Introduction
The prognosis of patients with oral squamous cell
carcinoma (OSCC) remains poor despite advances in diagnosis and
conventional treatment (1). OSCC,
the most common development in areas of carcinogen-exposed
epithelium, likely results from the accumulation of cellular and
genetic alteration, leading to aberrant expression of proteins
involved in cell growth regulation (2). Blocking or modifying the function of
these proteins may impede or delay the development of cancer.
Chemotherapy is the mainstay treatment for patients with recurrent
or metastatic OSCC, either alone or in combination with other
agents (3). Although this
conventional treatment of OSCC has been well advanced to date,
five-year survival rate of OSCC remains <50% (4). Therefore, discovery and development
of effective chemotherapeutic agents for OSCC might improve the
survival rate for patients with OSCC.
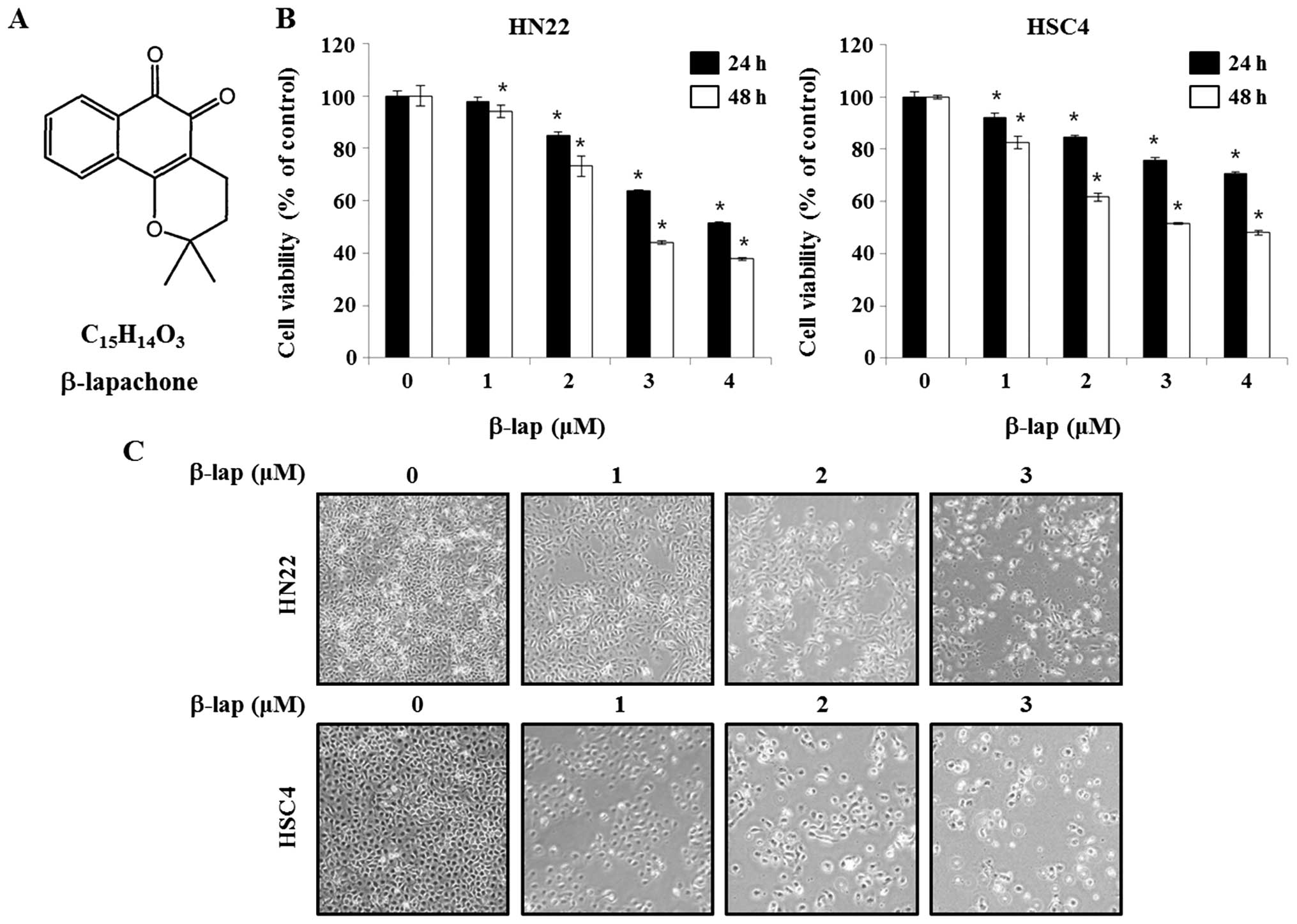
β-lapachone
(3.4-dihydro-2,2-dimethyl-2H-naphthol[1,2-b] pyran-5,6-dione)
(β-lap), a novel bio-reductive anticancer drug is a derivative of
naturally occurring substance lapachol (Fig. 1) found in the South American
lapacho tree (Tabebuia avellanedae) (5). β-lap is reported to have anticancer
(5), anti-Trypanosoma cruzi
(6), anti-inflammatory (7,8),
antibacterial (9), antifungal
(9), antiviral (10), and wound healing properties
(11). Previous studies also have
demonstrated that β-lap treatment induced cell death in a variety
of different human cancer cells (12,13).
In particular, β-lap is a topoisomerase I (14) and II (15) inhibitor that can repress telomerase
activity in human prostate carcinoma and lung carcinoma cells in
vitro, resulting in the induction of apoptosis (16).
Specificity protein 1 (Sp1), belonging to the
specificity protein/Krüppel-like factor family of transcription
factors that bind to GC-rich promoter element through three
Cys2His2-type zinc-fingers, plays an essential role in a variety of
cellular functions (17). Sp1 has
been reported to contribute to tumorigenesis through regulating
gene transcription related to growth and proliferation (18). In particular, Sp1 has been shown to
increase during the process of transformation in fibrosarcoma
transformation model. Inhibition of Sp1 by small interfering RNA
was shown to reduce tumor proliferation in nude mice (19). In this regard, other recent studies
suggest that Sp1-mediated functions are novel targets for cancer
therapy (20,21).
Although the anticancer effect of β-lap has been
demonstrated against several cancer cell lines and models, its
effects on oral cancer or mechanisms of β-lap induced apoptosis
remain poorly understood. Therefore, the objective of this study
was to examine the regulatory effect of β-lap on the growth and
apoptosis of OSCCs and investigate its anticancer mechanism in
relation to Sp1.
Materials and methods
Cell culture
Human oral squamous cell carcinoma cell lines HN22
and HSC4 were cultured in Hyclone Dulbecco’s modified Eagle’s
medium (DMEM) (Welgene, Daegu, Korea) containing 10%
heat-inactivated fetal bovine serum and 100 U/ml each of penicillin
and streptomycin (Gibco, Grand Island, NY, USA) at 37°C with 5%
CO2 in a humidified atmosphere.
Cell viability assay
The effect of β-lap on HN22 and HSC4 was estimated
using CellTiter96® Aqueous One Cell Proliferation assay
kit (Promega, Madison, WI, USA) according to the manual for
3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium
(MTS) assay. HN22 (3×103) and HSC4 (2×103)
cells were seeded into 96-well plates for 24 h and treated with
various concentrations of β-lap for 24 and 48 h. MTS solution was
added into 96-well plate and incubated for 2 h. The absorbance was
measured at 490 nm using Absorbance Microplate Reader (Biotek,
Winooski, VT, USA). Experiments were carried out in triplicates on
different days. The percentage of viability was calculated as:
(viable cells)% = (OD of β-lap-treated sample / OD of untreated
sample) × 100.
DAPI staining
In order to determine apoptosis induction, HN22 and
HSC4 treated by β-lap were detected by DAPI
(4′-6-diamidino-2-phenylindole dihydrochoride) (staining
techniques. Briefly, HN22 and HSC4 cells treated with 0–3 μM β-lap
for 48 h were fixed with 100% methanol for 30 min at room
temperature. After washing twice with PBS, fixed cells were
incubated with DAPI (Sigma-Aldrich, St. Louis, MO, USA) for 10 min.
Finally, slides were mounted with a mounting solution containing
10% glycerol and observed under a FluoView confocal laser
microscope (Fluoview FV10i, Olympus Corp., Tokyo, Japan).
Cell cycle analysis
Cell cycle analysis was performed using Muse™ Cell
Analyzer from Millipore (Billerica, MA, USA) following the
manufacturer’s instructions. Briefly, after the treatment with
β-lap 0, 1, 2 and 3 μM for 48 h, HN22 and HSC4 cells were
collected, washed with cold PBS, fixed in 70% ethanol overnight at
−20°C. Cells were then washed again with PBS before staining (150
μg/ml RNase A and 20 μg/ml propidium iodide (PI, Sigma-Aldrich) and
incubated at 37°C for 30 min. After staining, cells were processed
for cell cycle analysis.
Western blot analysis
After β-lap treatment, HN22 and HSC4 cells were
washed with ice-cold PBS and lysed in M-PER® Mammalian
Protein Extraction reagent (Thermo Scientific, Rockford, IL, USA)
containing a protease inhibitor cocktail (Roche, Switzerland).
Protein concentrations were measured using BCA protein assay kit
(Thermo Scientific). Protein samples were separated by 8 or 12%
SDS-poly-acrylamide gel electrophoresis and transferred to
polyvinylidene difluoride (PVDF) membranes (Millipore) using
standard procedures. After blocking with 5% (v/v) skim milk in
TBS-T buffer, membranes were incubated with primary antibody
overnight on a rocking platform at 4°C. The membrane was then
washed 5 times with TBS-T buffer for 10 min and incubated with
horseradish peroxidase conjugated anti-mouse or anti-rabbit IgG
antibodies. After washing in TBS-T buffer, membranes were
visualized using a chemiluminescent ECL detection kit (Thermo
Scientific). The primary antibodies used in this study were: Sp1,
β-actin, PARP, cleaved PARP, p27, p21, cyclin D1, survivin, Bcl-2,
Bcl-xl, Bax (Santa Cruz Biothchonolgy, Santa Cruz, CA, USA),
caspase-3 and cleaved caspase-3 antibodies (Cell Signaling
Technology, Danvers, MA, USA).
Statistical analysis
Data are reported as the average ± SD of triplicate
independent experiment. Statistical significance was assessed using
Student’s t-test. Compared to non-treated group, p<0.05 was
considered statistically significant.
Results
Growth inhibition effects of β-lap on
OSCC cells
β-lap was reported to have anti-proliferation with
antitumor properties in different cancers (12). Initially, to investigate the
efficacy of β-lap as an anticancer drug, HN22 and HSC4 cells were
treated by β-lap and cell viability was determined by MTS assay. As
shown in Fig. 1B, the efficiency
of β-lap in altering cell viability of HN22 and HSC4 cells were
assayed after 24 or 48 h of incubation in β-lap-containing medium
at different concentration (1, 2, 3 or 4 μM). The cell viability of
HN22 was 94.1±0.08, 73.3±0.05, 43.9±0.02 and 38.1±0.01% after
treated by 1, 2, 3 and 4 μM of β-lap for 48 h, respectively,
compared to untreated control cells. The cell viability of HSC4 was
82.5±0.05, 61.6±0.03, 52.0±0.01 and 48.1±0.02% after treated by 1,
2, 3 and 4 μM of β-lap for 48 h, respectively, compared to that of
untreated control cells.
Next, the morphological changes were observed under
an optical microscope after 48 h of treatment of β-lap. The
apoptotic phenotype included rounded cells with cytoplasmic
blebbing and irregular shape (Fig.
1C). These results showed that β-lap treatment effectively
inhibited cell growth of human OSCC cells.
β-lap treatment induces apoptosis in OSCC
cells
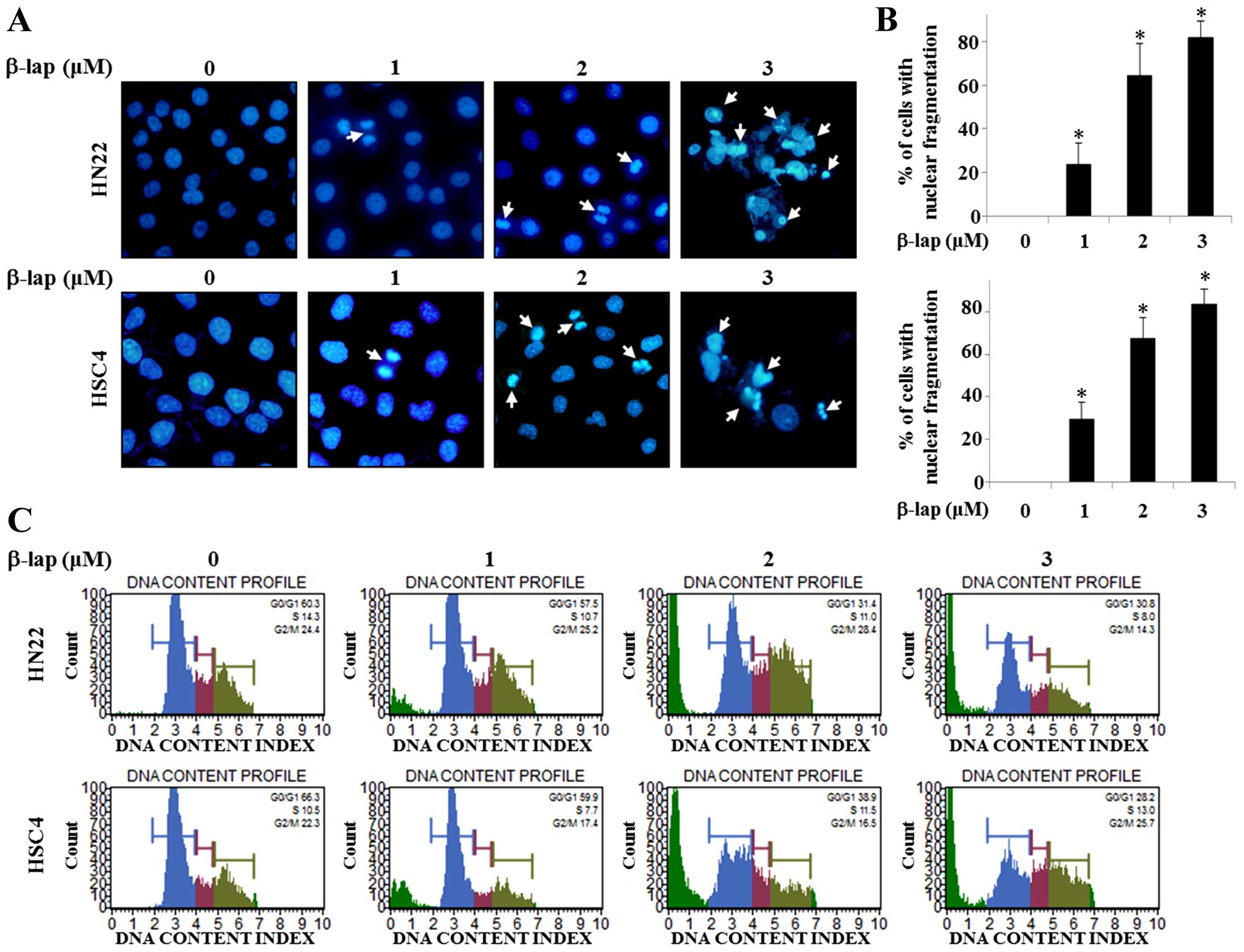
In order to evaluate the mechanisms of growth
suppression of HN22 and HSC4 cells by β-lap, we observed DNA
condensation and apoptotic bodies in the nucleus. Confocal laser
microscopic analysis of β-lap-treated HN22 or HSC4 cells was used
to demonstrate apoptotic morphological changes using DAPI staining.
The results presented in Fig. 2A
indicate that β-lap treatment induced DNA condensation and
decreased the cell number. Percentage of cells with DNA
condensation in β-lap-treated group compared to DMSO-treated group
are shown in Fig. 2B.
To gain insight into the mechanism of the
anti-proliferative activity of β-lap, its effects on cell cycle
distribution were analyzed by FACS analysis. As shown in Fig. 2C, significant increase of the
number of sub-G1 cells in HN22 and HSC4 treated by β-lap
compared to untreated control cells was found in a
concentration-dependent manner. These data showed that β-lap
treatment effectively inhibited cell proliferation, leading to
apoptotic cell death in OSCC cells.
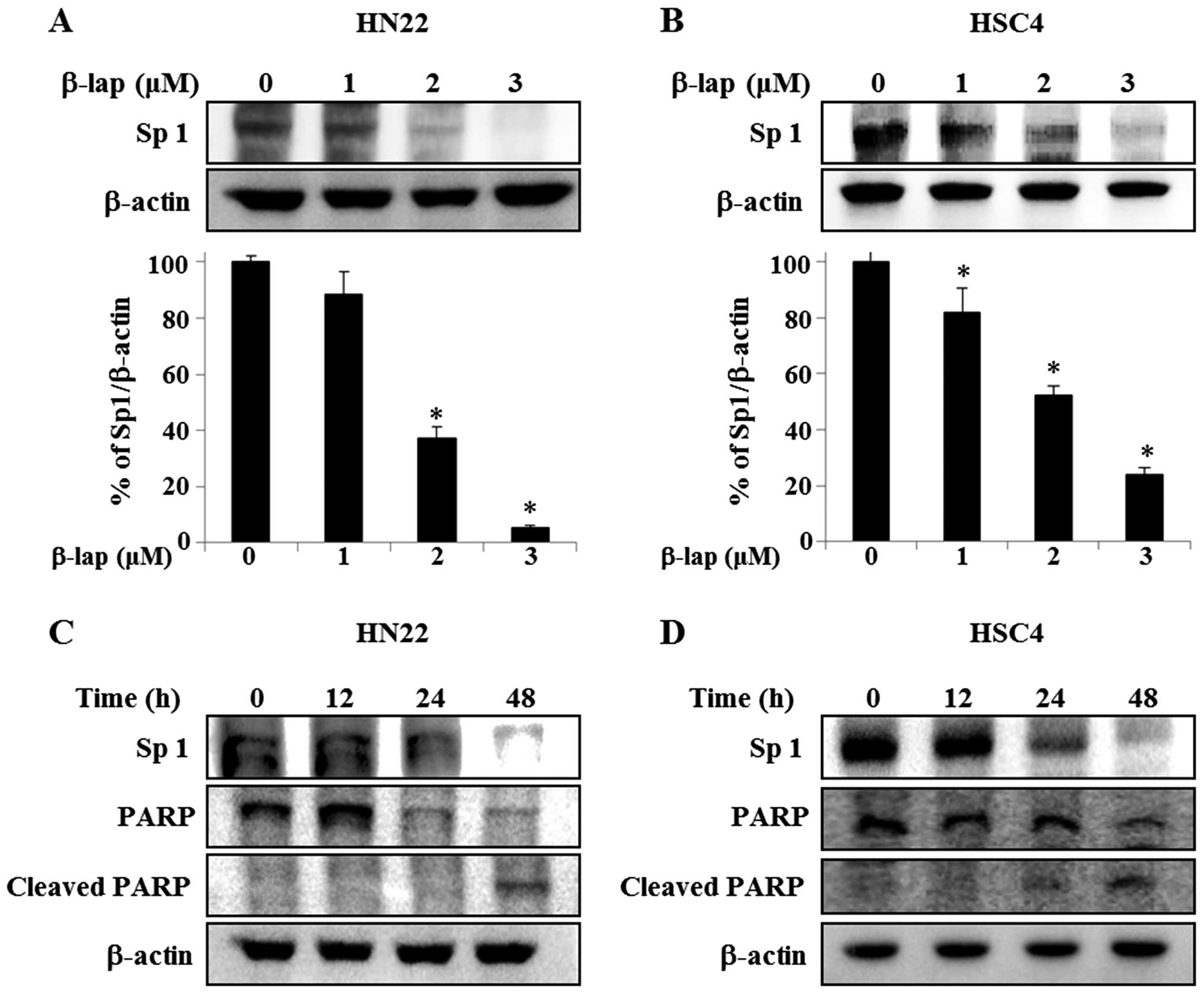
β-lap suppresses expression of Sp1 in
OSCC cells
Because Sp1 plays an important role in oncogenesis,
if the expression level of Sp1 protein is effectively modulated by
a chemotherapeutic agent, then the agent may be a potent candidate
as anticancer drug by suppressing tumor progression. We therefore
assessed the anti-proliferative response of β-lap in HN22 and HSC4
cells correlating its effect with Sp1. As shown in Fig. 3A and B, HN22 and HSC4 cells were
treated with vehicle (DMSO) or different concentrations of β-lap
for 48 h, Sp1 levels were dramatically decreased in treated cells,
with a maximum decrease of 94.7±0.01% of HN22 compared to untreated
group and 76.1±0.05% of decrease of HSC4 compared to untreated
group. To further verify the apoptotic effect of the downregulation
of Sp1 by β-lap, HN22 and HSC4 were treated with 3 μM β-lap for
different time periods (0, 12, 24 and 48 h) (Fig. 3C and D). To understand the cellular
effect of downregulation of Sp1 by β-lap in detail, we examined the
expression levels of PARP and cleaved PARP, as indicators of
apoptosis induction, in HN22 and HSC4 (Fig. 3C and D). Our results revealed that
the downregulation of Sp1 by β-lap treatment leads to apoptotic
cell death.
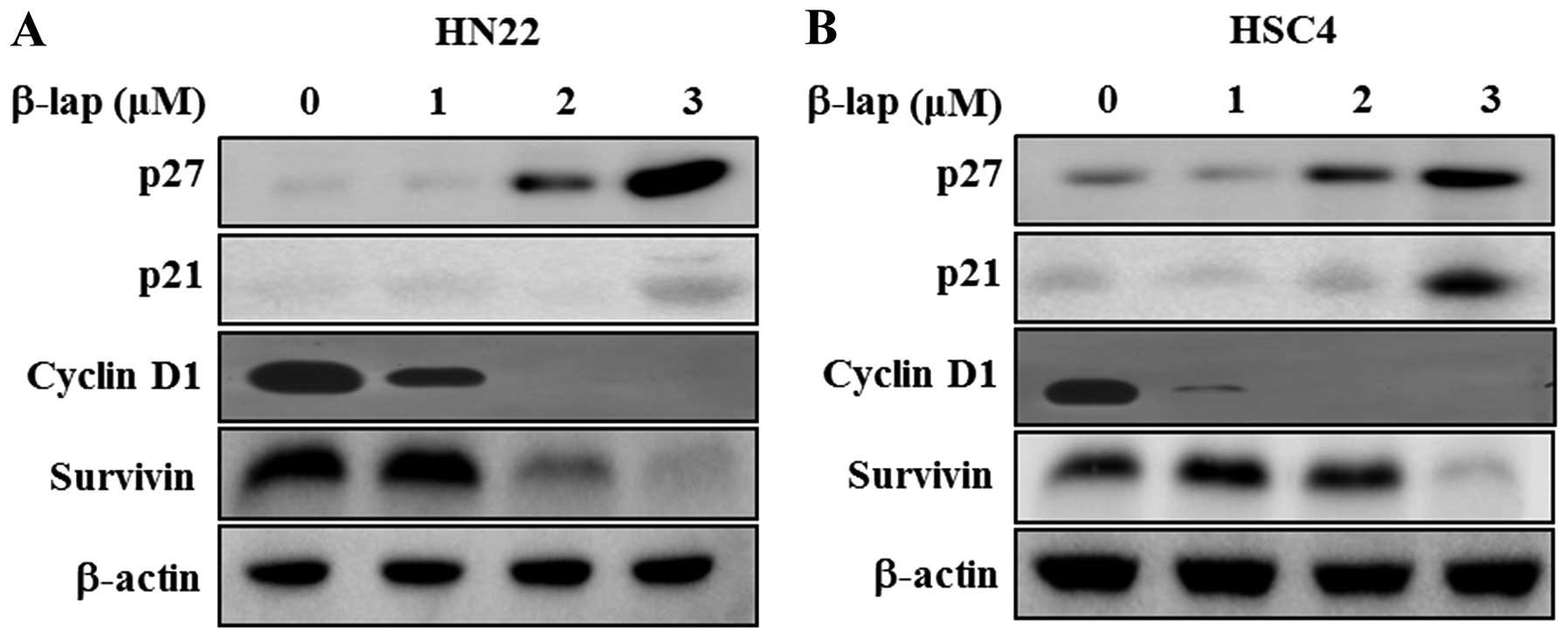
β-lap regulates cell cycle arrest- and
apoptosis regulating proteins
To determine the regulatory role of β-lap, we
focused on the expression levels of Sp1 downstream targets and
apoptosis-related proteins. We found that cell cycle arrest
proteins, including p27 and p21, were significantly enhanced in a
concentration-dependent manner by β-lap, whereas cell proliferation
and survival associated proteins, including cyclin D1 and survivin,
were decreased by β-lap treatment (Fig. 4). Additionally, we tested the
expression levels of several pro-apoptotic and anti-apoptotic
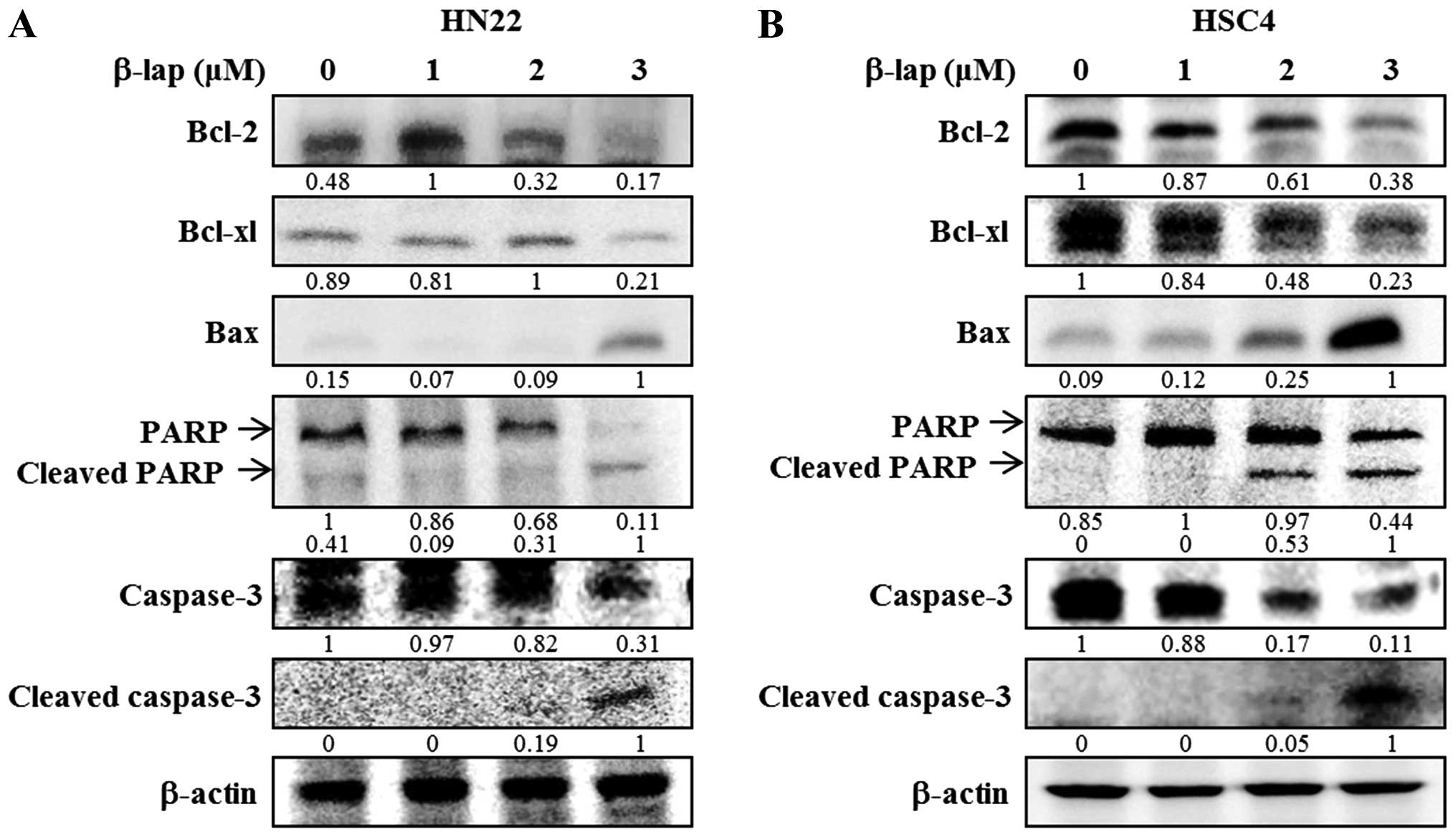
proteins in HN22 and HSC4 cell lines. As shown in Fig. 5, downregulation of Bcl-2 and Bcl-xL
and upregulation of Bax appeared to be involved in the apoptotic
cell death induced by β-lap. In addition, PARP and caspase were
decreased by β-lap treatment. Cleaved PARP and cleaved caspase-3
were induced by β-lap in a concentration-dependent manner. Our
results demonstrated that the treatment of OSCC cells with β-lap
induced the downregulation of Sp1, resulting in cell cycle arrest
and induction of apoptotic cell death.
Discussion
Natural derived or originated compounds are a rich
source of agents of value to medicine and have been the mainstay
for cancer chemotherapy for the past 30 years (22). This situation is accompanied by
increasing interest from drug companies and institutions devoted to
the search for new drugs (23).
Thus, the evaluation of natural products has been considered to
play an important role in development of chemotherapeutic agents.
Here, we focused on the anticancer effects of β-lap, a naturally
occurring quinone found in the bark of lapacho tree (Tabebuia
avellanedae) native to South America (5). β-lap has been also identified to be a
topoisomerase I and II inhibitor (14,15).
Previous studies have reported that inhibition of the enzymes of
topoisomerase I and II can strongly inhibit lymphocyte-,
neutrophil- and macrophage-associated joint inflammatory processes
and reduce the severity of collagen-induced arthritis (24,25).
Therefore, topoisomerase inhibitors can not only be used as
anticancer drugs to inhibit the proliferation of tumor cells, as
they have the potential to inhibit similar proliferative processes,
including rheumatoid arthritis (RA), synoviocytes and
angiogenesis.
Previously, several mechanisms involved in the
anticancer effect of β-lap were demonstrated. β-lap was reported to
induce sub-G1 cell population of cell cycle and induce
apoptosis in various cancer cells, including human leukemic, breast
carcinoma, prostate carcinoma, and lung carcinoma (5,26–29),
suggesting that β-lap could interfere with proliferation and induce
apoptosis in close association with sub-G1 arrest.
Nevertheless, the anticancer activities of β-lap on human OSCC
cells have not yet been fully characterized.
Here, we clearly demonstrated that β-lap could
induce apoptosis in human OSCC (HN22 and HSC4) cells. The induction
of apoptotic cell death by β-lap was also associated with
characteristic morphological changes, such as DNA condensation and
rounded cells, with cytoplasmic blebbing and irregularities in
shape. We observed an increase of DNA condensation and perinuclear
apoptotic bodies and sub-G1 population by β-lap
treatment in a concentration-dependent manner, supporting the
progress of apoptosis in β-lap-treated cells.
Sp1 is known as a transcription factor that is
regulated by the molecular target genes in various biological
processes, including survival, invasion, metastasis, and
angiogenesis (30). Previous
reports have shown that Sp1 is accumulated in cancer cells
(31). Transcriptional activity
primarily contributes to the increase of Sp1 during cancer
formation (32). Therefore, other
studies have examined whether downregulation of Sp1 by
anti-proliferative agents could modulate cell cycle- and
apoptosis-associated proteins and lead to growth inhibition and
apoptosis (33–36). For these reasons, Sp1 has been
suggested as therapeutic molecular target as well as potential
predictor of poor prognosis in cancer. Other studies have shown
that Sp1 level was highly increased and that the inhibition of Sp1
plays a role in growth inhibition and induction of apoptosis in
malignant pleural mesothelioma and oral cancer cells (20,21).
Our results showed that Sp1 was significantly reduced in
β-lap-treated cells and that apoptosis-related proteins were
regulated by Sp1.
Both p21 and p27 are negative regulator of
cyclin-dependent kinases and in this function they are negative
check-point regulators of the cell cycle. Their functional roles in
sub-G1 phase arrest result from the interaction of
cyclins and cyclin-dependent kinase (CDKs) complexes (37,38).
Cyclin D1 was also regulated by β-lap treatment. Cyclin D1 is the
first cyclin to be upregulated by growth factors during
G1 phase and it is considered to be a key intracellular
mediator of extracellular signal, that regulates proliferation
(39). Thus, its expression level
is related to tumorigenesis and cell maintenance (40). A previous study showed that
increased level of cyclin D1 is frequently observed in human
cancers (41). In addition, Sp1
was reported to be related to a variety of tumor-related genes. For
example, survivin contains Sp1 sites in the promoter region
(42). We confirmed that β-lap
could modulate expression of anti- and pro-apoptotic proteins
toward apoptosis. In addition, β-lap positively regulates p21 and
p27 and negatively regulates cyclin D1 and survivin in OSCCs,
resulting in the activation of caspase-dependent apoptosis pathway
through activated caspase-3 and PARP.
Based on the present data, we examined the cancer
chemoprevention effect and mechanisms of β-lap on OSCCs. Our
results indicated that β-lap was able to inhibit cell proliferation
and induce apoptosis by Sp1 through regulation of cell
cycle-related downstream target of Sp1 protein and apoptosis
associated proteins. Taken together, β-lap induced both
antiproliferative and apoptotic effects by inhibiting Sp1-regulated
gene products.
Acknowledgements
This study was carried out with the support of
‘Cooperative Research Program for Agriculture Science and
Technology Development (Project No. PJ009274)’ Rural Development
Administration, Republic of Korea.
References
|
1
|
Forastiere A, Koch W, Trotti A and
Sidransky D: Head and neck cancer. N Engl J Med. 345:1890–1900.
2001. View Article : Google Scholar
|
|
2
|
Jefferies S and Foulkes WD: Genetic
mechanisms in squamous cell carcinoma of the head and neck. Oral
Oncol. 37:115–126. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Herskovic A, Martz K, al-Sarraf M,
Leichman L, Brindle J, Vaitkevicius V, Cooper J, Byhardt R, Davis L
and Emami B: Combined chemotherapy and radiotherapy compared with
radiotherapy alone in patients with cancer of the esophagus. N Engl
J Med. 326:1593–1598. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Warnakulasuriya S: Global epidemiology of
oral and oropharyngeal cancer. Oral Oncol. 45:309–316. 2009.
View Article : Google Scholar
|
|
5
|
Moon DO, Kang CH, Kim MO, Jeon YJ, Lee JD,
Choi YH and Kim GY: Beta-lapachone (LAPA) decreases cell viability
and telomerase activity in leukemia cells: Suppression of
telomerase activity by LAPA. J Med Food. 13:481–488. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Ferreira SB, Salomão K, de Carvalho da
Silva F, Pinto AV, Kaiser CR, Pinto AC, Ferreira VF and de Castro
SL: Synthesis and anti-Trypanosoma cruzi activity of β-lapachone
analogues. Eur J Med Chem. 46:3071–3077. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
de Almeida ER, da Silva Filho AA, dos
Santos ER and Lopes CA: Antiinflammatory action of lapachol. J
Ethnopharmacol. 29:239–241. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Tzeng HP, Ho FM, Chao KF, Kuo ML,
Lin-Shiau SY and Liu SH: beta-Lapachone reduces endotoxin-induced
macrophage activation and lung edema and mortality. Am J Respir
Crit Care Med. 168:85–91. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Guiraud P, Steiman R, Campos-Takaki GM,
Seigle-Murandi F and Simeon de Buochberg M: Comparison of
antibacterial and antifungal activities of lapachol and
beta-lapachone. Planta Med. 60:373–374. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Li CJ, Zhang LJ, Dezube BJ, Crumpacker CS
and Pardee AB: Three inhibitors of type 1 human immunodeficiency
virus long terminal repeat-directed gene expression and virus
replication. Proc Natl Acad Sci USA. 90:1839–1842. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Kung HN, Yang MJ, Chang CF, Chau YP and Lu
KS: In vitro and in vivo wound healing-promoting activities of
beta-lapachone. Am J Physiol Cell Physiol. 295:C931–C943. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Planchon SM, Wuerzberger S, Frydman B,
Witiak DT, Hutson P, Church DR, Wilding G and Boothman DA:
Beta-lapachone-mediated apoptosis in human promyelocytic leukemia
(HL-60) and human prostate cancer cells: A p53-independent
response. Cancer Res. 55:3706–3711. 1995.PubMed/NCBI
|
|
13
|
Planchon SM, Pink JJ, Tagliarino C,
Bornmann WG, Varnes ME and Boothman DA: beta-Lapachone-induced
apoptosis in human prostate cancer cells: Involvement of NQO1/xip3.
Exp Cell Res. 267:95–106. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Li CJ, Averboukh L and Pardee AB:
beta-Lapachone, a novel DNA topoisomerase I inhibitor with a mode
of action different from camptothecin. J Biol Chem.
268:22463–22468. 1993.PubMed/NCBI
|
|
15
|
Krishnan P and Bastow KF: Novel mechanism
of cellular DNA topoisomerase II inhibition by the
pyranonaphthoquinone derivatives alpha-lapachone and
beta-lapachone. Cancer Chemother Pharmacol. 47:187–198. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Lee JH, Cheong J, Park YM and Choi YH:
Down-regulation of cyclooxygenase-2 and telomerase activity by
beta-lapachone in human prostate carcinoma cells. Pharmacol Res.
51:553–560. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Wierstra I: Sp1: emerging roles - beyond
constitutive activation of TATA-less housekeeping genes. Biochem
Biophys Res Commun. 372:1–13. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Black AR, Black JD and Azizkhan-Clifford
J: Sp1 and krüppel-like factor family of transcription factors in
cell growth regulation and cancer. J Cell Physiol. 188:143–160.
2001. View
Article : Google Scholar : PubMed/NCBI
|
|
19
|
Jiang Y, Wang L, Gong W, Wei D, Le X, Yao
J, Ajani J, Abbruzzese JL, Huang S and Xie K: A high expression
level of insulin-like growth factor I receptor is associated with
increased expression of transcription factor Sp1 and regional lymph
node metastasis of human gastric cancer. Clin Exp Metastasis.
21:755–764. 2004. View Article : Google Scholar
|
|
20
|
Chae JI, Jeon YJ and Shim JH:
Downregulation of Sp1 is involved in honokiol-induced cell cycle
arrest and apoptosis in human malignant pleural mesothelioma cells.
Oncol Rep. 29:2318–2324. 2013.PubMed/NCBI
|
|
21
|
Kim DW, Ko SM, Jeon YJ, Noh YW, Choi NJ,
Cho SD, Moon HS, Cho YS, Shin JC, Park SM, et al:
Anti-proliferative effect of honokiol in oral squamous cancer
through the regulation of specificity protein 1. Int J Oncol.
43:1103–1110. 2013.PubMed/NCBI
|
|
22
|
Mann J: Natural products in cancer
chemotherapy: Past, present and future. Nat Rev Cancer. 2:143–148.
2002. View
Article : Google Scholar
|
|
23
|
Wilson RM and Danishefsky SJ: Small
molecule natural products in the discovery of therapeutic agents:
The synthesis connection. J Org Chem. 71:8329–8351. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Jackson JK, Higo T, Hunter WL and Burt HM:
Topoisomerase inhibitors as anti-arthritic agents. Inflamm Res.
57:126–134. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Tudan C, Jackson JK, Higo TT and Burt HM:
The effect of inhibiting topoisomerase I and II on the
anti-apoptotic response associated with pro-inflammatory crystals
of calcium pyrophosphate dihydrate in human neutrophils. Inflamm
Res. 52:8–17. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Pink JJ, Wuerzberger-Davis S, Tagliarino
C, Planchon SM, Yang X, Froelich CJ and Boothman DA: Activation of
a cysteine protease in MCF-7 and T47D breast cancer cells during
beta-lapachone-mediated apoptosis. Exp Cell Res. 255:144–155. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Wuerzberger SM, Pink JJ, Planchon SM,
Byers KL, Bornmann WG and Boothman DA: Induction of apoptosis in
MCF-7:WS8 breast cancer cells by beta-lapachone. Cancer Res.
58:1876–1885. 1998.PubMed/NCBI
|
|
28
|
Choi YH, Kang HS and Yoo MA: Suppression
of human prostate cancer cell growth by beta-lapachone via
down-regulation of pRB phosphorylation and induction of Cdk
inhibitor p21(WAF1/CIP1). J Biochem Mol Biol. 36:223–229. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Dong GZ, Oh ET, Lee H, Park MT, Song CW
and Park HJ: Beta-lapachone suppresses radiation-induced activation
of nuclear factor-kappaB. Exp Mol Med. 42:327–334. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Safe S and Abdelrahim M: Sp transcription
factor family and its role in cancer. Eur J Cancer. 41:2438–2448.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Li L and Davie JR: The role of Sp1 and Sp3
in normal and cancer cell biology. Ann Anat. 192:275–283. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Hsu TI, Wang MC, Chen SY, Yeh YM, Su WC,
Chang WC and Hung JJ: Sp1 expression regulates lung tumor
progression. Oncogene. 31:3973–3988. 2012. View Article : Google Scholar :
|
|
33
|
Kavurma MM and Khachigian LM: Sp1 inhibits
proliferation and induces apoptosis in vascular smooth muscle cells
by repressing p21WAF1/Cip1 transcription and cyclin
D1-Cdk4-p21WAF1/Cip1 complex formation. J Biol Chem.
278:32537–32543. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Xu R, Zhang P, Huang J, Ge S, Lu J and
Qian G: Sp1 and Sp3 regulate basal transcription of the survivin
gene. Biochem Biophys Res Commun. 356:286–292. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Sankpal UT, Goodison S, Abdelrahim M and
Basha R: Targeting Sp1 transcription factors in prostate cancer
therapy. Med Chem. 7:518–525. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Chae JI, Jeon YJ and Shim JH:
Anti-proliferative properties of kahweol in oral squamous cancer
through the regulation specificity protein 1. Phytother Res.
28:1879–1886. 2014. View
Article : Google Scholar : PubMed/NCBI
|
|
37
|
Sherr CJ and Roberts JM: CDK inhibitors:
Positive and negative regulators of G1-phase progression. Genes
Dev. 13:1501–1512. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Murray AW: Recycling the cell cycle:
Cyclins revisited. Cell. 116:221–234. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Sherr CJ: G1 phase progression: Cycling on
cue. Cell. 79:551–555. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Ewen ME and Lamb J: The activities of
cyclin D1 that drive tumorigenesis. Trends Mol Med. 10:158–162.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Weinstein IB: Relevance of cyclin D1 and
other molecular markers to cancer chemoprevention. J Cell Biochem.
(Suppl 25): S23–S28. 1996. View Article : Google Scholar
|
|
42
|
Xu Q, Liu M, Xu N and Zhu H: Variation in
Sp1 binding sites correlates with expression of survivin in breast
cancer. Mol Med Rep. 10:1395–1399. 2014.PubMed/NCBI
|