Introduction
Malignant melanoma is the leading cause of skin
cancer-related death (80%) worldwide, mainly due to its high
proliferation capability, metastatic properties and lack of
effective treatment (1). According
to the World Health Organization (WHO), the incidence of melanoma
is rising faster than that of any other type of cancer worldwide
(2). There are a limited number of
efficacious therapies that are current available, including
interferon α-2b, interleukin (IL)-2, the anti-CTLA-4 antibody,
ipilimumab, the BRAF inhibitors, vemurafenib and dabrafenib, as
well as a MEK inhibitor, trametinib. In addition, either
significant immune related adverse effects or short-lived durable
responses of these available treatments further limited their
therapeutic application (3–5).
Therefore, there is still a need to find and identify potential low
toxicity and sensitive drugs, which recognize specific molecular
target to strengthen the efficacy in the treatment of melanoma.
Lewis Y (LeY) is a difucosylated oligosaccharide
with the chemical structure [Fucα1→2Galβ1→4(Fucα1→3)GlcNAcβ1→R],
carried by glycoconjugates (glycoproteins and glycolipids) on the
cell surface. LeY is a tumor-associated carbohydrate antigen (TACA)
and its abnormal expression is frequently found in various cancers,
such as breast, pancreas, gastric, ovarian and skin cancers
(6–9). Several reports suggested that
abnormal expression of LeY was associated with tumor growth,
metastasis, angiogenesis and drug resistance (10–13).
Monoclonal antibody 692/29 targeting LeY showed good antitumor
responses (14). The activation of
epidermal growth factor receptor (EGFR) in skin cancers is closely
related to the carcinogenic events including cell proliferation,
migration and invasion (15). LeY
oligosaccharide antigen linked to EGFR plays a critical role in
dimerzation and activation of EGFR as well as downstream of the
EGFR/MAPK signaling pathway. Therefore, the inhibition of LeY
synthesis and LeY mediated activation of EGFR/MAPK signaling
pathway play an important role in the treatment of cancer.
The synthesis of tumor-associated carbohydrate
antigens, including LeY, is controlled by the specific
glycosyltransferases. Fucosyltransferases (FUTs) are the key
enzymes catalyzing the synthesis of fucosylated glycans. FUTs gene
family contains 1, 2-, 1, 3/4-, and 1, 6-linkages, which catalyze
the transfer of L-fucose from GDP-fucose to their acceptors. At
least eight 1, 3/4-FUT genes have been identified: FUT3, 4, 5, 6,
7, 9, 10 and FUT11 (16,17). The 1, 3-fucosylation of LeY is
catalyzed by fucosyltransferase IV (FUT4). Increased FUT4
expression has been reported in many cancers, such as gastric,
colorectal, and lung cancer (18–20).
Our previous study showed that high proliferative ability skin
cancer A431 cells carried a higher level of FUT4 than low
proliferative capability SCC12 cells (21). Moreover, high expression of FUT4
promoted cell proliferation by augmenting the synthesis of LeY
(22), while suppressing the
expression of FUT4 by RNAi technology reduced the synthesis of LeY
and inhibited cell growth (9).
Therefore, it is critical to find effective drugs which can
decrease the synthesis of FUT4/LeY and inhibit tumor growth.
Ginsenoside Rg3 is the main active component in
ginseng. Rg3 has a wide range of pharmacological and therapeutic
effects, including anti-inflammation, anti-fatigue, immune
stimulation and anticancer. Previous reports showed that Rg3 had
anticancer effect in gastric, breast, colon and hepatocellular
cancers (23–26). Currently, Rg3 is used clinically to
treat late-stage non-small cell lung cancer (NSCLC) in China. The
underlying anticancer mechanisms of Rg3 contain anti-proliferation,
induce apoptosis, anti-metastasis and anti-angiogenesis (26–28).
However, the mechanism of Rg3 on melanoma cell proliferation, and
its potential role in regulation of FUT4 and LeY expression on cell
proliferation have not been reported.
In the present study, we found that Rg3
significantly inhibited melanoma cell growth through inhibiting
EGFR phosphorylation with FUT4/LeY low expression in vitro
and in vivo. We suggest that Rg3 deactivation of the
EGFR/MAPK pathway through downregulating FUT4/LeY expression
performs a key role in the treatment of melanoma.
Materials and methods
Ethics statement
All animal work performed in this study was approved
by the Animal Ethics Committee of Dalian Medical University.
Moreover, the detail protocols and experimental processes conformed
to the Experimental Animal Management Regulations of Dalian Medical
University.
Reagents and antibodies
The 20 (R)-Rg3 was provided by Dalian Fu Sheng
Pharmaceutical Co. (Dalian, China). A solution of Rg3 as freshly
prepared in DMEM (500 μg/ml) and filtered by 0.22-μm membranes. It
was diluted with cell culture media to final concentration in
different treatments. DMEM, fetal bovine serum (FBS), TRIzol and
Lipofectamine™ 2000 reagents were purchased from Invitrogen
(Camarillo, CA, USA). DMSO and MTT were purchased from
Sigma-Aldrich (St. Louis, MO, USA). The enhanced chemiluminescence
(ECL) assay kit was purchased from Amersham (Pittsburgh, PA, USA).
Anti-rabbit FUT4, PCNA, p-ERK1/2, ERK, β-actin, HRP-conjugated
anti-mouse IgM, HRP-conjugated anti-rabbit and anti-mouse IgG
antibodies were purchased from Proteintech group (Wuhan, China).
EGFR and p-EGFR were purchased from Cell Signaling Technology
(Boston, MA, USA). FITC conjugated goat anti-mouse IgG and TRITC
conjugated goat anti-rabbit IgG were purchased from Santa Cruz
Biotechnology (Santa Cruz, CA, USA). HRP-Ulex Europaeus (UEA)
lectin was purchased by EY Laboratories (San Mateo, CA, USA), which
preferentially recognizes the total fucose. Mouse anti-Giantin
Golgi marker antibody and mouse anti-LeY antibody (BG-8) were
purchased from Abcam (Cambridge, UK). AG1478 inhibitor was obtained
from Sigma (St. Louis, MO, USA). Coomassie protein assay reagent
was purchased from Bio-Rad (Hercules, CA, USA).
Cell culture
Human melanoma cell line A375 (original commercial
source from American Type Cell Culture, ATCC, Manassas, VA, USA)
was a kind gift from Dr Xiao-Qi Wang (Department of Dermatology,
Northwestern University, IL, USA) and cultured in DMEM with 10%
FBS, 100 U/ml penicillin and 100 μg/ml streptomycin; maintained at
37°C under 5% CO2 in humidified air.
Transient transfection
A375 cells (1×105) were trypsinized and
seeded onto 6-well plates. When cells reached 70–80% confluence,
FUT4 siRNA was transiently transfected into A375 cells using
Lipofectamine™ 2000 Reagent following the manufacturer’s
instructions (Invitrogen, Carlsbad, CA, USA). The transfection
reagent was removed after 5 h and the cells were harvested after 48
h.
Cell viability assay
MTT assay was performed to detect the effect of Rg3
on A375 melanoma cell proliferation. In brief, cells
(2×103/well) were plated in 96-well plates. Twenty-four
hours later, cells were treated with different concentrations of
Rg3 (0, 25, 50 and 100 μg/ml) for another 24 h before MTT was added
into the culture medium (0.5 mg/ml). After incubation for 4 h at
37°C, the cells were lysed with DMSO at room temperature for 10
min. The absorbance was measured at 490 nm on a microplate reader
(Bio-Rad). The test was repeated three times.
Colony forming assay
Cells (1×103 cells/well) were plated in
6-well plates containing DMEM with 10% FBS at 37°C. After 24 h,
cells were treated with different concentrations of Rg3 (0, 25, 50
and 100 μg/ml) for 24 h and then cells were allowed to grow for 7
days in the absence of Rg3. Cells were fixed and stained with
crystal violet (0.5%) for 20 min at room temperature. Images were
captured with the inverted microscope (Olympus IX71, Japan).
Immunoblotting
Cells were washed with PBS (pH 7.4), and incubated
with lysis buffer [1% Triton X-100, 150 mM NaCl, 10 mM Tris, pH
7.4, 1 mM ethylenediaminetetraacetic acid (EDTA), 1 mM
ethyleneglycol tetraacetic acid (EGTA), pH 8.0, 0.2 mM
Na3VO4, 0.2 mM phenylmethylsulfonyl fluoride,
0.5% Nonidet P-40] on ice for 15 min. The cell lysates were
clarified by centrifugation at 9000 × g for 10 min and the
supernatants were collected. Protein concentration was determined
with the Coomassie protein assay reagent (Bio-Rad) using BSA as a
standard. Cell lysates (50–100 μg/ml) were separated by an 8–12%
SDS-PAGE gel electrophoresis. Samples were transferred
electrophoretically to nitrocellulose membranes or PVDF (0.2 μm),
blocked with TTBS (50 mM Tris-HCl, pH 7.5, 0.15 M NaCl, 0.1%
Tween-20) containing 5% fat-free dry milk followed by overnight
incubation at 4°C with anti-FUT4 (1:1,000), anti-LeY (1:200),
anti-PCNA (1:1,000), anti-EGFR (1:500), anti-pEGFR (1:500),
anti-ERK1/2 (1:500) and anti-pERK1/2 (1:500). The specific antibody
binding was detected using HRP-conjugated anti-rabbit or anti-mouse
antibody (1:2,000) for 45 min at room temperature. β-actin antibody
(1:1,000) was used to confirm the equal loading. Immunoreactive
proteins were visualized with ECL detection system and data were
analyzed by Image Lab.
Indirect immunofluorescent staining
Cells were grown on glass coverslips. After washing
with PBS, cells were fixed in 4% paraformaldehyde-PBS for 30 min
and followed treatment with 0.1% Triton X-100 for 10 min at 4°C.
After being blocked with goat serum for 30 min (37°C), cells were
incubated with rabbit anti-FUT4 (1:100) and mouse anti-Giantin
Golgi marker antibody (1:100) at 4°C overnight. TRITC-conjugated
goat anti-rabbit IgG and FITC-conjugated goat anti-mouse IgG
secondary antibody were incubated for 1 h at room temperature.
Images were captured with the inverted and confocal microscope
(Olympus IX71, Japan and LEICA TCS SP5 II, Germany).
Xenograft tumor mouse model
Male nude mice (Balb/c-nu/nu) were obtained from
Animal Center (Dalian Medical University). The animals (4–6 weeks)
were maintained under sterile conditions during the entire
experimental period. A375 cells (2×106) suspended in 0.2
ml PBS were injected subcutaneously into the right flank. After 7
days of tumor development, mice were randomly divided into 5
different groups (n=6/group). Rg3 20 mg/kg of body weight, FUT4
siRNA plasmid (siFUT4) (6 mg/kg) or Rg3 (20 mg/kg) plus siFUT4 (6
mg/kg) were subcutaneously administered for 3 weeks with time
interval of 48 h. The mice treated with vehicle or empty vector
only served as controls. Tumor volume was measured by Vernier
calipers every other day after tumor inoculation. The tumor volume
was calculated according to the formula (volume = 1/2 length ×
width2). At the end of the experiment (the 30th day),
the tumor mass was weighed.
Immunohistochemical staining
The expression of FUT4 was analyzed by
immunohistochemistry (IHC) using paraffin-embedded melanoma patient
tissues. Serial sections (4 μm each) were prepared, deparaffinized
in xylene and rehydrated in a graded alcohol. After microwaved for
20 min in citrate buffer to expose the antigen and washed with PBS,
slides were incubated in 3% H2O2 for 10 min
at room temperature to block endogenous peroxidase activity.
Non-specific binding was blocked with goat serum at room
temperature for 30 min before incubation overnight at 4°C with
rabbit IgG FUT4 (1:100). After extensive washing with PBS, sections
were incubated with respect secondary antibody for 30 min at room
temperature. The signal was visualized with peroxidase-labeled
streptavidin complexes DAB and the sections were briefly
counterstained with hematoxylin. Yellowish-brown stain indicated a
positive result. The negative control was generated by replacing
the primary antibody with isotype IgG. Slides were mounted and
visualized on an inverted microscope (Nikon Ti-DS, Japan).
Statistical analysis
Each experiment was repeated 3 times and results
presented as the mean ± SEM. P<0.05 was considered to be
significant and P<0.01 was considered to be highly significant.
Statistical software SPSS ver. 16 was used for analyzing the
data.
Results
Rg3 inhibits cell growth in A375 melanoma
cells
In order to study the antitumor effect of Rg3
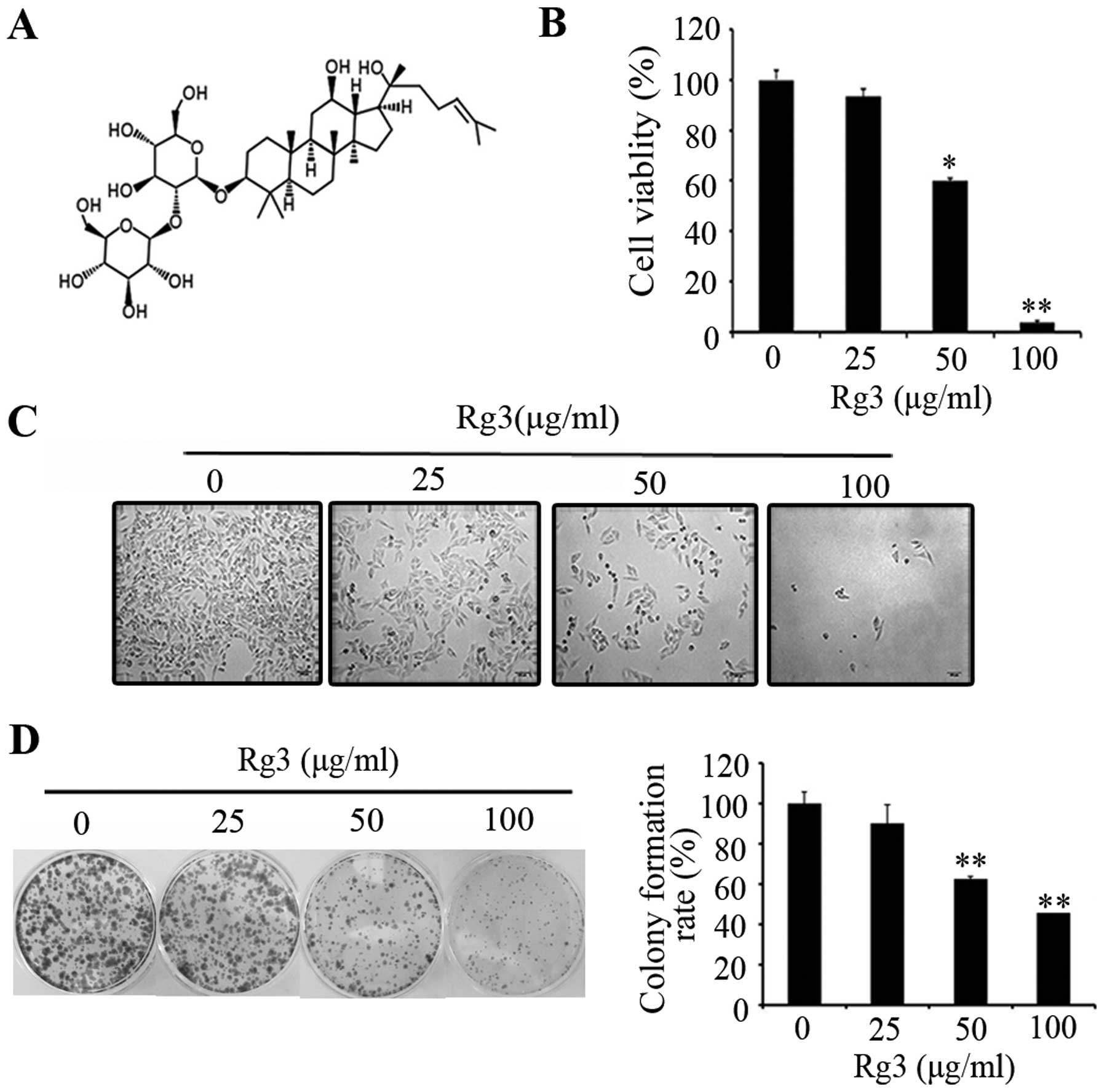
(structure is shown in Fig. 1A) on
cell proliferation, melanoma cells were treated with Rg3 at
different concentrations (0–100 μg/ml) for 24 h. We observed that
Rg3 significantly inhibited cell proliferation in a dose-dependent
manner as determined by MTT assay (Fig. 1B), representative images (Fig. 1C) of colony forming assay (Fig. 1D) are shown. There was a
significant inhibitory effect of Rg3 on cell proliferation as
compared with the untreated cells. These results demonstrate that
Rg3 inhibits human melanoma cell proliferation.
Rg3 decreases the expression of FUT4 and
LeY
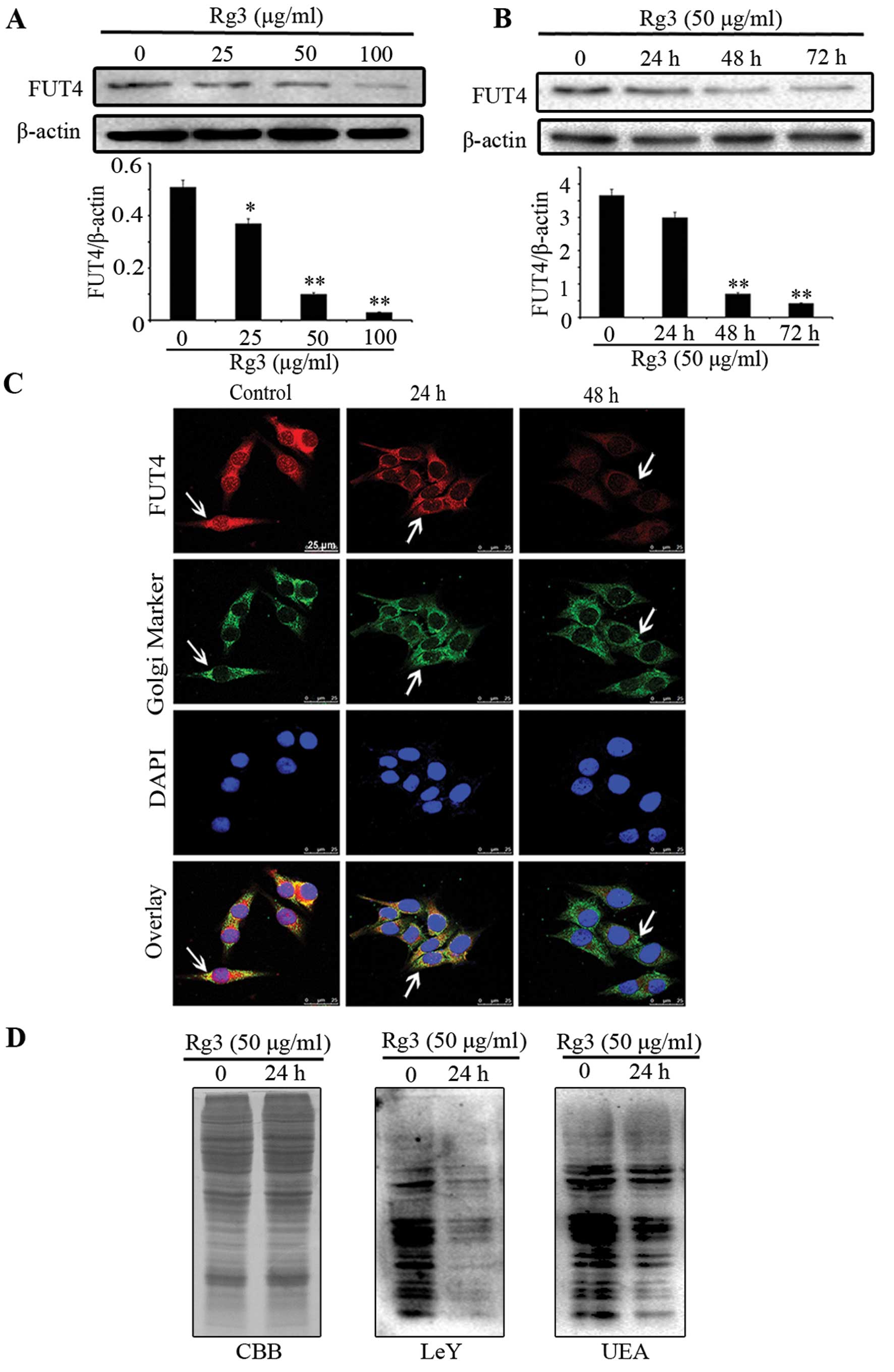
By western blotting (Fig. 2A and B), we found that Rg3
significantly inhibited FUT4 expression in a dose- and
time-dependent manner. Moreover, confocal staining further
confirmed the above results and showed that FUT4 was predominantly
co-localized at position of Golgi apparatus with Giantin (Fig. 2C). To detect the protein expression
of LeY and UEA lectin in cells treated without or with Rg3,
Coomassie brilliant blue (CBB) staining and western blotting were
employed (Fig. 2D). Western
blotting revealed that Rg3 significantly inhibited the protein
expression level of LeY and UEA lectin as compared with untreated
cells. These results indicate that Rg3 inhibits the expression of
FUT4 in a dose- and time-dependent manner. Furthermore, Rg3
effectively interferes with the synthesis of LeY antigen.
Downregulating FUT4 expression inhibits
A375 cell proliferation and decreases the expression of LeY
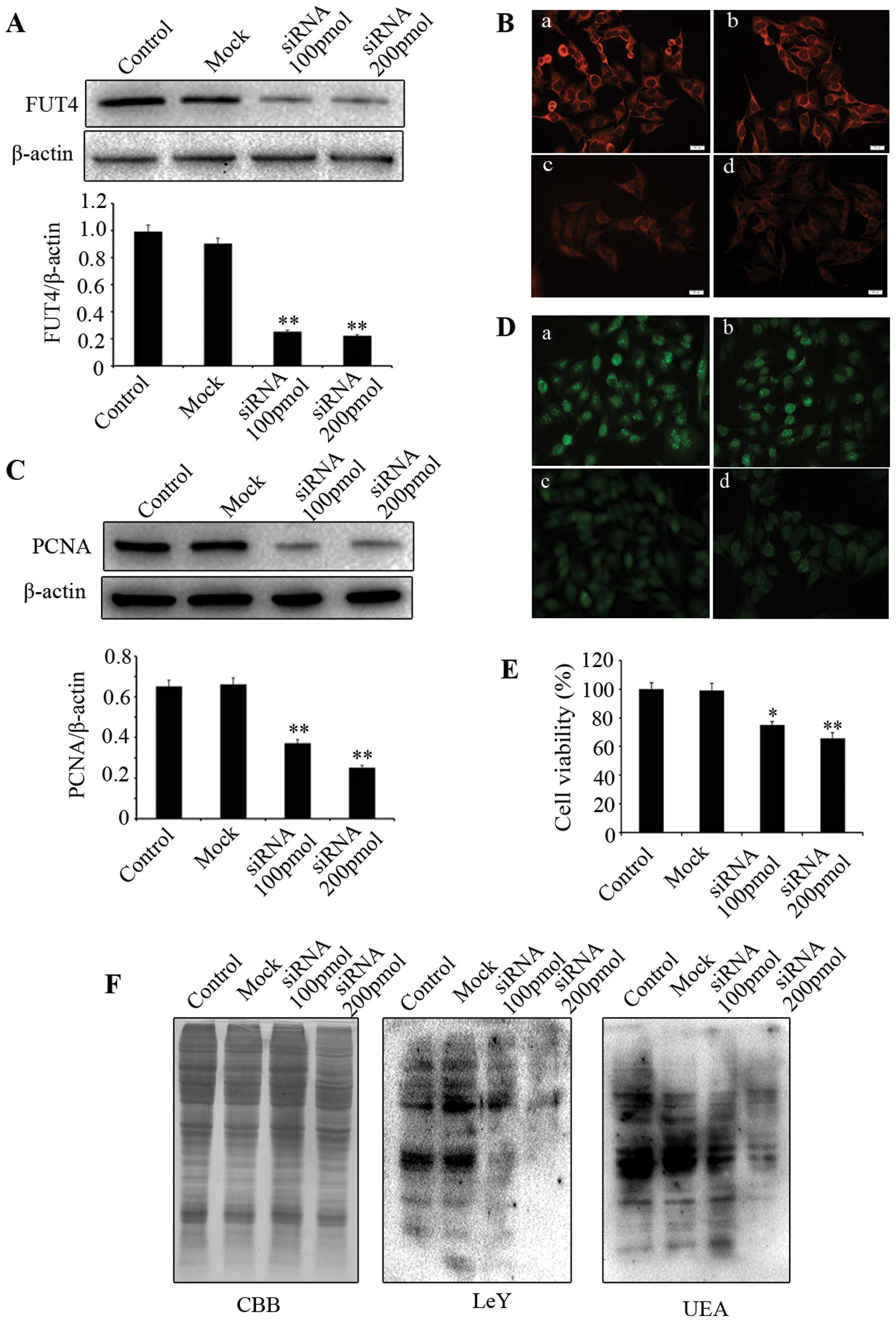
To investigate whether downregulating FUT4
expression affects cell proliferation, western blotting and
immunofluorescent staining were employed. PCNA is a commonly used
cell proliferation marker. We showed that knocking down FUT4
expression with FUT4 siRNA (Fig. 3A
and B) led to significant reduction of PCNA by western blotting
(Fig. 3C) and immunofluorescence
(Fig. 3D). MTT assay indicated
that cell viability in FUT4 knockdown cells was significantly
decreased in comparison with the mock treated and untransfected
cells (Fig. 3E). Moreover, FUT4
siRNA transfection showed significantly decreased expression of LeY
and UEA lectin as compared to mock treated and untreated cells
(Fig. 3F). These results indicate
that knockdown of FUT4 inhibits melanoma cell proliferation, which
correlates to its inhibitory effect on the synthesis of LeY.
Downregulating FUT4 expression decreases
the tyrosine phosphorylation of EGF-mediated EGFR/MAPK
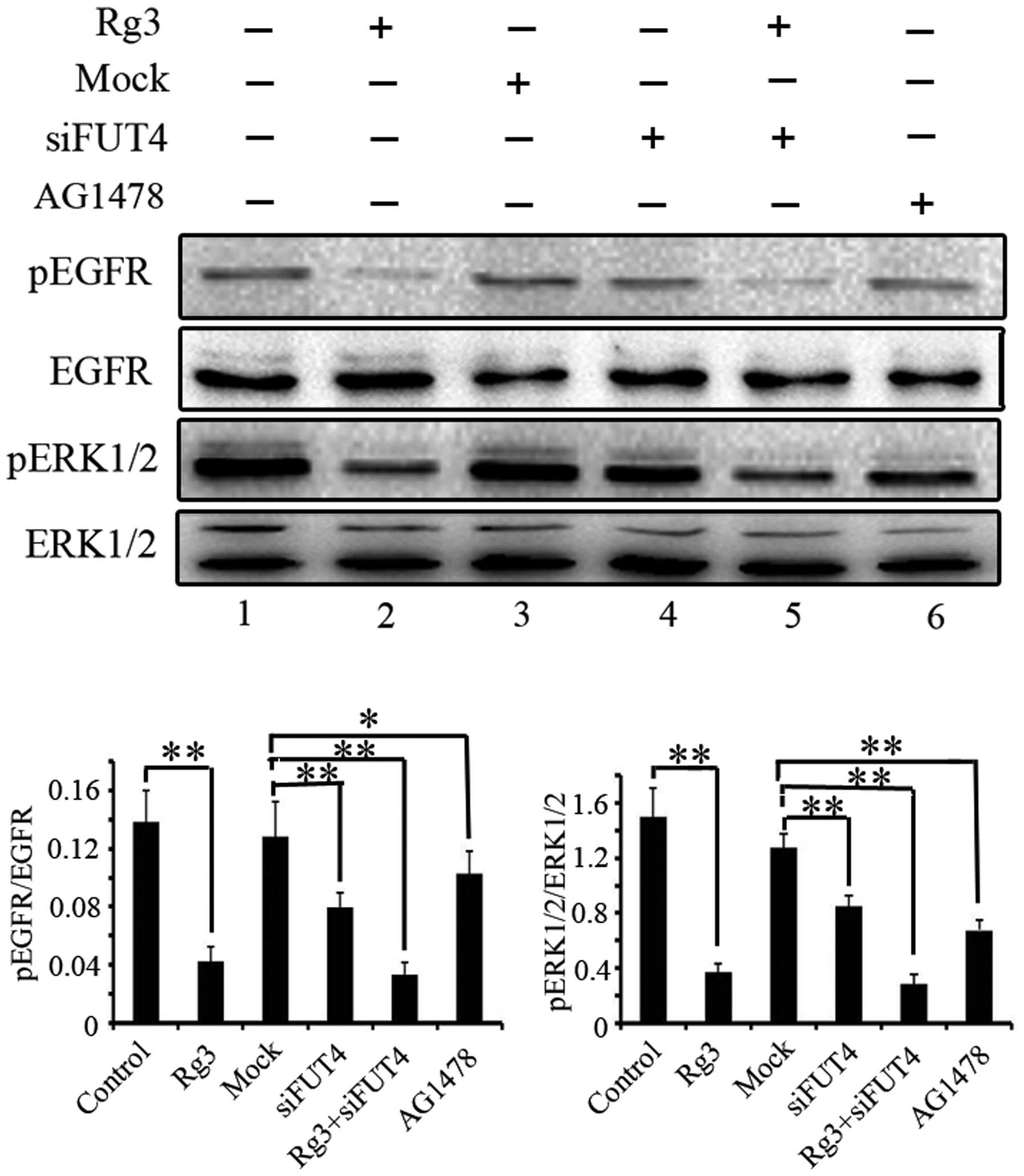
The phosphorylation of epidermal growth factor
receptor (EGFR) and extracellular signal-regulated kinases (ERK1/2)
were examined to determine the inhibitory role of Rg3 on EGFR/MAPK
pathway by western blotting. Cells were treated with Rg3, FUT4
siRNA and EGFR inhibitor (AG1478, 10−4 M) for 24 h.
Pretreated with FUT4 siRNA and followed by Rg3 treatment for 24 h.
Antibodies directed against pEGFR, EGFR, pERK1/2 and ERK, were used
in western blotting (Fig. 4).
Treatment with Rg3, FUT4 siRNA or Rg3 in combination with FUT4
siRNA significantly inhibited the phosphorylation of EGFR and
ERK1/2 (Fig. 4, lanes 2, 4 and 5
vs. lane 1). Moreover, the EGFR inhibitor (AG1478) was also used to
detect the EGFR/MAPK activation as an inhibitor control (Fig. 4, lane 6). The results indicate that
the inhibition of the EGFR/MAPK pathway is at least part of the
mechanism by which Rg3 inhibits melanoma cell proliferation.
Rg3 inhibits the growth of melanoma
xenograft tumors in vivo
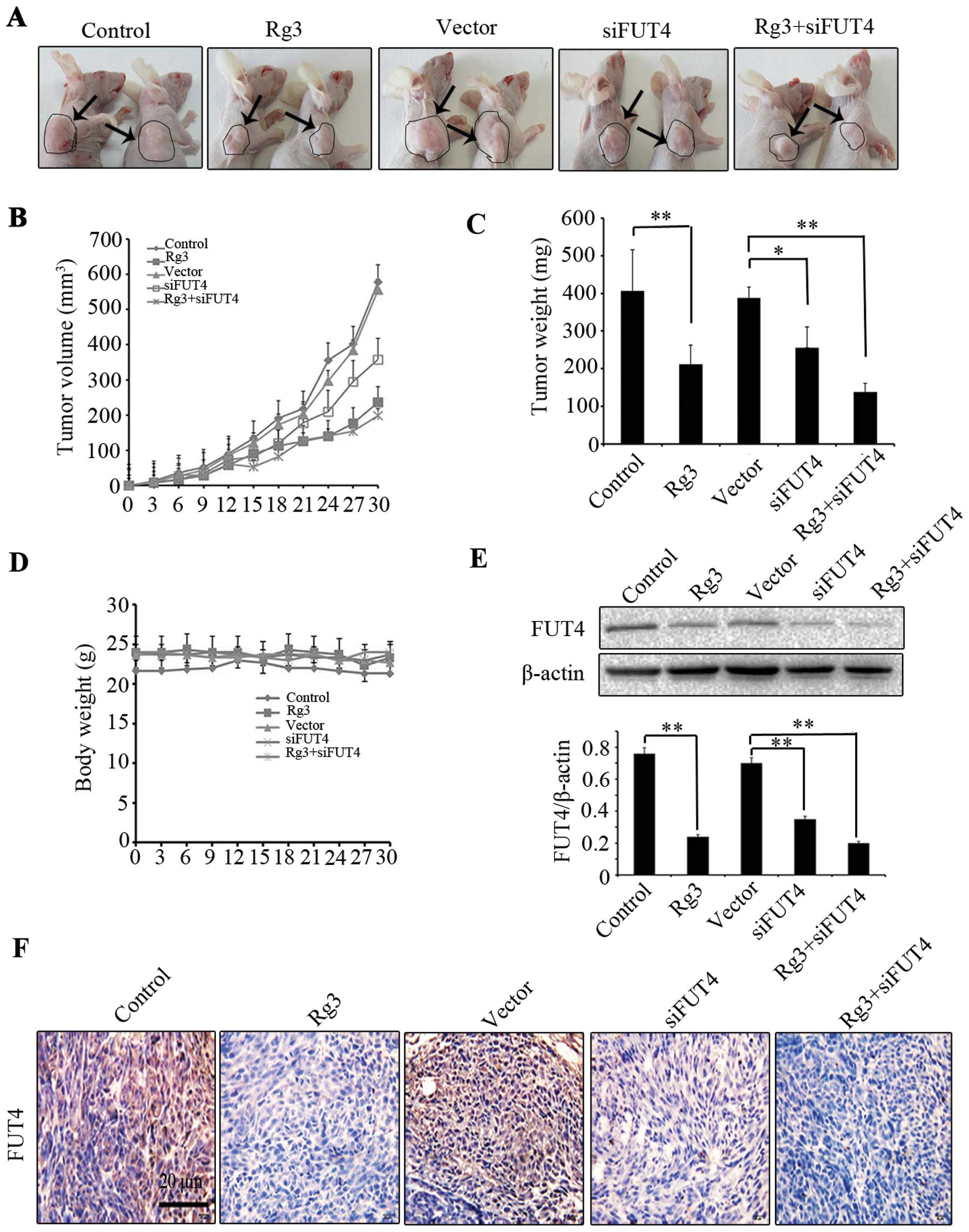
The antitumor effect of Rg3 on tumor growth was
further validated in vivo. Mice were randomly divided into
five groups for different treatments with six mice per group. Each
treatment group was analyzed clinically (tumor volume, tumor weight
and body weight) and each treatment on FUT4 expression was
determined by western blotting and immunohistochemical staining. We
found that Rg3 treatment group showed a significant inhibition of
xenograft tumor volume by 52.50% (P<0.05), and the siFUT4
treatment group inhibited tumor volume by 35.79% (P<0.05); while
Rg3 combined with siFUT4 treatment group inhibited tumor volume by
64.38% (P<0.01), indicating that greater inhibition was achieved
than Rg3 or siFUT4 treatment group alone (Fig. 5A and B). The mice were sacrificed
and tumor weight of each group on day 30 was compared. Rg3
(0.212±0.054 g) and siFUT4 (0.256±0.043 g) treatment groups showed
low tumor weight as compared to vector and non-treated controls
(0.406±0.110 g) (P<0.05). Moreover, combination treatment with
Rg3 and siFUT4 led to much lower tumor weight (0.138±0.052 g) as
compared to the mice treated with Rg3 or siFUT4 alone (Fig. 5C). There were no significant
differences in body weight between control and treatment group
(Fig. 5D). To investigate the
mechanism of Rg3 inhibition on melanoma tumor growth, we analyzed
FUT4 expression in Rg3, FUT4 siRNA or combination treatment group
by western blotting and immunohistochemical staining. We found that
Rg3 and siFUT4 treated group showed decreased FUT4 expression as
compared to non-treated and vector-treated control group, while
combinational treatment showed much lower expression of FUT4
compared with the mice treatment with Rg3 or siFUT4 alone (Fig. 5E and F). The results suggest that
Rg3 inhibit melanoma growth in vivo.
Discussion
Ginsenoside Rg3 is a monomer extracted from ginseng
roots and it has strong antitumor activity. Previous studies have
shown that Rg3 inhibits proliferation and induces apoptosis in
gastric, hepatic and colorectal cancers (23,26,29,30),
suppresses migration, invasion in lung cancer (28), enhances the susceptibility to
docetaxel in colon cancer (31)
and inhibits autophagy and sensitizes to doxorubicin in
hepatocellular carcinoma (29). In
the present study, we investigated the anticancer activity of Rg3
on human melanoma cells both in vitro and in vivo. We
found that Rg3 inhibited melanoma cell proliferation in a
dose-dependent manner. Moreover, Rg3 significantly reduced
xenograft melanoma volume and weight when compared to the control
group. These results indicate that Rg3 is a potential drug to
inhibit melanoma proliferation.
The antitumor effects of Rg3 have been reported in
many cancers, but whether Rg3 antitumor effect correlates to its
regulatory effect on fucosylation is unclear. Several reports have
shown that fucosyltransferases (FUTs) play a critical role on tumor
progression. Yang et al proved that overexpression of FUT4
promoted A431 cell proliferation and inhibited cell apoptosis
(32,33). Zhang et al found that
suppression of FUT1/FUT4 expression by siRNA inhibited tumor growth
(9). Ciolczyk-Wierzbicka et
al demonstrated that higher expression of fucosyltransferases
(FUT1, FUT4) played an important role in the formation of surface
structures that facilitate metastasis of melanoma (34). In our study, we found that Rg3
inhibited melanoma cell proliferation through downregulation of
FUT4 both in vitro and in vivo. Rg3 decreased the
expression of FUT4 in a dose- and time-dependent manner. Moreover,
Rg3 combined with FUT4 siRNA showed stronger effect than the
treatment with Rg3 or FUT4 siRNA alone in melanoma xenograft
models. These results suggest that Rg3 mediates inhibition of FUT4
expression and is involved in its inhibitory effect on cell
proliferation. Rg3 is a potential FUT4 inhibitor and Rg3 combined
with FUT4 siRNA may be a new therapy strategy in the treatment of
melanoma.
The synthesis of LeY can be regulated at the
transcriptional level by FUT4 (35). Abnormal elevation of LeY expression
can be seen in many cancers correlating to malignant
transformation. High expression of LeY promoted cell proliferation
in ovarian cancer (8), decreased
survival in lymph node negative breast carcinomas (36) and was also a strong risk factor for
chemotherapeutic drug resistance in ovarian carcinoma patients
(13,37). However, the antitumor effect of Rg3
on melanoma and its mechanism on FUT4 and LeY expression remains
unknown. In this study, we demonstrated that Rg3 decreased the
expression of FUT4/LeY and inhibited cell proliferation. Similar
results were also achieved by knocking down FUT4 with its siRNA.
Moreover, Rg3 combined with FUT4 siRNA showed greater inhibitory
effect than the treatment with Rg3 alone. These results indicate
that Rg3 inhibits FUT4 expression and FUT4 further reduces LeY
synthesis, by which to inhibit cell proliferation through EGFR/MAPK
signaling pathway. To our knowledge, we report for the first time
the inhibitory role of Rg3 on human melanoma growth by reducing
fucosylation.
EGFR can be directly or indirectly activated by
different growth factors, which promote aberrant EGFR signaling
activation and facilitate cell proliferation. Because of its role
on tumor progression, the EGFR has been intensely studied as a
therapeutic target (15). LeY is
one of the glycoproteins carried by EGFR, changes of EGFR
glycosylation may activate growth-factor receptor tyrosine kinases
and promote tumor proliferation. LeY antibody (IGN311) inhibited
EGFR-mediated MAPK signaling activation and prevented tumor growth
(38). We have previously reported
that FUT4 siRNA mediated deactivation of EGFR/MAPK pathway to
inhibit cancer cell proliferation (9). Herein, we investigated the role of
Rg3 regulated FUT4/LeY expression on EGFR-mediated MAPK signaling
pathway. We found that Rg3, FUT4 siRNA and Rg3 combined with FUT4
siRNA led to reduced activation of EGFR and ERK1/2 in A375 melanoma
cells.
In conclusion, our results suggest that Rg3 inhibits
cell proliferation by downregulating the EGFR/MAPK signaling
pathway through reducing FUT4/LeY expression. To our knowledge,
this is the first study to evaluate the molecular mechanism of Rg3
on melanoma proliferation and the role of Rg3 regulated
fucosylation changes on melanoma growth. Our results indicate that
Rg3 may be a promising therapeutic drug for melanoma treatment.
Acknowledgements
This study was supported by the China 973 grant no.
2012CB822100, National Natural Science Foundation of China Research
grants no. 30672753 and 31270866.
Abbreviations:
|
FUT4
|
fucosyltransferase IV
|
|
LeY
|
Lewis Y
|
|
EGFR
|
epidermal growth factor receptor
|
|
MAPK
|
mitogen-activated protein kinases
|
|
ERK
|
extracellular regulated protein
kinases
|
|
MTT
|
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide
|
|
TACA
|
tumor-associated carbohydrate
antigen
|
References
|
1
|
Ives NJ, Stowe RL, Lorigan P and Wheatley
K: Chemotherapy compared with biochemotherapy for the treatment of
metastatic melanoma: a meta-analysis of 18 trials involving 2,621
patients. J Clin Oncol. 25:5426–5434. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Balch CM, Gershenwald JE, Soong SJ, et al:
Final version of 2009 AJCC melanoma staging and classification. J
Clin Oncol. 27:6199–6206. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Menzies AM and Lon GV: Recent advances in
melanoma systemic therapy, BRAF inhibitors, CTLA4 antibodies and
beyond. Eur J Cancer. 49:3229–3241. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Ma C and Armstrong AW: Severe adverse
events from the treatment of advanced melanoma: a systematic review
of severe side effects associated with ipilimumab, vemurafenib,
interferon alfa-2b, dacarbazine and interleukin-2. J Dermatolog
Treat. 25:401–408. 2014. View Article : Google Scholar
|
|
5
|
Menaa F: Latest approved therapies for
metastatic melanoma: what comes next? J Skin Cancer. 1:1–10. 2013.
View Article : Google Scholar
|
|
6
|
Zhang S, Zhang HS, Cordon-Cardo C, Reuter
VE, Singhal AK, Lioyd KO and Livingston PO: Selection of tumor
antigens as targets for immune attack using immunohistochemistry:
II. Blood group-related antigens. Int J Cancer. 73:50–56. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Cao Y, Merling A, Karsten U and
Schwartz-Albiez R: The fucosylated histoblood group antigens H type
2 (blood group O, CD173) and Lewis Y (CD174) are expressed on
CD34+ hematopoietic progenitors but absent on mature
lymphocytes. Glycobiology. 11:677–683. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Escrevente C, Machado E, Brito C, Reis CA,
Stoeck A, Runz S, Marmé A, Altevogt P and Costa J: Different
expression levels of alpha3/4 fucosyltransferases and Lewis
determinants in ovarian carcinoma tissues and cell lines. Int J
Oncol. 29:557–566. 2006.PubMed/NCBI
|
|
9
|
Zhang Z, Sun P, Liu J, Fu L and Yan Q:
Suppression of FUT1/FUT4 expression by siRNA inhibits tumor growth.
Biochim Biophys Acta. 1783:287–296. 2008. View Article : Google Scholar
|
|
10
|
Wang YF, Liu JJ, Lin B, Wang CZ, Li Q, Liu
S, Yan L, Zhang S and Iwamori M: Study on the expression and
clinical significances of Lewis y antigen and integrin αv, β3 in
epithelial ovarian tumors. Int J Mol Sci. 12:3409–3421. 2011.
View Article : Google Scholar
|
|
11
|
Kuo CH, Chen PK, Chang BI, Sung MC, Shi
CS, Lee JS, Chang CF, Shi JY and Wu HL: The recombinant lectin-like
domain of thrombomodulin inhibits angiogenesis through interaction
with Lewis Y antigen. Blood. 119:1302–1313. 2012. View Article : Google Scholar
|
|
12
|
Gao J, Hu ZH, Liu JJ, Liu DW, Wang Y, Cai
M, Zhang D, Tan M and Lin B: Expression of CD147 and Lewis y
antigen in ovarian cancer and their relationship to drug
resistance. Med Oncol. 31:920–929. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Hu ZH, Gao J, Liu B, et al: High
expression of Lewis y antigen and CD44 is correlated with
resistance to chemotherapy in epithelial ovarian cancers. PLoS One.
8:e572502013. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Noble P, Spendlove I, Harding S, Parsons T
and Durrant LG: Durrant therapeutic targeting of Lewisy
and Lewisb with a novel monoclonal antibody 692/29. PLoS
One. 8:e548922013. View Article : Google Scholar
|
|
15
|
Zandi R, Larsen AB, Anderse P, Stockhausen
MT and Poulsen HS: Mechanism for oncogenic activation of the
epidermal growth factor receptor. Cell Signal. 19:2013–2023. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Weston BW, Nair RP, Larsen RD and Lowe JB:
Isolation of a novel human alpha (1,3)-fucosyltransferase gene and
molecular comparison to the human Lewis blood group alpha (1,3/1,4)
fucosyltransferase gene. Syntenic, homologous, nonallelic genes
encoding enzymes with distinct acceptor substrate specificities. J
Biol Chem. 267:4152–4160. 1992.PubMed/NCBI
|
|
17
|
Weston BW, Smith PL, Kelly RJ and Lowe JB:
Molecular cloning of a fourth member of a human alpha
(1,3)-fucosyltransferase gene family. Multiple homologous sequences
that determine expression of the Lewis x, sialyl Lewis x, and
difucosylsialyl Lewis x epitopes. J Biol Chem. 267:24575–24584.
1992.PubMed/NCBI
|
|
18
|
Petretti T, Schulze B, Schlag PM and
Kemmner W: Altered mRNA expression of glycosyltransferases in human
gastric carcinomas. Biochim Biophys Acta. 1428:209–218. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Ito H, Hiraiwa N, Kannaqi R, et al:
Altered mRNA expression of specific molecular species of fucosyl-
and sialyl-transferases in human colorectal cancer tissues. Int J
Cancer. 71:556–564. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Ogawa J, Inoue H and Koide S: Expression
of alpha-1, 3-fucosyltransferase type IV and VII genes is related
to poor prognosis in lung cancer. Cancer Res. 56:325–329.
1996.PubMed/NCBI
|
|
21
|
Li HY, Tong SM, Liu JW, Han L, Hou H, Yan
Q and Wang XQ: Differential fucosyltransferase IV expression in
squamous carcinoma cells is regulated by promoter methylation. Cell
Mol Biol Lett. 17:206–216. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Yang X, Zhang Z, Jia S, Liu Y, Wang X and
Yan Q: Overexpression of fucosyltransferase IV in A431 cell line
increases cell proliferation. Int J Biochem Cell Biol.
39:1722–1730. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Park EH, Kim YJ, Kang KS, et al: Stereo
specific anticancer effects of ginsenoside Rg3 epimers isolated
from heat-processed American ginseng on human gastric cancer cell.
J Ginseng Res. 38:22–27. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Chen XP, Qian LL, Hong JH and Chen JH:
Ginsenoside Rg3 inhibits CXCR4 expression and related migrations in
a breast cancer cell line. Int J Clin Oncol. 16:519–523. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Lee SY, Kim GT, Roh SH, Song JS, Kim HJ,
Hong SS, Kwon SW and Park JH: Proteomic analysis of the anti-cancer
effect of 20S-Ginsenoside Rg3 in human colon cancer cell lines.
Biosci Biotechnol Biochem. 73:811–816. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Zhang C, Liu LJ, Yu Y, Chen B, Tang C and
Li X: Antitumor effects of ginsenoside Rg3 on human hepatocellular
carcinoma cells. Mol Med Rep. 5:1295–1298. 2012.PubMed/NCBI
|
|
27
|
Kim JW, Jung SY, Kwon YH, Lee JH, Lee YM,
Lee BY and Kwon SM: Ginsenoside Rg3 attenuates tumor angiogenesis
via inhibiting bioactivities of endothelial progenitor cells.
Cancer Biol Ther. 13:504–515. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Kim YJ, Choi WI, Ko H, et al: Stereo
specific effects of ginsenoside 20-Rg3 inhibits TGF1-induced
epithelial-mesenchymal transition and suppresses lung cancer
migration, invasion and anoikis resistance. Toxicology. 322:23–33.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Kim DG, Jung KH, Kim YS, et al: 20
(S)-Ginsenoside Rg3 is a novel inhibitor of autophagy and
sensitizes hepatocellular carcinoma to doxorubicin. Oncotarget.
5:4438–4451. 2014.PubMed/NCBI
|
|
30
|
He BC, Gao JL, Zhou BQ, et al: Ginsenoside
Rg3 inhibits colorectal tumor growth through the down-regulation of
Wnt/β-catenin signaling. Int J Oncol. 38:437–445. 2011. View Article : Google Scholar
|
|
31
|
Kim SM, Lee SY, Yuk DY, Moon DC, Choi SS,
Kim Y, Han SB, Oh KW and Hong JT: Inhibition of NF-κB by
ginsenoside Rg3 enhances the susceptibility of colon cancer cells
to docetaxel. Arch Pharm Res. 32:755–765. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Yang XS, Liu S, Liu YJ, Liu JW, Liu TJ,
Wang XQ and Yan Q: Overexpression of fucosyltransferase IV promotes
A431 cell proliferation through activating MAPK and PI3K/Akt
signaling pathways. J Cell Physiol. 225:612–619. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Yang XS, Liu YJ, Liu JW, Wang XQ and Yan
Q: Cyclophosphamide-induced apoptosis in A431 cells is inhibited by
fucosyltransferase IV. J Cell Biochem. 112:1376–1383. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Ciolczyk-Wierzbicka D, Bodzioch M, Gil D,
Zmudzinska D, Dembinska-Kiec A and Laidler P: Expression of
fucosyltransferases contributes to melanoma invasive phenotype. Med
Chem. 3:418–424. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Azuma Y, Ito M, Taniguchi A and Matsumoto
K: Expression of cell surface Lewis X and Y antigens and FUT4 mRNA
is increased in Jurkat cells undergoing apoptosis. Biochim Biophys
Acta. 1672:157–163. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Madjd Z, Parsons T, Watson N, Spendlove I,
Ellis I and Durrant LG: High expression of Lewisy/b antigens is
associated with decreased survival in lymph node negative breast
carcinomas. Breast Cancer Res. 7:780–787. 2005. View Article : Google Scholar
|
|
37
|
Hu ZH, Gao S, Lin B, et al: Elevated
levels of Lewis Y and integrin α5β1 correlate with chemotherapeutic
drug resistance in epithelial ovarian carcinoma. Int J Mol Sci.
13:15588–15600. 2012. View Article : Google Scholar
|
|
38
|
Farhan H, Schuster C, Kircheis R, et al:
Inhibition of xenograft tumor growth and down-regulation of ErbB
receptors by an antibody directed against Lewis Y antigen. J
Pharmacology Exp Ther. 319:1459–1466. 2006. View Article : Google Scholar
|