Introduction
Cervical cancer is the fourth most common cancer
among women worldwide (1). An
estimated 12,360 new cases were diagnosed and 4,020 people died of
the disease in the United States in 2014 (2). The most important cause of cervical
cancer is human papillomavirus (HPV) persistent infection. HPV16
can be detected in approximately 55–60% of all cases of cervical
cancer worldwide (3). Although the
current treatment can cure 80–95% of early-stage and 60% of locally
advanced cancers, the recurrent and metastatic disease still
remains a major problem (4). As
the use of concurrent platinum-based chemoradiation often leads to
severe toxicity, complementary and alternative medicine (CAM) is
recently becoming a popular treatment for various cancers. Many
natural products have received increased attention as a potential
source of new therapeutic antitumor drugs (5,6).
Huaier is a kind of fungi and has been used for the
treatment of various diseases in China for many years. The
effective ingredient of Huaier extract is proteoglycan, which
contains 41.53% polysaccharides, 12.93% amino acids and 8.72% water
(7). The anticancer activity of
Huaier extract has been demonstrated in vitro and in
vivo in hepatocarcinoma (8–10),
breast cancer (7,11,12),
colorectal cancer (13), melanoma
(14), and ovarian cancer
(15). Huaier may cause anticancer
effects by various mechanisms, including inhibition of cell growth,
induction of apoptosis, inhibition of tumor-induced angiogenesis
and inactivation of the epithelial-mesenchymal transition (EMT)
(7–15).
However, the effects of Huaier on cervical cancer
cells are unknown. In the present study, we investigated the
antitumor mechanisms of Huaier in the cervical cancer SiHa cells
with HPV-16-positive, and C33A HPV-negative cells.
Materials and methods
Cell lines and cell culture
Two human cervical cancer cell lines SiHa and C33A
cells were purchased from the American Type Culture Collection
(ATCC, Manassas, VA, USA) and preserved in our laboratory. Both
cell types were cultured in minimum essential medium (MEM,
Invitrogen, Carlsbad, CA, USA) containing 10% fetal bovine serum
(FBS, Haoyang Biological Manufacture Co. Ltd., Tianjin, China) in
5% CO2 at 37°C.
Preparation of Huaier aqueous
extract
Electuary ointment of Huaier was provided by
Gaitianli Medicine Co. Ltd. (Jiangsu, China). Three grams of the
electuary ointment was dissolved in 30 ml of complete medium and
was sterilized with 0.22-μm filter to get the 100 mg/ml stock
solution for long storage at −20°C (11).
Cell viability assay
Cell viability was determined by
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT)
assay. SiHa cells (5×103 cells/well) and C33A cells
(8×103 cells/well) were seeded in a 96-well plate and
incubated for 24 h prior to drug treatment. Following treatment
with Huaier at different doses (0–12 mg/ml) for 48 or 72 h, 20 μl
of MTT solution was added to each well and the plates were
incubated at 37°C for an additional 4 h. The MTT solution was then
removed and 100 μl of dimethyl sulfoxide (DMSO) was added to each
well to dissolve the precipitated crystals. Cell viability was
assessed by measuring absorbance at 570 nm in a microplate reader
(Bio-Rad, Hercules, CA, USA). Dose response curves were then
created as a percentage of vehicle treated control cells using
Excel software.
Colony formation assay
Briefly, SiHa cells were plated at a density of 600
cells/well in 6-well plates and C33A cells were plated at 800
cells/well. After 24 h, the cells were exposed to various
concentrations of Huaier (0, 1 and 2 mg/ml) followed by incubation
at 37°C in a 5% CO2 incubator for 8 days. This was
followed by fixing the colonies with 4% paraformaldehyde and
staining with 1% crystal violet.
Cell cycle analysis
Cells were seeded in 6-well plates at a density of
5×105 cells/well and starved in serum-free medium at
37°C. After 24-h starvation, the cells were treated with various
concentrations of Huaier extract or complete medium as the negative
control for 24 h. The cells were then trypsinized, washed with
ice-cold PBS and resuspended in 1 ml staining solution (50 μg/ml
PI, 20 μg/ml RNase A). After incubation of 30 min at room
temperature, samples were analyzed using a FACSCalibur flow
cytometer (BD Biosciences).
Migration and invasion assay
Migration assays were performed using the 24-well
transwell chambers which contain polycarbonate filters with 8-μm
pores (BD Falcon, Bedford, MA, USA). SiHa cells (10×104
cells/well) and C33A cells (15×104 cells/well) were
suspended in 0.2 ml of fresh medium without FBS, then added to the
upper well of the chamber. Lower chambers were filled with the
culture medium containing 20% FBS as a chemo-attractant with or
without 2 mg/ml Huaier. After incubation at 37°C for 24 h, the
cells that had migrated to the lower surface were fixed with
methanol, stained with 0.2% crystal violet, and counted under an
Olympus light microscope. Invasion assay was performed in the same
way as the migration assay except that the membrane was coated with
Matrigel (BD Biosciences, San Jose, CA, USA). The chambers were
incubated at 37°C for 48 h before the cells were fixed.
Western blot analysis
The SiHa and C33A cells were seeded and treated with
different concentrations of Huaier extract. At different
time-points, cells were harvested and lysed on ice with
radioimmunoprecipitation assay (RIPA) buffer (PBS containing 1%
NP40, 0.1% SDS, 5 mM EDTA, 0.5% sodium deoxycholate, 1 mM sodium
orthovanadate and protease inhibitors). Subsequently, 30 μg of
total cellular protein from each sample were separated by 10%
SDS-PAGE and electrotransferred onto a polyvinylidene fluoride
(PVDF) membranes by using a semi-dry blotting apparatus (Bio-Rad).
After blocking with 5% non-fat milk, the membrane was incubated
overnight at 4°C with the primary antibody and then with
horseradish peroxidase-coupled secondary antibody. Signal was
detected with enhanced chemiluminescence (ECL) (Perkin-Elmer Inc.)
by Imagequant LAS 4000 (GE Healthcare, Japan). The first antibodies
used included β-actin (GeneTex) and three MAPKs (ERK, JNK and p38)
antibodies (Cell Signaling Technology).
Animal experiments
The present study was approved by the institutional
guidelines of the Animal Care and Use Committee at Shandong
University. Fourteen BALB/c nu/nu female mice, 4–6-week-old, were
purchased from Huafukang experimental animal limited company and
housed within a dedicated SPF facility at Laboratory Animal Center
of Qilu Hospital. SiHa cells (4×106) were subcutaneously
injected into the right flank of each mouse. Then, the mice were
randomly divided into 2 groups (n=7 mice/group). After 2 days, each
mouse was given 100 μl solution containing 50 mg Huaier extract
(test group) or medium only (control group) by gavage daily. Tumors
were measured once a week with a digital caliper. Tumor volume
(mm3) was determined by the length (a) and the width (b)
as V=ab2/2. After 59 days, the mice were surgically
excised, weighed.
Statistical evaluation
SSPS 16.0 software was used for statistical
analysis, and Student's t-test was used to analyze the statistical
difference. P<0.05 was set as a significant difference. Results
are reported as mean ± standard deviation (SD).
Results
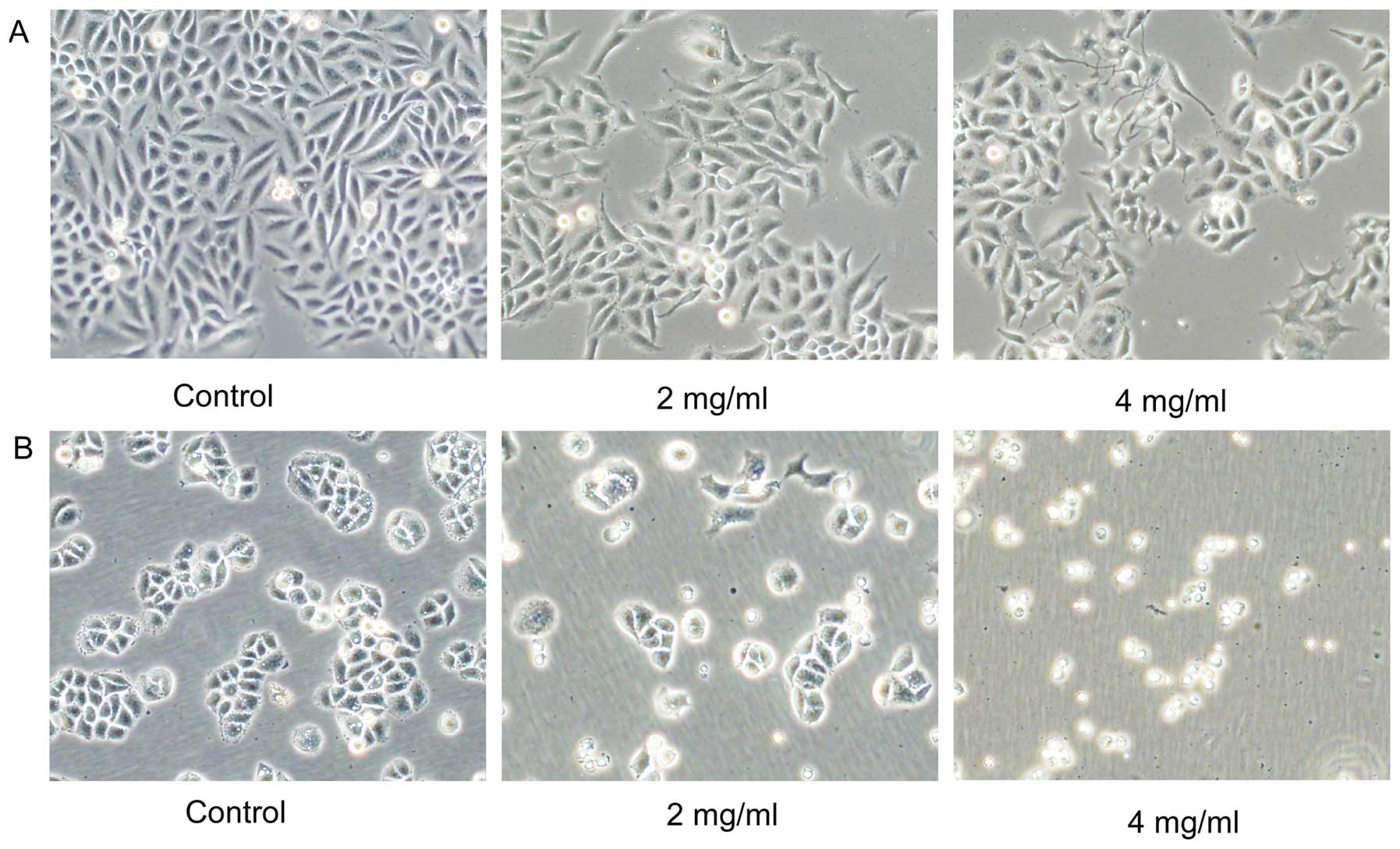
Huaier causes cell morphology changes and
inhibits cell viability in both SiHa and C33A cells
Morphological changes of SiHa and C33A cells induced
by Huaier were observed (Fig. 1).
Both cells were treated with various concentrations (0, 2 and 4
mg/ml) of Huaier extract for 72 h. Compared with the untreated
cells, the majority of the Huaier-treated SiHa cells changed from
spindle to star-shaped with sharp outlines, some of which become
the special ‘wiredrawing' morphology. However, most of the C33A
cells did not show obvious changes and were non-viable.
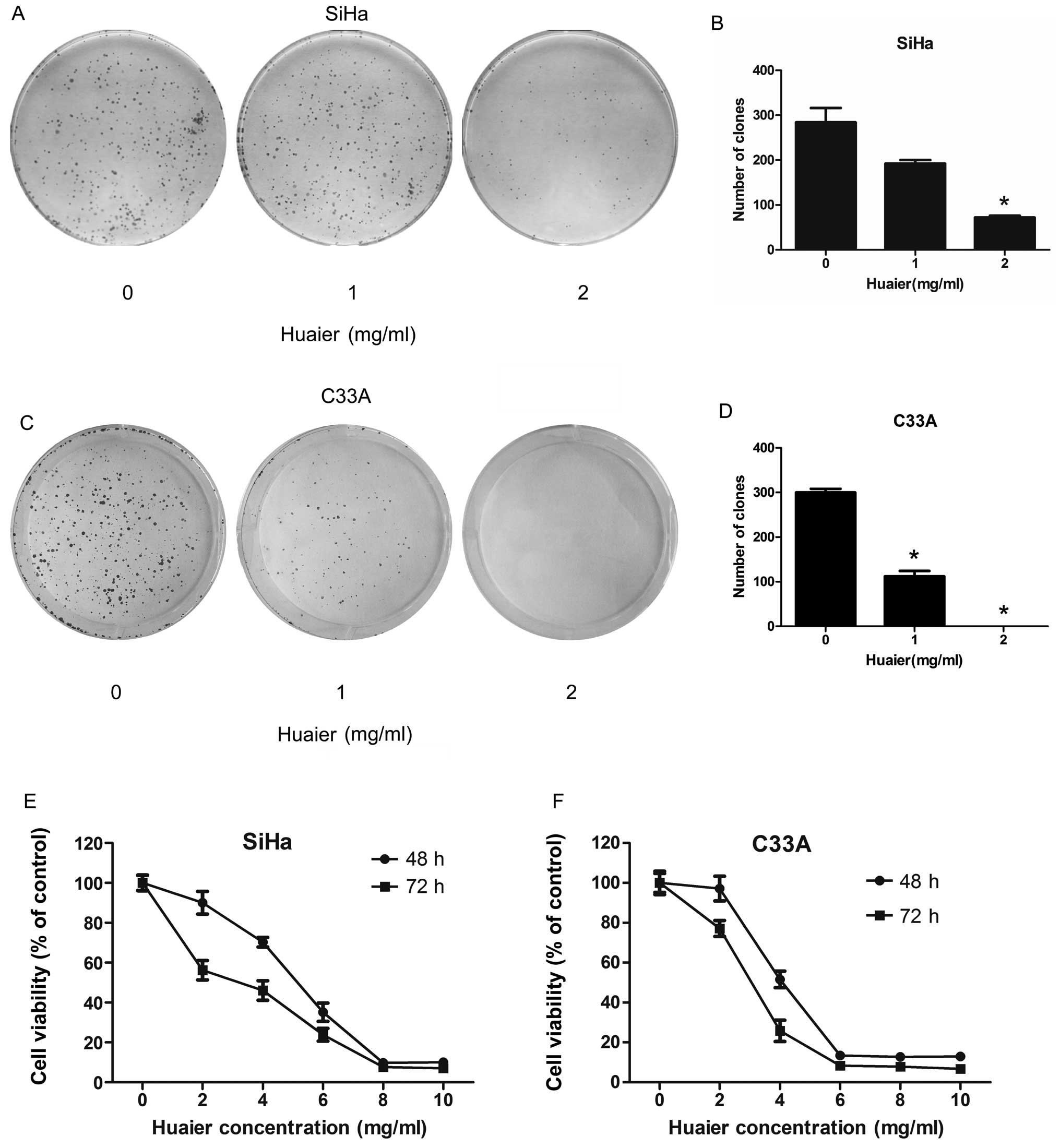
In colony formation assay, Huaier at concentrations
of 1 and 2 mg/ml significantly inhibit the colony forming abilities
of both SiHa and C33A cell. After 8 days continuous culture with
Huaier, the colony forming ability of SiHa cells was reduced to
67.6 and 25.4% relative to untreated cells, respectively (Fig. 2A and B). C33A cells exhibited even
more decrease in colony formation, and the number of colonies that
formed after 8-day treatment was reduced to 37.3 and 0%,
respectively (Fig. 2C and D).
According to the MTT assay (Fig. 2E and F), the proliferation of both
SiHa and C33A cells was inhibited after treatment with different
concentrations of Huaier for 48 and 72 h. The C33A cells were more
sensitive to the Huaier treatment than SiHa cells. The
IC50 values in SiHa and C33A cells treated with Huaier
for 48 h were 5.20 and 3.95 mg/ml, respectively. In addition, a
sharp decrease in cell viability was observed when the cells were
treated with 6 mg/ml Huaier in C33A cells compared with 8 mg/ml
Huaier in SiHa cells.
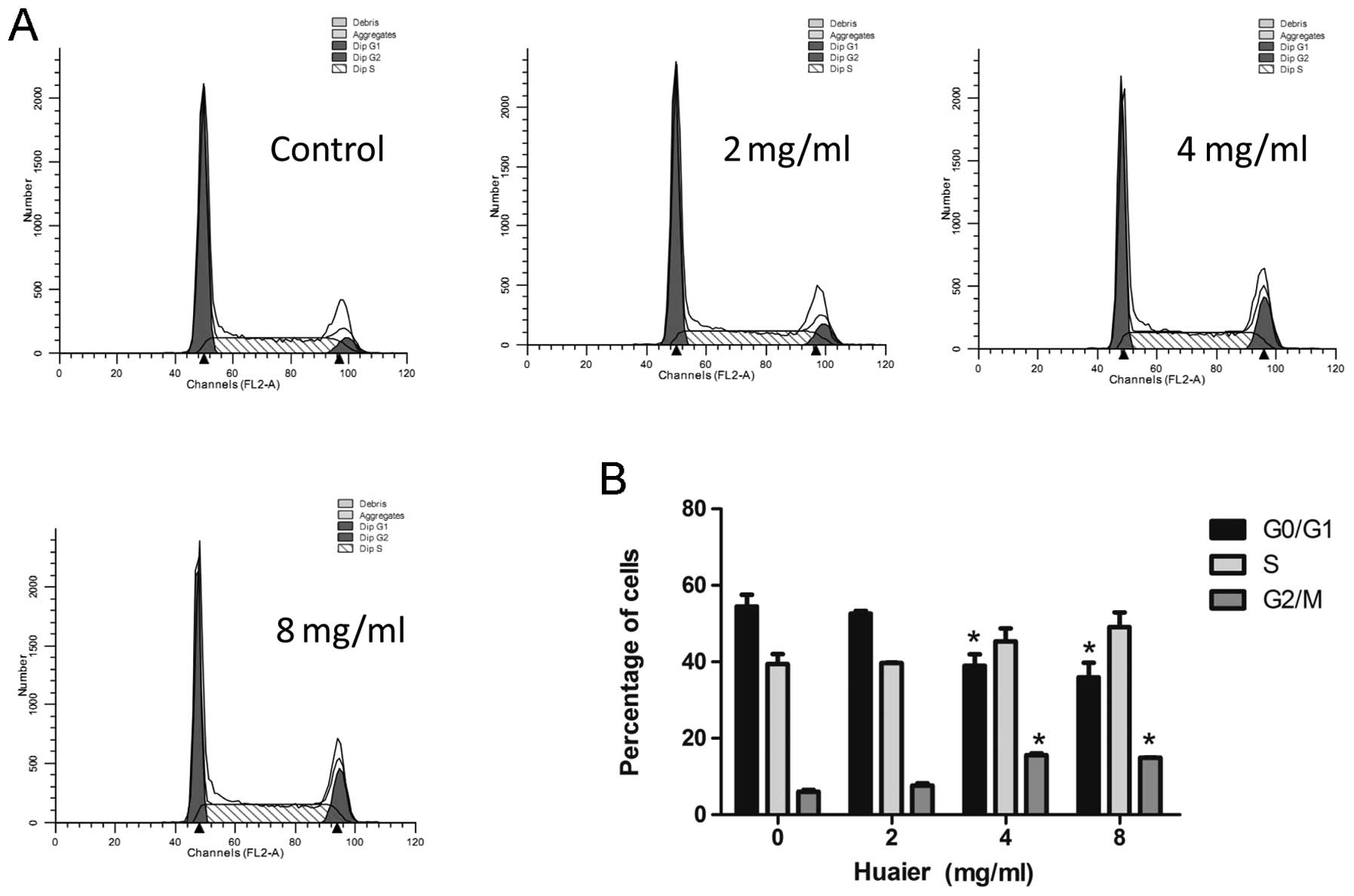
Huaier induces cell cycle arrest in C33A
cells but not in SiHa cells
In order to determine whether the anti-proliferative
effect of Huaier attributes to its induction of cell cycle arrest,
we treated cells with different concentrations of Huaier for 24 h
and analyzed the cell cycle distribution using flow cytometry. As
shown in Fig. 3, Huaier treatment
increased the percentage of cells at the G2/M phase in a
dose-dependent manner in C33A cells, while had no effect on cell
cycle in SiHa cells at any of the tested concentrations (data not
shown). It indicated that Huaier induced cell arrest at the G2/M
phase in HPV-negative cancer cells but not HPV-positive cells.
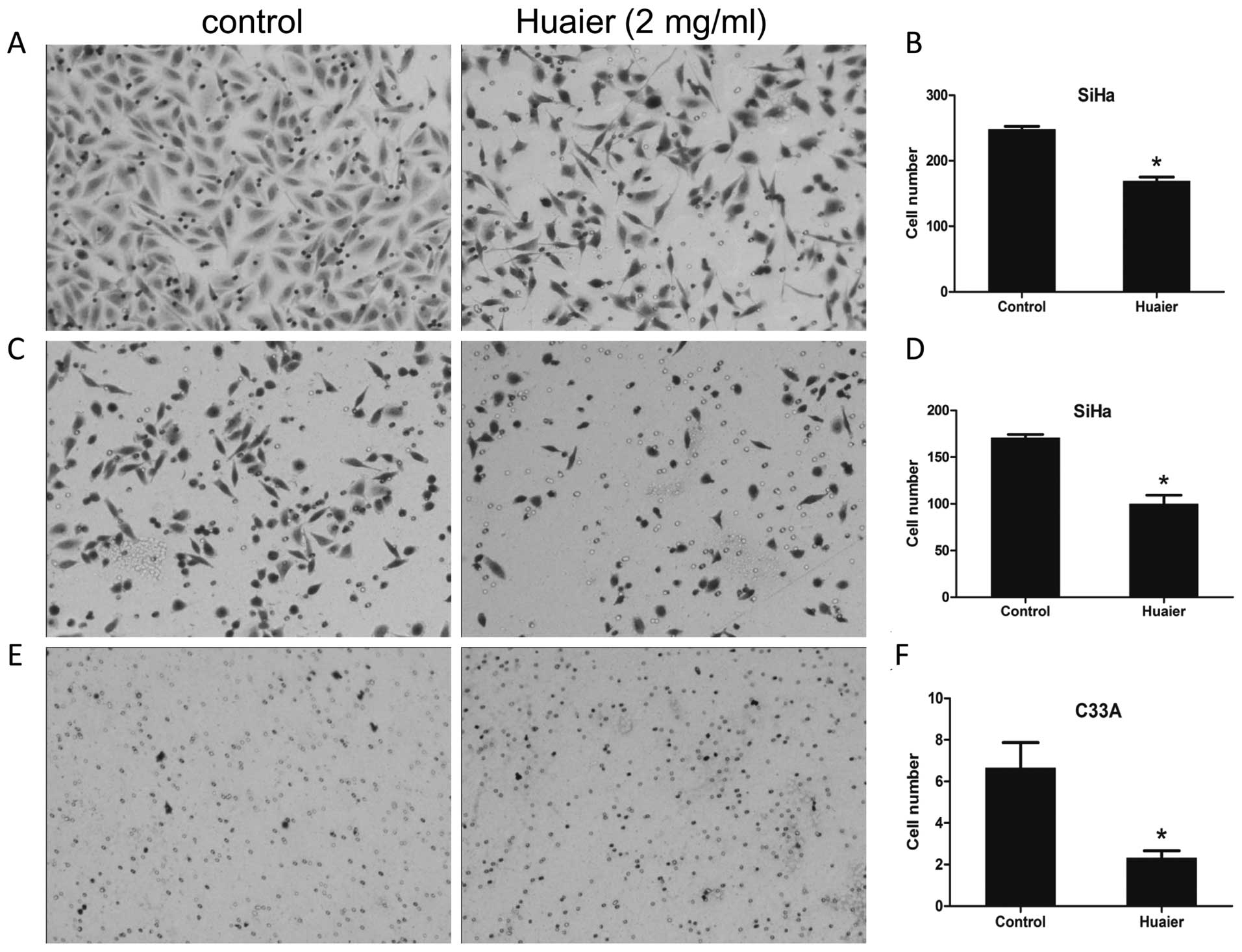
Cell motility was suppressed due to
exposure to Huaier
In this study, the effects of Huaier on the
abilities of cell mobility and invasion was determined using the
transwell migration assay. The medium containing 20% FBS was added
in the lower wells in the absence or presence of 2 mg/ml Huaier
extracts, and cells were allowed to migrate for 24 h. As shown in
Fig. 4C–F, the abilities of
migration of both SiHa and C33A cells were significantly inhibited
in the presence of Huaier. The number of migrated SiHa cells in the
treated group was 170.67±6.11 relative to 116.00±16.00 of untreated
control (P=0.0020) (Fig. 4C and
D), and the number of migrated C33A cells in the treated group
was 2.33±0.57 compared with the control cells (6.67±2.08, P=0.0255)
(Fig. 4E and F). Using the same
manner as the mobility assay except utilizing Matrigel-coated
inserts with 8-μm pore size, the invasive potential of
Huaier-treated and untreated SiHa cells was compared (Fig. 4A and B). It revealed that the
number of cells invading through the Matrigel-coated membrane upon
treatment with Huaier was significantly less than that of the
untreated control (169.33±10.06 versus 248.00±8.00, P=0.0004). C33A
cells have been reported to be poorly invasive cells (16), therefore, we did not perform an
invasive assay in C33A cells.
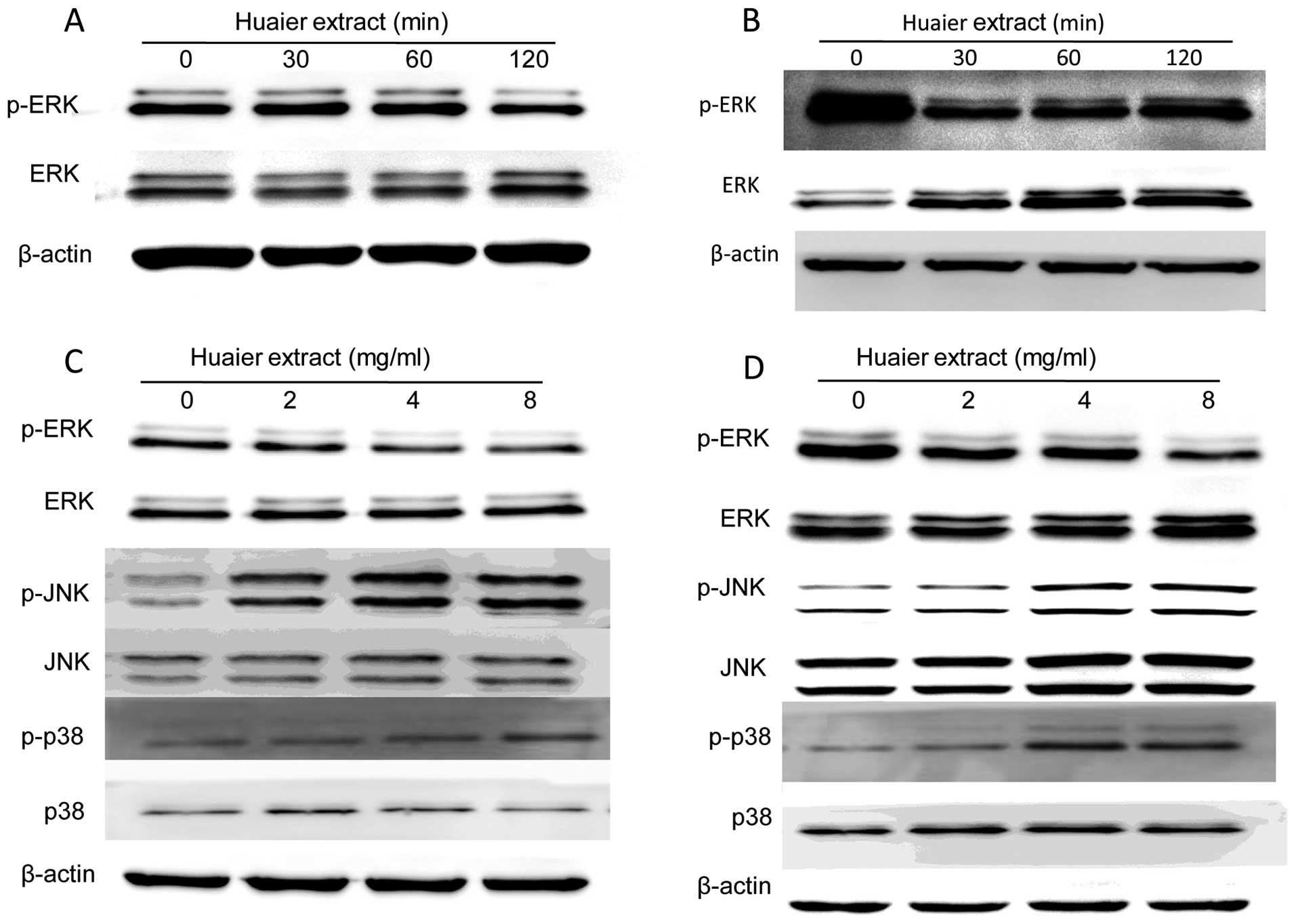
Huaier may exert its anti-cervical cancer
activity via MAPK signaling pathways
To confirm whether MAPK signaling pathways are
involved in the regulation of cervical cancer cell proliferation
and motility induced by Huaier, the activities of three major
members including the extracellular signal-regulated kinase (ERK),
the c-Jun NH2-terminal kinase (JNK) and the p38-MAPK were examined
by western blotting. It was shown that the levels of phosphorylated
ERK (p-ERK) in both cells treated with Huaier extract were
decreased in a time-dependent manner (Fig. 5A and B). Furthermore, the
expression of p-ERK in SiHa cells was greatly reduced at 120 min
after incubation with 8 mg/ml Huaier, while the sharp reduction in
C33A cells occurred at 30 min. Therefore, we investigated the
changes of p-ERK in SiHa and C33A cells treated with various
concentration of Huaier at 120 and 30 min, respectively. The
results showed that Huaier treatment downregulated the
phosphorylation of ERK without affecting overall ERK expression
levels (Fig. 5C and D). On the
other hand, the expression of phosphorylated JNK (p-JNK) and
phosphorylated p38 (p-p38) was increased with treatment of
increasing concentrations of Huaier (0, 2, 4 and 8 mg/ml) in both
SiHa and C33A cells (Fig. 5C and
D).
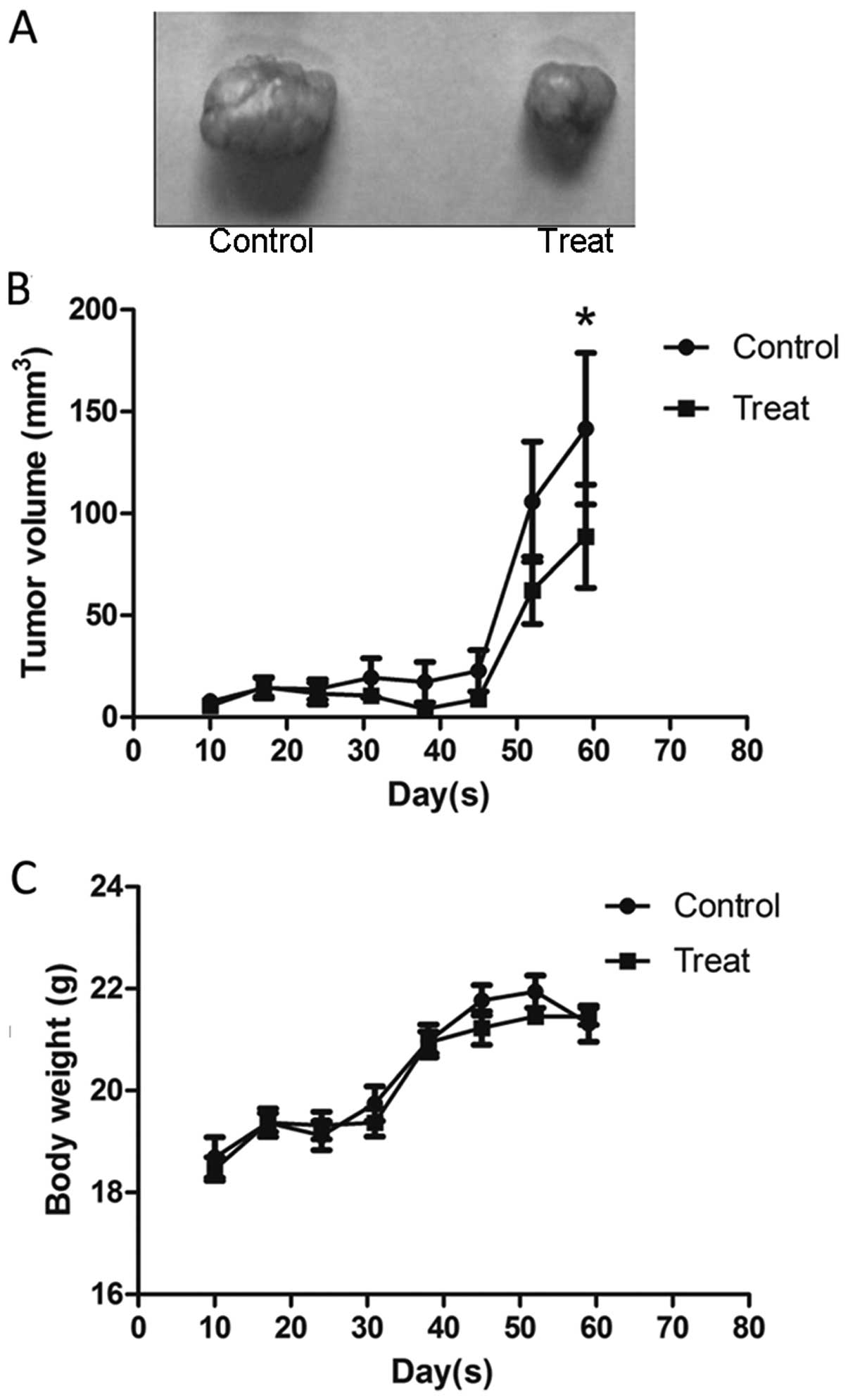
Huaier inhibits tumor growth in vivo
To further assess the effect of Huaier on tumor
growth in vivo, the SiHa cell subcutaneous tumor model was
established in BALB/c nu/un mice. Two days after the cells were
subcutaneously injected into the mice, Huaier was administered to
the mice by gavage at a concentration of 2.5 g/kg per day for 3
weeks. Data analysis was performed by considering the tumor size at
7-day intervals. As shown in Fig.
6B, a slight reduction in tumor volume in the mice treated with
Huaier occurred one week after the last gavage and the significant
reduction was found four weeks later, compared with the untreated
group (141.57±98.44 versus 88.71±66.97 mm3, P=0.0093).
Furthermore, there was no change in mouse body weight during the
experiment (Fig. 6C). Taken
together, our data suggest that Huaier is a potent suppressor of
cervical cancer growth, with no apparent signs of toxicity.
Discussion
Traditional Chinese medicines have attracted
increasing attention due to their promising anticancer potential.
Among them, Huaier extracts have been demonstrated to be effective
for many cancers including hepatocarcinoma (8–10),
breast cancer (7,11,12),
and ovarian cancer (15). However,
little is known about its effects on cervical cancer and its
anticancer mechanism is still not clear. In the present study, we
demonstrated the inhibitory effects of Huaier extract on human
cervical cancer growth in vitro and in vivo, and
investigated the possible mechanisms of action involved. We have
provided evidence that Huaier can inhibit the proliferation of
cervical cancer cells via the JNK/p38 pathway.
A previous study indicated that Huaier could inhibit
cell proliferation by inducing apoptosis (7). However in the present study we found
that Huaier treatment in cervical cancer cells did not work in the
same way as other cancer cells (data not shown). Our western blot
results showed that p-JNK and p-p38 increased in a dose-dependent
manner with the treatment of Huaier. It is known that JNK and p38
are members of mitogen activated protein kinase (MAPK) family which
are involved in many cellular responses such as proliferation,
differentiation and apoptosis. Their activation is induced by
several stress stimuli (i.e., oxidative stress, UV radiation,
hyperosmosis, and inflammatory cytokines) (17). Activation of both p-JNK and p-p38
was also observed in case of a few other anticancer agents such as
Aplidin™ (18), adaphostin
(19) and Abrus precatorius
(20).
Data show that some anticancer agents modulate JNK
activity followed by various pathways including: i) the
pro-apoptotic effect; ii) regulation of cell cycle arrest; iii)
autophagy. The first pathway has been considered an important
process inducing cell death and several drugs in clinical use have
been shown to play an role in this manner, for example etoposide,
paclitaxel, and Taxol (18).
However, our study showed that Huaier induced an accumulation of
C33A cells in the G2/M phase of the cell cycle and a decrease in
the population of cells in the G1 phase, but not in SiHa cells.
These data demonstrated that Huaier-induced cell death was mediated
by the cell cycle process, which may in turn be partly regulated by
the JNK/p38 pathway. In our study, the cell cycle arrest was
observed only in C33A cells treated with Huaier which are
HPV-negative. Therefore, we speculated that Huaier may inhibit the
growth of HPV-positive tumor cells via other mechanisms than the
pro-apoptotic process and cell cycle arrest. More cell lines need
to be studied to elucidate the phenomena.
Migration assay and invasion assay were used to
evaluate tumor cell motility. Our results showed that Huaier
significantly inhibited the migration and invasion in SiHa cells,
and migration in C33A cells. In order to reveal the potential
signaling pathways underlying the anti-metastasis activity of
Huaier extract, we investigated the expression of ERKs. The ERK1
and ERK2 (ERK1/2) are copiously present in all human tissues which
have >80% amino acid identity (21). It is well accepted that the ERK
pathway plays a significant role in MMP regulation which
facilitates tumor cell migration and invasion. In this study, we
found that the levels of p-ERK in cervical cancer SiHa and C33A
cells decreased by Huaier treatment in a time- and dose-dependent
manner. The result suggested that Huaier suppressed motility of
cervical cancer cells via downregulation of p-ERK.
In conclusion, our study showed that Huaier extract
had an inhibitory effect on cell proliferation in cervical cancer
cells via JNK/p38 pathway. We also revealed that cell cycle was
arrested in G2/M phase induced by the activation of p-JNK and p-p38
in HPV-negative cells. In HPV-positive cells Huaier extract can
also play an important role in cell proliferation and motility,
however, the mechanism is still not clear. Further studies should
be performed to clarify whether or not Huaier extract can be used
as an anti-HPV agent.
Acknowledgements
This study was supported by the National High
Technology Research and Development Program (‘863' Program) of
China (2014AA020605, 2012AA02A507), National Natural Science
Foundation of China (81272857), National Science and Technology
Project (2015BAI13B05).
References
|
1
|
Kamangar F, Dores GM and Anderson WF:
Patterns of cancer incidence, mortality, and prevalence across five
continents: Defining priorities to reduce cancer disparities in
different geographic regions of the world. J Clin Oncol.
24:2137–2150. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Siegel R, Ma J, Zou Z and Jemal A: Cancer
statistics, 2014. CA Cancer J Clin. 64:9–29. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
de Sanjose S, Quint WG, Alemany L, Geraets
DT, Klaustermeier JE, Lloveras B, Tous S, Felix A, Bravo LE, Shin
HR, et al; Retrospective International Survey and HPV Time Trends
Study Group. Human papillomavirus genotype attribution in invasive
cervical cancer: A retrospective cross-sectional worldwide study.
Lancet Oncol. 11:1048–1056. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Cornelio DB, Roesler R and Schwartsmann G:
Emerging therapeutic agents for cervical cancer. Recent Pat
Anticancer Drug Discov. 4:196–206. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Munagala R, Kausar H, Munjal C and Gupta
RC: Withaferin A induces p53-dependent apoptosis by repression of
HPV oncogenes and upregulation of tumor suppressor proteins in
human cervical cancer cells. Carcinogenesis. 32:1697–1705. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Yang Z, Garcia A, Xu S, Powell DR, Vertino
PM, Singh S and Marcus AI: Withania somnifera root extract inhibits
mammary cancer metastasis and epithelial to mesenchymal transition.
PLoS One. 8:e750692013. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Zhang N, Kong X, Yan S, Yuan C and Yang Q:
Huaier aqueous extract inhibits proliferation of breast cancer
cells by inducing apoptosis. Cancer Sci. 101:2375–2383. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Ren J, Zheng C, Feng G, Liang H, Xia X,
Fang J, Duan X and Zhao H: Inhibitory effect of extract of fungi of
Huaier on hepatocellular carcinoma cells. J Huazhong Univ Sci
Technolog Med Sci. 29:198–201. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Zheng J, Li C, Wu X, Liu M, Sun X, Yang Y,
Hao M, Sheng S, Sun Y, Zhang H, et al: Huaier polysaccharides
suppresses hepatocarcinoma MHCC97-H cell metastasis via
inactivation of EMT and AEG-1 pathway. Int J Biol Macromol.
64:106–110. 2014. View Article : Google Scholar
|
|
10
|
Zheng J, Li C, Wu X, Liu M, Sun X, Yang Y,
Hao M, Sheng S, Sun Y, Zhang H, et al: Astrocyte elevated gene-1
(AEG-1) shRNA sensitizes Huaier polysaccharide (HP)-induced
anti-metastatic potency via inactivating downstream P13K/Akt
pathway as well as augmenting cell-mediated immune response. Tumour
Biol. 35:4219–4224. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Wang X, Zhang N, Huo Q and Yang Q:
Anti-angiogenic and antitumor activities of Huaier aqueous extract.
Oncol Rep. 28:1167–1175. 2012.PubMed/NCBI
|
|
12
|
Wang X, Zhang N, Huo Q, Sun M, Dong L,
Zhang Y, Xu G and Yang Q: Huaier aqueous extract inhibits stem-like
characteristics of MCF7 breast cancer cells via inactivation of
hedgehog pathway. Tumour Biol. 35:10805–10813. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Zhang T, Wang K, Zhang J, Wang X, Chen Z,
Ni C, Qiu F and Huang J: Huaier aqueous extract inhibits colorectal
cancer stem cell growth partially via downregulation of the
Wnt/β-catenin pathway. Oncol Lett. 5:1171–1176. 2013.PubMed/NCBI
|
|
14
|
Zhang F, Zhang Z and Liu Z: Effects of
Huaier aqueous extract on proliferation and apoptosis in the
melanoma cell line A875. Acta Histochem. 115:705–711. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Yan X, Lyu T, Jia N, Yu Y, Hua K and Feng
W: Huaier aqueous extract inhibits ovarian cancer cell motility via
the AKT/ GSK3β/β-catenin pathway. PLoS One. 8:e637312013.
View Article : Google Scholar
|
|
16
|
Shin HJ, Rho SB, Jung DC, Han IO, Oh ES
and Kim JY: Carbonic anhydrase IX (CA9) modulates tumor-associated
cell migration and invasion. J Cell Sci. 124:1077–1087. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Cuenda A and Rousseau S: p38 MAP-kinases
pathway regulation, function and role in human diseases. Biochim
Biophys Acta. 1773:1358–1375. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Cuadrado A, Garcia-Fernandez LF, Gonzalez
L, Suarez Y, Losada A, Alcaide V, Martinez T, Fernandez-Sousa JM,
Sanchez-Puelles JM and Munoz A: Aplidin induces apoptosis in human
cancer cells via glutathione depletion and sustained activation of
the epidermal growth factor receptor, Src, JNK, and p38 MAPK. J
Biol Chem. 278:241–250. 2003. View Article : Google Scholar
|
|
19
|
Yu C, Rahmani M, Almenara J, Sausville EA,
Dent P and Grant S: Induction of apoptosis in human leukemia cells
by the tyrosine kinase inhibitor adaphostin proceeds through a
RAF-1/MEK/ ERK- and AKT-dependent process. Oncogene. 23:1364–1376.
2004. View Article : Google Scholar
|
|
20
|
Behera B, Mishra D, Roy B, Devi KS,
Narayan R, Das J, Ghosh SK and Maiti TK: Abrus precatorius
agglutinin-derived peptides induce ROS-dependent mitochondrial
apoptosis through JNK and Akt/P38/P53 pathways in HeLa cells. Chem
Biol Interact. 222C:97–105. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Reddy KB, Nabha SM and Atanaskova N: Role
of MAP kinase in tumor progression and invasion. Cancer Metastasis
Rev. 22:395–403. 2003. View Article : Google Scholar : PubMed/NCBI
|