Introduction
Pancreatic cancer is the fourth-leading cause of
cancer-related deaths in both males and females in the United
States (1), and it has been
postulated that the primary cause of death in cancer patients is
due to the consequences of metastasis (2). The overall 5-year survival rate for
over 100,000 pancreatic cancer patients diagnosed from 1985–1995
was 23.4% for patients who had surgical treatment due to metastasis
or cancer resurgence (3). Heparin
is an efficient anti-metastatic agent; it inhibits metastasis by
binding to P-selectin and blocking the adhesion between platelets
and the disseminated tumor cells in the blood. The use of heparin
as an anti-metastatic agent is limited due to the side effect of
bleeding. In our experiments we aimed to develop and test the
inhibitory effect of heparin derivatives that are devoid of
systemic anticoagulation on blood-borne metastasis and on
surgically induced metastasis.
P-selectin is among the selectins in a family of
cell adhesion molecules divided into groups E, L, and P found on
the surface of endothelial cells, leukocytes, and platelets,
respectively, and has been studied extensively. P-selectin was
found to be most relevant in the process of tumor metastasis. The
adhesions that form between tumor cells and platelets via
P-selectin are required to create the metastatic micro-thrombi
(4). Sialylated fucosylated
glycans are the ligands for P-selectin, and tumors with high
expression of these ligands typically have poor prognosis due to
high rates of metastasis (5,6).
Heparin was found to be an efficient ligand for
P-selectin and blocks its binding with tumor cells, and therefore
attenuates tumor metastasis in animal models (7,8). By
depriving the circulating tumor cells of their platelet shield,
they become more fragile in the harsh environment of the
circulatory system and are more readily cleared by the immune
system. The anti-metastatic properties of heparin are a result of
one or more of the following: inhibition of heparinase, blocking of
P- and L-selectins (9), inhibition
of tissue factor (10) and
inhibition of angiogenesis (11).
Low molecular weight heparin (LMWH) has been shown
to decrease tumor metastasis in animal experiments and clinical
trials (12), but the use of
heparin and its LMWH derivatives as anti-metastatic agents is
limited because of the risk of inducing adverse bleeding
complications. A meta-analysis performed in 2007 showed an increase
in bleeding in patients treated with LMWH as an anti-metastatic
(12). To overcome this
complication, we developed sulfated non-anticoagulant heparin
(NACH) derivatives (13). Several
experimental studies have shown that certain heparin derivatives
devoid of systemic anticoagulant activities can reduce the
incidence of experimental metastasis (14). Hence, NACH is expected to have the
same anti-metastatic effect as LMWH because it carries all
properties of heparin except the anticoagulant activity. In our
laboratory we developed a novel sulfated form of NACH, named
S-NACH, and it was found to be effective and safe in a mouse model
of tumor growth and tumor angiogenesis (15).
Tumor excision of a primary cancer with no
metastasis has been the cornerstone treatment for most cancers, but
scientific evidence has revealed that tumor manipulation during
resection can increase the risk of metastasis (16). Clinical research has shown that
heparin is effective in reducing metastasis in surgical patients.
In 1995 a study was done to evaluate the use of heparin to reduce
surgically induced venous thromboembolism. Re-analysis of the
survival data comparing patients who received heparin to those who
did not showed that the three-year mortality from disseminated
malignancy was reduced in half (9.2 vs. 21.4%) (17). In 2000, von Tempelhoff et al
studied the effect of the LMWH certoparin in a randomized, double
blind study of ovarian cancer patients with follow-up for 2 years
after surgery (18). The death
rate with administration of certoparin vs. unfractionated heparin
was 21.4 vs. 37.5% deaths, respectively, at 2 years.
In our current investigation, we aimed to test the
inhibitory effect of heparin derivatives on post-surgical
metastasis using orthotopically implanted pancreatic cancer in a
mouse model. We chose to work with a pancreatic cancer cell line
due to its aggressive nature and, by trying to inhibit the
surgically induced metastasis in an animal model, we were aiming to
develop a study model for increasing the survival rate in human
cancer patients after surgical treatment.
Materials and methods
Tumor cells and test compounds
Luciferase-labeled pancreatic cancer cell line
Mpanc96-luc was provided by Dr Thiruvengadam Arumugam (M.D.
Anderson Cancer Center, Houston, TX, USA). Tinzaparin, an LMWH, was
obtained from Leo Pharma Inc. (Ballerup, Denmark). S-NACH was
synthesized at Rensselaer Polytechnic Institute (Rensselaer, NY,
USA). Both tinzaparin and S-NACH were solubilized in PBS at
concentrations of 10–20 mg/ml. E-cadherin and secondary antibodies
were purchased from Santa Cruz Biotechnology Inc. (Dallas, TX,
USA).
Animals
Immune-deficient female NCr nude homozygous mice,
aged 5–6 weeks and weighing between 18 and 20 g, were purchased
from Harlan Laboratories (Indianapolis, IN, USA). Experiments were
performed in compliance with Public Health Service Policy on Humane
Care and Use of Laboratory Animals and approved by the Albany VA
Medical Center (Albany, NY, USA) IACUC. All animal studies were
conducted in the Albany VA Animal Facility, and mice were
maintained under specific pathogen-free conditions, with controlled
conditions of temperature (20–24°C) and humidity (60–70%) and a
12-h light/dark cycle with ad libitum access to water and
food.
Liver metastasis after splenic
implantation of tumor cells
Mice were randomly distributed into a control group
and 2 treatment groups, with up to 8 mice per group. They were
anesthetized with inhaled isoflurane and received subcutaneous
(s.c.) injection of PBS (the control) or test compounds (tinzaparin
or S-NACH) according to the group. Thirty minutes later, a left
lateral abdominal incision was made, and one million Mpanc96-luc
cells (suspended in 30 μl DMEM media) were injected into the
spleen. Animals in the 2 treatment groups received daily s.c.
injections (10 or 20 mg/kg) of the test compounds and were
euthanized after 4 weeks in the first trial and after 2 weeks in a
second trial. IVIS images were taken once per week to evaluate the
extent of metastasis and to assess the best timing of termination
of the experiment, and after termination.
Liver metastasis after excision of
pancreatic tumor
Mice were randomly distributed into a control group
and 2 treatment groups, with up to 8 mice per group. Mice were
anesthetized with inhaled isoflurane, and a half million
Mpanc96-luc cancer cells (suspended in 30 μl DMEM media) were
injected into the pancreatic tail through an abdominal incision.
One week later, the pancreatic tumor was surgically removed.
Animals received PBS (control) or the test compounds (tinzaparin,
S-NACH) 30 min before the tumor excision surgery and daily after
that for 3 weeks until they were euthanized. In the first trial
animals received 10 mg/kg of either tinzaparin or S-NACH, but
S-NACH concentration was increased to 20 mg/kg in the second trial.
The spread of cancer cells was monitored using IVIS imaging once
per week and after termination.
Quantitation of metastasis using IVIS
imaging
The IVIS imaging system (Caliper Life Sciences,
Hopkinton, MA, USA) is an in vivo imaging technology that
was used to measure tumor metastasis (19). The system operates by capturing
light emitted from a luminescent source, such as luciferin, in this
case Mpanc96-luc. Light is measured by a highly sensitive camera
and software. Photographic and luminescence images were taken at
constant exposure time. Xenogen IVIS Living Image software (Caliper
Life Sciences, version 3.2) was used to quantify non-saturated
bioluminescence in regions of interest (ROI). Bioluminescence was
quantified as photons/second for each ROI (15).
Bleeding time
Bleeding time in mice was tested as described by
Dejana et al (20) and in
our previous work [Alshaiban et al (21)] for the above two groups of mice
during the period of receiving test compounds. After being on
treatment for 1 week, and 24 h after the last treatment, the mice
were anesthetized with isoflurane inhalation. Using a scalpel, 0.5
cm of the distal end of the tail was transected. The remaining
length of tail was immersed immediately into a 37°C solution of
saline. Bleeding time was measured from the time of tail
transection until visible bleeding could no longer be observed.
Histopathology
Tumor specimens were fixed in 10% buffered formalin,
processed, and embedded in paraffin. After fixation the specimens
were transferred into the embedding chambers to hold the specimens
in position until the paraffin became solid to prevent further
rotation. Four-micrometer serial sections were cut and then stained
using haematoxylin and eosin. Sections were evaluated for various
pathologic parameters using a light microscope (Leica, Buffalo
Grove, IL, USA).
Western blot analysis
MPanc96-luc cells were incubated with S-NACH (20
μg/ml) and cultured for 48 h. Proteins were collected, and
concentrations were determined by the Bradford assay using bovine
serum albumin as a standard (Protein Assay kit, Bio-Rad
Laboratories, Hercules, CA, USA). Total protein extracts (50 μg)
were mixed with SDS sample buffer (6.25 mM Tris-HCl, pH 6.8, 2.3%
SDS, 10% glycerol, 5% β-mercaptoethanol, 0.005% bromophenol blue)
and resolved by SDS-PAGE on 10–20% gradient acrylamide gels.
Proteins (50 μg) were detected immunologically following semi-dry
electro-transfer (Trans-Blot SD system, Bio-Rad Laboratories) onto
PVDF membranes (Millipore, Billerica, MA, USA). The membranes were
blocked with 5% non-fat dry milk in Tris-buffered saline with
Tween-100 for 30 min at room temperature and incubated overnight at
4°C with anti-E-cadherin. After washing 3 times in 0.5% non-fat dry
milk in Tris-buffered saline with Tween-100, blots were incubated
with horseradish peroxidase-conjugated secondary antibody for 1 h
at room temperature. Band intensities were measured using ImageJ
software.
Statistical analysis
An overall comparison of the means for all groups
(control, tinzaparin, and S-NACH) was carried out using a one-way
ANOVA. Tukey confidence intervals were used to test for differences
in means for each experimental group (tinzaparin and S-NACH) versus
the control group. A value of p<0.05 indicated a statistically
significant difference.
Results
Liver metastasis after splenic
implantation of tumor cells
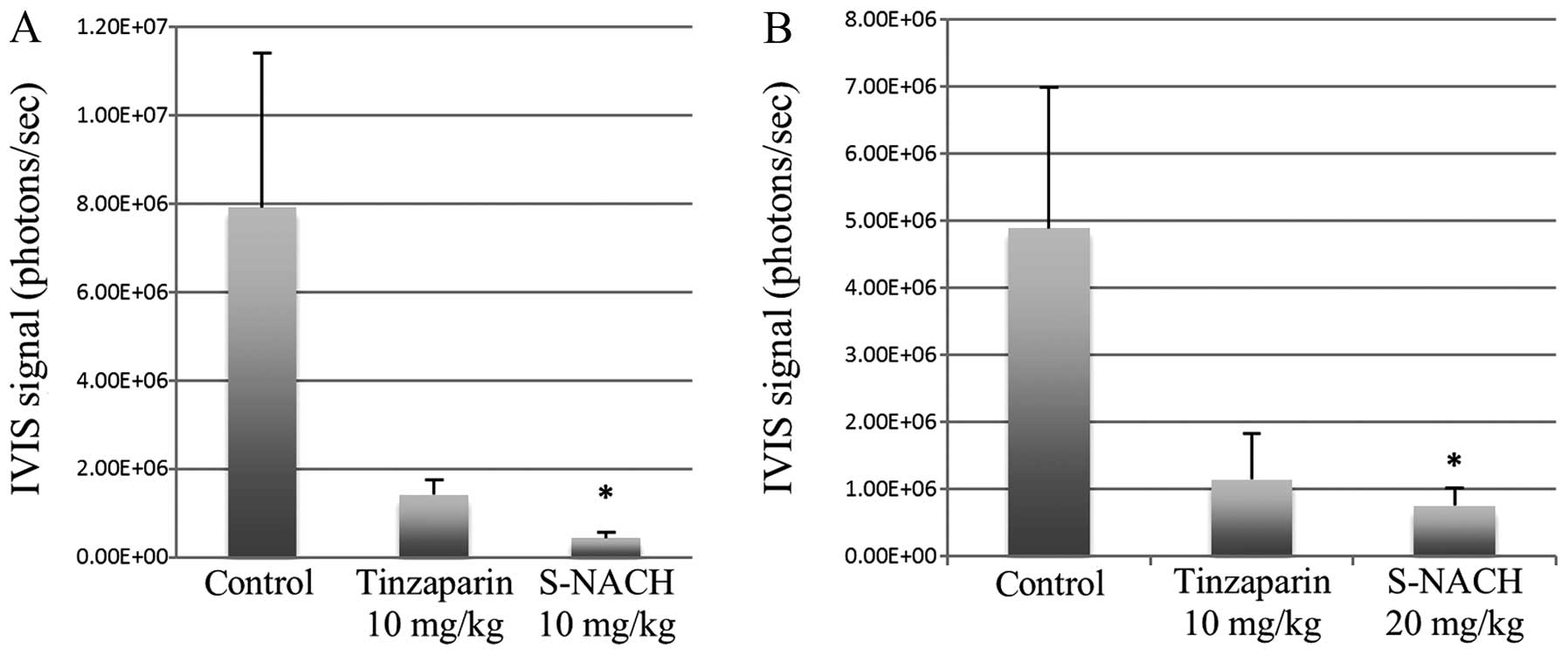
In the first trial of the experiment we compared
tinzaparin (10 mg/kg) and S-NACH (10 mg/kg) to the control
(Fig. 1A). We were able to
significantly inhibit the tumor metastasis of Mpanc96-luc cancer
cells from spleen to liver for S-NACH relative to the control group
(p<0.05). For tinzaparin relative to control, a trend for
metastasis inhibition was evident but not statistically significant
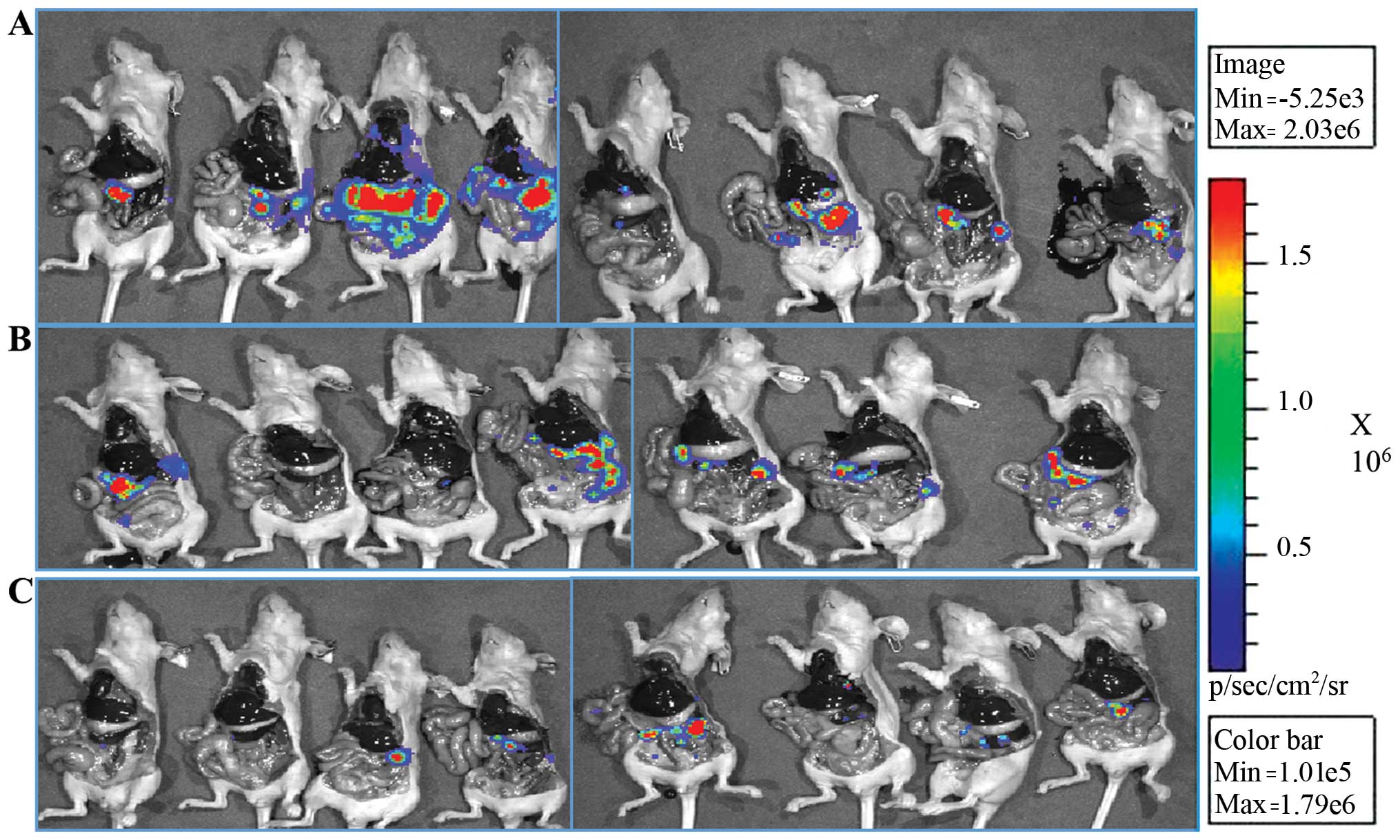
(p=0.1). The IVIS images after animal sacrifice are shown in
Fig. 2, and both tinzaparin and
S-NACH groups had less light intensity of metastasized cancer cells
compared to the control group. In the second trial we increased the
dose of the well-tolerated S-NACH to 20 mg/kg s.c. daily. We were
again able to show a statistically significant inhibition of tumor
metastasis to the liver for S-NACH relative to the control group
(p=0.02, Fig. 1B). For tinzaparin
relative to control, a strong trend for metastasis inhibition was
evident but did not approach statistical significance (p=0.08).
There were no animal deaths in the S-NACH group, but there were 4
deaths (~50%) in the tinzaparin group, most probably because of
internal bleeding after the surgery.
Liver metastasis after excision of
pancreatic tumor
Treatment with 10 mg/kg of tinzaparin or S-NACH
resulted in decreased metastasis to the liver after pancreatic
tumor excision, but it was not statistically significant compared
to control (p=0.90 for tinzaparin, p=0.19 for S-NACH). However, 10
mg/kg of S-NACH was able to significantly decrease metastasis to
the kidneys compared to control (p=0.005, data not shown) and to
decrease the recurrence of local tumor after surgery compared to
control (p<0.05, Fig. 3A).
Treatment with 20 mg/kg of S-NACH resulted in a decrease in tumor
recurrence relative to control (p=0.1 Fig. 3B) and a decrease in liver
metastasis relative to control (p=0.08, Fig. 3C). Although these latter results
were not statistically significant, the increase in S-NACH
concentration from 10 to 20 mg/kg led to a greater percentage
decrease in metastasis (66 vs. 82%, respectively). The percentage
of death in the tinzaparin group reached 50% but did not exceed 11%
in the S-NACH group.
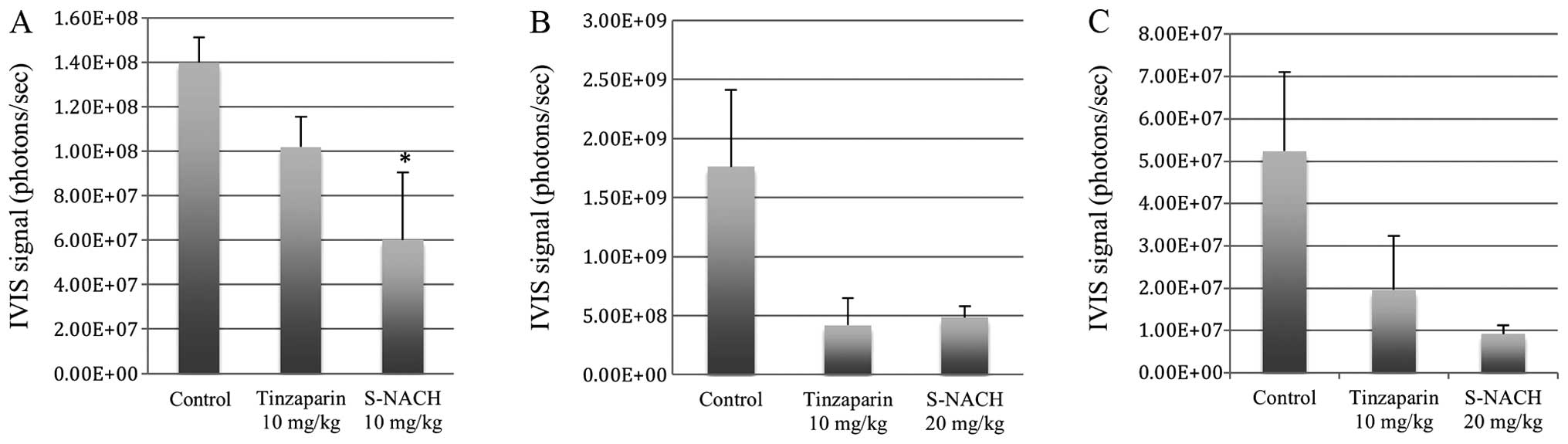 | Figure 3Effect of tinzaparin or S-NACH
treatment on tumor recurrence and metastasis after surgical
excision of pancreatic tumor. Mice were sacrificed after 3 weeks,
and IVIS imaging was used to quantify tumor recurrence or
metastasis. (A) A dose of 10 mg/kg S-NACH inhibited tumor
recurrence and showed a significant decrease in the number of
cancer cells relative to the control (p<0.05), n=8, 4, and 6 for
control, tinzaparin, and S-NACH, respectively. (B) A dose of 20
mg/kg S-NACH inhibited tumor recurrence and showed a decrease in
the number of cancer cells relative to control, but did not reach
statistical significance (p=0.1), n=6, 4, and 6 for control,
tinzaparin and S-NACH, respectively. (C) A dose of 20 mg/kg S-NACH
showed a decrease in number of cancer cells metastasizing to the
liver, but did not reach statistical significance (p=0.08), n=6, 4,
and 6 for control, tinzaparin, and S-NACH, respectively. Data
represent mean ± SEM. *p<0.05. |
Bleeding time
Tinzaparin treatment of 10 mg/kg doubled the
bleeding time (124±21 sec) compared to the control group (60±18
sec, p<0.05), and S-NACH treatment of 10 mg/kg had no effect on
bleeding time compared to control. When the dose of S-NACH was
increased to 20 mg/kg, there was still no difference in mean
bleeding time (64±19 sec, p=0.74).
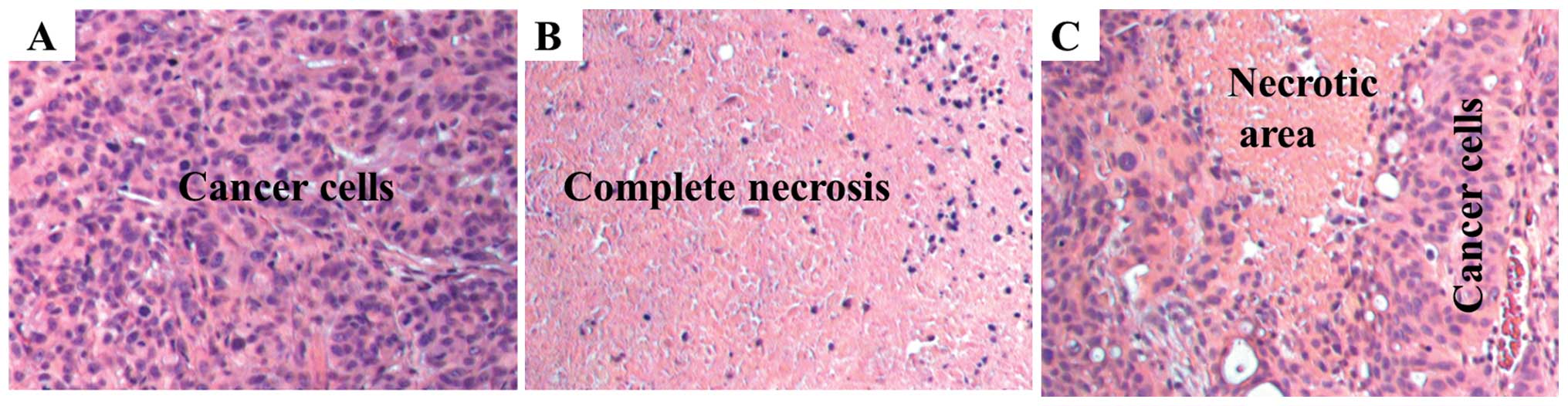
Histopathology
Histology showed that untreated animals have
high-grade (anaplastic) features as common to advanced stage
pancreatic cancer (Fig. 4A). In
S-NACH treated animals, tumors showed large regions (50%) of
necrosis (p<0.01, Fig. 4B) when
compared to control group (15%). In contrast, tinzaparin treatment
resulted in modest increase in necrotic area (30%) (Fig. 4C) as compared to control group.
Necrotic areas included showed both early stage (fragmented and
small nucleus) and late stage (ghost cells without nucleus) areas
indicating that S-NACH had effects on early and later aspects of
cell death. Tumor necrosis induced by S-NACH was inversely
proportional to the bioluminescent signal in the tumor, since only
live cells show bioluminescent signal.
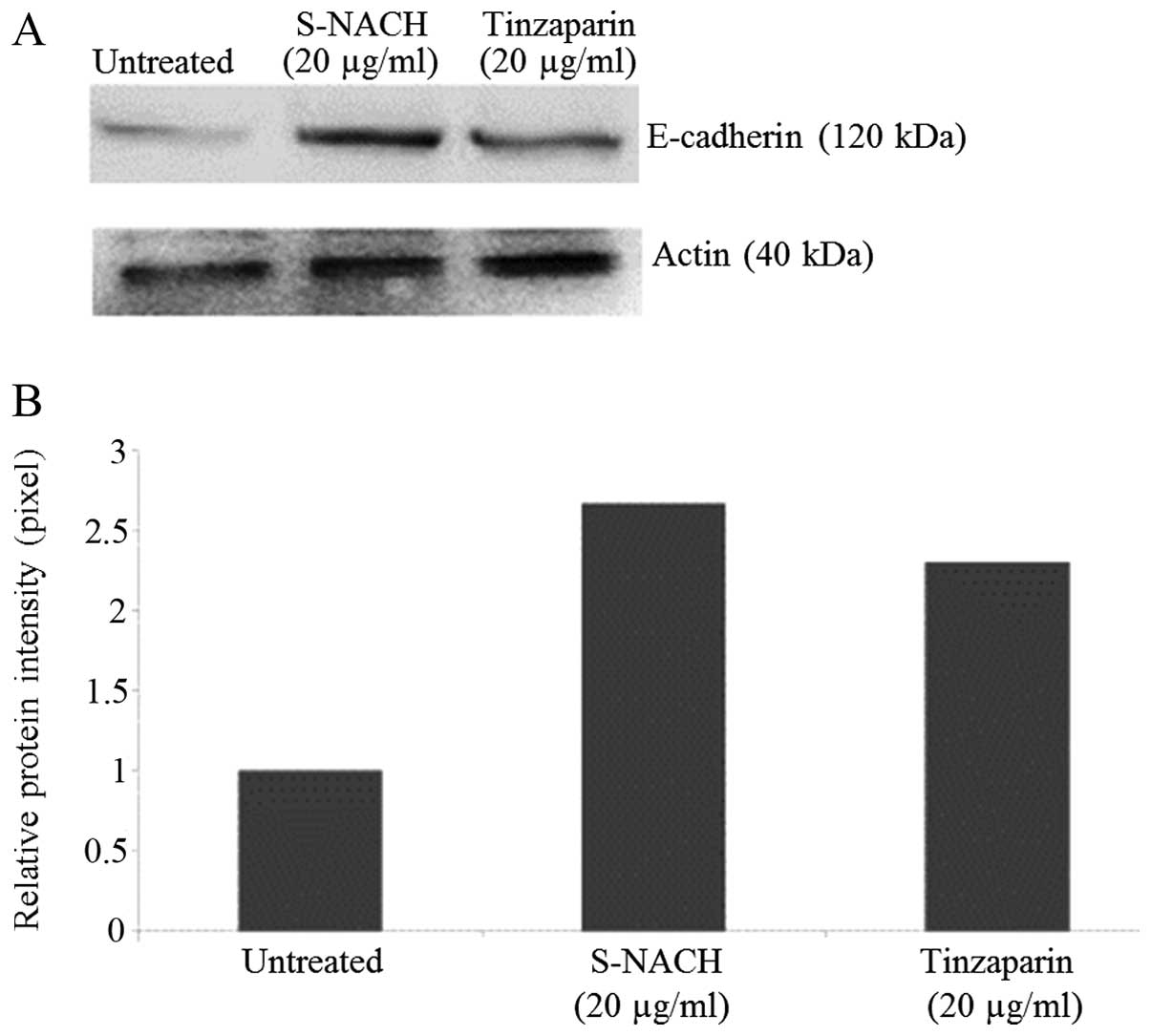
Western blot analysis
When MPanc96-luc cells were incubated with either
S-NACH or tinzaparin, the expression of E-cadherin was increased by
2.0- to 2.5-fold as compared to control untreated cells (Fig. 5).
Discussion
The strong involvement of platelets with cancer
metastasis was proven a long time ago in studies that showed that
thrombocytopenia was consistently associated with a decreased
incidence of distant metastases (22). More recently, P-selectin was found
to be the link between platelets and cancer cells. P-selectin
deficient mice were injected intravenously with cancer cells, lung
metastasis decreased, and then injecting those mice with heparin
did not show a synergistic effect, thus indicating that heparin and
P-selectin work under the same mechanism (4,23).
A meta-analysis in 2007 showed that LMWH decreased
mortality in cancer patients ≤13.3% compared to warfarin, which
non-significantly reduced mortality to 5.8% (12). It was shown that the effect of
S-NACH in inhibiting metastasis is not related to the heparin
anticoagulant property as reported from our laboratory, and by
others (14,15,24).
These results indicate that the beneficial use of LMWH and its
derivatives is not due to its anticoagulant activity. In contrast,
the anticoagulant property is a drawback in using heparin, and as
shown in our animal model, heparin caused higher mortality in mice
due to internal bleeding compared to other groups.
The development of NACH provided a heparin
derivative but without the side effect of increased bleeding.
Different types of NACH have been prepared and tested; they vary in
their efficacy. Kragh et al was able to demonstrate that
NACH inhibited spontaneous metastasis by 48% compared to 12% for
LMWH (25). In our laboratory,
S-NACH demonstrated potent inhibition of pancreatic cancer
adhesion, invasion, and metastasis (experimental metastasis) in
addition to its inhibitory effects on tumor growth and tumor
angiogenesis, which is not the case with other non-anticoagulant
heparins (24). In our present
experiments we were able to prove again the efficacy of S-NACH in
inhibiting experimental metastasis; there was a 72–82% decrease in
liver metastasis compared to control group. S-NACH was also safe
and did not increase the death rate among the mice even after
increasing the dose.
Although heparin and its derivatives work best on
inhibiting hematogenous spread of tumor cells via
P-selectin-mediated platelets - cancer cell adhesion, heparin
possesses other biological activities such as inhibition of
angiogenesis and lymphogenesis. Lymphogenesis is one of the
mechanisms enhanced by cancer cells to facilitate metastasis.
Lymphatic vessels provide a route to local lymph nodes, after which
metastases often travel through the blood (26). The increased production of vascular
endothelial growth factor (VEGF) stimulates the growth of new blood
and lymph vessels. The gastrointestinal carcinomas are known to
metastasize first to lymph nodes (27), and inhibiting angiogenesis and
lymphogenesis is another biological activity of heparin that aids
in preventing metastasis (28).
In vitro studies demonstrated that tumor
cells could be shed during surgical manipulation of the primary
tumor (29). Retrospective
clinical studies showed more favorable results for patients
receiving perioperative LMWH (17). Von Tempelhoff et al showed
the clinically beneficial effect of preoperative heparin treatment
(18). Here, treatment with LMWH
or S-NACH before performing the surgery on the mice allowed the
test compounds time to bind to the P-selectin platelets and inhibit
them from binding to the disseminated cancer cells. The results we
obtained using S-NACH showed a decrease in cancer recurrence at the
site of surgical removal compared to control (p<0.05), and
although it showed a trend in decreasing liver metastasis, the
number did not reach statistical significance (p=0.06). This was
probably due to the small number of mice that survived after
undergoing two surgeries in a week (implanting the cancer cells and
excising the tumor). S-NACH demonstrated distinct upregulation of
key junctional adhesion molecule E-cadherin; its upregulation is
known to limit cancer cell migration and invasion (30).
The benefits of heparin derivative in inhibiting
metastasis can be introduced into clinical practice and can be used
synergistically with current chemotherapies. LMWH and S-NACH were
shown to increase the uptake of chemotherapeutics in treating
breast cancer in a mouse model (31). This effect was also shown in a
retrospective study of combining heparin and chemotherapy for
optimal results in treating lung cancer patients (32). Research has shown that a brief
course of subcutaneous low molecular weight heparin favorably
influences the survival in patients with advanced malignancy, and
it deserves additional clinical evaluation (33). Lebeau et al carried out a
randomized, multicenter clinical trial in 1994 to study the
positive influence of anticoagulant treatment in small cell lung
cancer. The study included 277 patients, and the results showed
better survival rates for patients treated with subcutaneous
heparin for 5 weeks compared to control group at 1, 2, and 3 years
(40 vs. 30, 11 vs. 9 and 9 vs. 6%, respectively) (34).
The average dose of the LMWH dalteparin as an
antimetastatic agent in human clinical trials was 5,000 IU,
equivalent to 3–5 mg/kg in humans (12), and the average dose of enoxaparin
in humans is 1.0–1.5 mg/kg. In our experiment we used 10 mg/kg for
LMWH (tinzaparin) and as high as 20 mg/kg for S-NACH. S-NACH was
safely administered and did not increase the bleeding time compared
to control group. The number of mouse deaths in all experiments was
calculated at the end of the project, and we noted a high
percentage of mice dying in the LMWH group due to internal
bleeding, whereas the percentage of death in the S-NACH group was
statistically similar to the control. Further studies might be
required to firm these trends with a larger sample size and perhaps
reducing the dose of tinzaparin to 5 mg/kg.
Potential mechanisms for the anticancer efficacy of
heparin derivatives, including S-NACH or tinzaparin, might be due
to their multimodal mechanisms contributing to anticancer and
anti-metastasis efficacy. This might include their effective
antiangiogenesis efficacy (via the release of endogenous
endothelial TFPI), inhibition of cancer cell adhesion
(anti-selectin), inhibition of cancer cell invasion (inhibition of
heparinases, matrix degrading enzymes, and through other cell
adhesion molecules), anti-inflammatory efficacy, and possibly other
mechanisms (35–38). It was also suggested that combining
the effect of heparin and its derivative S-NACH with current
adjuvant or neo-adjuvant therapy will lead to a decrease in the
required chemotherapy dose and increased tumor chemo-responsiveness
based on reported studies from our laboratories (31).
These data suggest that S-NACH is an effective
anti-metastatic agent in our mouse model; it decreased distal
metastasis and surgically induced metastasis. S-NACH was also found
to be safe in terms of bleeding tendencies compared to LMWH. S-NACH
warrants further clinical evaluation.
Acknowledgements
We appreciate the excellent editing by Dr Kelly
Keating [Pharmaceutical Research Institute (PRI) at the Albany
College of Pharmacy and Health Sciences] and the technical support
by staff of the PRI. We thank Alexander Durant in the Animal
Laboratory of the Albany Stratton VA Medical Center, (Albany, NY,
USA); Dr Thiruvengadam Arumugam (M.D. Anderson Cancer Center,
Houston, TX, USA) for supplying the Mpanc96-luc cell line; and Dr
Robert J. Linhardt (Rensselaer Polytechnic Institute, Rensselaer,
NY, USA) for supplying S-NACH. S.A. Mousa holds a US patent on
S-NACH (13).
References
|
1
|
Siegel R, Ward E, Brawley O and Jemal A:
Cancer statistics, 2011: the impact of eliminating socioeconomic
and racial disparities on premature cancer deaths. CA Cancer J
Clin. 61:212–236. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Chambers AF, Groom AC and MacDonald IC:
Dissemination and growth of cancer cells in metastatic sites. Nat
Rev Cancer. 2:563–572. 2002. View
Article : Google Scholar : PubMed/NCBI
|
|
3
|
Sener SF, Fremgen A, Menck HR and
Winchester DP: Pancreatic cancer: a report of treatment and
survival trends for 100,313 patients diagnosed from 1985–1995,
using the National Cancer Database. J Am Coll Surg. 189:1–7. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Borsig L, Wong R, Feramisco J, Nadeau DR,
Varki NM and Varki A: Heparin and cancer revisited: mechanistic
connections involving platelets, P-selectin, carcinoma mucins, and
tumor metastasis. Proc Natl Acad Sci USA. 98:3352–3357. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Stone JP and Wagner DD: P-selectin
mediates adhesion of platelets to neuroblastoma and small cell lung
cancer. J Clin Invest. 92:804–813. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Mannori G, Crottet P, Cecconi O, et al:
Differential colon cancer cell adhesion to E-, P-, and L-selectin:
role of mucin-type glycoproteins. Cancer Res. 55:4425–4431.
1995.PubMed/NCBI
|
|
7
|
Nelson RM, Cecconi O, Roberts WG, Aruffo
A, Linhardt RJ and Bevilacqua MP: Heparin oligosaccharides bind L-
and P-selectin and inhibit acute inflammation. Blood. 82:3253–3258.
1993.PubMed/NCBI
|
|
8
|
Koenig A, Norgard-Sumnicht K, Linhardt R
and Varki A: Differential interactions of heparin and heparan
sulfate glycosaminoglycans with the selectins. Implications for the
use of unfractionated and low molecular weight heparins as
therapeutic agents. J Clin Invest. 101:877–889. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Zacharski LR and Loynes JT: The heparins
and cancer. Curr Opin Pulm Med. 8:379–382. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Kasthuri RS, Taubman MB and Mackman N:
Role of tissue factor in cancer. J Clin Oncol. 27:4834–4838. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Capila I and Linhardt RJ: Heparin-protein
interactions. Angew Chem Int Ed Engl. 41:391–412. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Kuderer NM, Khorana AA, Lyman GH and
Francis CW: A meta-analysis and systematic review of the efficacy
and safety of anticoagulants as cancer treatment: impact on
survival and bleeding complications. Cancer. 110:1149–1161. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Mousa SA: Oxidized heparin fractions and
their use in inhibiting angiogenesis. US patent 8,071,569.
2011:December 06–2011
|
|
14
|
Lapierre F, Holme K, Lam L, et al:
Chemical modifications of heparin that diminish its anticoagulant
but preserve its heparanase-inhibitory, angiostatic, anti-tumor and
anti-metastatic properties. Glycobiology. 6:355–366. 1996.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Sudha T, Phillips P, Kanaan C, Linhardt
RJ, Borsig L and Mousa SA: Inhibitory effect of non-anticoagulant
heparin (S-NACH) on pancreatic cancer cell adhesion and metastasis
in human umbilical cord vessel segment and in mouse model. Clin Exp
Metastasis. 29:431–439. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
van der Bij GJ, Oosterling SJ, Beelen RH,
Meijer S, Coffey JC and van Egmond M: The perioperative period is
an underutilized window of therapeutic opportunity in patients with
colorectal cancer. Ann Surg. 249:727–734. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Kakkar A, Hedges R, Williamson R and
Kakkar V: Perioperative heparin-therapy inhibits late death from
metastatic cancer. Int J Oncol. 6:885–888. 1995.PubMed/NCBI
|
|
18
|
von Tempelhoff GF, Harenberg J, Niemann F,
Hommel G, Kirkpatrick CJ and Heilmann L: Effect of low molecular
weight heparin (Certoparin) versus unfractionated heparin on cancer
survival following breast and pelvic cancer surgery: a prospective
randomized double-blind trial. Int J Oncol. 16:815–824.
2000.PubMed/NCBI
|
|
19
|
Lim E, Modi KD and Kim J: In vivo
bioluminescent imaging of mammary tumors using IVIS spectrum. J Vis
Exp. 26:12102009.PubMed/NCBI
|
|
20
|
Dejana E, Callioni A, Quintana A and de
Gaetano G: Bleeding time in laboratory animals. II - A comparison
of different assay conditions in rats. Thromb Res. 15:191–197.
1979. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Alshaiban A, Muralidharan-Chari V, Nepo A
and Mousa SA: Modulation of sickle red blood cell adhesion and its
associated changes in biomarkers by sulfated non-anticoagulant
heparin derivative. Clin Appl Thromb Hemost. (In press).
|
|
22
|
Gasic GJ, Gasic TB and Stewart CC:
Antimetastatic effects associated with platelet reduction. Proc
Natl Acad Sci USA. 61:46–52. 1968. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Kim YJ, Borsig L, Varki NM and Varki A:
P-selectin deficiency attenuates tumor growth and metastasis. Proc
Natl Acad Sci USA. 95:9325–9330. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Mousa SA, Linhardt R, Francis JL and
Amirkhosravi A: Antimetastatic effect of a non-anticoagulant
low-molecular-weight heparin versus the standard
low-molecular-weight heparin, enoxaparin. Thromb Haemost.
96:816–821. 2006.PubMed/NCBI
|
|
25
|
Kragh M, Binderup L, Vig Hjarnaa PJ, Bramm
E, Johansen KB and Frimundt Petersen C: Non-anti-coagulant heparin
inhibits metastasis but not primary tumor growth. Oncol Rep.
14:99–104. 2005.PubMed/NCBI
|
|
26
|
Bacac M and Stamenkovic I: Metastatic
cancer cell. Annu Rev Pathol. 3:221–247. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Kopfstein L and Christofori G: Metastasis:
cell-autonomous mechanisms versus contributions by the tumor
microenvironment. Cell Mol Life Sci. 63:449–468. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Achen MG, Mann GB and Stacker SA:
Targeting lymphangiogenesis to prevent tumour metastasis. Br J
Cancer. 94:1355–1360. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Ito S, Nakanishi H, Hirai T, et al:
Quantitative detection of CEA expressing free tumor cells in the
peripheral blood of colorectal cancer patients during surgery with
real-time RT-PCR on a LightCycler. Cancer Lett. 183:195–203. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Wang F, Sloss C, Zhang X, Lee SW and
Cusack JC: Membrane-bound heparin-binding epidermal growth factor
like growth factor regulates E-cadherin expression in pancreatic
carcinoma cells. Cancer Res. 67:8486–8493. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Phillips PG, Yalcin M, Cui H, et al:
Increased tumor uptake of chemotherapeutics and improved
chemoresponse by novel non-anticoagulant low molecular weight
heparin. Anticancer Res. 31:411–419. 2011.PubMed/NCBI
|
|
32
|
Lebeau B, Baud M, Masanes MJ, Febvre M,
Mokhtari T and Chouaid C: Optimization of small-cell lung cancer
chemotherapy with heparin: a comprehensive retrospective study of
239 patients treated in a single specialized center. Chemotherapy.
57:253–258. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Klerk CP, Smorenburg SM, Otten HM, et al:
The effect of low molecular weight heparin on survival in patients
with advanced malignancy. J Clin Oncol. 23:2130–2135. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Lebeau B, Chastang C, Brechot JM, et al:
Subcutaneous heparin treatment increases survival in small cell
lung cancer. ‘Petites Cellules’ Group. Cancer. 74:38–45. 1994.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Mousa SA and Petersen LJ: Anti-cancer
properties of low-molecular-weight heparin: preclinical evidence.
Thromb Haemost. 102:258–267. 2009.PubMed/NCBI
|
|
36
|
Mousa SA: Comparative pharmacodynamic
assessment of the antiangiogenesis activity of heparin and
low-molecular-weight heparin fractions: structure-function
relationship. Clin Appl Thromb Hemost. 19:48–54. 2013. View Article : Google Scholar
|
|
37
|
Yu CJ, Ye SJ, Feng ZH, et al: Effect of
Fraxiparine, a type of low molecular weight heparin, on the
invasion and metastasis of lung adenocarcinoma A549 cells. Oncol
Lett. 1:755–760. 2010.PubMed/NCBI
|
|
38
|
Ilan N, Elkin M and Vlodavsky I:
Regulation, function and clinical significance of heparanase in
cancer metastasis and angiogenesis. Int J Biochem Cell Biol.
38:2018–2039. 2006. View Article : Google Scholar : PubMed/NCBI
|