significantly
lower in miR-221 inhibitor transfected groups than in the control
groups (P<0.05, Fig. 7).
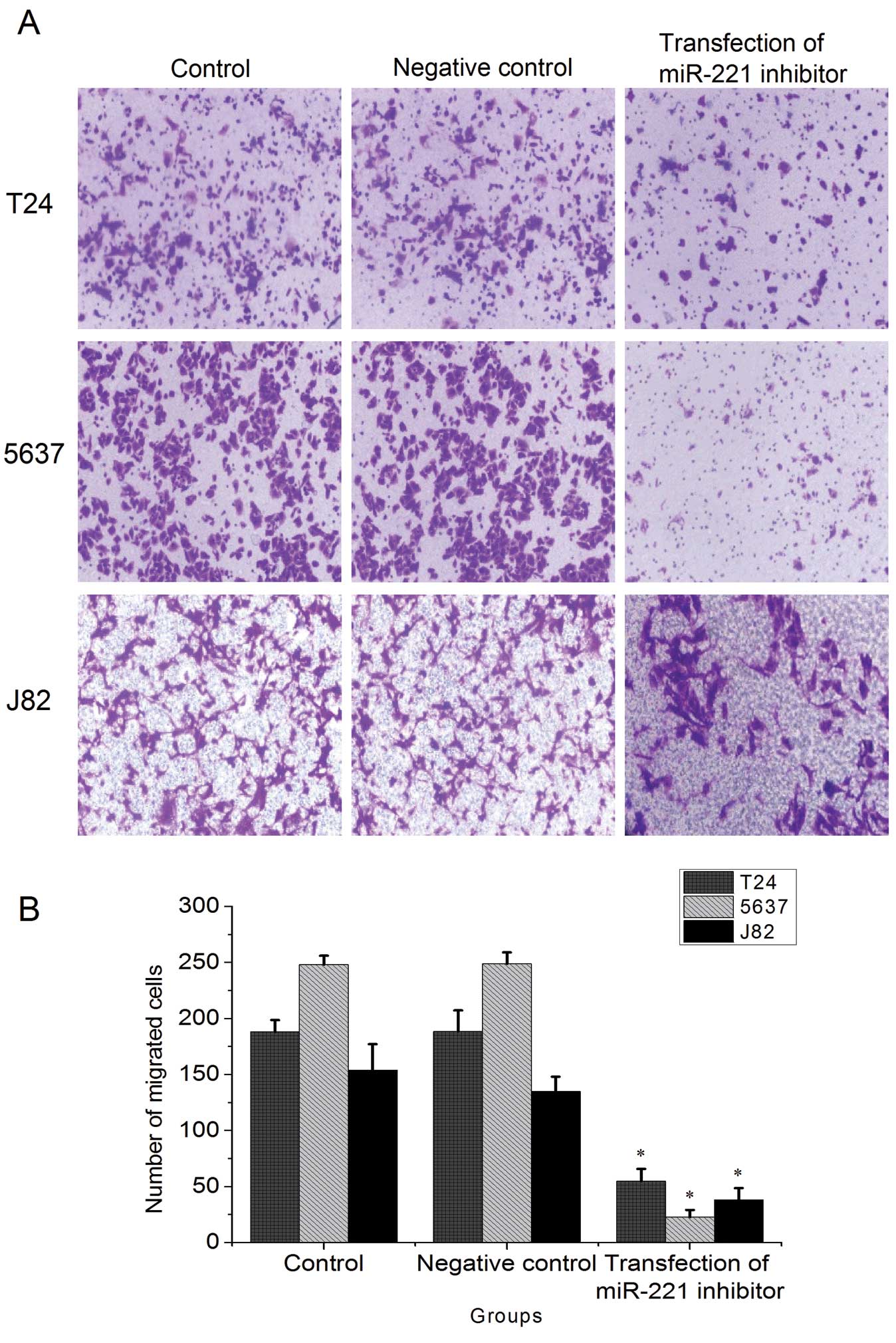
miR-221 inhibitor reduces the
invasiveness of bladder cancer cells
Transwell assays showed that in T24, 5637 and J82
cells, the invasiveness of bladder cancer cells was significantly
reduced in miR-221 inhibitor transfected groups than in the control
groups (P<0.05, Fig. 8). There
was no significant difference in invasive capability between the
control groups and negative control groups.
Discussion
Interactions between the immune system and tumor
cells play a very important role in tumorigenesis. Failure of the
immune system to recognize or kill cancer cells leads to tumor
occurrence and development. Immune killing of tumor cells involves
the transmission of apoptotic signals from immune cells to tumor
cells, as well as the subsequent transduction of the apoptotic
signal in tumor cells. However, most of the research on immune
evasion of tumor cells focuses on the ability of cancer cells to
block the apoptotic signal as it is transmitted from immune cells
to tumor cells (29). In fact,
transmission of the apoptotic signal from immune cells to tumor
cells is actually not blocked completely, which means that tumor
cells do receive attenuated apoptotic signals from immune cells
(9–11). Therefore, abnormal intracellular
transduction of apoptotic signals in tumor cells may be another
important factor for immune evasion.
Apoptosis is a process of programmed autonomous cell
death. It is induced by a variety of factors inside and outside the
body and is controlled by strict and complex regulation of
signaling networks (30–32). Currently, three pathways of cell
apoptosis have been characterized. The first is the intrinsic
apoptotic pathway, namely the mitochondrial apop-totic pathway, in
which the intracellular signal transduction involves the
interaction of pro-apoptotic factors and the anti-apoptotic Bcl-2
protein family. The second apoptosis pathway is the endoplasmic
reticulum signaling pathway, which does not yet have a clear
mechanism. The third apoptosis pathway is the extrinsic apoptosis
pathway, also termed as dead receptor pathway, which induces
apoptosis by activating the corresponding ligands and initiating
the death receptors on the cell surfaces (33–38).
The three pathways are interrelated. For instance, the extrinsic
apoptotic pathway can activate the mitochondrial apoptotic pathway
by activation of Bid (39).
PUMA was first reported by Nakano in 2001 (74) as a member of the BH3-only subfamily
in the Bcl-2 family. It is a pro-apoptosis factor. The
pro-apoptosis function of PUMA mainly depends on its BH3 domain and
the orderliness and integrity of the 43 amino acid residues at its
C-terminus (34,39–41).
Previous studies have shown that the pro-apoptotic mechanism of
PUMA acts through various mechanisms, ultimately causing
depolarization of the mitochondrial membrane potential and
initiating the mitochondrial apoptosis pathway. This results in the
release of cytochrome c and Smac/DIABLO into the cytoplasm
and the activation of apoptosis (15,42–44).
However, the expression of PUMA is regulated by transcription
factors (p53, c-Myc and FoxO3a) as well as post-transcriptional
regulation (miR-221/miR-222) (44–48).
It has been reported that miR-221 expression is
upregulated in bladder cancer tissues compared to adjacent bladder
tissues and that miR-221 can specifically target the 3′ non-coding
region of the mature PUMA mRNA and silence PUMA expression
(12,44,49).
In this study, miR-221 inhibitor (modified by 2′ O-methylation and
FAM) and a negative control sequence were transfected into bladder
cancer cells. After transfection with miR-221 inhibitor for 24, 48
and 72 h, cell viability was measured using the MTT assay. Results
indicated that miR-221 inhibitor significantly impedes the
proliferation of bladder cancer cells. Furthermore, the inhibition
of proliferation is time-dependent, indicating that a decrease in
miR-221 suppresses cell proliferation. Floating cell debris after
our 72-h transfection made it more difficult to meet the
requirements of subsequent experiments, so we chose to transfect
miR-221 inhibitor for 48 h in subsequent experiments. Taking our
qRT-PCR and western blotting results together, we have shown that,
48 h after transfection of miR-221 inhibitor, miR-221 mRNA levels
were significantly inhibited, resulting in an increase in PUMA and
Bax mRNA and protein, but a decrease in Bcl-2 mRNA and protein.
Flow cytometry and AO-EB staining assays confirmed that miR-221
inhibition promotes apoptosis in bladder cancer cells.
Collectively, the overexpression of endogenous miR-221 is an
important factor for the inhibition of apoptosis in bladder cancer
cells.
Tumor cells capable of immune evasion are not only
able to resist apoptosis, but can also release immunosuppressive
factors into the extracellular environment by autocrine or
paracrine secretion, which results in deep immunosuppressive
regions formed locally in tumor cells. The formation of these
regions is one of the important mechanisms of tumor cell immune
evasion. Among these immunosuppressive factors, VEGF and MMP play
important roles in tumor invasion and metastasis.
There are seven VEGF isoforms, namely VEGF-A,
VEGF-B, VEGF-C, VEGF-D, VEGF-E, VEGF-F and PIGF (50). VEGF-C plays an important role in
the regulation of tumor metastasis and infiltration. Previous
studies have shown that VEGF-C is a protein precursor that must be
activated by converting enzyme (proprotein convertases, PC) 5 and 7
(51,52). The associated receptors of VEGF-C
include VEGFR-2, VEGFR-3 and Nrp-2, among which VEGFR-3 is the
receptor that promotes lymphangiogenesis and tumor cell migration,
invasion and metastasis. Study of Nrp-2 has suggested that Nrp-2
only plays a regulatory role and is not a necessary receptor during
lymphangiogenesis (53–57). In human tumors, abnormal VEGFR-3
expression has been found in gastric cancer (58), lung cancer (59), colorectal cancer (60,61),
head and neck cancer (62),
bladder cancer (63), and breast
cancer (64). Based on these
previous studies, we confirmed that VEGF-C overexpression in tumor
cells is the premise and one of the early events of tumor cell
microme-tastasis into the lymph node. Studies on immune evasion
have confirmed that VEGF-C is a potent immunosuppressive factor,
which not only impedes immune cells from recognizing tumor cells,
but can also destroy the biological functions of immune cells
(65). In this study, after
transfecting miR-221 inhibitor into T24, 5637 and J82 bladder
cancer cells, VEGF-C mRNA and protein expression levels decreased
significantly. It remains to be further studied whether this
phenomenon is due to the upregulation of pro-apoptotic genes PUMA
and Bax, the downregulation of anti-apoptotic Bcl-2, or other
mechanisms, but it is clear that miR-221 did not directly regulate
the effects of VEGF-C. Taken together, decreased miR-221 in bladder
cancer reduces the effects of VEGF-C in promoting the formation of
microlymphatic vessels and capillary vessels at tumor sites, as
well as the ability of VEGF-C to inhibit immune cell activity.
There are many members in the family of MMPs, among
which MMP-2 and MMP-9 are closely associated with tumor invasion
and development. Their main mechanism of action include: i) MMP-2
and MMP-9 degrade the extracellular matrix and basement membrane
and destroy cell structure. In addition, MMP-2 has the potential
capability to activate extracellular matrix structural proteins and
plays an important role in the chemotaxis of inflammatory cells as
well as the spontaneous stimulation of tumor cells for migration
and invasion; ii) MMPs promote tumor angiogenesis. As blood vessel
formation is a prerequisite for the tumor cell growth, metastasis
and invasion, MMP-2 promotes angiogenesis after interacting with
their corresponding substrates. At the initial stage of tumor
angiogenesis in tumor nodules, MMP-2 secretion is essential for
tumor cells (66–70). In this study, after transfecting
miR-221 inhibitor into bladder cancer T24, 5637 and J82 cells for
48 h, we found that both the mRNA and protein levels of MMP-2 and
MMP-9 decreased. Although we only detected total MMP-2 and MMP-9
protein, rather than their phosphorylated isoforms which are the
activated forms, our Transwell results indicate that cancer cell
invasiveness was significantly decreased after reducing miR-221.
Furthermore, MMP-2 and MMP-9 are closely related to tumor cell
invasion (71–73). Therefore, the overexpression of
MMP-2 and MMP-9 in bladder cancer is likely an important factor for
the invasion and metastasis of bladder cancer cells.
In conclusion, this study showed that the
overexpression of miR-221 in bladder cancer cells inhibits the
expression of the pro-apoptotic gene PUMA, thereby inhibiting the
trans-duction of apoptotic signals. Meanwhile, the downregulation
of miR-221 in bladder cancer cells reduced the expression of
VEGF-C, MMP-2 and MMP-9, resulting in reduced invasion of bladder
cancer cells. Therefore, miR-221-induced PUMA silencing is a key
factor for the immune evasion of bladder cancer.
Acknowledgements
This study was supported by the National Natural
Science Foundation of China (grant no. 81160272) and the Natural
Science Foundation of Jiangxi (grant no. 800GZY0039) and the
Jiangxi Province Science Foundation for Youths (grant no.
2010JX02761) and The Science and Technology Development Fund of
Macao Special Administrative Region (grant no. 064/2012/A).
References
|
1
|
Abedinpour P, Baron VT, Welsh J, et al:
Regression of prostate tumors upon combination of hormone ablation
therapy and celecoxib in vivo. Prostate. 71:813–823. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Ahmad A, Aboukameel A, Kong DJ, et al:
Phosphoglucose isomerase/autocrine motility factor mediates
epithelial-mesenchymal transition regulated by miR-200 in breast
cancer cells. Cancer Res. 71:3400–3409. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Akkoc A, Inan S and Sonmez G: Matrix
metalloproteinase (MMP-2 and MMP-9) and steroid receptor
expressions in feline mammary tumors. Biotech Histochem.
87:312–319. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Amente S, Zhang J, Lavadera ML, et al: Myc
and PI3K/AKT signaling cooperatively repress FOXO3a-dependent PUMA
and GADD45a gene expression. Nucleic Acids Res. 39:9498–9507. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Asirvatham AJ, Gregorie CJ, Hu Z, et al:
MicroRNA targets in immune genes and the Dicer/Argonaute and ARE
machinery components. Mol Immunol. 45:1995–2006. 2008. View Article : Google Scholar
|
|
6
|
Bean GR, Ganesan YT, Dong YY, et al: PUMA
and BIM are required for oncogene inactivation-induced apoptosis.
Sci Signal. 6:ra202013.PubMed/NCBI
|
|
7
|
Campone M, Noel B, Couriaud C, et al:
c-Myc dependent expression of proapoptotic Bim renders
HER2-overexpressing breast cancer cells dependent on anti-apoptotic
Mcl-1. Mol Cancer. 10:1102011. View Article : Google Scholar
|
|
8
|
Catto JW, Alcaraz A, Bjartell AS, et al:
MicroRNA in prostate, bladder, and kidney cancer: a systematic
review. Eur Urol. 59:671–681. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Chang LY, Lin YC, Mahalingam J, et al:
Tumor-derived chemokine CCL5 enhances TGF-beta-mediated killing of
CD8(+) T cells in colon cancer by T-regulatory cells. Cancer Res.
72:1092–1102. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Chen L, Zhang J, Han L, et al:
Downregulation of miR-221/222 sensitizes glioma cells to
temozolomide by regulating apoptosis independently of p53 status.
Oncol Rep. 27:854–860. 2012.
|
|
11
|
Chopin D, Barei-Moniri R, Maille P, et al:
Human urinary bladder transitional cell carcinomas acquire the
functional Fas ligand during tumor progression. Am J Pathol.
162:1139–1149. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Di Martino MT, Gulla A, Cantafio ME, et
al: In vitro and in vivo antitumor activity of miR-221/222
inhibitors in multiple myeloma. Oncotarget. 4:242–255.
2013.PubMed/NCBI
|
|
13
|
Eissa S, Badr S, Elhamid SA, et al: The
value of combined use of survivin mRNA and matrix metalloproteinase
2 and 9 for bladder cancer detection in voided urine. Dis Markers.
34:57–62. 2013. View Article : Google Scholar :
|
|
14
|
Eissa S, Shabayek MI, Ismail MF, et al:
Diagnostic evaluation of apoptosis inhibitory gene and tissue
inhibitor matrix metalloproteinase-2 in patients with bladder
cancer. IUBMB Life. 62:394–399. 2010.PubMed/NCBI
|
|
15
|
Errami Y, Naura AS, Kim H, et al:
Apoptotic DNA fragmentation may be a cooperative activity between
caspase-activated deoxy-ribonuclease and the poly(ADP-ribose)
polymerase-regulated DNAS1L3, an endoplasmic reticulum-localized
endonuclease that translocates to the nucleus during apoptosis. J
Biol Chem. 288:3460–3468. 2013. View Article : Google Scholar :
|
|
16
|
Follis AV, Chipuk JE, Fisher JC, et al:
PUMA binding induces partial unfolding within BCL-xL to disrupt p53
binding and promote apoptosis. Nat Chem Biol. 9:163–168. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Foster RR, Satchell SC, Seckley J, et al:
VEGF-C promotes survival in podocytes. Am J Physiol Renal Physiol.
291:F196–F207. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Garcia-Lora A, Algarra I and Garrido F:
MHC class I antigens, immune surveillance, and tumor immune escape.
J Cell Physiol. 195:346–355. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Gottardo F, Liu CG, Ferracin M, et al:
Micro-RNA profiling in kidney and bladder cancers. Urol Oncol.
25:387–392. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Holoch PA and Griffith TS: TNF-related
apoptosis-inducing ligand (TRAIL): a new path to anti-cancer
therapies. Eur J Pharmacol. 625:63–72. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Igney FH and Krammer PH: Immune escape of
tumors: apoptosis resistance and tumor counterattack. J Leukoc
Biol. 71:907–920. 2002.PubMed/NCBI
|
|
22
|
Inman BA, Sebo TJ, Frigola X, et al: PD-L1
(B7-H1) expression by urothelial carcinoma of the bladder and
BCG-induced granulomata: associations with localized stage
progression. Cancer. 109:1499–1505. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Jayasinghe C, Simiantonaki N,
Michel-Schmidt R, et al: Endothelial VEGFR-3 expression in
colorectal carcinomas is associated with hematogenous metastasis.
Oncol Rep. 22:1093–1100. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Jayasooriya RG, Choi YH, Moon SK, et al:
Methanol extract of Hydroclathrus clathratus suppresses matrix
metalloproteinase-9 in T24 bladder carcinoma cells by suppressing
the NF-kappaB and MAPK pathways. Oncol Rep. 27:541–546. 2012.
|
|
25
|
Jemal A, Bray F, Center MM, et al: Global
cancer statistics. CA Cancer J Clin. 61:69–90. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Jin X, Xiao LJ, Zhang XS, et al: Apotosis
in ovary. Front Biosci (Schol Ed). 3:680–697. 2011. View Article : Google Scholar
|
|
27
|
Kajiya K, Sawane M, Huggenberger R, et al:
Activation of the VEGFR-3 pathway by VEGF-C attenuates UVB-induced
edema formation and skin inflammation by promoting
lymphangiogenesis. J Invest Dermatol. 129:1292–1298. 2009.
View Article : Google Scholar
|
|
28
|
Khong HT and Restifo NP: Natural selection
of tumor variants in the generation of ‘tumor escape’ phenotypes.
Nat Immunol. 3:999–1005. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Krebs R, Tikkanen JM, Ropponen JO, et al:
VEGF-C/VEGFR-3 signaling regulates inflammatory response in
development of obliterative airway disease. J Heart Lung Transpl.
30:S1182011. View Article : Google Scholar
|
|
30
|
Langers AMJ, Verspaget HW, Hawinkels LJAC,
et al: MMP-2 and MMP-9 in normal mucosa are independently
associated with outcome of colorectal cancer patients. Br J Cancer.
106:1495–1498. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Li XQ, Dang XG and Sun XB: Expression of
survivin and VEGF-C in breast cancer tissue and its relation to
lymphatic metastasis. Eur J Gynaecol Oncol. 33:178–182.
2012.PubMed/NCBI
|
|
32
|
Li Y, Yang K, Mao Q, et al: Inhibition of
TGF-beta receptor I by siRNA suppresses the motility and
invasiveness of T24 bladder cancer cells via modulation of
integrins and matrix metallopro-teinase. Int Urol Nephrol.
42:315–323. 2010. View Article : Google Scholar
|
|
33
|
Lund AW, Duraes FV, Hirosue S, et al:
VEGF-C promotes immune tolerance in B16 melanomas and
cross-presentation of tumor antigen by lymph node lymphatics. Cell
Rep. 1:191–199. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Martins SF, Garcia EA, Luz MA, et al:
Clinicopathological correlation and prognostic significance of
VEGF-A, VEGF-C, VEGFR-2 and VEGFR-3 expression in colorectal
cancer. Cancer Genomics Proteomics. 10:55–67. 2013.PubMed/NCBI
|
|
35
|
Min Y, Ghose S, Boelte K, et al:
C/EBP-delta regulates VEGF-C autocrine signaling in
lymphangiogenesis and metastasis of lung cancer through HIF-1
alpha. Oncogene. 30:4901–4909. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Neal MD, Sodhi CP, Jia H, et al: The P53
upregulated modulator of apoptosis (Puma) regulates Tlr4-mediated
enterocyte apopotosis in the pathogenesis of necrotizing
enterocolitis. Shock. 35:60. 2011.
|
|
37
|
Newton MR, Askeland EJ, Andresen ED, et
al: Anti-interleukin-10R1 monoclonal antibody in combination with
BCG is protective against bladder cancer metastasis in a murine
orthotopic tumor model and demonstrates systemic specific antitumor
immunity. Clin Exp Immunol. 177:261–268. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Niederkorn JY: Immune escape mechanisms of
intraocular tumors. Prog Retin Eye Res. 28:329–347. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Okada A: Roles of matrix
metalloproteinases and tissue inhibitor of metalloproteinase (TIMP)
in cancer invasion and metastasis. Gan To Kagaku Ryoho.
26:2247–2252. 1999.
|
|
40
|
Okada R, Nagaosa K, Kuraishi T, et al:
Apoptosis-dependent externalization and involvement in apoptotic
cell clearance of DmCaBP1, an endoplasmic reticulum protein of
Drosophila. J Biol Chem. 287:3138–3146. 2012. View Article : Google Scholar :
|
|
41
|
Olofsson B, Jeltsch M, Eriksson U, et al:
Current biology of VEGF-B and VEGF-C. Curr Opin Biotech.
10:528–535. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Piazzolla G, Nuzzaci M, Vitti A, et al:
Apoptotic effects of a chimeric plant virus carrying a mimotope of
the hepatitis C virus hypervariable region 1: role of caspases and
endoplasmic reticulum-stress. J Clin Immunol. 32:866–876. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Planaguma L, Liljestrom M, Alameda F, et
al: Matrix metalloproteinase-2 and matrix metalloproteinase-9
codistribute with transcription factors RUNX1/AML1 and ETV5/ERM at
the invasive front of endometrial and ovarian carcinoma. Hum
Pathol. 42:57–67. 2011. View Article : Google Scholar
|
|
44
|
Poyet C, Banzola I, Linto T, et al:
Bladder cancer micro-environment influences maturation signature in
lymphatic endothelial cells (LECs) by VEGF-C. Eur Urol (Suppl).
11:E906–U905. 2012. View Article : Google Scholar
|
|
45
|
Rabinovich GA, Gabrilovich D and Sotomayor
EM: Immunosuppressive strategies that are mediated by tumor cells.
Annu Rev Immunol. 25:267–296. 2007. View Article : Google Scholar
|
|
46
|
Saharinen P, Eklund L, Pulkki K, et al:
VEGF and angiopoietin signaling in tumor angiogenesis and
metastasis. Trends Mol Med. 17:347–362. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Sato H, Takino T and Miyamori H: Roles of
membrane-type matrix metalloproteinase-1 in tumor invasion and
metastasis. Cancer Sci. 96:212–217. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Seiler R, Thalmann GN and Fleischmann A:
MMP-2 and MMP-9 in lymph-node-positive bladder cancer. J Clin
Pathol. 64:1078–1082. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Senger DR, Van de Water L, Brown LF, et
al: Vascular permeability factor (VPF, VEGF) in tumor biology.
Cancer Metastasis Rev. 12:303–324. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Siddle H, Kreiss A, Tovab C, et al: Immune
escape strategies of a contagious cancer, devil facial tumour
disease. Mol Immunol. 51:302012. View Article : Google Scholar
|
|
51
|
Siriwardena BSMS, Kudo Y, Ogawa I, et al:
VEGF-C is associated with lymphatic status and invasion in oral
cancer. J Clin Pathol. 61:103–108. 2008. View Article : Google Scholar
|
|
52
|
Stanton MJ, Dutta S, Zhang H, et al:
Autophagy control by the VEGF-C/NRP-2 axis in cancer and its
implication for treatment resistance. Cancer Res. 73:160–171. 2013.
View Article : Google Scholar :
|
|
53
|
Sullu Y, Demirag GG, Yildirim A, et al:
Matrix metalloproteinase-2 (MMP-2) and MMP-9 expression in invasive
ductal carcinoma of the breast. Pathol Res Pract. 207:747–753.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Takano S: Glioblastoma angiogenesis: VEGF
resistance solutions and new strategies based on molecular
mechanisms of tumor vessel formation. Brain Tumor Pathol. 29:73–86.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Takizawa H, Kondo K, Fujino H, et al: The
balance of VEGF-C and VEGFR-3 mRNA is a predictor of lymph node
metastasis in non-small cell lung cancer. Br J Cancer. 95:75–79.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Torii A, Kodera Y, Ito M, et al: Matrix
metalloproteinase 9 in mucosally invasive gastric cancer. Gastric
Cancer. 1:142–145. 1998. View Article : Google Scholar
|
|
57
|
Tsubata T: Apotosis of mature B cells. Int
Rev Immunol. 18:347–365. 1999. View Article : Google Scholar
|
|
58
|
Valtola R, Salven P, Heikkila P, et al:
VEGFR-3 and its ligand VEGF-C are associated with angiogenesis in
breast cancer. Am J Pathol. 154:1381–1390. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Vasala K, Paakko P and
Turpeenniemi-Hujanen T: Matrix metalloproteinase-9 (MMP-9)
immunoreactive protein in urinary bladder cancer: a marker of
favorable prognosis. Anticancer Res. 28:1757–1761. 2008.PubMed/NCBI
|
|
60
|
Vasala K and Turpeenniemi-Hujanen T: Serum
tissue inhibitor of metalloproteinase-2 (TIMP-2) and matrix
metalloproteinase-2 in complex with the inhibitor (MMP-2:TIMP-2) as
prognostic markers in bladder cancer. Clin Biochem. 40:640–644.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Wang F, Li HM, Wang HP, et al:
siRNA-mediated knockdown of VEGF-A, VEGF-C and VEGFR-3 suppresses
the growth and metastasis of mouse bladder carcinoma in vivo. Exp
Ther Med. 1:899–904. 2010.PubMed/NCBI
|
|
62
|
Wang Z, Li R, Zhou B, et al: Relationships
of human laryngeal squamous cell carcinomas with the expression of
VEGF-C and VEGFR-3. Sheng Wu Yi Xue Gong Cheng Xue Za Zhi.
26:842–846. 2009.(In Chinese). PubMed/NCBI
|
|
63
|
Wu K, Wang X, Xie Z, et al: Glutathione
S-transferase P1 gene polymorphism and bladder cancer
susceptibility: an updated analysis. Mol Biol Rep. 40:687–695.
2013. View Article : Google Scholar
|
|
64
|
Yerlikaya A, Okur E and Ulukaya E: The
p53-independent induction of apoptosis in breast cancer cells in
response to proteasome inhibitor bortezomib. Tumor Biol.
33:1385–1392. 2012. View Article : Google Scholar
|
|
65
|
Yonemura Y, Fushida S, Bando E, et al:
Lymphangiogenesis and the vascular endothelial growth factor
receptor (VEGFR)-3 in gastric cancer. Eur J Cancer. 37:918–923.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Yu J and Zhang L: No PUMA, no death:
implications for p53-dependent apoptosis. Cancer Cell. 4:248–249.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Yu J, Zhang L, Hwang PM, et al: PUMA
induces the rapid apoptosis of colorectal cancer cells. Mol Cell.
7:673–682. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Zhang C, Zhang J, Zhang A, et al: PUMA is
a novel target of miR-221/222 in human epithelial cancers. Int J
Oncol. 37:1621–1626. 2010.PubMed/NCBI
|
|
69
|
Zhang CZ, Zhang JX, Zhang AL, et al:
MiR-221 and miR-222 target PUMA to induce cell survival in
glioblastoma. Mol Cancer. 9:2292010. View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Zhang LN, Li JY and Xu W: A review of the
role of Puma, Noxa and Bim in the tumorigenesis, therapy and drug
resistance of chronic lymphocytic leukemia. Cancer Gene Ther.
20:1–7. 2013. View Article : Google Scholar
|
|
71
|
Zhao ZW, Wang JJ, Tang JS, et al: JNK- and
Akt-mediated Puma expression in the apoptosis of
cisplatin-resistant ovarian cancer cells. Biochem J. 444:291–301.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
72
|
Zheng M, Zhang Q, Joe Y, et al: Curcumin
induces apoptotic cell death of activated human CD4+ T
cells via increasing endoplasmic reticulum stress and mitochondrial
dysfunction. Int Immunopharmacol. 15:517–523. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
73
|
Zhu X, Tai W, Shi W, et al: Matrix
metalloproteinase-9 silencing by RNA interference promotes the
adhesive-invasive switch in HT1080 human fibrosarcoma cells. Clin
Lab. 58:313–322. 2012.PubMed/NCBI
|
|
74
|
Nakano K and Vousden KH: PUMA, a novel
proapoptotic gene, is induced by p53. Mol Cell. 7:683–694. 2001.
View Article : Google Scholar : PubMed/NCBI
|