Introduction
Epithelial cancer cells are surrounded by the tumor
microenvironment, which consists of various non-transformed cells,
soluble factors, signaling molecules and extracellular matrix.
Interactions between tumor cells and their surrounding environment
are critical for tumor growth, invasion, metastasis and therapeutic
resistance (1). Tumors actively
recruit cells, including mesenchymal stem cells (MSCs), and these
cells may play a role in facilitating cancer progression (2).
MSCs are non-hematopoietic stem cells found in
several different tissues, including umbilical cord blood,
placenta, adipose tissue and bone marrow. They have an innate
ability to self-renew and differentiate into multi-lineages,
including osteoblasts, chondrocytes, myocytes and adipocytes
(3–7). Recent studies have shown that MSCs
have the unique ability to travel to injured tissue and actively
participate in tissue repair (8,9).
Interactions between MSCs and breast cancer cells
were reported to modify distinct proliferative and morphological
cancer cell changes (10,11). More recently, an important study
reported the interactions between cancer cells and hMSCs in tumor
progression and metastasis by demonstrating that ovarian
cancer-derived exosomes contributed to the generation of
tumor-associated myofibroblasts from MSCs in tumor stroma (12).
Cancer cell invasion and metastasis is often
facilitated by transdifferentiation through the
epithelial-to-mesenchymal transition (EMT) (13). The EMT endows cancer cells with
migratory, invasive and stem cell properties. At the molecular
level, EMT is defined by downregulation of the epithelial
differentiation marker E-cadherin and upregulation of mesenchymal
markers such as Snail, N-cadherin, Twist, vimentin and
β-catenin.
One of the main inducers of EMT in various cancers
is TGF-β, which has been described to induce EMT in ovarian cancer
(14). The intact TGF-β signaling
pathway appears to be necessary for metastatic phenotypes in an
endometrial cancer model (15). In
cancer, TGF-β exerts the tumor promoter by enhancing cell invasion
and metastasis in breast cancer (16). In addition to TGF-β, the production
of growth factors, cytokines, chemokines, matrix-degrading enzymes
and immunomodulatory mechanisms by MSCs augment tumor progression
by providing a suitable environment (17).
EMT studies regarding the interactions between MSCs
and tumor cells are insufficient for gynecologic cancer. In the
present study, we investigate the growth pattern and potent inducer
of EMT in gynecologic cancer cell lines (SKOV-3, IGROV-1 and
Ishikawa) after co-culture with human MSCs (amnion, deciduas and
bone marrow-derived). The co-cultured media were analyzed for 36
cytokines (C5a, CD40 ligand, G-CSF, GM-CSF, GROα, I-309, sICAM-1,
IFN-γ, IL-1α, IL-1β, IL-1ra, IL-2, IL-4, IL-5, IL-6, IL-8, IL-10,
IL-12p70, IL-13, IL-16, IL-17, IL-17E, IL-23, IL-27, IL-32α, IP-10,
I-TAC, MCP-1, MIF, MIP-1α, MIP-1β, serpin E1, RANTES, SDF-1, TNFα
and sTREM-1) to identify which expressed more cytokine than a
monoculture of the cancer cell line. By using specific cytokines,
we report whether a cytokine acts as a potent inducer of EMT using
differentiation markers and morphologic changes. In addition, we
analyze matrix metalloproteinases (MMP-2 and MMP-9) using western
blot analysis. MMPs are well known as extracellular matrix
degrading enzymes, and their activity is associated with tumor
invasiveness and the migratory capacity of ovarian cancer cells
(18). We also performed Matrigel
invasion assay and wound healing assay for cell migration and
invasion, respectively.
Materials and methods
Culture of cancer cell lines
The human ovarian cancer cell lines SKOV-3 and
IGROV-1 were cultured in RPMI-1640 supplemented with heat
inactivated 10% fetal bovine serum (FBS). The human endometrial
cancer cell line Ishikawa was cultured in minimum essential medium
(MEM) supplemented with 5% FBS. All cells in the first passage were
obtained from the American Type Culture Collection (ATCC; Manassas,
VA, USA) and cultured in an incubator at 37°C, 5%
CO2.
Isolation and culture of human
mesenchymal stem cells (hMSCs)
The human mesenchymal stem cells (hMSCs) used
originated from amnion, decidua and bone marrow. Bone marrow
derived hMSCs in the first passage were obtained from the
Department of Orthopedic Surgery of Guro Hospital. Placentas were
obtained from clinically normal pregnant patients after vaginal or
caesarean deliveries. The Institutional Review Board of Korea
University Guro Hospital approved the procedure.
The amnion and decidua were mechanically peeled from
the placenta and washed with phosphate-buffered saline (PBS)
several times to remove blood. To collect mononuclear cells, the
tissues were incubated with 1 mg/ml type I collagenase for 3 h.
After centrifugation, cells were washed with PBS and resuspended in
α-minimum essential medium (α-MEM; Invitrogen, Grand Island, NY,
USA) supplemented with 10% FBS and 10 ng/ml basic fibroblast growth
factor (bFGF). The cells were seeded into a T75 flask and cultures
were maintained at 37°C in a humidified atmosphere with 5%
CO2. The medium was replaced twice a week.
Direct co-culture
Primary hMSCs (5×104
cells/cm2) were seeded and allowed to adhere overnight.
Cancer cell lines were then seeded at a density of 5×104
cells/cm2 onto the monolayer of hMSCs. All cell types
were cultured individually in parallel as controls. Cells were
maintained in Dulbecco's modified Eagle's medium (DMEM;
Invitrogen). After 7 days, the media was harvested and epithelial
cells were retrieved as described below.
Human cytokine array
A cytokine array was performed in each conditioned
medium after 3–4 days of culture (cancer cell line monoculture and
co-culture with hMSCs). Human cytokine array panel A (R&D
Systems, Minneapolis, MN, USA) was used according to the
manufacturer's instructions and analyzed 36 cytokines at a time.
The 36 cytokines included C5a, CD40 ligand, G-CSF, GM-CSF, GROα,
I-309, sICAM-1, IFN-γ, IL-1α, IL-1β, IL-1ra, IL-2, IL-4, IL-5,
IL-6, IL-8, IL-10, IL-12p70, IL-13, IL-16, IL-17, IL-17E, IL-23,
IL-27, IL-32α, IP-10, I-TAC, MCP-1, MIF, MIP-1α, MIP-1β, serpin E1,
RANTES, SDF-1, TNFα and sTREM-1.
Treatment of TGF-β1 and IL-6
In the human cytokine array, IL-6 was found to
increase in all co-culture groups. For this reason, cancer cells
were treated with IL-6 and EMT markers were checked. In addition,
TGF-β1 was added to each cancer cell line as a positive control.
Each cancer cell line was treated with 10 ng/ml TGF-β1 (Abcam,
Milan, Italy) and 50 ng/ml IL-6 (Invitrogen) separately for 2 days.
For all experiments, cells were cultured to 80% confluency and
serum-starved for 24 h before treatment with TGF-β1 and IL-6. Cell
cultures were maintained at 37°C and 5% CO2.
Total RNA isolation and
reverse-transcriptase reaction
Total RNA extraction and purification were performed
using RNeasy mini kit (Qiagen, Valencia, CA, USA) according to the
manufacturer's protocol. Transcript amplification was performed
using 1 μg/μl of total RNA and reverse transcriptase polymerase
chain reaction using the SuperScript™ III First-Strand Synthesis
System for RT-PCR kit (Invitrogen). The cycling conditions were
25°C for 10 min, 37°C for 120 min and 85°C for 5 min.
Quantitative real-time PCR analysis
Real-time PCR was used to quantify E-cadherin,
N-cadherin, Snail and Twist. Expression was normalized using the
GAPDH housekeeping gene product as an endogenous reference. The
primers and probes were designed for humans using Primer Express
2.0 (Applied Biosystems, Foster City, CA, USA). E-cadherin,
N-cadherin, Snail and Twist mRNA levels were quantified using
TaqMan Real-Time PCR with an ABI 7300 system (Applied Biosystems).
cDNAs were amplified by PCR using gene-specific probes and primer
pairs as follows: E-cadherin (Assays-on-Demand, Hs01023894_m1;
Applied Biosystems), N-cadherin (Hs00983056_m1), Snail
(Hs00195591_m1) and Twist (Hs00361186_m1). The amplification
conditions were 50°C for 2 min, 95°C for 10 min, 40 cycles of
amplication at 95°C for 15 sec, and 60°C for 1 min. Data are
presented as mean fold changes in gene expression relative to the
control in 3 different experiments.
Western blot analysis
Total cell lysates of TGF-β1 and IL-6 treated and
untreated cancer cell lines were obtained by lysing the cells in
RIPA buffer containing 50 mM Tris-HCl, 150 mM NaCl, 1% Triton
X-100, 0.1% SDS, 1% NaDeoxycholate (pH7.4), and protease inhibitor
cocktail (1 mM phenylmethylsulfonyl fluoride, 10 μg/ml peptasin A,
10 μg/ml aprotinin and 5 μg/ml leupeptin).
Protein concentrations were measured using Bio-Rad
protein assay kits (Bio-Rad Laboratories, Hercules, CA, USA). The
protein lysates were then separated by SDS-PAGE and transferred
onto nitrocellulose membrane (Hybond™-P; Amersham Biosciences,
Piscataway, NJ, USA). After blocking with PBS containing 0.2%
Tween-20 and 5% non-fat dry milk at 4°C overnight, membranes were
incubated with the primary antibodies anti-E-cadherin (BD
Biosciences, San Diego, CA, USA), anti-N-cadherin (Abcam,
Cambridge, UK), anti-Snail (Abcam), anti-twist (Abcam), anti-MMP-2
(Cell Signaling Technology, Beverly, MA, USA) and anti-MMP-9 (Cell
Signaling Technology). Protein detection was done with a
chemiluminescence detection system (Pierce Chemical Co., Rockford,
IL, USA).
Immunofluorescence
TGF-β1 and IL-6 treated and untreated cancer cell
lines were fixed by treatment with 3.7% paraformaldehyde,
permeabilized with 0.5% Triton X-100 for 10 min, and blocked with
3% bovine serum albumin for 30 min at room temperature. After
blocking, cells were incubated overnight at 4°C with primary
antibodies, washed with PBS, incubated for 30 min at room
temperature with Alexa Fluor-594 or -488-conjugated secondary
antibody (Molecular Probes, Eugene, OR, USA), and co-stained with 2
μM 4′6-diamidino-2-phenylindole (DAPI) fluorescence (Molecular
Probes) at 37°C. After washing three times with PBS, the slides
were mounted with Vectashield mounting medium (Vector Laboratories,
Burlingame, CA, USA) and observed under confocal microscopy (LSM
700; Zeiss, Wetzlar, Germany).
Wound-healing assay
Cells were seeded at 1×105 cells/well in
12-well plates and preincubated for 24 h in serum-free RPMI
(Invitrogen). A monolayer of cells at 90% confluence wounded with a
plastic tip was allowed to close the wound in culture medium that
was untreated, or treated with TGF-β1 and IL-6. Cell migration into
the wound surface was then monitored by microscopy after 24 h and
reported as the estimated ratio of remaining wounded area relative
to the initial wound area. Quantitation of monolayer closure was
performed using the NIH Imaging program, and results are expressed
as the percentage of wound closure. This assay was repeated three
times independently.
Matrigel invasion assay
Cells (1×105/well) were seeded in the
upper chamber, which was coated with Matrigel (Calbiochem, La
Jolla, CA, USA) and serum-free medium containing different
concentrations of the drugs was added to the lower chamber. After
48 h of incubation, non-migration cells were removed from the upper
chamber with a cotton swab and the cells present on the lower
surface of the insert were stained with Diff-Quick stain (Bio
Chemical Sciences Inc. Science, Swedesboro, NJ, USA). Invading
cells were then measured by microscopy. All experiments were
repeated three times to confirm results.
Statistical analyses
A Student's t-test was performed to determine
statistically significant differences between groups, and a P-value
<0.05 was considered significant.
Results
Co-culture with hMSCs alters the
proliferation of gynecologic cancer cells
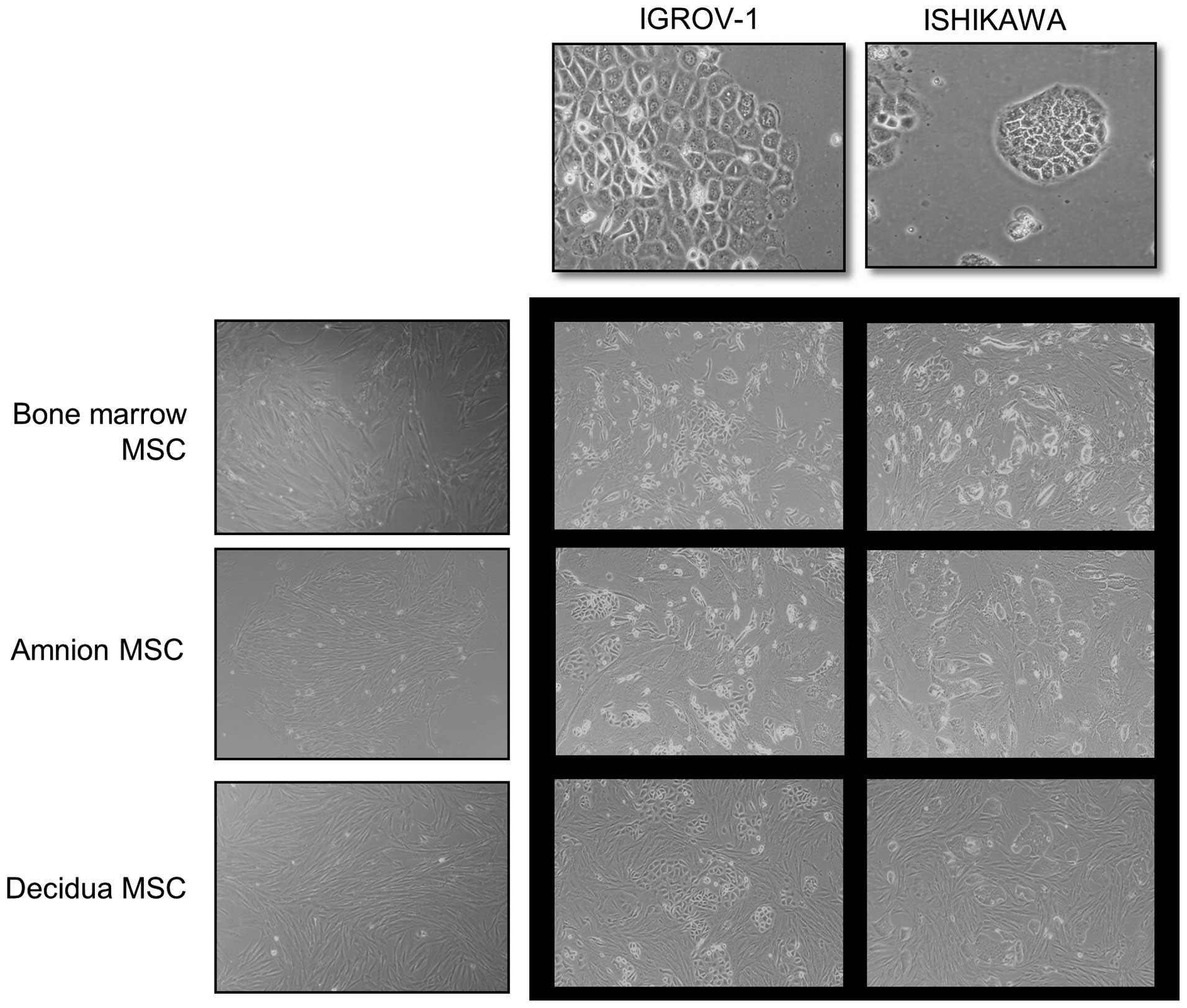
Gynecologic cancer cell line cultures grew rapidly
into cohesive monolayers on a plastic substratum. The cancer cells
had typical cobblestone-like epithelial morphology. Amnion, decidua
and bone marrow derived hMSCs formed a monolayer of homogeneous
bipolar spindle-like cells with a whirlpool-like array. To
investigate interactions between hMSCs and cancer cells, cancer
cells were directly co-cultured with hMSCs (Fig. 1). IGROV-1 and Ishikawa in
co-cultures were compared with IGROV-1 and Ishikawa in
monocultures. Cancer cells in co-cultures with hMSCs had altered
morphology and growth patterns in response to hMSCs. The
cobblestone shaped cancer cell colony dissociated and a
spindle-like, fibroblastic appearance took its place. The change
was contact-dependent.
Cytokine expression in each
co-cultivation
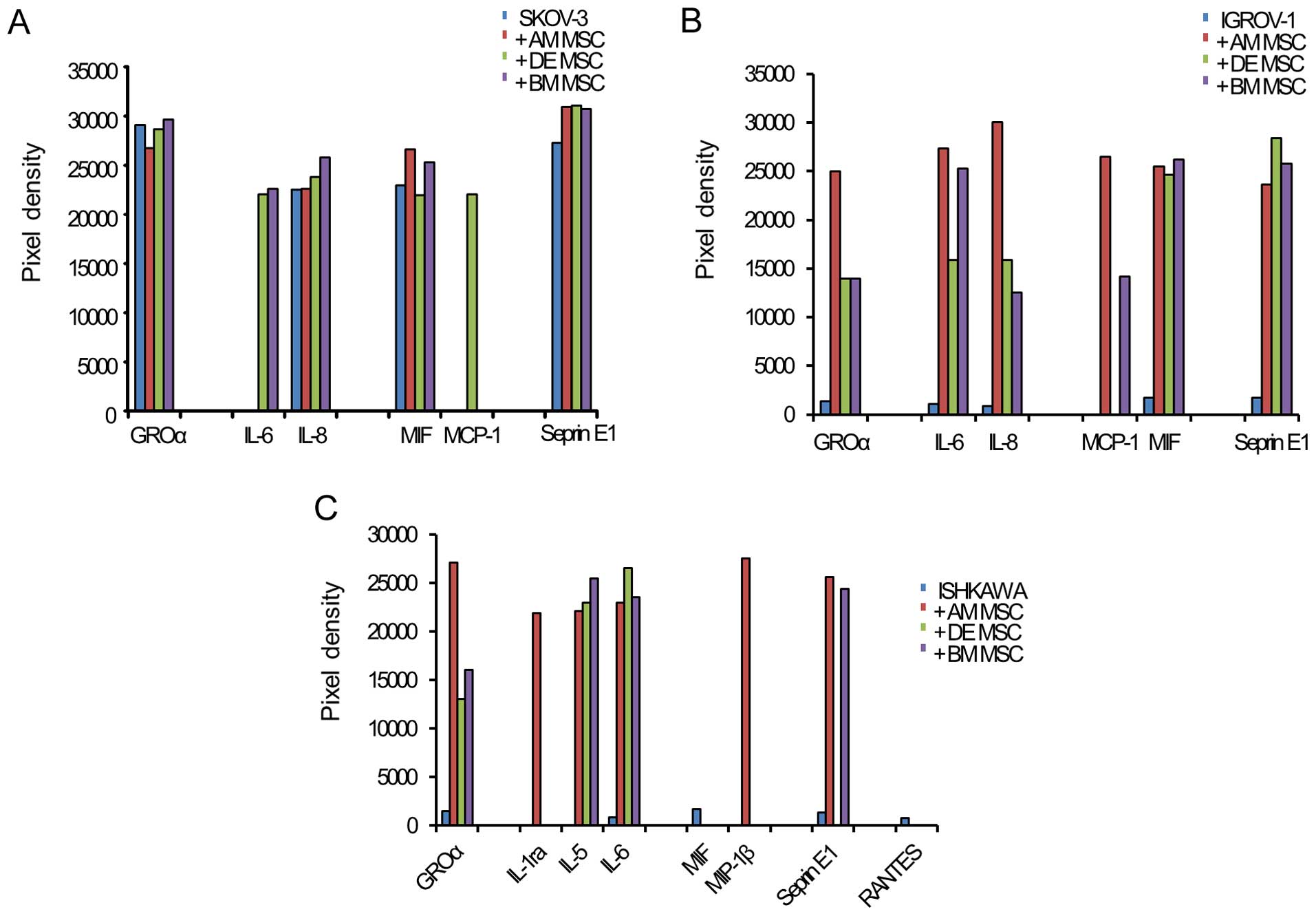
After analyzing for 36 cytokines, a distinction was
found in the cytokine array between cancer cell monoculture and
cancer cells cocultured with hMSCs. With each hMSC, SKOV-3 secreted
GROα (CXCL1), IL-6, IL-8 (CXCL8), MIF (GIF, DER6), MCP-1 (CCL2) and
Serpin E1 (PAI-1). With each hMSC, IGROV-1 secreted GROα (CXCL1),
IL-6, IL-8 (CXCL8), MIF (GIF and DER6), MCP-1 (CCL2) and serpin E1
(PAI-1). With each hMSC, Ishikawa secreted GROα (CXCL1), IL-1ra,
IL-5, IL-6, MIF (GIF, DER6), MIP-1β, serpinE1 and RANTES. In all
cancer cell lines with hMSCs, only IL-6 increased more
significantly than in monoculture (Fig. 2). This result shows that IL-6 was
an inducer of EMT in the interactions of hMSCs and cancer
cells.
TGF-β1 and IL-6 treatment induces
mesenchymal morphologic changes associated with EMT
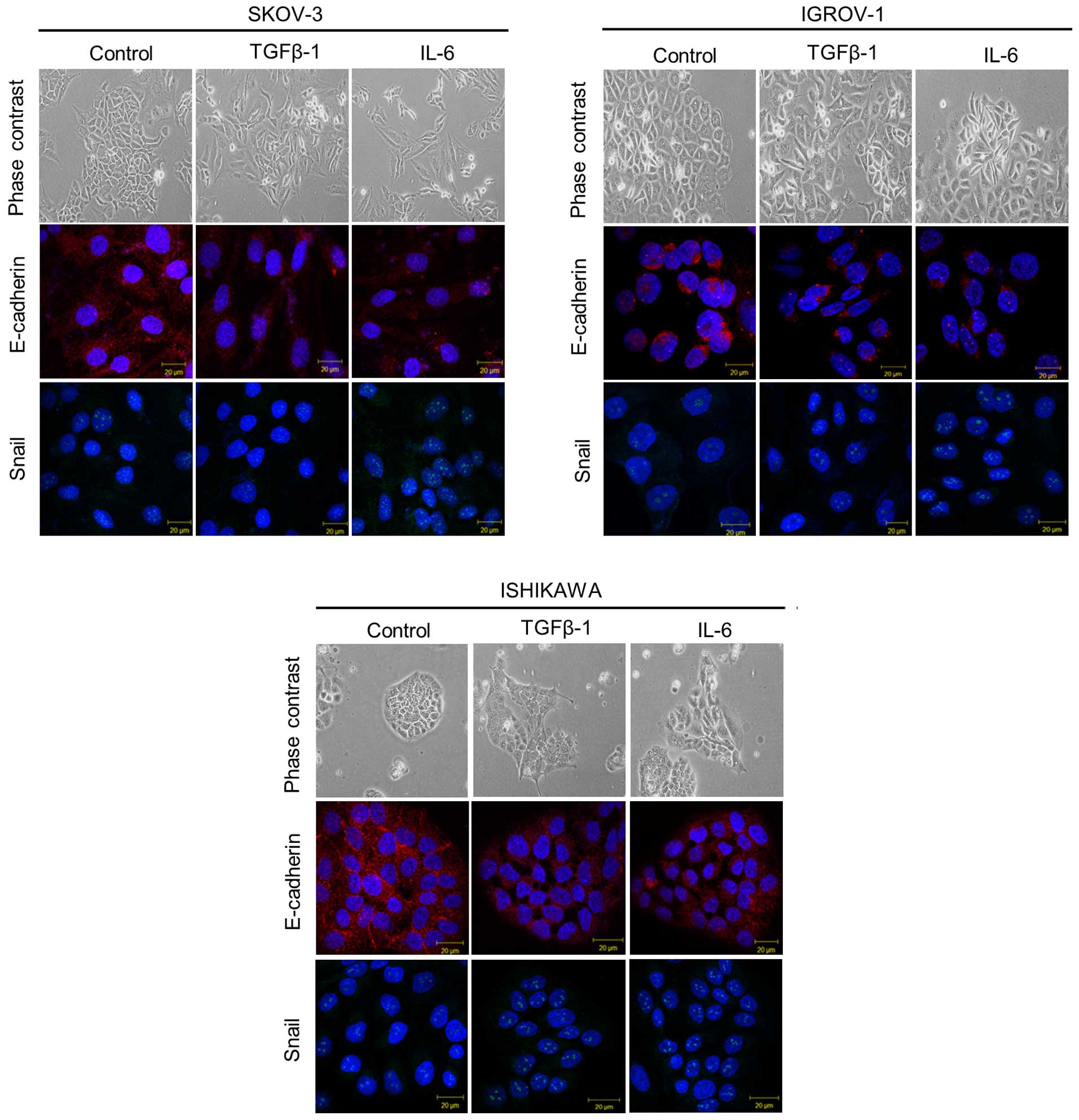
To investigate the role of IL-6 in promoting EMT,
cancer cell lines were cultured with IL-6. In ddition, TGF-β1 was
added to each cancer cell line as a positive control. When TGF-β1
was added to each cancer cell type, cell dissociation and
fibroblastic morphologic changes were observed, as shown in EMT. A
similar morphologic change occurred when IL-6 was added. To study
whether the shift in morphology occurred as a consequence of EMT,
assays were performed by immunofluorescence for epithelial and
mesenchymal markers. As shown in Fig.
3, untreated cancer cells strongly stained for the epithelial
marker E-cadherin on the cell surface, while few stained for the
mesenchymal marker Snail in the nucleus. In contrast, IL-6 and
TGF-β1 treated cancer cells had significantly increased Snail
expression and decreased E-cadherin expression.
To determine whether changes in gene expression were
detected at the protein level, western blot analysis was performed
(Fig. 4A). In SKOV-3 cultured with
TGF-β1, E-cadherin expression decreased while N-cadherin and Snail
expression increased. When IL-6 was added to SKOV-3, similar
changes in E-cadherin, N-cadherin and Snail levels were observed.
Decreased E-cadherin and increased Snail were also observed in each
TGF-β1 and IL-6 treated IGROV-1. In Ishikawa, Snail increased while
E-cadherin decreased in both treated groups.
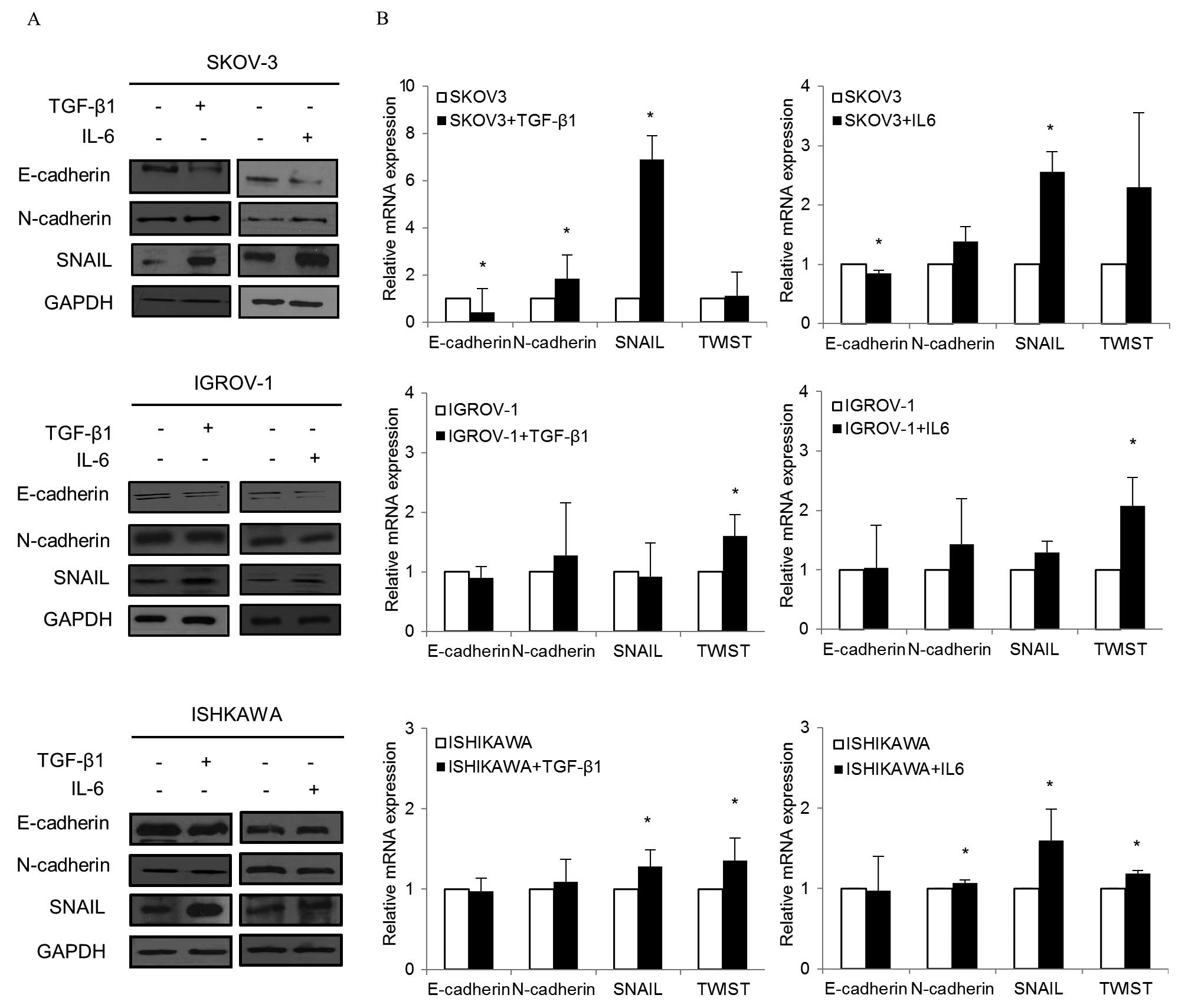 | Figure 4EMT markers after TGF-β1 and IL-6
treatment. (A) Cells treated with TGF-β1 (10 ng/ml) and IL-6 (50
ng/ml) for 48–72 h. Western blotting was performed with
anti-E-cadherin, anti-N-cadherin or anti-Snail. In SKOV-3 cultured
with TGF-β1, E-cadherin expression decreased while N-cadherin and
Snail expression increased noticeably. When IL-6 was added to
SKOV-3, similar changes in E-cadherin, N-cadherin and Snail levels
were observed. Decreased E-cadherin and increased Snail were also
observed in TGF-β1 and IL-6 treated IGROV-1. Snail increased in
Ishikawa while E-cadherin decreased in both treated groups. (B)
Cells treated with TGF-β1 and IL-6 for 48 h were collected, and RNA
was subjected to quantitative real-time PCR using specific primers
for E-cadherin, N-cadherin and Snail. Relative mRNA levels were
normalized to corresponding GAPDH mRNA expression. Bars represent
the standard deviation of three independent experiments conducted
in triplicate. When TGF-β1 was added to SKOV-3, E-cadherin
decreased while N-cadherin and Snail significantly increased. For
IL-6, significant decreases in E-cadherin and increases in Snail
were noted. In IGROV-1, a significant increase in Twist was
observed in both the TGF-β1 and IL-6 treatment groups. When TGF-β1
was added to Ishikawa, significant increases in Snail and Twist
were found. Similarly, significant increases in N-cadherin, Snail,
and Twist were noted in Ishikawa with IL-6. *P<0.05,
compared to cells treated and control. |
In quantitative real-time PCR, there were
significant changes in EMT markers in both TGF-β1 and IL-6
treatment groups (Fig. 4B). When
TGF-β1 was added to SKOV-3, E-cadherin decreased while mesenchymal
markers such as N-cadherin and Snail significantly increased. With
IL-6, significant decreases in E-cadherin and increases in Snail
were noted. In IGROV-1, a significant increase in Twist, a
mesenchymal marker, was observed in both the TGF-β1 and IL-6
treatment groups. When TGF-β1 was added to Ishikawa, significant
increases in Snail and Twist were detected. Similarly, significant
increases in N-cadherin, Snail and Twist were noted in Ishikawa
with IL-6.
TGF-β1 and IL-6 treatment promotes
metastatic potential of gynecologic cancer cells by EMT
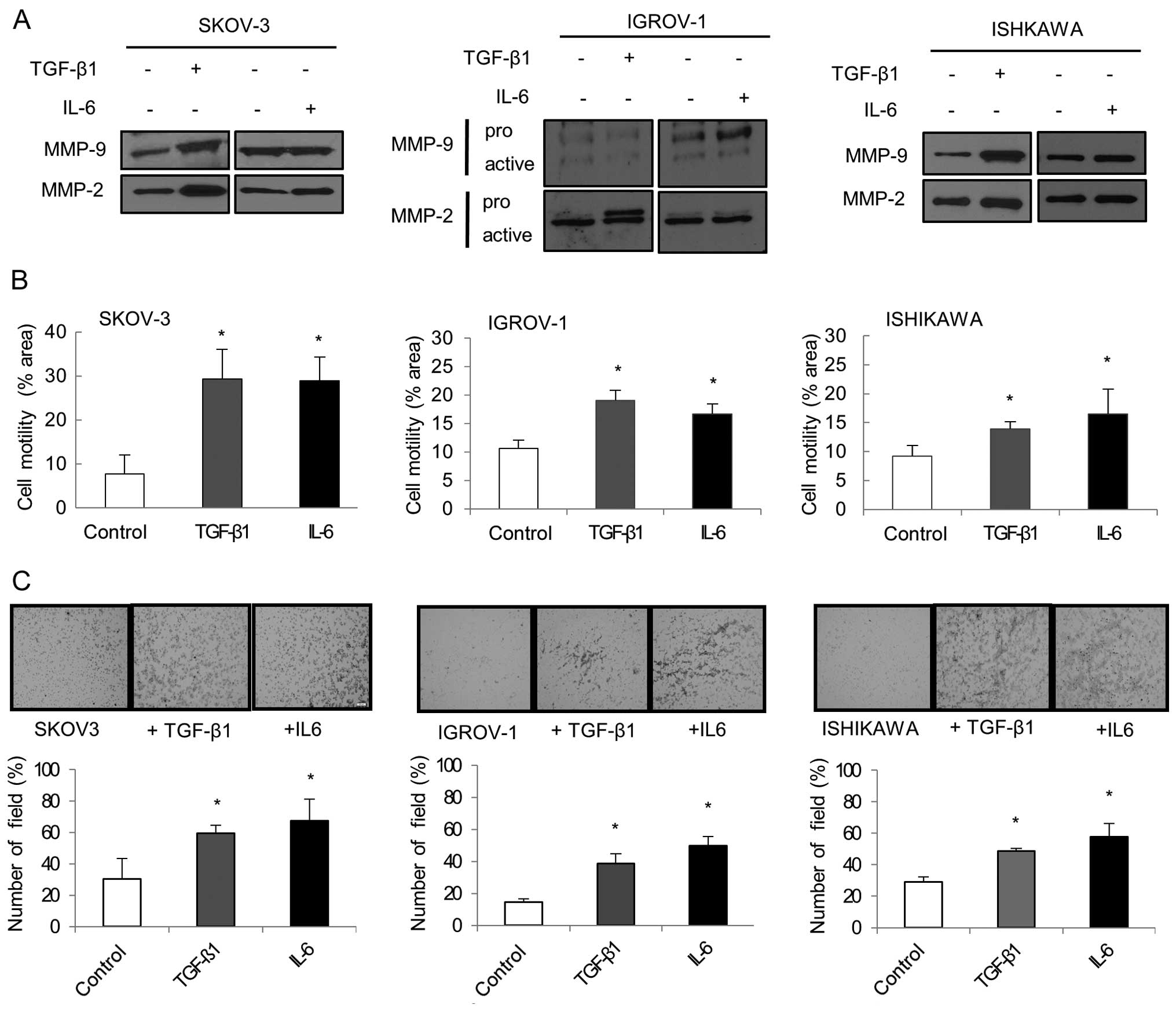
Cell migration and invasion were evaluated by
analyzing the overexpression of MMPs. Western blot analysis showed
that MMP-2 and MMP-9 expression increased in each TGF-β1 and IL-6
treated cell line (Fig. 5A).
Another important aspect of EMT is an increase in cellular
motility. Separate treatment with TGF-β1 and IL-6 significantly
enhanced the migration ability of cancer cells compared to
untreated cells (P<0.05; Fig.
5B). Matrigel invasion assay showed that IL-6 and TGF-β1 were
both effective at increasing cancer cell invasion. IL-6 and TGF-β1
treated cancer cells displayed significantly increased invasiveness
compared to untreated cancer cells (P<0.05; Fig. 5C).
Discussion
Developing tumors recruit MSCs through releasing
endocrine and paracrine signals (19). MSCs are multipotent and have the
ability to preferentially migrate towards the tumor
microenvironment. Several reports have implicated MSCs in promoting
tumor growth and metastasis (13,20,21).
Increasing evidence suggests that dynamic interaction between
cancer cells and their microenvironment supports tumor growth and
metastasis. However, the role of MSCs in gynecologic tumor
microenvironment and the underlying mechanisms remain unclear. The
present study was performed to better understand these
interactions.
Notably, MSCs have been reported to promote breast
cancer metastasis by stimulating EMT (2). EMT is a complex morphogenetic process
in which epithelial cells lose their innate characteristics and
gain new mesenchymal properties. It is an important biological
process responsible for cancer cell invasion and metastasis. In
addition, this morphogenetic process is commonly observed in
gynecologic cancer patients (14,22,23).
In these experiments, we showed that gynecologic
cancer cells co-cultured with hMSCs had altered morphology and
growth patterns in response to hMSCs. The compact cancer cell
colony organization dissociated and transformed into fibroblastic
spindle-shaped appearances. This change was contact-dependent and
similar to that of a breast cancer model (2). MSCs are thought to affect cancer cell
growth and differentiation.
Various signaling pathways that are associated with
cytokines and growth factors in the tumor microenvionment trigger
the EMT process (24). This
happens by activating variable signaling pathways and their target
genes (25). For example TGF-β,
and growth factors such as hepatocyte growth factor (HGF),
epidermal growth factor (EGF), and fibroblast growth factors (FGFs)
can induce EMT in vitro after activating their receptors in
specific cell types (26). The
TGF-β family is one of the best characterized EMT inducers in
cancer pathogenesis. TGF-β is a potent regulator of EMT and cell
stemness in breast cancer stem cells (16).
TGF-β1 and interleukin-like EMT inducer
independently promote an EMT phenotype in mouse mammary epithelial
cells (27). This was confirmed in
our experiment. In the gynecologic cancer cell lines (SKOV-3,
IGROV-1 and Ishikawa), IL-6 increased in co-culture with hMSCs more
than in monoculture. Interactions between hMSCs and cancer cells
released IL-6 into the tumor microenvironment. On the basis of this
result, we focused on IL-6 and further investigated whether IL-6
could be an inducer of EMT. This was done using a number of
specific EMT differentiation markers and morphologic changes.
After each TGF-β1 and IL-6 treatment of gynecologic
cancer cell lines, both groups appeared to have morphological
changes similar to those of mesenchymal cells. The cancer cell
colony dissociated into spindle-shaped cells like those in the EMT
process. We also observed that IL-6 and TGF-β1 treated cancer cells
significantly increased Snail expression and decreased E-cadherin
expression using immunofluorescence. IL-6 seems to mediate the EMT
process by decreasing epithelial markers and increasing mesenchymal
markers.
EMT differentiation markers were also examined after
IL-6 treatment by quantitative RT-PCR and western blot analysis. In
agreement with previous reports on other cancers (27,28),
we showed that IL-6 induced EMT in gynecologic cancer cell lines by
acquiring a mesenchymal morphology with increased expression of
mesenchymal markers. However, there were differences between each
cancer cell line in the expression of EMT markers. SKOV-3 with IL-6
had significantly decreased E-cadherin and increased Snail on
RT-PCR, with decreased E-cadherin and increased Snail and
N-cadherin on western blot analysis. IGROV-1 with IL-6 had
noticeably increased Twist on RT-PCT, with decreased E-cadherin and
increased Snail on western blot analysis. Ishikawa with IL-6 had
significantly increased Snail, N-cadherin and Twist on RT-PCT, with
decreased E-cadherin and increased Snail on western blot
analysis.
Cancer cells underwent EMT in response to IL-6 and
TGF-β1, which enhanced invasion and metastasis. We observed that
IL-6 induces the release of matrix metal-loproteinases (MMPs) from
gynecologic cancer cell lines (SKOV-3, IGROV-1 and Ishikawa).
Matrix metalloproteinases (MMPs) are zinc-dependent endopeptidases
that cleave extracellular matrix (ECM) molecules. MMP-2 and MMP-9
are expressed by epithelial cells, particularly cancer cells, and
can degrade type IV collagen of basement membranes (29). The degree of enzymatic degradation
of basement membrane type IV collagen may correlate with the
metastatic potential and modulating tumor invasion of carcinoma
(30,31). High MMP-2/MMP-9 expression was
associated with metastatic potential in several human
carcinomas.
IL-6 is a pleiotropic cytokine involved in the acute
phase of inflammation (32). The
tumor-promoting action of IL-6 has been shown in experimental
cancer models. In ovarian cancer, IL-6 enhances tumor cell
proliferation and increases resistance to chemotherapy via the
JAK/STAT signaling pathway (33).
IL-6 and its receptor are also overexpressed in malignant ascites
from ovarian cancer and correlate with poor prognosis (28). The endogenous expression of IL-6 by
MSCs increased breast cancer cell growth and metastasis (34). MSCs stimulated tumor growth through
the paracrine production of secreted IL-6. In breast cancer cells
co-cultured with MSCs, paracrine IL-6 was found to be the principal
mediator of STAT3 phosphorylation. STAT3 phosphorylation was lost
when IL-6 was depleted from MSC conditioned media or when the IL-6
receptor was blocked in tumor cells (35).
The present study may confirm that expression of
inter-leukin-6 by interactions between gynecologic cancer cells and
hMSCs induces EMT. Our results suggest that IL-6 plays a critical
role in oncogenic EMT mediated by the interaction of hMSCs and
cancer cells. IL-6 can serve as a key regulator of invasion and
metastasis in gynecologic cancer. Future studies should address the
role of IL-6 in mechanotransduction, which modulates changes in
gene expression to alter gynecologic cancer cell differentiation
and metastatic potential.
Acknowledgements
The present study was supported by the National
Research Foundation of Korea (NRF) grant funded by the Korea
government (MEST) (no. 2010-0021906).
References
|
1
|
Swartz MA, Iida N, Roberts EW, Sangaletti
S, Wong MH, Yull FE, Coussens LM and DeClerck YA: Tumor
microenvironment complexity: Emerging roles in cancer therapy.
Cancer Res. 72:2473–2480. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Martin FT, Dwyer RM, Kelly J, Khan S,
Murphy JM, Curran C, Miller N, Hennessy E, Dockery P, Barry FP, et
al: Potential role of mesenchymal stem cells (MSCs) in the breast
tumour microenvironment: Stimulation of epithelial to mesenchymal
transition (EMT). Breast Cancer Res Treat. 124:317–326. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Lu LL, Liu YJ, Yang SG, Zhao QJ, Wang X,
Gong W, Han ZB, Xu ZS, Lu YX, Liu D, et al: Isolation and
characterization of human umbilical cord mesenchymal stem cells
with hematopoiesis-supportive function and other potentials.
Haematologica. 91:1017–1026. 2006.PubMed/NCBI
|
|
4
|
Prockop DJ, Sekiya I and Colter DC:
Isolation and characterization of rapidly self-renewing stem cells
from cultures of human marrow stromal cells. Cytotherapy.
3:393–396. 2001. View Article : Google Scholar
|
|
5
|
In't Anker PS, Scherjon SA, Kleijburg-van
der Keur C, de Groot-Swings GM, Claas FH, Fibbe WE and Kanhai HH:
Isolation of mesenchymal stem cells of fetal or maternal origin
from human placenta. Stem Cells. 22:1338–1345. 2004. View Article : Google Scholar
|
|
6
|
Pittenger MF, Mackay AM, Beck SC, Jaiswal
RK, Douglas R, Mosca JD, Moorman MA, Simonetti DW, Craig S and
Marshak DR: Multilineage potential of adult human mesenchymal stem
cells. Science. 284:143–147. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Deans RJ and Moseley AB: Mesenchymal stem
cells: Biology and potential clinical uses. Exp Hematol.
28:875–884. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Chen J, Zhang ZG, Li Y, Wang L, Xu YX,
Gautam SC, Lu M, Zhu Z and Chopp M: Intravenous administration of
human bone marrow stromal cells induces angiogenesis in the
ischemic boundary zone after stroke in rats. Circ Res. 92:692–699.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Spaeth E, Klopp A, Dembinski J, Andreeff M
and Marini F: Inflammation and tumor microenvironments: Defining
the migratory itinerary of mesenchymal stem cells. Gene Ther.
15:730–738. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Hombauer H and Minguell JJ: Selective
interactions between epithelial tumour cells and bone marrow
mesenchymal stem cells. Br J Cancer. 82:1290–1296. 2000. View Article : Google Scholar
|
|
11
|
Fierro FA, Sierralta WD, Epuñan MJ and
Minguell JJ: Marrow-derived mesenchymal stem cells: Role in
epithelial tumor cell determination. Clin Exp Metastasis.
21:313–319. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Cho JA, Park H, Lim EH, Kim KH, Choi JS,
Lee JH, Shin JW and Lee KW: Exosomes from ovarian cancer cells
induce adipose tissue-derived mesenchymal stem cells to acquire the
physical and functional characteristics of tumor-supporting
myofibroblasts. Gynecol Oncol. 123:379–386. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Karnoub AE, Dash AB, Vo AP, Sullivan A,
Brooks MW, Bell GW, Richardson AL, Polyak K, Tubo R and Weinberg
RA: Mesenchymal stem cells within tumour stroma promote breast
cancer metastasis. Nature. 449:557–563. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Vergara D, Merlot B, Lucot JP, Collinet P,
Vinatier D, Fournier I and Salzet M: Epithelial-mesenchymal
transition in ovarian cancer. Cancer Lett. 291:59–66. 2010.
View Article : Google Scholar
|
|
15
|
Lei X, Wang L, Yang J and Sun LZ: TGFbeta
signaling supports survival and metastasis of endometrial cancer
cells. Cancer Manag Res. 2009:15–24. 2009.PubMed/NCBI
|
|
16
|
Imamura T, Hikita A and Inoue Y: The roles
of TGF-β signaling in carcinogenesis and breast cancer metastasis.
Breast Cancer. 19:118–124. 2012. View Article : Google Scholar
|
|
17
|
Spaeth EL, Dembinski JL, Sasser AK, Watson
K, Klopp A, Hall B, Andreeff M and Marini F: Mesenchymal stem cell
transition to tumor-associated fibroblasts contributes to
fibrovascular network expansion and tumor progression. PLoS One.
4:e49922009. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Do TV, Kubba LA, Du H, Sturgis CD and
Woodruff TK: Transforming growth factor-beta1, transforming growth
factor-beta2, and transforming growth factor-beta3 enhance ovarian
cancer metastatic potential by inducing a Smad3-dependent
epithelial-to-mesenchymal transition. Mol Cancer Res. 6:695–705.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Stagg J: Mesenchymal stem cells in cancer.
Stem Cell Rev. 4:119–124. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Shinagawa K, Kitadai Y, Tanaka M, Sumida
T, Kodama M, Higashi Y, Tanaka S, Yasui W and Chayama K:
Mesenchymal stem cells enhance growth and metastasis of colon
cancer. Int J Cancer. 127:2323–2333. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Albarenque SM, Zwacka RM and Mohr A: Both
human and mouse mesenchymal stem cells promote breast cancer
metastasis. Stem Cell Res (Amst). 7:163–171. 2011. View Article : Google Scholar
|
|
22
|
Lee MY and Shen MR: Epithelial-mesenchymal
transition in cervical carcinoma. Am J Transl Res. 4:1–13.
2012.PubMed/NCBI
|
|
23
|
Castilla MÁ, Moreno-Bueno G, Romero-Pérez
L, Van De Vijver K, Biscuola M, López-García MÁ, Prat J,
Matías-Guiu X, Cano A, Oliva E, et al: Micro-RNA signature of the
epithelial-mesenchymal transition in endometrial carcinosarcoma. J
Pathol. 223:72–80. 2011. View Article : Google Scholar
|
|
24
|
Francí C, Takkunen M, Dave N, Alameda F,
Gómez S, Rodríguez R, Escrivà M, Montserrat-Sentís B, Baró T,
Garrido M, et al: Expression of Snail protein in tumor-stroma
interface. Oncogene. 25:5134–5144. 2006.PubMed/NCBI
|
|
25
|
Hogan NM, Dwyer RM, Joyce MR and Kerin MJ:
Mesenchymal stem cells in the colorectal tumor microenvironment:
Recent progress and implications. Int J Cancer. 131:1–7. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Morel AP, Lièvre M, Thomas C, Hinkal G,
Ansieau S and Puisieux A: Generation of breast cancer stem cells
through epithelial-mesenchymal transition. PLoS One. 3:e28882008.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Sullivan NJ, Sasser AK, Axel AE, Vesuna F,
Raman V, Ramirez N, Oberyszyn TM and Hall BM: Interleukin-6 induces
an epithelial-mesenchymal transition phenotype in human breast
cancer cells. Oncogene. 28:2940–2947. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Yadav A, Kumar B, Datta J, Teknos TN and
Kumar P: IL-6 promotes head and neck tumor metastasis by inducing
epithelial-mesenchymal transition via the JAK-STAT3-SNAIL signaling
pathway. Mol Cancer Res. 9:1658–1667. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Chen P and Parks WC: Role of matrix
metalloproteinases in epithelial migration. J Cell Biochem.
108:1233–1243. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Zeng ZS, Cohen AM and Guillem JG: Loss of
basement membrane type IV collagen is associated with increased
expression of metalloproteinases 2 and 9 (MMP-2 and MMP-9) during
human colorectal tumorigenesis. Carcinogenesis. 20:749–755. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Thomas GT, Lewis MP and Speight PM: Matrix
metalloproteinases and oral cancer. Oral Oncol. 35:227–233. 1999.
View Article : Google Scholar
|
|
32
|
Kishimoto T: Interleukin-6: Discovery of a
pleiotropic cytokine. Arthritis Res Ther. 8(Suppl 2): S22006.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Duan Z, Foster R, Bell DA, Mahoney J,
Wolak K, Vaidya A, Hampel C, Lee H and Seiden MV: Signal
transducers and activators of transcription 3 pathway activation in
drug-resistant ovarian cancer. Clin Cancer Res. 12:5055–5063. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Shah K: Mesenchymal stem cells engineered
for cancer therapy. Adv Drug Deliv Rev. 64:739–748. 2012.
View Article : Google Scholar :
|
|
35
|
Sasser AK, Sullivan NJ, Studebaker AW,
Hendey LF, Axel AE and Hall BM: Interleukin-6 is a potent growth
factor for ER-alpha-positive human breast cancer. FASEB J.
21:3763–3770. 2007. View Article : Google Scholar : PubMed/NCBI
|