Introduction
Epithelial ovarian cancer (EOC) is the world’s most
lethal gynecological cancer, and the World Health Organization
Global Database listed EOC as the 7th leading form of cancer in
women in 2008 (1).
Although the mean 5-year survival rate for EOC has
improved significantly over the past 30 years, the prognosis
remains poor, with a 46% 5-year survival rate (2). The prognosis for EOC is closely
related to the cancer clinical stage at diagnosis. The mean 5-year
survival rate in advanced stages (FIGO stage III or IV) is as low
as 11–41% (2). More than 70% of
EOCs are detected in the advanced stages mainly because of a lack
of early warning signs and reliable diagnostic tests. Cytoreductive
surgery followed by adjuvant chemotherapy is recommended as the
primary treatment for advanced EOC. Postoperatively, a taxane and
carboplatin combination is used as the first-line chemotherapy. EOC
is highly responsive to initial anticancer treatment, but
approximately half of the advanced cases recur within 2 years and
result in poor prognosis due to a decreased response to
chemotherapy (3). Therefore, new
clinically useful biomarkers and new targets for EOC treatment need
to be identified in order to initiate intensive treatment.
Carbonyl reductase 1 (CBR1) is an NADPH-dependent
oxidoreductase with broad specificity for carbonyl compounds, which
reduces aldehydes and ketones (4).
CBR1 has been isolated from various organs such as the liver,
kidney, breast, ovary, and vascular endothelial cells (5), and has been studied for its function
in the metabolism of a variety of drugs such as anthracycline,
daunorubicin, haloperidol, and doxorubicin (6,7).
Another important CBR1 function is to convert prostaglandin (PG)
E2 to PGF2α (8,9).
PGE2 have been demonstrated not only to modulate
apoptosis and Bcl-2 expression (10), but also to induce angiogenesis
(11).
CBR1 has been reported to relate to tumor
progression (12–14). Suppression of CBR1 expression was
associated with poor prognosis in uterine endometrial cancer and
uterine cervical squamous cell carcinoma (12,13).
Our previous studies showed that decreased CBR1 expression is
associated with lymph node metastasis and poor prognosis in ovarian
cancer (14), and induction of
CBR1 expression in ovarian tumors leads to a spontaneous decrease
in tumor size (15).
In vitro experiments showed that CBR1
suppression enhanced uterine squamous cell carcinoma and
endometrial carcinoma malignant behavior (12,13).
A significant inverse relationship between vascular endothelial
growth factor (VEGF) and CBR1 expression was observed in cancer
tissues (16). The epithelial
mesenchymal transition (EMT) has been associated with tumor
progression and poor prognosis in various human cancers.
Predominantly, the functional loss of E-cadherin in epithelial
cells is a common feature of EMT (17). Earlier studies showed that CBR1 is
likely to be associated with EMT in endometrial adenocarcinoma
(13). Furthermore, the
extracellular matrix (ECM) degradation is necessary for cancer
cells to invade. Matrix metalloproteinases (MMPs) are enzymes that
resolve ECM. Loss of E-cadherin and activation of MMP-9 have been
reported to correlate with poor prognosis in ovarian cancer
(18). Because CBR1 molecular
mechanisms remain unclear in ovarian cancer, it may be of interest
to investigate the relationship between altered expression of CBR1
and molecules such as VEGF, E-cadherin, and MMPs that affect
malignant behavior.
In this study, we investigated the effect of
decreased CBR1 expression on proliferation of ovarian cancer cells
and growth of ovarian cancer, and aimed to elucidate its mechanisms
of action.
Materials and methods
Cell line and culture
OVCAR-3 was obtained from the American Type Culture
Collection (Rockville, MD, USA). OVCAR-3 cells have been commonly
used to produce xenografted solid tumor (16) and are derived from human ovarian
cancer. They were cultured in RPMI-1640 medium supplemented with
10% fetal calf serum (FCS), 100 U/ml penicillin and 100 mg/ml
streptomycin at 37°C in a water-saturated atmosphere with 5%
CO2/95% air.
Animal experiments
Animal experiments were conducted in accordance with
the Guidelines for Animal Experimentation, Hirosaki University
(Aomori, Japan). Eight-week-old female BALB/c nu/nu mice were used
in this study. All mice were group housed in plastic cages with
stainless steel grid tops in an air-conditioned and 12-h light/dark
cycle-maintained room in the Institute for Animal Experiments of
Hirosaki University and fed with water and food ad
libitum.
Plasmid DNA preparation
To optimize and obtain highly efficient
transfection, we used a pCMV6-AC-GFP vector (OriGene Technologies,
Inc., Rockville, MD, USA) that encodes the human CBR1, GFP and
ampicillin-resistant gene. For amplification, pCMV6-AC-GFP was
transformed into E. coli-DH5α competent cells by heat shock
transformation according to standard laboratory protocols. The
transformed bacteria were amplified in LB-ampicillin medium. The
plasmids were purified from cultured transformed bacteria using a
PureLink HiPure Plasmid Filter Miniprep DNA purification kit
(Invitrogen Life Technologies, Carlsbad, CA, USA) according to the
manufacturer’s protocol. Plasmid DNA (pDNA) was diluted in sterile
water at a concentration of 3 μg/μl.
Small interfering RNA preparation
The sequences of small interfering RNA (siRNA)
duplexes specific to CBR1 were synthesized commercially by
Invitrogen Life Technologies. CBR1 siRNA sense,
5′-AUACGUUCACCACUCUCCCTT-3′ and antisense,
5′-GGGAGAGUGGUGAACGUAUTT-3′ were designed to target different
coding regions of the human CBR1 mRNA sequence (GeneBank Accession
no. NM_001757).
Transfection
OVCAR-3 cells were trypsinized at a density of
5.0×105 cells/plate and were rinsed twice with
serum-free RPMI-1640. The cells were then transferred into an
electroporation cuvette. Afterwards, 10 μl of human CBR1 pDNA was
added to the cells and electroporated with the square wave program
with poring pulses and transfer pulses using a NEPA21 (Nepa Gene
Co., Ltd., Chiba, Japan). Electroporated OVCAR-3 cells were then
immediately transferred to the culture plates containing RPMI-1640
with fetal bovine serum (FBS). Transfected cells were harvested 4
days later. The control was only given electric stimulation by
electroporation.
Cell proliferation
OVCAR-3 cells were cultured in 6-well plates as
described in the cell line and culture paragraph above. Cell counts
were performed 24 h in a logarithmic growth phase after
transfection of OVCAR-3 cells with CBR1-DNA or CBR1-siRNA. To
distinguish live and dead cells, cells were stained with 0.3%
trypan blue solution (Wako Pure Chemicals Industries, Ltd., Osaka,
Japan). Cells were counted using a hemocytometer. The cell count
was performed in triplicate and the total cell counts are presented
as averages.
Invasion assay
The tumor cell invasiveness was determined using the
CytoSelect 24-Well Cell Invasion Assay kit (basement membrane,
colorimetric format; Cell Biolabs, Inc., San Diego, CA, USA)
according to the manufacturer’s instructions. For invasion assays,
the cells were cultured in serum-free culture medium at a density
of 1.0×105 cells/well in the upper chamber, which had an
8.0-μm pore size membrane coated with a uniform layer of basement
membrane matrix solution, and the lower chamber was filled with the
culture medium with 10% FBS. After incubation for 48 h, invasive
cells on the bottom of the membrane were stained with Diff-Quick
(Sysmex Corp., Hyōgo, Japan) and quantified using an absorption
photometer (OD=560 mm).
Xenograft mouse model
The mice were divided into three groups (n=5 for
each group). OVCAR-3 cells or OVCAR-3 expressing the CBR1-DNA
(5.0×105 cells) were inoculated subcutaneously in 0.2 ml
of RPMI-1640 medium in the back region of nude mice. All the mice
were numbered, housed separately, and tumor development was
examined for 10 days. The examination started once the longer
diameter of the tumor reached 5 mm (day 0). The control group and
the CBR1-DNA group were intratumorally administered 5% glucose
solution, while the CBR1-siRNA group was intratumorally injected
with the CBR1-siRNA using Invivofectamine (Invitrogen Life
Technologies) at a dose of 80 μg twice a week on day 0 and 7. Mice
were monitored every day for tumor growth until day 14. The tumor
dimensions were measured every day using caliper and tumor volume
was calculated using the equation: V (mm3) = A ×
B2/2, where A is the largest diameter and B is the
smallest diameter (19). The mice
were sacrificed on day 14 to remove the tumor and lungs for
pathologic and biochemical studies.
Western blot analysis
Cell lysates (50 μg protein) were prepared from
tumor tissues, electrophoresed through a 12.5% sodium dodecyl
sulfate polyacrylamide gel, and blotted as described previously
(16). The protein concentration
was determined using Bradford’s method. The blots were probed with
the following diluted antibodies for 2 h: CBR1 at 1:200, human
VEGF-C at 1:200 (both from Santa Cruz Biotechnology, Inc., Santa
Cruz, CA, USA), E-cadherin at 1:100,000 (GeneTex, Inc., Irvine, CA,
USA), MMP-9 at 1:500 (Abnova, Walnut, CA, USA), and β-actin at
1:2,000 (Sigma-Aldrich, St. Louis, MO, USA). The membranes were
then incubated for 1 h with the appropriate biotinylated secondary
antibodies, transferred to avidin-biotin-peroxidase complex
reagent, and incubated in this solution for 30 min.
Diaminobenzidine was used as a substrate.
Statistical analysis
The Tukey-Kramer test was used to assess differences
in the number of living cells between the control, CBR1-DNA, and
CBR1-siRNA group. Differences in the invasion assay between the
three groups were evaluated using Student’s t-test. Differences in
tumor volume between the control, CBR1-DNA, and CBR1-siRNA group
were evaluated using the Mann-Whiney U test. A probability value of
P<0.05 was considered to be significant.
Results
Comparison of CBR1 expression levels
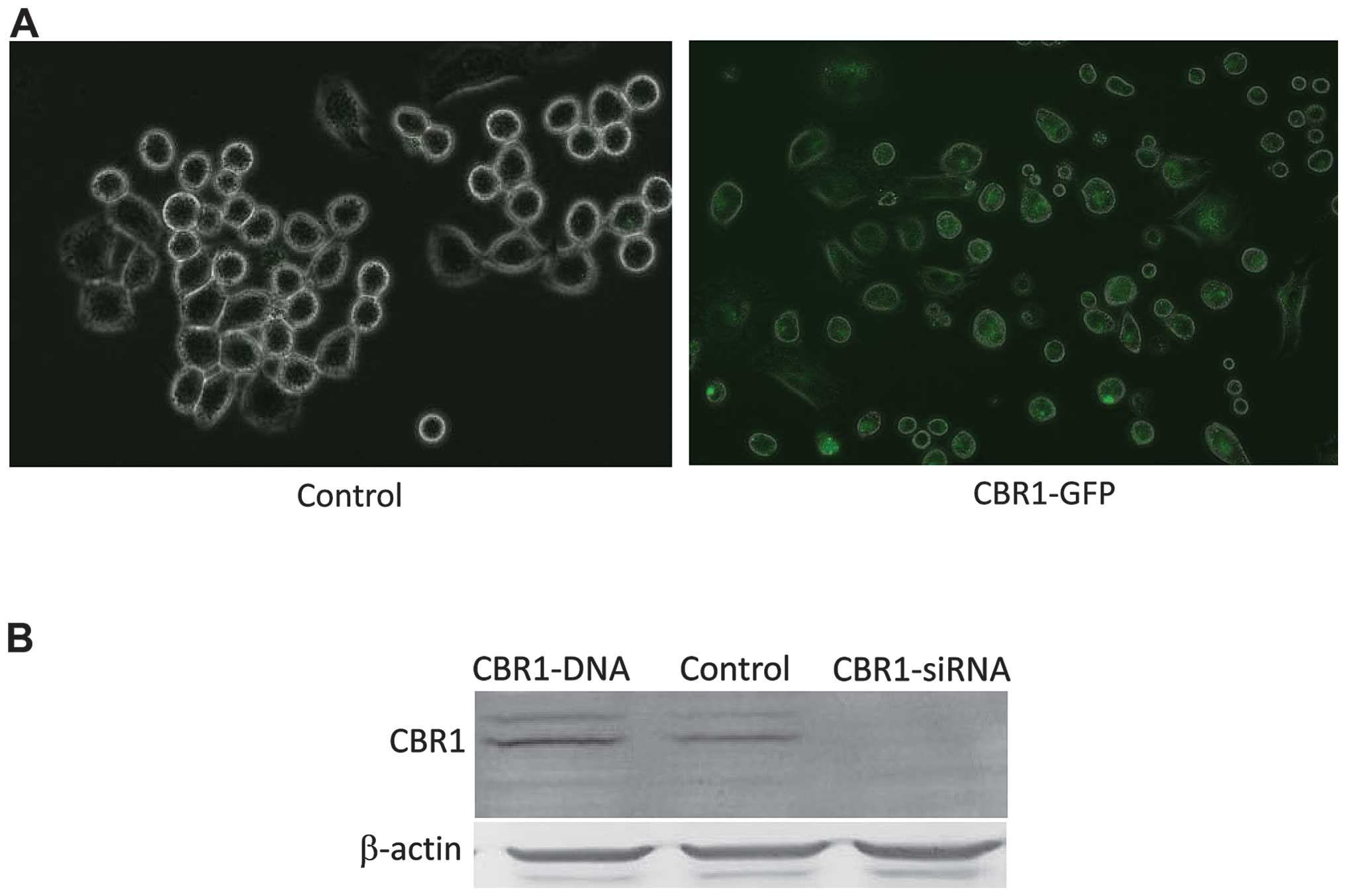
CBR1 expression levels were compared in OVCAR-3
cells transfected with CBR1-DNA or CBR1-siRNA. CBR1-GFP protein
fluorescence was clearly detected in CBR1-DNA-tranfected cells
(Fig. 1A). Western blot analysis
showed that CBR1 expression level was higher in
CBR1-DNA-transfected cells and lower in CBR1-siRNA-tranfected cells
when compared to the control cells (Fig. 1B).
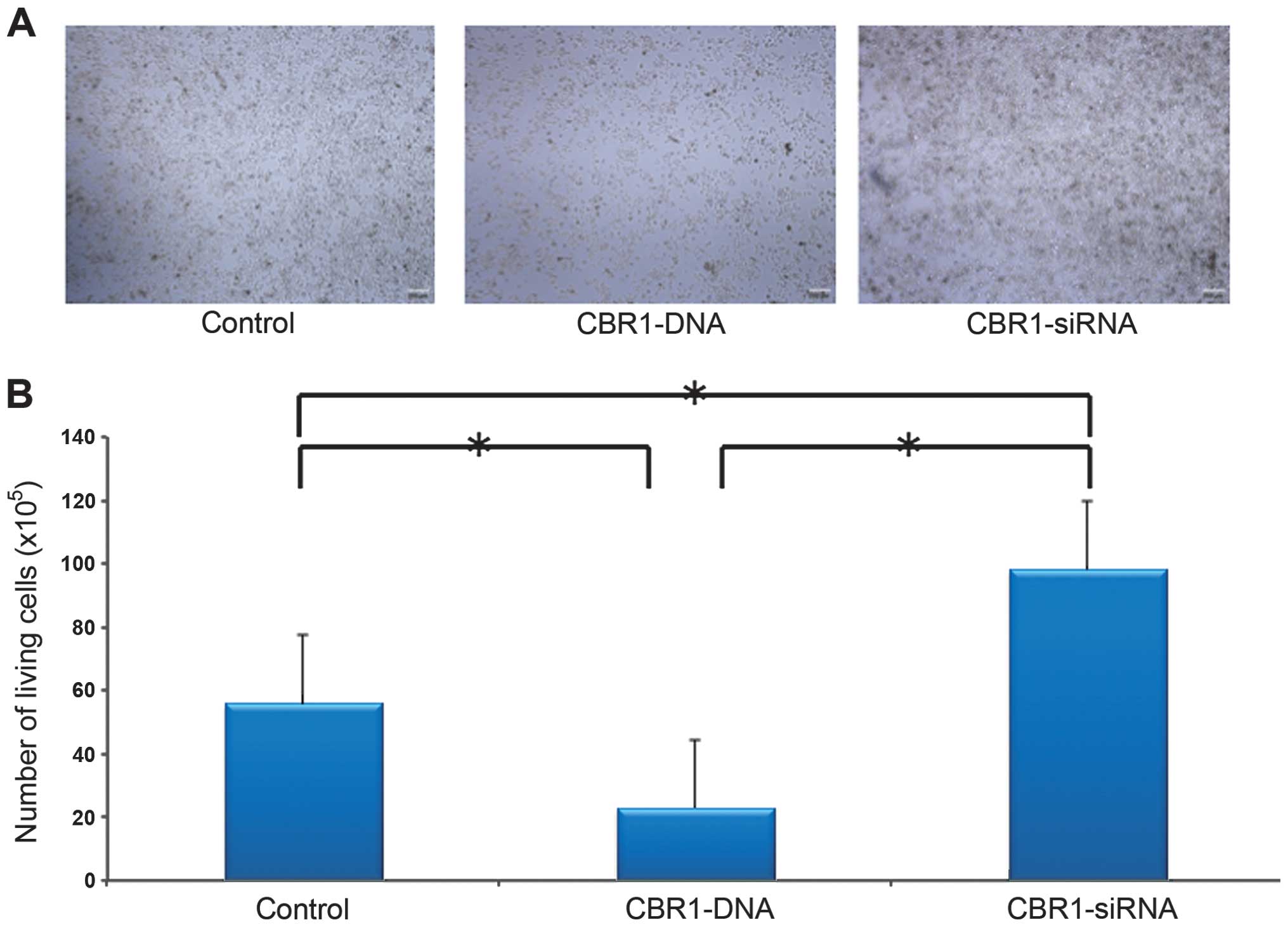
Cell proliferation
Difference in cell density was demonstrated among
the three groups as shown in Fig.
2A. The CBR1-siRNA-tranfected cells grew in multilayers. Cell
density was lower in CBR1-DNA-transfected cells and higher in
CBR1-siRNA-transfected cells when compared to the control cells
(Fig. 2A). The cell counts were
55.6×105 cells for the control group,
22.8×105 cells for the CBR1-DNA group, and
98.1×105 cells for the CBR1-siRNA group, respectively.
The number of living cells was significantly higher in the
CBR1-siRNA group than in the other two groups and significantly
lower in CBR1-DNA group than in the control group (Fig. 2B, P<0.001 each).
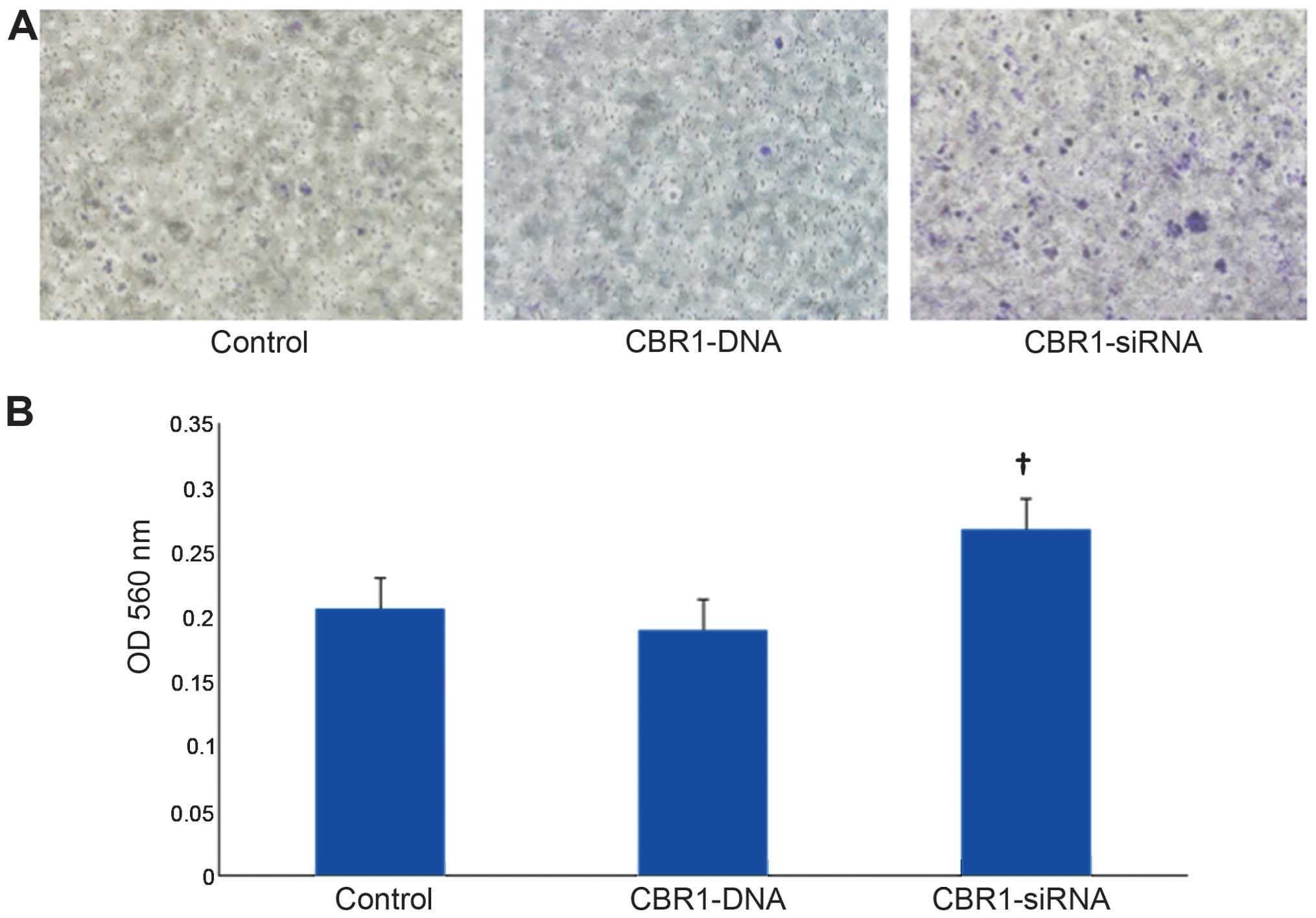
Cell invasion
Fig. 3A indicates
the appearance of invasive cells 48 h after transfection. Invasive
cells were stained in blue. Cell invasion was significantly higher
in CBR1-siRNA-transfected cells than in both the control and the
CBR1-DNA-transfected cells (Fig.
3B, P<0.05).
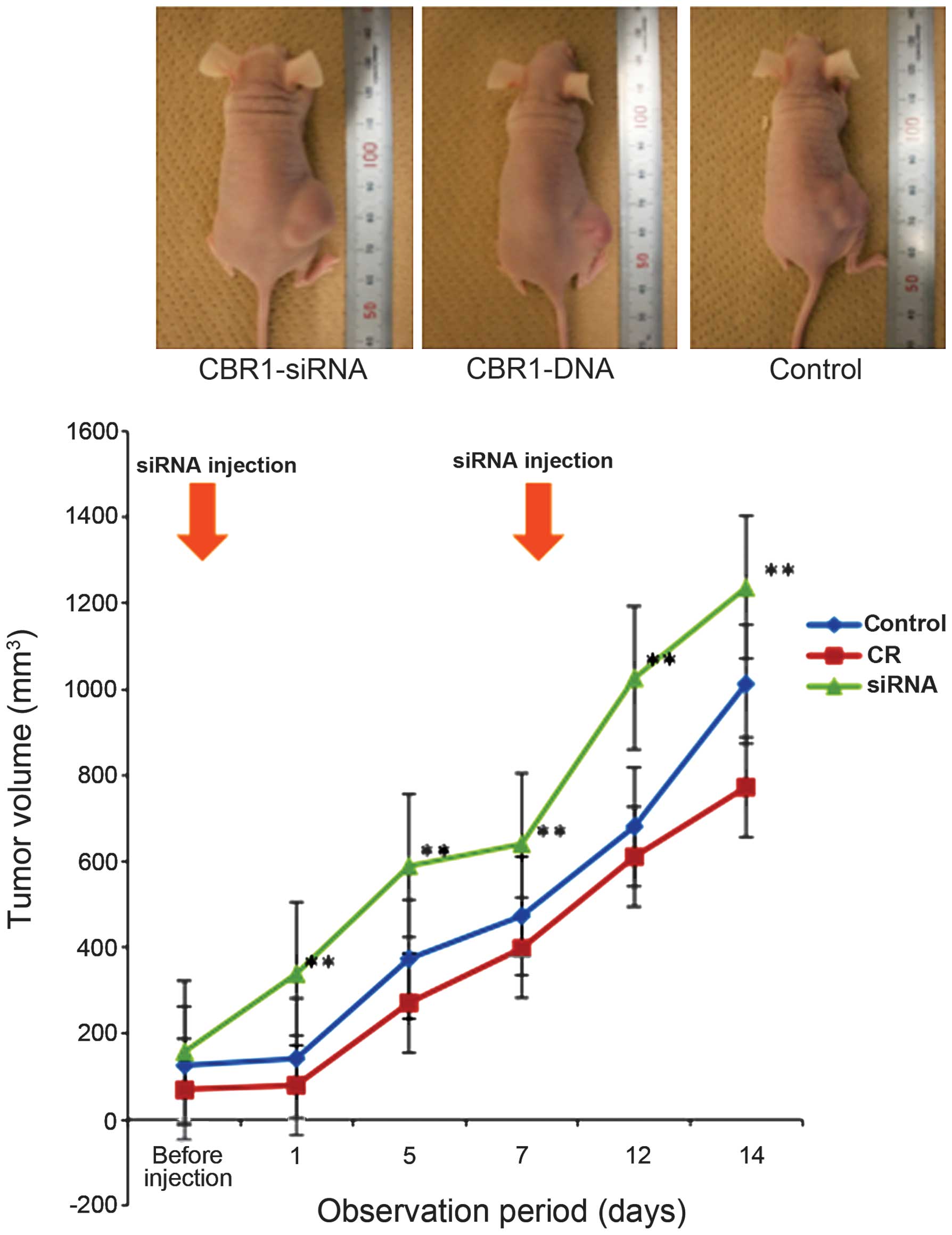
Tumor growth
In the xenograft mouse model, tumor volumes were
significantly reduced from the first day after initial infection
with the CBR1-siRNA in the CBR1-siRNA group (n=5) compared to the
control (n=5) and CBR1-DNA group (n=5) (Fig. 4, P<0.05). The same trend lasted
until they were sacrificed at day 14. Although tumor growth in the
CBR1-DNA group was suppressed compared to the control group, there
was no significant difference between the two groups. Inset photos
are representatives of each group at day 14.
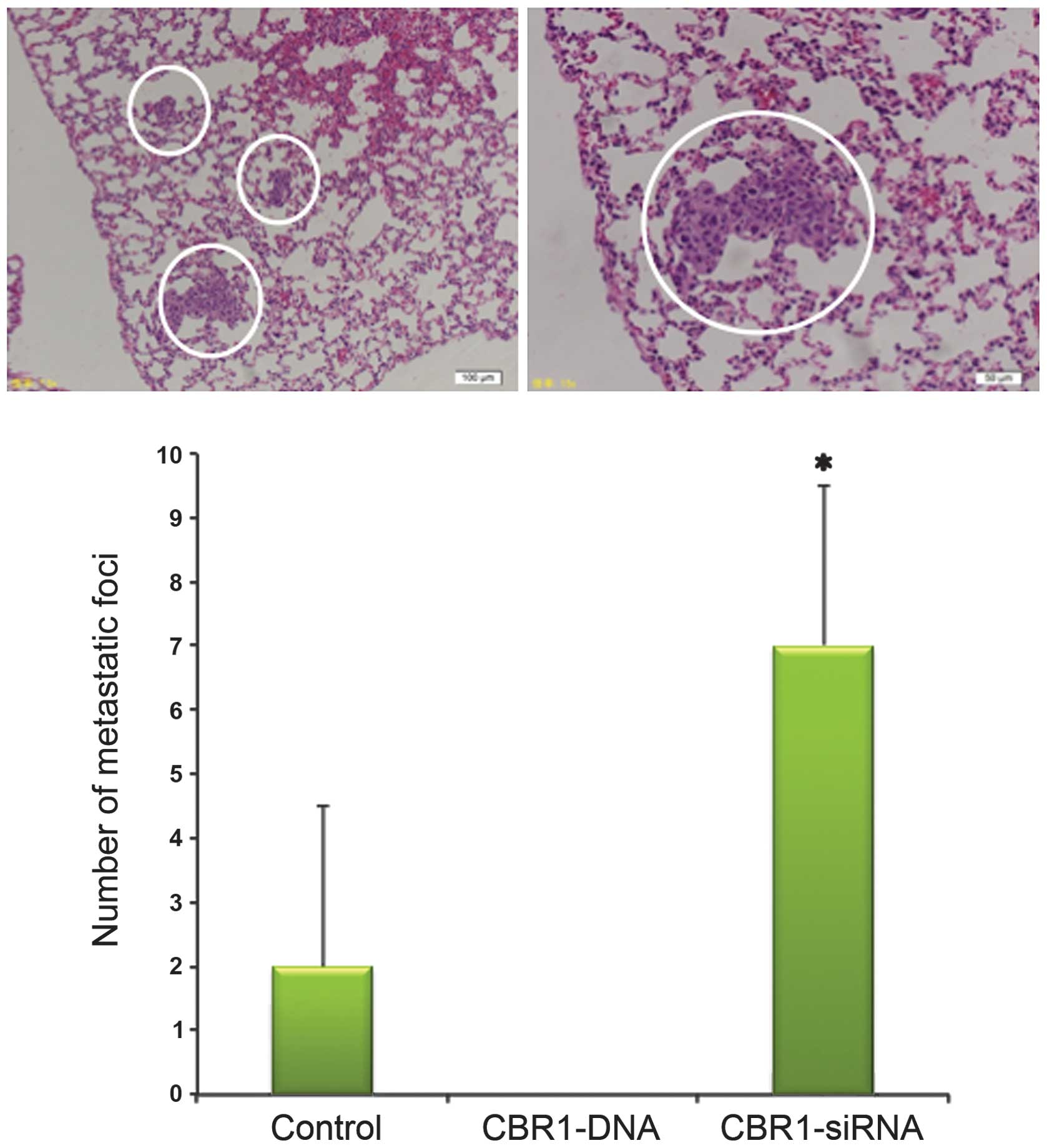
Tumor invasion and metastasis
The mice were sacrificed on day 14 to remove the
tumor and lungs and to observe the abdominal cavity. The number of
metastatic foci in the lungs was 7.0±2.0, 0, and 2.0±2.0 in mice
bearing CBR1-siRNA-injected, CBR1-DNA, and the control tumors,
respectively. Metastatic foci to the lungs were significantly
increased in CBR1-siRNA group compared with the other two groups
(Fig. 5, P<0.05). There was no
metastatic lesion in the lungs of CBR1-DNA tumor-bearing mice. Most
of the mice in the CBR1-siRNA group presented a deep invasion of
subcutaneous tumors into the abdominal cavity (data not shown).
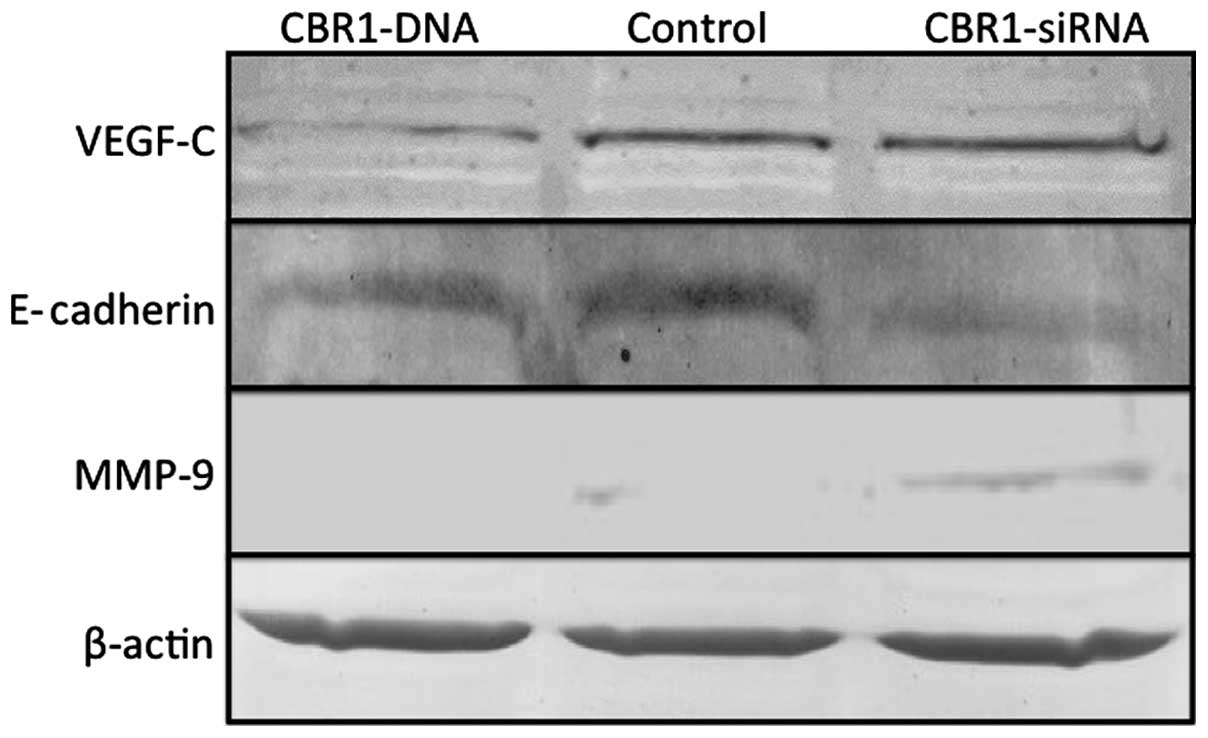
Altered expression of VEGF-C, E-cadherin,
and MMP-9 in the tumors according to CBR1 expression levels
Western blot analysis showed that, while VEGF-C
expression was decreased in the CBR1-DNA tumors and was stable in
the CBR1-siRNA tumors, E-cadherin-decreased expression and
MMP-9-increased expression were observed in the CBR1-siRNA tumors
compared to the other two groups (Fig.
6).
Discussion
In this study using ovarian cancer cells, CBR1
suppression by siRNA transfection showed a significantly higher
proliferative ability and invasive activity that caused rapid tumor
growth and lung metastasis than the control cells. In addition,
CBR1 suppression led to decreased expression of E-cadherin and
increased expression of MMP-9 in tumors, whereas VEGF-C expression
remained stable. Results of the present study were consistent with
earlier findings obtained in clinical (12–14)
and animal studies (20). Umemoto
et al reported that CBR1 loss or decrease was significantly
related to retroperitoneal lymph node metastasis and poor outcome
in EOC (14). Murakami et
al showed a significantly close relationship between decreased
CBR1 expression and progression-free survival as well as overall
survival in uterine cervical or endometrial cancer (12,13).
Ismail et al, on the other hand, showed that mouse cancer
cells in which CBR1 was knocked down by transfection of an
antisense CBR1 cDNA acquired a potent metastatic potential
(20). In addition, our previous
study showed that ovarian tumors derived from CBR1 sense
cDNA-transfected cells grew up to the second week, but then
decreased continuously until the fifth week of observation
(15). We have shown that the
spontaneous regression was due to increased necrosis through
phagocytosis of apoptotic cells by phagocytes attracted by
increased eat-me-signal induced by CBR1 (15). Furthermore, CBR1 expression vector
transfection into mouse ovarian cancer cells induced a significant
reduction of PGE2 level and VEGF expression (16). These findings confirm that CBR1
expression is involved in cancer cell growth and strongly indicate
that CBR1 expression influences cancer cell acquisition of
malignant and metastatic potential.
In this study, CBR1 suppression in ovarian cancer
induced a decrease in E-cadherin expression and an increase in
MMP-9 expression. The loss of E-cadherin is known as a marker of
EMT that is associated with carcinoma progression and poor
prognosis in malignant tumors (21). Because MMPs resolve ECM, the MMP
increase is necessary to acquire a potent invasive potential. Among
MMP subtypes, MMP-9 is closely associated with poor outcome in
ovarian cancer (22) and its
expression level is significantly stronger as lesions progressed
from a benign tumor to advanced carcinoma (23,24).
Earlier study showed that MMP-9 overexpression led to a loss of
E-cadherin and promoted a migratory and invasive phenotype in
ovarian cancer cells (25),
supporting the present results. On the other hand, in this study,
although VEGF-C expression was decreased in the CBR1-DNA tumors,
its expression in CBR1-siRNA tumors was similar to that of the
control ovarian cancer cell tumors. VEGF subtypes are commonly
known to be involved in cancer metastasis (26). VEGF receptor (VEGFR)-3, a tyrosine
kinase receptor, is involved in lymphangiogenesis and distant
metastasis. VEGF-C is a specific ligand of VEGFR-3 (27). Stable expression of VEGF-C/VEGFR-3
increased distant metastasis, including lungs and lymph node
metastasis in various types of malignant tumor (28–31).
The present study showed that lung metastasis was significantly
more frequent in CBR1-siRNA tumors than in the other two groups.
Additionally, lung metastasis did not occur in CBR1-DNA tumors. The
present results suggest that the increase in MMP-9 and decrease in
E-cadherin induced by reducing CBR1 expression may enhance
malignant behavior such as invasion and metastasis in ovarian
cancer under stable expression of VEGF-C.
CBR1 exists in various tissues in humans (5). It is important to confirm whether
CBR1 actually functions as an enzyme in cancer cells. Our previous
study showed that PGE2 levels were reduced in OVCAR-3
cells transfected with CBR1 sense cDNA (16) and that the increase of E-cadherin
expression was blocked by quercetin, which inhibits CBR1 enzymatic
activities (12), suggesting that
the effects of CBR1 are due to its enzymatic activities rather than
its structural effects.
In conclusion, it emerged that CBR1 loss or decrease
promoted tumor proliferation and growth as well as invasion and
metastasis in this study, suggesting that CBR1 might become a new
candidate for molecular targeting therapy.
Acknowledgements
This study was supported by a Grant-in-Aid for
Cancer Research from the Ministry of Education, Culture, Sports,
Science and Technology (Tokyo, Japan) (no. 20591935 to Dr Y.
Yokoyama).
References
|
1
|
Siegel R, Naishadham D and Jemal A: Cancer
statistics, 2013. CA Cancer J Clin. 63:11–30. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Heintz AP, Odicino F, Maisonneuve P, et
al: Carcinoma of the ovary. FIGO 26th Annual Report on the Results
of Treatment in Gynecological Cancer. Int J Gynaecol Obstet.
95(Suppl 1): S161–S192. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Yokoyama Y, Futagami M, Watanabe J, et al:
Redistribution of resistance and sensitivity to platinum during the
observation period following treatment of epithelial ovarian
cancer. Mol Clin Oncol. 2:212–218. 2014.PubMed/NCBI
|
|
4
|
Gonzalez-Covarrubias V, Ghosh D, Lakhman
SS, Pendyala L and Blanco JG: A functional genetic polymorphism on
human carbonyl reductase 1 (CBR1 V88I) impacts on catalytic
activity and NADPH binding affinity. Drug Metab Dispos. 35:973–980.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Wermuth B, Bohren KM, Heinemann G, von
Warthburg JP and Gabbay KH: Human carbonyl reductase. Nucleotide
sequence analysis of a cDNA and amino acid sequence of the encoded
protein. J Biol Chem. 263:16185–16188. 1988.PubMed/NCBI
|
|
6
|
Plebuch M, Soldan M, Hungerer C, Koch L
and Maser E: Increased resistance of tumor cells to daunorubicin
after transfection of cDNAs coding for anthracycline inactivating
enzymes. Cancer Lett. 255:49–56. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Olson LE, Bedja D, Alvey SJ, Cardounel AJ,
Gabrielson KL and Reeves RH: Protection from doxorubicin-induced
cardiac toxicity in mice with a null allele of carbonyl reductase
1. Cancer Res. 63:6602–6606. 2003.PubMed/NCBI
|
|
8
|
Schieber A, Frank RW and Ghisla S:
Purification and properties of prostaglandin 9-ketoreductase from
pig and human kidney. Identity with human carbonyl reductase. Eur J
Biochem. 206:491–502. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Waclawik A and Ziecik AJ: Differential
expression of prostaglandin (PG) synthesis enzymes in conceptus
during peri-implantation period and endometrial expression of
carbonyl reductase/PG 9-ketoreductase in the pig. J Endocrinol.
194:499–510. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Sheng H, Shao J, Morrow JD, Beauchamp RD
and DuBois RN: Modulation of apoptosis and Bcl-2 expression by
prostaglandin E2 in human colon cancer cells. Cancer Res.
58:362–366. 1998.PubMed/NCBI
|
|
11
|
Tsujii M, Kawano S, Tsuji S, Sawaoka H,
Hori M and DuBois RN: Cyclooxygenase regulates angiogenesis induced
by colon cancer cells. Cell. 93:705–716. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Murakami A, Fukushima C, Yoshidomi K, et
al: Suppression of carbonyl reductase expression enhances malignant
behaviour in uterine cervical squamous cell carcinoma: carbonyl
reductase predicts prognosis and lymph node metastasis. Cancer
Lett. 311:77–84. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Murakami A, Yakabe K, Yoshidomi K, et al:
Decreased carbonyl reductase 1 expression promotes malignant
behaviours by induction of epithelial mesenchymal transition and
its clinical significance. Cancer Lett. 323:69–76. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Umemoto M, Yokoyama Y, Sato S, Tsuchida S,
Al-Mulla F and Saito Y: Carbonyl reductase as a significant
predictor of survival and lymph node metastasis in epithelial
ovarian cancer. Br J Cancer. 85:1032–1036. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Wang H, Yokoyama Y, Tsuchida S and
Mizunuma H: Malignant ovarian tumors with induced expression of
carbonyl reductase show spontaneous regression. Clin Med Insights
Oncol. 6:107–115. 2012.PubMed/NCBI
|
|
16
|
Yokoyama Y, Xin B, Shigeto T, et al:
Clofibric acid, a peroxisome proliferator-activated receptor alpha
ligand, inhibits growth of human ovarian cancer. Mol Cancer Ther.
6:1379–1386. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Yang J and Weinberg RA:
Epithelial-mesenchymal transition: at the crossroads of development
and tumor metastasis. Dev Cell. 14:818–829. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Alshenawy HA: Immunohistochemical
expression of epidermal growth factor receptor, E-cadherin, and
matrix metalloproteinase-9 in ovarian epithelial cancer and
relation to patient deaths. Ann Diagn Pathol. 14:387–395. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Wakui M, Yokoyama Y, Wang H, Shigeto T,
Futagami M and Mizunuma H: Efficacy of a methyl ester of
5-aminolevulinic acid in photodynamic therapy for ovarian cancers.
J Cancer Res Clin Oncol. 136:1143–1150. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Ismail E, Al-Mulla F, Tsuchida S, et al:
Carbonyl reductase: a novel metastasis-modulating function. Cancer
Res. 60:1173–1176. 2000.PubMed/NCBI
|
|
21
|
Cano A, Pérez-Moreno MA, Rodrigo I, et al:
The transcription factor snail controls epithelial-mesenchymal
transitions by repressing E-cadherin expression. Nat Cell Biol.
2:76–83. 2000. View
Article : Google Scholar : PubMed/NCBI
|
|
22
|
Li LN, Zhou X, Gu Y and Yan J: Prognostic
value of MMP-9 in ovarian cancer: a meta-analysis. Asian Pac J
Cancer Prev. 14:4107–4113. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Ozalp S, Tanir HM, Yalcin OT, Kabukcuoglu
S, Oner U and Uray M: Prognostic value of matrix
metalloproteinase-9 (gelatinase-B) expression in epithelial ovarian
tumors. Eur J Gynaecol Oncol. 24:417–420. 2003.PubMed/NCBI
|
|
24
|
Sillanpää S, Anttila M, Voutilainen K, et
al: Prognostic significance of matrix metalloproteinase-9 (MMP-9)
in epithelial ovarian cancer. Gynecol Oncol. 104:296–303. 2007.
View Article : Google Scholar
|
|
25
|
Cowden Dahl KD, Symowicz J, Ning Y, et al:
Matrix metalloproteinase 9 is a mediator of epidermal growth
factor-dependent e-cadherin loss in ovarian carcinoma cells. Cancer
Res. 68:4606–4613. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Yokoyama Y, Charnock-Jones DS, Licence D,
et al: Vascular endothelial growth factor-D is an independent
prognostic factor in epithelial ovarian carcinoma. Br J Cancer.
88:237–244. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Tammela T and Alitalo K:
Lymphangiogenesis: molecular mechanisms and future promise. Cell.
140:460–476. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Abbasi MM, Monfaredan A, Hamishehkar H,
Seidi K and Jahanban-Esfahlan R: Novel DOX-MTX nanoparticles
improve oral SCC clinical outcome by down regulation of lymph
dissemination factor VEGF-C expression in vivo: oral and IV
modalities. Asian Pac J Cancer Prev. 15:6227–6232. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Ikeda K, Oki E, Saeki H, et al:
Intratumoral lymphangiogenesis and prognostic significance of VEGFC
expression in gastric cancer. Anticancer Res. 34:3911–3915.
2014.PubMed/NCBI
|
|
30
|
Gyftopoulos K, Lilis I, Kourea H and
Papadaki H: The expression of vascular endothelial growth factor-C
correlates with lymphatic microvessel density and lymph node
metastasis in prostate carcinoma: an immunohistochemical study.
Urol Ann. 6:224–230. 2014. View Article : Google Scholar
|
|
31
|
Peppicelli S, Bianchini F and Calorini L:
Inflammatory cytokines induce vascular endothelial growth factor-C
expression in melanoma-associated macrophages and stimulate
melanoma lymph node metastasis. Oncol Lett. 8:1133–1138.
2014.PubMed/NCBI
|