Introduction
Chronic myelogenous leukemia (CML) is one of the
neoplasms characterized by abnormally elevated white blood cells
(WBCs) (1). The incidence of CML
in the United States is ~5,000 cases per year, and its prevalence
has been increasing annually since 2000, owing to the low annual
mortality rate of 1–2% (2). CML
has a pivotal place in oncology because >95% of affected
individuals express fusion Bcr-Abl protein, a genetic rearrangement
formed by reciprocal translocation between chromosomes 9 and 22,
which results in a shortened 22q-the Philadelphia chromosome (Ph)
(3). The subsequent deregulation
of Bcr-Abl leads to enhanced proliferation, resistance to
apoptosis, and altered adhesion through hyper-activation of various
signaling pathways, including MAPK/Erk and PI3K/Akt (4). The universal presence of Bcr-Abl
fusion protein in CML patients has led to the development of small
molecule kinase inhibitors to target Bcr-Abl.
Imatinib, the first generation powerful tyrosine
kinase inhibitor (TKI), revolutionized the treatment of CML
(5). Imatinib has demonstrated
excellent treatment for CML due to a cumulative complete
cytogenetic response (CCyR) rate of 87% and the projected overall
and progression-free survival rates of 89% in a newly diagnosed
patient with CML-chronic phase (CML-CP) (6). Unfortunately, resistance to Imatinib
is encountered, and an increasing number of recent studies have
documented a lack of patient response to Imatinib (7,8).
Imatinib resistance might to be due to amplification and point
mutation in a kinase domain of Bcr-Abl (9,10).
More than 40 kinds of different point mutations have
been classified to be related with clinical resistance to Imatinib,
and especially 20% of all the point mutations are identified to be
a stubborn point mutation, namely the T315I point mutation, which
is an amino acid transformation in the Abl 315th position (11). Although, the second generation
Bcr-Abl TKIs, such as Nilotinib and Dasatinib, have been developed
and circumvented several types of point mutations (E255K, M351T),
those have not overcome T315I mutation as well (12,13).
These TKIs are not curative since most patients who discontinue
therapy due to drug resistance will exhibit rapid progression. To
overcome this resistance, more effective tyrosine kinase inhibitors
are being developed, however, drug resistance and side effects of
new agents are still important issues to be considered. Therefore,
the search for other novel targets and new strategies for the
management of CML is urgent.
Plant-derived natural products play an important
role in the cure of various diseases. Numerous natural products are
under investigation for their clinical efficacy in the prevention
and treatment of a wide array of diseases including cancer
(14,15). Among these, Yellow poplar or tulip
tree, Liriodendron tulipifera L. (Magnoliaceae), is a fast
growing native timber tree with a tall and straight trunk in North
America (16). The bark of
Liriodendron tulipifera L. was widely used by the Native
Americans as a tonic, stimulant, and its diaphoretic properties
have been considered effective in treating chronic rheumatism,
dyspepsia and avian malaria (17).
To date, the phytochemical investigation of Liriodendron
tulipifera L. has yielded numerous constituents, including
antiplasmodial alkaloids, various bioactive lignans, and cytotoxic
sesquiterpene lactones (18–21).
A recent study has reported that partenolide, one of
the sesquiterpene lactones isolated from Liriodendron
tulipifera L. and its semi-synthetic derivatives, showed a
cytotoxic effect in leukemia cells (22). These facts imply that the extract
of Liriodendron tulipifera L. has the potential to be an
anticancer agent in leukemia. For this reason, Liriodendron
tulipifera L., a rich source of metabolites with
a-methylene-r-lactone moiety, was chosen to be investigated for
anti-leukemic potential. In this study, we extracted CD-200 with
rich sesquiterpene lactones from Liriodendron tulipifera L.
and investigated its anticancer effect and mechanism of action in
BaF3/T315I or BaF3/WT leukemic cells. Our present study
demonstrated that CD-200 inhibited the Bcr-Abl pathway and induced
apoptosis in BaF3/WT cells, and also T315I-mutated Bcr-Abl cells
with resistance to Imatinib.
Materials and methods
Extraction of CD-200
Trees of Liriodendron tulipifera L., yellow
poplar (~0.9–1.4 meters in height), were collected from Kangjin,
Chonnam Province, Korea. Air-dried trees (1.0 kg) were chopped off
before extraction. The chopped plant materials were exhaustively
extracted three times with either ethyl acetate or ethanol. Each
extract was separately filtered and concentrated by a rotary
evaporator. The resulting concentrates were completely dried via
freeze dryer to yield brownish extract (11.3 g). In order to
isolate the active ingredient, sesquiterpene lactones, including
epi-Tulipinolide from ethyl acetate or ethanol extract, further
reversed-phase high-performance liquid chromatography (HPLC) with a
stepwise gradient solvent system from H2O to methanol
was performed.
Cells and materials
The BaF3/WT and BaF3/T315I cells were provided by Dr
M. Deininger (Huntsman Cancer Institute, Salt Lake City, UT, USA).
The cells expressed wild-type Bcr-Abl and Bcr-Abl with the T315I
mutation, respectively. BaF3/WT and BaF3/T315I cells were grown in
Roswell Park Memorial Institute Medium-1640 (RPMI-1640), containing
10% fetal bovine serum (FBS) and 1% penicillin/streptomycin.
RPMI-1640, FBS, and penicillin/streptomycin were purchased from
Gibco (Grand Island, NY, USA). Imatinib was purchased from LC
Laboratories (Woburn, MA, USA), and sodium
2,3-bis(2-methoxy-4-nitro-5-sulfophe sodium
2,3-bis(2-methoxy-4-nitro-5-sulfophenyl)-2H-tetrazolium-5-carboxanilide
inner salt (XTT) assays were purchased from WelGene Inc. (Daegu,
Korea).
Cell viability assay
Cell viability of corresponding compounds was
determined by XTT assay. The cells were seeded and treated onto
96-well plates at a density of 1×103 cells per well and
incubated at 37°C for 48 h. The cells were then treated with either
CD-200 or Imatinib at the indicated concentrations (0.1–10 μg/ml)
or (0.01–10 μM), respectively. Then, 10 μl of XTT labeling mixture
[1 ml of XTT/20 μl of phenazinemethosulfate (PMS)] was added to
each well. After incubation for 4 h, optical density (OD) was
determined using a microplate reader by measuring the absorbance at
wavelengths 540 nm and 620 nm. The absorbance rates for each well
were calculated as OD540–OD620.
Western blotting
After the cells were treated with various
concentrations of either CD-200 or Imatinib and incubated at 37°C
for various times, they were collected and washed with cold
phosphate-buffered saline (PBS). Then, the cells were lysed with a
RIPA buffer (Biosesang, Seongnam, Korea) containing protease and
phosphatase inhibitor cocktails (GenDEPOT, Barker, TX, USA). The
proteins were resolved by sodium dodecyl sulfate-polyacrylamide gel
electrophoresis, and transferred onto the nitrocellulose membranes.
The blots were immunostained with appropriate primary antibodies
followed by secondary antibodies conjugated to horseradish
peroxidase. Antibody binding was detected with an enhanced
chemiluminescence reagent (Bio-Rad, Hercules, CA, USA). Primary
monoclonal antibodies against the following factors were used;
p-Bcr-Abl (Tyr177), p-Crkl (Tyr207), p-Stat5
(Tyr694), Bcr-Abl, Crkl, Stat5, cleave caspase-3,
cleaved PARP, survivin, Mcl-1 and β-actin (Cell Signaling
Technology, Danvers, MA, USA). Secondary antibodies were purchased
from Santa Cruz Biotechnology (Dallas, TX, USA).
Immunofluorescence
BaF3/T315I cells were plated in 48-well plates with
RPMI-1640 medium and treated with CD-200 for various
concentrations. The cells were then suspended on poly-l-lysine-coated slides, followed
by Shandon Cytospin 3 (Akribis Scientific, Cheshire, WA, USA) for 3
min at 1000 rpm. Thereafter, the cells were fixed in 4%
paraformaldehyde (PFA) for 15 min at room temperature and washed
twice with PBS. The cells were blocked in 5% horse and goat serums
in PBS for 1 h at room temperature, then incubated in a humidified
chamber at 4°C overnight with p-Bcr-Abl (Tyr177)
antibody (Cell Signaling Technology). After washing twice with PBS,
the cells were incubated with rabbit tetramethyl rhodamine
isothiocyanate (TRITC) secondary antibody (Dianova, Germany) for 1
h at room temperature. They were also stained with
4,6-diamidino-2-phenylindole (DAPI) to visualize the nuclei. The
slides were then washed twice with PBS and covered with Dako
(Carpinteria, CA, USA) before viewing with a confocal laser
scanning microscope (Olympus, Tokyo, Japan).
Moreover, after deparaffinization, immunostaining
was performed, using 8-μm-thick sections of the tumor samples. The
tissue sections were then blocked with a normal goat or horse serum
(Vector Laboratories, Burlingame, CA, USA) for 1 h, and were
incubated at 4°C overnight with the primary antibody. After washing
twice with PBS, the cells were incubated with the rabbit TRITC
secondary antibody for 1 h at room temperature. They were also
stained with DAPI to visualize the nuclei. The slides were then
washed twice with PBS and covered with Dako before viewing with a
confocal laser scanning microscope.
Cell cycle analysis
BaF3/T315I cells were plated in 10-cm dishes with an
RPMI-1640 medium and treated with CD-200 at the indicated
concentrations (0–5 μg/ml). The cells were collected and fixed in
cold 70% ethanol at −20°C overnight. After washing with PBS, the
cells were subsequently stained with 50 μg/ml propidium iodide (PI)
and 100 μg/ml RNase A for 30 min at room temperature in the dark;
then a flow cytometric analysis was performed to determine the
percentage of cells in specific sub-G1 phases, using a
FACS Calibur flow cytometry (BD Biosciences, San Jose, CA,
USA).
TUNEL staining
BaF3/T315I cells were plated in 48-well plates with
RPMI-1640 medium and treated with various concentrations of CD-200.
The cells were then suspended on poly-l-lysine-coated slides, followed
by Shandon Cytospin 3 for 3 min at 1000 rpm. They were then fixed
in 4% PFA for 15 min at room temperature and washed twice with PBS.
The terminal deoxynucleotidyl transferase-mediated nick end
labeling (TUNEL) was subsequently performed using a TUNEL kit
(Merck Millipore, Temecula, CA, USA) in accordance to the
manufacturer’s instructions.
Measurement of mitochondrial membrane
potential
Mitochondrial membrane potential (MMP, Δψ)
was assessed using the Mitochondrial Membrane Potential Detection
kit (BD Biosciences). BaF3/T315I cells were then treated with
various concentrations of CD-200 for 8 h. The cells were incubated
with JC-1 working solution at 37°C. After washing two times, the
cells were suspended in 0.5 ml of 1X assay buffer prior to flow
cytometry. The results were analyzed using FlowJo software (Tree
Star, Ashland, OR, USA).
Analysis of cytochrome c
localization
BaF3/T315I cells were treated with various
concentrations for 8 h. To label the mitochondria, the cells were
incubated with 500 nM mitochondrion-specific dye
(MitoTracker® Green FM; Molecular Probes Inc., Eugene,
OR, USA) for 45 min at 37°C prior to fixation. The cells were then
suspended on poly-l-lysine-coated slides, followed
by Shandon Cytospin 3 for 3 min at 1000 rpm. They were then fixed
in 4% PFA for 15 min at room temperature and washed with PBS. The
cells were incubated at 4°C overnight with cytochrome c antibody
(Santa Cruz Biotechnology). After washing twice with PBS, the cells
were incubated with mouse TRITC secondary antibody (Dianova). The
cells were also stained with DAPI to visualize the nuclei. The
slides were then washed twice with PBS and covered with Dako before
viewing with a confocal laser scanning microscope.
Tumor xenograft study
All animal experiments were performed in accordance
to the guidelines of the INHA Institutional Animal Care and Use
Committee (INHA IACUC) at Inha University Medical School. The cells
were harvested and mixed in PBS (200 μl/mouse). Six weeks old male
BALB/c nude mice (Orient Bio, Seoul, Korea) were inoculated with
1×105 cells in the flank. When the tumor size reached
approximately 50–100 mm3, they were randomly divided
into 3 groups, with 5 mice in each. Then, these mice were given
CD-200 (50 mg/kg) or Imatinib (50 mg/kg) intra-peritoneally once a
day for 11 days, and the control group was fed vehicle. The tumor
size was measured every 2 days, and it was calculated using the
formula, 0.5 × length × width2.
Immunohistochemistry
The tissue sections were blocked with normal goat or
horse serum (Vector Laboratories) for 1 h, and incubated at 4°C
overnight in 1:50 dilutions of p-Bcr-Abl, Ki-67, and cleaved
caspase-3 (Cell Signaling Technology). The sections were then
incubated with biotinylated secondary antibodies (1:100) for 1 h.
The sections were visualized by an avidin-biotin peroxidase complex
solution using an ABC kit (Vector Laboratories), which were then
washed in PBS and developed with a diaminobenzidine
tetrahydrochloride substrate for 15 min, then counterstained with
hematoxylin. At least 3 random fields of each section were examined
at ×400 magnification and analyzed using a computer image analysis
system (Media Cybernetics, Rockville, MD, USA).
Statistical analysis
Data were expressed as the mean ± standard deviation
(SD). Statistical analysis was performed using ANOVA and unpaired
Student’s t-tests. Statistical significance was set to
p<0.05.
Results
CD-200 inhibits the proliferation of
BaF3/WT and BaF3/T315I cells
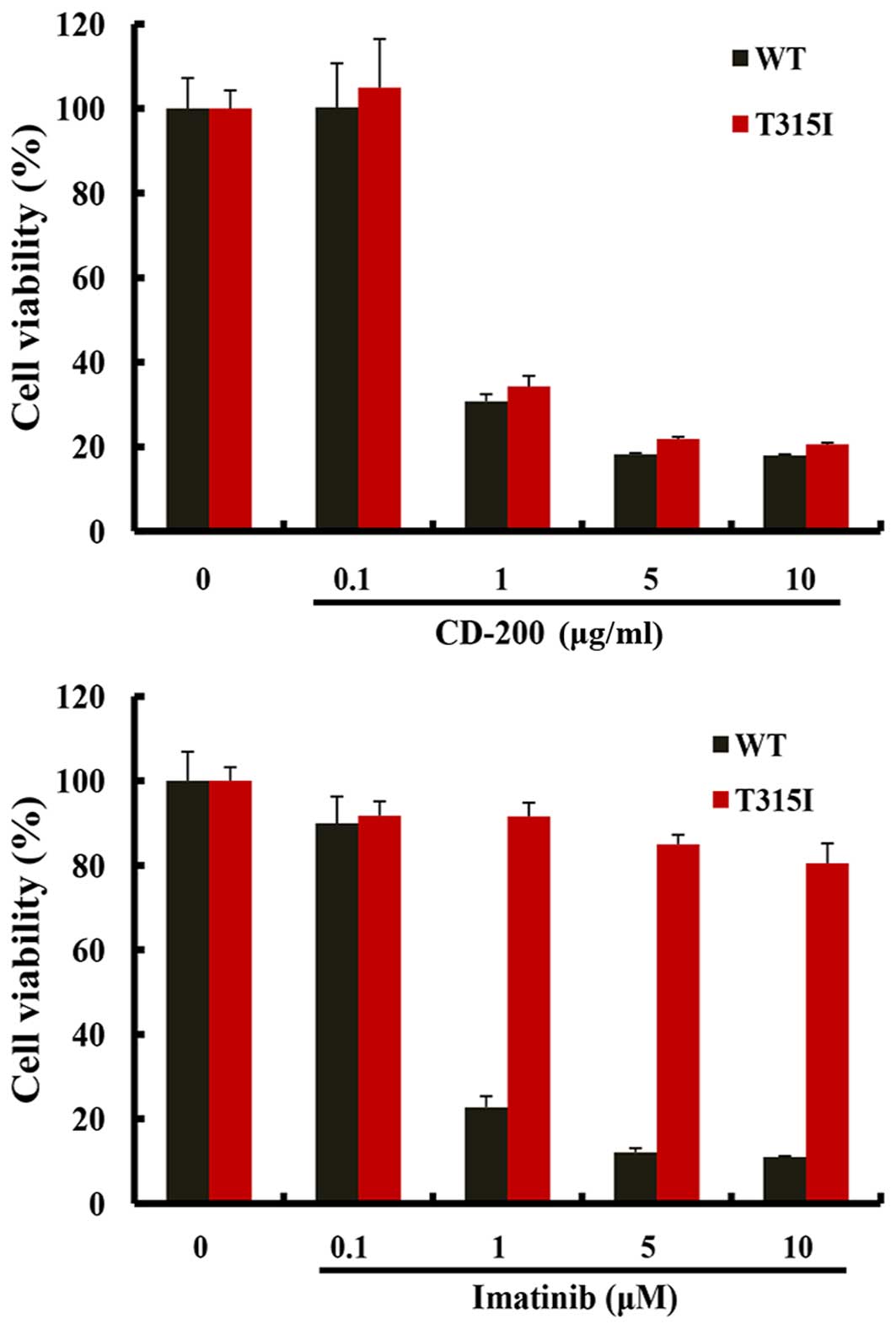
XTT assays were performed to evaluate the effect of
CD-200 on the growth of BaF3/WT and BaF3/T315I cells. The cells
were treated with various concentrations of CD-200 and Imatinib for
48 h. As shown in Fig. 1, both
CD-200 and Imatinib treatments reduced the cell viability of
BaF3/WT cells; the IC50 values were about 0.6 μg/ml for
CD-200 and 0.4 μM for Imatinib. In addition, CD-200 significantly
reduced the cell viability of BaF3/T315I cells (IC50≅0.8
μg/ml), while Imatinib had little effect. These data suggest that
CD-200 exhibits the potent inhibitory activity in BaF3 cells,
expressing Bcr-Abl T315I mutation resistance to Imatinib, as well
as wild- type Bcr-Abl leukemic cells.
CD-200 inhibits Bcr-Abl signaling
pathways in BaF3/T315I cells
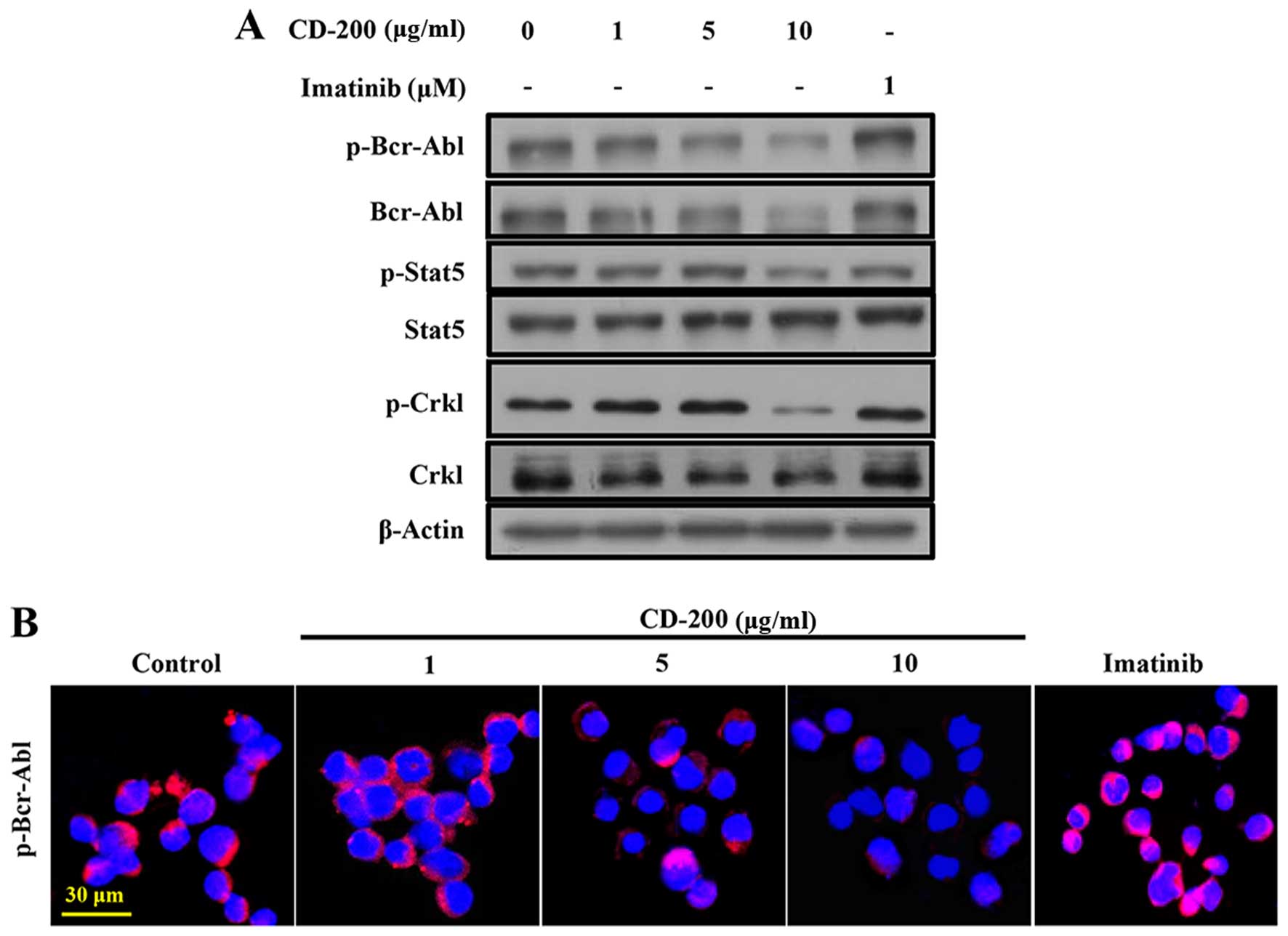
In order to assess the effects of CD-200 on the
inhibition of Bcr-Abl activity, phosphorylation of Bcr-Abl and its
respective downstream signals, Crkl and Stat5, were measured by
western blotting. As shown in Fig.
2A, CD-200 strongly inhibited the phosphorylation of Bcr-Abl
(Tyr177) in BaF3/T315I cells. Likewise, the
phosphorylation levels of Crkl (Tyr207) and Stat5
(Tyr694) were effectively suppressed. In contrast,
Imatinib did not alter the phosphorylation levels of Bcr-Abl, Crkl,
and Stat5 in BaF3/T315I cells. The expression of p-Bcr-Abl was
confirmed by confocal fluorescent microscopy (Fig. 2B).
CD-200 induces apoptotic cell death in
BaF3/T315I cells
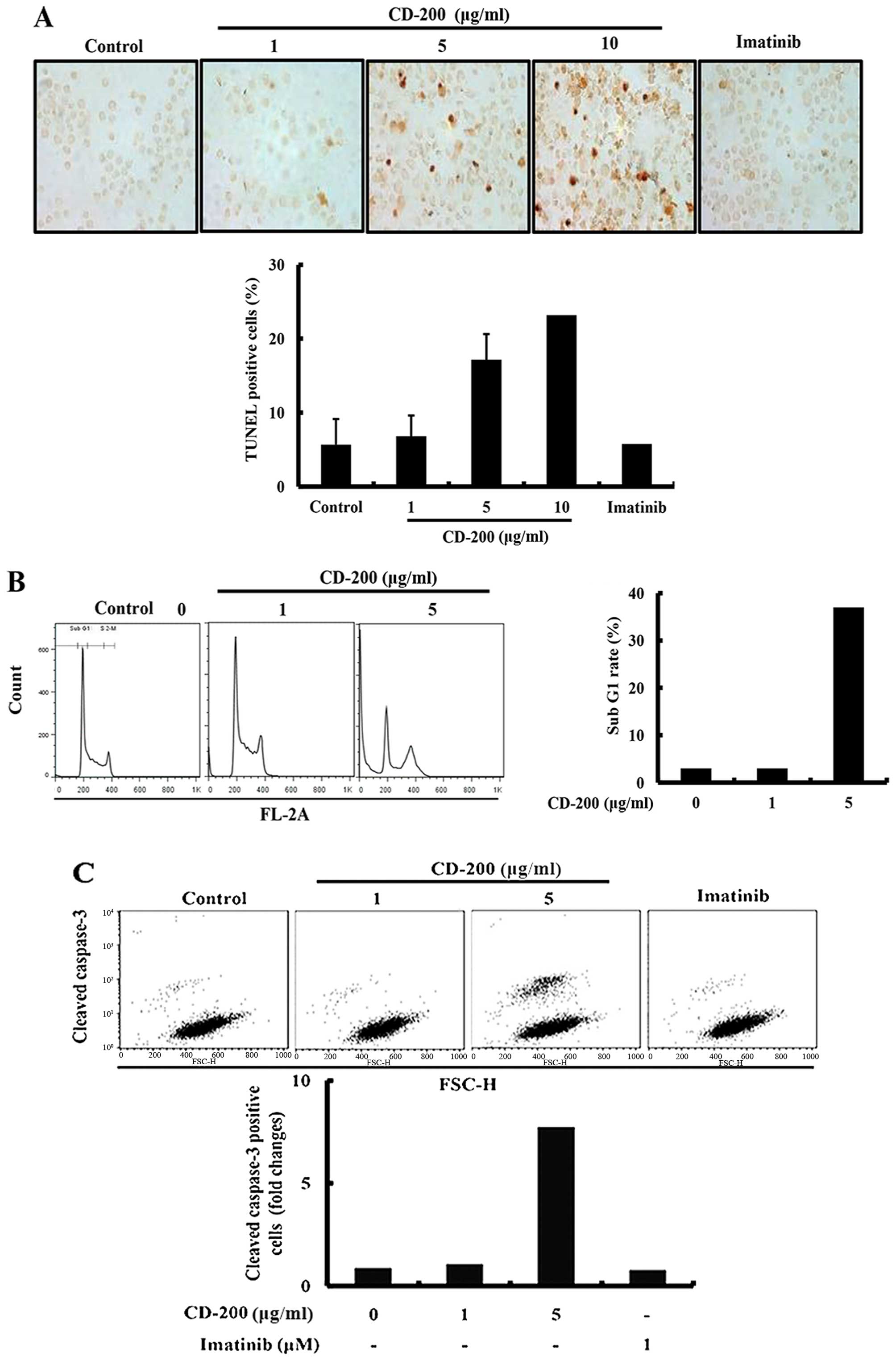
In order to investigate whether the anticancer
effect of CD-200 in BaF3/T315I cells was associated with the
induction of apoptosis, we performed several cell-based apoptosis
assays. We first identified the nuclear morphology on 12 h after
treatment with CD-200 by using TUNEL apoptosis assay kits. As a
result, CD-200-treated cells were presented with more prominent DNA
fragmentation in comparison to the control group (Fig. 3A). Next, we assessed the cell cycle
distribution by a flow cytometric analysis. After 24 h treatment
with CD-200, the cells were collected and stained with PI, then
analyzed by FACS. As shown in the Fig.
3B, CD-200 increased the number of cells in the
sub-G1 phase, associated with early apoptosis without
changes of the cell cycle arrest (Fig.
3B). In support of apoptotic effect of CD-200, we observed that
CD-200 also increased cleaved caspase-3 positive cells by FACS
(Fig. 3C).
CD-200 induces mitochondria-dependent
apoptosis in BaF3/T315I cells
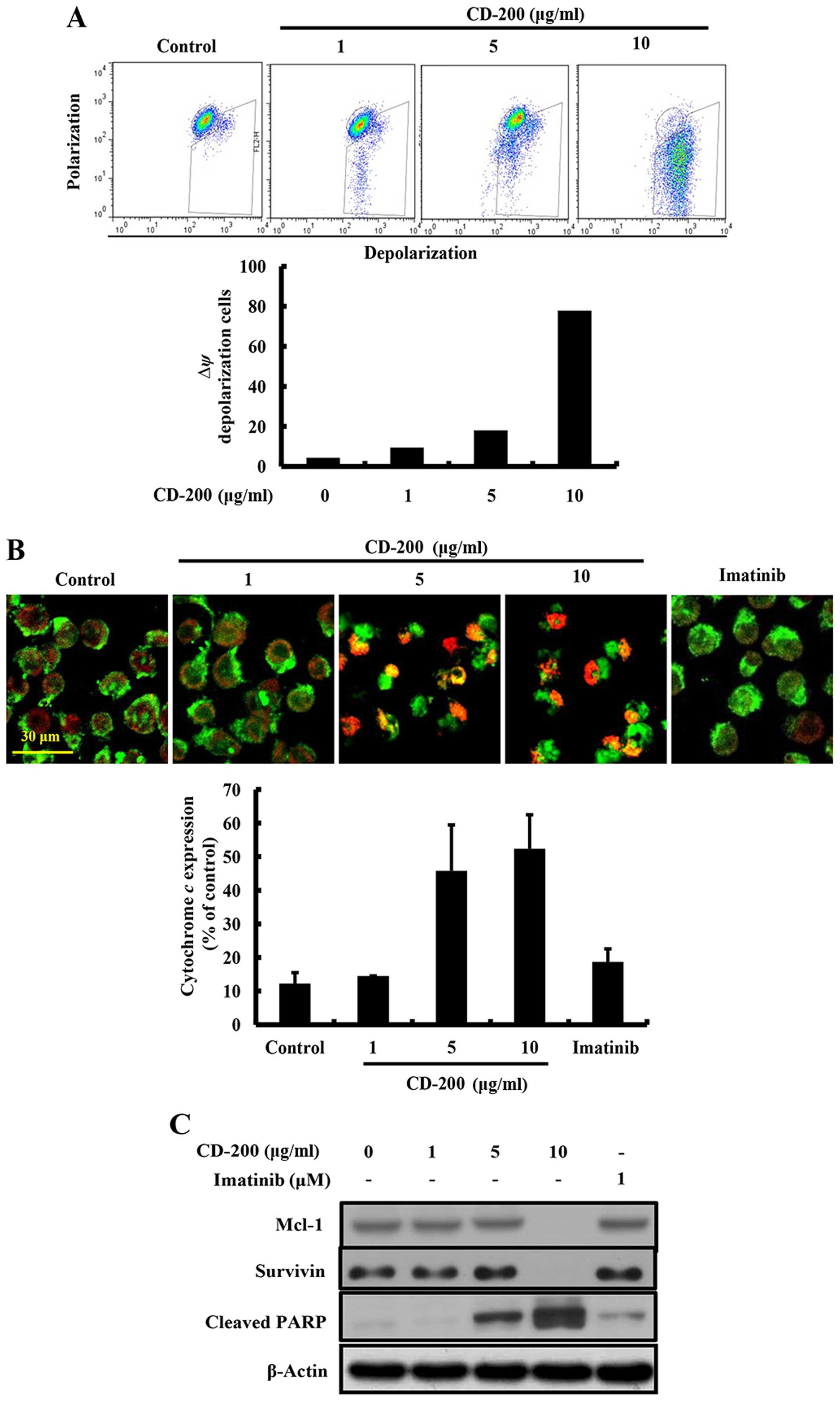
To gain further insight into the mechanism underling
apoptosis induced by CD-200, we examined the mitochondrial
potential change, which plays an important role in the regulation
of apoptosis. Since a loss of MMP induces the transition of
mitochondrial permeability and release of cytochrome c from the
mitochondria to cytosol (23), we
measured the MMP and cytochrome c release in CD-200-treated
BaF3/T315I cells. As shown in Fig.
4A, CD-200 significantly reduced the fluorescence intensity
reflecting MMP, while no changes were observed in the
Imatinib-treated group. Moreover, we observed that the treatment of
CD-200 increased the release of cytochrome c by immunostaining
(Fig. 4B). In addition, CD-200
inhibited the expression of mitochondria-mediated protein families,
such as Mcl-1, and survivin (Fig.
4C) along with that of cleaved PARP. These results indicated
that CD-200 induced apoptosis through the mitochondria-mediated
intrinsic pathways in BaF3/T315I cells.
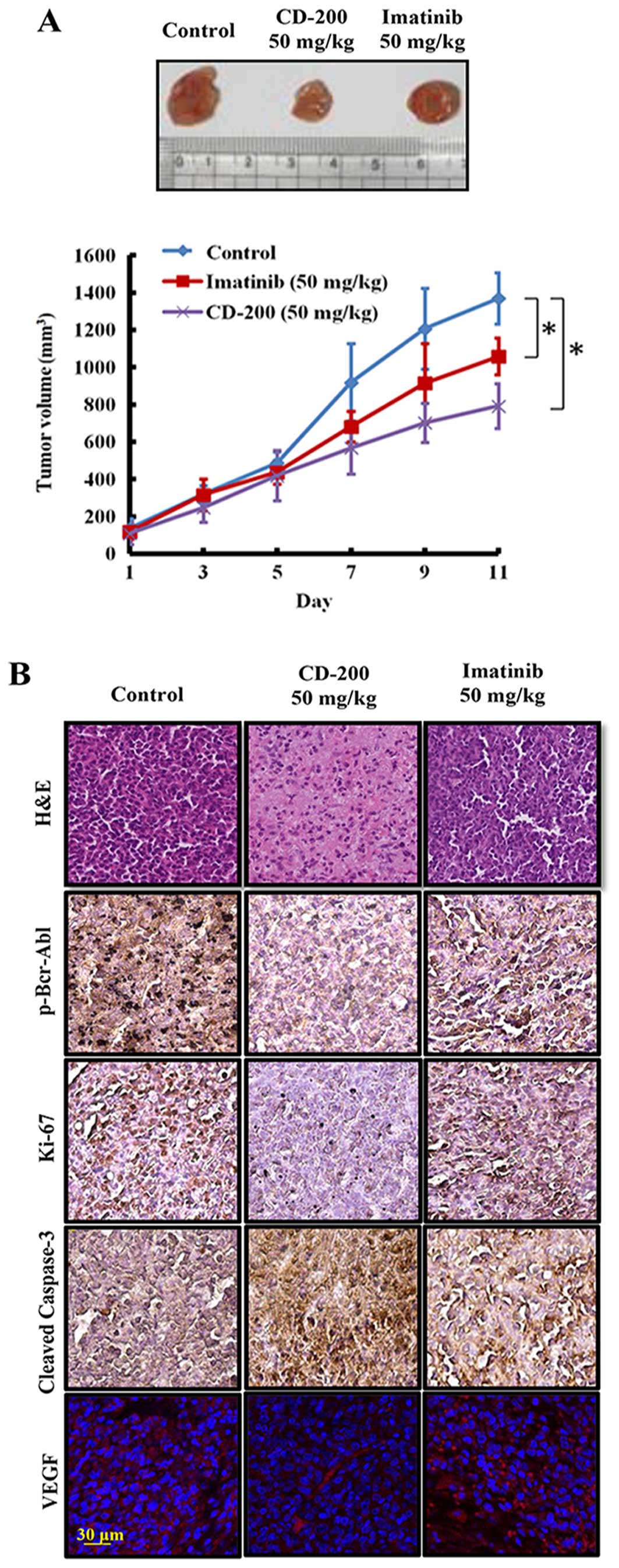
CD-200 inhibits tumor growth in mouse
xenograft models
We extended our study to an in vivo mouse
xenograft model. After inoculation with BaF3/T315I cells, mice were
intraperitoneally injected with CD-200, at doses of 50 mg/kg and
Imatinib, once a day for 11 days. CD-200 potently inhibited the
progression of tumor growth, which became more noticeable and
significant on day 11 as compared to the control group, whereas
Imatinib treatment did not show significant anticancer effect in
this BaF3/T315I cell xenograft model (Fig. 5A). No significant changes in body
weight or adverse effect were observed in any of the groups (data
not shown). To further confirm whether CD-200 inhibits tumor growth
through the induction of apoptosis and inhibition of proliferation,
we identified the expression of cleaved caspase-3 and Ki-67 in
tumor tissues. As expected, CD-200-treated tumor showed an
increased expression of cleaved caspase-3 and decreased Ki-67
expression compared to the control and Imatinib groups (Fig. 5B). Moreover, the treatment with
CD-200 decreased the phosphorylation of p-Bcr-Abl (Fig. 5B). Taken together, these results
demonstrate that CD-200 has antitumor potency in the mouse
xenograft model bearing BaF3/T315I cells.
Discussion
For over 40 years, natural products have played a
very important role in cancer chemotherapy, either as unmodified or
synthetically modified forms (24). For instance, plant-derived
compounds are widely used as anticancer agents, such as bisindole
(vinca) alkaloids, camptothecins, epipodophyllotoxins, and taxanes
(25). The focus in cancer control
has been on the search for safer anticancer agents, with higher
patient acceptability. Thus, the use of herbal/natural products is
more popular over synthetic drugs, and provides alternative
treatment options for patients (26,27).
Natural products have been investigated for cancer prevention and
treatment (27,28). In this study, we extracted CD-200
from Liriodendron tulipifera L., with abundant sesquiterpene
lactones. Based on a previous study where sesquiterpene lactones,
isolated from Liriodendron tulipifera L., showed cytotoxic
effect in leukemia cells (22), we
set out to identify the anticancer effects of CD-200, and its
mechanism of action in BaF3/WT and BaF3/T315I leukemia cells with
Imatinib resistance. Herein, we report for the first time that
CD-200 inhibited the Bcr-Abl signaling pathway, which may lead to
the inhibition of cell growth and induction of apoptosis in
vitro and in vivo models of Imatinib-resistant CML.
Imatinib has been used as a major TKI to target the
Bcr-Abl tyrosine kinase activity in CML patients for decades
(29). However, Imatinib
resistance develops over time and is an emerging problem for CML
patients. Although, Imatinib strongly inhibits the phosphorylation
of tyrosine in the wild-type Bcr-Abl, it does not act on Bcr-Abl
with T315I mutations (30).
Although the new second-generation Abl kinase inhibitors, such as
AMN107, Dasatinib, INNO-406 and PD166326, have been developed, they
do not show any positive effects in Imatinib-resistant patients
with T315I mutation (12,13,31,32).
Thus, it is important to develop alternative treatment strategies.
In this study, we aimed to identify effective chemotherapy with the
use of natural products against CML cells with T315I-mutant Bcr-Abl
that confers resistance to Imatinib. To carry this out, we
extracted CD-200 from Liriodendron tulipifera L., and
investigated whether CD-200 had potent activity in BaF3-expressing
wild-type Bcr-Abl and T315I-mutated Bcr-Abl cells. CD-200 strongly
inhibited the cell proliferation in both BaF3/WT and BaF3/T315I
cells. However, Imatinib failed to inhibit the proliferation of
BaF3/T315I cells, since Imatinib did not inhibit cell growth even
at a high concentration (10 μM).
The Bcr-Abl kinase signals affects multiple
downstream survival pathways, including Ras/Raf/Mek, PI3K/Akt, and
Jak/Stat, which contribute to the pathogenesis of CML (33,34).
Especially, Stat5 pathway, a surrogate marker of Bcr-Abl activity
in primary CML cells, contributes to leukemic cell proliferation
and survival (35). Furthermore,
the Stat5 activation is mediated by an adaptor protein, Crk-like
protein (Crkl) (36). In addition,
Stat5 activity appears to play a major role in anti-apoptotic and
proliferative abilities of Bcr-Abl transformed cells (37). Thus, we investigated whether CD-200
could suppress the Bcr-Abl signaling pathway. As expected, CD-200
inhibited the phosphorylation of Bcr-Abl and the phosphorylation of
Bcr-Abl downstream target Stat5 in BaF3/T315I cells. Also, the
phosphorylation of Crkl, another Bcr-Abl downstream target, was
clearly reduced by CD-200 treatment. On the contrary, Imatinib did
not inhibit the phosphorylation of the Bcr-Abl pathways, such as
Bcr-Abl, Stat5 and Crkl in BaF3/T315I cells. These results reveal
that the decrease of phosphorylation of Crkl and Stat5 by CD-200
indicate an effective inhibition of Bcr-Abl carrying T315I highly
resistant mutation, whereas no inhibition of phosphorylation was
induced by Imatinib treatment.
Apoptosis is a controlled form of cell death with
the ability to contribute to the inhibition of cell growth in
cancer cells. To date, the molecular mechanisms by which anticancer
drugs induce apoptosis have been reported to involve the activation
of various apoptotic signaling or inhibition of survival signaling
(38,39). Bcr-Abl is known as a potent cell
death inhibitor and inhibits apoptosis, as well as the enhancement
of cell proliferation in CML (40,41).
Bcr-Abl positive cells continue to signal biochemically to prevent
apoptosis induced by chemotherapeutic therapy (42). Previous studies have shown that
Bcr-Abl prevents apoptosis through the inhibition of mitochondrial
cytochrome c release (41). In
keeping with these reports, we examined whether CD-200 has an
effect on the mitochondrial membrane potential and induction of
apoptosis in BaF3/T315I cells. In this study, we observed that
CD-200 increased cytochrome c release and decreased the expression
of Mcl-1 and survivin, which has been proposed to bind to caspases
to inhibit apoptosis with mitochondria potential-related molecules
(43). CD-200 induced an increased
expression of cleaved PARP, as well as TUNEL positive apoptotic
bodies.
Previously, anticancer effects of several
sesquiterpene lactones, a main ingredient of CD-200, isolated from
various plants have been reported to modulate various molecular
signal transduction pathways (44–46).
Especially, Yeh et al have reported that sesquiterpene
lactones induced apoptosis by increasing caspase-3 through a
modulation of the Stat signaling pathway in lung cancer (47). Similarly, CD-200, including
sesquiterpene lactones, also induced apoptosis by changing the
mitochondria potential in CML cells, although their source were
different. These events were supported by in vivo results,
showing that CD-200 inhibited the tumor growth and induced
apoptosis by increasing the expression of cleaved caspase-3 in
tumor tissues of xenograft mouse BaF3/T315I cells. In contrast,
Imatinib did not change the expression of apoptosis-related
molecules in vitro and in vivo. Considering all the
above results, CD-200 induced apoptosis via a
mitochondria-dependent pathway in BaF3/T315I cells, suggesting that
apoptosis by CD-200 may be accomplished by inhibiting the Bcr-Abl
signaling pathways.
In conclusion, our study demonstrated that CD-200
suppressed tumor growth and induced mitochondria-mediated apoptosis
by downregulating the Bcr-Abl signaling pathway. Our findings could
be considered for future clinical investigations in CML patients
with Imatinib resistance. We suggest that CD-200 may have great
potential in overcoming T315I mutation-induced Imatinib resistance
in patients with CML.
Acknowledgements
This study was supported by Inha University Grant
and Medical Research Center (2014009392) funded by MSIP, Korea.
References
|
1
|
Savona M and Talpaz M: Chronic myeloid
leukemia: changing the treatment paradigms. Oncology (Williston
Park). 20:707–711; discussion 712–704, 719, 724. 2006.
|
|
2
|
Huang X, Cortes J and Kantarjian H:
Estimations of the increasing prevalence and plateau prevalence of
chronic myeloid leukemia in the era of tyrosine kinase inhibitor
therapy. Cancer. 118:3123–3127. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Kurzrock R, Gutterman JU and Talpaz M: The
molecular genetics of Philadelphia chromosome-positive leukemias. N
Engl J Med. 319:990–998. 1988. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Tomoda K, Kato JY, Tatsumi E, Takahashi T,
Matsuo Y and Yoneda-Kato N: The Jab1/COP9 signalosome subcomplex is
a downstream mediator of Bcr-Abl kinase activity and facilitates
cell-cycle progression. Blood. 105:775–783. 2005. View Article : Google Scholar
|
|
5
|
Druker BJ, Talpaz M, Resta DJ, Peng B,
Buchdunger E, Ford JM, Lydon NB, Kantarjian H, Capdeville R,
Ohno-Jones S, et al: Efficacy and safety of a specific inhibitor of
the BCR-ABL tyrosine kinase in chronic myeloid leukemia. N Engl J
Med. 344:1031–1037. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Druker BJ, Guilhot F, O’Brien SG, Gathmann
I, Kantarjian H, Gattermann N, Deininger MW, Silver RT, Goldman JM,
Stone RM, et al; IRIS Investigators. Five-year follow-up of
patients receiving imatinib for chronic myeloid leukemia. N Engl J
Med. 355:2408–2417. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Shah NP, Nicoll JM, Nagar B, Gorre ME,
Paquette RL, Kuriyan J and Sawyers CL: Multiple BCR-ABL kinase
domain mutations confer polyclonal resistance to the tyrosine
kinase inhibitor imatinib (STI571) in chronic phase and blast
crisis chronic myeloid leukemia. Cancer Cell. 2:117–125. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Gorre ME, Mohammed M, Ellwood K, Hsu N,
Paquette R, Rao PN and Sawyers CL: Clinical resistance to STI-571
cancer therapy caused by BCR-ABL gene mutation or amplification.
Science. 293:876–880. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Al-Achkar W, Wafa A, Moassass F, Klein E
and Liehr T: Multiple copies of BCR-ABL fusion gene on two
isodicentric Philadelphia chromosomes in an imatinib
mesylate-resistant chronic myeloid leukemia patient. Oncol Lett.
5:1579–1582. 2013.PubMed/NCBI
|
|
10
|
Hochhaus A, Kreil S, Corbin AS, La Rosée
P, Müller MC, Lahaye T, Hanfstein B, Schoch C, Cross NC, Berger U,
et al: Molecular and chromosomal mechanisms of resistance to
imatinib (STI571) therapy. Leukemia. 16:2190–2196. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
O’Hare T, Walters DK, Stoffregen EP, Jia
T, Manley PW, Mestan J, Cowan-Jacob SW, Lee FY, Heinrich MC,
Deininger MW, et al: In vitro activity of Bcr-Abl inhibitors AMN107
and BMS-354825 against clinically relevant imatinib-resistant Abl
kinase domain mutants. Cancer Res. 65:4500–4505. 2005. View Article : Google Scholar
|
|
12
|
Martinelli G, Iacobucci I, Soverini S,
Palandri F, Castagnetti F, Rosti G and Baccarani M: Nilotinib: A
novel encouraging therapeutic option for chronic myeloid leukemia
patients with imatinib resistance or intolerance. Biologics.
1:121–127. 2007.PubMed/NCBI
|
|
13
|
Shah NP, Tran C, Lee FY, Chen P, Norris D
and Sawyers CL: Overriding imatinib resistance with a novel ABL
kinase inhibitor. Science. 305:399–401. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Goh SS, Woodman OL, Pepe S, Cao AH, Qin C
and Ritchie RH: The red wine antioxidant resveratrol prevents
cardiomyocyte injury following ischemia-reperfusion via multiple
sites and mechanisms. Antioxid Redox Signal. 9:101–113. 2007.
View Article : Google Scholar
|
|
15
|
Winkelmann I, Diehl D, Oesterle D, Daniel
H and Wenzel U: The suppression of aberrant crypt multiplicity in
colonic tissue of 1,2-dimethylhydrazine-treated C57BL/6J mice by
dietary flavone is associated with an increased expression of Krebs
cycle enzymes. Carcinogenesis. 28:1446–1454. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Lafon CW: Stand dynamics of a
yellow-poplar (Liriodendron tulipifera L.) forest in the
Appalachian Mountains, Virginia, USA. Dendrochronologia. 22:43–52.
2004. View Article : Google Scholar
|
|
17
|
Rafinesque CS: Medical Flora or Manual of
the Medical Botany of the United States of North America. In two
volumes. Philadelphia: 1828
|
|
18
|
Graziose R, Rathinasabapathy T, Lategan C,
Poulev A, Smith PJ, Grace M, Lila MA and Raskin I: Antiplasmodial
activity of aporphine alkaloids and sesquiterpene lactones from
Liriodendron tulipifera L. J Ethnopharmacol. 133:26–30. 2011.
View Article : Google Scholar
|
|
19
|
Li WJ, Lin YC, Wu PF, Wen ZH, Liu PL, Chen
CY and Wang HM: Biofunctional constituents from Liriodendron
tulipifera with antioxidants and anti-melanogenic properties. Int J
Mol Sci. 14:1698–1712. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Doskotch RW and el-Feraly FS: Antitumor
agents. II Tulipinolide, a new germacranolide sesquiterpene, and
constunolide Two cytotoxic substances from Liriodendron tulipifera
L. J Pharm Sci. 58:877–880. 1969. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Moon MK, Oh HM, Kwon BM, Baek NI, Kim SH,
Kim JS and Kim DK: Farnesyl protein transferase and tumor cell
growth inhibitory activities of lipiferolide isolated from
Liriodendron tulipifera. Arch Pharm Res. 30:299–302. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Neelakantan S, Nasim S, Guzman ML, Jordan
CT and Crooks PA: Aminoparthenolides as novel anti-leukemic agents:
Discovery of the NF-kappaB inhibitor, DMAPT (LC-1). Bioorg Med Chem
Lett. 19:4346–4349. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Kaufmann SH and Hengartner MO: Programmed
cell death: Alive and well in the new millennium. Trends Cell Biol.
11:526–534. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Kinghorn AD, Chin YW and Swanson SM:
Discovery of natural product anticancer agents from biodiverse
organisms. Curr Opin Drug Discov Devel. 12:189–196. 2009.PubMed/NCBI
|
|
25
|
Balunas MJ, Su B, Landini S, Brueggemeier
RW and Kinghorn AD: Interference by naturally occurring fatty acids
in a noncellular enzyme-based aromatase bioassay. J Nat Prod.
69:700–703. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Zhao J: Nutraceuticals, nutritional
therapy, phytonutrients, and phytotherapy for improvement of human
health: A perspective on plant biotechnology application. Recent
Pat Biotechnol. 1:75–97. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Hong SW, Jung KH, Lee HS, Choi MJ, Son MK,
Zheng HM and Hong SS: SB365 inhibits angiogenesis and induces
apoptosis of hepatocellular carcinoma through modulation of
PI3K/Akt/mTOR signaling pathway. Cancer Sci. 103:1929–1937. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
de Oliveira LZ, Farias IL, Rigo ML,
Glanzner WG, Gonçalves PB, Cadoná FC, Cruz IB, Farias JG, Duarte
MM, Franco L, et al: Effect of Uncaria tomentosa extract on
apoptosis triggered by oxaliplatin exposure on HT29 cells. Evid
Based Complement Alternat Med. 2014:2747862014. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Deininger M, Buchdunger E and Druker BJ:
The development of imatinib as a therapeutic agent for chronic
myeloid leukemia. Blood. 105:2640–2653. 2005. View Article : Google Scholar
|
|
30
|
O’Hare T, Corbin AS and Druker BJ:
Targeted CML therapy: Controlling drug resistance, seeking cure.
Curr Opin Genet Dev. 16:92–99. 2006. View Article : Google Scholar
|
|
31
|
Jabbour E, Cortes J and Kantarjian H:
Nilotinib for the treatment of chronic myeloid leukemia: An
evidence-based review. Core Evid. 4:207–213. 2009. View Article : Google Scholar
|
|
32
|
Quintás-Cardama A, Kantarjian H and Cortes
J: Flying under the radar: The new wave of BCR-ABL inhibitors. Nat
Rev Drug Discov. 6:834–848. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Steelman LS, Pohnert SC, Shelton JG,
Franklin RA, Bertrand FE and McCubrey JA: JAK/STAT, Raf/MEK/ERK,
PI3K/Akt and BCR-ABL in cell cycle progression and leukemogenesis.
Leukemia. 18:189–218. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Kirchner D, Duyster J, Ottmann O, Schmid
RM, Bergmann L and Munzert G: Mechanisms of Bcr-Abl-mediated
NF-kappaB/Rel activation. Exp Hematol. 31:504–511. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Klejman A, Schreiner SJ,
Nieborowska-Skorska M, Slupianek A, Wilson M, Smithgall TE and
Skorski T: The Src family kinase Hck couples BCR/ABL to STAT5
activation in myeloid leukemia cells. EMBO J. 21:5766–5774. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Hemmeryckx B, van Wijk A, Reichert A,
Kaartinen V, de Jong R, Pattengale PK, Gonzalez-Gomez I, Groffen J
and Heisterkamp N: Crkl enhances leukemogenesis in BCR/ABL P190
transgenic mice. Cancer Res. 61:1398–1405. 2001.PubMed/NCBI
|
|
37
|
Hoelbl A, Schuster C, Kovacic B, Zhu B,
Wickre M, Hoelzl MA, Fajmann S, Grebien F, Warsch W, Stengl G, et
al: Stat5 is indispensable for the maintenance of bcr/abl-positive
leukaemia. EMBO Mol Med. 2:98–110. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Cabot MC, Giuliano AE, Han TY and Liu YY:
SDZ PSC 833, the cyclosporine A analogue and multidrug resistance
modulator, activates ceramide synthesis and increases vinblastine
sensitivity in drug-sensitive and drug-resistant cancer cells.
Cancer Res. 59:880–885. 1999.PubMed/NCBI
|
|
39
|
Selzner M, Bielawska A, Morse MA, Rüdiger
HA, Sindram D, Hannun YA and Clavien PA: Induction of apoptotic
cell death and prevention of tumor growth by ceramide analogues in
meta-static human colon cancer. Cancer Res. 61:1233–1240.
2001.PubMed/NCBI
|
|
40
|
Bedi A, Zehnbauer BA, Barber JP, Sharkis
SJ and Jones RJ: Inhibition of apoptosis by BCR-ABL in chronic
myeloid leukemia. Blood. 83:2038–2044. 1994.PubMed/NCBI
|
|
41
|
Deming PB, Schafer ZT, Tashker JS, Potts
MB, Deshmukh M and Kornbluth S: Bcr-Abl-mediated protection from
apoptosis downstream of mitochondrial cytochrome c release. Mol
Cell Biol. 24:10289–10299. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Bueno-da-Silva AE, Brumatti G, Russo FO,
Green DR and Amarante-Mendes GP: Bcr-Abl-mediated resistance to
apoptosis is independent of constant tyrosine-kinase activity. Cell
Death Differ. 10:592–598. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Altieri DC: Survivin and IAP proteins in
cell-death mechanisms. Biochem J. 430:199–205. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Kreuger MR, Grootjans S, Biavatti MW,
Vandenabeele P and D’Herde K: Sesquiterpene lactones as drugs with
multiple targets in cancer treatment: Focus on parthenolide.
Anticancer Drugs. 23:883–896. 2012.PubMed/NCBI
|
|
45
|
Saikali M, Ghantous A, Halawi R, Talhouk
SN, Saliba NA and Darwiche N: Sesquiterpene lactones isolated from
indigenous Middle Eastern plants inhibit tumor promoter-induced
transformation of JB6 cells. BMC Complement Altern Med. 12:892012.
View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Viennois E, Xiao B, Ayyadurai S, Wang L,
Wang PG, Zhang Q, Chen Y and Merlin D: Micheliolide, a new
sesquiterpene lactone that inhibits intestinal inflammation and
colitis-associated cancer. Lab Invest. 94:950–965. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Yeh CT, Huang WC, Rao YK, Ye M, Lee WH,
Wang LS, Tzeng DT, Wu CH, Shieh YS, Huang CY, et al: A
sesquiterpene lactone antrocin from Antrodia camphorata negatively
modulates JAK2/STAT3 signaling via microRNA let-7c and induces
apoptosis in lung cancer cells. Carcinogenesis. 34:2918–2928. 2013.
View Article : Google Scholar : PubMed/NCBI
|