Introduction
Hepatocellular carcinoma (HCC) is a common cancer
with high morbidity and mortality rates (1). Although prognosis of patients with
HCC has improved due to the development of techniques for early
detection and treatment, the overall survival rates in patients
with poorly differentiated HCC remain low (2).
Fucoidan is a water-soluble dietary fiber derived
from brown seaweed. It is composed of polysaccharides containing
L-fucose and sulfate ester groups (3). Fucoidan has various biological
activities including anti-inflammatory, anti-viral and
antihypertensive actions (4,5).
Furthermore, fucoidan exerts anticancer activity and is reported to
inhibit the proliferation of colon, breast and prostate cancer cell
lines (6–8). Although fucoidan suppresses HCC
proliferation, most of the cell lines used in previous studies,
including HepG2 and Huh-7 cells, were well-differentiated (9–14).
Thus, the anticancer activity of fucoidan against poorly
differentiated HCC cells, such as the HLF cell line, remains
unclear.
The mechanisms of fucoidan-mediated hepatoma
inhibition have been investigated. However, Zhu et al
reported that fucoidan does not inhibit vascular endothelial growth
factor, basic fibroblast growth factor, interleukin-8, or
heparanase expression in HCC cells and/or tumor tissues, and that
it had no effect on angiogenesis and apoptosis in vivo
(12). Thus, the mechanisms of
fucoidan-mediated hepatoma inhibition remain unclear. Adenosine
monophosphate-activated protein kinase (AMPK) is a sensor of
cellular energy and nutrient status and can crosstalk with
signaling pathways that promote proliferation (15). Activated AMPK regulates molecules
associated with protein, glucose and lipid metabolism and the cell
cycle, thereby suppressing cancer proliferation (16,17).
However, the effects of fucoidan on AMPK and its downstream
molecules are unknown.
The aim of this study was to investigate the effects
of fucoidan on the proliferation of the poorly differentiated HCC
cell line HLF. Additionally, we examined the effects of fucoidan on
AMPK and its downstream metabolism and cell cycle-associated
molecules in HLF cells.
Materials and methods
Reagents and antibodies
Fucoidan from Fucus vesiculosus was purchased
from Sigma-Aldrich Co. LLC (St. Louis, MO, USA). All other reagents
were purchased from Wako Pure Chemical Industries, Ltd. (Osaka,
Japan) unless otherwise indicated. Antibodies against AMPK,
phosphorylated (p)-AMPK (Thr172), tuberous sclerosis complex (TSC)
2, p-TSC2 (Thr1462), mammalian target of rapamycin (mTOR), p-mTOR
(Ser2448), p-eukaryotic elongation factor (eEF) 2 (Thr56),
eukaryotic elongation factor 2 kinase (eEF2K), p-eEF2K (Ser366),
glycogen synthase kinase (GSK) 3β, p-GSK3α (Ser21), p-GSK3β (Ser9),
adenosine triphosphate (ATP)-citrate lyase (ACL), p-ACL (Ser454),
acetyl-CoA carboxylase (ACC), p-ACC (Ser79), retinoblastoma (Rb),
p16, p53, p-p53 (Ser9), p-p53 (Ser15), cyclin D1, cyclin-dependent
kinase (CDK) 4, CDK6 and glyceraldehyde-3-phosphate dehydrogenase
(GAPDH) were purchased from Cell Signaling Technology, Inc.
(Danvers, MA, USA). Antibodies against p21 were purchased from BD
Biosciences (San Jose, CA, USA).
Cell lines
HLF cells were maintained in Dulbecco’s modified
Eagle’s medium supplemented with 10% fetal bovine serum (Life
Technologies; Japan, Tokyo, Japan), penicillin (100 U/ml) and
streptomycin (100 U/ml) at 37°C in a humidified atmosphere
containing 5% CO2.
Fucoidan treatment
HLF cells were seeded (1×104 cells) in
10-cm dishes. Fucoidan was dissolved in phosphate buffered saline
(PBS; 130 mM NaCl, 2 mM NaH2PO4 and 7 mM
Na2HPO4, pH 7.4), and 10, 50 or 100 μg/ml of
fucoidan was added 2 h after seeding. The culture medium containing
fucoidan was replaced every 24 h, until 96 h.
Cell proliferation analysis
Cell proliferation was evaluated by counting cells.
Cells were counted 0, 24, 48, 72 and 96 h after treatment with
fucoidan (n=4 per condition) or PBS (control; n=4). Cells were
trypsinized after washing with PBS. Then, cell numbers were
determined using an automated cell counter (CDA-500; Sysmex Corp.,
Kobe, Japan) as previously described (18).
Immunoblotting analysis
After washes with PBS, cells were lysed in lysis
buffer (50 mM HEPES, 250 mM NaCl, 20 mM EDTA and 0.1% Nonidet P-40,
pH 7.5) containing 1 mM phenylmethylsulfonyl fluoride, a protease
inhibitor cocktail (Sigma-Aldrich Co. LLC.), 10 nM NaF and 1 mM
Na3VO4. Cell lysates were centrifuged at
12,000 × g for 20 min at 4°C, and the supernatant was collected.
The protein concentration was determined by a Bio-Rad protein assay
kit (Bio-Rad Laboratories, Inc., Hercules, CA, USA). The samples
were then mixed with an equal volume of 2X sample loading buffer
containing 2% sodium dodecyl sulfate (SDS) and 2-mercaptoethanol,
and were incubated at 95°C for 5 min. Samples were loaded on
SDS-polyacrylamide gels and transferred onto equilibrated
polyvinylidene difluoride membranes (Bio-Rad Laboratories, Inc.).
The membranes were blocked for 1 h at room temperature with 5% skim
milk, and then incubated with primary antibodies overnight at 4°C.
The membranes were washed and incubated with horseradish
peroxidase-labeled secondary antibodies (GE Healthcare UK Ltd.,
Buckinghamshire, UK) for 1 h at room temperature. After several
washes, the membranes were incubated with chemiluminescent reagents
(ECL Advanced Western Blotting Detection kit; GE Healthcare UK
Ltd.), and specific bands were visualized by an image analyzer
LAS-1000 plus (Fuji Film, Tokyo, Japan) as previously described
(19).
Cell cycle analysis
After treatment with PBS or 100 μg/ml fucoidan for
24 or 48 h, the cells were trypsinized, washed with PBS, and
incubated in 0.2% Triton X-100 for 20 min at 37°C. Then, DNA
content was assessed by staining ethanol-fixed cells with propidium
iodide and monitoring by FACS-Calibur (Becton Dickinson, Franklin
Lakes, NJ, USA). The flow cytometry data were collected, and cell
cycle distributions were analyzed with Cell Quest software (Becton
Dickinson) as previously described (20).
Statistical analysis
All data are expressed as the mean ± SD. Comparisons
among multiple groups were made using one-way analyses of variance,
followed by Fisher’s protected least-significant-difference
post-hoc test as previously described (21). A P-value <0.05 was considered
statistically significant.
Results
Effects of fucoidan on HLF cell
proliferation
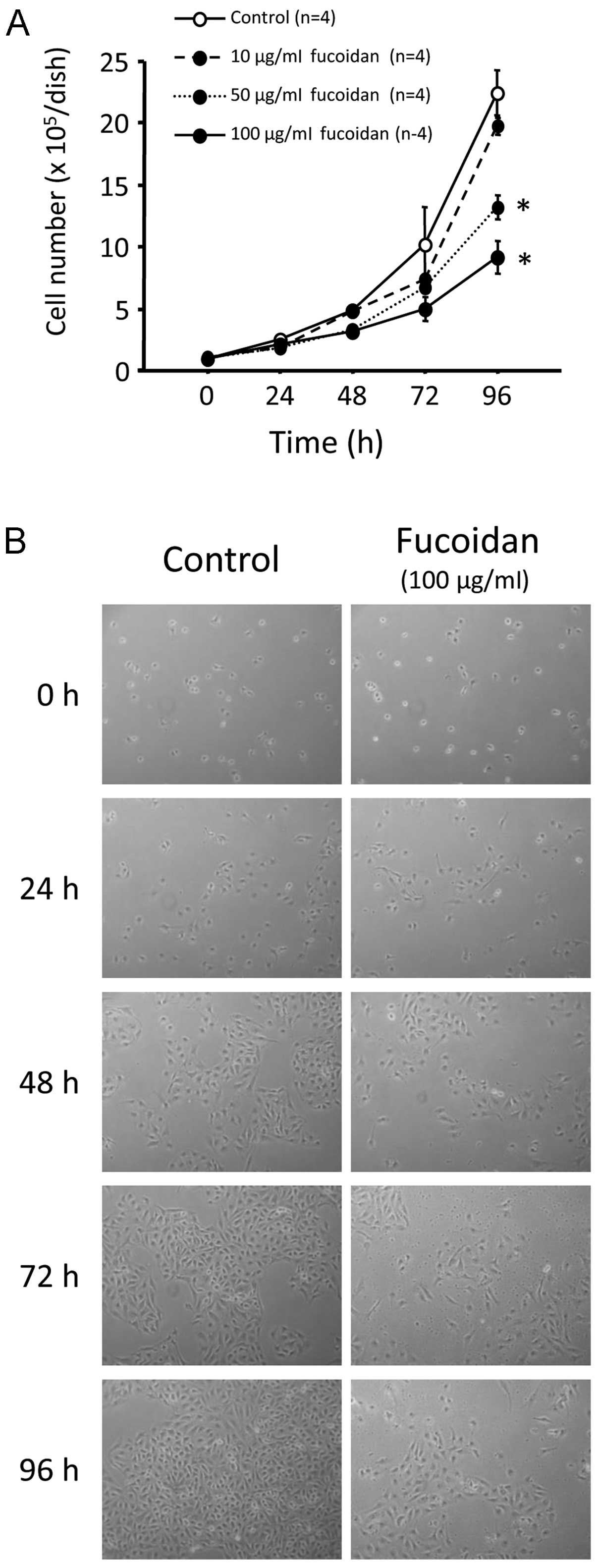
The cell number did not differ between the control
cells and those treated with 10 μg/ml fucoidan. However, treatment
with 50 and 100 μg/ml fucoidan significantly and dose- and
time-dependently suppressed cell proliferation (Fig. 1A and B, P<0.0001). Cell number
was ~50% lower 96 h after treatment with 100 μg/ml fucoidan, as
compared to the controls (22.4±1.8×105 vs.
0.9±0.2×105 cells/dish; Fig. 1A and B). Cell viability was
evaluated by trypan blue staining. Cell death was rarely observed
beyond 96-h fucoidan treatment at any dose (data not shown).
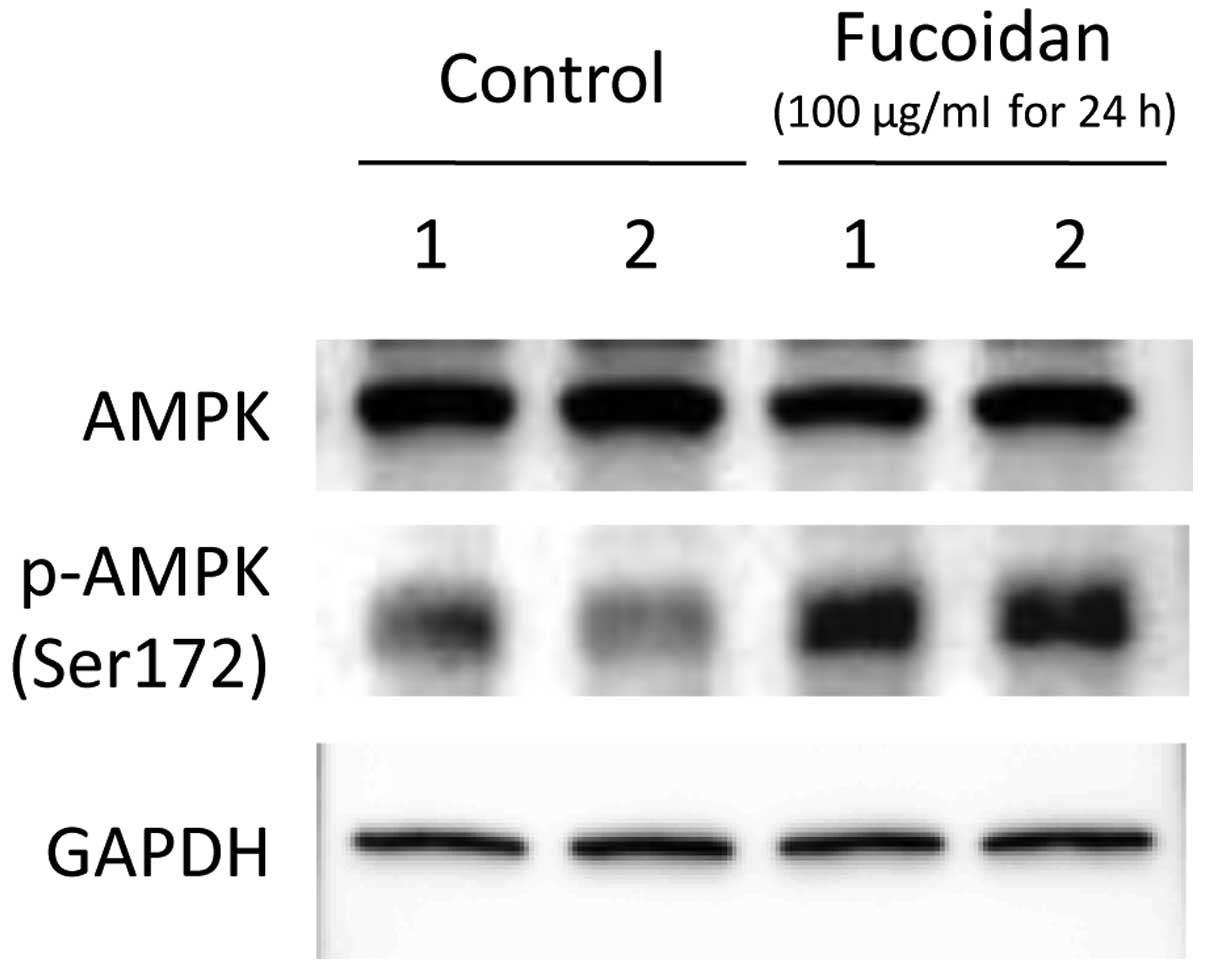
Effects of fucoidan on AMPK expression
and phosphorylation in HLF cells
After 24 h of treatment with PBS or 100 μg/ml
fucoidan, AMPK expression and Ser172 phosphorylation were evaluated
by immunoblotting. Although AMPK protein expression level did not
differ between the controls and fucoidan-treated HLF cells,
treatment with fucoidan enhanced Ser172 phosphorylation, as
compared to control cells (Fig.
2).
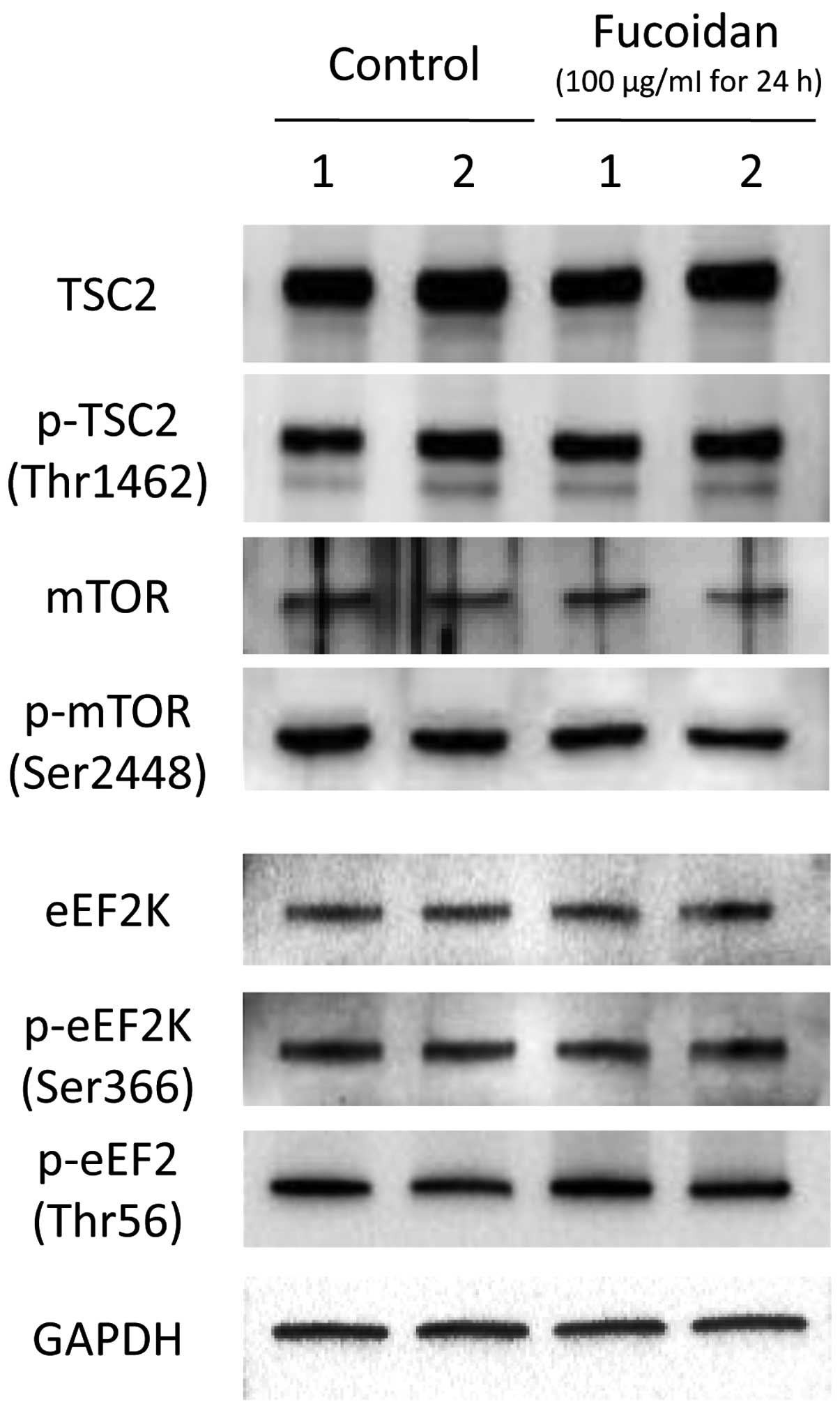
Effects of fucoidan on
metabolism-associated molecule expression and phosphorylation in
HLF cells
The effects of fucoidan on metabolism-associated
molecules, such as TSC2/mTOR and eEF2/eEF2K were examined by
immunoblotting. TSC2 and mTOR expression was not altered by
fucoidan treatment (Fig. 3).
Furthermore, fucoidan treatment did not affect TSC2 Thr1462 or mTOR
Ser2448 phosphorylation in HLF cells (Fig. 3). Similarly, eEF2K expression and
EF2K Ser366 and eEF2 Thr56 phosphorylation were not altered by
fucoidan treatment (Fig. 3).
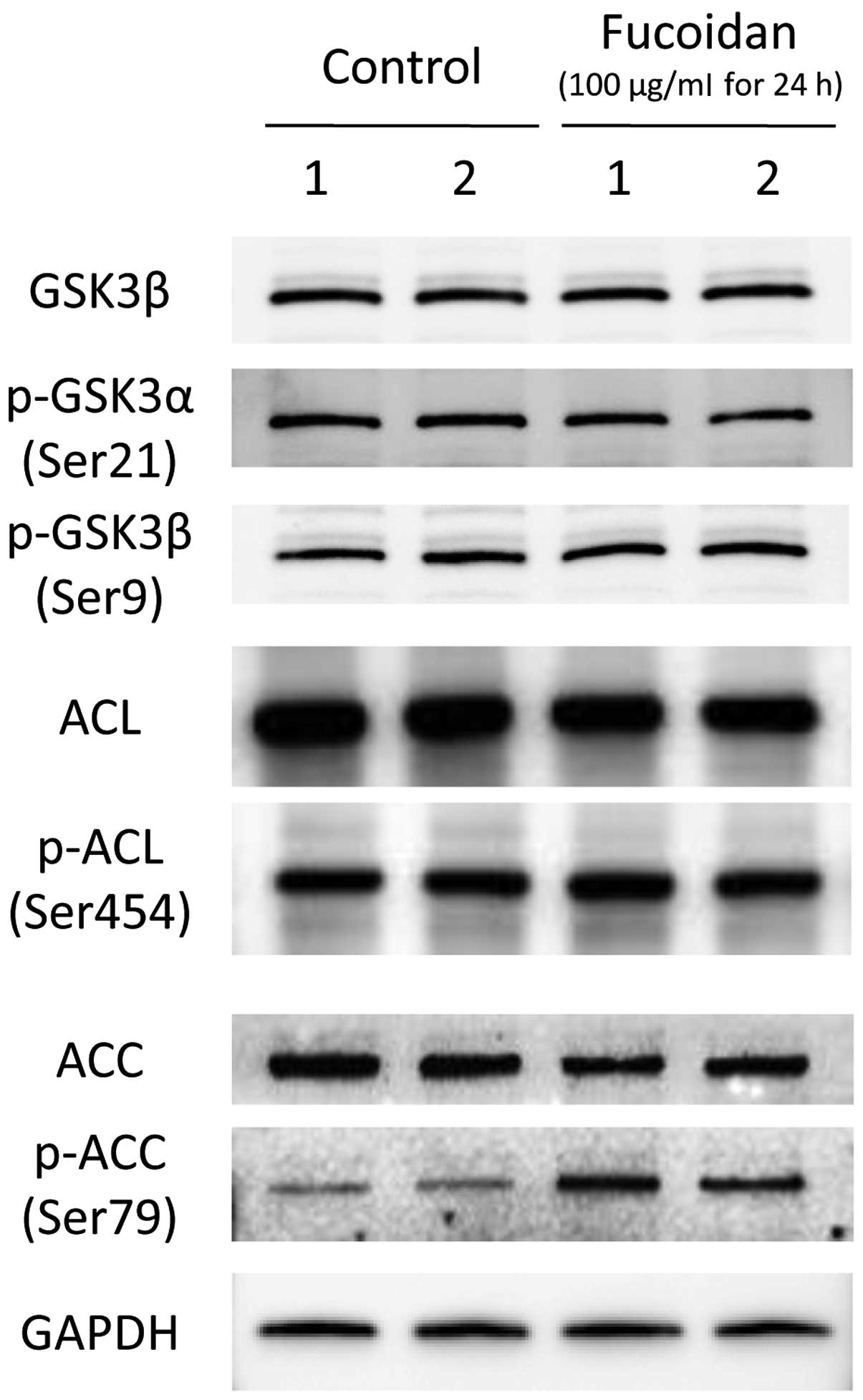
Effects of fucoidan on glucose and lipid
metabolism-associated molecule expression in HLF cells
We next assessed the effects of fucoidan on the
expression and phosphorylation of glucose and lipid
metabolism-associated molecules, including GSK3β, ACL and ACC, by
immunoblotting. The expression of GSK3β, and the phosphorylation of
GSK3β at Ser9 and GSK3α at Ser21 were not altered by fucoidan
treatment (Fig. 4). Additionally,
ACL expression and phosphorylation at Ser366 did not differ between
the control and fucoidan-treated HLF cells (Fig. 4). Although there was no difference
in ACC expression after fucoidan treatment, fucoidan enhanced ACC
phosphorylation at Ser79 (Fig.
4).
Effects of fucoidan on cell
cycle-associated molecule expression and phosphorylation in HLF
cells
The effects of fucoidan on cell cycle-associated
molecule expression and phosphorylation were examined by
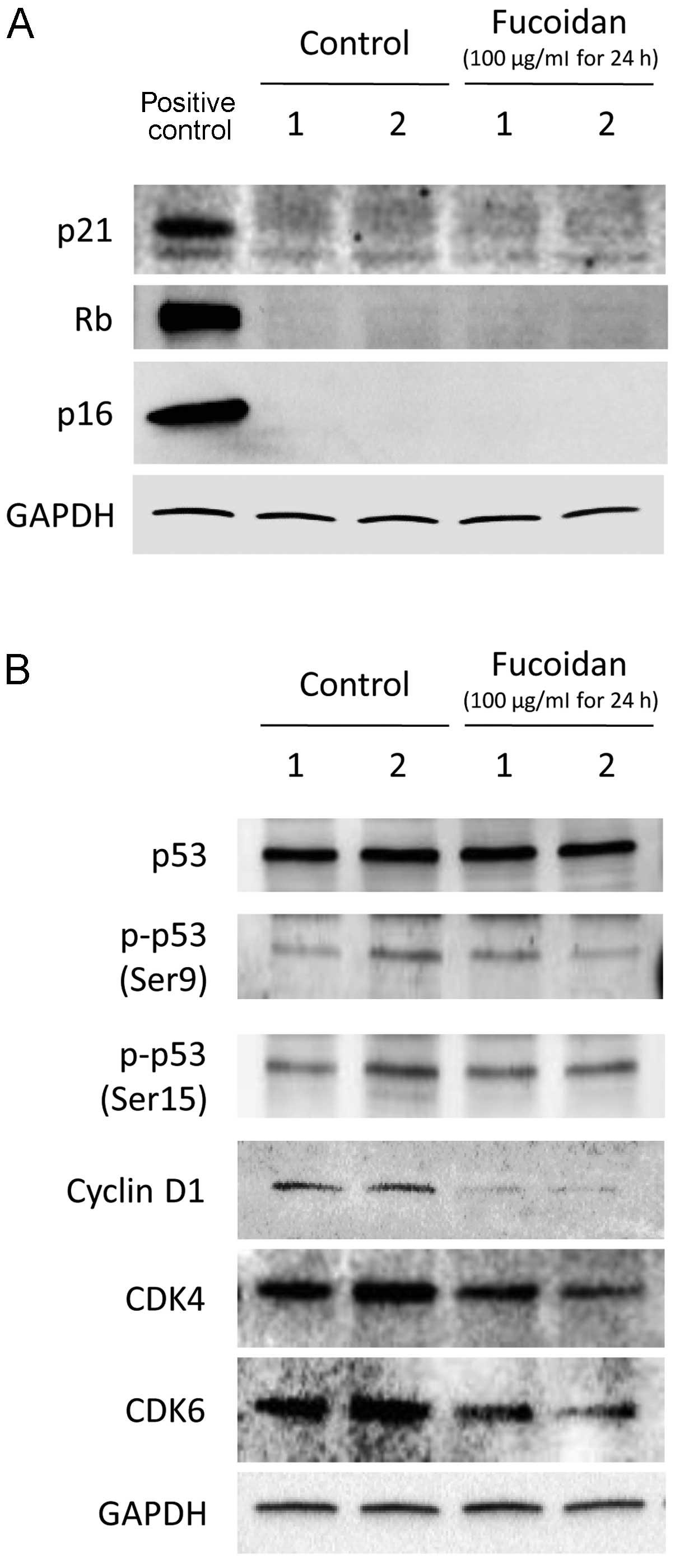
immunoblotting. Fucoidan did not alter p21, Rb and p16 expression
in HLF cells (Fig. 5A).
Furthermore, p53 expression and phosphorylation at Ser9 and Ser15
were similar in the control and fucoidan-treated HLF cells
(Fig. 5B). In contrast, fucoidan
decreased the expression of cyclin D1, CDK4 and CDK6 (Fig. 5B).
Effects of fucoidan on HLF cell
cycle
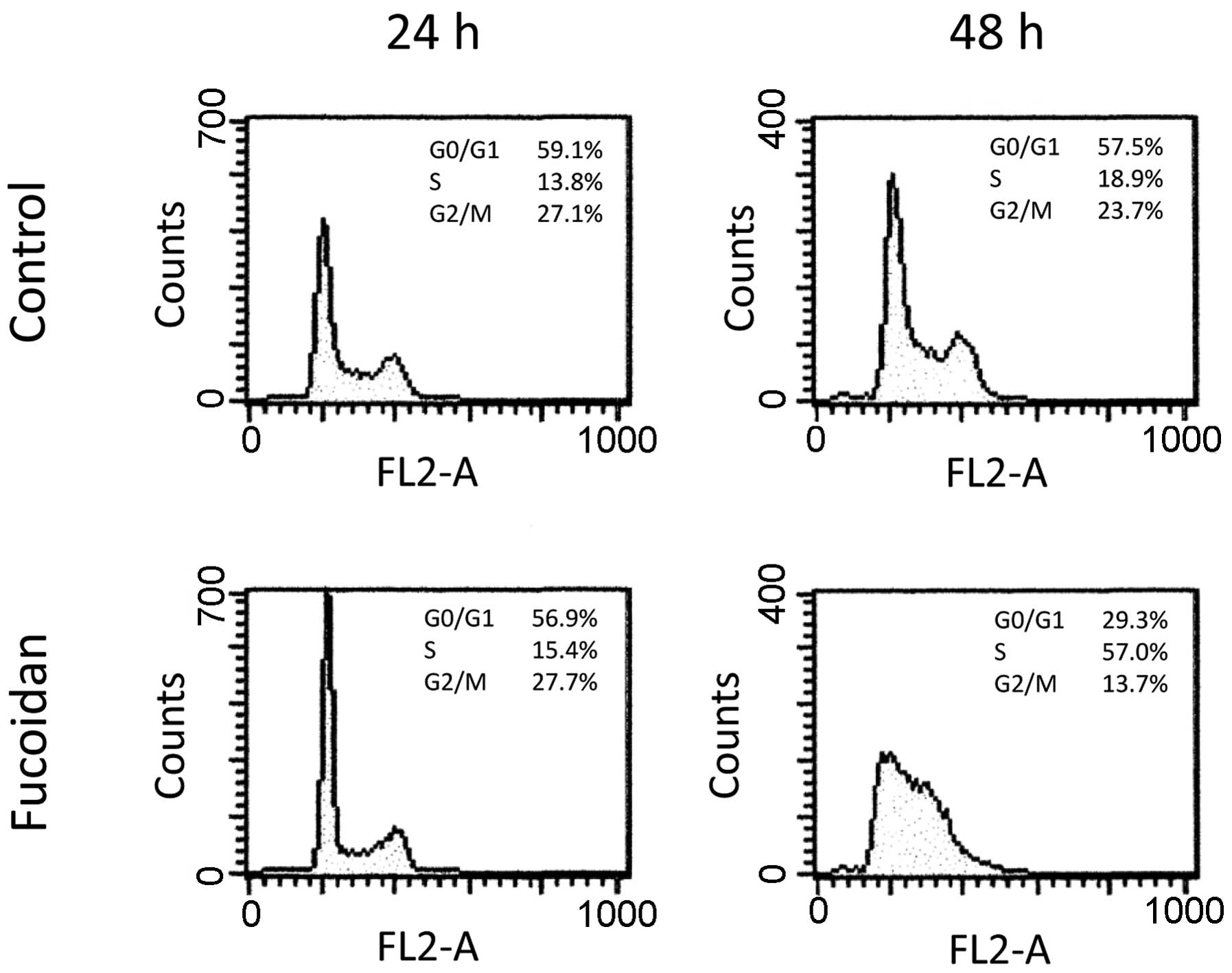
At 24 h after initial treatment, there was no
significant difference in cell cycle between the control and
fucoidan-treated HLF cells (Fig.
6). However, 48 h after initial treatment, the percentage of
cells in the G0/G1 phase was 57.4 and 29.3%, that in the S phase
was 18.9 and 57.0%, and that in the G2/M phase was 23.7 and 13.7%
in the control and fucoidan-treated HLF cells, respectively
(Fig. 6). Thus, 100 μg/ml fucoidan
treatment arrested HLF cells in the G1/S phase. Apoptosis was not
observed in the control or fucoidan-treated HLF cells (Fig. 6).
Discussion
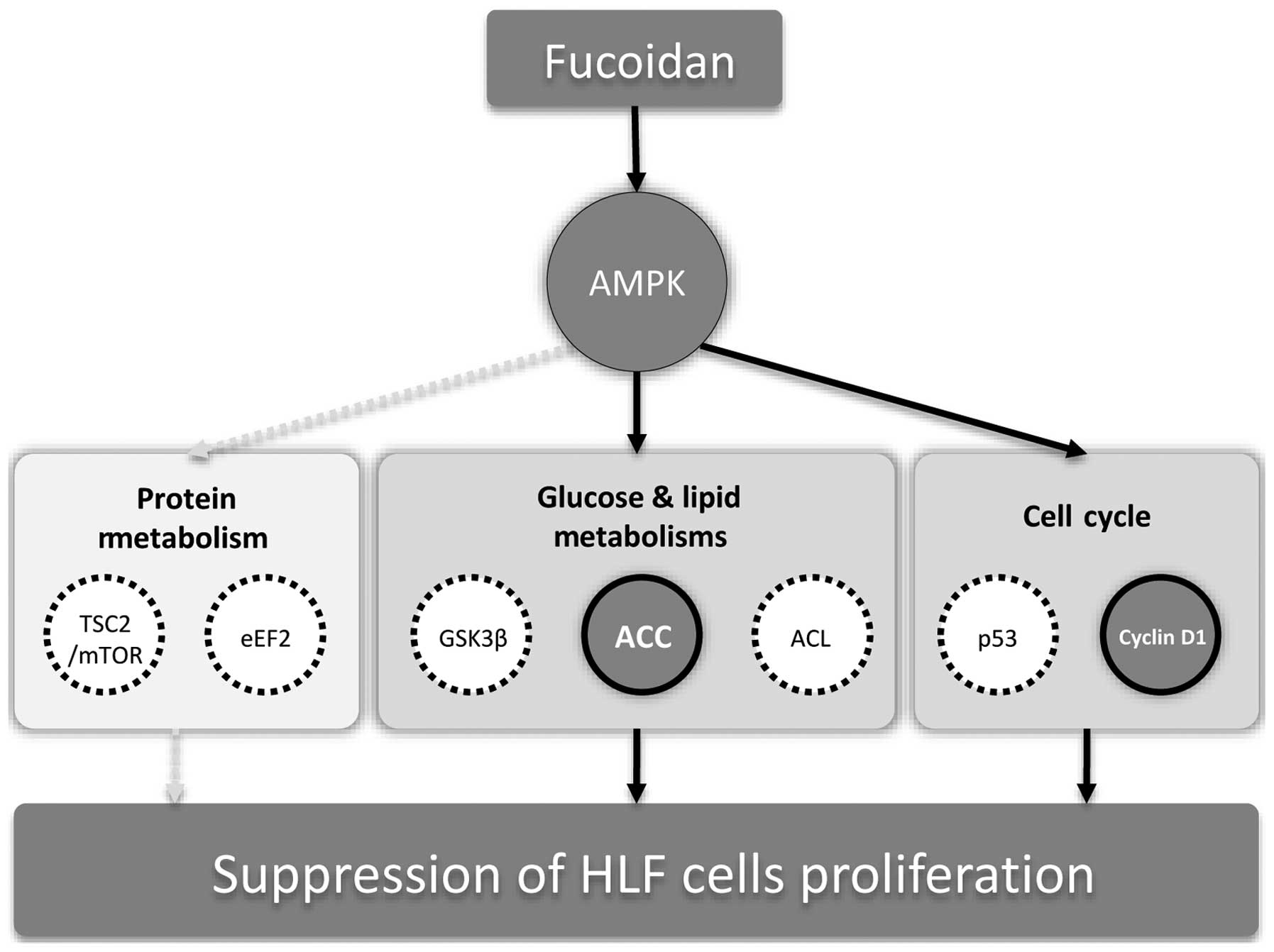
We demonstrated that fucoidan suppressed the
proliferation of HLF cells, a poorly differentiated HCC. We also
showed that fucoidan induces AMPK accompanied by ACC
phosphorylation and downregulation of cyclin D1, CDK4 and CDK6
expression. These findings suggest that fucoidan inhibits
proliferation via AMPK-associated suppression of fatty acid
synthesis and G1/S transition in HLF cells.
In this study, we showed that fucoidan suppressed
proliferation, but not apoptosis, in HLF cells. In contrast,
previous studies reported that fucoidan induces apoptosis in
several human HCC cell lines, including HepG2, Huh7 and SMMC-7721
cells (9,11,13,14).
It is unclear why fucoidan did not induce apoptosis in HLF cells;
however, one possibility is that there are different phenotypes
between HCC cell lines. HLF cells were established from poorly
differentiated HCC (22) and are
resistant to apoptosis due to low tumor necrosis factor-related
apoptosis-inducing ligand-receptor 2 expression (23). Thus, the mechanisms mediating
fucoidan-induced proliferation suppression may differ depending on
HCC phenotype.
AMPK is a central regulator of proliferation and
exerts its effects via the regulation of metabolism and the cell
cycle. Thus, AMPK is a potential therapeutic target for cancer
(16,17). However, the effects of fucoidan on
AMPK have not been reported. We first demonstrated that fucoidan
phosphorylated AMPK in HLF cells. Although the mechanisms
regulating fucoidan-induced AMPK activation remain unclear, AMPK is
a member of the metabolite-sensing protein kinase family and is
activated by depletion of intracellular ATP levels (16,17).
Fucoidan causes mitochondrial dysfunction in cancer cells (24). Since mitochondria produce ATP,
fucoidan may activate AMPK through depletion of ATP via
mitochondrial injury.
Phosphorylated AMPK inhibits protein synthesis,
thereby suppressing cell proliferation through downregulation of
the TCS2/mTOR (17) and eEF2/eEF2K
pathways (25). Additionally, Lee
et al reported that fucoidan inhibits human lung cancer cell
migration and invasion via mTOR regulation (26). However, fucoidan did not affect the
TCS2/mTOR or eEF2/eEF2K pathways in this study, suggesting that
impairment of protein synthesis is not a major mechanism for
fucoidan-induced suppression of HLF cell proliferation.
Phosphorylated AMPK also inhibits gluconeogenesis
and lipogenesis, thereby suppressing proliferation through GSK3β,
ACL and ACC (10,17,27).
In this study, fucoidan did not affect the expression or
phosphorylation of GSK3β and ACL; however, fucoidan induced ACC
phosphorylation at Ser79. Phosphorylation at Ser79 inhibits
malonyl-CoA production, an initial step of fatty acid synthesis
(28). Various cancer cells
exhibit a markedly increased rate of de novo fatty acid
synthesis (17). Cancer cells
activate fatty acid biosynthesis to sustain an increasing demand
for phospholipids for membrane biogenesis (29). Moreover, high levels of arachidonic
acid can promote breast cancer cell proliferation (30). Taken together, the suppression of
fatty acid synthesis through AMPK-induced ACC phosphorylation may
mediate fucoidan-induced proliferation suppression (Fig. 7). AMPK activation phosphorylates
ACC, resulting in the suppression of prostate, breast and ovarian
cancer cell proliferation (31–33),
supporting our hypothesis.
AMPK is reported to regulate proliferation via the
cell cycle machinery (17).
Phosphorylated AMPK suppresses proliferation through p21, Rb, p16
and p53 (17). However, these
molecules were not expressed in HLF cells, and fucoidan did not
alter the expression or phosphorylation of p53. In contrast,
fucoidan downregulated cyclin D1, CDK4 and CDK6 expression in HLF
cells. Cyclin D1 is required for cell cycle G1/S transition
(34) and G1/S arrest was seen in
this study. Sikka et al reported that AMPK activation
downregulates cyclin D1, CDK4 and CDK6, thereby inhibiting squamous
cancer cell proliferation (35).
Similar findings were also reported in myeloma cells (36). Moreover, Banafa et al
demonstrated that fucoidan decreases cyclin D1 and CDK4 gene
expression in human breast cancer MCF-7 cells (37). Thus, our findings, along with
previous studies, suggest that AMPK-induces G1/S phase arrest via
downregulation of cyclin D1, CDK4 and CDK6, thereby mediating
fucoidan-induced suppression of HLF cell proliferation (Fig. 7).
In conclusion, we showed that fucoidan inhibited the
proliferation of HLF cells, a poorly differentiated HCC. In
addition, we demonstrated that fucoidan phosphorylated AMPK
accompanied by phosphorylation of ACC and downregulates cyclin D1,
CDK4 and CDK6 expression. These findings suggest that fucoidan
suppresses proliferation via AMPK-mediated inhibition of fatty acid
synthesis and cell cycle G1/S transition in HLF cells.
Acknowledgements
This study was supported, in part, by Health and
Labour Sciences Research Grants for Research on Hepatitis from the
Ministry of Health, Labour and Welfare of Japan.
Abbreviations:
|
HCC
|
hepatocellular carcinoma
|
|
AMPK
|
adenosine monophosphate-activated
protein kinase
|
|
p
|
phosphorylated
|
|
TSC
|
tuberous sclerosis complex
|
|
mTOR
|
mammalian target of rapamycin
|
|
eEF
|
eukaryotic elongation factor
|
|
eEF2K
|
eukaryotic elongation factor 2
kinase
|
|
GSK
|
glycogen synthase kinase
|
|
ATP
|
adenosine triphosphate
|
|
ACL
|
ATP-citrate lyase
|
|
ACC
|
acetyl-CoA carboxylase
|
|
Rb
|
retinoblastoma
|
|
CDK
|
cyclin-dependent kinase
|
|
GAPDH
|
glyceraldehyde-3-phosphate
dehydrogenase
|
|
PBS
|
phosphate buffered saline
|
References
|
1
|
Burroughs A, Hochhauser D and Meyer T:
Systemic treatment and liver transplantation for hepatocellular
carcinoma: Two ends of the therapeutic spectrum. Lancet Oncol.
5:409–418. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Kluger MD, Salceda JA, Laurent A, Tayar C,
Duvoux C, Decaens T, Luciani A, Van Nhieu JT, Azoulay D and Cherqui
D: Liver resection for hepatocellular carcinoma in 313 western
patients: tumor biology and underlying liver rather than tumor size
drive prognosis. J Hepatol. Dec 18–2014.(Epub ahead of print).
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Tanaka K and Sorai S: Hydrolysis of
fucoidan by abalone liver alpha-L-fucosidase. FEBS Lett. 9:45–48.
1970. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Cui W, Zheng Y, Zhang Q, Wang J, Wang L,
Yang W, Guo C, Gao W, Wang X and Luo D: Low-molecular-weight
fucoidan protects endothelial function and ameliorates basal
hypertension in diabetic Goto-Kakizaki rats. Lab Invest.
94:382–393. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Fitton JH: Therapies from fucoidan;
multifunctional marine polymers. Mar Drugs. 9:1731–1760. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Boo HJ, Hong JY, Kim SC, Kang JI, Kim MK,
Kim EJ, Hyun JW, Koh YS, Yoo ES, Kwon JM, et al: The anticancer
effect of fucoidan in PC-3 prostate cancer cells. Mar Drugs.
11:2982–2999. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Kim EJ, Park SY, Lee JY and Park JH:
Fucoidan present in brown algae induces apoptosis of human colon
cancer cells. BMC Gastroenterol. 10:962010. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Xue M, Ge Y, Zhang J, Wang Q, Hou L, Liu
Y, Sun L and Li Q: Anticancer properties and mechanisms of fucoidan
on mouse breast cancer in vitro and in vivo. PLoS One.
7:e434832012. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Min EY, Kim IH, Lee J, Kim EY, Choi YH and
Nam TJ: The effects of fucodian on senescence are controlled by the
p16INK4a-pRb and p14Arf-p53 pathways in hepatocellular carcinoma
and hepatic cell lines. Int J Oncol. 45:47–56. 2014.PubMed/NCBI
|
|
10
|
Yuan HD and Piao GC: An active part of
Artemisia sacrorum Ledeb. suppresses gluconeogenesis through AMPK
mediated GSK3β and CREB phosphorylation in human HepG2 cells.
Biosci Biotechnol Biochem. 75:1079–1084. 2011. View Article : Google Scholar
|
|
11
|
Roshan S, Liu YY, Banafa A, Chen HJ, Li
KX, Yang GX, He GY and Chen MJ: Fucoidan induces apoptosis of HepG2
cells by down-regulating p-Stat3. J Huazhong Univ Sci Technolog Med
Sci. 34:330–336. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Zhu C, Cao R, Zhang SX, Man YN and Wu XZ:
Fucoidan inhibits the growth of hepatocellular carcinoma
independent of angiogenesis. Evid Based Complement Alternat Med.
2013:6925492013. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Nagamine T, Hayakawa K, Kusakabe T, Takada
H, Nakazato K, Hisanaga E and Iha M: Inhibitory effect of fucoidan
on Huh7 hepatoma cells through downregulation of CXCL12. Nutr
Cancer. 61:340–347. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Fukahori S, Yano H, Akiba J, Ogasawara S,
Momosaki S, Sanada S, Kuratomi K, Ishizaki Y, Moriya F, Yagi M, et
al: Fucoidan, a major component of brown seaweed, prohibits the
growth of human cancer cell lines in vitro. Mol Med Rep. 1:537–542.
2008.PubMed/NCBI
|
|
15
|
Hardie DG: AMPK - sensing energy while
talking to other signaling pathways. Cell Metab. 20:939–952. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Lacher MD, Pincheira R, Zhu Z,
Camoretti-Mercado B, Matli M, Warren RS and Castro AF: Rheb
activates AMPK and reduces p27Kip1 levels in Tsc2-null cells via
mTORC1-independent mechanisms: Implications for cell proliferation
and tumorigenesis. Oncogene. 29:6543–6556. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Motoshima H, Goldstein BJ, Igata M and
Araki E: AMPK and cell proliferation - AMPK as a therapeutic target
for atherosclerosis and cancer. J Physiol. 574:63–71. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Hashimoto O, Shinkawa M, Torimura T,
Nakamura T, Selvendiran K, Sakamoto M, Koga H, Ueno T and Sata M:
Cell cycle regulation by the Wee1 inhibitor PD0166285, pyrido
[2,3-d] pyimidine, in the B16 mouse melanoma cell line. BMC Cancer.
6:2922006. View Article : Google Scholar
|
|
19
|
Kawaguchi T, Yoshida T, Harada M, Hisamoto
T, Nagao Y, Ide T, Taniguchi E, Kumemura H, Hanada S, Maeyama M, et
al: Hepatitis C virus down-regulates insulin receptor substrates 1
and 2 through up-regulation of suppressor of cytokine signaling 3.
Am J Pathol. 165:1499–1508. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Selvendiran K, Koga H, Ueno T, Yoshida T,
Maeyama M, Torimura T, Yano H, Kojiro M and Sata M: Luteolin
promotes degradation in signal transducer and activator of
transcription 3 in human hepatoma cells: An implication for the
antitumor potential of flavonoids. Cancer Res. 66:4826–4834. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Kawaguchi T, Sakisaka S, Mitsuyama K,
Harada M, Koga H, Taniguchi E, Sasatomi K, Kimura R, Ueno T, Sawada
N, et al: Cholestasis with altered structure and function of
hepatocyte tight junction and decreased expression of canalicular
multispecific organic anion transporter in a rat model of colitis.
Hepatology. 31:1285–1295. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Matsumoto A, Ono M, Fujimoto Y, Gallo RL,
Bernfield M and Kohgo Y: Reduced expression of syndecan-1 in human
hepatocellular carcinoma with high metastatic potential. Int J
Cancer. 74:482–491. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Yamamoto T, Nagano H, Sakon M, Wada H,
Eguchi H, Kondo M, Damdinsuren B, Ota H, Nakamura M, Wada H, et al:
Partial contribution of tumor necrosis factor-related
apoptosis-inducing ligand (TRAIL)/TRAIL receptor pathway to
antitumor effects of interferon-alpha/5-fluorouracil against
hepatocellular carcinoma. Clin Cancer Res. 10:7884–7895. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Park HY, Kim GY, Moon SK, Kim WJ, Yoo YH
and Choi YH: Fucoidan inhibits the proliferation of human urinary
bladder cancer T24 cells by blocking cell cycle progression and
inducing apoptosis. Molecules. 19:5981–5998. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Leprivier G, Remke M, Rotblat B, Dubuc A,
Mateo AR, Kool M, Agnihotri S, El-Naggar A, Yu B, Somasekharan SP,
et al: The eEF2 kinase confers resistance to nutrient deprivation
by blocking translation elongation. Cell. 153:1064–1079. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Lee H, Kim JS and Kim E: Fucoidan from
seaweed Fucus vesiculosus inhibits migration and invasion of human
lung cancer cell via PI3K-Akt-mTOR pathways. PLoS One.
7:e506242012. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Migita T, Okabe S, Ikeda K, Igarashi S,
Sugawara S, Tomida A, Taguchi R, Soga T and Seimiya H: Inhibition
of ATP citrate lyase induces an anticancer effect via reactive
oxygen species: AMPK as a predictive biomarker for therapeutic
impact. Am J Pathol. 182:1800–1810. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Hopkins TA, Dyck JR and Lopaschuk GD:
AMP-activated protein kinase regulation of fatty acid oxidation in
the ischaemic heart. Biochem Soc Trans. 31:207–212. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Scaglia N, Chisholm JW and Igal RA:
Inhibition of stearoylCoA desaturase-1 inactivates acetyl-CoA
carboxylase and impairs proliferation in cancer cells: Role of
AMPK. PLoS One. 4:e68122009. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Chang NW, Wu CT, Chen DR, Yeh CY and Lin
C: High levels of arachidonic acid and peroxisome
proliferator-activated receptor-alpha in breast cancer tissues are
associated with promoting cancer cell proliferation. J Nutr
Biochem. 24:274–281. 2013. View Article : Google Scholar
|
|
31
|
Hadad SM, Hardie DG, Appleyard V and
Thompson AM: Effects of metformin on breast cancer cell
proliferation, the AMPK pathway and the cell cycle. Clin Transl
Oncol. 16:746–752. 2014. View Article : Google Scholar
|
|
32
|
Lin VC, Tsai YC, Lin JN, Fan LL, Pan MH,
Ho CT, Wu JY and Way TD: Activation of AMPK by pterostilbene
suppresses lipogenesis and cell-cycle progression in p53 positive
and negative human prostate cancer cells. J Agric Food Chem.
60:6399–6407. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Rattan R, Giri S, Hartmann LC and Shridhar
V: Metformin attenuates ovarian cancer cell growth in an AMP-kinase
dispensable manner. J Cell Mol Med. 15:166–178. 2011. View Article : Google Scholar
|
|
34
|
Santra MK, Wajapeyee N and Green MR: F-box
protein FBXO31 mediates cyclin D1 degradation to induce G1 arrest
after DNA damage. Nature. 459:722–725. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Sikka A, Kaur M, Agarwal C, Deep G and
Agarwal R: Metformin suppresses growth of human head and neck
squamous cell carcinoma via global inhibition of protein
translation. Cell Cycle. 11:1374–1382. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Zi FM, He JS, Li Y, Wu C, Yang L, Yang Y,
Wang LJ, He DH, Zhao Y, Wu WJ, et al: Metformin displays
anti-myeloma activity and synergistic effect with dexamethasone in
in vitro and in vivo xenograft models. Cancer Lett. 356:443–453.
2015. View Article : Google Scholar
|
|
37
|
Banafa AM, Roshan S, Liu YY, Chen HJ, Chen
MJ, Yang GX and He GY: Fucoidan induces G1 phase arrest and
apoptosis through caspases-dependent pathway and ROS induction in
human breast cancer MCF-7 cells. J Huazhong Univ Sci Technolog Med
Sci. 33:717–724. 2013. View Article : Google Scholar : PubMed/NCBI
|