Introduction
Pituitary adenomas represent ~10–25% of the
intrinsic central nervous system (CNS) tumors (1). Although most pituitary tumors are
benign and slow growing, some of them are aggressive and can cause
morbidity and premature mortality. Pituitary adenomas exhibit a
wide range of clinical behavior including brain structure
compression and abnormal pituitary hormone production (2). A number of pituitary adenomas are
invasive and expand to adjacent tissues and structures such as
sphenoid, cavernous sinus and dura mater. These tumors are often
rapidly growing, readily recurring after surgical resection,
resistant to radiation and pharmacotherapy, and are thus a great
medical challenge (3). Despite
extensive research efforts, how pituitary adenomas become rapidly
growing and invasive remains to be elucidated.
The Wnt/β-catenin signaling pathway plays a critical
role in pituitary development and organogenesis via regulating the
transcription factors Pitx2, Nr5a1 (Sf1) and Tcf712 (Tcf4)
(4–6). This pathway has been demonstrated to
control the maintenance of progenitor/stem cells and secure the
balance between stem cell differentiation and self-renewal in
various tissues and organs (7,8).
Deregulation of this balance leads to a number of cancers (9–12),
and evidence for a possible role of pituitary progenitor/stem cells
in the genesis of pituitary tumors has been shown in an animal
study (13). In a previous study,
we found that β-catenin was overexpressed in human pituitary
adenomas, and its expression level correlated to tumor invasion
(14). However, the underlying
mechanisms remain to be elucidated.
In normal cells, β-catenin is either bound to
E-cadherin to maintain cell adhesion or resides unbound in the
cytoplasm (15). Cytoplasmic
β-catenin binds to adenomatous polyposis coli (APC), axin1 and
glycogen synthase kinase-3β (GSK-3β), resulting in β-catenin
phosphorylation which promotes its degradation (8,16–18).
The accumulation of β-catenin in the cytoplasm leads to its
translocation into the nucleus and binding to TCF/LEF transcription
factors to regulate several target genes (19). Increasing evidence suggests that
excessive accumulation of β-catenin is involved in tumor invasion
and proliferation (20–22). In human colorectal cancer,
β-catenin expression levels are found to be associated with
aggressive morphological features, epithelial-mesenchymal
transition and a poor prognosis (23). In human astrocytomas, overexpressed
β-catenin binds to a Lef/Tcf protein to promote cell proliferation.
However, little is known about the downstream molecules that are
activated by β-catenin in invasive pituitary adenomas (24).
In the present study, we created a stable β-catenin
knockdown pituitary cell line to investigate the role of β-catenin
and its downstream molecules in the tumorgenesis of pituitary
adenomas. Our results showed that knockdown of β-catenin with shRNA
significantly inhibited pituitary adenoma cell proliferation and
migration, and this change was accompanied by decreased expression
of AKT, STAT3, cyclin D1, CDK4, MMP-2 and MMP-9. Our results
suggest that β-catenin may regulate multiple downstream molecules
to enhance pituitary adenoma cell proliferation and invasion.
Materials and methods
Cell culture
The mouse growth hormone pituitary adenoma cell line
GT1.1 (purchased from the China Academy of Medical Sciences Cell
Culture Center, Beijing, China) was cultured in DMEM-F12 medium
(Gibco, Carlsbad, CA, USA) supplemented with 10% fetal bovine serum
(FBS; Gibco) and maintained at 37°C in a humidified atmosphere with
5% CO2.
Plasmid transfection with β-catenin
shRNA
For β-catenin knockdown experiment, shRNA plasmid
(sc-29210-SH) and copGFP control plasmid (sc-108083) were purchased
from Santa Cruz Biotechnology (Santa Cruz, CA, USA). According to
the manufacturer’s instructions, the cells at 70–80% confluence
were subjected to shRNA plasmid transfection using transfection
reagent (Santa Cruz Biotechnology). Puromycin (5 μg/ml) was used to
kill non-transfected cells and transfection efficiency was checked
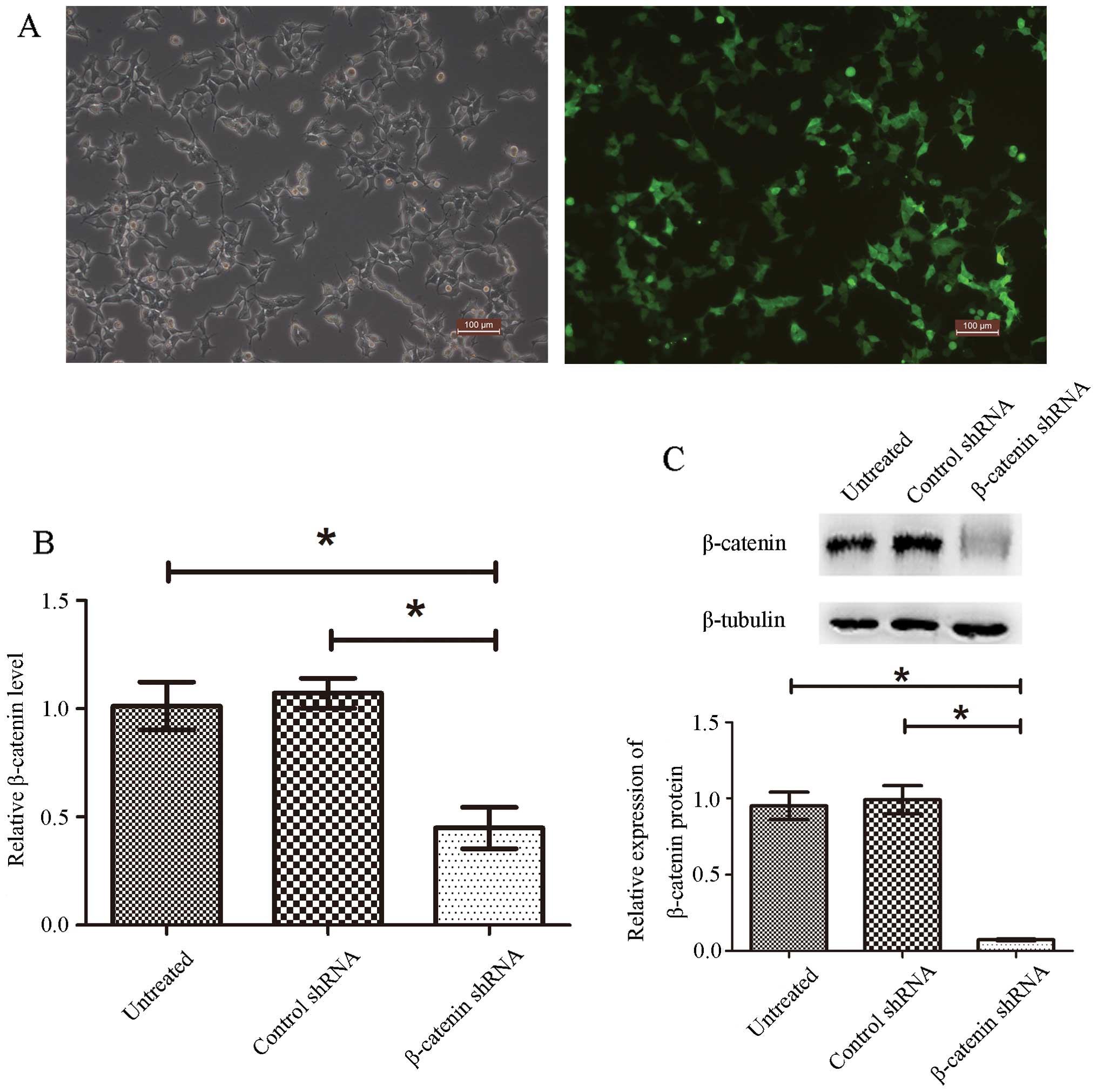
under a fluorescence microscope. More than 90% of the cells showed
green fluorescence (Fig. 1A) and
were used for the indicated experiments.
Total RNA extraction and real-time
quantitative polymerase chain reaction (RT-qPCR)
Total RNA was extracted using the RNAiso Plus
(Takara Bio, Shiga, Japan). Total RNA (2 μg) was reverse
transcribed using PrimeScript™ RT Master Mix (Takara Bio) according
to manufacturer’s instructions. The primer sequences used for
RT-qPCR were as follows: mouse β-catenin: 5′-TGG CAA TCA AGA GAG
CAA GC-3′ (forward) and 5′-GAC AGA CAG CAC CTT CAG CA-3′ (reverse);
mouse β-actin: 5′-GGA GAT TAC TGC CCT GGC TCC TA-3′ (forward) and
5′-GAC TCA TCG TAC TCC TGC TTG CTG-3′ (reverse); mouse MMP-2:
5′-TGT GTT CTT TGC AGG GAA TGA AT-3′ (forward) and 5′-TGT CTT CTT
GTT TTT GCT CCA GTT A-3′ (reverse); mouse MMP-9: 5′-CCT CTG GAG GTT
CGA CGT GA-3′ (forward) and 5′-TAG GCT TTC TCT CGG TAC TGG AA-3′
(reverse). RT-qPCR was performed on ABI 7300 Real-Time PCR system
(Applied Biosystems, Foster City, CA, USA) using the SYBR Premix Ex
Taq kit (Takara Bio) following the cycling profile: 95°C for 30
sec, followed by 40 cycles of amplification (95°C for 5 sec and
60°C for 20 sec). The target mRNA expression levels were normalized
to β-actin. The Ct value for each sample was calculated using the
Ct-method and results were expressed as 2−ΔΔCT.
Western blot analysis
The cells were lysed in RIPA lysis buffer
(sc-364162; Santa Cruz Biotechnology) containing 10 μl/ml protease
inhibitor cocktail (sc-364162; Santa Cruz Biotechnology) and
incubated on ice for 30 min. Protein concentration was quantified
using the BCA protein assay kit (Pierce, Rockford, IL, USA).
Protein samples (30 μg/lane) were diluted in 5X SDS-PAGE sample
loading buffer and electrophoretically separated on 10% SDS-PAGE
gel. After transferring onto polyvinylidene difluoride membranes
(Millipore, Bedford, MA, USA), the membranes were blocked in 5%
non-fat skim milk and incubated overnight at 4°C in primary
antibodies. After being washed 3 times for 5 min, membranes were
incubated in suitable secondary antibodies (1:5,000; Millipore) for
1 h at room temperature. Signals were detected using the Immobilon
Western Chemiluminescent HRP Substrate (Millipore) and Alliance
Imaging systems (Uvitec, Cambridge, UK). Antibodies were obtained
as follows: primary antibodies, monoclonal rabbit anti-mouse
β-catenin (1:1000; Abcam, Cambridge, UK), monoclonal rabbit
anti-mouse STAT3 (1:1,000; Cell Signaling Technology, Beverly, MA,
USA), monoclonal rabbit anti-mouse cyclin D1 (1:800; Millipore),
monoclonal rabbit anti-mouse CDK4 (1:200; Santa Cruz
Biotechnology), monoclonal rabbit anti-mouse β-tubulin (1:500;
Abcam); secondary antibody, goat anti-rabbit IgG, peroxidase
conjugated (1:5,000; Millipore).
Gelatin zymography
MMP-2 and MMP-9 levels in culture media and in
cellular extracts were examined using gelatin zymography assay.
GT1.1 cells were seeded on 6-well plates at 1×106
cells/well and incubated at 37°C in 2 ml of serum-free DMEM-F12 for
24 h before collecting the supernatants. The cells were lysed in
RIPA lysis buffer as described above. Protein (30 μg) or 20 μl
supernatant per lane was mixed with equal volume of 2X sample
buffer (62.5 mM Tris-HCl containing 10% glycerol, 0.00125%
bromophenol blue and 12% SDS) without reducing agent and were
electrophoresed on a 10% sodium dodecyl sulfate-polyacrylamide gel
with 0.1% gelatin (Invitrogen, Grand Island, NY, USA) incorporated
as a substrate for gelatinolytic proteases under non-reducing
condition at 120 V for 2 h. Gels were washed two times in 25 ml/l
Triton X-100 for 30 min and subsequently incubated at 37°C for 36 h
in reaction buffer (50 mmol/l Tris-HCl, pH 7.6, 50 mmol/l NaCl, 5
mmol/l CaCl2, 1 μmol/l ZnCl2, 0.02% Brij-35),
and stained with 0.5% coomassie brilliant blue R-250 (Bio-Rad
Laboratories, Hercules, CA, USA). Gelatinolytic activity was
measured as clear areas using Gel Doc XR+ imaging system (Bio-Rad
Laboratories).
Cell proliferation assay
GT1.1 cells were seeded into 96-well plates at 2,000
cells/well and incubated for 8 h to allow cell adhesion. Cell
proliferation was assessed at indicated incubation time-points
after cell adhesion using Cell Counting kit-8 (Dojindo
Laboratories, Kumamoto, Japan). All measurements were conducted in
triplicate.
Transwell assay
For tumor invasion assay, cold serum-free DMEM-F12
medium was mixed with Matrigel (1:5; BD Biosciences, NJ, USA). The
upper chambers (8 μm pore size; Corning, Franklin Lakes, NY, USA)
were coated with 100 μl Matrigel mixture and allowed to solidify at
37°C for 4 h, then 2×105 cells were seeded into the
upper chambers. The lower chamber contained 1 ml DMEM-F12 and 10%
FBS as a chemoattractant. After 24-h incubation, non-invaded cells
in the upper chambers were removed with a cotton swab, and invaded
cells were fixed in 70% methanol. Fixed cells were stained with
0.1% crystal violet for 10 min, washed twice with PBS and counted
(5 random ×100 fields/well). Data were expressed as the mean cell
number of three independent experiments.
Statistical analysis
The statistical analyses were performed using SPSS
16.0 statistics software. All data are presented as means ± SEM.
The significance of difference between the groups was determined by
using a one-way analysis of variance (ANOVA) followed by Tukey’s
post hoc test. A value of P<0.05 was considered as a
statistically significant difference.
Results
β-catenin is downregulated in GT1.1 cells
transfected with β-catenin shRNA plasmid
To determine whether β-catenin affects the
proliferation and invasion of pituitary adenoma, we created a
stable-knockdown cell line by transfecting β-catenin shRNA plasmid
into GT1.1 cells. The efficiency of stable transfection in GT1.1
cells was examined by fluorescent microscopy, and >90% of the
cells were positively transfected as indicated by the expression of
the reporter gene GFP (Fig. 1A).
In addition, real-time RT-PCR and western blot analysis were
performed to determine the efficacy of β-catenin shRNA in knocking
down β-catenin. Fig. 1B shows that
β-catenin shRNA transfection significantly decreased β-catenin mRNA
expression (57% reduction), while control shRNA had no effect when
compared with untreated cells. Accordingly, β-catenin protein
levels were also significantly reduced in β-catenin shRNA
transfected cells (Fig. 1C). These
results clearly indicate that we successfully created a stable
β-catenin knockdown pituitary adenoma cell line by shRNA plasmid
transfection.
Knockdown of β-catenin inhibits GT1.1
cell proliferation
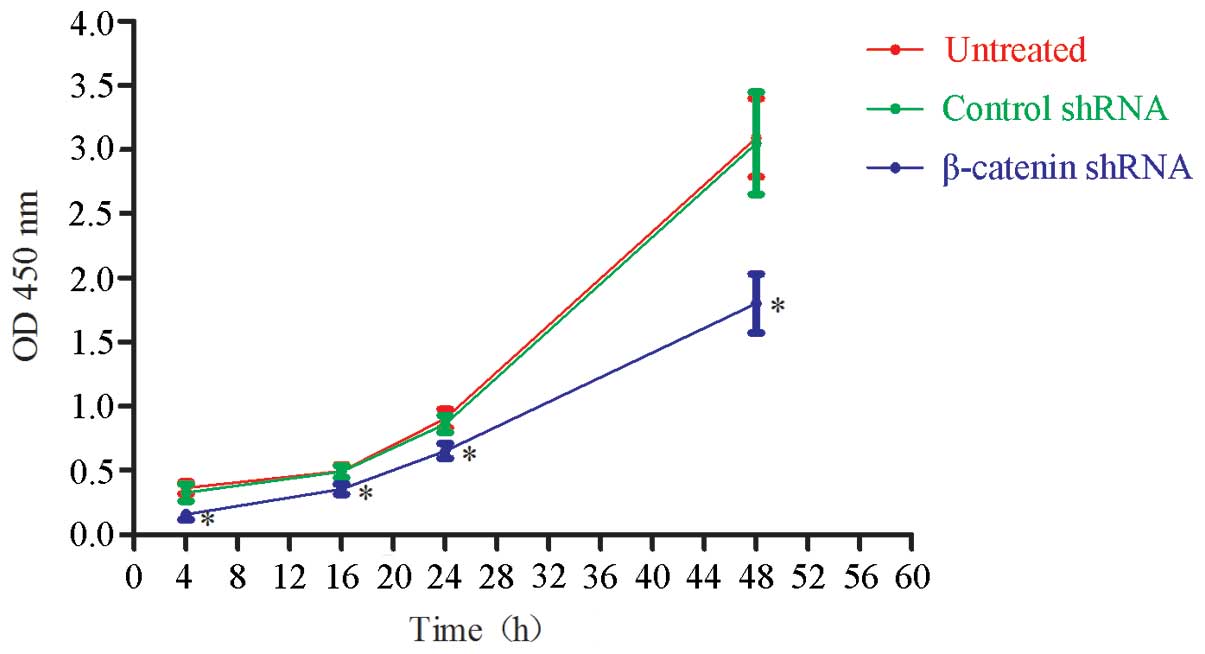
Cell Counting kit-8 (CCK-8) assay was performed to
assess the effect of β-catenin knockdown on cell proliferation. The
cells were seeded at a density of 2,000 cells/well in 96-well
plates and were allowed to attach for 8 h before measuring cell
proliferation at 4, 16, 24 and 48 h. As shown in Fig. 2, β-catenin shRNA transfection
significantly, but not completely, inhibited GT1.1 cell
proliferation when compared with control shRNA (P<0.05). As
expected, there was no difference between control shRNA-treated and
untreated cells. This result indicated that knockdown of β-catenin
significantly inhibited GT1.1 cell proliferation.
Knockdown of β-catenin attenuates
pituitary adenoma cell invasion
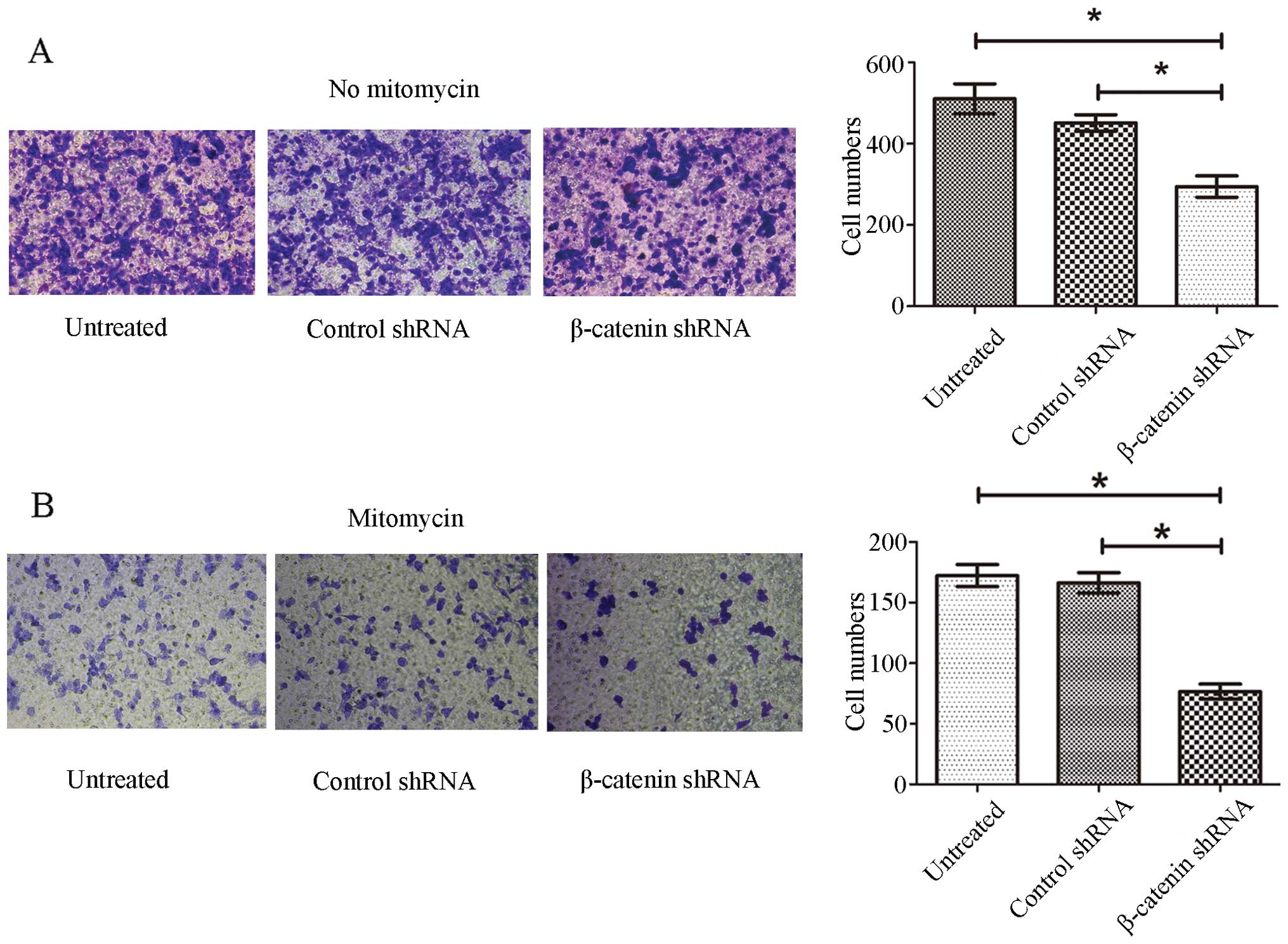
Transwell invasion assay was applied to determine
whether β-catenin knockdown could inhibit the invasion of GT1.1
cells. As shown in Fig. 3A,
β-catenin shRNA transfection significantly reduced the number of
cells that migrated to the lower surface of the inserts compared
with control shRNA-treated cells and untreated cells (P<0.05),
and there was no difference between control shRNA-treated and
untreated cells. As β-catenin shRNA transfection inhibited cell
proliferation, there was a possibility that the reduced number of
β-catenin shRNA-treated cells detected in the lower surface of the
insert might result from inhibited proliferation. To exclude this
possibility, we inhibited cell proliferation with 25 μg/ml
mitomycin half an hour before assessing invasion. Similarly, a
significant reduction in cell invasion was observed for β-catenin
shRNA transfected cells (P<0.05), and there was no difference
between control shRNA-treated and untreated cells (Fig. 3B). The results showed that
knockdown of β-catenin inhibited pituitary adenoma cell
invasion.
Knockdown of β-catenin inhibits the
expression STAT3 and AKT in GT1.1 cells
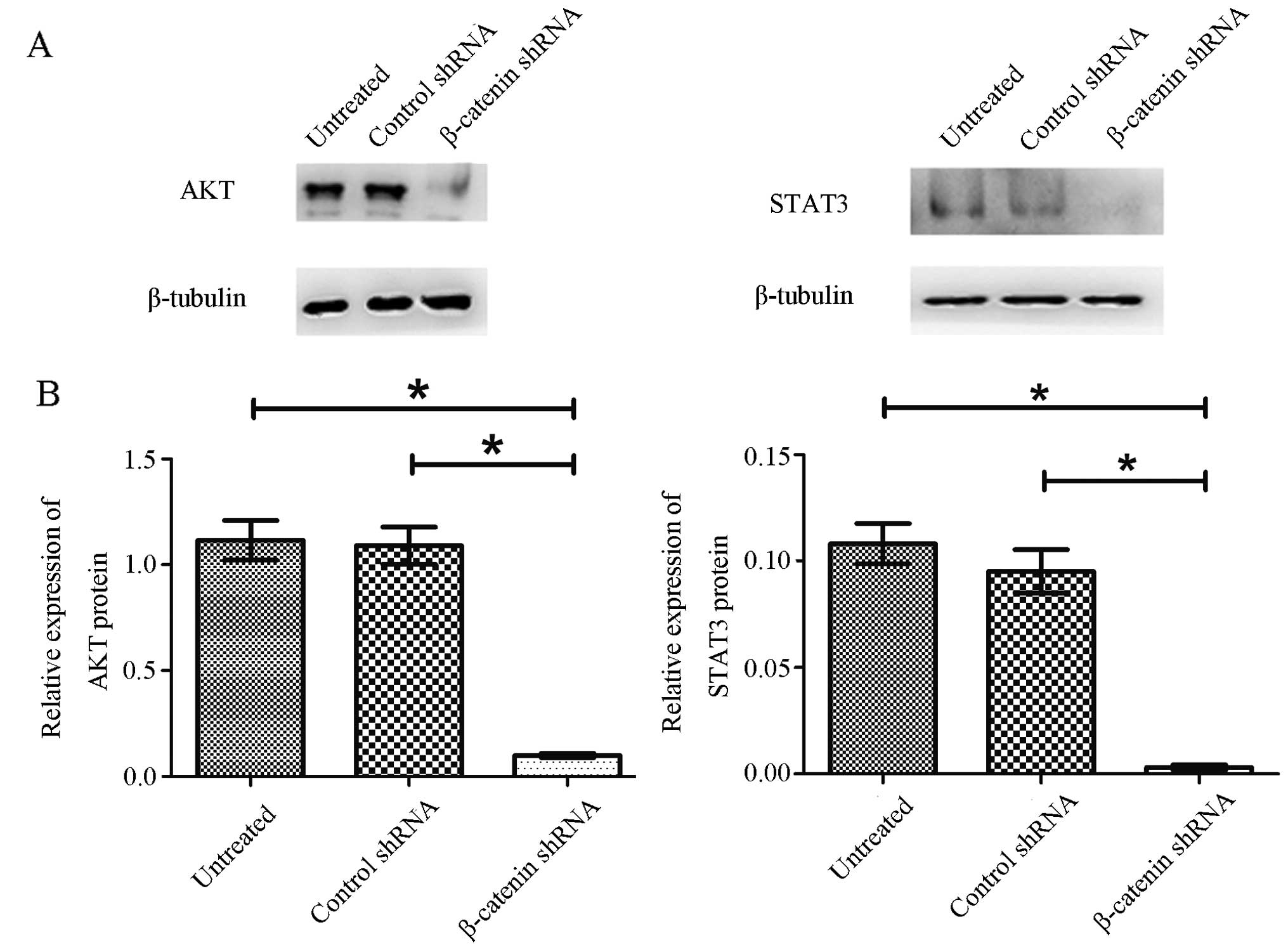
To further understand how β-catenin knockdown
inhibited pituitary adenoma cell proliferation and invasion, we
assessed the changes of the important signal molecules STAT3 and
AKT in GT1.1 cells that critically promote tumor invasion and
metastasis in various cancers (25,26).
Western blot analysis was performed to evaluate STAT3 and AKT
proteins, and the data are shown in Fig. 4. Compared with control
shRNA-transfected cells and untreated cells, both STAT3 and AKT
protein levels were drastically reduced (>90%) in β-catenin
shRNA-transfected cells. These results demonstrated that knockdown
of β-catenin inhibited the expression of STAT3 and AKT in pituitary
adenoma cells.
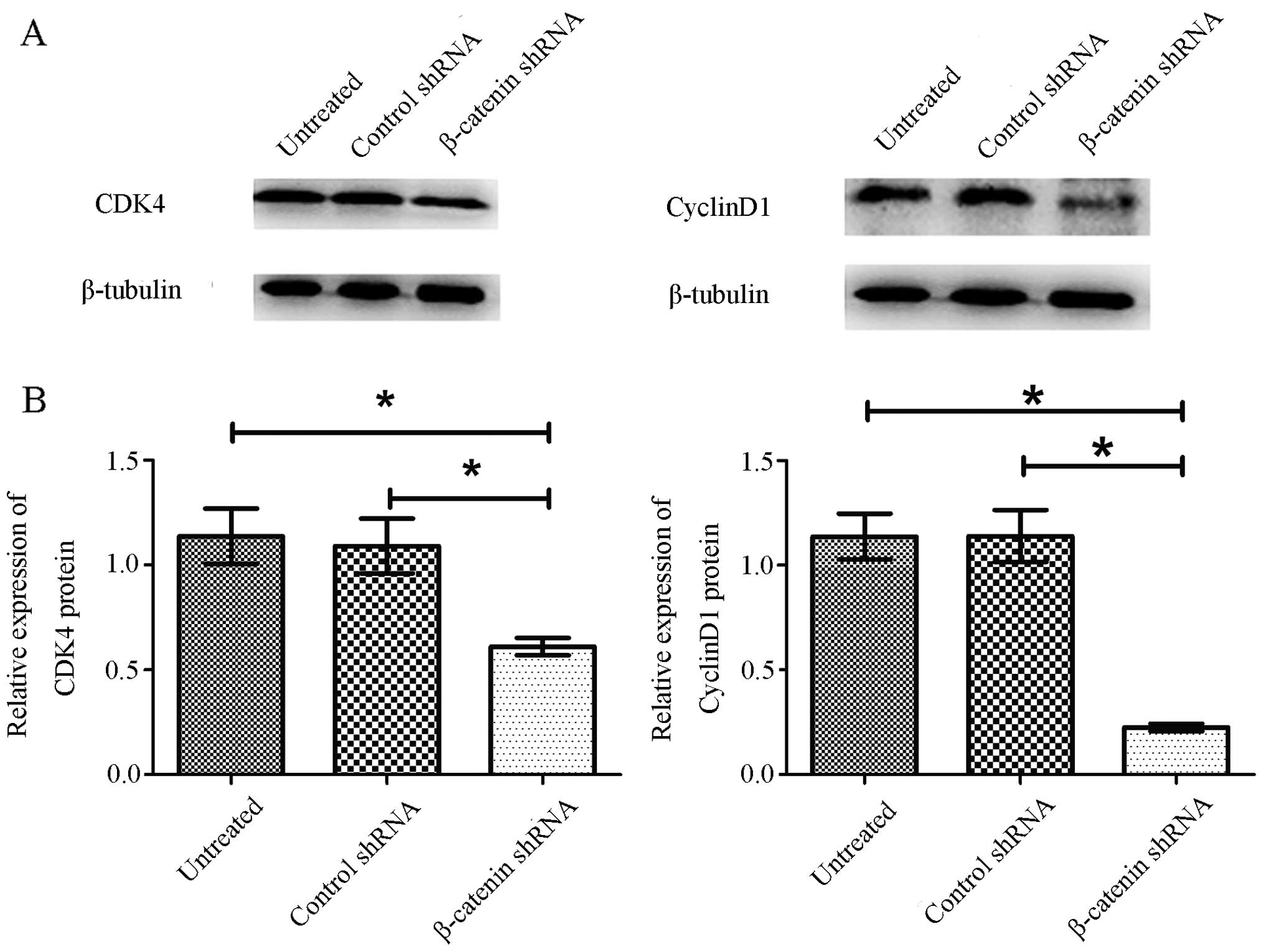
Knockdown of β-catenin inhibits the
expression of cyclin D1 and CDK4 in GT1.1 cells
To further elucidate the growth suppression effect
of β-catenin shRNA on GT1.1 cells, we assessed the changes of
cyclin D1 and CDK4, two important cell cycle-related molecules,
using western blot analysis. As shown in Fig. 5, β-catenin shRNA transfection
downregulated cyclin D1 and CDK4 expression in GT1.1 cells in
comparison to control shRNA. As expected, control shRNA had no
effect on cyclin D1 and CDK4 compared to untreated cells. The
results suggest that β-catenin may promote cell cycle progression
to contribute to cell proliferation in GT1.1 cells.
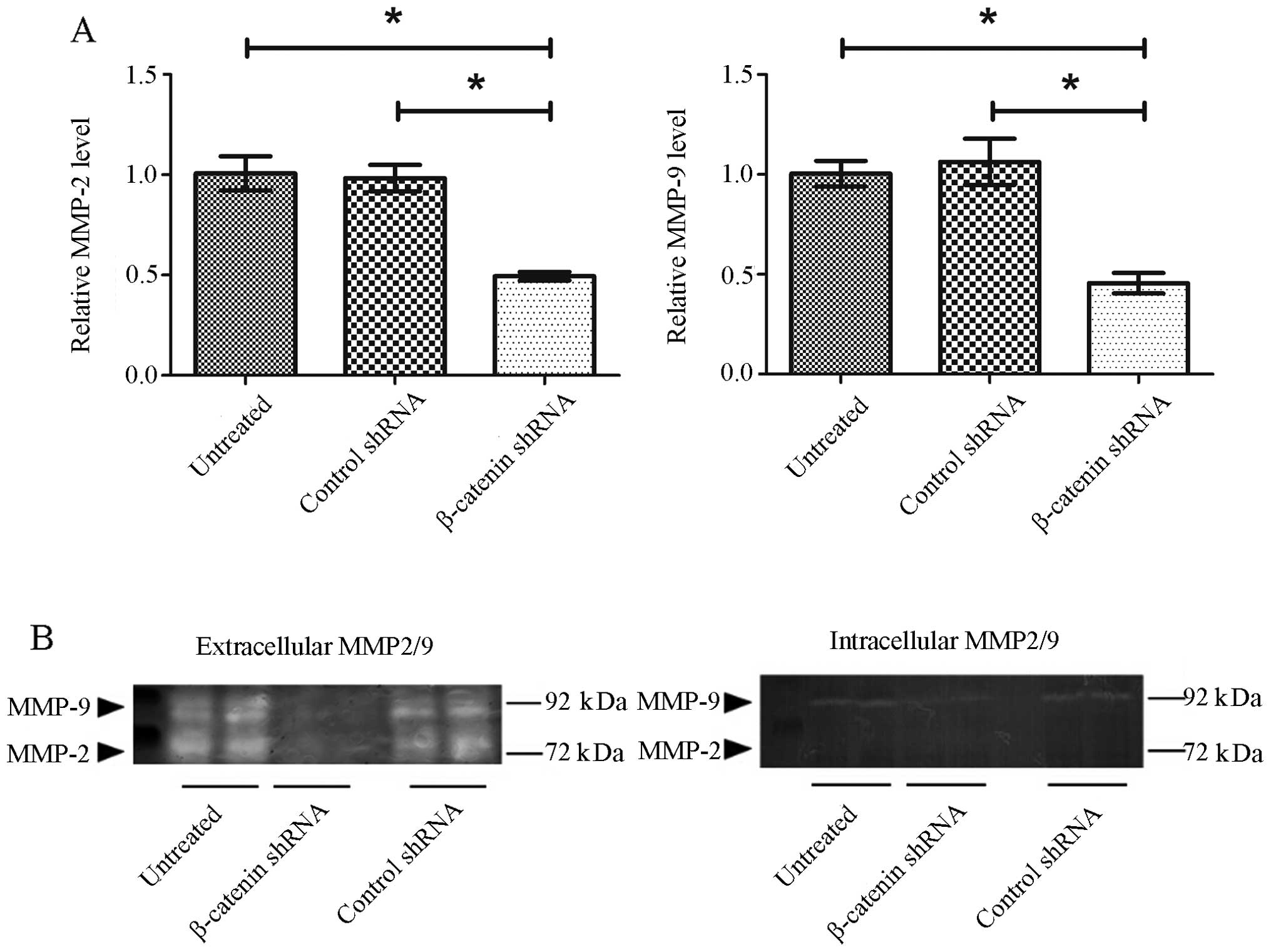
Knockdown of β-catenin suppresses MMP-2
and MMP-9 expression in GT1.1 cells
MMP-2 and -9 are key gelatin proteolytic enzymes
that break down and remodel the extracellular matrix to promote and
facilitate cancer metastasis (27,28).
To study the mechanism of β-catenin knockdown on the inhibition of
GT1.1 cell invasion, we quantified MMP-2 and MMP-9 mRNA expression
in GT1.1 cells with RT-qPCR. The results showed that MMP-2 and
MMP-9 mRNA expression were suppressed by 51 and 55% in β-catenin
shRNA-transfected cells comparred to control shRNA (Fig. 6A). No significant difference was
observed for either MMP-2 or MMP-9 mRNA levels between control
shRNA group and untreated group. Gel gelatin zymography was
performed to assess MMP-2 and MMP-9 protein levels. As shown in
Fig. 6B, high levels of MMP-2 and
MMP-9 were found in the supernatants of untreated GT1.1 cells,
while no MMP-2 and -9 bands were detected when β-catenin was
knocked down by shRNA. Apparently, the inhibitory effect of
β-catenin shRNA on MMP-2/9 mRNA expression was less than that of
MMP-2 and -9 secretions. We speculated that β-catenin shRNA might
not only down-regulate MMP-2/9 expression (or synthesis) but also
inhibits their secretion at extracellular MMP-2/9 levels. To test
this possibility, we measured intracellular MMP-2/9 protein levels
to see whether there was MMP-2/9 accumulation in the cells
transfected with β-catenin shRNA. MMP-2 band was not detectable in
the cellular extracts (Fig. 6B).
There was a weak MMP-9 band detected in the cellular extracts of
untreated cells and control shRNA-transfected cells, and no MMP-9
band was detected for β-catenin shRNA-transfected cells (Fig. 6B). These results suggested that
β-catenin shRNA may reduce extracellular MMP-2/9 via interrupting
MMP-2/9 synthesis rather than their secretion.
Discussion
In our previous study, we found that Wnt4 and its
downstream gene β-catenin were overexpressed in FSH-omas, GH-omas,
PRL-omas, TSH-omas and NF-omas, but not in ACTH-omas. Moreover, the
level of β-catenin expression was correlated with the degree of
tumor invasion (14). However, the
mechanisms underlying this correlation have not been established.
In the present study, we showed that knockdown of β-catenin
suppressed the proliferation and invasion of pituitary adenoma
cells, and this change was accompanied by decreased expression of
AKT, STAT3, cyclin D1, CDK4, MMP-2 and MMP-9. Our results suggest
that β-catenin may regulate multiple downstream molecules to
enhance pituitary adenoma cell proliferation and invasion.
Invasive pituitary adenomas that exhibit relatively
higher mitotic activity with a MIB-1 labeling index (LI) >3% or
show extensive p53 immunoreactivity are classified as ‘atypical
adenomas’ (29). The prognosis for
invasive pituitary adenomas is often unpredictable because they are
resistant to radiation and chemotherapy and cannot be fully
resected. Currently, the mechanisms underlying the invasive
phenotype of pituitary adenoma remain largely unknown. Deregulation
of Wnt/β-catenin pathway is involved in development and progression
of various tumors. Here, our data showed that knocking down
β-catenin by shRNA dramatically repressed the aggressive behavior
of pituitary adenoma cells, i.e. proliferation and invasion.
AKT, also known as protein kinase B (PKB), plays a
critical role in multiple cellular processes such as apoptosis,
proliferation, transformation and migration. The activation of the
PI3K/AKT pathway has been found in a wide range of cancers
(30). AKT inhibition has been
reported to decrease the expression of cyclin D1, MMP-2 and MMP-9,
resulting in suppressed proliferation and invasion in U251 glioma
cells (31). In the present study,
we found that pituitary adenoma cell line GT1.1 expresses high
level of AKT, and knockdown of β-catenin drastically (>90%)
inhibited AKT expression.
Besides AKT, STAT3 has also been reported to be a
downstream molecule of the β-catenin pathway (32). STAT3 plays an important role in the
proliferation of tumor cells by regulating cell cycle-related gene
transcription (33–35). Of note, in this study, we found
that knockdown of β-catenin almost completely blocked STAT3
expression in pituitary adenoma cells. STAT3 has been reported to
act on cyclin D1 and CDK4 to promote cell proliferation (36), thus, we assessed the effect of
β-catenin on cyclin D1 and CDK4. As expected, both proteins were
downregulated in pituitary adenoma cells when β-catenin was knocked
down by shRNA. These results suggest that the suppressed expression
of AKT, STAT3, cyclin D1 and CDK4 by β-catenin shRNA may account
for the observed inhibition on the proliferation of pituitary
adenoma cells.
Matrix metalloproteinases (MMPs) are a family of
single-chain zinc-containing proteolytic enzymes that regulate the
degradation of extracellular matrix in both physiological and
pathological processes including angiogenesis, neoplasia and
inflammation. Aberrant expression of MMPs has proved to contribute
to tumor invasion and metastasis by breaking down and remodeling
the extracellular matrix (27,28).
Several studies reported that there might be a possible correlation
between invasive pituitary tumor phenotype and expression or
activity of MMP-2 and MMP-9 (37,38).
In the present study, we showed that β-catenin shRNA transfection
almost completely inhibited MMP-2/9 synthesis in pituitary adenoma
cells, which may account for the reduced invasion of β-catenin
shRNA transfected cells. It is worth pointing out that β-catenin
shRNA inhibits MMP-2/9 mRNA expression to a lesser extent comparing
its inhibition on MMP-2/9 protein synthesis. This discrepancy
suggests that β-catenin may both inhibit MMP-2/9 transcription and
translation, and further studies are warranted to investigate this
possibility.
These in vitro data seem to be contradictory
to our previous findings that β-catenin expression levels were
reversely correlated with tumor grade in pituitary adenomas
(14). However, in our previous
study, we only compared β-catenin expression in invasive pituitary
adenomas, but did not measure β-catenin levels in non-invasive
pituitary adenomas. Using a stable β-catenin knockdown pituitary
adenoma cell line, here we clearly showed that β-catenin
contributes to pituitary adenoma cell proliferation and
invasion.
In conclusion, the present study demonstrates that
knockdown of β-catenin inhibits the proliferation and invasion of
pituitary adenoma cells probably through inhibiting AKT, STAT3,
cyclin D1, CDK4, MMP-2 and MMP-9, and targeting β-catenin signaling
pathway might be a novel strategy for pituitary adenoma
therapy.
Acknowledgements
This study was supported by grants from Shenzhen
Science and Technology Innovation Commission (Shenzhen Key
Laboratory of Neurosurgery - grant no. ZDSYS20140509173142601, and
Basic Research Grant - grant no. JCYJ20130401113737900).
References
|
1
|
Vandeva S, Jaffrain-Rea ML, Daly AF,
Tichomirowa M, Zacharieva S and Beckers A: The genetics of
pituitary adenomas. Best Pract Res Clin Endocrinol Metab.
24:461–476. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Melmed S: Pathogenesis of pituitary
tumors. Nat Rev Endocrinol. 7:257–266. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Asa SL and Ezzat S: The pathogenesis of
pituitary tumors. Annu Rev Pathol. 4:97–126. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Douglas KR, Brinkmeier ML, Kennell JA, et
al: Identification of members of the Wnt signaling pathway in the
embryonic pituitary gland. Mamm Genome. 12:843–851. 2001.
View Article : Google Scholar
|
|
5
|
Brinkmeier ML, Potok MA, Cha KB, et al:
TCF and Groucho-related genes influence pituitary growth and
development. Mol Endocrinol. 17:2152–2161. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Kioussi C, Briata P, Baek SH, et al:
Identification of a Wnt/Dvl/beta-Catenin → Pitx2 pathway mediating
cell-type-specific proliferation during development. Cell.
111:673–685. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Brinkmeier ML, Potok MA, Davis SW and
Camper SA: TCF4 deficiency expands ventral diencephalon signaling
and increases induction of pituitary progenitors. Dev Biol.
311:396–407. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Zeng YA and Nusse R: Wnt proteins are
self-renewal factors for mammary stem cells and promote their
long-term expansion in culture. Cell Stem Cell. 6:568–577. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Polakis P: Wnt signaling and cancer. Genes
Dev. 14:1837–1851. 2000.PubMed/NCBI
|
|
10
|
Reed KR, Athineos D, Meniel VS, et al:
B-catenin deficiency, but not Myc deletion, suppresses the
immediate phenotypes of APC loss in the liver. Proc Natl Acad Sci
USA. 105:18919–18923. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Nelson WJ and Nusse R: Convergence of Wnt,
beta-catenin, and cadherin pathways. Science. 303:1483–1487. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Willert K and Jones KA: Wnt signaling: is
the party in the nucleus? Genes Dev. 20:1394–1404. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Gaston-Massuet C, Andoniadou CL, Signore
M, et al: Increased Wingless (Wnt) signaling in pituitary
progenitor/stem cells gives rise to pituitary tumors in mice and
humans. Proc Natl Acad Sci USA. 108:11482–11487. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Li W, Zhang Y, Zhang M, Huang G and Zhang
Q: Wnt4 is over-expressed in human pituitary adenomas and is
associated with tumor invasion. J Clin Neurosci. 21:137–141. 2014.
View Article : Google Scholar
|
|
15
|
Benjamin JM and Nelson WJ: Bench to
bedside and back again: molecular mechanisms of alpha-catenin
function and roles in tumorigenesis. Semin Cancer Biol. 18:53–64.
2008. View Article : Google Scholar
|
|
16
|
Kalani MY, Cheshier SH, Cord BJ, et al:
Wnt-mediated self-renewal of neural stem/progenitor cells. Proc
Natl Acad Sci USA. 105:16970–16975. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Nusse R: Wnt signaling and stem cell
control. Cell Res. 18:523–527. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Logan CY and Nusse R: The Wnt signaling
pathway in development and disease. Annu RevCell Dev Biol.
20:781–810. 2004. View Article : Google Scholar
|
|
19
|
Cadigan KM and Peifer M: Wnt signaling
from development to disease: insights from model systems. Cold
Spring Harb Perspect Biol. 1:a0028812009. View Article : Google Scholar :
|
|
20
|
Park GB, Kim DJ, Kim YS, Lee HK, Kim CW
and Hur DY: Silencing of galectin-3 represses osteosarcoma cell
migration and invasion through inhibition of FAK/Src/Lyn activation
and beta-catenin expression and increases susceptibility to
chemotherapeutic agents. Int J Oncol. 46:185–194. 2015.
|
|
21
|
Bai XL, Zhang Q, Ye LY, et al: Myocyte
enhancer factor 2C regulation of hepatocellular carcinoma via
vascular endothelial growth factor and Wnt/beta-catenin signaling.
Oncogene. Oct 20–2014.(Epub ahead of print). View Article : Google Scholar
|
|
22
|
Salaroli R, Ronchi A, Buttarelli FR, et
al: Wnt activation affects proliferation, invasiveness and
radiosensitivity in medulloblastoma. J Neurooncol. Sep
28–2014.(Epub ahead of print). PubMed/NCBI
|
|
23
|
Gao ZH, Lu C, Wang MX, Han Y and Guo LJ:
Differential beta-catenin expression levels are associated with
morphological features and prognosis of colorectal cancer. Oncol
Lett. 8:2069–2076. 2014.PubMed/NCBI
|
|
24
|
Sareddy GR, Panigrahi M, Challa S,
Mahadevan A and Babu PP: Activation of Wnt/beta-catenin/Tcf
signaling pathway in human astrocytomas. Neurochem Int. 55:307–317.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Trovato M, Torre ML, Ragonese M, et al:
HGF/c-met system targeting PI3K/AKT and STAT3/phosphorylated-STAT3
pathways in pituitary adenomas: an immunohistochemical
characterization in view of targeted therapies. Endocrine.
44:735–743. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Ma PC, Tretiakova MS, Nallasura V,
Jagadeeswaran R, Husain AN and Salgia R: Downstream signalling and
specific inhibition of c-MET/HGF pathway in small cell lung cancer:
implications for tumour invasion. Br J Cancer. 97:368–377. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Liotta LA and Kohn EC: The
microenvironment of the tumour-host interface. Nature. 411:375–379.
2001. View
Article : Google Scholar : PubMed/NCBI
|
|
28
|
Coussens LM and Werb Z: Inflammation and
cancer. Nature. 420:860–867. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
de Aguiar PH, Aires R, Laws ER, et al:
Labeling index in pituitary adenomas evaluated by means of MIB-1:
is there a prognostic role? A critical review. Neurol Res.
32:1060–1071. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Cully M, You H, Levine AJ and Mak TW:
Beyond PTEN mutations: the PI3K pathway as an integrator of
multiple inputs during tumorigenesis. Nat Rev Cancer. 6:184–192.
2006. View
Article : Google Scholar : PubMed/NCBI
|
|
31
|
Fu Y, Zhang Q, Kang C, et al: Inhibitory
effects of adenovirus mediated COX-2, Akt1 and PIK3R1 shRNA on the
growth of malignant tumor cells in vitro and in vivo. Int J Oncol.
35:583–591. 2009.PubMed/NCBI
|
|
32
|
Yue X, Lan F, Yang W, et al: Interruption
of beta-catenin suppresses the EGFR pathway by blocking multiple
oncogenic targets in human glioma cells. Brain Res. 1366:27–37.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Dechow TN, Pedranzini L, Leitch A, et al:
Requirement of matrix metalloproteinase-9 for the transformation of
human mammary epithelial cells by Stat3-C. Proc Natl Acad Sci USA.
101:10602–10607. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Silver DL, Naora H, Liu J, Cheng W and
Montell DJ: Activated signal transducer and activator of
transcription (STAT) 3: localization in focal adhesions and
function in ovarian cancer cell motility. Cancer Res. 64:3550–3558.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Senft C, Priester M, Polacin M, et al:
Inhibition of the JAK-2/STAT3 signaling pathway impedes the
migratory and invasive potential of human glioblastoma cells. J
Neurooncol. 101:393–403. 2011. View Article : Google Scholar
|
|
36
|
Wang J, Li X, Lu X and Pi L: The
regulation of stat3 signal transduction pathway to G1 to S phase of
laryngocarcinoma cell. Lin Chung Er Bi Yan Hou Tou Jing Wai Ke Za
Zhi. 22:699–703. 2008.(In Chinese). PubMed/NCBI
|
|
37
|
Liu W, Kunishio K, Matsumoto Y, Okada M
and Nagao S: Matrix metalloproteinase-2 expression correlates with
cavernous sinus invasion in pituitary adenomas. J Clin Neurosci.
12:791–794. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Gong J, Zhao Y, Abdel-Fattah R, et al:
Matrix metallopro-teinase-9, a potential biological marker in
invasive pituitary adenomas. Pituitary. 11:37–48. 2008. View Article : Google Scholar
|