Introduction
Lung cancer is the leading cause of cancer-related
death worldwide. More than 80% of lung cancers are non-small cell
lung cancers (NSCLCs), and lung adenocarcinoma is the most common
type of NSCLC. The median survival of patients with metastatic
NSCLC treated with cytotoxic chemotherapy agents is 10–12 months
(1,2).
Epidermal growth factor receptor (EGFR), a member of
the family of growth factor receptor tyrosine kinases, is expressed
in a variety of solid cancers. EGFR somatic mutations were
identified in 5–40% of NSCLCs, and is especially common in
never-smokers, women, Asians, and patients with adenocarcinoma
(3–6). NSCLCs harboring-activated EGFR
mutations are addicted to EGFR signaling, and treatment with
small-molecule EGFR-tyrosine kinase inhibitors (TKIs) such as
gefitinib and erlotinib demonstrated dramatic responses to lung
adenocarcinomas with EGFR mutations (7,8).
However, almost all lung adenocarcinoma patients with EGFR
mutations who respond to EGFR-TKIs ultimately develop resistance to
these agents. Therefore, to prolong the survival time of advanced
NSCLC patients with EGFR mutations, conventional cytotoxic
chemotherapy is necessary regardless of whether it is administered
before or after treatment with EGFR-TKIs.
At present, the combination of platinum with one of
several chemotherapeutic agents [docetaxel, paclitaxel,
gemcitabine, vinorelbine (VNR), irinotecan, pemetrexed or
FT-5-chloro-2,4-dihydroxypyridine-potassium oxonate (S-1)] is
considered a standard chemotherapy for advanced NSCLC (1,2,9,10).
However, non-platinum combinations of third-generation drugs such
as gemcitabine + VNR have less toxicity and almost equivalent
efficacy compared to platinum-based chemotherapy (11). Therefore, non-platinum combination
chemotherapy can be an option as a first-line treatment, even in
patients with advanced NSCLC harboring EGFR mutations.
VNR, which is widely used to treat solid tumors such
as NSCLC and breast cancer, is a semisynthetic vinca-alkaloid
derived from vinblastine. This chemotherapeutic agent is one of the
standard treatment agents for elderly patients with NSCLC (12), and, in combination with cisplatin,
VNR is the only third-generation drug that demonstrated a
consistent improvement of survival in the adjuvant setting of
resected NSCLC (13–15).
UFT is an oral anticancer agent combining tegafur
(FT) and uracil at a molar ratio of 1:4. FT is a prodrug of
5-fluorouracil (5-FU), and uracil is a competitive and reversible
inhibitor of dihydropyrimidine dehydrogenase (DPD), the
rate-limiting enzyme responsible for the catabolism of 5-FU. S-1 is
a novel oral fluorouracil antitumor drug that combines FT,
5-chloro-2,4-dihydroxypyridine (which inhibits DPD activity), and
potassium oxonate (which reduces gastrointestinal toxicity). UFT
and S-1 are referred to as dehydrogenase-inhibitory
fluoropyrimidine (DIF).
UFT is effective in prolonging the survival of
patients with NSCLC after surgical resection (16,17).
In a recent phase III trial, the combination chemotherapy of S-1
with carboplatin was not inferior in terms of overall survival (OS)
compared with a standard chemotherapy, carboplatin and paclitaxel,
for patients with advanced NSCLC (9). These results suggest the potential of
DIF as a chemotherapeutic agent for advanced NSCLC.
We reported the schedule-dependent synergistic
effect of VNR and subsequent 5-FU or UFT on NSCLC in vitro
and in an animal model (18).
Based on these preclinical data, we conducted two phase II studies
of VNR + DIF, under which VNR was infused on days 1 and 8, and 600
mg/day UFT or 80 mg/m2/day S-1 was administered daily
from day 2 to 6 and from day 9 to 13 in a 3-week cycle. The
combination therapy of VNR + UFT was shown to be feasible and
active in the treatment of elderly patients with advanced NSCLC
(19). Promising results were also
observed in unselected advanced NSCLC patients treated with the
combination of VNR + S-1 (20).
In the process of clinical trials and clinical
practice applying the combination treatment of VNR + DIF for
advanced NSCLC, we noticed that patients exhibiting long-term
stable disease tended to harbor EGFR mutations. This finding
raised a hypothesis that the combination treatment of VNR + DIF may
be specifically effective in NSCLC patients with EGFR
mutations.
In the present study, we retrospectively compared
the efficacy of the combination treatment of VNR + DIF with that of
the standard platinum-based chemotherapy in patients with lung
adenocarcinoma harboring EGFR mutations. We also sought to
identify the mechanism by which the combination chemotherapy of VNR
+ DIF was more favorable than platinum-based chemotherapy in NSCLC
harboring EGFR mutations in vitro.
Materials and methods
Comparison of the effects of
chemotherapies
We retrospectively reviewed 39 lung adenocarcinoma
patients harboring EGFR mutations who were diagnosed from
November, 2004 to March, 2013 at Tottori University Hospital in
Yonago, Japan and who received the combination therapy of VNR + DIF
or platinum-based chemotherapy for the first cytotoxic
chemotherapy. The presence of EGFR mutation was evaluated by
the polymerase chain reaction (PCR)-invader method (BML, Inc.,
Tokyo, Japan). EGFR mutation analyses were not performed in
four cases. These patients achieved long-term progression-free
survival (PFS) times of >6 months with gefitinib treatment. The
PFS was <6 months in >95% of the EGFR
mutation-negative patients (21).
Thus, we considered these four patients as EGFR
mutation-positive cases.
The differences between the two groups were compared
by the Mann-Whitney test and χ2 or Fisher’s exact test
for numerical and categorized data, respectively. Tumor response
was evaluated according to the Response Evaluation Criteria in
Solid Tumors (RECIST) (22). The
OS and PFS times following the first-line cytotoxic chemotherapy
was assessed using the Kaplan-Meier method and compared by the
log-rank test. P<0.05 was considered significant.
Chemicals and reagents
VNR (Kyowa Hakko Kirin Co., Ltd., Tokyo, Japan) was
dissolved in distilled water and stored at −20°C. A stock solution
of cisplatin (CDDP) (Nippon Kayaku Co., Ltd., Tokyo, Japan) was
reconstituted with water, diluted in 0.9% sodium chloride solution,
and stored at −20°C. Gefitinib (AstraZeneca, Cheshire, UK) and 5-FU
(KyowaHakko Kirin Co., Ltd.) were dissolved in dimethyl sulfoxide
and stored at −20°C.
3-(4,5-Dimethylhiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT)
(Wako Pure Chemical Industries, Ltd., Osaka, Japan) was dissolved
in phosphate-buffered saline (PBS) and stored at −20°C.
Cell lines and cultures
The human NSCLC cell line PC9, which harbors an
EGFR exon 19 deletion mutation (ΔE746-A750) (23) was obtained from the RIKEN
BioResource Center (Ibaraki, Japan). The fibroblast cell line 1BR3,
stably transfected with a mutant EGFR construct with an
L858R replacement in exon 21 (1BR3-LR), was a generous gift from Dr
David J. Chen (24). The PC9 cells
were maintained in RPMI-1640 medium supplemented with 10% fetal
bovine serum (FBS) and antibiotics (100 U/ml penicillin and 100
μg/ml streptomycin). 1BR3-LR cells were maintained in MEM-α medium
supplemented with 10% FBS and antibiotics (100 U/ml penicillin, 100
μg/ml streptomycin, and 2 μg/ml blasticidin). These cells were
grown in a humidified atmosphere of 5% CO2/95% air at
37°C.
MTT assay
The cell growth inhibition by chemotherapeutic
agents was determined by an MTT assay. Cells counted with a
hematocytometer were plated in 96-well flat-bottom multiplates
(Nalge Nunc International Corp., Rochester, NY, USA) in 100 μl of
medium and incubated overnight to permit cell attachment. The
medium was then removed from each well and replaced with 100 μl
medium containing the drugs for the indicated time. After 72 h, 10
μg of MTT in 10 μl PBS was added to each well, and incubation was
continued for an additional 4 h. Thereafter, 100 μl of 0.04 N HCl
in 2-propanol was added, and the multiplates were incubated
overnight to solubilize the MTT formazan crystal. The absorbance of
each well was measured at 570 nm wavelength (reference 650 nm)
using a Tecan Sunrise scanning multiwell spectrometer (Tecan Japan
Co., Ltd., Kanagawa, Japan). Each experiment was performed in
triplicate for each drug concentration and was independently
performed three times.
Immunoprecipitation and western blot
analysis
Cells were incubated in 6-well tissue culture plates
overnight and washed with ice-cold PBS and lysed in lysis buffer
[1% NP-40, 0.25% sodium deoxycholate, 150 mM NaCl, pH 7.4, 50 mM
Tris-HCl, 1 mM EDTA, 1 mM NaF, 1 mM sodium orthovanadate
(Na3VO4)] including 1 mM phenylmethylsulfonyl
fluoride, 1 μg/ml leupeptin, 1 μg/ml aprotinin, and 1 μg/ml
pepstatin. After 5 min on ice, lysates were centrifuged at 13,000 ×
g for 10 min at 4°C, and the supernatant was then collected.
Protein was measured using the Bio-Rad Protein Assay reagent
(Bio-Rad Laboratories, Hercules, CA, USA), and protein lysates
containing 20 μg of total cellular protein or immunoprecipitates
with the indicated antibodies were subjected to discontinuous
sodium dodecyl sulfate-polyacrylamide gel electrophoresis
(SDS-PAGE).
Proteins were electrotransferred to a polyvinylidene
fluoride (PVDF) membrane (GE Healthcare Japan, Tokyo, Japan) for 60
min at 4°C at 100 V. Non-specific binding was blocked by incubation
with 5% non-fat milk in Tris-buffered saline containing 0.1%
Tween-20 (TBST) for 1 h at room temperature. After blocking, the
membrane was incubated in primary antibody (1X PBST containing 1%
milk, 1:2,000) overnight at 4°C. The membrane was then washed three
times with PBST. The immunoblots were incubated for 1 h in a
1:10,000 dilution of goat anti-rabbit or anti-mouse IgG coupled
with horseradish peroxidase as a secondary antibody (GE Healthcare
Japan) in TBST containing 1% milk.
Finally, each protein was detected using an enhanced
chemiluminescence detection system (ECL prime) and captured with an
ImageQuant LAS 400 (both from GE Healthcare Japan). The antibody
against EGFR was purchased from Santa Cruz Biotechnology, Inc.
(Santa Cruz, CA, USA). Anti-phosphotyrosine antibody (4G10) was
purchased from Merck Millipore (Darmstadt, Germany), and
anti-β-actin antibody was purchased from Sigma-Aldrich Japan
(Tokyo, Japan).
Assessment of combination effect
A combination index (CI) was calculated using the
Chou-Talalay method (25) and used
to evaluate the combination effect of the two drugs. The CI
quantitatively depicts synergism (CI<1), addictive effect
(CI=1), and antagonism (CI>1).
Results
The characteristics of patients and
efficacy of VNR + DIF and platinum-based chemotherapy
A total of 39 patients were included in this
retrospective study. The ages of the 39 patients ranged from 35 to
84 years (median age, 65 years), with 16 males (41.0%) and 23
females (59%). All tumors were adenocarcinomas, and 31 patients had
stage IV disease (79.5%). Seven patients received gefitinib prior
to cytotoxic chemotherapy.
At the first cytotoxic chemotherapy, 24 patients
received VNR + DIF chemotherapy (VNR + UFT, n=5; VNR + S-1, n=19)
and the other 15 patients received platinum-based chemotherapy. Of
the 15 patients in the platinum group, eight patients received
CDDP-based chemotherapy (CDDP + gemcitabine, n=4; CDDP + docetaxel,
n=4), and the seven others received carboplatin-based chemotherapy
(carboplatin + paclitaxel, n=5; carboplatin + pemetrexed, n=1;
carboplatin + gemcitabine, n=1).
Table I shows the
patient characteristics according to the first-line chemotherapy
regimen (VNR + DIF vs. platinum). There was no significant
difference between the two regimen groups with regard to age,
gender, disease stage, smoking status, EGFR mutation type, Eastern
Cooperative Oncology Group (ECOG) performance status (PS), and
chemotherapy line. As a later line of cytotoxic chemotherapy, seven
(29.2%) patients in the VNR + DIF group received platinum-based
chemotherapy, and four (26.7%) patents in the platinum group
received VNR + DIF treatment.
 | Table ICharacteristics of the 39 lung
adenocarcinoma patients harboring EGFR mutations. |
Table I
Characteristics of the 39 lung
adenocarcinoma patients harboring EGFR mutations.
| VNR + DIF
(n=24) | Platinum
(n=15) | P-value |
|---|
| Age (years) | | | 0.31a |
| Median
(range) | 66.5 (50–84) | 64 (35–74) | |
| Sex | | | 0.92b |
| Male | 10 | 6 | |
| Female | 14 | 9 | |
| Disease stage | | | 0.84c |
| IIIA | 1 | 0 | |
| IIIB | 3 | 2 | |
| IV | 19 | 12 | |
| Recurrence | 1 | 1 | |
| Histology |
|
Adenocarcinoma | 24 | 15 | |
| Smoking status | | | 0.74c |
| Current | 3 | 3 | |
| Former | 5 | 4 | |
| Never | 16 | 8 | |
| EGFR mutation
type | | | 0.41c |
| Exon 19
deletion | 13 | 6 | |
| Exon 21 point
mutation | 7 | 7 | |
| Minor
mutation | 2 | 0 | |
| Unknown | 2 | 2 | |
| Performance
status | | | 0.44c |
| 0 | 14 | 8 | |
| 1 | 10 | 6 | |
| 2 | 0 | 1 | |
| Chemotherapy
line | | | 0.87b |
| First-line | 20 | 12 | |
| Second-line
(gefitinib as first-line) | 4 | 3 | |
Both the objective response rate (ORR) and the
disease control rate (DCR) of the VNR + DIF patients were favorable
compared with those of the platinum group, although the differences
were not significant (54.2 vs. 42.9%, p=0.74 and 87.5 vs. 71.4%,
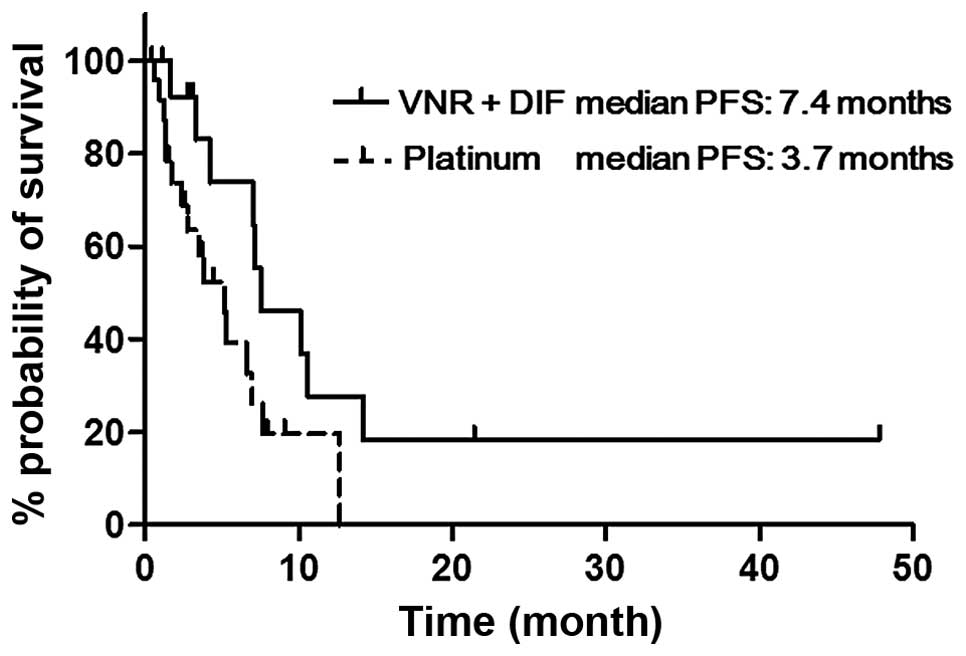
p=0.39; Table II). Fig. 1 shows the Kaplan-Meier curves for
PFS in the VNR + DIF and platinum groups. The median PFS of the VNR
+ DIF group was significantly longer than that of the platinum
group (7.4 vs. 3.7 months, p=0.02). The median OS was not
significantly different between the two groups (36.6 vs. 35.4
months, p=0.34; Table II).
 | Table IIComparison of efficacy parameters
between the combination of VNR + DIF and platinum-based
chemotherapy. |
Table II
Comparison of efficacy parameters
between the combination of VNR + DIF and platinum-based
chemotherapy.
| Confidence interval
(95%) | VNR + DIF
(n=24) | Platinum
(n=15) | P-value |
|---|
| ORR | 54.2
(32.0–76.4) | 42.9
(29.6–56.1) | 0.74a |
| DCR | 87.5
(80.7–94.3) | 71.4
(59.4–83.5) | 0.39a |
| mPFS (months) | 7.4 (6.2–8.7) | 3.7 (2.9–4.6) | 0.02b |
| mOS (months) | 36.6
(27.2–46.0) | 35.4
(31.0–39.7) | 0.34b |
The cell growth inhibition and effect of
VNR, CDDP and 5-FU on EGFR phosphorylation in PC9 cells
Based on the results of the retrospective study, we
speculated that VNR or DIF may have an effect on EGFR activity. To
address this speculation, we performed in vitro experiments
using PC9 cells harboring an active form of EGFR
mutation.
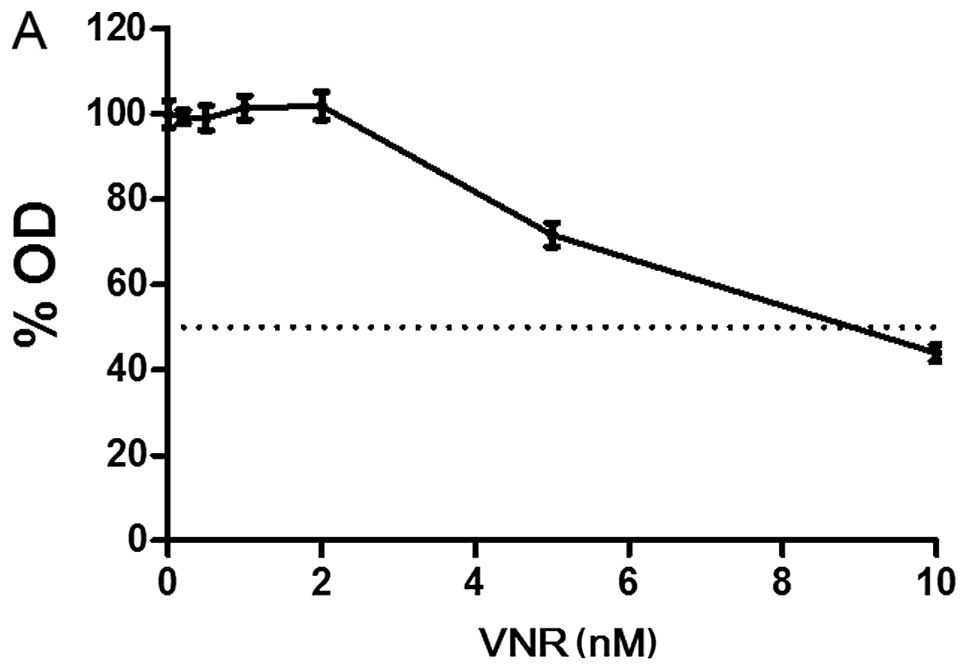
We first evaluated the sensitivity of PC9 cells to
VNR, 5-FU, and CDDP. The half-life of VNR in plasma after
intravenous injection is ~20 h (26), and CDDP is almost completely
eliminated within 24 h from plasma (27). In previous studies, DIF was orally
administered to patients for 5 days in the combination of VNR + DIF
(19,20), and the 5-FU concentration in plasma
stayed roughly constant during an oral intake of DIF (28,29).
We therefore exposed PC9 cells to VNR, CDDP and 5-FU for 24, 24 and
72 h, respectively, and 72 h after the start of drug exposure, we
performed an MTT assay to evaluate the inhibition of cell
proliferation. The concentration of VNR producing a 50% inhibition
of cell growth (IC50) was 8.1 nM, that of CDDP was 0.59
μM, and that of 5-FU was 13.8 μM (Fig.
2), and these are clinically achievable concentrations
(26–29).
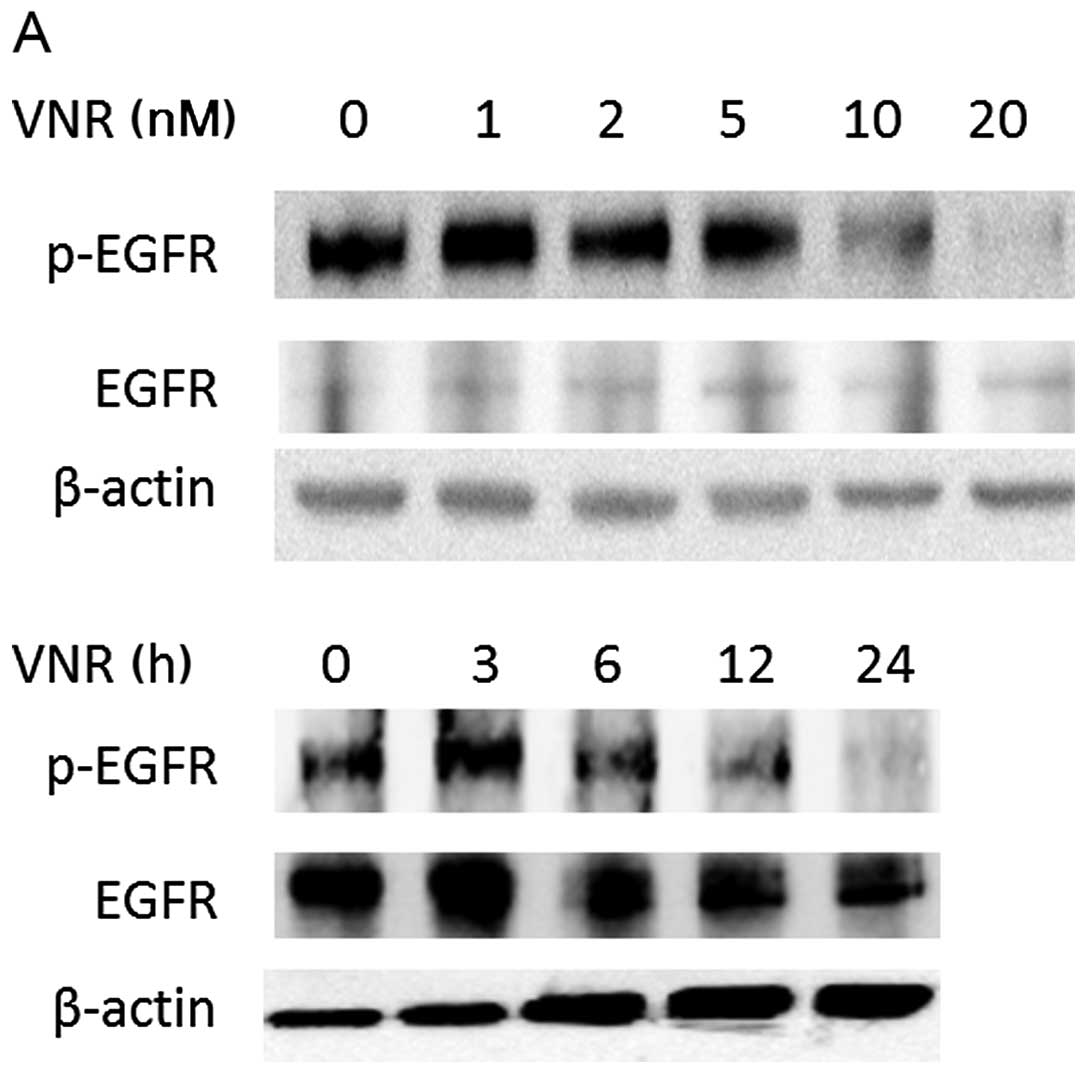
We evaluated the phosphorylation of EGFR after the
treatment with each drug at the concentrations up to ~2-fold higher
than the IC50. After the treatment with a 10 nM or
higher concentration of VNR for 24 h, the phosphorylation of EGFR
was clearly decreased. In the PC9 cells, this EGFR
dephosphorylation induced by VNR appeared 12–24 h after the start
of the exposure to 20 nM VNR (Fig.
3A), whereas such dephosphorylation of EGFR was not detected in
the 24-h treatment with 5-FU or CDDP at the concentrations tested
(Fig. 3B and C).
The cell growth inhibition and effects of
gefitinib, VNR, CDDP and 5-FU on EGFR phosphorylation in 1BR3-LR
cells
To elucidate whether the suppression of EGFR
phosphorylation induced by VNR functions as an anti-proliferative
mechanism of VNR, we used 1BR3 cells (in which EGFR is not
expressed), stably transfected with the L858R mutant EGFR
(1BR3-LR).
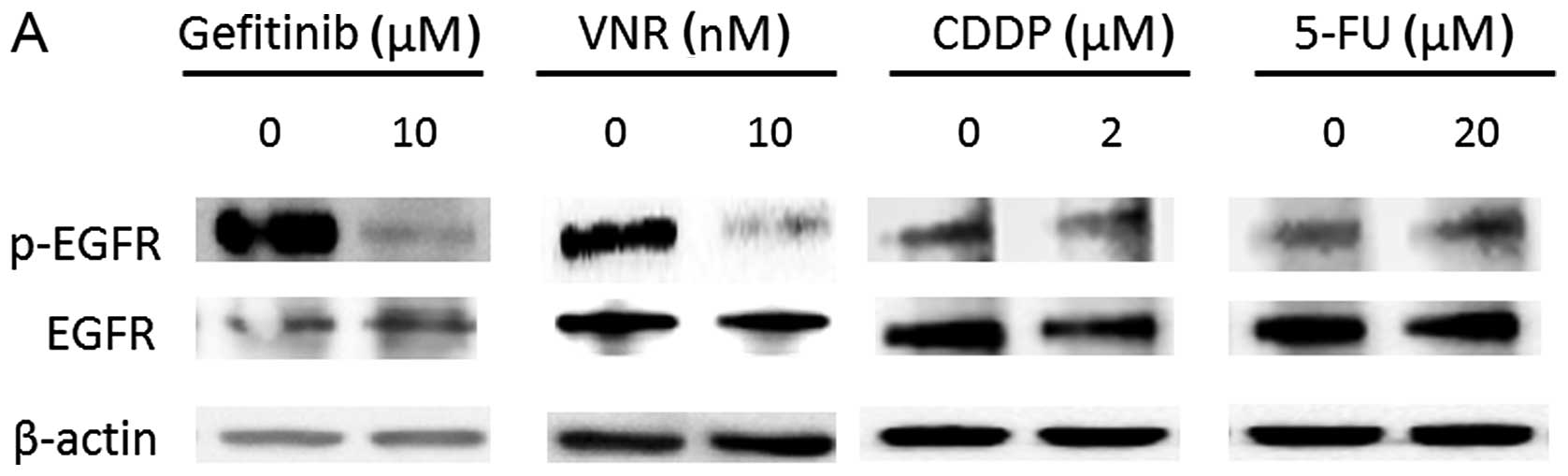
We determined the effects of gefitinib, VNR, CDDP
and 5-FU on EGFR phosphorylation in 1BR3-LR cells. As shown in
Fig. 4A, the treatment with 10 nM
VNR for 24 h suppressed EGFR phosphorylation as well as gefitinib
did, a selective EGFR-TKI in 1BR3-LR cells. Similar to the PC9
cells, neither CDDP nor 5-FU induced the dephosphorylation of
EGFR.
 | Figure 4Effects of vinorelbine (VNR),
cisplatin (CDDP), and 5-fluorouracil (5-FU) on epidermal growth
factor receptor (EGFR) phosphorylation and cell growth inhibition
in 1BR3-LR cells. (A) 1BR3-LR cells were treated with the indicated
concentrations of gefitinib, VNR, CDDP, or 5-FU for 24 h. Total
cellular protein (1 mg) from cell lysate was immunoprecipitated
using anti-EGFR antibody and subjected to a western blot analysis
with anti-phosphotyrosine (p-EGFR, upper panel), and the membrane
was stripped of bound antibodies and re-probed with anti-EGFR
antibody (middle panel). Total cellular protein (20 μg) of the same
lysate was subjected to a western blot analysis with β-actin (lower
panel). (B–E) 1BR3-LR cells were treated with the indicated
concentrations of gefitinib, VNR, CDDP, or 5-FU for 72 h in the
medium containing 10% (solid line) or 0.5% (dotted line) fetal
bovine serum (FBS). The survival cell fraction is expressed as the
percentage of optical density (% OD) in reference to the OD of the
untreated cells in an
3-(4,5-dimethylhiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT)
assay. Data are presented as means ± SD of three separate
experiments. |
We evaluated the cell growth inhibition by these
drugs in 1BR3-LR cells. In 1BR3-LR cells cultured in 10%
FBS-containing medium, gefitinib slightly promoted cell growth,
although it effectively suppressed EGFR phosphorylation. Gefitinib
inhibited the cell growth concentration dependently in the medium
containing 0.5% FBS (Fig. 4B),
indicating that the proliferation or survival of 1BR3-LR cells is
dependent on EGFR-mediated signaling in low-serum condition.
We compared the growth inhibitory activities of VNR,
5-FU, and CDDP in 1BR3-LR cells between normal (10%) and low (0.5%)
serum conditions, and we found that the cell growth inhibition by
VNR was enhanced in the low-serum condition compared to that in the
normal-serum condition (Fig. 4C).
The sensitivity of 1BR3-LR cells to CDDP did not clearly differ by
serum concentration (Fig. 4D). In
the low-serum condition, 1BR3-LR cells tended to be resistant to
5-FU-induced cell growth inhibition (Fig. 4E).
The effect of
Na3VO4 on EGFR phosphorylation and gefitinib-
and VNR-induced cell growth inhibition
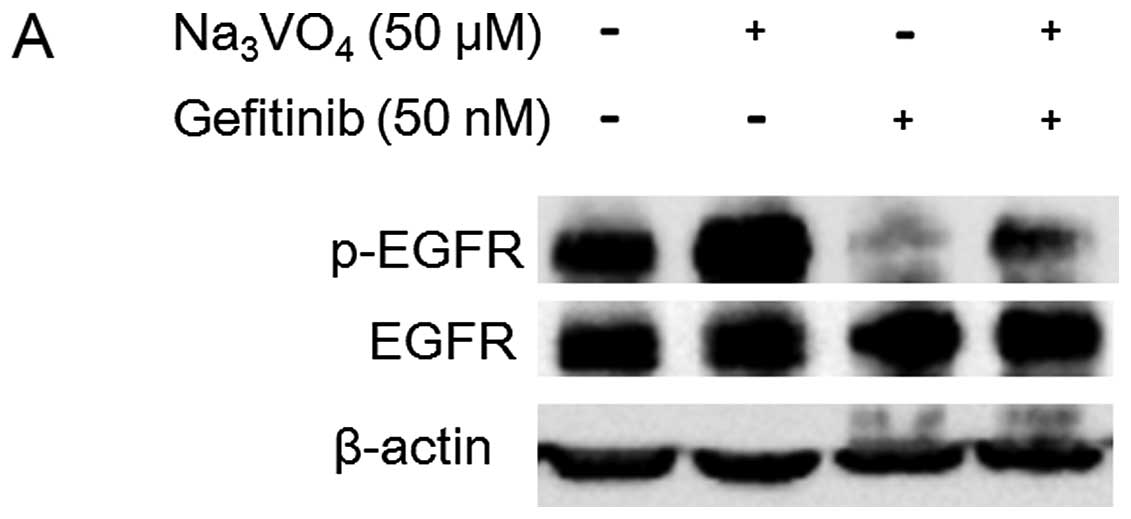
To further test whether the EGFR dephosphorylation
induced by VNR was related to anti-proliferative effect of VNR, we
tested whether Na3VO4, an inhibitor of
protein tyrosine phosphatases, can interfere with the gefitinib- or
VNR-induced dephosphorylation of EGFR and affect the cell growth
inhibition by gefitinib or VNR in PC9 cells. We treated PC9 cells
with 50 nM gefitinib or 20 nM VNR in the presence or absence of 50
μM Na3VO4 for 24 h and then evaluated the
EGFR phosphorylation. The EGFR dephosphorylation caused by
gefitinib or VNR was clearly inhibited in the presence of
Na3VO4 (Fig. 5A
and B).
The cell growth inhibition of PC9 cells by gefitinib
or VNR was compared in the presence or absence of
Na3VO4. As shown in Fig. 5C and D, the cell growth inhibitory
activity of both gefitinib and VNR was greatly interfered with by
Na3VO4.
Synergistic cell growth inhibition by the
combination of gefitinib or VNR with 5-FU in PC9 cells
In our previous study, the combination treatment of
VNR and subsequent 5-FU synergistically inhibited cell growth in
three NSCLC cell lines (18). In
the present study, to reproduce this synergism and to clarify
whether EGFR suppression by VNR is related to this interaction, we
evaluated the combination effects using the CI and the simultaneous
combination of gefitinib and 5-FU, or the sequential treatment of
VNR followed by 5-FU. Since gefitinib suppressed EGFR activity
within 1 h in vitro (6),
gefitinib and 5-FU were combined simultaneously.
We treated PC9 cells with the indicated
concentrations of gefitinib + 5-FU for 72 h or VNR for 24 h and
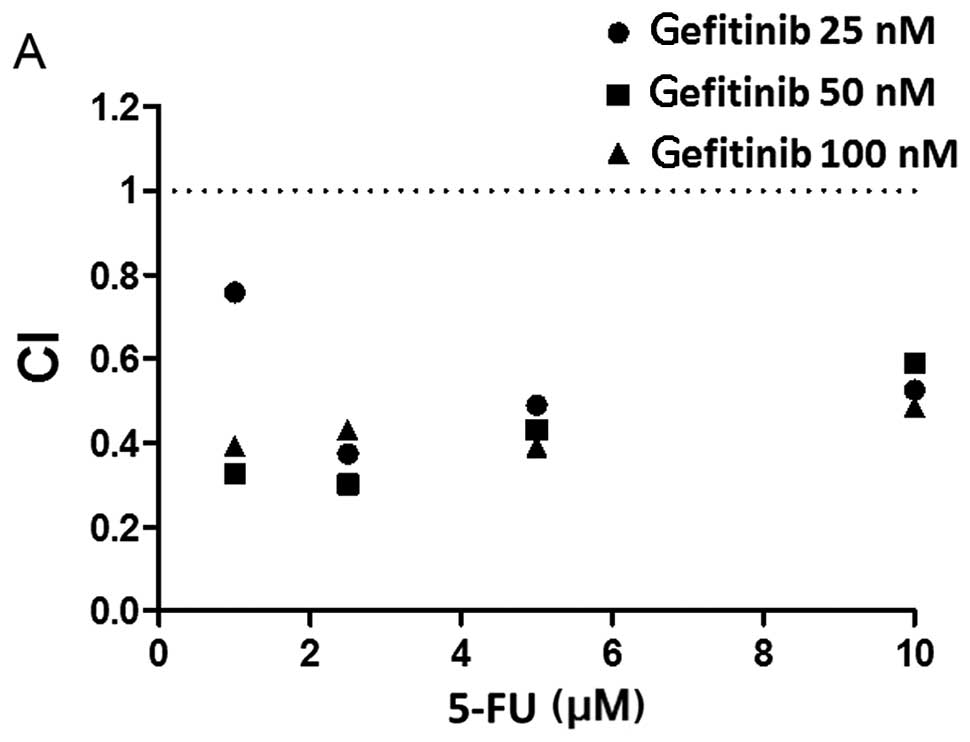
5-FU for the following 72 h, and we calculated the CI (Fig. 6). As shown in the Fig., the CI
values for the combination of gefitinib and 5-FU were all <1.0,
indicating that this simultaneous combination showed synergistic
cell growth inhibitory activity against PC9 cells. Similar results
were achieved for sequential exposure to VNR followed by 5-FU with
CI<0.3, which implied strong synergism (Fig. 6B).
Discussion
The aim of this study was to evaluate whether the
combination of VNR + DIF is a more effective treatment compared
with the standard platinum-based chemotherapy in
EGFR-mutated lung adenocarcinoma patients, and then to
clarify the underlying mechanism by which VNR + DIF was efficacious
in such patients. In the retrospective analysis, the PFS of the
patients who received VNR + DIF chemotherapy was longer than that
of the patients who received platinum-based chemotherapy. Using
mutated EGFR-expressing cells, we found that VNR induced EGFR
dephosphorylation and that this effect of VNR may be related to its
cell growth inhibitory activity. We propose that EGFR inhibition by
VNR may be one of the mechanisms of the synergistic effect by the
sequential treatment of VNR and subsequent 5-FU.
In this retrospective study, the characteristics of
the patients who received VNR + DIF chemotherapy were not
significantly different from those who received platinum-based
chemotherapy. Nevertheless, the PFS of the VNR + DIF treatment
group was significantly longer than that of the platinum-based
chemotherapy group. The RR and DCR values of the VNR + DIF
chemotherapy patients also tended to be better than those of the
platinum-based chemotherapy patients, although the difference was
not significant. These results suggest that the combination of VNR
+ DIF may be more effective than platinum-based chemotherapy, at
least in terms of the antitumor effect in lung adenocarcinomas with
EGFR-activating mutations.
Despite the significant difference in PFS, the OS of
the present two regimen groups was not significantly different.
Over one-quarter of the patients in each group crossed over to the
other regimen as a later-line treatment. The comparison of OS was
performed between small groups (n=24 for VNR + DIF, n=15 for
platinum), and thus the statistical power was low. We suspect that
the lack of a significant difference in OS was due to these
reasons. In a proportional hazard analysis performed in another
study, we found that the application of the VNR + DIF combination
but not platinum-based chemotherapy was a significant and
independent factor to prolong survival in lung adenocarcinoma
patients with EGFR mutations (unpublished data). These
results suggest that VNR + DIF chemotherapy may be superior to
platinum-based chemotherapy in the treatment of lung adenocarcinoma
patients with EGFR mutations.
To clarify the mechanisms by which VNR + DIF
chemotherapy was favorable in the treatment of EGFR-mutated
lung adenocarcinoma, we focused on the effects of VNR and 5-FU on
EGFR phosphorylation. In EGFR-mutated PC9 cells, VNR induced
EGFR dephosphorylation 12–24 h after drug exposure at the
concentration of 10 nM or higher. In the treatment of NSCLC, when
20–30 mg/m2 of VNR is administered, a VNR concentration
>10 nM is maintained in peripheral blood for 12–24 h (26). Thus, an EGFR-dephosphorylating
concentration of VNR is clinically achievable.
The sufficiently cell growth-inhibiting and
clinically relevant concentration of CDDP and 5-FU (27–29)
did not affect the EGFR phosphorylation in PC9 cells. Our
observation in terms of EGFR dephosphorylation by VNR is in accord
with the result of a previous investigation. Wu et al
reported that in esophageal cancer cells, the disruption of the
microtubule network induced by microtubule-targeting drugs such as
docetaxel and vincristine, another vinca-alkaloid, was associated
with EGFR dephosphorylation and the subsequent inhibition of Akt
and Erk (30). VNR is a
semisynthetic vinca-alkaloid, a member of the family of
microtubule-targeting drugs. Although the precise mechanism is
still unknown, EGFR-suppressing activity may thus be a common
property among taxanes and vinca-alkaloids.
To test whether the EGFR dephosphorylation induced
by VNR is associated with its anti-proliferative effect, we took
advantage of 1BR3-LR cells, which express an active form of EGFR.
Parental 1BR3 cells do not express EGFR. Although we observed that
1BR3-LR cells were completely resistant to gefitinib in normal
culture medium containing 10% FBS, gefitinib showed cell growth
inhibition against 1BR3-LR cells in the medium containing 0.5% FBS.
These results indicate that the growth or survival of 1BR3-LR cells
is at least partially dependent on EGFR signaling in a low-serum
condition.
We also found that the growth inhibition of 1BR3-LR
cells by VNR was enhanced in the low-serum condition, although such
changes of drug sensitivity were not observed in CDDP- or
5-FU-treated cells. These findings strongly support the
interpretation that the enhanced sensitivity to VNR in the
low-serum condition is not a non-specific effect but rather is due
to the suppression of EGFR signaling, since both gefitinib and VNR
(and not CDDP or 5-FU) suppressed EGFR phosphorylation.
This interpretation is further supported by our
finding that Na3VO4 interfered with the EGFR
dephosphorylation induced by gefitinib and VNR, and suppressed the
cell growth inhibition by these agents in PC9 cells. Taken
together, our results led us to conclude that VNR-induced EGFR
dephosphorylation is associated with the anti-proliferative effect
of VNR in lung adenocarcinoma cell lines harboring EGFR
mutations.
We found previously that the combination of VNR
followed by 5-FU resulted in synergistic cell growth inhibition in
three NSCLC cell lines (18). The
synergism was also observed in PC9 cells harboring an EGFR
mutation with the sequential treatment of VNR and then 5-FU.
Therefore, although it still remains to be determined whether the
EGFR suppression by VNR itself may lead to a better antitumor
effect of VNR in EGFR-mutated lung adenocarcinoma, it is
possible that this synergism also contributed to the favorable
antitumor activity observed in patients treated with VNR + DIF.
In addition, as in an earlier study (31), the simultaneous combination of
gefitinib and 5-FU showed synergistic cell growth inhibition in PC9
cells in the present study. Therefore, the synergism of VNR
followed by 5-FU may be attributable, at least in part, to the
EGFR-suppressing activity of VNR.
The important therapeutic target of 5-FU is
thymidylate synthase (TS), and the downregulation of TS would be
expected to enhance the cytotoxicity of 5-FU (32). EGFR signal transduction has been
shown to be involved in the expression of TS genes (33,34),
and in our previous study, VNR as well as gefitinib was shown to
suppress TS expression (18).
Thus, the decrease of TS caused by EGFR suppression may be a common
mechanism of the synergism by the combination of VNR or gefitinib
with 5-FU.
The identification of activating mutations of the
EGFR gene in a subset of NSCLC patients led to a change in
the treatment of the disease (6),
and the presence of EGFR mutations is a predictive marker of
response to EGFR-TKI (3,4). It has been reported that the effect
of cytotoxic chemotherapy is not different between patients with
and without EGFR mutations (35,36).
Thus, the cytotoxic agents for NSCLC patients with EGFR
mutations are not different from those used for EGFR
wild-type patients. To our knowledge, there has been no prospective
study attempting to identify which agents or combination
chemotherapy is specifically effective in EGFR-mutated
NSCLC.
The identification of such cytotoxic agents or
combination chemotherapy is expected to improve the survival of
NSCLC patients harboring EGFR mutations. In the present
study, we observed favorable PFS by the combination of VNR + DIF
and the potential mechanism of this good treatment outcome. We
propose that the combination chemotherapy of VNR and DIF can be a
promising strategy for NSCLC patients harboring EGFR
mutations. Since our observations were retrospective and
experimental, there are several limitations. To establish the
optimal VNR + DIF combination chemotherapy in NSCLC patients with
EGFR mutations, we are performing a prospective phase II
trial of this treatment targeting such patients.
In conclusion, the PFS afforded by the VNR + DIF
combination treatment was significantly longer compared to that of
platinum-based chemotherapy in lung adenocarcinoma patients with
EGFR mutations. VNR suppressed EGFR phosphorylation in PC9
cells, and this activity may be related with cell growth inhibition
of VNR, and the synergistic cell growth inhibition when VNR was
combined with 5-FU. The combination chemotherapy of VNR + DIF may
be a promising treatment for NSCLC patients with EGFR
mutations.
References
|
1
|
Schiller JH, Harrington D, Belani CP, et
al: Comparison of four chemotherapy regimens for advanced
non-small-cell lung cancer. N Engl J Med. 346:92–98. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Ohe Y, Ohashi Y, Kubota K, et al:
Randomized phase III study of cisplatin plus irinotecan versus
carboplatin plus paclitaxel, cisplatin plus gemcitabine, and
cisplatin plus vinorelbine for advanced non-small-cell lung cancer:
Four-Arm Cooperative Study in Japan. Ann Oncol. 18:317–323. 2007.
View Article : Google Scholar
|
|
3
|
Paez JG, Jänne PA, Lee JC, et al: EGFR
mutations in lung cancer: correlation with clinical response to
gefitinib therapy. Science. 304:1497–1500. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Mitsudomi T and Yatabe Y: Mutations of the
epidermal growth factor receptor gene and related genes as
determinants of epidermal growth factor receptor tyrosine kinase
inhibitors sensitivity in lung cancer. Cancer Sci. 98:1817–1824.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Nana-Sinkam SP and Powell CA: Molecular
biology of lung cancer: diagnosis and management of lung cancer,
3rd ed: American College of Chest Physicians evidence-based
clinical practice guidelines. Chest. 143(Suppl 5): e30S–e39S. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Lynch TJ, Bell DW, Sordella R, et al:
Activating mutations in the epidermal growth factor receptor
underlying responsiveness of non-small-cell lung cancer to
gefitinib. N Engl J Med. 350:2129–2139. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Mitsudomi T, Morita S, Yatabe Y, et al:
Gefitinib versus cisplatin plus docetaxel in patients with
non-small-cell lung cancer harbouring mutations of the epidermal
growth factor receptor (WJTOG3405): an open label, randomised phase
3 trial. Lancet Oncol. 11:121–128. 2010. View Article : Google Scholar
|
|
8
|
Maemondo M, Inoue A, Kobayashi K, et al:
Gefitinib or chemotherapy for non-small-cell lung cancer with
mutated EGFR. N Engl J Med. 362:2380–2388. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Okamoto I, Yoshioka H, Morita S, et al:
Phase III trial comparing oral S-1 plus carboplatin with paclitaxel
plus carboplatin in chemotherapy-naïve patients with advanced
non-small-cell lung cancer: results of a west Japan oncology group
study. J Clin Oncol. 28:5240–5246. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Hanna N, Shepherd FA, Fossella FV, et al:
Randomized phase III trial of pemetrexed versus docetaxel in
patients with non-small-cell lung cancer previously treated with
chemotherapy. J Clin Oncol. 22:1589–1597. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Pujol JL, Barlesi F and Daurès JP: Should
chemotherapy combinations for advanced non-small cell lung cancer
be platinum-based? A meta-analysis of phase III randomized trials.
Lung Cancer. 51:335–345. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Gridelli C: The ELVIS trial: a phase III
study of single-agent vinorelbine as first-line treatment in
elderly patients with advanced non-small cell lung cancer. Elderly
Lung Cancer Vinorelbine Italian Study. Oncologist. 6(Suppl 1):
S4–S7. 2001. View Article : Google Scholar
|
|
13
|
Butts CA, Ding K, Seymour L, et al:
Randomized phase III trial of vinorelbine plus cisplatin compared
with observation in completely resected stage IB and II
non-small-cell lung cancer: updated survival analysis of JBR-10. J
Clin Oncol. 28:29–34. 2010. View Article : Google Scholar :
|
|
14
|
Dunant A, Pignon JP and Le Chevalier T:
Adjuvant chemotherapy for non-small cell lung cancer: contribution
of the International Adjuvant Lung Trial. Clin Cancer Res.
11:5017s–5021s. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Douillard JY, Rosell R, De Lena M, et al:
Adjuvant vinorelbine plus cisplatin versus observation in patients
with completely resected stage IB–IIIA non-small-cell lung cancer
(Adjuvant Navelbine International Trialist Association [ANITA]): a
randomised controlled trial. Lancet Oncol. 7:719–727. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Nakagawa M, Tanaka F, Tsubota N, Ohta M,
Takao M and Wada H; West Japan Study Group for Lung Cancer Surgery.
A randomized phase III trial of adjuvant chemotherapy with UFT for
completely resected pathological stage I non-small-cell lung
cancer: the West Japan Study Group for Lung Cancer Surgery (WJSG) -
the 4th study. Ann Oncol. 16:75–80. 2005. View Article : Google Scholar
|
|
17
|
Kato H, Ichinose Y, Ohta M, et al: A
randomized trial of adjuvant chemotherapy with uracil-tegafur for
adenocarcinoma of the lung. N Engl J Med. 350:1713–1721. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Matsumoto S, Igishi T, Hashimoto K, et al:
Schedule-dependent synergism of vinorelbine and 5-fluorouracil/UFT
against non-small cell lung cancer. Int J Oncol. 25:1311–1318.
2004.PubMed/NCBI
|
|
19
|
Igishi T, Shigeoka Y, Yasuda K, et al: UFT
plus vinorelbine in advanced non-small cell lung cancer: a phase I
and an elderly patient-directed phase II study. J Thorac Oncol.
4:376–382. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Kodani M, Kinoshita N, Ueda Y, Suyama H,
Sumikawa T, Makino H, Kurai J, Matsumoto S, Igishi T and Shimizu E:
Phase II study of S-1 and vinorelbine in patients with advanced
non-small cell lung cancer. Eur J Cancer. 47(Suppl 1): S6202011.
View Article : Google Scholar
|
|
21
|
Mok TS, Wu YL, Thongprasert S, et al:
Gefitinib or carboplatin-paclitaxel in pulmonary adenocarcinoma. N
Engl J Med. 361:947–957. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Eisenhauer EA, Therasse P, Bogaerts J, et
al: New response evaluation criteria in solid tumours: revised
RECIST guideline (version 1.1). Eur J Cancer. 45:228–247. 2009.
View Article : Google Scholar
|
|
23
|
Takata M, Chikumi H, Miyake N, et al: Lack
of AKT activation in lung cancer cells with EGFR mutation is a
novel marker of cetuximab sensitivity. Cancer Biol Ther.
13:369–378. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Das AK, Chen BP, Story MD, Sato M, Minna
JD, Chen DJ and Nirodi CS: Somatic mutations in the tyrosine kinase
domain of epidermal growth factor receptor (EGFR) abrogate
EGFR-mediated radioprotection in non-small cell lung carcinoma.
Cancer Res. 67:5267–5274. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Chou TC and Talalay P: Quantitative
analysis of dose-effect relationships: the combined effects of
multiple drugs or enzyme inhibitors. Adv Enzyme Regul. 22:27–55.
1984. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Khayat D, Rixe O, Brunet R, et al:
Pharmacokinetic linearity of i.v. vinorelbine from an intra-patient
dose escalation study design. Cancer Chemother Pharmacol.
54:193–205. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Dickgreber NJ, Fink TH, Latz JE, Hossain
AM, Musib LC and Thomas M: Phase I and pharmacokinetic study of
pemetrexed plus cisplatin in chemonaive patients with locally
advanced or metastatic malignant pleural mesothelioma or non-small
cell lung cancer. Clin Cancer Res. 15:382–389. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Hirata K, Horikoshi N, Aiba K, et al:
Pharmacokinetic study of S-1, a novel oral fluorouracil antitumor
drug. Clin Cancer Res. 5:2000–2005. 1999.PubMed/NCBI
|
|
29
|
Muggia FM, Wu X, Spicer D, et al: Phase I
and pharmacokinetic study of oral UFT, a combination of the
5-fluorouracil prodrug tegafur and uracil. Clin Cancer Res.
2:1461–1467. 1996.PubMed/NCBI
|
|
30
|
Wu X, Sooman L, Lennartsson J, Bergström
S, Bergqvist M, Gullbo J and Ekman S: Microtubule inhibition causes
epidermal growth factor receptor inactivation in oesophageal cancer
cells. Int J Oncol. 42:297–304. 2013.
|
|
31
|
Okabe T, Okamoto I, Tsukioka S, et al:
Synergistic antitumor effect of S-1 and the epidermal growth factor
receptor inhibitor gefitinib in non-small cell lung cancer cell
lines: role of gefitinib-induced down-regulation of thymidylate
synthase. Mol Cancer Ther. 7:599–606. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Wada Y, Yoshida K, Suzuki T, et al:
Synergistic effects of docetaxel and S-1 by modulating the
expression of metabolic enzymes of 5-fluorouracil in human gastric
cancer cell lines. Int J Cancer. 119:783–791. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Hanada N, Lo HW, Day CP, Pan Y, Nakajima Y
and Hung MC: Co-regulation of B-Myb expression by E2F1 and EGF
receptor. Mol Carcinog. 45:10–17. 2006. View Article : Google Scholar
|
|
34
|
Ginsberg D: EGFR signaling inhibits
E2F1-induced apoptosis in vivo: implications for cancer therapy.
Sci STKE. 2007:pe42007. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Lee KH, Han SW, Hwang PG, et al: Epidermal
growth factor receptor mutations and response to chemotherapy in
patients with non-small-cell lung cancer. Jpn J Clin Oncol.
36:344–350. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Takano T, Fukui T, Ohe Y, et al: EGFR
mutations predict survival benefit from gefitinib in patients with
advanced lung adenocarcinoma: a historical comparison of patients
treated before and after gefitinib approval in Japan. J Clin Oncol.
26:5589–5595. 2008. View Article : Google Scholar : PubMed/NCBI
|