Introduction
Soft tissue sarcomas are a heterogeneous group of
solid malignant tumours which represent ~1% of all new cancer cases
in Europe and the United States (1). Fibrosarcomas are rare soft tissue
sarcomas originating from the intra- and intermuscular fibrous
tissues, fascia and tendons and account for ~3% of all soft tissue
sarcomas. Therapy for fibrosarcomas should be individualised and
multimodal. The therapy of choice involves surgical resection with
a wide margin of healthy tissue, usually followed by radiation
treatment in order to decrease local recurrence (2,3).
Unfortunately, ~50% of all patients develop distant metastases and
are ineligible for surgical treatment (4,5). In
cases of advanced metastatic disease the median survival time with
and without chemotherapy treatment is <12 months (6,7). Few
agents such as doxorubicin, ifosfamide and dacarbazine have proven
to be effective in the therapy of soft tissue sarcomas (2). However, the results of these
treatments are poor and often exhibit no significant improvements
in overall survival (8).
Doxorubicin, which has been the most frequently used
chemotherapeutic agent in the treatment of soft tissue sarcomas,
demonstrates response rates of 20–30% in disseminated disease
(9,10). The combination of doxorubicin with
ifosfamide is more effective, exhibiting higher response rates than
doxorubicin alone, but is associated with severe short- and
long-term toxicities, including cardiomyopathy and bone marrow
suppression (11–13). The recently published EORTC 62012
trial which involved 455 patients with locally advanced,
unresectable or metastatic high-grade soft tissue sarcomas
concluded that an intensified therapy with doxorubicin and
ifosfamide is not suitable for palliation of advanced soft tissue
sarcomas because of the severe side-effects and should only be used
when the specific goal is tumour shrinkage (13). Further, the utility of the
first-line cytostatic doxorubicin is limited by dose-related and
cumulative myocardial toxicity, especially in elderly patients with
pre-existing cardiac disease (14). However, age is an important
determinant of sarcoma occurrence and the incidence of soft tissue
sarcomas increases dramatically at ages >50 years and above
which are naturally associated with higher prevalence of cardiac
diseases (15). To date, there are
no effective and well-tolerated cytostatics for the palliative
treatment of patients who are not suitable for aggressive
anthracycline-based chemotherapy. Hence, there is still a need for
alternative and well-tolerated compounds that exhibit
antineoplastic effects in sarcoma cells.
Within the scope of this trial, we investigated the
effects of the natural pine bark extract pycnogenol on human
fibrosarcoma cells. Pycnogenol is a brand name for an extract
obtained from the bark of the Pinus pinaster pine tree by a
standardised process. It is manufactured by Horphag Research, Ltd.
(Geneva, Switzerland) and is available as a nutritional supplement
in the United States and in Europe. Pycnogenol is primarily
composed of a mixture of flavonoids, mainly procyanidins and
phenolic acids. It is standardised to contain ~65–75% procyanidins
that consist of taxifolin, catechin and epicatechin subunits of
varying chain length (16). Other
constituents are polyphenolic monomers, cinnamic acids and their
glycosides (17).
Since pycnogenol is a naturally occurring compound
that is very well-tolerated with a high oral bioavailability, it
has been highly studied for the treatment of many diseases
including cancer (17,18). Several in vitro studies
demonstrated the anticancer activity of pycnogenol in a wide range
of malignant cell lines including leukemia, ovarian and breast
cancer cells (19–21). Moreover, pycnogenol has been
reported to alleviate adverse effects of oncologic treatment in a
clinical trial with 64 chemotherapy patients (22). Patients receiving pycnogenol during
chemotherapy treatment had a significant decreased incidence of
side-effects such as nausea, vomiting, diarrhoea and weight loss
when compared with patients from the control group.
Inspired by these findings, we examined in the
following study the apoptosis-inducing activity of pycnogenol and
its constituents on human fibrosarcoma cells.
Materials and methods
Volunteers
Ten healthy female (six) and male (four) subjects
aged 18–31 years (mean age 24.8±6 years) participated in this
study. All participants gave written informed consent. The study
was reviewed and approved by the Ethics Committee of the
BG-University Hospital Bergmannsheil, Ruhr-University Bochum,
Germany with the permit no. 3162–08.
Protocol of pycnogenol intake
After a 24-h diet free of flavonoids (no vegetables,
fruits, marmalades, tea, coffee, cocoa, wine and beer) blood
samples were taken from the 10 volunteers. Subsequently, the
volunteers received a single dose of 300 mg pycnogenol
(Pycnogenol®; Horphag Research, Ltd., London, UK) per
os with 200 ml water. Flavonoid-free diet was continued by the
volunteers for another 6 h. Blood samples were taken again 6 h
after pycnogenol intake. All blood samples were centrifuged and
plasma was aliquoted, frozen and stored at −80°C until further
analysis.
Cell line
Human fibrosarcoma cells, HT1080, were purchased
from American Type Culture Collection (ATCC) (cell line CCI 121;
Wesel, Germany) and maintained in modified Eagle’s medium (MEM) and
non-essential amino acids (NEAA) + 10% fetal bovine serum (FBS)
supplemented with 1% penicillin (100 U/ml) and streptomycin (100
μg/ml), 1% sodium pyruvate and 1% L-glutamine. The cells were
cultured in a humidified atmosphere at 37°C with 5% CO2
in 25 cm2 flasks.
Reagents
Pycnogenol was obtained from Horphag Research, Ltd.
(Geneva, Switzerland). Catechin, epicatechin and taxifolin were
obtained from Sigma-Aldrich (St. Louis, MO, USA) and dissolved in
distilled water obtaining a concentration of 11.25 ng/ml
(catechin), 6.25 ng/ml (epicatechin) and 6.25 ng/ml (taxifolin).
The concentrations of catechin and taxifolin are achievable mean
concentrations in human plasma several hours after single oral
intake of catechin and taxifolin, respectively (16,23).
There were no data available regarding pharmacokinetics and
achievable concentrations of epicatechin in human plasma after oral
intake.
Cell treatment
For every drug experiment, 80 μl of 3×106
cells/ml were placed in 6-well plates containing the medium. After
24 h, the medium was replaced and the drugs (catechin, epicatechin,
taxifolin) or diluted plasma samples were added to each well at the
above-mentioned concentrations. Different time points were chosen
to identify the possible time dependency of the effects. All
experiments were repeated for each of three consecutive
passages.
Flow cytometric analysis
At the indicated incubation time, the floating cells
were collected together with the supernatant and adherent cells,
which were harvested by trypsinisation. The cells were pelleted by
centrifugation, resuspended in 195 μl binding buffer (Bender
MedSystems, Vienna, Austria) and incubated with 5 μl Annexin V (BD
Biosciences, Heidelberg, Germany) and 10 μl propidium iodide (PI)
(Bender MedSystems) following the manufacturer’s instructions. The
cells were analysed immediately using a FACSCalibur flow cytometer
(BD Biosciences). For each measurement, 20,000 cells were counted.
Dot plots and histograms were analysed using CellQuest Pro Software
(BD Biosciences). Annexin V binds phosphatidylserine on the outer
membranes of cells, which then becomes exposed on the surface of
apoptotic cells. Thus, the Annexin V-positive cells are considered
apoptotic. PI is an intercalating agent that cannot permeate
through the cell membranes of viable or early apoptotic cells.
Therefore, PI stains only the DNA of necrotic or very late
apoptotic cells. In this study, Annexin V- and PI-positive cells
were termed necrotic. Annexin V- and PI-negative cells were counted
as viable.
Cell morphology
The morphology of the adherent and suspended cells
was examined and documented using a phase contrast Zeiss Axiovert
25 microscope (Carl Zeiss, Jena, Germany).
Statistical analysis
The results of FACS analysis were used to determine
the percentages of viable, apoptotic and necrotic cells, which are
expressed as the means ± SD from at least three independent
experiments and consecutive passages. In this study, comparisons
between the experimental groups were performed using one-way
measures of variance (one-way ANOVAs) over all time points (Tukey’s
test). Results were considered statistically significant for
p≤0.05.
Oligonucleotide microarray analysis
To identify the changes in gene expression levels
caused by the treatment with the tested substances or plasma
samples, total RNA was purified from the cells after incubation
with the appropriate agent for 6 h using a RNeasy kit from Qiagen
(Hilden, Germany) as specified by the manufacturer. RNA integrity
was assessed using an Agilent 2100 Bioanalyzer (Agilent
Technologies, Inc., Santa Clara, CA, USA). For microarray analyses,
we applied the methods previously described by Daigeler et
al (24). We used the
Affymetrix GeneChip platform, employing a standard protocol for
sample preparation and microarray hybridisation. A one-way ANOVA
model followed by Tukey’s honestly significant difference (HSD)
test was used to verify the hypothesis that there were no
differences in expression between the drug-treated and the control
group. The multiplicity correction was performed using
Benjamini-Hochberg procedure to control the false discovery rate
(FDR) at 0.05%. In a pair-wise comparison of the differentially
expressed genes between the control and the treated cells
identified by the ANOVA analyses, a subset of genes was identified
that displayed a conjoint regulation in the treated cells. Genes
were placed in this latter group if they exhibited a mean ≥2-fold
increase or decrease compared to the control cells. This subset of
genes was subjected to the GeneTrail (25) software to identify any
over-representation of genes associated with the regulatory
pathways that are represented in the Kyoto Encyclopaedia of Genes
and Genomes (KEGG) and TRANSPATH databases. Microarray data are
deposited in the GEO public database (accession no. GSE59704).
These methods fulfilled the MIAME criteria (http://www.mged.org/miame).
Results
Single applications of catechin,
epicatechin and taxifolin are not effective in reducing cell
viability of HT1080 fibrosarcoma cells
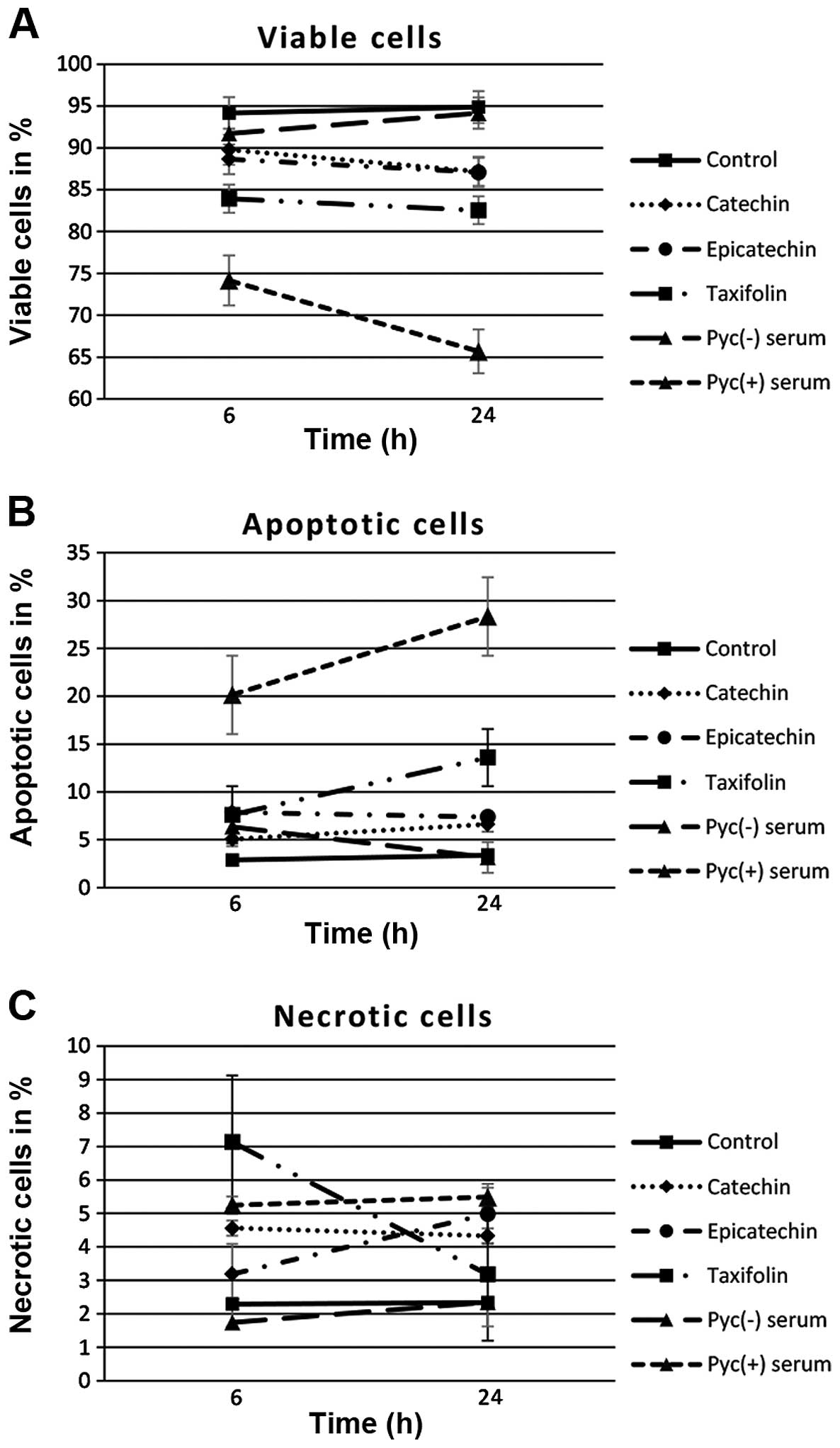
The viability of the HT1080 cells was moderately but
significantly reduced by single treatment with taxifolin (Fig. 1). A total of 82.5±1.7% (mean ± SD)
of the cells were detected as viable after 24 h treatment with
taxifolin (vs. 94.9±0.6% in the control group, p=0.001). Single
treatment with catechin led also to significant reduction of viable
cells after 24 h of incubation, but only a slight decrease in cell
viability was observed; the percentage of viable cells was reduced
to 87.2±1.0% (p<0.001). Exposure to epicatechin alone decreased
cell viability likewise to 87.1±0.9% (p<0.001).
Plasma samples obtained after pycnogenol intake
induced significantly apoptotic cell death. Application of plasma
samples before pycnogenol intake had no significant effect on cell
viability over all time points (Fig.
1). After 24-h treatment with pycnogenol-negative human plasma
94.2±1.0% of the cells were detected as viable. In contrast, the
viability of untreated control cells was 94.9±1.4%. Strikingly,
treatment of HT1080 cells with plasma samples after pycnogenol
intake resulted in significant apoptotic cell death. The first
significant apoptotic response was observed after 6 h of incubation
with 20.1±2.9% of the cells left apoptotic and 74.2±1.4% remaining
viable (p<0.001). Apoptosis reached a maximum after 24 h of
treatment. Here, 28.3±6.0% were observed to be apoptotic (vs.
3.4±1.4% in control group, p<0.001) and 65.7±4.0% were left
viable whereas the percentage of necrotic cells was only
5.5±3.6%.
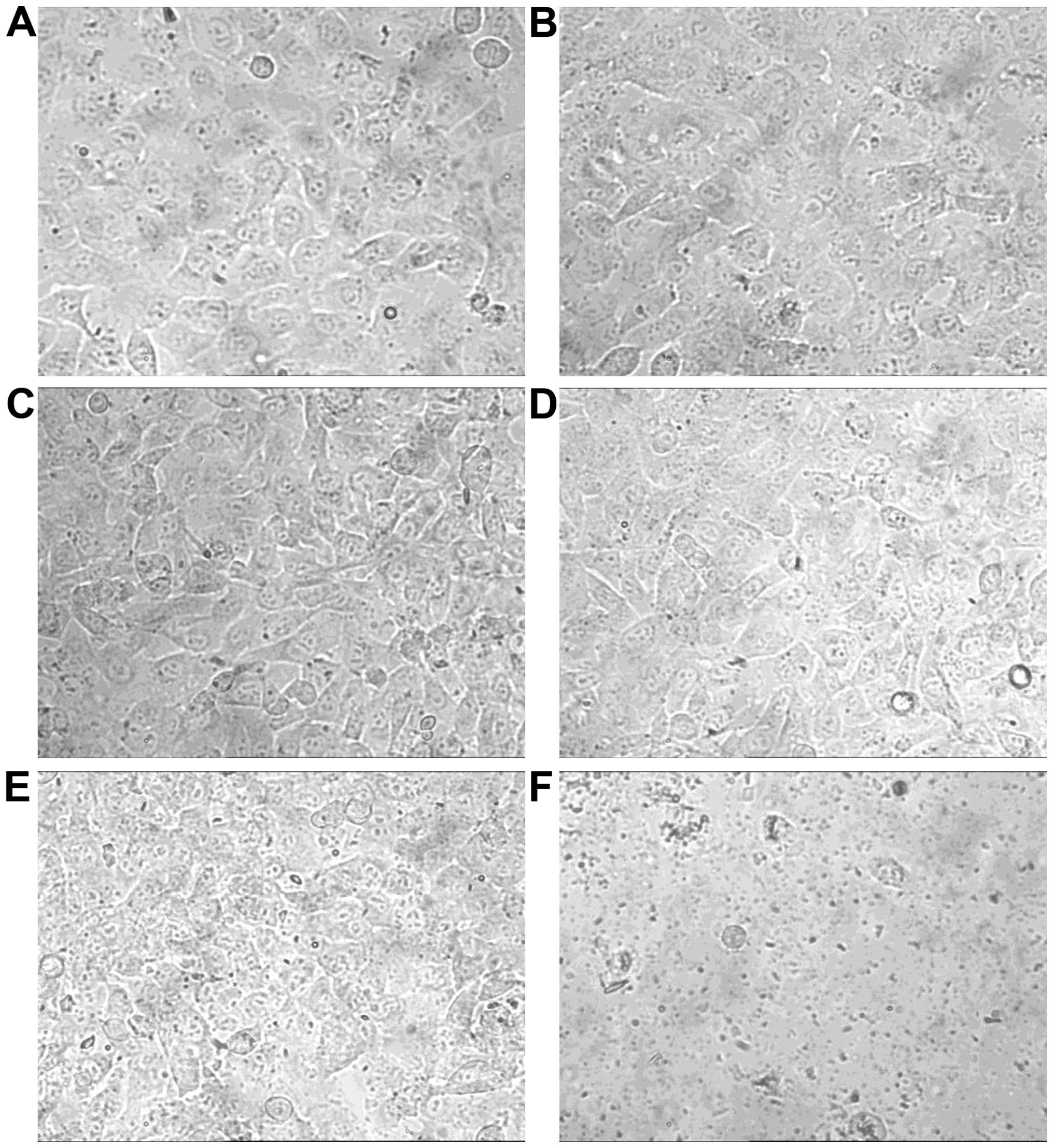
Only addition of plasma samples after pycnogenol
intake induced morphological changes and cell detachment. Catechin,
epicatechin, taxifolin and plasma samples before pycnogenol intake
did not alter cell morphology and density as observed using
bright-field microscopy (Fig. 2).
However, plasma samples after pycnogenol intake reduced cell
density of HT1080 fibrosarcoma cells indicating decreased rates of
cell division and proliferation respectively. Further, it led to
shrinkage of cells and dissolution of confluent cell groups
followed by complete cell detachment. Longer incubation resulted in
obvious morphological aberrations.
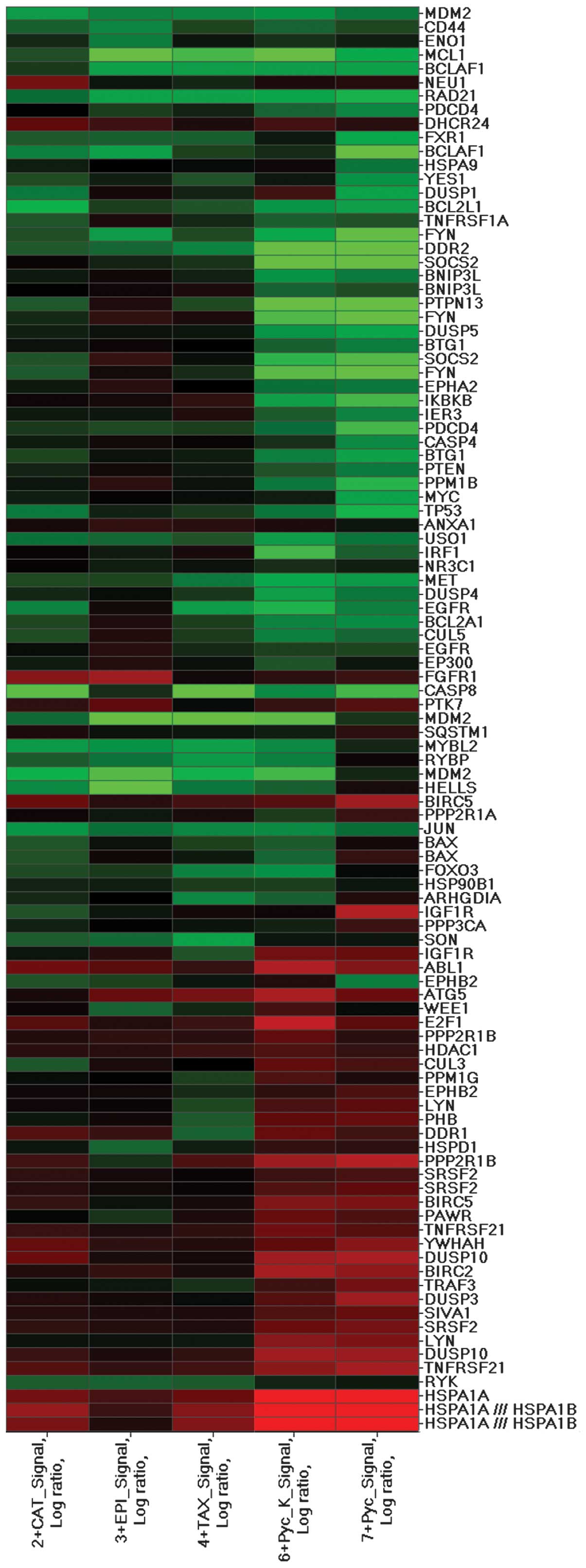
Microarray analysis revealed differential gene
expression patterns of HT1080 cells treated with plasma samples
after pycnogenol intake. Based on comparison analysis algorithm,
pycnogenol remarkably altered the expression levels of different
combinations of probe sets. In cells treated with plasma samples
after pycnogenol intake, microarray analyses identified noticeable
expression changes in 1,128 genes. Of these, 57.5% (649) were
downregulated and 42.5% (479) upregulated.
To obtain an overview of the biological processes
affected by pycnogenol, we analysed the regulated targets of the
pathways that were over-represented in our data set using the
GeneTrail application (25).
Significant over-representation was detected in several pathway
categories that included apoptosis, MAPK signalling pathway,
pathways in cancer, p53 signalling, cell adhesion and metabolic
pathways (Table I). To understand
the molecular details underlying the diverse modes of cell death in
fibrosarcoma cells, we focused on the differentially expressed
apoptosis-associated genes that were altered by plasma samples
after pycnogenol intake (Fig. 3,
Table II).
 | Table IMicroarray analysis using GeneTrail
software identified significant changes in the following computed
KEGG pathway categories with p<0.05 and FDR adjustments
(Benjamini-Hochberg). |
Table I
Microarray analysis using GeneTrail
software identified significant changes in the following computed
KEGG pathway categories with p<0.05 and FDR adjustments
(Benjamini-Hochberg).
| Selection of
significant subcategories (α=0.05, FDR adjusted) | p-values | Expected no. of
regulated genes | Observed no. of
regulated genes |
|---|
| Apoptosis | 0.002 | 55 | 84 |
| MAPK signalling
pathway | 0.028 | 12 | 22 |
| Pathways in
cancer | 0.002 | 16 | 33 |
| P53 signalling | 0.002 | 13 | 27 |
| Cell adhesion | 0.002 | 29 | 51 |
| Metabolic
pathways | 0.004 | 54 | 32 |
 | Table IISummary of the expression changes of
apoptosis-related genes for cells treated with human plasma samples
after pycnogenol intake compared to untreated cells. |
Table II
Summary of the expression changes of
apoptosis-related genes for cells treated with human plasma samples
after pycnogenol intake compared to untreated cells.
| Gene ID | Gene title | Oncological
relevance | Signal log ratio
(compared to untreated cells) | Refs. |
|---|
| FN1 | Fibronectin 1 | Promotes pulmonary
metastasis of human fibrosarcoma HT1080 cells in nude mice.
Upregulation is associated with increased proliferation, adhesion
and invasion of fibrosarcoma cells in vitro | −1.1 | 37,38 |
| CTNNA1 | Catenin α1 | Associated with
increased cell survival of synovial sarcoma cells | −1.1 | 39 |
| LAMC1 | Laminin γ1 | Contributes to
cancer cell migration and invasion in prostate cancer | −1.0 | 40 |
| RHOA | Rho GDP
dissociation inhibitor α | Enhances metastatic
potential of different sarcoma cell lines in vivo | −0.9 | 47,48 |
| ITGB1 | Integrin, β1
(fibronectin receptor, β polypeptide) | Promotes human lung
cancer cell invasion and metastasis in vitro and in
vivo. Promotes proliferation and cell survival of colorectal
carcinoma cells | −0.9 | 49,50 |
| JAK1 | Janus kinase 1 | Inactivation of
JAK1 in fibrosarcoma cells leads to loss of invasion in
vitro and metastasis in vivo | −0.8 | 46 |
| PIK3CB |
Phosphatidylinositol-4,5- bisphosphate
3-kinase, catalytic subunit β | Required for growth
of phosphatase and tensin homolog (PTEN)-deficient coloncarcinoma
cells | −0.3 | 51 |
| PIK3RI |
Phosphoinositide-3-kinase, regulatory
subunit 1 (α) | Downregulation
results in decreased proliferation, migration, and invasion in
different malignant cell lines | −0.3 | 52,53 |
| AKT3 | V-akt murine
thymoma viral oncogene homolog 3 (protein kinase B, γ) | Contributes to
invasive migration and tumour metastasis in various
malignancies | −0.3 | 54 |
| KRAS | V-Ki-ras2 Kirsten
rat sarcoma viral oncogene homolog | Overexpression
promotes progression of metastatic fibrosarcoma in vivo | −0.3 | 55 |
| DUSP1 | Dual specificity
phosphatase 1 | Inhibits
proliferation and induces apoptosis in human hepatocellular and
pancreatic carcinoma | 1.0 | 56,57 |
| BCLAF | BCL2-associated
transcription factor 1 | Upregulation is
associated with increased apoptosis and growth inhibition in
bladder cancer cell lines | 1.0 | 58 |
| COX3 | Cytochrome c
oxidase III | Decreased
expression is associated with apoptosis resistance in colon cancer
cells | 0.7 | 59 |
| MAPK8 | Mitogen-activated
protein kinase 8 | Contributes to
apoptosis induced by cytostatics in different sarcoma cell
lines | 0.5 | 60 |
Discussion
Fibrosarcomas are rare tumours within the
heterogeneous group of soft tissue sarcomas and respond poorly to
conventional treatments, such as chemotherapy and radiation.
Despite excellent rates of local disease control, treatment options
in distant metastatic disease, especially in pulmonary locations,
are very limited and have an associated median survival of <12
months (6,7). Due to the rarity of fibrosarcomas,
the development of new therapeutics has been difficult, and the
lack of novel chemotherapy protocols remains a major problem.
Additionally, elderly patients with cardiac subdisease are
ineligible for doxorubicin-based chemotherapy which is still
considered as first-line treatment at metastatic disease stage. For
these reasons, there was increasing interest in assessing whether
the cardiotoxicity of doxorubicin could be mitigated by antioxidant
compounds. In past studies, the maritime pine bark pycnogenol as
well as its main constituent catechin were found to protect
cardiomyocytes against doxorubicin-induced free radicals
attenuating its cardiotoxicity in mice (26–28).
Interestingly, pycnogenol and its metabolites are also known to
exhibit anticancer activity in a wide range of human cancer cell
lines (19–21). Because pycnogenol is extremely
well-tolerated and no severe side-effects were ever reported, it is
categorized as a nutritional supplement in most of the European
countries and readily available. The numerous advantages of
pycnogenol inspired us to analyse its anticancer activity in human
fibrosarcoma cells.
In our study, plasma samples after pycnogenol intake
significantly induced apoptosis in HT1080 cells ex vivo
whereas plasma samples before pycnogenol intake did not exhibit any
effect. Moreover, it led to decreased cell division and distinct
morphological changes. Interestingly, pycnogenol was more effective
in apoptosis induction than its main constituents catechin,
epicatechin and taxifolin indicating that the metabolised
components of pycnogenol interact synergistically.
To further elucidate the actions of metabolised
pycnogenol on a molecular basis, we analysed changes in expression
of apoptosis-related genes using microarray technology.
Notable gene alterations induced by pycnogenol were
found in members of the PI3K/Akt signalling pathway (Table II). Interestingly, the PI3K/Akt
pathway is widely dysregulated in many solid malignancies including
several soft tissue sarcoma subtypes and many studies have shown
this pathway to be vital to the growth and survival of cancer cells
(29–32). Here, multiple mechanisms have been
found to induce PI3K/Akt signalling, such as activating mutations
of key genes such as phosphatidylinositol-4,5-bisphosphate
3-kinase, catalytic subunit β (PIK3CB),
phosphoinositide-3-kinase, regulatory subunit 1 (PIK3RI) and
V-akt murine thymoma viral oncogene homolog 3 (AKT3)
(33). In the current study,
plasma samples after pycnogenol intake led to a downregulation of
PIK3CB, PIK3RI and AKT3 when compared to untreated cells or cells
treated with plasma samples before pycnogenol intake suggesting
that the PI3K/Akt signalling pathway may play a role in apoptosis
induction in human fibrosarcoma cells (Table II). The only experimental study
assessing the impact of PI3K/Akt pathway in fibrosarcoma cells
demonstrated that inhibition of PI3K via small molecular inhibitors
decreased remarkably the invasive potential of HT1080 cells in
vitro (34). However, the role
of PI3K/Akt pathway in human fibrosarcoma is still unknown and
warrants further research because the novel and well-tolerated
group of PI3K inhibitors could be potentially useful therapeutic
options.
Interestingly, we found a correlation between
apoptotic efficacy of metabolised pycnogenol and downregulation of
several genes encoding for cell adhesion proteins such as
fibronectin 1 (FN1), catenin α1 (CTNNA1) and laminin γ1 (LAMC1)
(Table II). Cell adhesion and its
underlying pathways play a crucial role in growth, metastasis and
development of fibrosarcomas (35,36).
In past experimental studies, upregulation of FN1 was shown to
increase the invasive potential of HT1080 cells in vitro and
to promote their pulmonary metastasis in vivo whereas
overexpression of CTNNA1 and LAMC1 were associated with increased
cell survival of different malignant cell lines (37–40).
However, retrospectively we cannot conclude whether downregulation
of these cell adhesion proteins itself led to apoptosis or vice
versa. Thus, the appealing hypothesis that disruption of cell
adhesion leads to apoptosis in human fibrosarcoma cells requires
further experimental support.
Plasma samples with metabolised pycnogenol led also
to a downregulation of Janus kinase 1 (JAK1). The JAK/STAT
signalling pathway is a key signal transduction pathway implicated
in the pathogenesis of many human cancers including several soft
tissue sarcoma subtypes (41,42).
Constitutive JAK/STAT activity has been demonstrated to cause
tumourigenic inflammation and increased proliferation in a wide
range of malignant diseases, including malignant fibrous
histiocytoma (43–45). In fibrosarcomas, inactivation of
JAK1 led to loss of invasion in vitro and metastasis in
vivo (46). Recently,
pharmacological inhibition of JAK1 was shown to induce apoptosis in
rhabdomyosarcoma cells in vivo (42). However, understanding the complex
role of JAK1 in sarcoma cell death may provide new opportunities
for rational pathway-based therapies and drug development. Several
novel JAK/STAT-inhibitors have been tested in clinical trials which
could be promising agents in the therapy of metastatic soft tissue
sarcomas.
In conclusion, this in vitro study
demonstrates that the natural pine bark extract pycnogenol has the
potential to induce apoptosis and alter gene expression in
fibrosarcoma cells. Although a wide variety of genes and pathways
were involved, the PIK3/Akt signalling pathway appears to play a
key role in mediating apoptosis of HT1080 cells via pycnogenol
metabolites. Pycnogenol is not meant to replace doxorubicin-based
chemotherapy in patients with metastatic fibrosarcoma, but it could
be a potential mild therapeutic option for patients that are not
suitable for chemotherapy and have to undergo palliative treatment.
The encouraging results of this study provide experimental support
for in vivo trials assessing the effect of pycnogneol in
soft tissue sarcomas.
References
|
1
|
Hoos A, Lewis JJ and Brennan MF: Soft
tissue sarcoma: prognostic factors and multimodal treatment.
Chirurg. 71:787–794. 2000.(In German). View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Patrikidou A, Domont J, Cioffi A and Le
Cesne A: Treating soft tissue sarcomas with adjuvant chemotherapy.
Curr Treat Options Oncol. 12:21–31. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Kaushal A and Citrin D: The role of
radiation therapy in the management of sarcomas. Surg Clin North
Am. 88:629–646. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
O’Brien GC, Cahill RA, Bouchier-Hayes DJ
and Redmond HP: Co-immunotherapy with interleukin-2 and taurolidine
for progressive metastatic melanoma. Ir J Med Sci. 175:10–14. 2006.
View Article : Google Scholar
|
|
5
|
Solomon LR, Cheesbrough JS, Bhargava R, et
al: Observational study of need for thrombolytic therapy and
incidence of bacteremia using taurolidine-citrate-heparin,
taurolidine-citrate and heparin catheter locks in patients treated
with hemodialysis. Semin Dial. 25:233–238. 2012. View Article : Google Scholar
|
|
6
|
Karavasilis V, Seddon BM, Ashley S,
Al-Muderis O, Fisher C and Judson I: Significant clinical benefit
of first-line palliative chemotherapy in advanced soft-tissue
sarcoma: retrospective analysis and identification of prognostic
factors in 488 patients. Cancer. 112:1585–1591. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Billingsley KG, Lewis JJ, Leung DH, Casper
ES, Woodruff JM and Brennan MF: Multifactorial analysis of the
survival of patients with distant metastasis arising from primary
extremity sarcoma. Cancer. 85:389–395. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Pezzi CM, Pollock RE, Evans HL, et al:
Preoperative chemotherapy for soft-tissue sarcomas of the
extremities. Ann Surg. 211:476–481. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Donato Di Paola E and Nielsen OS; EORTC
Soft Tissue and Bone Sarcoma Group. The EORTC soft tissue and bone
sarcoma group. European Organisation for Research and Treatment of
Cancer. Eur J Cancer. 38(Suppl 4): S138–S141. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Nedea EA and DeLaney TF: Sarcoma and skin
radiation oncology. Hematol Oncol Clin North Am. 20:401–429. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Brodowicz T, Schwameis E, Widder J, et al:
Intensified adjuvant IFADIC chemotherapy for adult soft tissue
sarcoma: a prospective randomized feasibility trial. Sarcoma.
4:151–160. 2000. View Article : Google Scholar
|
|
12
|
Frustaci S, Gherlinzoni F, De Paoli A, et
al: Adjuvant chemotherapy for adult soft tissue sarcomas of the
extremities and girdles: results of the Italian randomized
cooperative trial. J Clin Oncol. 19:1238–1247. 2001.PubMed/NCBI
|
|
13
|
Judson I, Verweij J, Gelderblom H, et al:
Doxorubicin alone versus intensified doxorubicin plus ifosfamide
for first-line treatment of advanced or metastatic soft-tissue
sarcoma: a randomised controlled phase 3 trial. Lancet Oncol.
15:415–423. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Swain SM, Whaley FS and Ewer MS:
Congestive heart failure in patients treated with doxorubicin: a
retrospective analysis of three trials. Cancer. 97:2869–2879. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Burningham Z, Hashibe M, Spector L and
Schiffman JD: The epidemiology of sarcoma. Clin Sarcoma Res.
2:142012. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Grimm T, Skrabala R, Chovanová Z, et al:
Single and multiple dose pharmacokinetics of maritime pine bark
extract (pycnogenol) after oral administration to healthy
volunteers. BMC Clin Pharmacol. 6:42006. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Rohdewald P: A review of the French
maritime pine bark extract (Pycnogenol), a herbal medication with a
diverse clinical pharmacology. Int J Clin Pharmacol Ther.
40:158–168. 2002. View
Article : Google Scholar : PubMed/NCBI
|
|
18
|
Packer L, Rimbach G and Virgili F:
Antioxidant activity and biologic properties of a procyanidin-rich
extract from pine (Pinus maritima) bark, pycnogenol. Free Radic
Biol Med. 27:704–724. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Huang WW, Yang JS, Lin CF, Ho WJ and Lee
MR: Pycnogenol induces differentiation and apoptosis in human
promyeloid leukemia HL-60 cells. Leuk Res. 29:685–692. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Buz’Zard AR and Lau BH: Pycnogenol reduces
talc-induced neoplastic transformation in human ovarian cell
cultures. Phytother Res. 21:579–586. 2007. View Article : Google Scholar
|
|
21
|
Huynh HT and Teel RW: Selective induction
of apoptosis in human mammary cancer cells (MCF-7) by pycnogenol.
Anticancer Res. 20:2417–2420. 2000.PubMed/NCBI
|
|
22
|
Belcaro G, Cesarone MR, Genovesi D, et al:
Pycnogenol may alleviate adverse effects in oncologic treatment.
Panminerva Med. 50:227–234. 2008.PubMed/NCBI
|
|
23
|
Chow HH, Hakim IA, Vining DR, et al:
Effects of dosing condition on the oral bioavailability of green
tea catechins after single-dose administration of Polyphenon E in
healthy individuals. Clin Cancer Res. 11:4627–4633. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Daigeler A, Brenzel C, Bulut D, et al:
TRAIL and Taurolidine induce apoptosis and decrease proliferation
in human fibro-sarcoma. J Exp Clin Cancer Res. 27:822008.
View Article : Google Scholar
|
|
25
|
Backes C, Keller A, Kuentzer J, et al:
GeneTrail - advanced gene set enrichment analysis. Nucleic Acids
Res. 35:W186–W192. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Feng WH, Wei HL and Liu GT: Effect of
PYCNOGENOL on the toxicity of heart, bone marrow and immune organs
as induced by antitumor drugs. Phytomedicine. 9:414–418. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Abd El-Aziz TA, Mohamed RH, Pasha HF and
Abdel-Aziz HR: Catechin protects against oxidative stress and
inflammatory- mediated cardiotoxicity in adriamycin-treated rats.
Clin Exp Med. 12:233–240. 2012. View Article : Google Scholar
|
|
28
|
Du Y and Lou H: Catechin and
proanthocyanidin B4 from grape seeds prevent doxorubicin-induced
toxicity in cardiomyocytes. Eur J Pharmacol. 591:96–101. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Raynaud FI, Eccles S, Clarke PA, et al:
Pharmacologic characterization of a potent inhibitor of class I
phosphatidylinositide 3-kinases. Cancer Res. 67:5840–5850. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Willems L, Tamburini J, Chapuis N, Lacombe
C, Mayeux P and Bouscary D: PI3K and mTOR signaling pathways in
cancer: new data on targeted therapies. Curr Oncol Rep. 14:129–138.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Guo S, Lopez-Marquez H, Fan KC, et al:
Synergistic effects of targeted PI3K signaling inhibition and
chemotherapy in liposarcoma. PLoS One. 9:e939962014. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Hernando E, Charytonowicz E, Dudas ME, et
al: The AKT-mTOR pathway plays a critical role in the development
of leiomyo-sarcomas. Nat Med. 13:748–753. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Cheung M and Testa JR: Diverse mechanisms
of AKT pathway activation in human malignancy. Curr Cancer Drug
Targets. 13:234–244. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Ito S, Koshikawa N, Mochizuki S and
Takenaga K: 3-Methy-ladenine suppresses cell migration and invasion
of HT1080 fibrosarcoma cells through inhibiting phosphoinositide
3-kinases independently of autophagy inhibition. Int J Oncol.
31:261–268. 2007.PubMed/NCBI
|
|
35
|
Weinspach D, Seubert B, Schaten S, et al:
Role of L1 cell adhesion molecule (L1CAM) in the metastatic
cascade: promotion of dissemination, colonization, and metastatic
growth. Clin Exp Metastasis. 31:87–100. 2014. View Article : Google Scholar
|
|
36
|
Nikitovic D, Berdiaki A, Banos A,
Tsatsakis A, Karamanos NK and Tzanakakis GN: Could growth
factor-mediated extracellular matrix deposition and degradation
offer the ground for directed pharmacological targeting in
fibrosarcoma? Curr Med Chem. 20:2868–2880. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Gong M, Ueda Y, Kanazawa Y, Tsuchiya H and
Ma YG: Association of gene FN1 with pulmonary metastasis of human
fibrosarcoma. Zhonghua Zhong Liu Za Zhi. 29:14–16. 2007.(In
Chinese). PubMed/NCBI
|
|
38
|
Ying L, Lau A, Alvira CM, et al:
LC3-mediated fibronectin mRNA translation induces fibrosarcoma
growth by increasing connective tissue growth factor. J Cell Sci.
122:1441–1451. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Sato H, Hasegawa T, Kanai Y, et al:
Expression of cadherins and their undercoat proteins (alpha-,
beta-, and gamma-catenins and p120) and accumulation of
beta-catenin with no gene mutations in synovial sarcoma. Virchows
Arch. 438:23–30. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Nishikawa R, Goto Y, Kojima S, et al:
Tumor-suppressive microRNA-29s inhibit cancer cell migration and
invasion via targeting LAMC1 in prostate cancer. Int J Oncol.
45:401–410. 2014.PubMed/NCBI
|
|
41
|
Shouda T, Hiraoka K, Komiya S, et al:
Suppression of IL-6 production and proliferation by blocking STAT3
activation in malignant soft tissue tumor cells. Cancer Lett.
231:176–184. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Yan S, Li Z and Thiele CJ: Inhibition of
STAT3 with orally active JAK inhibitor, AZD1480, decreases tumor
growth in Neuroblastoma and Pediatric Sarcomas in vitro and in
vivo. Oncotarget. 4:433–445. 2013.PubMed/NCBI
|
|
43
|
Li N, Grivennikov SI and Karin M: The
unholy trinity: inflammation, cytokines, and STAT3 shape the cancer
microenvironment. Cancer Cell. 19:429–431. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Redell MS, Ruiz MJ, Alonzo TA, Gerbing RB
and Tweardy DJ: Stat3 signaling in acute myeloid leukemia:
ligand-dependent and -independent activation and induction of
apoptosis by a novel small-molecule Stat3 inhibitor. Blood.
117:5701–5709. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Senft C, Priester M, Polacin M, et al:
Inhibition of the JAK-2/STAT3 signaling pathway impedes the
migratory and invasive potential of human glioblastoma cells. J
Neurooncol. 101:393–403. 2011. View Article : Google Scholar
|
|
46
|
Teng Y, Xie X, Walker S, White DT, Mumm JS
and Cowell JK: Evaluating human cancer cell metastasis in
zebrafish. BMC Cancer. 13:4532013. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Miyamoto C, Maehata Y, Ozawa S, et al:
Fasudil suppresses fibrosarcoma growth by stimulating secretion of
the chemokine CXCL14/BRAK. J Pharmacol Sci. 120:241–249. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Kosla J, Paňková D, Plachý J, et al:
Metastasis of aggressive amoeboid sarcoma cells is dependent on
Rho/ROCK/MLC signaling. Cell Commun Signal. 11:512013. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Wang XM, Li J, Yan MX, et al: Integrative
analyses identify osteopontin, LAMB3 and ITGB1 as critical
pro-metastatic genes for lung cancer. PLoS One. 8:e557142013.
View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Song J, Zhang J, Wang J, et al: β1
integrin modulates tumor growth and apoptosis of human colorectal
cancer. Oncol Rep. 32:302–308. 2014.PubMed/NCBI
|
|
51
|
Wee S, Wiederschain D, Maira SM, et al:
PTEN-deficient cancers depend on PIK3CB. Proc Natl Acad Sci USA.
105:13057–13062. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Weber GL, Parat MO, Binder ZA, Gallia GL
and Riggins GJ: Abrogation of PIK3CA or PIK3R1 reduces
proliferation, migration, and invasion in glioblastoma multiforme
cells. Oncotarget. 2:833–849. 2011.PubMed/NCBI
|
|
53
|
Fu Y, Zhang Q, Kang C, et al: Inhibitory
effects of adenovirus mediated COX-2, Akt1 and PIK3R1 shRNA on the
growth of malignant tumor cells in vitro and in vivo. Int J Oncol.
35:583–591. 2009.PubMed/NCBI
|
|
54
|
Chin YR and Toker A: Function of Akt/PKB
signaling to cell motility, invasion and the tumor stroma in
cancer. Cell Signal. 21:470–476. 2009. View Article : Google Scholar :
|
|
55
|
Algarra I, Perez M, Serrano MJ, Garrido F
and Gaforio JJ: c-K-ras overexpression is characteristic for
metastases derived from a methylcholanthrene-induced fibrosarcoma.
Invasion Metastasis. 18:261–270. 1999. View Article : Google Scholar
|
|
56
|
Calvisi DF, Pinna F, Meloni F, et al:
Dual-specificity phosphatase 1 ubiquitination in extracellular
signal-regulated kinase-mediated control of growth in human
hepatocellular carcinoma. Cancer Res. 68:4192–4200. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Gil-Araujo B, Toledo Lobo MV,
Gutiérrez-Salmerón M, et al: Dual specificity phosphatase 1
expression inversely correlates with NF-κB activity and expression
in prostate cancer and promotes apoptosis through a p38 MAPK
dependent mechanism. Mol Oncol. 8:27–38. 2014. View Article : Google Scholar
|
|
58
|
Yoshitomi T, Kawakami K, Enokida H, et al:
Restoration of miR-517a expression induces cell apoptosis in
bladder cancer cell lines. Oncol Rep. 25:1661–1668. 2011.PubMed/NCBI
|
|
59
|
Payne CM, Holubec H, Bernstein C, et al:
Crypt-restricted loss and decreased protein expression of
cytochrome C oxidase subunit I as potential hypothesis-driven
biomarkers of colon cancer risk. Cancer Epidemiol Biomarkers Prev.
14:2066–2075. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Koyama T, Mikami T, Koyama T, et al:
Apoptosis induced by chemotherapeutic agents involves c-Jun
N-terminal kinase activation in sarcoma cell lines. J Orthop Res.
24:1153–1162. 2006. View Article : Google Scholar : PubMed/NCBI
|