Introduction
Interferon regulatory factor (IRF)-5 is a
transcription factor member of the IRF family that regulates the
expression of genes induced by viral infection (1). While IRF-5 was identified as a
regulator of type I interferon (IFN) (2), further studies indicated that IRF-5
plays essential roles in the regulation of genes involved in the
stimulation of the immune system, cell growth, apoptosis and
oncogenesis (3–6). Overexpression of IRF-5 has been
associated with autoimmune diseases such as systemic lupus
erythematosus and rheumatoid arthritis (7,8).
IRF-5 is a direct target of p53 (9) and displays some tumor suppressor
properties as it can induce p21, Bak, Bax and
caspase-8 genes (6,10). Downregulation of IRF-5 by
hypermethylation has been reported in hepatocellular carcinoma and
gastric cancer (11,12). In contrast, IRF-5 is upregulated in
thyroid cancer where it contributes to cell proliferation and
survival (13). High-level
expression of IRF-5 has been also detected in Hodgkin’s lymphoma
cells and considered to be crucial for their survival (14).
Human T-cell leukemia virus type 1 (HTLV-1) causes
either adult T-cell leukemia (ATL) or chronic inflammatory
disorders, such as HTLV-1-associated myelopathy/tropical spastic
paraparesis, uveitis and arthritis (15,16).
The HTLV-1 genome encodes the transactivator Tax protein that plays
essential regulatory roles in HTLV-1 replication and oncogenic
transformation of T lymphocytes (17,18).
Tax modulates the activation of host signaling pathways to mediate
cellular transformation and hyperstimulates the immune system
(17,18). Therefore, viral Tax protein is
considered to initiate the ATL-related leukemogenesis process,
which subsequently progresses towards the ultimate leukemic stage
through additional events occurring in Tax absence. In addition,
Tax plays a central role in the pathophysiology of chronic
inflammatory disorders (19).
With regard to the IRF family of transcription
factors, several novel putative targets for Tax-mediated gene
activation have been identified. IRF-3, a transcription factor
critical in innate immunity to viral infection, is constitutively
activated in a Tax-dependent manner (20). On the contrary, oncogenic IRF-4,
another member in the IRF family of transcription factors, is
overexpressed in lymphocytes of patients with ATL and
HTLV-1-transformed T cells (21–25).
Tax also induces the expression of IRF-4 (21,25–27).
However, the expression of IRF-5 and related regulatory mechanisms
have not been fully determined in HTLV-1-infected T cells. In this
study, we determined the expression level of IRF-5 in
HTLV-1-infected T cells. The results showed that IRF-5 expression
was induced by Tax and that it regulated the expression of tumor
necrosis factor (TNF) family cytokines.
Materials and methods
Cell culture
The HTLV-1-infected MT-2, MT-4, C5/MJ, SLB-1,
HUT-102, MT-1, TL-OmI and ED-40515(−) T-cell lines, and the
negative control uninfected human leukemia Jurkat, MOLT-4 and
CCRF-CEM T-cell lines, were grown in Roswell Park Memorial
Institute-1640 medium supplemented with 10% heat-inactivated fetal
bovine serum and antibiotics. JPX-9 cells are derivatives of Jurkat
with Tax gene, which is stably integrated under the control
of a metallothionein promoter (28). To induce Tax expression, JPX-9
cells were cultured in the presence of 20 μM CdCl2. The
human acute monocytic leukemia cell line, THP-1, was set as a
positive control for IRF-5 expression. TY8-3/MT-2 was established
from the interleukin (IL)-2-dependent human T-cell line, TY8-3,
co-cultured with mitomycin C (MMC)-treated MT-2 cells, and was
capable of growth completely independent of IL-2 (29). Jurkat cells were stimulated with
1,000 U/ml of recombinant human IFN-α for the indicated time
intervals.
HTLV-1 infection by co-cultivation
Peripheral blood mononuclear cells (PBMC) from a
healthy donor were isolated from the heparinized blood sample by
centrifugation over a Ficoll-Paque layer (GE Healthcare
Bio-Sciences AB, Uppsala, Sweden). MT-2 cells were pretreated with
200 μg/ml of MMC for 60 min, pipetted vigorously, and washed three
times with phosphate-buffered saline. PBMC and MMC-treated MT-2
cells were co-cultured in the presence of 10 ng/ml of IL-2. The
culture medium was half-changed with fresh medium supplemented with
IL-2 every 3 days. Since MT-2 cells were pretreated extensively
with MMC, no discernible MT-2 cells were found.
RT-PCR
Total RNA was extracted from cells with TRIzol
(Invitrogen Life Technologies, Carlsbad, CA, USA) according to the
protocol provided by the manufacturer. The RNA was reverse
transcribed into cDNA using a PrimeScript™ RT-PCR kit (Takara Bio
Inc., Otsu, Japan). The sequences of the primers for IRF-5, IFN-α,
IFN-β, IFN-γ, 2′,5′-oligoadenylate synthetase (2–5 AS), MxA, TNF-α,
lymphotoxin (LT)-β, β-actin, glyceraldehyde-3-phosphate
dehydrogenase (GAPDH), Tax in HTLV-1-infected T cells, and Tax in
JPX-9 cells are summarized in Table
I (2,30–36).
 | Table IPrimer sequences used in RT-PCR. |
Table I
Primer sequences used in RT-PCR.
| Name | Forward (5′) | Reverse (3′) |
|---|
| IRF-5 |
GCCTTGTTATTGCATGCCAGC |
AGACCAAGCTTTTCAGCCTGG |
| IRF-5 (V1/4) |
CCTGGCGCAGCCACGCAGGCGCA |
CCAAAAGAGTAATCCTCAGGG |
| IRF-5 (V2) |
GCGCCTGGAAAGCGAGCTCG |
CCAAAAGAGTAATCCTCAGGG |
| IRF-5 (V3) |
CTAGGCAGGTGCAACCCCAAAA |
CCAAAAGAGTAATCCTCAGGG |
| IFN-α |
CAGGAGGAGTTTGATGGCAACCAG |
GACAACCTCCCAGGCACAAGGGC |
| IFN-β |
ATGACCAACAAGTGTCTCCTCCAAA |
GTTTCGGAGGTAACCTGTAAGTCTG |
| IFN-γ |
ATGAAATATACAAGTTATATCTTGGCTTT |
GATGCTCTTCGACCTCGAAACAGCAT |
| 2–5 AS |
CCAGGAAATTAGGAGACAGC |
TGGCAGGGAGGAAGCAGGAG |
| MxA |
GCATCCCACCCTCTATTACT |
TGTCTTCAGTTCCTTTGTCC |
| TNF-α |
ATGAGCACTGAAAGCATGATC |
TCACAGGGCAATGATCCCAAAGTAGACCTGCCC |
| LT-β |
AAGCTGCCAGAGGAGGAGCC |
TCCCGCTCGTCAGAAACGCC |
| Tax in
HTLV-1-infected T cells |
CCGGCGCTGCTCTCATCCCGGT |
GGCCGAACATAGTCCCCCAGAG |
| Tax in JPX-9
cells |
ATCGGCTCAGCTCTACAGTTCCT |
ATTCGCTTGTAGGGAACATTGGT |
| β-actin |
GTGGGGCGCCCCAGGCACCA |
CTCCTTAATGTCACGCACGATTTC |
| GAPDH |
GCCAAGGTCATCCATGACAACTTTGG |
GCCTGCTTCACCACCTTCTTGATGTC |
Protein extraction and immunoblot
analysis
Immunoblot analysis was performed on whole cell
lysate, and nuclear and cytoplasmic fractions. Each protein was
extracted as previously described (37–39),
and subjected to sodium dodecyl sulfate-polyacrylamide gels and
transferred to polyvinylidene difluoride membranes. The membranes
were then probed for IRF-5 (Abnova Corp., Taipei, Taiwan), actin
(NeoMarkers Inc., Fremont, CA, USA), lamin B (Santa Cruz
Biotechnology Inc., Santa Cruz, CA, USA) or Myc-Tag (Wako Pure
Chemical Industries, Ltd., Osaka, Japan). Mouse monoclonal antibody
to Tax, Lt-4, was previously described (40). The bands were visualized using
enhanced chemiluminescence kit (Amersham Biosciences Corp.,
Piscataway, NJ, USA).
Immunofluorescence assays
Cells were fixed in 4% paraformaldehyde and
permeabilized with Triton X-100. Then, the cells were stained with
mouse anti-IRF-5 antibody (Abnova Corp.). For immunofluorescence
studies, washed cells were incubated with anti-mouse secondary
antibody conjugated with Alexa Fluor 488 (Invitrogen Life
Technologies). The nuclei were stained with Hoechst 33342 (Wako
Pure Chemical Industries, Ltd.). After final washing, the cells
were examined under a Leica DMI6000 microscope (Leica Microsystems,
Wetzlar, Germany). Mounted coverslips were viewed through a 63× oil
immersion lens (NA1.4) on a Leica TCS confocal system.
Immunohistochemical analysis
The diagnosis of ATL was based on clinical features,
hematological findings and the presence of anti-HTLV-1 antibodies
in the serum. Biopsy samples were taken from the lesional skin and
lymph nodes of four patients with ATL. IRF-5 immunohistochemistry
was performed using an anti-IRF-5 antibody (Abnova Corp.) after
pretreatment of the deparafinized tissue sections with ready-to-use
proteinase K (Dako, Carpinteria, CA, USA). The sections were
counterstained with methyl green, hydrated in ethanol, cleaned in
xylene, and mounted. The stained cells were examined under a light
microscope (Axicoskop 2 Plus) with an Achroplan 40×/0.65 lens (both
from Zeiss, Jena, Germany). Images were acquired with an AxioCam
MRc camera and AxioVision 4.7 software (Zeiss). A signed consent
form was obtained from each tissue donor.
Plasmids
The IRF-5 P-V3 promoter fragment was cloned into the
KpnI and XhoI sites of pGL3-Basic vector (Promega
Corp., Madison, WI, USA) (30).
Details of the plasmid expressing the HTLV-1 Tax through β-actin
promoter were published previously (41). The coding sequences of IRF-5 were
cloned into the pcDNA4/myc-His A vector (Invitrogen Life
Technologies).
Transfection and luciferase assay
Jurkat cells were transfected at 5×106
cells with the luciferase reporter plasmid, together with 0.5–5 μg
of the Tax expression vector by electroporation (250 V, 960 μF).
Each transfection included the phRL-TK plasmid (Promega Corp.) as
an internal control for variation in transfection efficiency. After
48 h, transfected cells were collected by centrifugation, washed
with phosphate-buffered saline, and lysed in reporter lysis buffer
(Promega Corp.). The luciferase activity was determined by the
Dual-Luciferase Reporter system (Promega Corp.) using the protocol
supplied by the manufacturer.
Microarray analysis
Jurkat cells were transfected with a control
pcDNA4/myc-His A vector or IRF-5 expression vector using
MicroPorator MP-100 (Digital Bio Technology Co., Ltd., Seoul,
Korea), pulsed three times at 1,325 V for 10 msec each. After 24 h,
total RNA samples were prepared using the RNeasy Plus Mini kit
(Qiagen, Hilden, Germany) and confirmed to be of good quality by
Agilent 2100 Bioanalyzer (Agilent Technologies, Inc., Waldbronn,
Germany). Microarray analysis using a SurePrint G3 Human GE 8×60 K
Microarray kit version 2.0 (Agilent Technologies, Inc.) was
performed as previously described (42).
Results
Expression of IRF-5 in HTLV-1-infected
T-cell lines
To investigate IRF-5 expression in both
HTLV-1-infected and -uninfected T-cell lines, we collected three
uninfected (Jurkat, MOLT-4 and CCRF-CEM), five HTLV-1-transformed
(MT-2, MT-4, C5/MJ, SLB-1 and HUT-102) and three ATL-derived [MT-1,
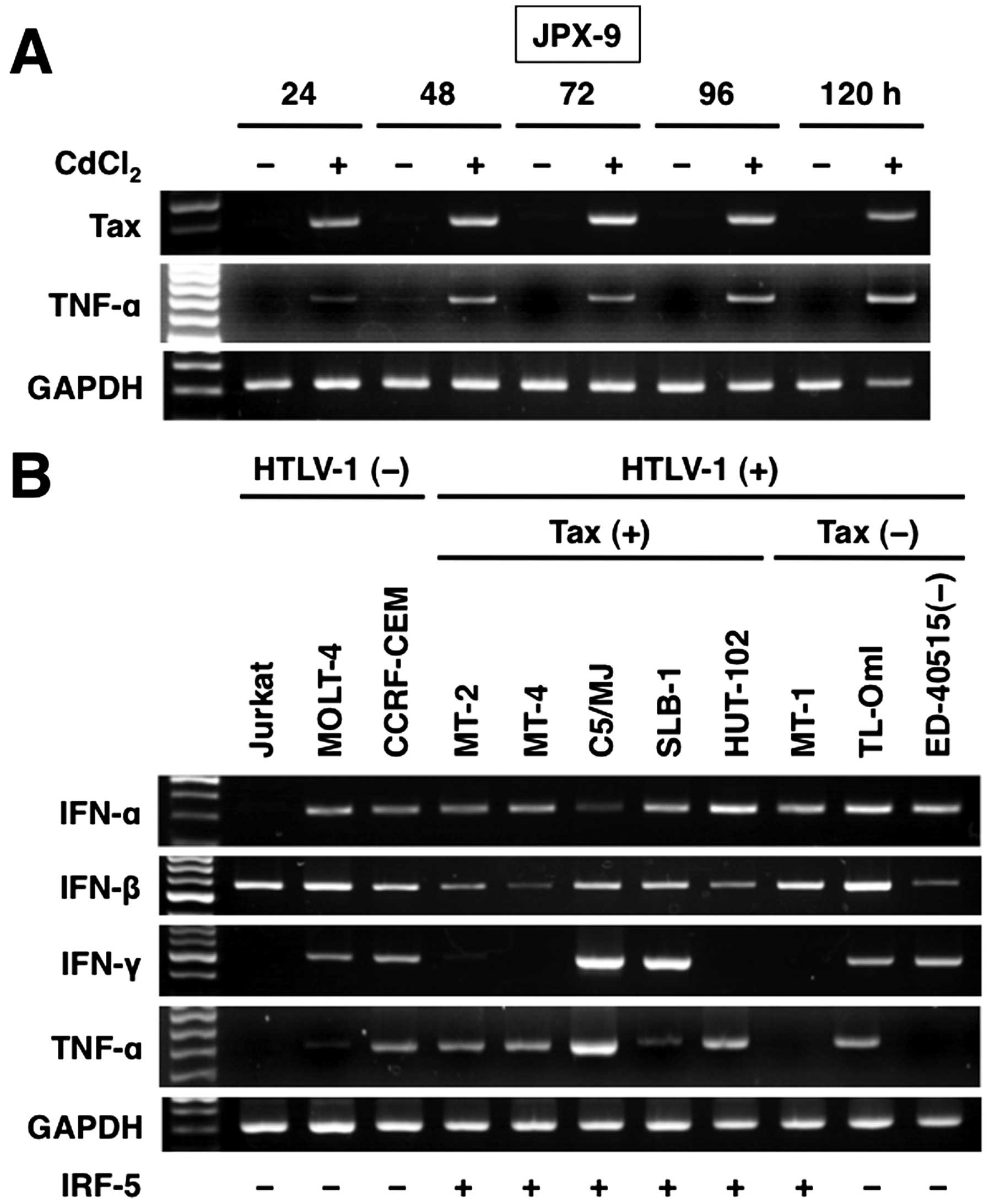
TL-OmI and ED-40515(−)] T-cell lines. All HTLV-1-transformed T-cell
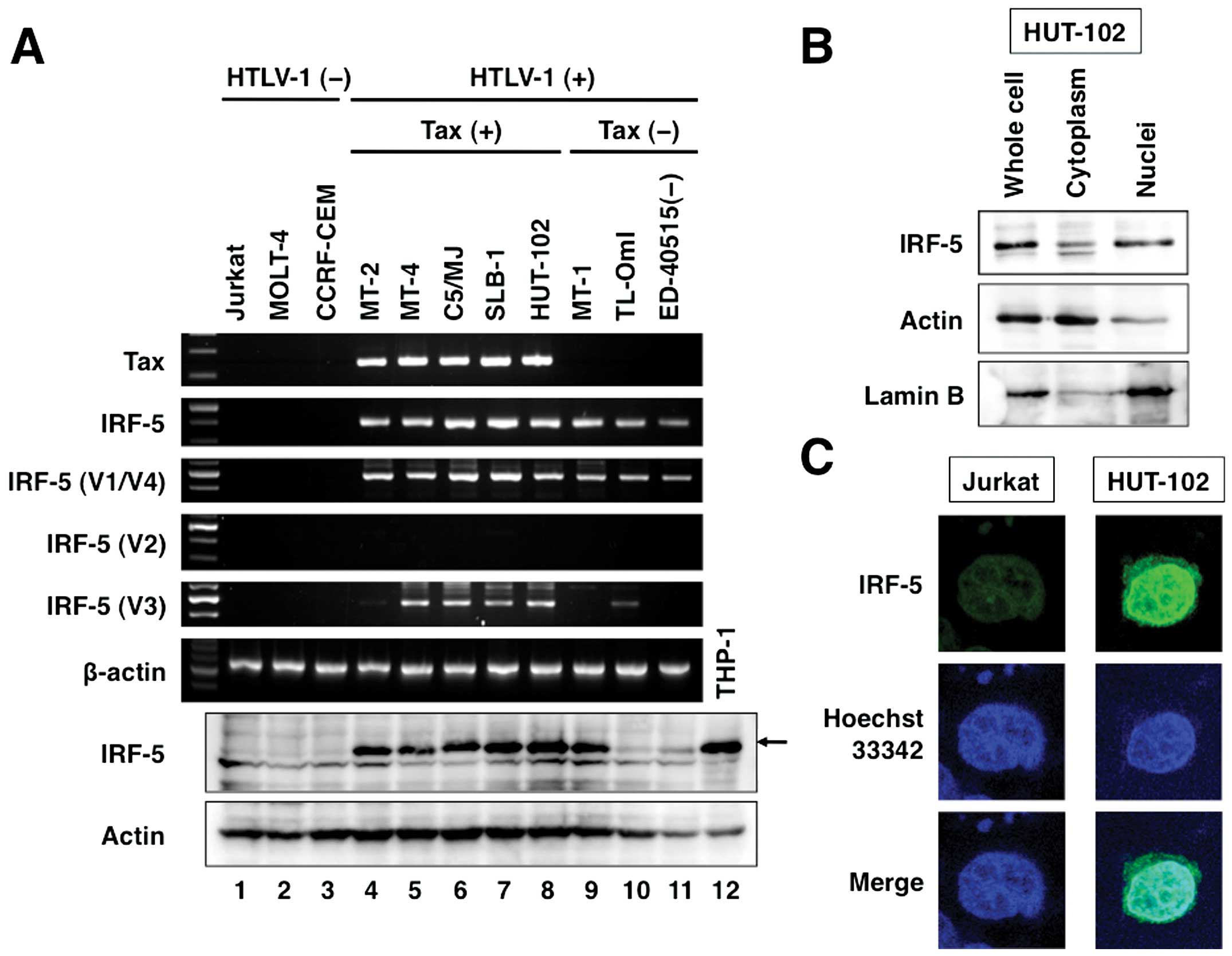
lines constitutively expressed Tax mRNA (Fig. 1A, panel 1). Expression of IRF-5
mRNA in 11 T-cell lines was analyzed by reverse-transcription
polymerase chain reaction (RT-PCR) using IRF-5 specific primers.
All eight of the HTLV-1-positive T-cell lines examined (lanes 4–11)
strongly expressed IRF-5 mRNA, whereas the three HTLV-1-negative
T-cell lines (lanes 1–3) did not (Fig.
1A, panel 2). IRF-5 is transcribed into various distinct
alternatively spliced isoforms (30), and the IRF-5 variant 1 (V1), V2 and
V3 transcripts have different noncoding first exons, whereas V1 and
V4 share the same first exon. Primer sets that specifically
recognize exon 1 of each isoform (Ex1V1, Ex1V2 and Ex1V3) and a
common region in exon 4 of IRF-5 were optimized (Table I).
PCR amplification using the exon 1-specific sense
primers was isoform-specific. We next examined the levels of
constitutive exon 1-specific IRF-5 isoform expression in several
T-cell lines (Fig. 1A, panels
3–5). Ex1V1 transcripts were detected in all HTLV-1-infected T-cell
lines (Fig. 1A, panel 3). In
comparison, transcripts associated with Ex1V2 could not be detected
(Fig. 1A, panel 4). IRF-5 V3
transcript levels were specifically high in Tax-positive
HTLV-1-transformed T-cell lines (lanes 5–8), excluding MT-2,
suggesting a possible role for Tax in enhanced IRF-5 Ex1V3
transcript levels (Fig. 1A, panel
5). High level of IRF-5 protein expression was evident in
Tax-positive HTLV-1-transformed T-cell lines and ATL-derived MT-1
(Fig. 1A, panel 7, lanes 4–9).
Intracellular mapping by immunoblotting and immunofluorescence
staining showed high levels of IRF-5 in the nuclei of
HTLV-1-infected HUT-102 cells (Fig. 1B
and C). In contrast, almost no IRF-5 was detected in uninfected
Jurkat cells (Fig. 1C).
Abundant IRF-5 expression in ATL cells in
lymph nodes and skin lesions
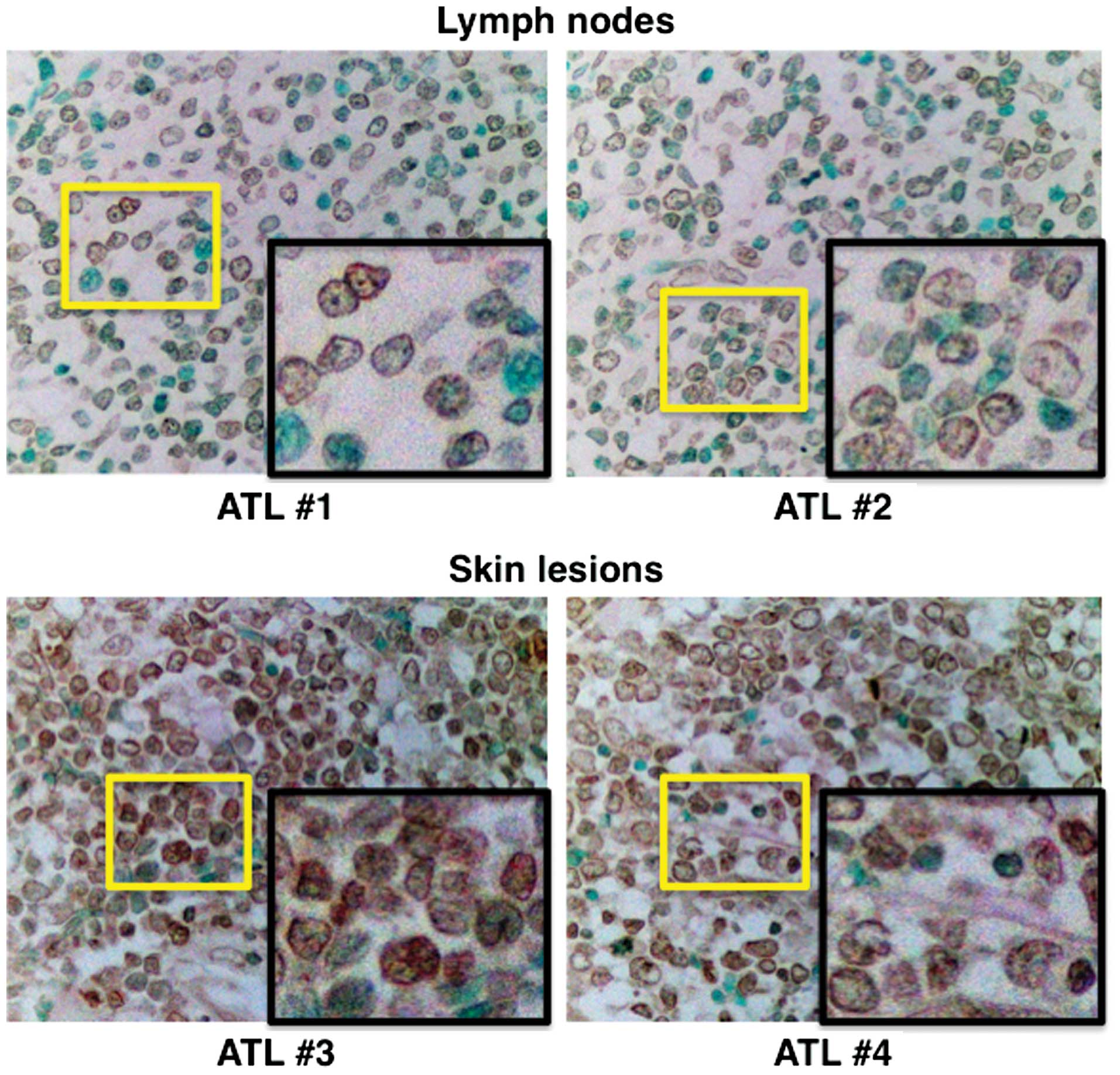
Immunohistochemical staining of ATL cells in
archived lymph nodes and skin tissue samples showed abundant IRF-5
in the nuclei of these cells (Fig.
2).
IRF-5 expression during HTLV-1
infection
To examine whether HTLV-1 infection induces IRF-5
expression, studies were performed using the parental TY8-3 cell
line and TY8-3 cells infected with HTLV-1 (TY8-3/MT-2). TY8-3/MT-2
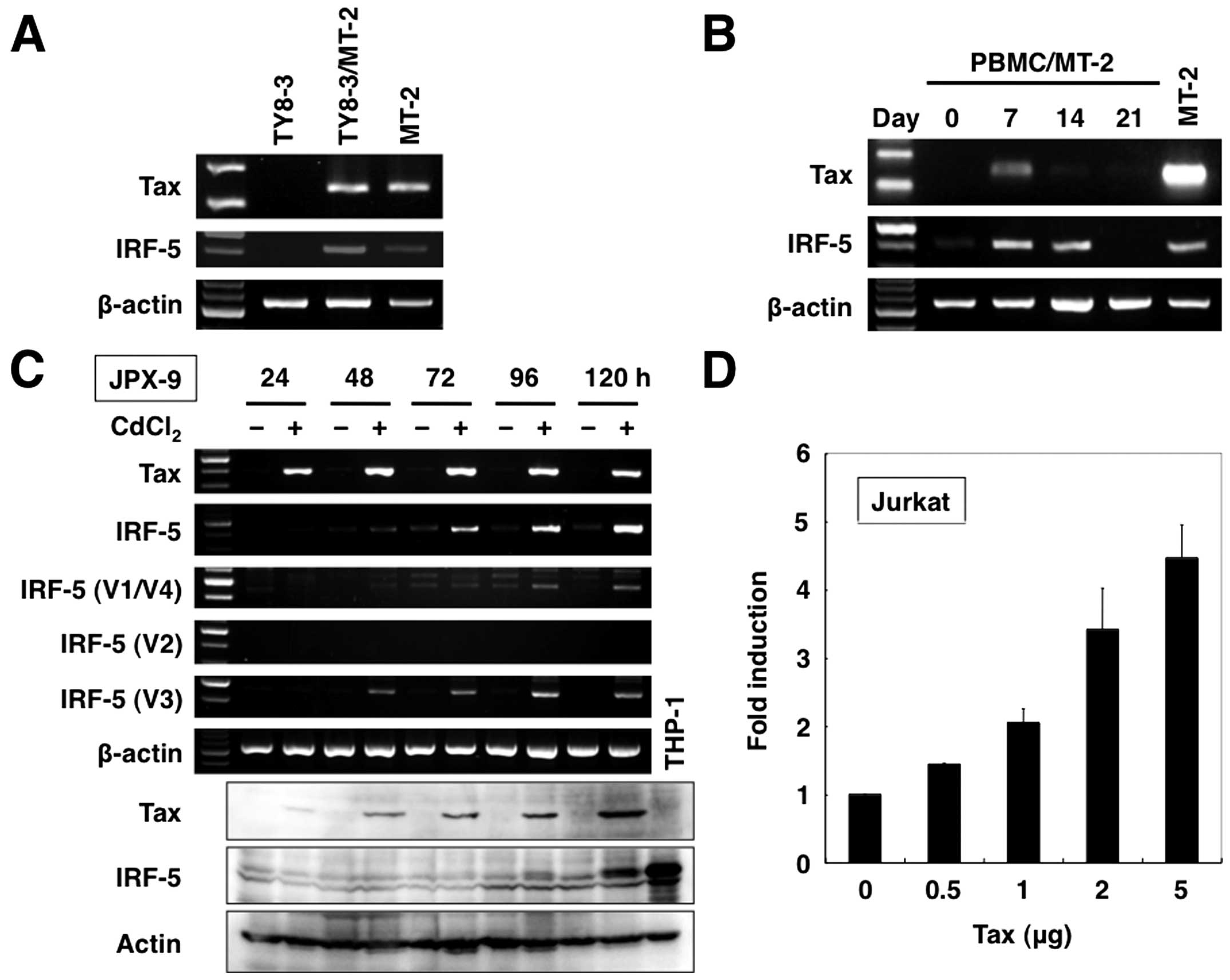
cells strongly expressed Tax mRNA (Fig. 3A). RT-PCR analysis also
demonstrated upregulation of IRF-5 mRNA in TY8-3/MT-2 cells. To
substantiate HTLV-1 control of IRF-5 expression in PBMC, we
co-cultured PBMC and MMC-treated MT-2 cells. At seven days after
co-cultivation, PBMC were harvested for assessment of expression of
HTLV-1 viral gene by RT-PCR. PBMC co-cultured with MMC-treated MT-2
cells expressed Tax mRNA (Fig.
3B). Furthermore, IRF-5 expression levels increased in these
cells following induction of HTLV-1 gene. Noteworthy, IRF-5
expression was still detected in PBMC at 14 days after
co-cultivation, which expressed Tax at a very low level (Fig. 3B). These results indicate that
HTLV-1 infection induces the expression of IRF-5 in PBMC as well as
T-cell line.
Direct effect of Tax on expression of
IRF-5
Tax gene product is the primary viral
transactivator protein that modulates the expression of both viral
and cellular genes (17,18). To test whether Tax directly induces
IRF-5 expression, we used JPX-9, the Jurkat subline carrying Tax
under the control of the metallothionein gene promoter
(28). This cell line has been
widely used to examine the effect of Tax on the expression of
various cellular genes (28). The
results are shown in Fig. 3C.
Treatment of JPX-9 with CdCl2 rapidly induced Tax mRNA
expression (panel 1). Similarly, Tax protein expression was induced
within 24 h after addition of CdCl2 and reached a
maximal level within 120 h (panel 7). Expression of the
IRF-5 gene was not detected at 24 h but became evident
within 48 h after addition of CdCl2 (panel 2). A
significant increase in the level of expression of IRF-5 protein
(panel 8) was also noted; the protein level began to rise 96 h
after the addition of CdCl2 with the maximal level
observed at 120 h.
We next examined IRF-5 isoform expression in
CdCl2-treated and -untreated JPX-9 cells. As shown in
Fig. 3C, CdCl2
upregulated Ex1 V1-associated transcript levels (panel 3), whereas
V2 transcript levels were not detected in treated or untreated
samples (panel 4). In comparison, CdCl2 upregulated
IRF-5 V3 transcript levels (panel 5).
We also examined the effects of Tax on IRF-5 isoform
3 promoter (P-V3). The activities of the reporter construct
containing the entire P-V3 region were analyzed in transient
transfection assays in Jurkat cells (Fig. 3D). Co-transfection of the P-V3
promoter construct in Jurkat cells with Tax expression plasmid
enhanced promoter activity in Tax dose-dependent manner. Considered
collectively, the above results indicate that Tax can activate
IRF-5 P-V3 promoter.
Type I IFN does not regulate IRF-5
Type I IFN is a well-known transcriptional inducer
of IRF-5 (10). However, its
efficacy on IRF-5 induction in T cells is still unknown. Jurkat
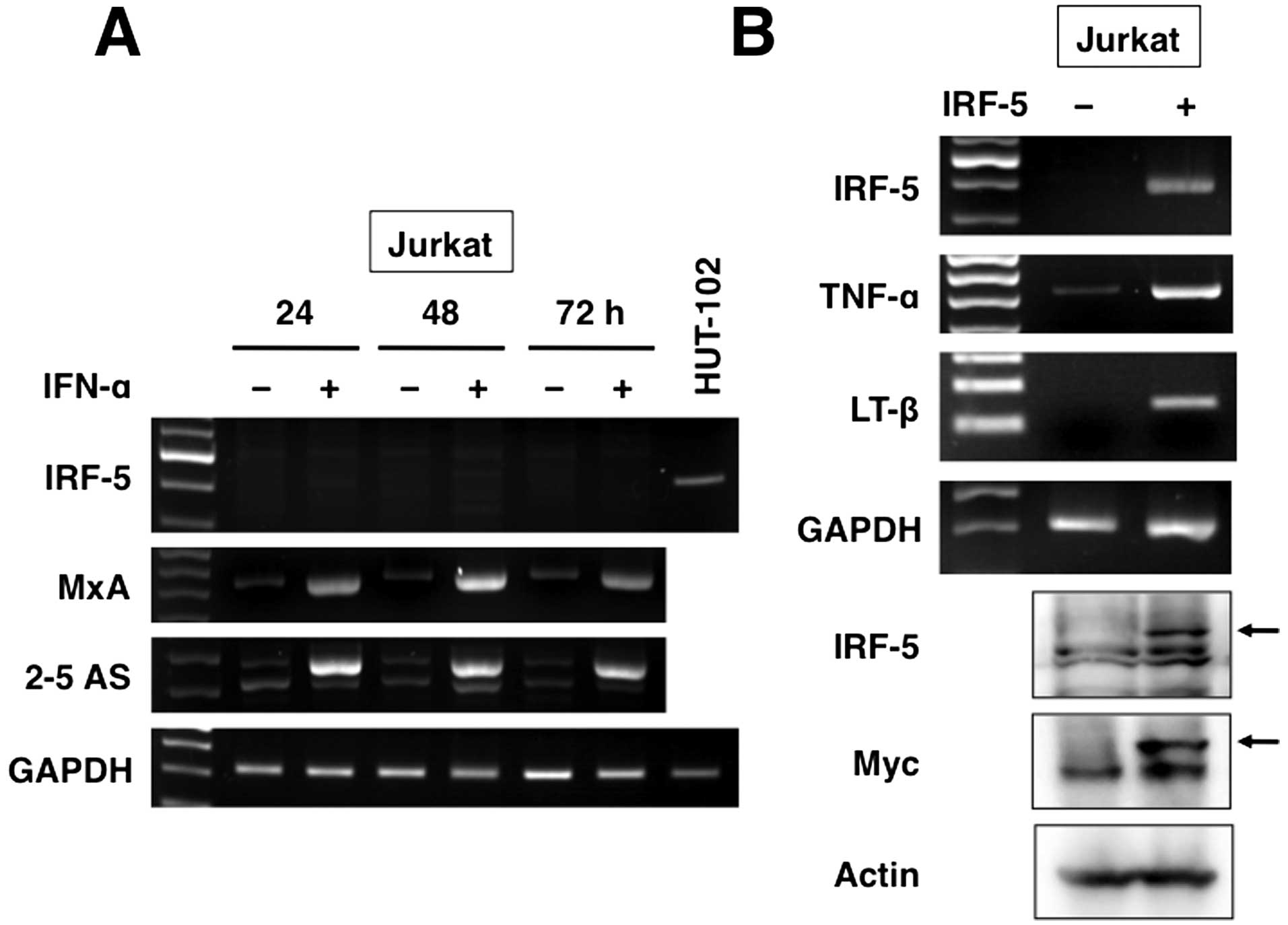
cells were exposed to IFN-α before RT-PCR. Unexpectedly, IFN-α
failed to induce IRF-5 (Fig. 4A).
However, treatment of these cells with IFN-α significantly
increased the levels of other IFN-stimulated genes, MxA and
2–5 AS.
IRF-5 targets TNF family cytokine
genes
To assess the potential role of IRF-5 in initiating
HTLV-1-infected cell-characteristic gene expression in T cells,
Jurkat cells were transfected with control or IRF-5 expression
plasmid. After 24 h of transfection, RT-PCR and immunoblotting were
performed using specific primers, and anti-IRF-5 and anti-Myc
antibodies to confirm transgene expression (Fig. 4B). In these experiments, changes in
IRF-5-induced gene expression were analyzed by microarray analysis.
The analysis showed significant enrichment of the up- and
downregulated genes in IRF-5-transfected T cells. Table II shows genes that were
upregulated by at least 5-fold in the presence of IRF-5. Previous
studies reported that HTLV-1 infection induces T-cell activation
and in vitro spontaneous lymphocyte proliferation, leading
to the production of high levels of TNF-α in non-stimulated PBMC
(43). Typical features of
HTLV-1-infected T cells were recapitulated by IRF-5 activity, such
as upregulation of TNF-α and LT-β (Table II and Fig. 4B).
 | Table IIIRF-5-mediated genes with ≥5 fold
change. |
Table II
IRF-5-mediated genes with ≥5 fold
change.
| Symbol | Accession no. | Gene | Fold change |
|---|
| IL17F | NM_052872 | Interleukin
17F | 8.69 |
| AZU1 | NM_001700 | Azurocidin 1 | 7.34 |
| TNF | NM_000594 | Tumor necrosis
factor | 7.24 |
| PYY | NM_004160 | Peptide YY | 7.03 |
| TEK | NM_000459 | TEK tyrosine
kinase, endothelial | 6.82 |
| LTB | NM_002341 | Lymphotoxin β (TNF
superfamily, member 3) | 5.84 |
| SLC24A4 | NM_153646 | Solute carrier
family 24 (sodium/potassium/calcium exchanger), member 4 | 5.76 |
| ISPD | NM_001101426 | Isoprenoid synthase
domain containing | 5.76 |
| NRXN2 | NM_138732 | Neurexin 2 | 5.67 |
| TMEM200A | NM_052913 | Transmembrane
protein 200A | 5.65 |
| SFRP1 | NM_003012 | Secreted
frizzled-related protein 1 | 5.47 |
| KIR2DS4 | NM_012314 | Killer cell
immunoglobulin-like receptor, two domains, short cytoplasmic tail,
4 | 5.42 |
| SDCBP2 | NM_080489 | Syndecan binding
protein (syntenin) 2 | 5.42 |
| KLHDC7B | NM_138433 | Kelch domain
containing 7B | 5.38 |
| FAM176A | NM_032181 | Family with
sequence similarity 176, member A | 5.19 |
| THADA | NM_001083953 | Thyroid adenoma
associated | 5.18 |
| GALNTL1 | NM_020692 |
UDP-N-acetyl-α-D-galactosamine
(polypeptide N-acetylgalactosaminyltransferase-like 1) | 5.10 |
| CGA | NM_000735 | Glycoprotein
hormones, α polypeptide | 5.02 |
| OR10A7 | NM_001005280 | Olfactory receptor,
family 10, subfamily A, member 7 | 5.00 |
Tax can also induce TNF-α expression
HTLV-1-infected T-cell lines constitutively secrete
TNF-α (44). Furthermore, TNF-α
expression is activated in the arthritic joints of Tax transgenic
mice compared with the normal joints of non-transgenic mice
(45). In the next set of
experiments, we examined the effects of Tax on TNF-α expression in
T cells. As expected, TNF-α was induced in Tax-expressing JPX-9
(Fig. 5A). Next, we used several
T-cell lines to analyze the role of IRF-5 in the expression of type
I and II IFN, and TNF-α. Compared to control T-cell lines,
HTLV-1-infected T-cell lines expressed high levels of TNF-α
(Fig. 5B). Similar to control
T-cell lines, HTLV-1-infected T-cell lines also constitutively
expressed IFN-α and -β. IFN-γ expression was not associated with
HTLV-1 infection. There was no correlation between IFN and Tax
expression in HTLV-1-infected T-cell lines. The constitutive
expression of TNF-α tended to be associated with IRF-5 or Tax
expression.
Discussion
IRF-5 mRNA expression has been detected in B cells,
dendritic cells, monocytes and natural killer cells but not in T
cells (30). In the present study,
we demonstrated that T cells acquire high levels of IRF-5
expression during HTLV-1 infection. Our results are consistent with
those of oligonucleotide microarray reported by Baba et al
(46), who identified IRF-5 to be
one of the genes that were upregulated more than 40-fold in three
HTLV-1-infected T-cell lines, including MT-2, compared with
uninfected T-cell line MOLT-4. Our results also showed that the
oncoprotein Tax activated the IRF-5 V3 promoter, and that IRF-5 was
expressed in ATL cells infiltrating the lymph nodes and skin.
Whether the expression of IRF-5 is present in PBMC samples from
patients with ATL containing leukemic cells (which do not express
Tax protein) was not examined and thus remains a very interesting
question. Importantly, ATL-derived MT-1 cells, which also do not
express Tax protein, expressed high levels of IRF-5 protein. These
results suggest that Tax-independent IRF-5 expression mechanisms
may also exist in ATL cells.
To establish the effects of endogenous IRF-5 in
HTLV-1-infected T cells, we silenced its expression using the siRNA
in HUT-102 cells (data not shown). Although IRF-5 silencing was
confirmed by RT-PCR and western blot analysis, reduced IRF-5
expression did not affect cell growth (data not shown). In this
study, the role of IRF-5 in T cells was investigated using
IRF-5-expressing Jurkat cells and cDNA array technology. The
results showed that IRF-5 expression does not affect the expression
of cell growth- and apoptosis-related genes (data not shown).
Furthermore, IRF-5 overexpression did not increase the
proliferation of Jurkat cells (data not shown). These studies
confirmed that IRF-5 does not directly modulate cell growth.
However, the exact IRF-5 function in cell proliferation needs to be
further investigated using IRF-5 stable transfectants.
IRF-5 exists in multiple alternatively spliced
isoforms that are expressed in a cell type-specific manner
(30). The present results
demonstrated that the induction of IRF-5 expression by Tax is
isoform-specific. Tax-positive HTLV-1-infected T-cell lines
specifically upregulated Ex1V3 transcripts, and the Ex1V3-specific
transcripts were upregulated by Tax expression. Indeed, promoter
reporter assays demonstrated that Tax enhanced P-V3 promoter
activity.
IRF-5 is a central mediator that controls the
expression of type I IFN (2).
However, there was no link between IRF-5 and type I IFN expression
in HTLV-1-infected T cell lines. In addition, IFN-α did not
upregulate IRF-5 in Jurkat T cells. Although this finding does not
completely exclude IFN-α-induced IRF-5 expression in primary
peripheral T cells, the expression of type I IFN seems to be
independent of IRF-5 in HTLV-1-infected T cells. On the contrary,
IRF-5 expression induced other TNF family genes. TNF-α is a major
cytokine involved in the promotion of inflammatory responses, and
it plays a crucial role in the pathogenesis of various
inflammatory, autoimmune and malignant diseases (47). Genetic polymorphisms leading to
increased TNF-α production enhance susceptibility to not only ATL
(48), but also to uveitis,
another HTLV-1-related disease (49). TNF-α is suggested to contribute to
the high levels of organ infiltration by leukemic cells in ATL
(50). Tax can activate mouse
TNF-α promoter through nuclear factor-κB (NF-κB) activation
(51). Furthermore, IRF-5 can
specifically interact with NF-κB RelA, and sustain TNF-α secretion
in human dendritic cells (52).
These findings suggest that Tax/NF-κB/IRF-5 may cooperate in
HTLV-1-induced TNF-α promoter activation.
In summary, the present study indicates that
high-level IRF-5 expression is specific to HTLV-1-infected T cells.
The main function of IRF-5 in ATL and other HTLV-1-related disease
should be investigated.
Acknowledgements
The authors thank Keisuke Kidoguchi and Takano Ohta
for their excellent assistance. We also thank Drs Martin Schmidt,
Kayoko Matsumoto and Yuetsu Tanaka for providing expression vectors
for IRF-5 and Tax, and Tax antibody, as well as Dr Masataka
Nakamura for providing JPX-9, Dr Michiyuki Maeda for providing
ED-40515(−), and Fujisaki Cell Center, Hayashibara Biochemical
Laboratories, Inc. (Okayama, Japan) for providing C5/MJ, HUT-102
and MT-1. Recombinant human IL-2 was kindly provided by Takeda
Pharmaceutical Company Ltd. (Osaka, Japan). This work was supported
in part by JSPS KAKENHI grant numbers 90542358 and 25461428.
References
|
1
|
Battistini A: Interferon regulatory
factors in hematopoietic cell differentiation and immune
regulation. J Interferon Cytokine Res. 29:765–780. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Barnes BJ, Moore PA and Pitha PM:
Virus-specific activation of a novel interferon regulatory factor,
IRF-5, results in the induction of distinct interferon α genes. J
Biol Chem. 276:23382–23390. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Takaoka A, Tamura T and Taniguchi T:
Interferon regulatory factor family of transcription factors and
regulation of oncogenesis. Cancer Sci. 99:467–478. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Yanai H, Chen H-M, Inuzuka T, Kondo S, Mak
TW, Takaoka A, Honda K and Taniguchi T: Role of IFN regulatory
factor 5 transcription factor in antiviral immunity and tumor
suppression. Proc Natl Acad Sci USA. 104:3402–3407. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Tamura T, Yanai H, Savitsky D and
Taniguchi T: The IRF family transcription factors in immunity and
oncogenesis. Annu Rev Immunol. 26:535–584. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Hu G and Barnes BJ: IRF-5 is a mediator of
the death receptor-induced apoptotic signaling pathway. J Biol
Chem. 284:2767–2777. 2009. View Article : Google Scholar
|
|
7
|
Graham RR, Kozyrev SV, Baechler EC, Reddy
MVPL, Plenge RM, Bauer JW, Ortmann WA, Koeuth T, González Escribano
MF, et al; Argentine and Spanish Collaborative Groups. A common
haplotype of interferon regulatory factor 5 (IRF5) regulates
splicing and expression and is associated with increased risk of
systemic lupus erythematosus. Nat Genet. 38:550–555. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Dawidowicz K, Allanore Y, Guedj M, Pierlot
C, Bombardieri S, Balsa A, Westhovens R, Barrera P, Alves H,
Teixeira VH, et al: ECRAF: The interferon regulatory factor 5 gene
confers susceptibility to rheumatoid arthritis and influences its
erosive phenotype. Ann Rheum Dis. 70:117–121. 2011. View Article : Google Scholar
|
|
9
|
Mori T, Anazawa Y, Iiizumi M, Fukuda S,
Nakamura Y and Arakawa H: Identification of the interferon
regulatory factor 5 gene (IRF-5) as a direct target for p53.
Oncogene. 21:2914–2918. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Barnes BJ, Kellum MJ, Pinder KE, Frisancho
JA and Pitha PM: Interferon regulatory factor 5, a novel mediator
of cell cycle arrest and cell death. Cancer Res. 63:6424–6431.
2003.PubMed/NCBI
|
|
11
|
Shin SH, Kim B-H, Jang J-J, Suh KS and
Kang GH: Identification of novel methylation markers in
hepatocellular carcinoma using a methylation array. J Korean Med
Sci. 25:1152–1159. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Yamashita M, Toyota M, Suzuki H, Nojima M,
Yamamoto E, Kamimae S, Watanabe Y, Kai M, Akashi H, Maruyama R, et
al: DNA methylation of interferon regulatory factors in gastric
cancer and noncancerous gastric mucosae. Cancer Sci. 101:1708–1716.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Massimino M, Vigneri P, Fallica M, Fidilio
A, Aloisi A, Frasca F and Manzella L: IRF5 promotes the
proliferation of human thyroid cancer cells. Mol Cancer. 11:212012.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Kreher S, Bouhlel MA, Cauchy P, Lamprecht
B, Li S, Grau M, Hummel F, Köchert K, Anagnostopoulos I, Jöhrens K,
et al: Mapping of transcription factor motifs in active chromatin
identifies IRF5 as key regulator in classical Hodgkin lymphoma.
Proc Natl Acad Sci USA. 111:E4513–E4522. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Kannian P and Green PL: Human T
lymphotropic virus type 1 (HTLV-1): Molecular biology and
oncogenesis. Viruses. 2:2037–2077. 2010. View Article : Google Scholar
|
|
16
|
Hasunuma T, Sumida T and Nishioka K: Human
T cell leukemia virus type-I and rheumatoid arthritis. Int Rev
Immunol. 17:291–307. 1998. View Article : Google Scholar
|
|
17
|
Grassmann R, Aboud M and Jeang K-T:
Molecular mechanisms of cellular transformation by HTLV-1 Tax.
Oncogene. 24:5976–5985. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Currer R, Van Duyne R, Jaworski E, Guendel
I, Sampey G, Das R, Narayanan A and Kashanchi F: HTLV tax: A
fascinating multifunctional co-regulator of viral and cellular
pathways. Front Microbiol. 3:4062012. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Ohsugi T: A transgenic mouse model of
human T cell leukemia virus type 1-associated diseases. Front
Microbiol. 4:492013. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Suzuki S, Zhou Y, Refaat A, Takasaki I,
Koizumi K, Yamaoka S, Tabuchi Y, Saiki I and Sakurai H: Human T
cell lymphotropic virus 1 manipulates interferon regulatory signals
by controlling the TAK1-IRF3 and IRF4 pathways. J Biol Chem.
285:4441–4446. 2010. View Article : Google Scholar :
|
|
21
|
Sharma S, Mamane Y, Grandvaux N, Bartlett
J, Petropoulos L, Lin R and Hiscott J: Activation and regulation of
interferon regulatory factor 4 in HTLV type 1-infected T
lymphocytes. AIDS Res Hum Retroviruses. 16:1613–1622. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Tsuboi K, Iida S, Inagaki H, Kato M,
Hayami Y, Hanamura I, Miura K, Harada S, Kikuchi M, Komatsu H, et
al: MUM1/IRF4 expression as a frequent event in mature lymphoid
malignancies. Leukemia. 14:449–456. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Imaizumi Y, Kohno T, Yamada Y, Ikeda S,
Tanaka Y, Tomonaga M and Matsuyama T: Possible involvement of
interferon regulatory factor 4 (IRF4) in a clinical subtype of
adult T-cell leukemia. Jpn J Cancer Res. 92:1284–1292. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Ramos JC, Ruiz P Jr, Ratner L, Reis IM,
Brites C, Pedroso C, Byrne GE Jr, Toomey NL, Andela V, Harhaj EW,
et al: IRF-4 and c-Rel expression in antiviral-resistant adult
T-cell leukemia/lymphoma. Blood. 109:3060–3068. 2007.
|
|
25
|
Yamagata T, Nishida J, Tanaka S, Sakai R,
Mitani K, Yoshida M, Taniguchi T, Yazaki Y and Hirai H: A novel
interferon regulatory factor family transcription factor,
ICSAT/Pip/LSIRF, that negatively regulates the activity of
interferon-regulated genes. Mol Cell Biol. 16:1283–1294.
1996.PubMed/NCBI
|
|
26
|
Sharma S, Grandvaux N, Mamane Y, Genin P,
Azimi N, Waldmann T and Hiscott J: Regulation of IFN regulatory
factor 4 expression in human T cell leukemia virus-I-transformed T
cells. J Immunol. 169:3120–3130. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Grumont RJ and Gerondakis S: Rel induces
interferon regulatory factor 4 (IRF-4) expression in lymphocytes:
Modulation of interferon-regulated gene expression by rel/nuclear
factor κB. J Exp Med. 191:1281–1292. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Ohtani K, Nakamura M, Saito S, Nagata K,
Sugamura K and Hinuma Y: Electroporation: Application to human
lymphoid cell lines for stable introduction of a transactivator
gene of human T-cell leukemia virus type I. Nucleic Acids Res.
17:1589–1604. 1989. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Yoshida T, Miyagawa E, Yamaguchi K,
Kobayashi S, Takahashi Y, Yamashita A, Miura H, Itoyama Y and
Yamamoto N: IL-2 independent transformation of a unique human T
cell line, TY8-3, and its subclones by HTLV-I and -II. Int J
Cancer. 91:99–108. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Mancl ME, Hu G, Sangster-Guity N,
Olshalsky SL, Hoops K, Fitzgerald-Bocarsly P, Pitha PM, Pinder K
and Barnes BJ: Two discrete promoters regulate the alternatively
spliced human interferon regulatory factor-5 isoforms. Multiple
isoforms with distinct cell type-specific expression, localization,
regulation, and function. J Biol Chem. 280:21078–21090. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Saikh KU, Lee JS, Kissner TL, Dyas B and
Ulrich RG: Toll-like receptor and cytokine expression patterns of
CD56+ T cells are similar to natural killer cells in
response to infection with Venezuelan equine encephalitis virus
replicons. J Infect Dis. 188:1562–1570. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Iki S, Yokota S, Okabayashi T, Yokosawa N,
Nagata K and Fujii N: Serum-dependent expression of promyelocytic
leukemia protein suppresses propagation of influenza virus.
Virology. 343:106–115. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Yokosawa N, Kubota T and Fujii N: Poor
induction of interferon-induced 2′,5′-oligoadenylate synthetase
(2–5 AS) in cells persistently infected with mumps virus is caused
by decrease of STAT-1α. Arch Virol. 143:1985–1992. 1998. View Article : Google Scholar
|
|
34
|
Brenner CA, Tam AW, Nelson PA, Engleman
EG, Suzuki N, Fry KE and Larrick JW: Message amplification
phenotyping (MAPPing): A technique to simultaneously measure
multiple mRNAs from small numbers of cells. Biotechniques.
7:1096–1103. 1989.PubMed/NCBI
|
|
35
|
Subrata LS, Lowes KN, Olynyk JK, Yeoh GCT,
Quail EA and Abraham LJ: Hepatic expression of the tumor necrosis
factor family member lymphotoxin-β is regulated by interleukin
(IL)-6 and IL-1β: Transcriptional control mechanisms in oval cells
and hepatoma cell lines. Liver Int. 25:633–646. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Hieshima K, Nagakubo D, Nakayama T,
Shirakawa AK, Jin Z and Yoshie O: Tax-inducible production of CC
chemokine ligand 22 by human T cell leukemia virus type 1
(HTLV-1)-infected T cells promotes preferential transmission of
HTLV-1 to CCR4-expressing CD4+ T cells. J Immunol.
180:931–939. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Mori N and Prager D: Transactivation of
the interleukin-1α promoter by human T-cell leukemia virus type I
and type II Tax proteins. Blood. 87:3410–3417. 1996.PubMed/NCBI
|
|
38
|
Ishikawa C, Kawakami H, Uchihara J-N,
Senba M and Mori N: CD69 overexpression by human T-cell leukemia
virus type 1 Tax transactivation. Biochim Biophys Acta.
1833:1542–1552. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Suzuki K, Bose P, Leong-Quong RY, Fujita
DJ and Riabowol K: REAP: A two minute cell fractionation method.
BMC Res Notes. 3:2942010. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Tanaka Y, Yoshida A, Takayama Y, Tsujimoto
H, Tsujimoto A, Hayami M and Tozawa H: Heterogeneity of antigen
molecules recognized by anti-tax1 monoclonal antibody Lt-4 in cell
lines bearing human T cell leukemia virus type I and related
retro-viruses. Jpn J Cancer Res. 81:225–231. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Matsumoto K, Shibata H, Fujisawa J-I,
Inoue H, Hakura A, Tsukahara T and Fujii M: Human T-cell leukemia
virus type 1 Tax protein transforms rat fibroblasts via two
distinct pathways. J Virol. 71:4445–4451. 1997.PubMed/NCBI
|
|
42
|
Kimura R, Ishikawa C, Rokkaku T, Janknecht
R and Mori N: Phosphorylated c-Jun and Fra-1 induce matrix
metalloproteinase-1 and thereby regulate invasion activity of 143B
osteosarcoma cells. Biochim Biophys Acta. 1813:1543–1553. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Santos SB, Porto AF, Muniz AL, de Jesus
AR, Magalhães E, Melo A, Dutra WO, Gollob KJ and Carvalho EM:
Exacerbated inflammatory cellular immune response characteristics
of HAM/TSP is observed in a large proportion of HTLV-I asymptomatic
carriers. BMC Infect Dis. 4:72004. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Tschachler E, Robert-Guroff M, Gallo RC
and Reitz MS Jr: Human T-lymphotropic virus I-infected T cells
constitutively express lymphotoxin in vitro. Blood. 73:194–201.
1989.PubMed/NCBI
|
|
45
|
Iwakura Y, Saijo S, Kioka Y,
Nakayama-Yamada J, Itagaki K, Tosu M, Asano M, Kanai Y and Kakimoto
K: Autoimmunity induction by human T cell leukemia virus type 1 in
transgenic mice that develop chronic inflammatory arthropathy
resembling rheumatoid arthritis in humans. J Immunol.
155:1588–1598. 1995.PubMed/NCBI
|
|
46
|
Baba M, Okamoto M, Hamasaki T, Horai S,
Wang X, Ito Y, Suda Y and Arima N: Highly enhanced expression of
CD70 on human T-lymphotropic virus type 1-carrying T-cell lines and
adult T-cell leukemia cells. J Virol. 82:3843–3852. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Bazzoni F and Beutler B: The tumor
necrosis factor ligand and receptor families. N Engl J Med.
334:1717–1725. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Tsukasaki K, Miller CW, Kubota T, Takeuchi
S, Fujimoto T, Ikeda S, Tomonaga M and Koeffler HP: Tumor necrosis
factor α polymorphism associated with increased susceptibility to
development of adult T-cell leukemia/lymphoma in human
T-lymphotropic virus type 1 carriers. Cancer Res. 61:3770–3774.
2001.PubMed/NCBI
|
|
49
|
Seki N, Yamaguchi K, Yamada A, Kamizono S,
Sugita S, Taguchi C, Matsuoka M, Matsumoto H, Nishizaka S, Itoh K,
et al: Polymorphism of the 5′-flanking region of the tumor necrosis
factor (TNF)-α gene and susceptibility to human T-cell lymphotropic
virus type I (HTLV-I) uveitis. J Infect Dis. 180:880–883. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Watters KM, Dean J, Hasegawa H, Sawa H,
Hall W and Sheehy N: Cytokine and growth factor expression by
HTLV-1 Lck-tax transgenic cells in SCID mice. AIDS Res Hum
Retroviruses. 26:593–603. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Albrecht H, Shakhov AN and Jongeneel CV:
trans activation of the tumor necrosis factor alpha promoter by the
human T-cell leukemia virus type I Tax1 protein. J
Virol. 66:6191–6193. 1992.PubMed/NCBI
|
|
52
|
Krausgruber T, Saliba D, Ryzhakov G,
Lanfrancotti A, Blazek K and Udalova IA: IRF5 is required for
late-phase TNF secretion by human dendritic cells. Blood.
115:4421–4430. 2010. View Article : Google Scholar : PubMed/NCBI
|