Introduction
Colon cancer is one of the most common malignancies
and causes considerable morbidity and mortality (1). Substantial progress has been made in
colon cancer treatment and diagnosis, but the treatment of colon
cancer is still challenging (2).
Thus, there is an urgent clinical need to explore new agents or
adjuvants for colon cancer treatment.
Natural products and their derived active
components, including semi-synthetic and synthetic analogs, have
served as one of the major sources for anticancer agents (3–6). A
few plant derived compounds have been used for anticancer drugs for
some years (7), such as
vinblastine, vincristine, etoposide, teniposide, taxol and
camptothecin. Hence, natural products play a major role in cancer
chemotherapy.
Tetrandrine (Tet), a bis-benzylisoquinoline
alkaloid, is extracted from the dried root of Stephania
tetrandra S. Moore (Chinese herb hang fang ji). It has
been reported that Tet can exert various effects, such as
anti-inflammatory, immunosuppressive and anti-hypertensive
(8–10). Also, it has been found that Tet was
able to inhibit proliferation and induce apoptosis in many cancer
cells, including breast, lung, colon cancer and neuroblastoma
(11–14). Mechanistically, it has been
reported that a few signaling pathways or critical factors are
involved in the anticancer activity for Tet, such as
mitogen-activated protein kinases (MAPKs) (15), Wnt/β-catenin (13), PI3K/Akt (16) and p53 (17). We have also found that Tet can
inhibit the proliferation of human colon cancer cell by targeting
Wnt/β-catenin (13), but the exact
mechanism of this effect is not fully understood yet.
Insulin-like growth factor (IGF) signaling pathway
plays an essential role in controlling cell differentiation,
proliferation, apoptosis and aging (18). The IGF signaling is well regulated
by IGF binding proteins (IGFBPs), which contains seven members
(IGFBP-1 to IGFBP-7). It has been reported that IGF signaling was
associated with colon cancer (19), such as IGFBP-2 and IGFBP-5
(20,21). IGFBP-5 has been reported
associating with many types of cancer (21), such as breast cancer (22, 23), urothelial carcinoma (24), neuroblastoma (25) and osteosarcoma (26), but the role of IGFBP-5 in colon
cancer remains unknown.
In the present study, we investigated the anticancer
activity of Tet in human colon cancer cells, and dissected the
possible molecular mechanism of this effect. Our results strongly
indicate that IGFBP-5 may play an important role in colon cancer
development, the anticancer activity of Tet in colon cancer may be
mediated by inhibiting Wnt/β-catenin signaling transduction partly
through downregulating the expression of IGFBP-5.
Materials and methods
Chemicals and drug preparations
Tetrandrine (Tet) and DMH were from Sigma-Aldrich
(St. Louis, MO, USA). The LoVo cell line was from the American Type
Culture Collection (ATCC, Manassas, VA, USA). All antibodies were
purchased from Santa Cruz Biotechnology, Inc. Cells were maintained
in Dulbecco’s modified Eagle’s medium (DMEM) with 10% fetal bovine
serum (FBS), 100 U/ml of penicillin and 100 μg/ml of streptomycin
at 37°C in 5% CO2. Tet was prepared as previously
reported for in vitro assay (27), or prepared with 0.5%
carboxymethylcellulose sodium (CMC-Na) as a suspension for in
vivo experiments.
Crystal violet viability assay
Crystal violet assay was conducted as previously
reported (28). Briefly, LoVo
cells were seeded in 24-well plates and treated with different
concentrations of Tet. At 24, 48 or 72 h after treatment, cells
were washed carefully with cold PBS (4°C) and stained with 0.5%
crystal violet formalin solution at room temperature for 20 min.
The stained cells were washed with water and air dried for imaging.
For quantification, crystal violet in the stained cells was
extracted with 1 ml 20% acetic acid at room temperature for 20 min
with gentle shaking. Absorbance at 570 nm was measured (29). Each assay was done in
triplicate.
Reverse transcription (RT) and polymerase
chain reaction (PCR) analysis
The total RNA were extracted with TRIzol
(Invitrogen), followed by RT to generate cDNA templates. Then, the
cDNA products were used for semi-quantitative PCR templates to
detect the expression level of target genes. All samples were
normalized with the expression level of GAPDH. The primer sequences
are available upon request.
Western blot assay
Sub-confluent LoVo cells were seeded in 6-well
plates, then treated with different concentrations of Tet and/or
combined with corresponding recombinant adenovirus. At the
scheduled time-point, cells lysates were collected and boiled for
10 min. For nucleus protein extraction, the protein was harvested
as introductions of the kit (#78833; Thermo Fisher Scientific,
Rockford, IL, USA). All samples were subjected to SDS-PAGE and
transfered to polyvinylidene fluoride membranes, blotted with
primary antibodies and secondary antibodies conjugated with
horseradish peroxidase successively. Finally, the target bands were
developed with SuperSignal West Femto Substrate (#34095; Thermo
Fisher Scientific). Each assay was done in triplicate.
Immunohistochemical staining
Tumor slides were deparaffinized and then rehydrated
as previously reported (13). The
deparaffinized slides were subjected to antigen retrieval and
probed with an anti-IGFBP-5 antibody, or goat IgG as control,
followed by incubation with biotin labled secondary antibodies and
streptavidin-HRP. The target proteins were visualized by
3,3′-diaminobenzidine staining and imaged under a microscope.
Recombinant adenoviral constructs for
IGFBP-5, siIGFBP-5 and GFP
The recombinant adenovirual vectors were carried out
following the AdEasy system (30,31).
Briefly, the coding sequence (CDS) of human IGFBP-5 was amplified
and sub-cloned into the shuttle vector pAdTrace-TO4, and the
siRNA-knockdown oligo cassettes were cloned into the pSES1 shuttle
vector (the siRNA for IGFBP-5 were designed with siDesign
software). Then, the shuttle vectors were transfected in HEK293
cells to package the recombinant adenoviruses, which were
designated as AdIGFBP-5, AdsiIGFBP-5 and AdGFP. All recombinant
adenoviruses were tagged with green fluorescent protein (GFP) and
AdGFP was used as the vector control.
Flow cytometric analysis for apoptosis
and cell cycle
Sub-confluent LoVo cells were seeded in 6-well
plates and treated with different concentrations of Tet and/or
combined with infection of AdIGFBP-5, AdsiIGFBP5 or AdGFP for 48 h.
For apoptosis analysis, cells were harvested and washed with cold
phosphate-buffered saline (PBS; 4°C), followed by incubating with
Annexin V-EGFP (#KGA104; Nanjing KeyGen Biotech Co., Ltd., Nanjing,
China) and propidium iodide (PI). Finally, the cells were analyzed
with fluorescence activated cell sorting (FACS). For cell cycle
analysis, cells were harvested and washed with PBS, fixed with cold
(4°C) 70% ethanol, washed with 50 and 30% ethanol and PBS. Finally,
cells were stained with 1 ml of 20 mg/ml PI containing RNase (1
mg/ml) in PBS for 30 min followed by flow cytometric analysis. Each
assay was done in triplicate.
Orthotopic colon cancer model
The colon cancer model used in the present study was
initialized by subcutaneous injection of DMH plus 1% DSS as
previously reported (32).
Briefly, Sprague-Dawely rats (weight 140±10 g, half male and half
female) were from the Animal Centre of Chongqing Medical University
(Chongqing, China). All experiments were approved by the
Institutional Animal Care and Use Committee (IACUC) of the
Chongqing Medical University. Rats were given three subcutaneous
injections of DMH (40 mg/kg) in the groin in a week (the same
volume normal saline was used as control), followed by free access
to drinking water containing 1% DSS for one week, and then changed
to regular water and food at the beginning of the 3rd week. At the
end of the 3rd week, the DMH treated rats were divided into 3
groups randomly (26 per group) and treated with different doses of
Tet (25 and 50 mg/kg) or solvent by intragastic administration,
five times a week up to the 20th week. At the end of the 10th week,
10 rats of each group were sacrificed to detect the formation of
ACF with methylene blue staining, and the number of ACF was counted
in the colorectum, and the images analysed under a microscope. At
the end of the 20th week, all rest rats were sacrificed to check
the formation of colon cancer and retrieved the tumor masses for
histological evaluation.
Xenograft colon cancer model
Sub-confluent LoVo cells were harvested and prepared
for subcutaneous injection to the flank of athymic nude mice
(female nude mice from the Animal Center of Chongqing Medical
University; five mice per group). One week after injection, the
animals were treated the with Tet (40 or 80 mg/kg) or solvent
intragastric administration. The animals were sacrificed after 4
weeks, and the tumor masses were retrieved, fixed and
paraffin-embedded. Serial sections were performed with hematoxylin
and eosin (H&E) staining for histological evaluation.
Luciferase reporter assay
Firefly reporter assay was carried out as previously
reported (30,33). Cells were seeded in T25 flasks and
transfected with 3.0 μg per flask of pTOP-Luc using Lipofectamine.
Twelve hours later, cells were seeded in 24-well plates and treated
with different concentrations of Tet and/or combined with
corresponding recombinant adenovirus. Twenty-four hours later,
cells were lysed and subjected to luciferase activity assay
following the manual of the kit (E1500; Promega, Madison, WI, USA).
Luciferase activity was normalized with total cellular protein
concentrations of the samples. Each assay was done in
triplicate.
Statistical analysis
Microsoft Excel was employed to calculate the
standard deviations. The differences were analyzed using the
Student’s t-test.
Results
Tet inhibits the proliferation of LoVo
cells
To validate whether Tet can function as a
chemotherapeutic agent for human colon cancers, we first test the
proliferation inhibitory effect of Tet on human colon cancer cells.
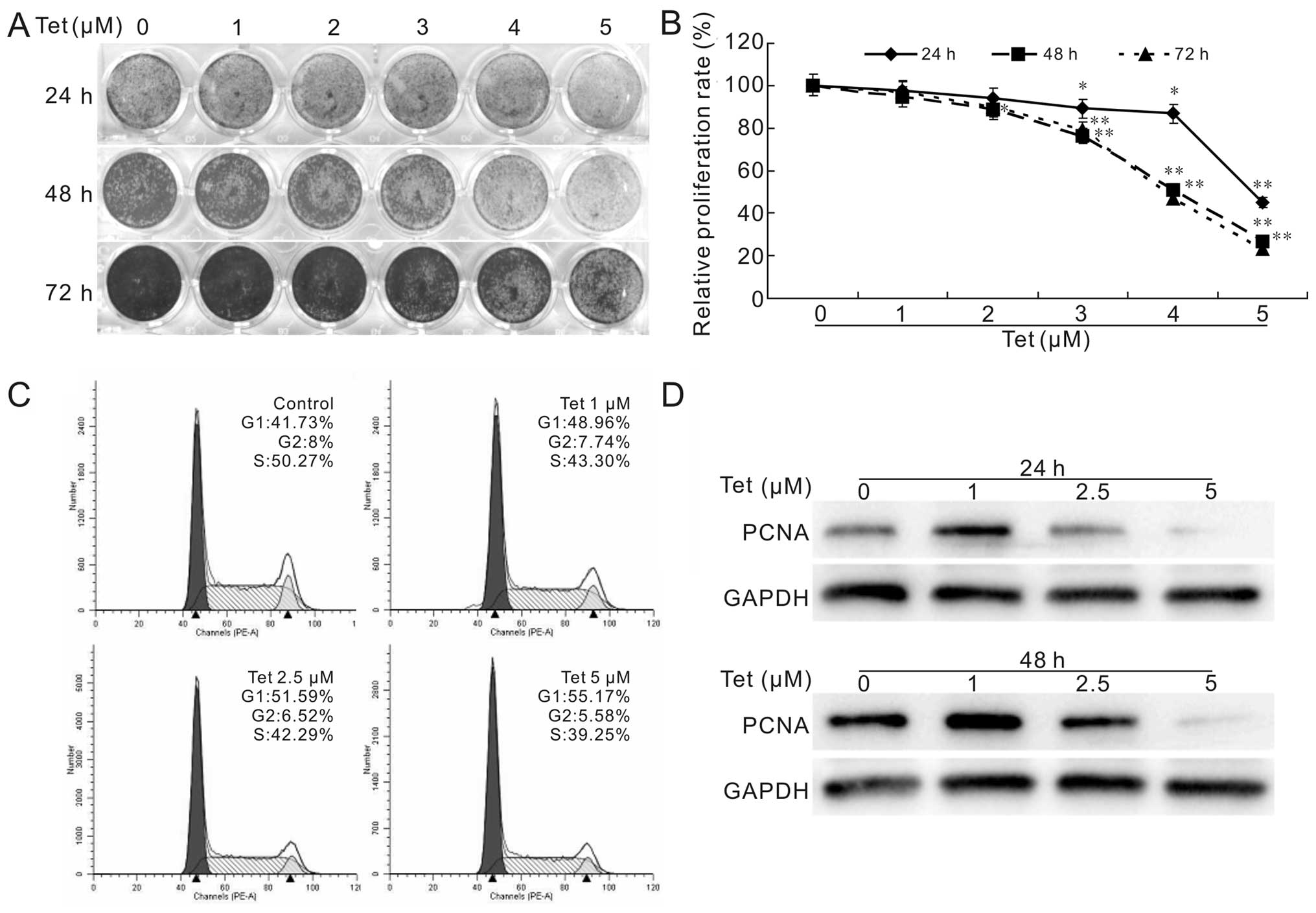
The result shows that Tet inhibits the proliferation of LoVo cells
effectively and concentration-dependently (Fig. 1A), even at the minimum
concentration of 5 μM. This result is consistent with the flow
cytometric assay for cell cycle analysis and western blot assay for
the proliferating cell nuclear antigen (PCNA) (Fig. 1C and D). Our result in HCT116 cells
was similar (13) (data not
shown). These data suggest that Tet may be used as a chemotherapy
agent or adjuvant for colon cancer treatment.
Tet induces apoptosis in LoVo cells
We next conducted further analyses to demonstrate
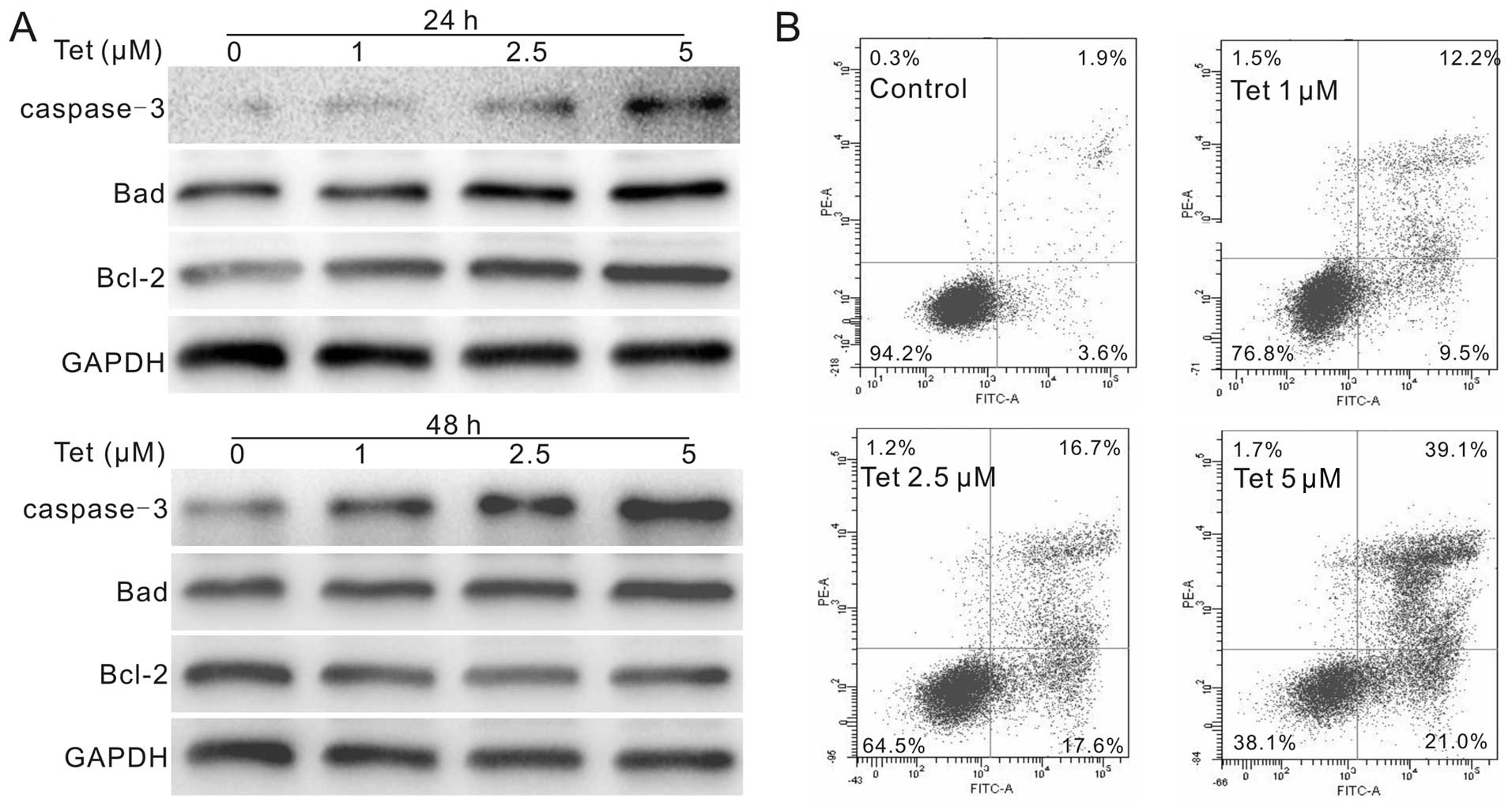
whether Tet could induce colon cancer cells to undergo apoptosis.
Western blot assay results show that Tet upregulates the protein
level of caspase-3 and Bad, but downregulates the protein level of
Bcl-2 (Fig. 2A), which is more
prominent when treated the cells with Tet for 48 h. The results of
flow cytometric analysis show that the percentage of apoptotic
cells increased concentration-dependently after Tet treatment
(Fig. 2B), similarly to the result
in HCT116 cells (13) (data not
shown). Thus, these results strongly suggest that Tet can induce
apoptosis in LoVo cells, a notable characteristic shared by most of
the agents currently used for cancer chemotherapy.
Tet prevents DMH plus DSS induced ACF and
colon cancer formation
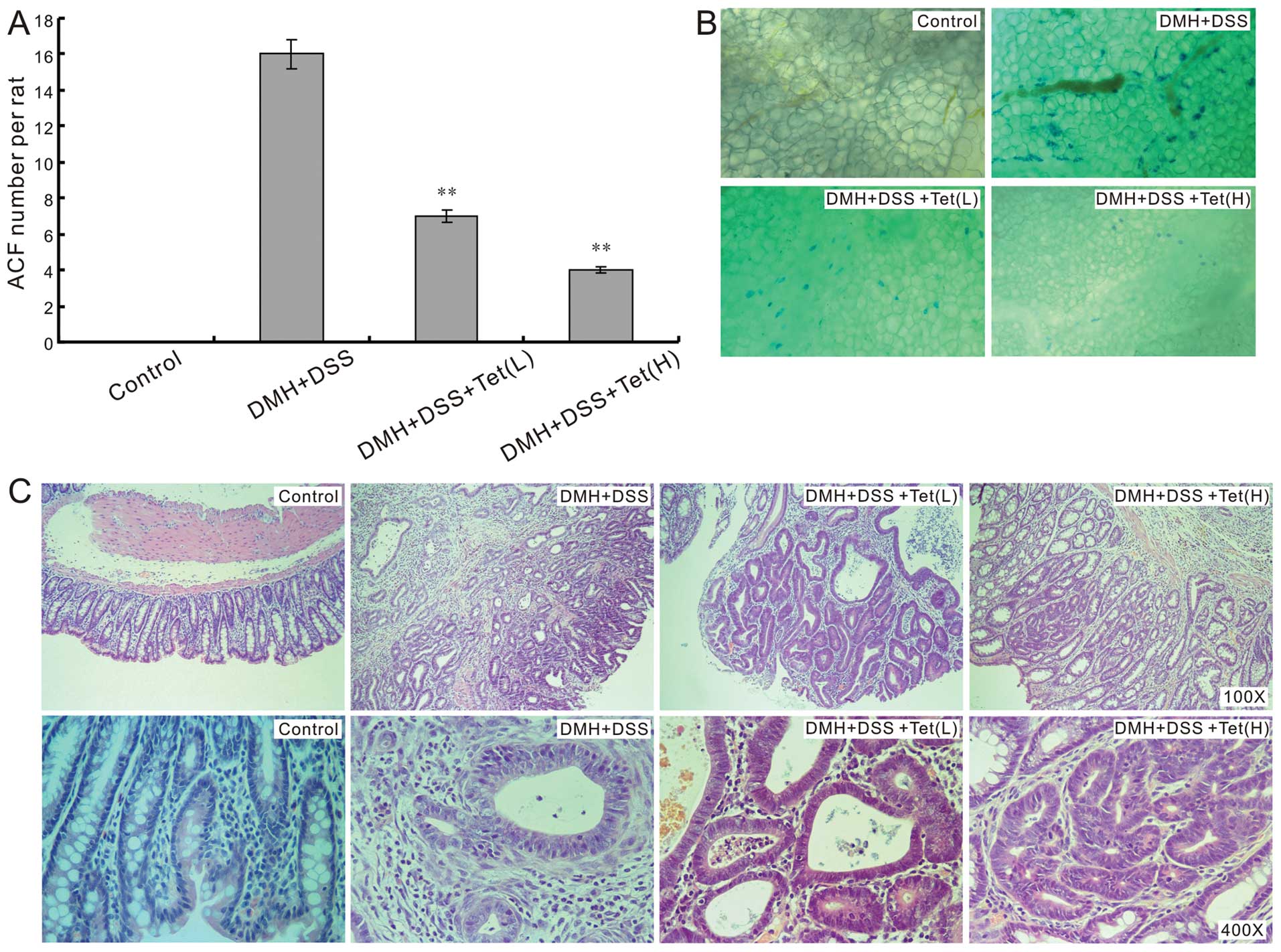
We next investigate whether Tet could prevent the
ACF and colon cancer formation induced by DMH and DSS in
vivo. No ACF was found in the control group in our experiments,
and the ACF number per rat in Tet treated groups was fewer than
that of the model group (Fig. 3A and
B). The colon cancers induced by DMH and DSS in model group was
more aggressive than those of Tet treated groups, and no cancer was
found in the control group. Although Tet can not block the colon
cancer formation initiated by DMH and DSS thoroughly, it was able
to attenuate the cancer grade dose-dependently (Fig. 3C). These results suggest that Tet
may prevent the initiation of colon cancer.
Tet decreases the expression of IGFBP-5
and inhibits the in vivo tumor growth in the xenograft human colon
cancer model
IGF plays an important role in tumorigenesis, and
the inhibitor of IGF signaling has been reported as a therapeutic
target for cancer (34,35). IGFBP-5 is an important regulatory
of IGF signaling and plays an important role in osteosarcoma
(26). But, its role in colon
cancer is not clear yet. Thus, we investigated whether IGFBP-5 was
involved in the anti-proliferation effect of Tet in human colon
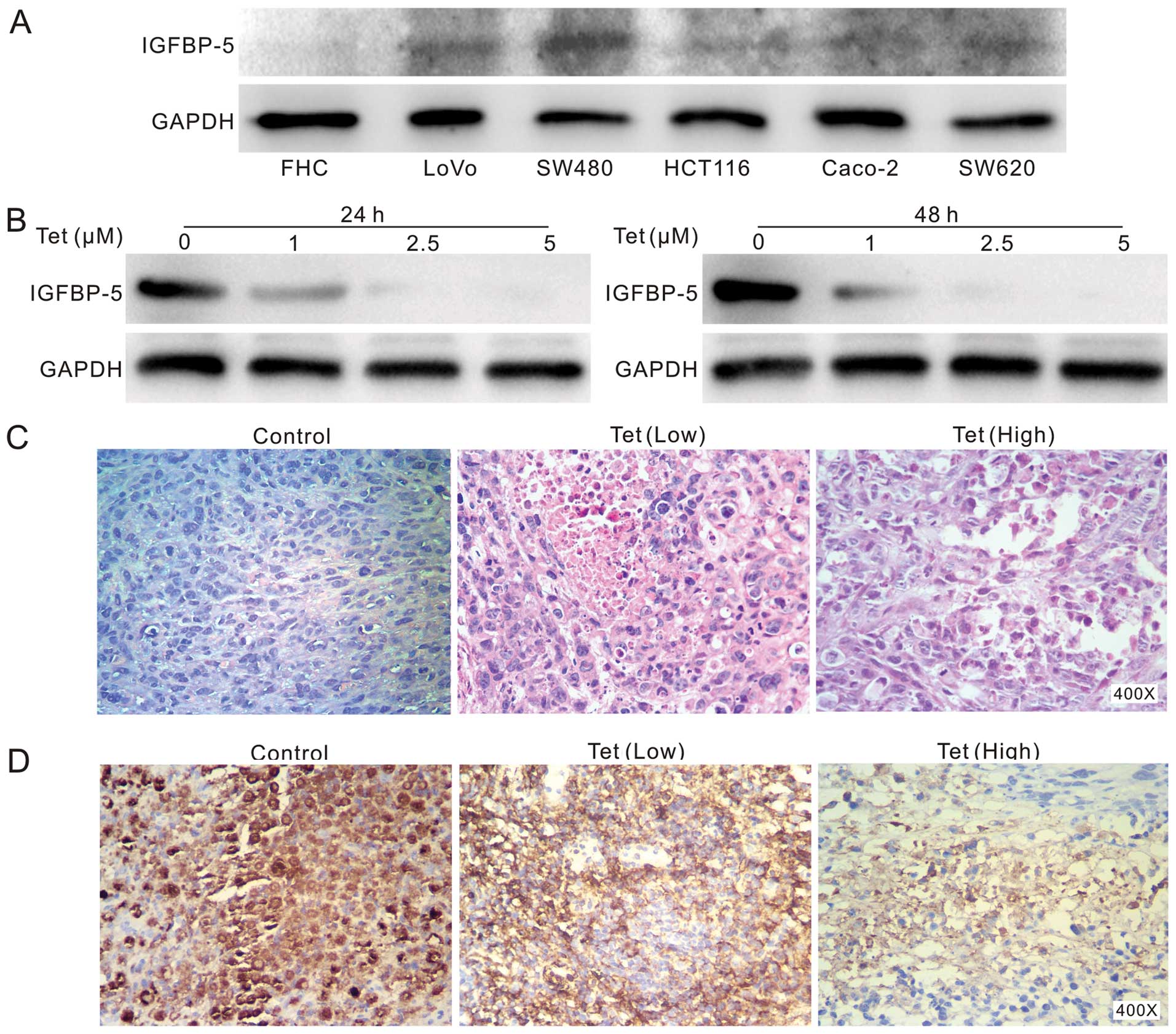
cancer. We test the endogenous IGFBP-5 expression in colon cancer
cell lines and fetal colon cell line (FHC), the results show that
IGFBP-5 is detectable in all colon cancer cell lines and are more
prominent than that in FHC cells (Fig.
4A). This result indicates that IGFBP-5 may be involved in
colon cancer development. Thus, we further investigated whether Tet
can regulate the expression of IGFBP-5. Western blot analysis
showed that Tet can inhibit the expression of IGFBP-5 apparently in
LoVo cells (Fig. 4B), and similar
result was obtained in HCT116 cells (data not shown). In the
xenograft colon cancer model assay, H&E staining showed a
decreased cellularity in tumor masses from Tet treated group
(Fig. 4C). Immunohistochemical
staining results show that Tet decreases IGFBP-5 protein level
concentration-dependently in the tumor masses (Fig. 4D). These results suggest that
IGBFP-5 may play an important role in colon cancer.
IGFBP-5 downregulation participates in
the anti-proliferation effect of Tet on LoVo cells
As Tet can downregulate the expression of IGFBP-5,
we performed further investigation to clarify how IGFBP-5 affects
the anti-proliferation and apoptosis inducing effects of Tet in
colon cancer cells. We constructed recombinant adenoviruses for
expressing the CDS and siRNA oligo fragments of IGFBP-5,
respectively. Tet treated LoVo cells, or Tet combined with
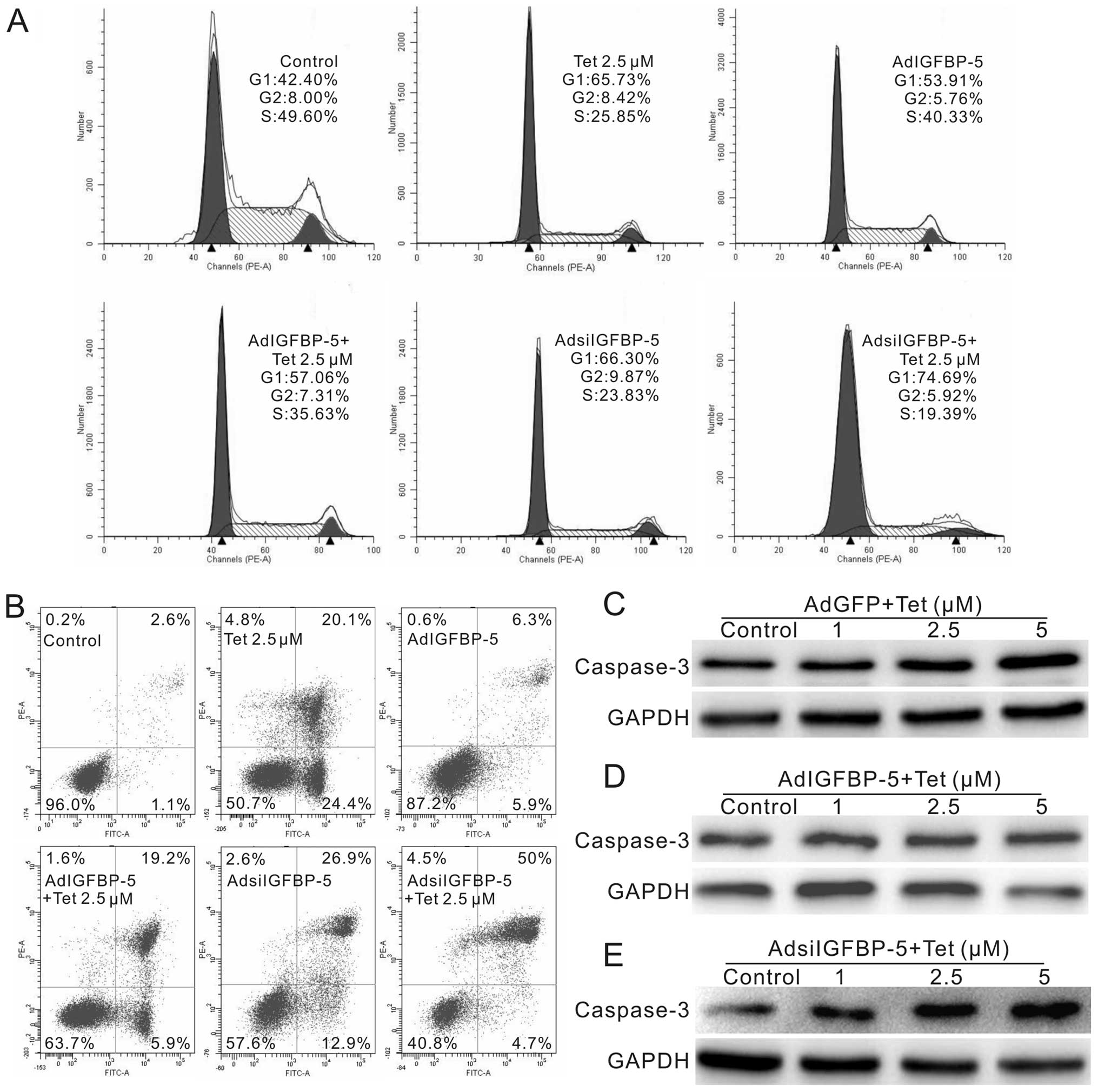
AdIGFBP-5 or AdsiIGFBP-5, were collected for cell cycle and
apoptosis analysis, and total protein was harvested for the
detection of caspase-3. The results show that exogenous expression
of IGFBP-5 can reverse the cell cycle arrest effect of Tet in LoVo
cells, while knockdown of IGFBP-5 enhances prominently the cell
cycle arrest effect, especially the G1 phase arrest (Fig. 5A). Apoptosis analyses showed that
exogenous expression of IGFBP-5 decreases the percentage of
apoptotic cells induced by Tet, whereas knockdown of IGFBP-5
increases the percentage of necrotic cells (Fig. 5B). Western blot analyses indicated
that Tet increases the protein level of caspase-3 (Fig. 5C), exogenous expression of IGFBP-5
has no obvious effect on the level of caspase-3 (Fig. 5D), but knockdown of IGFBP-5
enhances substantially the caspase-3 protein level (Fig. 5E). Thus, our data indicate that
downregulation of IGFBP-5 may play a crucial role in Tet induced
proliferation inhibition and apoptosis in LoVo cells.
IGFBP-5 affects Tet-induced
downregulation of Wnt/β-catenin signal transduction partly in LoVo
cells
Wnt/β-catenin signaling is one of the important
signaling pathways for embryonic development, proliferation and
differentiation. The aberrant activation of Wnt/β-catenin signaling
plays a critical role in the development of colorectal
tumorigenesis (36). Our previous
data show that Tet can target Wnt/β-catenin signaling to inhibit
the proliferation of human colon cancer cells, but the exact
mechanism is unknown. Thus, we further investigate whether IGFBP-5
can affect the Tet-induced inactivation of Wnt/β-catenin signaling
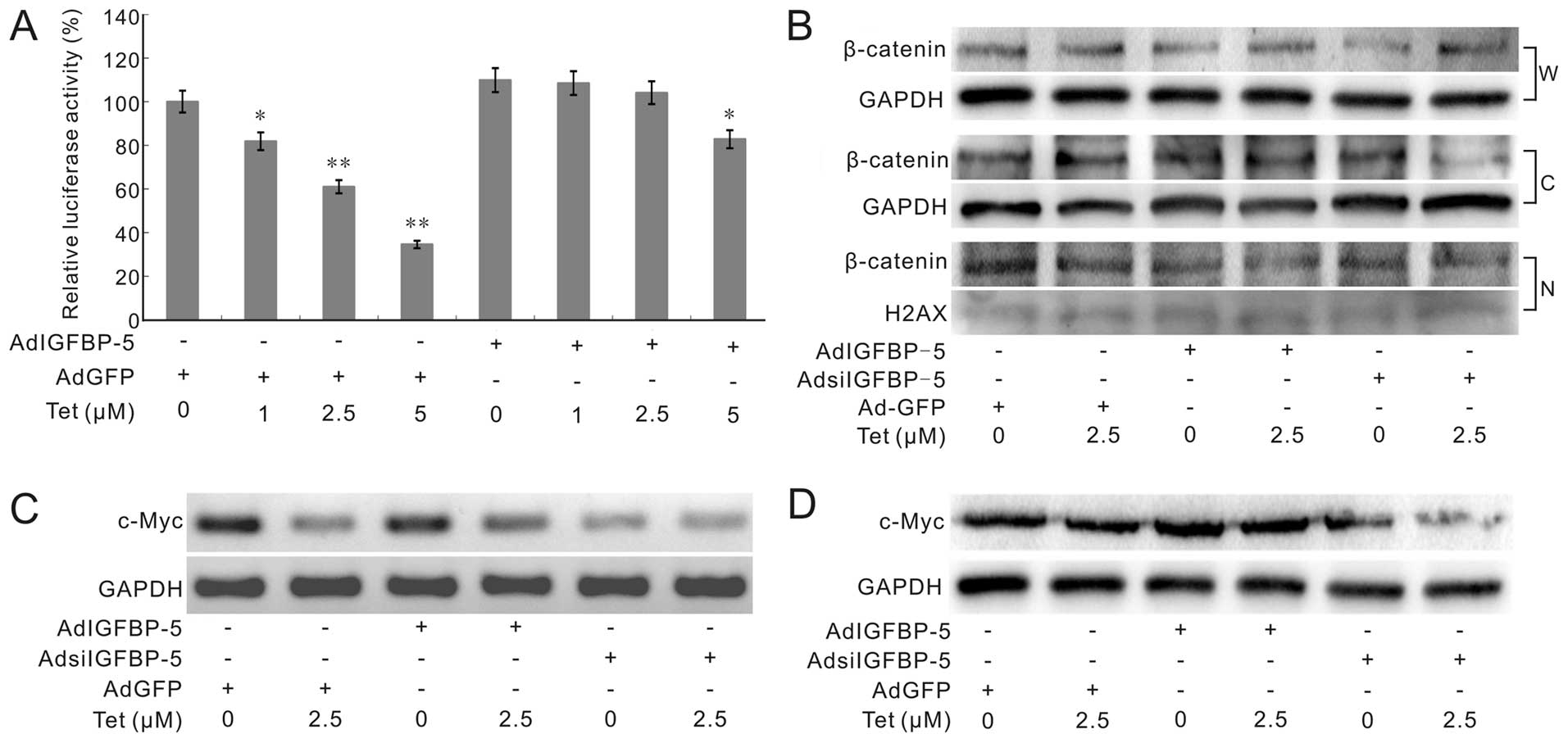
transduction or not. We took pTOP-luc reporter assay to test the
LEF/Tcf transcriptional activity in LoVo cells. Cells were treated
with different concentrations of Tet and combined with AdGFP or
AdIGFBP-5. The result shows that Tet can effectively inhibit the
luciferase activities of pTOP-Luc reporter, but exogenous
expression of IGFBP-5 can partly reverse this effect (Fig. 6A). Western blot analysis shows that
Tet, or Tet combined with AdIGFBP-5 or AdsiIGFBP-5 has no
substantial effects on β-catenin, either in cytoplasm or in the
nucleus (Fig. 6B). Further
analyses indicated that Tet can downregulate the expression of
c-Myc, one of the important downstream targets of Wnt/β-catenin
signaling (37), either the mRNA
expression or protein level. This effect can also be reversed by
exogenous expression of IGFBP-5, and enhanced by knockdown IGFBP5
(Fig. 6C and D). These results
indicate that Tet may target Wnt/β-catenin signaling through partly
downregulating IGFBP-5, although the detailed mechanisms remain
unknown.
Discussion
Tet, a bis-benzylisoquinoline alkaloid extracted
from the dried root of Chinese herb hang fang ji, has been
validated as a potential agent to inhibit proliferation and induce
apoptosis in many cancer cells, such as breast, lung, colon cancer
and neuroblastoma (11–14). In the present study, we
demonstrated that the anticancer activity of Tet in colon cancer
may be mediated by downregulating IGFBP-5 expression to inactivate
the canonical Wnt signaling transduction, although the detail
mechanism is still unknown.
IGF signaling pathway is a very complex system,
comprising two cell surface receptors (IGF1R and IGF2R), two
ligands (IGF1 and IGF2), seven binding proteins (IGFPB-1 to
IGFBP-7) with high affinity to IGFs and IGFBPs degrading enzymes.
The axis is very important for regulating the development and
normal physiological function, including regulating cell
differentiation, proliferation and apoptosis (38–41).
Thus, the aberrant IGF signaling has been implicated with the
development of cancer (42–45),
including human colon cancer (19). IGFBP-5, as one essential member of
IGF axis, has been reported implicated in cancer, but its role is
controversial. IGFBP-5 inhibited breast cancer cells (22), and suppressed human osteosarcoma
cell growth (26). However,
exogenous IGFBP-5 also protects breast cancer from apoptosis
induced by ceramide (23),
prolongs breast cancer cell survival (46), and associates with metastasis and
aggressiveness of breast cancer (47). Knockdown of IGFBP-5 can inhibit the
proliferation and differentiation of neuroblastoma cells (25), while overexpression of IGFBP-5
indicates a poor prognosis in urothelial carcinoma (24). The diverse functions of IGFBP-5 may
be dependent on the specific cellular context, cell type and the
localization in cells (21), as
well as the functional state of each domain (48). It is unquestionable that IGFBP-5 is
implicated with cancer. However, little is known about its role in
colon cancer. Thus, we investigated whether IGFBP-5 is involved in
the anticancer activity of Tet in colon cancer cells. Our results
show that the endogenous expression of IGFBP-5 in colon cancer
cells, including LoVo, SW480, HCT116, Caco-2 and SW620, is
detectable and more prominent than that of Fetal human cells (FHC),
(Fig. 4A). Tet can downregulate
the expression of IGFBP-5 in a concentration-dependent manner
(Fig. 4B), which was confirmed by
immunohistochemical staining for tumor masses (Fig. 4D). It has been reported that DMH
plus DSS-induced colon cancer is characteristic of IGFBP-5
upregulation (49). Hence, we
propose a hypothesis that IGFBP-5 may participate in the anticancer
activity of Tet in human colon cancer. The cell cycle analysis
shows that Tet can arrest cell cycle at G1 phase, and IGFBP-5
knockdown combined with Tet can enhance this effect dramatically.
On the contrary, over-expression of IGFBP-5 has no substantial
effect on cell cycle arrest, but it can almost reverse Tet induced
cell cycle arrest in LoVo cells. These results are consistent with
the apoptosis analysis (Fig. 5B and
C). These data collectively support that downregulation of
IGFBP-5 may play an important role in the anticancer effect of Tet
in colon cancer cells.
Wnt signaling pathway is important for embryogenesis
and cancer. The aberrant activation of Wnt signaling is an
important etiological cause of colon cancer (50). For canonical Wnt pathway (also
called Wnt/β-catenin signaling pathway), β-catenin is as a critical
factor. In the absence of Wnt ligands, axin, GSK-3β and protein APC
can be assembled as a destruct complex, which can promote the
proteolytic degradation of β-catenin and block the signaling
transduction. In the presence of Wnt ligands, the complex will be
disassembled and the β-catenin becomes stable in the cytoplasm and
then translocates into the nucleus. Finally, β-catenin interacts
with TCF/LEF transcription factors to regulate the expression of
downstream target genes (51). Our
results show that Tet can inhibit the β-catenin/TCF transcription
activity, while Tet combined with exogenous expression of IGFBP-5
can partly reverse this effect (Fig.
6A). Tet can inhibit DMH plus DSS-induced colon cancer, which
is characteristic of β-catenin mutation and IGFBP-5 upregulation
(49,52,53).
Thus, the inhibitory effect of Tet on Wnt/β-catenin signaling
transduction may not fully promote the degradation of β-catenin.
This was confirmed by our western blot assay, which shows that Tet,
or Tet combined with IGFBP-5 overexpression or knockdown exerted no
apparent effect on β-catenin protein level in the nucleus (Fig. 6B). Further analysis showed that Tet
can also downregulate the mRNA and protein expression of c-Myc, an
important downstream target of canonical Wnt signaling pathway
(37). This can be partly reversed
by exogenous expression of IGFBP-5, but enhanced by IGFBP-5
knockdown (Fig. 6C and D). These
data suggest that the inhibitory effect of Tet on Wnt/β-catenin
signaling may be mediated through downregulating the expression of
IGFBP-5, which may regulate the interaction of β-catenin and other
important transcriptional factors to determine the expression of
Wnt target genes.
Taken together, the present study strongly suggest
that Tet can be used as a potent anticancer agent or adjuvant for
colon cancer treatment. IGFBP-5 may be an essential factor
necessary for β-catenin to interact with TCF, LEF or other
essential transcription factors for Wnt/β-catenin signaling
transduction. The anticancer activity of Tet in colon cancer cells
may be mediated partly by downregulating the expression of IGFBP-5,
through which to inactivate Wnt/β-catenin signaling transduction.
Further investigations are needed to clarify the molecular
mechanism of how Tet downregulates IGFBP-5, and how IGFBP-5
modulates the Wnt/β-catenin signaling transduction, as well as the
clinical significance of IGFBP-5 in colon cancer.
Acknowledgements
The authors thank Bert Vogelstein (Johns Hopkins
Oncology Center, Baltimore, USA) for his kind provision of the
pTOP-Luc reporter vectors. This project was supported partly by the
Natural Science Foundation of China (NSFC, 81071462 and 81372120 to
Bai-Cheng He).
References
|
1
|
Cunningham D, Atkin W, Lenz HJ, et al:
Colorectal cancer. Lancet. 375:1030–1047. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
McKeown E, Nelson DW, Johnson EK, et al:
Current approaches and challenges for monitoring treatment response
in colon and rectal cancer. J Cancer. 5:31–43. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
da Rocha AB, Lopes RM and Schwartsmann G:
Natural products in anticancer therapy. Curr Opin Pharmacol.
1:364–369. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Mann J: Natural products in cancer
chemotherapy: past, present and future. Nat Rev Cancer. 2:143–148.
2002. View
Article : Google Scholar
|
|
5
|
Koehn FE and Carter GT: The evolving role
of natural products in drug discovery. Nat Rev Drug Discov.
4:206–220. 2005. View
Article : Google Scholar : PubMed/NCBI
|
|
6
|
Corson TW and Crews CM: Molecular
understanding and modern application of traditional medicines:
triumphs and trials. Cell. 130:769–774. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Wang HL, Zhang XH and Chang TH: Effects of
tetrandrine on smooth muscle contraction induced by mediators in
pulmonary hypertension. Acta Pharmacol Sin. 23:1114–1120.
2002.PubMed/NCBI
|
|
8
|
Shen YC, Chou CJ, Chiou WF and Chen CF:
Anti-inflammatory effects of the partially purified extract of
radix Stephaniae tetrandrae: comparative studies of its active
principles tetrandrine and fangchinoline on human polymorphonuclear
leukocyte functions. Mol Pharmacol. 60:1083–1090. 2001.PubMed/NCBI
|
|
9
|
Li SY, Ling LH, Teh BS, Seow WK and Thong
YH: Anti-inflammatory and immunosuppressive properties of the
bis-benzylisoquinolines: in vitro comparisons of tetrandrine and
berbamine. Int J Immunopharmacol. 11:395–401. 1989. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Wang H and Chen X: Tetrandrine ameliorates
cirrhosis and portal hypertension by inhibiting nitric oxide in
cirrhotic rats. J Huazhong Univ Sci Technolog Med Sci. 24:385–388.
3952004. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Xu W, Debeb BG, Lacerda L, Li J and
Woodward WA: Tetrandrine, a compound common in Chinese traditional
medicine, preferentially kills breast cancer tumor initiating cells
(TICs) in vitro. Cancers (Basel). 3:2274–2285. 2011. View Article : Google Scholar
|
|
12
|
Liu W, Zhang J, Ying C, et al: Tetrandrine
combined with gemcitabine and Cisplatin for patients with advanced
non-small cell lung cancer improve efficacy. Int J Biomed Sci.
8:28–35. 2012.PubMed/NCBI
|
|
13
|
He BC, Gao JL, Zhang BQ, et al:
Tetrandrine inhibits Wnt/beta-catenin signaling and suppresses
tumor growth of human colorectal cancer. Mol Pharmacol. 79:211–219.
2011. View Article : Google Scholar :
|
|
14
|
Chen Y, Chen JC and Tseng SH: Effects of
tetrandrine plus radiation on neuroblastoma cells. Anticancer Res.
29:3163–3171. 2009.PubMed/NCBI
|
|
15
|
Wu JM, Chen Y, Chen JC, Lin TY and Tseng
SH: Tetrandrine induces apoptosis and growth suppression of colon
cancer cells in mice. Cancer Lett. 287:187–195. 2010. View Article : Google Scholar
|
|
16
|
Nomura M, Yamazaki R, Takaya M, et al:
Inhibition of tetrandrine on epidermal growth factor-induced cell
transformation and its signal transduction. Anticancer Res.
27:3187–3193. 2007.PubMed/NCBI
|
|
17
|
Meng LH, Zhang H, Hayward L, Takemura H,
Shao RG and Pommier Y: Tetrandrine induces early G1
arrest in human colon carcinoma cells by down-regulating the
activity and inducing the degradation of G1-S-specific
cyclin-dependent kinases and by inducing p53 and
p21Cip1. Cancer Res. 64:9086–9092. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Kim KS, Seu YB, Baek SH, et al: Induction
of cellular senescence by insulin-like growth factor binding
protein-5 through a p53-dependent mechanism. Mol Biol Cell.
18:4543–4552. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Vanamala J, Reddivari L, Radhakrishnan S
and Tarver C: Resveratrol suppresses IGF-1 induced human colon
cancer cell proliferation and elevates apoptosis via suppression of
IGF-1R/Wnt and activation of p53 signaling pathways. BMC Cancer.
10:2382010. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Ben-Shmuel A, Shvab A, Gavert N, Brabletz
T and Ben-Ze’ev A: Global analysis of L1-transcriptomes identified
IGFBP-2 as a target of ezrin and NF-kappaB signaling that promotes
colon cancer progression. Oncogene. 32:3220–3230. 2013. View Article : Google Scholar
|
|
21
|
Gullu G, Karabulut S and Akkiprik M:
Functional roles and clinical values of insulin-like growth
factor-binding protein-5 in different types of cancers. Chin J
Cancer. 31:266–280. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Butt AJ, Dickson KA, McDougall F and
Baxter RC: Insulin-like growth factor-binding protein-5 inhibits
the growth of human breast cancer cells in vitro and in vivo. J
Biol Chem. 278:29676–29685. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Akkiprik M, Feng Y, Wang H, et al:
Multifunctional roles of insulin-like growth factor binding protein
5 in breast cancer. Breast Cancer Res. 10:2122008. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Liang PI, Wang YH, Wu TF, et al: IGFBP-5
overexpression as a poor prognostic factor in patients with
urothelial carcinomas of upper urinary tracts and urinary bladder.
J Clin Pathol. 66:573–582. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Cesi V, Giuffrida ML, Vitali R, et al:
C/EBP alpha and beta mimic retinoic acid activation of IGFBP-5 in
neuroblastoma cells by a mechanism independent from binding to
their site. Exp Cell Res. 305:179–189. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Su Y, Wagner ER, Luo Q, et al:
Insulin-like growth factor binding protein 5 suppresses tumor
growth and metastasis of human osteosarcoma. Oncogene.
30:3907–3917. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Chen Y, Chen JC and Tseng SH: Tetrandrine
suppresses tumor growth and angiogenesis of gliomas in rats. Int J
Cancer. 124:2260–2269. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
He BC, Chen L, Zuo GW, et al: Synergistic
antitumor effect of the activated PPARgamma and retinoid receptors
on human osteosarcoma. Clin Cancer Res. 16:2235–2245. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Ishiyama M, Tominaga H, Shiga M, Sasamoto
K, Ohkura Y and Ueno K: A combined assay of cell viability and in
vitro cytotoxicity with a highly water-soluble tetrazolium salt,
neutral red and crystal violet. Biol Pharm Bull. 19:1518–1520.
1996. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
He TC, Zhou S, da Costa LT, Yu J, Kinzler
KW and Vogelstein B: A simplified system for generating recombinant
adenoviruses. Proc Natl Acad Sci USA. 95:2509–2514. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Luo J, Deng ZL, Luo X, et al: A protocol
for rapid generation of recombinant adenoviruses using the AdEasy
system. Nat Protoc. 2:1236–1247. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Onose J, Imai T, Hasumura M, Cho YM and
Hirose M: A new medium-term rat colon bioassay applying neoplastic
lesions as endpoints for detection of carcinogenesis modifiers -
validation with known modifiers. Cancer Lett. 232:272–278. 2006.
View Article : Google Scholar
|
|
33
|
Zhou L, An N, Haydon RC, et al: Tyrosine
kinase inhibitor STI-571/Gleevec down-regulates the beta-catenin
signaling activity. Cancer Lett. 193:161–170. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Fidler MJ, Shersher DD, Borgia JA and
Bonomi P: Targeting the insulin-like growth factor receptor pathway
in lung cancer: problems and pitfalls. Ther Adv Med Oncol. 4:51–60.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Arcaro A: Targeting the insulin-like
growth factor-1 receptor in human cancer. Front Pharmacol.
4:302013. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Kinzler KW and Vogelstein B: Lessons from
hereditary colorectal cancer. Cell. 87:159–170. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
You Z, Saims D, Chen S, et al: Wnt
signaling promotes oncogenic transformation by inhibiting
c-Myc-induced apoptosis. J Cell Biol. 157:429–440. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Gluckman P, Klempt N, Guan J, et al: A
role for IGF-1 in the rescue of CNS neurons following
hypoxic-ischemic injury. Biochem Biophys Res Commun. 182:593–599.
1992. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Valentinis B and Baserga R: IGF-I receptor
signalling in transformation and differentiation. Mol Pathol.
54:133–137. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Fu P, Thompson JA, Leeding KS and Bach LA:
Insulin-like growth factors induce apoptosis as well as
proliferation in LIM 1215 colon cancer cells. J Cell Biochem.
100:58–68. 2007. View Article : Google Scholar
|
|
41
|
LeRoith D, Werner H, Beitner-Johnson D and
Roberts CT Jr: Molecular and cellular aspects of the insulin-like
growth factor I receptor. Endocr Rev. 16:143–163. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
LeRoith D and Roberts CT Jr: The
insulin-like growth factor system and cancer. Cancer Lett.
195:127–137. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Weroha SJ and Haluska P: The insulin-like
growth factor system in cancer. Endocrinol Metab Clin North Am.
41:335–350. vi2012. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Wang Z, Wang Z, Liang Z, et al: Expression
and clinical significance of IGF-1, IGFBP-3, and IGFBP-7 in serum
and lung cancer tissues from patients with non-small cell lung
cancer. Onco Targets Ther. 6:1437–1444. 2013.PubMed/NCBI
|
|
45
|
Chen D, Siddiq A, Emdad L, et al:
Insulin-like growth factor-binding protein-7 (IGFBP7): a promising
gene therapeutic for hepatocellular carcinoma (HCC). Mol Ther.
21:758–766. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Sureshbabu A, Okajima H, Yamanaka D, et
al: IGFBP5 induces cell adhesion, increases cell survival and
inhibits cell migration in MCF-7 human breast cancer cells. J Cell
Sci. 125:1693–1705. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Wang H, Arun BK, Wang H, et al: IGFBP2 and
IGFBP5 overexpression correlates with the lymph node metastasis in
T1 breast carcinomas. Breast J. 14:261–267. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Luther GA, Lamplot J, Chen X, et al:
IGFBP5 domains exert distinct inhibitory effects on the
tumorigenicity and metastasis of human osteosarcoma. Cancer Lett.
336:222–230. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Femia AP, Luceri C, Toti S, Giannini A,
Dolara P and Caderni G: Gene expression profile and genomic
alterations in colonic tumours induced by 1,2-dimethylhydrazine
(DMH) in rats. BMC Cancer. 10:1942010. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Paez D, Gerger A, Zhang W, et al:
Association of common gene variants in the WNT/beta-catenin pathway
with colon cancer recurrence. Pharmacogenomics J. 14:142–150. 2014.
View Article : Google Scholar
|
|
51
|
Kim JH, Liu X, Wang J, et al: Wnt
signaling in bone formation and its therapeutic potential for bone
diseases. Ther Adv Musculoskelet Dis. 5:13–31. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Tucker E, Buda A, Janghra B, et al:
Abnormalities of the cadherin-catenin complex in chemically-induced
colo-rectal carcinogenesis. Proc Nutr Soc. 62:229–236. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Imai T, Fukuta K, Hasumura M, et al:
Significance of inflammation-associated regenerative mucosa
characterized by Paneth cell metaplasia and beta-catenin
accumulation for the onset of colorectal carcinogenesis in rats
initiated with 1,2-dimethylhydrazine. Carcinogenesis. 28:2199–2206.
2007. View Article : Google Scholar : PubMed/NCBI
|