Introduction
The human health and life are under constant serious
threat of cancer. Surgery, radiation, and chemotherapy have been
the most commonly utilized methods for therapy of ovarian cancer.
Recently, chemotherapy has become the predominant mode of
treatment. Platinum drugs, such as cisplatin and its analogues, are
the most frequently used agents for the treatment of human cancer.
Initially, cisplatin responsiveness is high, however, a large
number of cancer patients will relapse with cisplatin-resistant
disease due to many mechanisms including increased detoxification
of the drug, changes in cellular efflux and uptake of the drug,
increased DNA repair and inhibition of apoptosis (1). Moreover, it causes unacceptable rates
of normal cell toxicity and a number of side-effects, such as
nephrotoxicity, neurotoxicity, ototoxicity electrolyte disturbance,
myelotoxicity, hemolytic anemia, nausea and vomiting (2–5).
Therefore, novel therapies and more selective drugs are needed for
the treatment of human cancer (6).
The cell cycle is the series of events that take
place in a cell leading to its division and duplication, resulting
in two daughter cells. It can be divided into three periods:
interphase (G1, S and G2 phases), the mitotic phase (M phase) and
cytokinesis. A dysregulation of cell cycle components may lead to
tumor formation (7), and
investigational anticancer drugs have recently focused on the
molecular targets involved in cell cycle control mechanisms
(8,9).
Apoptosis is a process of programmed cell death
(PCD) that occurs in multicellular organisms. Excessive apoptosis
causes atrophy, whereas defective apoptotic processes result in
uncontrolled cell proliferation, implicated in a wide variety of
diseases such as cancer. It has been reported that a decreased
susceptibility of cancer cells to apoptosis was tightly associated
with drug resistance. Therefore, inducing apoptosis in cancer cells
may be a promising strategy to overcome resistance (6). Many mechanisms are connected to the
failure to develop apoptosis in cancer cells, including the
expression of P-glycoprotein and p53 mutations. Two major pathways
leading to apoptosis, the intrinsic (mitochondrial) and extrinsic
(receptor-mediated) pathways, have been delineated. The Bcl-2
protein family regulates the intrinsic apoptotic pathway by
controlling the permeability of the mitochondrial membrane and the
release of the pro-apoptotic factors (10). Tumor necrosis factor-related
apoptosis-inducing ligand (TRAIL), including Apo2L/TRAIL, regulates
the extrinsic apoptotic pathway by engaging its receptor.
Flavonoids are natural polyphenols present in a
variety of foods, especially fruits and vegetables. The anticancer
activity of flavonoids isolated from plants have been widely
studied. It has been reported that flavonoids display anticancer
characteristics and might be able to prevent oxidation and
inflammation, diminish angiogenesis and cell proliferation, and
induce apoptosis (11). Myricetin
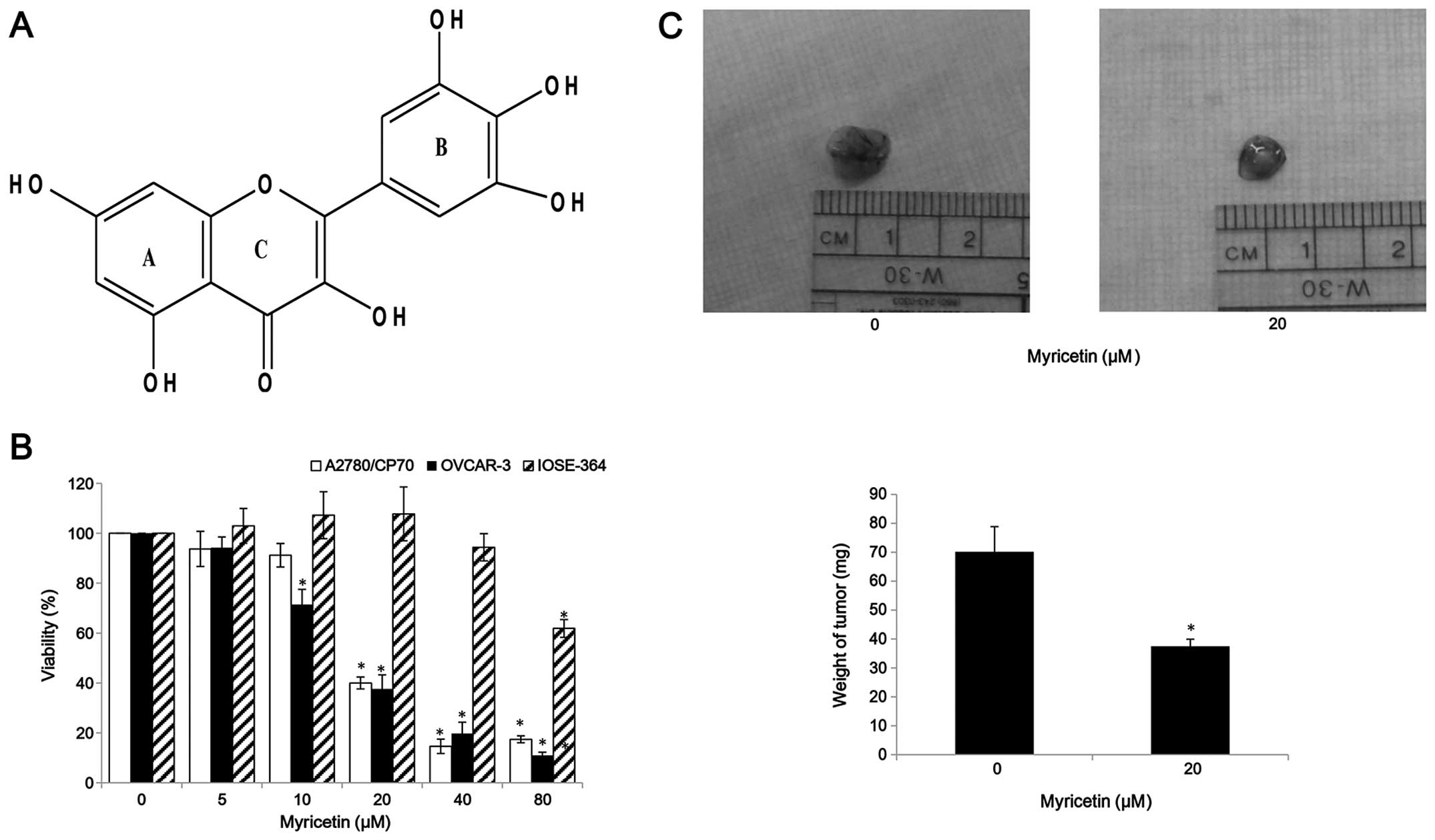
(Fig. 1A) is a member of the
flavonoid class of phenolic compounds with antioxidant properties
(12). It can be found in fruits,
vegetables, nuts, berries, tea and red wine (13,14).
Previous studies have reported myricetin induces apoptosis in
various cancer cells, such as in hepatoma, pancreatic cancer,
esophageal cancer and colon carcinoma cells (15–18).
In this study, we examined the effects of myricetin
apoptosis induction and cell cycle arrest in two platinum-resistant
ovarian cancer cell lines: A2780/CP70 and OVCAR-3. The mechanisms
involved in the effects of myricetin were also investigated.
Materials and methods
Cell culture and reagents
Human ovarian cancer cell lines, OVCAR-3 and
A2780/CP70, were kindly provided by Dr B. Jiang, Department of
Microbiology, Immunology, and Cell Biology, West Virginia
University, Morgantown, WV, USA. IOSE-364, normal ovarian surface
epithelial cells from healthy women, but immortalized with SV40
T/t, were a gift from Dr N. Auersperg at the University of British
Columbia, Canada (19). All cell
lines were maintained in RPMI-1640 medium (Sigma, St. Louis, MO,
USA) supplemented with 10% US-qualified fetal bovine serum
(Invitrogen, Grand Island, NY, USA). All cells were maintained in a
humidified incubator with 5% CO2 at 37°C. Myricetin was
purchased from J&K Chemical Technology (Beijing, China). It was
dissolved in dimethyl sulfoxide (DMSO) to make stock solutions of
100 mM, and equal amounts of DMSO were included in controls for
every experiment. The primary antibodies against caspase-3, -7, -8,
and -9, Bax, Bcl-2, DR5, Puma, FADD, and p21 were purchased from
Cell Signaling Technology, Inc. (Danvers, MA, USA). The primary
antibodies against p53, cmyc, Bcl-xl and GAPDH were purchased from
Santa Cruz Biotechnology Inc. (Santa Cruz, CA, USA).
Cell viability
Cell growth inhibition was determined by measuring
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT)
dye absorbance. The cells (1×104) were seeded into
96-well, incubated at 37°C. Cells were incubated overnight for
attaching to the bottom and then treated with different
concentrations of myricetin (5–80 μM) or DMSO (as vehicle). After
24 h, the medium was removed, and 100 μl MTT (1 mg/ml) were added
to each well and then incubated at 37°C for 4 h in the dark. After
removing the supernatant, the formed formazan crystals were
dissolved in 200 μl DMSO, and absorbance was measured at 570
nm.
Chicken chorioallantoic membrane
assay
Specific pathogen-free fertile chicken eggs (Charles
River Laboratories, North Franklin, CT, USA) were slowly turned by
an automatic egg turner (G.Q.F. Manufacturing Co., Savannah, GA,
USA) and incubated at 37.5°C. The cells (1.2×106) were
suspended in 20 μl FBS-free medium, mixed with 80 μl of Matrigel
(BD Bioscience) and 0- or 20-μM myricetin, incubated on an
autoclaved silicone mat for 30 min for gelation, and implanted into
the chorioallantoic membrane (CAM) of a 9-day-old chicken embryo.
After incubating another 5 days, the tumor implants were measured
for tumor weight.
Flow cytometry analysis of cell
apoptosis
The apoptosis effects of myricetin on ovarian cells
were determined by Alexa Fluor 488 Annexin V/Dead Cell Apoptosis
kit from Invitrogen. A2780/CP70, OVCAR-3 and IOSE-364 cells
(106/dish) treated with myricetin or DMSO for 24 h were
washed with cold PBS twice and re-suspended in binding buffer. An
aliquot of 100 μl of the cell solution (1×105 cells) was
transferred to a 5-ml tissue culture tube. Subsequently, 5 μl of
FITC Annexin V and 1 μl propidium iodide (PI) were added to the
cells. The cells were gently vortexed and incubated for 15 min at
room temperature in the dark. The next step involved the addition
of 400 μl of 1× binding buffer to each tube. The samples were
analyzed by flow cytometry (FACSCalibur system, BD Biosciences, San
Jose, CA, USA).
Apoptosis assessment by Hoechst 33342
staining
OVCAR-3 cells (106/dish) treated with
various concentrations of myricetin and pifithrin-α (PFT-α) (Sigma)
for 24 h were washed with cold PBS and stained with 10 μg/ml
Hoechst 33342 (Sigma) in PBS for 10 min in the dark at 37°C. Cell
apoptosis was examined under a fluorescence microscope (Zeiss).
Flow cytometry analysis of the cell
cycle
Cells treated with various concentrations of
myricetin for 24 h were digested by trypsin, collected by 1,000 rpm
centrifugation for 10 min, and then washed with cold PBS. The cell
pellets were suspended with 70% ethanol, stored at −20°C. After
centrifugation at 1,000 rpm for 6 min, the cell pellets were
re-suspended in PBS, collected by centrifugation, and incubated
with 180 μg/ml RNase A at 37°C for 15 min. Flow cytometry
(FACSCalibur system, BD Biosciences) was used for detection after
50 μg/ml propidium iodide (final concentration) was added to cell
pellets for 15-min staining. Data were plotted and analyzed by
using FCS software (De Novo Software, Los Angeles, CA, USA).
Caspase-3/7 and caspase-9 assay
A2780/CP70 and OVCAR-3 cells were seeded into
96-well plates (1×104/well) and incubated overnight at
37°C. Cells were treated with different concentrations of myricetin
(5–20 μM) or DMSO for 4 h. After treatment, Caspase-Glo 3/7 or
Caspase-Glo 9 Assay kit (Promega) was used to detect the
caspase-3/7 or caspase-9 enzymatic activities in both ovarian
cancer cell lines. Enzymatic activities were normalized by total
protein levels and were expressed as a percentage of the untreated
control.
Western blotting
Ovarian cancer cells were seeded in 60-mm dishes at
the concentration of 106/dish, incubated overnight, and
then treated with various concentrations of myricetin for 24 h. The
cells were lysed in 100 μl mammalian protein extraction reagent
including 1 μl Halt protease, 1 μl phosphatase inhibitor, and 2 μl
EDTA (M-PER, Pierce, Rockford, IL, USA). Total protein levels were
determined by a BCA Protein Assay kit (Pierce). The cell lysates
were separated by SDS-PAGE and blotted onto a nitrocellulose
membrane with a Mini-Protean 3 system (Bio-Rad, Hercules, CA, USA).
The membranes were blocked in 5% non-fat milk (dissolved in
Tris-buffer saline containing 0.1% Tween-20) for 1 h at room
temperature. The membranes were incubated with the primary
antibodies and secondary antibody dilutions, washed with TBST. The
antigen-antibody complex was visualized with the SuperSignal West
Dura Extended Duration Substrate (Pierce).
Transfection with small interfering RNA
(siRNA)
OVCAR-3 cells were seeded in 60-mm dishes at
5×105/dish and incubated overnight before transfection
with p53 siRNA, p21 siRNA or control siRNA (Santa Cruz) using
jetPRIME™ DNA and siRNA transfection reagent (VWR International,
Radnor, PA, USA) according to the manufacturer's protocol. After 24
h, cells were treated with myricetin or DMSO. Cell lysates were
collected for western blotting to test Bad, Bax, Bcl-xl, cmyc, p53
and p21 proteins.
Statistical analysis
The experiments were performed at least three times.
Results were expressed as mean ± standard error of mean (SEM) using
Microsoft Excel (2007). SPSS (Version 18.0 for Windows) was used to
perform statistical assessment. The results were analyzed using
post hoc test (2-sided Dunnett's test) and one-way analysis of
variance (ANOVA) to test differences between each treatment and
control. A p-value of <0.05 was considered statistically
significant.
Results
Effect of myricetin on ovarian cancer
cell proliferation
MTT assay was performed after treatment of ovarian
cancer and normal ovarian cells with myricetin to investigate the
effect of myricetin on the viability of platinum-resistant ovarian
cancer cells. We observed that compared with controls (myricetin 0
μM), myricetin significantly reduced the viability of A2780/CP70
and OVCAR-3. Cell viability with myricetin treatment (5–80 μM) for
24 h ranged from 93.8 to 17.4% (p<0.05) for A2780/CP70 cells
(Fig. 1B). Similarly, myricetin
also reduced viability of OVCAR-3 cells at concentrations of 10 μM
myricetin and above. Cell viability was significantly decreased
from 71.5% at a concentration of 10 μM myricetin to 12.5% at a
concentration of 80 μM myricetin (p<0.05) (Fig. 1B). We also examined the
growth-inhibitory activity of myricetin on IOSE-364 normal ovarian
cells (Fig. 1B). Myricetin had
higher level of cytotoxicity in ovarian cancer cells A2780/CP70 and
OVCAR-3 than on normal ovarian cells IOSE-364, indicating that
cisplatin-resistant ovarian cancer cells were more sensitive to
myricetin than normal ovarian cells. In CAM models, myricetin
reduced typical tumor growth of OVCAR-3 cells. The tumors treated
with 20 μM myricetin were smaller and lighter than corresponding
controls (Fig. 1C).
Myricetin induces apoptosis in A2780/CP70
and OVCAR-3 cells
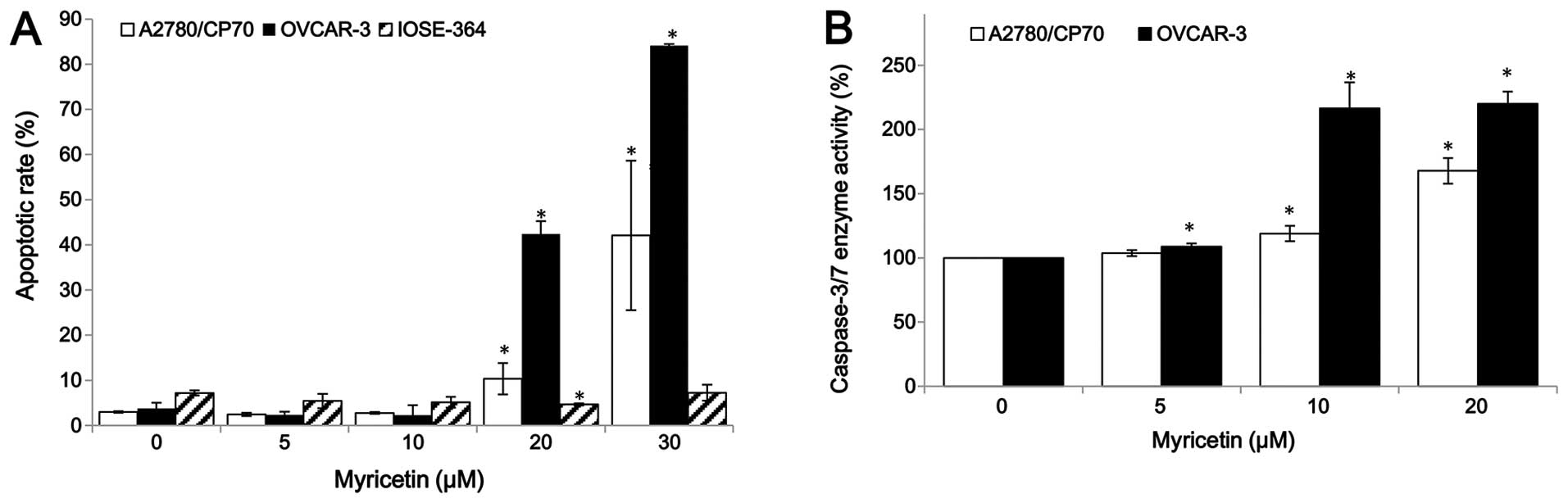
For the purpose of investigating whether myricetin
inhibited cell growth by inducing apoptosis, the apoptosis rates of
ovarian cancer cell lines A2780/CP70, OVCAR-3, and normal ovarian
cells IOSE-364 were treated with myricetin (5–30 μM) for 24 h, and
analyzed by flow cytometry after Annexin V and propidium iodide
(PI) staining. As shown in Fig.
2A, myricetin significantly induced apoptosis in the two
ovarian cancer cells, especially in OVCAR-3, but it did not induce
apoptosis in the normal ovarian cells IOSE-364. For A2780/CP70 and
OVCAR-3, the apoptosis rates were 3.0 and 3.8% when not treated
with myricetin, which was increased to the maximum apoptosis rates
of 42.1 and 84.2% at the concentration of 30 μM myricetin. However,
the treatment of myricetin did not increase the IOSE-364 cell
apoptosis rate (Fig. 2A).
Caspase-Glo 3/7 Assay kit was used to confirm that myricetin
induces apoptosis in ovarian cancer cells. As shown in Fig. 2B, compared to controls, the
caspase-3/7 enzymatic activities were maximally increased to 1.68-
and 2.20-fold in A2780/CP70 and OVCAR-3 when treated with 20 μM
myricetin for 4 h. These results indicate that myricetin inhibited
cell growth of ovarian cancer cells at least partly through the
induction of apoptosis.
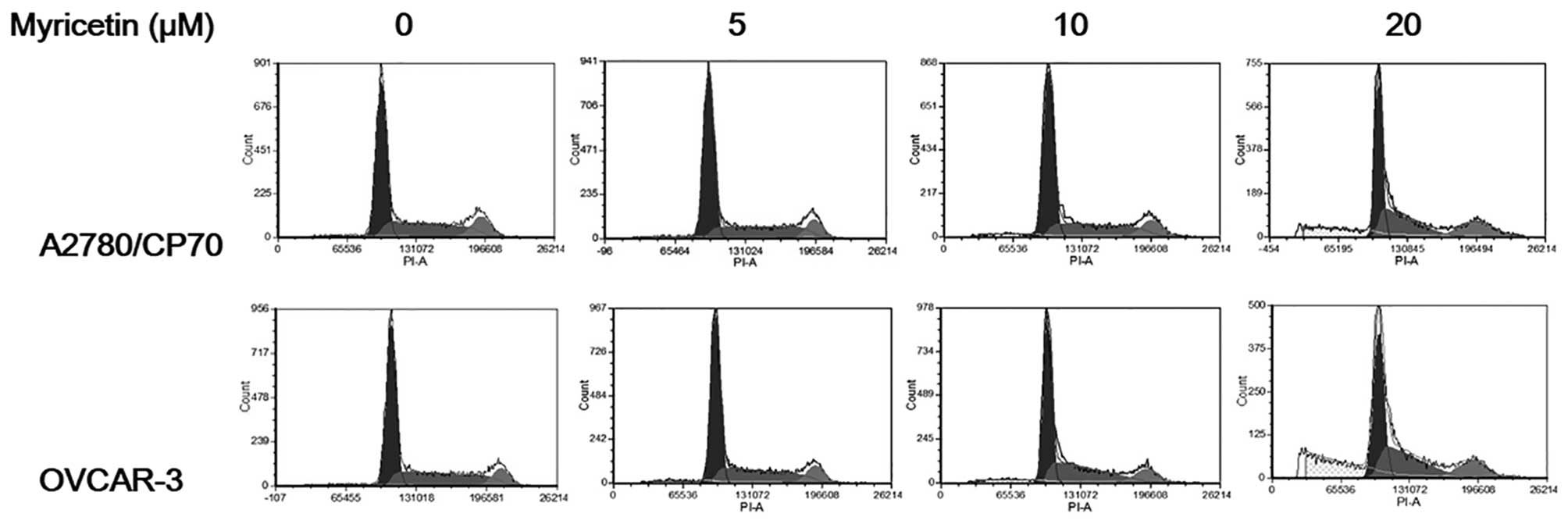
Myricetin does not induce cell cycle
arrest in A2780/CP70 and OVCAR-3 cells
To investigate whether the growth-inhibitory effect
of myricetin on ovarian cancer cells was caused by cell cycle
arrest, cell cycle phase distribution of cells treated with
myricetin (5–20 μM) for 24 h was analyzed by flow cytometry after
PI staining. As shown in Fig. 3,
myricetin had little effect on inducing cell cycle arrest in
A2780/CP70 and OVCAR-3 cells. These results indicate that myricetin
inhibited ovarian cancer cell growth through a mechanism separate
from cell cycle arrest.
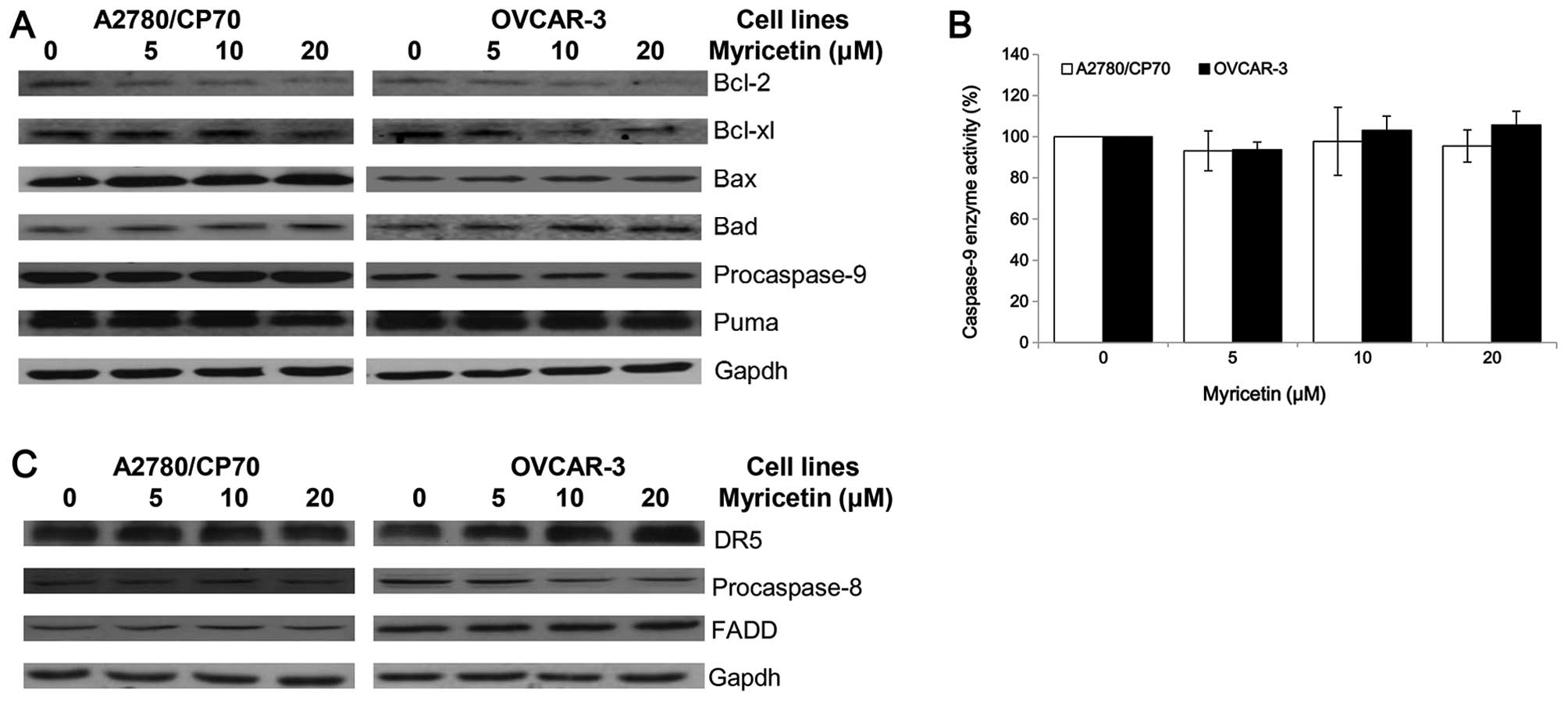
Effect of myricetin on the intrinsic and
extrinsic apoptotic pathway
The intrinsic apoptotic pathway and extrinsic
apoptotic pathway are the two main pathways which induce apoptosis
in cancer cells. An investigation was conducted to determine
whether myricetin induces apoptosis through the intrinsic pathway
in ovarian cancer cell lines A2780/CP70 and OVCAR-3. The expression
of Puma, Bcl-2, Bcl-xl, Bax, Bad and caspase-9 proteins were
detected by western blotting. As shown in Fig. 4A, the expression of Bax and Bad
proteins was increased, while the Bcl-2 and Bcl-xl protein levels
were decreased when treated with myricetin. However, the
procaspase-9 and Puma protein levels were not changed by treatment
with myricetin. The cleaved caspase-9 protein was not detected,
suggesting that it may be degraded after activation from
procaspase-9. Caspase-Glo 9 Assay kit was used to detect changes in
caspase-9 enzymatic activities when treated with myricetin. The
test was found to be consistent with the western blotting result,
which shows that myricetin had no effect on the caspase-9 enzymatic
activities of A2780/CP70 and OVCAR-3 cells (Fig. 4B). These results indicate that
myricetin might induce the intrinsic pathway in A2780/CP70 and
OVCAR-3 cells through Bcl-2 family proteins.
Next, the role of the extrinsic pathway in
myricetin-induced apoptosis was determined. Myricetin increased the
expression of matured DR5 and decreased the levels of procaspase-8
in OVCAR-3 cells but not A2780/CP70 cells (Fig. 4C). FADD protein levels were not
changed in both ovarian cancer cell lines treated with myricetin.
Myricetin might induce the extrinsic pathway in OVCAR-3 cells
through a DR5 and caspase-8 dependent pathway. However, the
induction of apoptosis in A2780/CP70 cells might be through an
extrinsic-independent pathway.
Role of p53 in the myricetin-induced
apoptosis in OVCAR-3 cells
P53, a tumor suppressor protein, is involved in
several cellular outcomes such as apoptosis and angiogenesis
(20). Therefore, we examined some
proteins which are associated with p53 to clarify whether myricetin
inhibits viability in A2780/CP70 and OVCAR-3 cells via the p53
protein (Fig. 5A). We found that
myricetin upregulated expression of the p53 and p21 proteins and
downregulated expression of the oncogene cmyc protein.
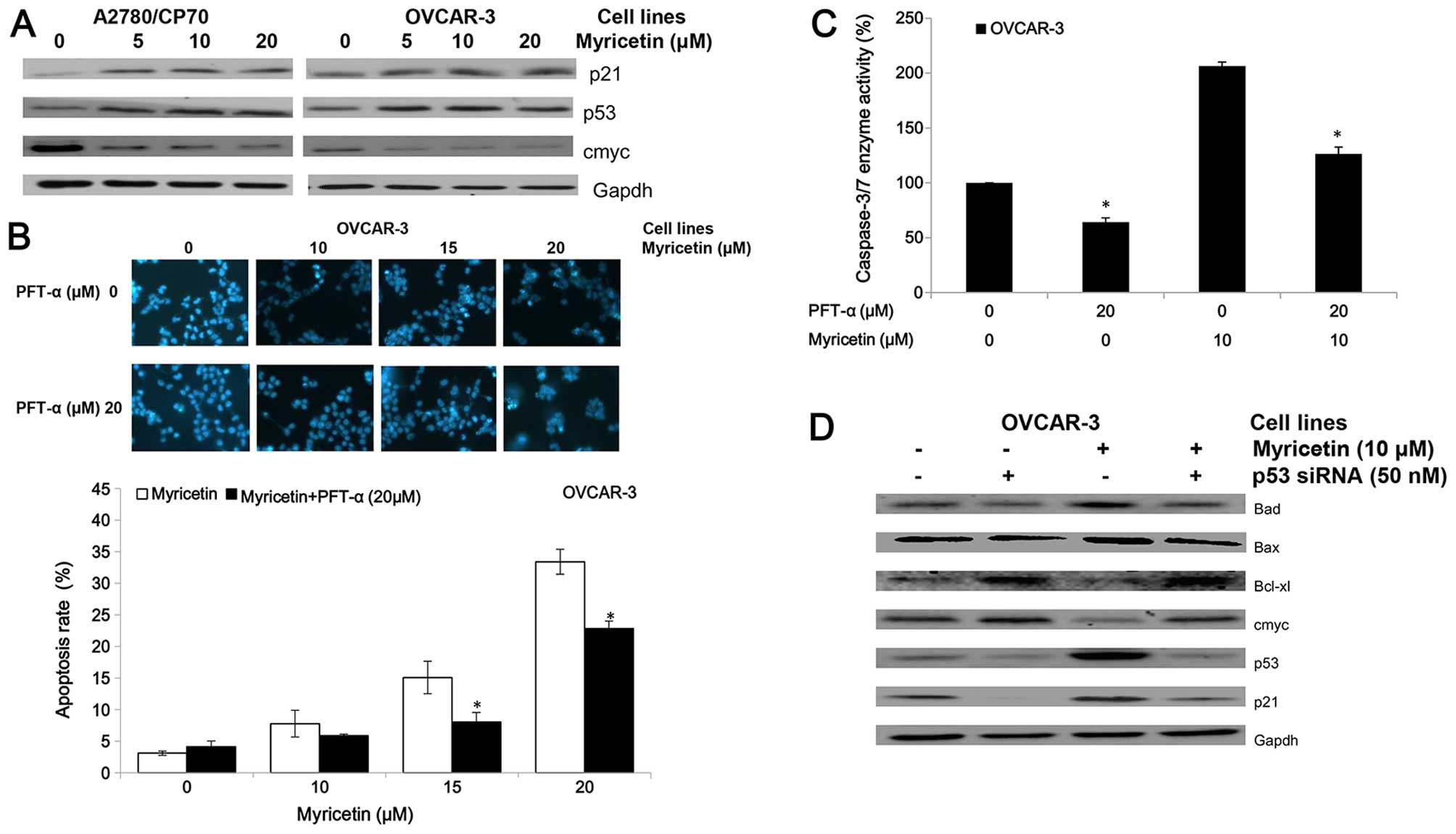 | Figure 5Myricetin induces apoptosis on
ovarian cancer cells in a p53-dependent pathway. (A) Myricetin
decreased the expression of protein cmyc, and increased levels of
protein p21 and p53. (B) Cells were treated with myricetin and p53
inhibitor pifithrin-α (PFT-α) for 24 h, stained with Hoechst 33342,
apoptotic rate measured by fluorescence microscope.
*p<0.05 as compared to myricetin-treated control. (C)
Cells were treated with myricetin and PFT-α for 4 h, and
caspase-3/7 enzymatic activity was determined using Caspase-Glo 3/7
assay kit. *p<0.05 as compared to myricetin-treated
control. (D) Knockdown p53 resulted in abrogation of
myricetin-increased levels of Bad, Bax, p21 and p53 and decreased
levels of cmyc, Bcl-2 and Bcl-xl in OVCAR-3 cells. Cells were
seeded in 60-mm dishes, incubated overnight, and then transfected
with p53 siRNA or control siRNA. After 24 h, cells were treated
with myricetin or DMSO. Cell lysates were collected for western
blotting. |
The inhibitor of pifithrin-α (PFT-α) was used to
investigate the role of p53 on myricetin-induced apoptosis in
OVCAR-3 cells. Treatment with 20 μM pifithrin-α significantly
reduced the apoptotic rates and caspase-3/7 enzymatic activities in
OVCAR-3 cells induced by myricetin (Fig. 5B and C). Fig. 5D shows knockdown of p53 by specific
siRNA (50 nM) resulted in abrogation of myricetin-increased levels
of Bax, Bad, and p21 proteins and myricetin-decreased levels of
cmyc and Bcl-xl proteins. These results indicate that
p53-associated intrinsic pathways were, at least partly, involved
in myricetin-induced apoptosis in ovarian cancer cells.
Role of p21 in the myricetin-induced
apoptosis in OVCAR-3 cells
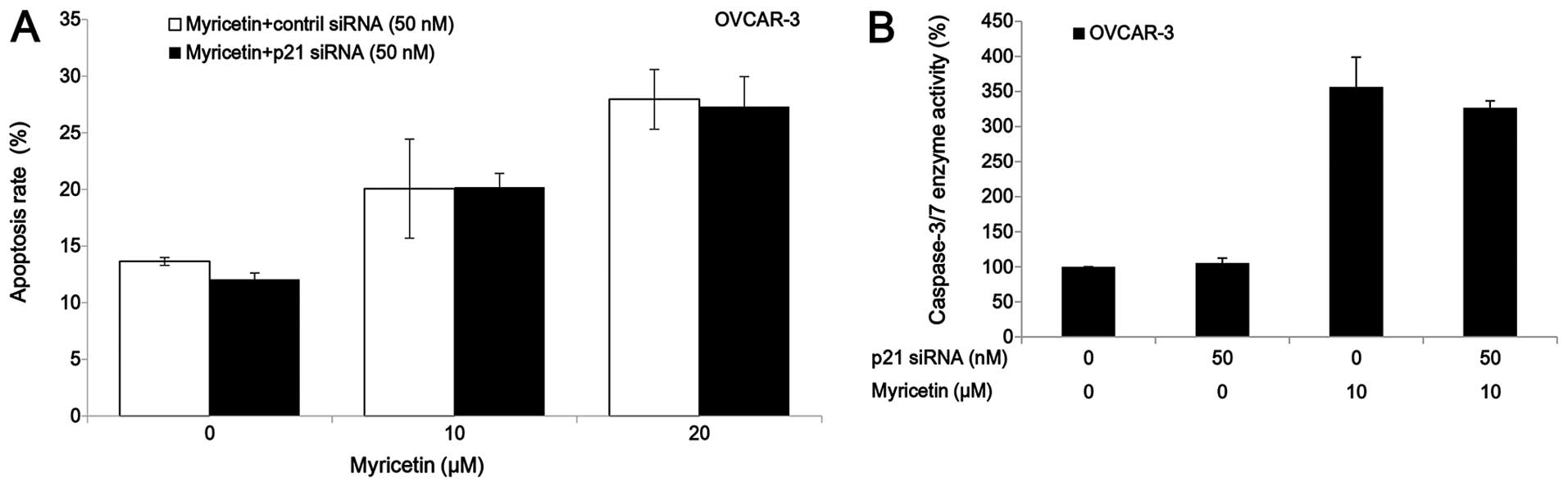
The p21 siRNA was used to investigate the role of
p21 on myricetin-induced apoptosis in OVCAR-3 cells. Knockdown of
p21 by siRNA (50 nM) did not abrogate the apoptosis and caspase-3/7
enzymatic activation in OVCAR-3 cells which was induced by
treatment of myricetin (Fig. 6).
These results indicate that myricetin induced apoptosis in OVCAR-3
cells through a p21-independent pathway.
Discussion
A pressing problem in cancer treatment is that
platinum-based chemotherapy is seriously hampered by high rates of
adverse effects and chemoresistance (21). There exists a dire need for more
selective drugs in the treatment of cancer. Previous studies have
reported that myricetin induces apoptosis in various cancer cells,
such as hepatoma, pancreatic cancer, esophageal cancer, and colon
carcinoma cells (15–18). However, its effects on ovarian
cancer are currently unknown. In this study, it was found that
myricetin was more cytotoxic to two cisplatin-resistant ovarian
cancer cell lines A2780/CP70 and OVCAR-3 than normal ovarian cells
IOSE-364, and that myricetin had stronger anticancer activity than
cisplatin in the two ovarian cancer cell lines when compared to our
previous study (19).
The mechanism through which cancer cells develop
resistance to chemotherapy is associated with increased resistance
to apoptosis. In pre-clinical disease models, agents that induce
apoptosis have been reported to sensitize tumor cells to
chemotherapy and radiotherapy (22). There are two major pathways leading
to apoptosis: the intrinsic or mitochondrial and extrinsic or
receptor-mediated pathways. The permeability of the mitochondria
and release of cytochrome c into the cytoplasm will be
increased when the intrinsic apoptotic pathway is activated. After
that, cytochrome c forms a multi-protein complex, known as
the apoptosome, initiating activation of caspase-9. Bcl-2 protein
family plays an important role in the regulation of the intrinsic
apoptotic pathway through controlling the permeability of the
mitochondrial membrane and the release of pro-apoptotic factors
(10). Whether or not cells will
undergo apoptosis is dependent on the balance between the pro-
(such as Bax and Bad) and anti-apoptotic (such as Bcl-xl and Bcl-2)
proteins of the family members. In the extrinsic pathway, tumor
necrosis factor-related apoptosis-inducing ligand (TRAIL),
including the Apo2L/TRAIL, regulates the extrinsic apoptotic
pathway by engaging its receptor, such as DR5. The receptor
homotypically binds to FAS-associated death domain protein (FADD)
to form death inducing signaling complex (DISC), activating
caspase-8 and -10. Activation of either the intrinsic pathway of
apoptosis or the extrinsic pathway results in activation of
caspase-3 and -7 culminating in apoptosis. In this study, myricetin
was found to induce intrinsic apoptosis through the Bcl-2 protein
but not the caspase-9 enzyme in A2780/CP70 and OVCAR-3 cells. A
previous study indicated that myricetin induces apoptosis in colon
cancer cells through increasing the ratio of Bax/Bcl-2 proteins
(18), which agrees with the
results obtained here. The effect of myricetin on the extrinsic
apoptotic pathway was also examined by testing the expression of
DR5, FADD, and caspase-8 proteins. It was found that myricetin
increased the levels of DR5 protein and decreased the levels of
procaspase-8 in the OVCAR-3 but not the A2780/CP70 cells,
suggesting that myricetin induces apoptosis in OVCAR-3 cells though
a DR5-associated extrinsic pathway. The alterations in the balance
of Bcl-2/Bax proteins was associated with the differential
induction of apoptosis in cancer versus normal cells (23). Evidence from clinical trials has
indicated that normal cells are resistant to the tumor necrosis
factor-related apoptosis-inducing ligand (TRAIL), and targeting DR5
selectively eliminates tumor cells while sparing normal cells
(24). This study has shown that
myricetin induced apoptosis in ovarian cancer cells A2780/CP70 and
OVCAR-3 but not normal ovarian cells IOSE-364, which might be due
to its effects on the expression of the Bcl-2 family and DR5
protein.
The cell cycle represents a series of events that
allow the cell to replicate into two daughter cells. Many cancer
cells have defective G1 checkpoint mechanisms and are more
dependent on the G2 checkpoint during replication than normal
cells. Cancer represents a dysregulation of the cell cycle, such as
an overexpression of cyclins or insufficient expression of CDKIs,
which result in cell growth and tumor formation (7). Therefore, the innovative strategy of
cell cycle arrest, which activates the apoptotic cascade and leads
to cell death, was developed. Novel anticancer drugs have been
focused on as the target of cell cycle control mechanisms (8,9).
Previous studies indicated that myricetin induces G2/M blockage in
human squamous cell carcinoma cell lines SCC-25 and human colon
cancer cell lines HCT116 (25,26).
However, in the present study, the cell cycle in human ovarian
cancer cells was not affected by treatment of myricetin, which
means myricetin inhibited ovarian cancer cell growth through a
mechanism separate from inducing cell cycle arrest.
p53, as a multifunctional tumor suppressor,
regulates cell cycle arrest, transcription, DNA repair, genomic
instability, senescence, differentiation, angiogenesis, apoptosis,
and glucose metabolism (20). If
the p53 gene is damaged, tumor suppression will be under serious
threat. As shown in previous studies, p53 alterations, such as loss
of function dominant-negative activity and gain of oncogene
function, has been connected with the failure of chemotherapy and
radiotherapy in a number of cancers (27). More than 50% of human tumors
contain p53 gene that is mutated or deleted (28), and people who have only one
functional copy of the p53 gene will probably suffer from tumors in
early adulthood. Therefore, increasing the amount of p53 might be a
new strategy for treatment of tumors. The intrinsic pathways of
apoptosis such as Bax, Puma, and Noxa are regulated by p53
(29). It has been reported that
the Bcl-2 family genes are regulated by p53, and cancer cells with
loss of p53 function are expected to contain relatively low levels
of Bax, Bad and high levels of Bcl-2 and Bcl-xl (30,31).
In ovarian cancer cells, p53 also has an effect on cell apoptosis
(32). In the present study,
myricetin was observed to increase p53 protein expression in the
ovarian cancer cells. In OVCAR-3 cells, the myricetin-induced
balance alterations of Bcl-2 family proteins were associated with
increased p53 protein, indicating that p53 played an important role
in the myricetin-activated intrinsic pathway of apoptosis. The
transcription factor cmyc protein is encoded by a regulator. It is
a nuclear protein which plays a role in the cell cycle, apoptosis,
progression, and cellular transformation. Previous studies
indicated that p53-dependent repression of cmyc was involved in
cell cycle arrest and apoptosis (33,34).
In this study, myricetin decreased the cmyc protein expression in
A2780/CP70 and OVCAR-3 cells, and knockdown of protein p53
neutralized the repressive effect myricetin had on cmyc expression
in OVCAR-3 cells. Therefore, it is reasonable to think that p53
induced apoptosis through a cmyc-dependent manner. Of course, more
studies are needed to clarify the role of cmyc in myricetin-induced
apoptosis in ovarian cancer cells. Additionally, it was seen that
myricetin increased the expression of p21 protein in both ovarian
cancer cell lines, and it was mediated by p53 in OVCAR-3 cells.
Therefore, it was inferred that p21 might be involved in
myricetin-induced apoptosis and the inhibition of angiogenesis in
OVCAR-3 cells.
P21, known as CDK-interacting protein 1 or
cyclin-dependent kinase inhibitor 1, regulates cell cycle
progression, tightly regulated by p53 protein (35). It has been reported that myricetin
increased levels of p21 protein in HepG2 cells which resulted in
cell cycle arrest at the G2/M phase (36). However, in the present study, the
increased levels of p21 protein had little effect on the cell cycle
in A2780/CP70 and OVCAR-3 cells. Some previous studies indicated
that p21 was a positive regulator of apoptosis in either
p53-dependent or independent pathways, while other studies showed
p21 inhibited p53-dependent apoptosis (37). In our study, although myricetin
increased levels of p21 protein in OVCAR-3 cells, it was not
associated with apoptosis.
In conclusion, the present study suggests that
myricetin, which exhibited higher cytotoxicity to two
cisplatin-resistant ovarian cancer cell lines than in normal
ovarian cells, might be a potential candidate for the
chemoprevention of ovarian cancer. It induces apoptosis in both
ovarian cancer cells through a Bcl-2 family protein-associated
intrinsic pathway. In OVCAR-3, myricetin also induces DR-5
associated extrinsic apoptosis. P53 plays an important role in
Bcl-2 family protein-dependent apoptosis, induced by myricetin, in
OVCAR-3 cells. Ostensibly, further studies in animal models and
human trials are needed to determine the efficacy of this compound
for the treatment of ovarian cancer.
Acknowledgements
We thank Dr Kathy Brundage from the Flow Cytometry
Core at West Virgina University for providing technical help on
apoptosis and cell cycle analysis, and Yu Fu for giving a critical
review of the manuscript. This study was supported by a West
Virginia Experimental Program to Stimulate Competitive Research
grant and NIH grants (P20RR016477 and P20GM103434) from the
National Institutes of Health awarded to the West Virginia IDeA
Network of Biomedical Research Excellence. This study was also
supported by the Chinese National Key Technologies R&D Program
of 12th Five-year Plan (2012BAD31B06).
Abbreviations:
|
TRAIL
|
tumor necrosis factor-related
apoptosis-inducing ligand
|
|
PFT-α
|
pifithrin-α
|
|
DMSO
|
dimethyl sulfoxide
|
|
PBS
|
phosphate-buffered saline
|
|
siRNA
|
small interfering RNA
|
|
DR5
|
death receptor 5
|
References
|
1
|
Stordal B and Davey M: Understanding
cisplatin resistance using cellular models. IUBMB Life. 59:696–699.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Loehrer PJ and Einhorn LH: Drugs five
years later. Cisplatin Ann Intern Med. 100:704–713. 1984.
View Article : Google Scholar
|
|
3
|
Milosavljevic N, Duranton C, Djerbi N,
Puech PH, Gounon P, Lagadic-Gossmann D, Dimanche-Boitrel MT, Rauch
C, Tauc M, Counillon L, et al: Nongenomic effects of cisplatin:
Acute inhibition of mechanosensitive transporters and channels
without actin remodeling. Cancer Res. 70:7514–7522. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Windsor RE, Strauss SJ, Kallis C, Wood NE
and Whelan JS: Germline genetic polymorphisms may influence
chemotherapy response and disease outcome in osteosarcoma: A pilot
study. Cancer. 118:1856–1867. 2012. View Article : Google Scholar
|
|
5
|
Levi JA, Aroney RS and Dalley DN:
Haemolytic anaemia after cisplatin treatment. Br Med J (Clin Res
Ed). 282:2003–2004. 1981. View Article : Google Scholar
|
|
6
|
Hall MD, Okabe M, Shen DW, Liang XJ and
Gottesman MM: The role of cellular accumulation in determining
sensitivity to platinum-based chemotherapy. Annu Rev Pharmacol
Toxicol. 48:495–535. 2008. View Article : Google Scholar
|
|
7
|
Champeris Tsaniras S, Kanellakis N,
Symeonidou IE, Nikolopoulou P, Lygerou Z and Taraviras S: Licensing
of DNA replication, cancer, pluripotency and differentiation: An
interlinked world? Semin Cell Dev Biol. 30:174–180. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Buolamwini JK: Cell cycle molecular
targets in novel anticancer drug discovery. Curr Pharm Des.
6:379–392. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Diaz-Moralli S, Tarrado-Castellarnau M,
Miranda A and Cascante M: Targeting cell cycle regulation in cancer
therapy. Pharmacol Ther. 138:255–271. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Brunelle JK and Letai A: Control of
mitochondrial apoptosis by the Bcl-2 family. J Cell Sci.
122:437–441. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Gates MA, Vitonis AF, Tworoger SS, Rosner
B, Titus-Ernstoff L, Hankinson SE and Cramer DW: Flavonoid intake
and ovarian cancer risk in a population-based case-control study.
Int J Cancer. 124:1918–1925. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Ong KC and Khoo HE: Biological effects of
myricetin. Gen Pharmacol. 29:121–126. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Ross JA and Kasum CM: Dietary flavonoids:
Bioavailability, metabolic effects, and safety. Annu Rev Nutr.
22:19–34. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Basli A, Soulet S, Chaher N, Mérillon JM,
Chibane M, Monti JP and Richard T: Wine polyphenols: Potential
agents in neuro-protection. Oxid Med Cell Longev. 2012:8057622012.
View Article : Google Scholar
|
|
15
|
Zhang XH, Chen SY, Tang L, Shen YZ, Luo L,
Xu CW, Liu Q and Li D: Myricetin induces apoptosis in HepG2 cells
through Akt/p70S6K/bad signaling and mitochondrial apoptotic
pathway. Anticancer Agents Med Chem. 13:1575–1581. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Phillips PA, Sangwan V, Borja-Cacho D,
Dudeja V, Vickers SM and Saluja AK: Myricetin induces pancreatic
cancer cell death via the induction of apoptosis and inhibition of
the phosphatidylinositol 3-kinase (PI3K) signaling pathway. Cancer
Lett. 308:181–188. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Zang W, Wang T, Wang Y, Li M, Xuan X, Ma
Y, Du Y, Liu K, Dong Z and Zhao G: Myricetin exerts
anti-proliferative, anti-invasive, and pro-apoptotic effects on
esophageal carcinoma EC9706 and KYSE30 cells via RSK2. Tumour Biol.
35:12583–12592. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Kim ME, Ha TK, Yoon JH and Lee JS:
Myricetin induces cell death of human colon cancer cells via
BAX/BCL2-dependent pathway. Anticancer Res. 34:701–706.
2014.PubMed/NCBI
|
|
19
|
Li B, Gao Y, Rankin GO, Rojanasakul Y,
Cutler SJ, Tu Y and Chen YC: Chaetoglobosin K induces apoptosis and
G2 cell cycle arrest through p53-dependent pathway in
cisplatin-resistant ovarian cancer cells. Cancer Lett. 356:418–433.
2015. View Article : Google Scholar
|
|
20
|
Darcy KM, Brady WE, McBroom JW, Bell JG,
Young RC, McGuire WP, Linnoila RI, Hendricks D, Bonome T and Farley
JH; Gynecologic Oncology Group. Associations between p53
overexpression and multiple measures of clinical outcome in
high-risk, early stage or suboptimally-resected, advanced stage
epithelial ovarian cancers A Gynecologic Oncology Group study.
Gynecol Oncol. 111:487–495. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Yang YI, Kim JH, Lee KT and Choi JH:
Costunolide induces apoptosis in platinum-resistant human ovarian
cancer cells by generating reactive oxygen species. Gynecol Oncol.
123:588–596. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Wilson TR, Johnston PG and Longley DB:
Anti-apoptotic mechanisms of drug resistance in cancer. Curr Cancer
Drug Targets. 9:307–319. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Liu T, Hannafon B, Gill L, Kelly W and
Benbrook D: Flex-Hets differentially induce apoptosis in cancer
over normal cells by directly targeting mitochondria. Mol Cancer
Ther. 6:1814–1822. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Ozören N and El-Deiry WS: Cell surface
death receptor signaling in normal and cancer cells. Semin Cancer
Biol. 13:135–147. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Maggioni D, Nicolini G, Rigolio R, Biffi
L, Pignataro L, Gaini R and Garavello W: Myricetin and naringenin
inhibit human squamous cell carcinoma proliferation and migration
in vitro. Nutr Cancer. 66:1257–1267. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Shiomi K, Kuriyama I, Yoshida H and
Mizushina Y: Inhibitory effects of myricetin on mammalian DNA
polymerase, topoisomerase and human cancer cell proliferation. Food
Chem. 139:910–918. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Kong D, Ma S, Liang B, Yi H, Zhao Y, Xin
R, Cui L, Jia L and Liu X and Liu X: The different regulatory
effects of p53 status on multidrug resistance are determined by
autophagy in ovarian cancer cells. Biomed Pharmacother. 66:271–278.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Hollstein M, Sidransky D, Vogelstein B and
Harris CC: p53 mutations in human cancers. Science. 253:49–53.
1991. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Kuo YC, Kuo PL, Hsu YL, Cho CY and Lin CC:
Ellipticine induces apoptosis through p53-dependent pathway in
human hepatocellular carcinoma HepG2 cells. Life Sci. 78:2550–2557.
2006. View Article : Google Scholar
|
|
30
|
Basu A and Haldar S: The relationship
between BcI2, Bax and p53: Consequences for cell cycle progression
and cell death. Mol Hum Reprod. 4:1099–1109. 1998. View Article : Google Scholar
|
|
31
|
Mihara M, Erster S, Zaika A, Petrenko O,
Chittenden T, Pancoska P and Moll UM: p53 has a direct apoptogenic
role at the mitochondria. Mol Cell. 11:577–590. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Guan YQ, Li Z, Yang A, Huang Z, Zheng Z,
Zhang L, Li L and Liu JM: Cell cycle arrest and apoptosis of
OVCAR-3 and MCF-7 cells induced by co-immobilized TNF-α plus IFN-γ
on polystyrene and the role of p53 activation. Biomaterials.
33:6162–6171. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Ho JS, Ma W, Mao DY and Benchimol S:
p53-Dependent transcriptional repression of c-myc is required for
G1 cell cycle arrest. Mol Cell Biol. 25:7423–7431. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Hermeking H and Eick D: Mediation of
c-Myc-induced apoptosis by p53. Science. 265:2091–2093. 1994.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Gartel AL and Radhakrishnan SK: Lost in
transcription: p21 repression, mechanisms, and consequences. Cancer
Res. 65:3980–3985. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Zhang XH, Zou ZQ, Xu CW, Shen YZ and Li D:
Myricetin induces G2/M phase arrest in HepG2 cells by inhibiting
the activity of the cyclin B/Cdc2 complex. Mol Med Rep. 4:273–277.
2011.PubMed/NCBI
|
|
37
|
Piccolo MT and Crispi S: The dual role
played by p21 may influence the apoptotic or anti-apoptotic fate in
cancer. J Cancer Res Updates. 1:189–202. 2012.
|