Introduction
Gliomas represent the most common primary brain
tumors in adults (1). The average
survival time for high-grade glioma patients is ~14 months
(2). Even with successful surgical
resecstion, the prognosis for a patient whose tumor is not
sensitive to chemotherapeutic drugs is poor. Cisplatin is well
established for use as a chemotherapeutic drug in gliomas (3). However, the molecular mechanisms
underlying the chemoresistance of gliomas are largely unclear.
MicroRNAs (miRNAs) are a class of small endogenous
noncoding RNAs of 18–25 nucleotides that negatively regulate their
target genes by interacting with the 3′-untranslated region (3′UTR)
of the mRNAs (4). The differential
expression of miRNA between cancers and their normal counterparts
have been explored in various cancers, including glioma (5–9), and
these results suggest critical roles for miRNAs in the pathogenesis
of the cancers. Recently, accumulating evidence has indicated that
miRNAs are implicated in the chemoresistance of gliomas. Let-7b was
found to be significantly downregulated in cisplatin-resistant U251
cells compared to wild-type cells and has the ability to improve
the therapeutic effect of cisplatin in glioma cells (10). Knockdown of miR-106a enhanced the
temozolomide-induced apoptosis in glioma cells (11). Overexpression of miR-136 sensitizes
glioma cells to cisplatin by targeting E2F1 and Bcl-2 (12,13).
To date, only a few studies have reported the function of miR-873
(14–16). In particular, the effect of miR-873
on the chemoresistance of glioma cells has not been reported.
In this study, we found that hsa-miR-873-5p
(miR-873) was downregulated in cisplatin-resistant glioma cells
compared to wild-type cells. Furthermore, exposing wild-type cells
to cisplatin decreased their expression of miR-873. Further
experiments demonstrated that miR-873 decreased the activity of
cisplatin-resistant glioma cells and sensitized cisplatin-resistant
glioma cells to cisplatin by directly targeting Bcl-2, an
anti-apoptotic protein expressed in a wide variety of cancers.
Ectopic expression of Bcl-2 in cisplatin-resistant glioma cells
could prevent the cisplatin-sensitizing effect of miR-873. These
results indicated that miR-873 might be a promising therapeutic
strategy to overcome cisplatin resistance in gliomas.
Materials and methods
Patients and samples
Twelve high-grade glioma tissues and seven normal
brain tissues were collected between 2011 and 2014 from the
Department of Neurosurgery, Renmin Hospital of Wuhan University.
The samples were obtained at the time of surgery and were
immediately snap-frozen in liquid until use. The project was
approved by the ethics committee of Renmin Hospital.
Cell lines and cell culture
The human glioma cell lines U87 and U251 were
purchased from American Type Culture Collection (ATCC). The cells
were cultured in Dulbecco's modified Eagle's medium (DMEM, Hyclone,
Logan, UT, USA) containing 10% fetal bovine serum (FBS, Gibco,
Grand Island, NY, USA) at 37°C in a 5% CO2 humidified
incubator.
The cisplatin-resistant U87 (U87/DDP) and U251
(U251/DDP) cell lines were established by prolonged exposure to
increasing concentrations of cisplatin (Sigma-Aldrich, St. Louis,
MO, USA). The cisplatin concentrations were increased stepwise from
0.1 to 10 μg/ml when the cells resumed growth similar to the
untreated cells. U87 cells with the ability to grow in 5 μg/ml of
cisplatin and U251 cells with the ability to grow in 0.5 μg/ml of
cisplatin were obtained 4 months after the initial drug exposure
and were designated U87/DDP and U251/DDP, respectively. The MTT
assay results demonstrated that the drug sensitivity of these cells
was changed.
RNA extraction and qPCR
Total RNA was extracted from human tissues using
TRIzol reagent (Invitrogen, San Diego, CA, USA). Reverse
transcription was performed using RevertAid First Strand cDNA
Synthesis kit (Thermo Scientific, Waltham, MA, USA). To detect
miR-873, the Bulge-Loop™ miRNA qRT-PCR Primer kits (Ribobio,
Guangzhou, China) were utilized following the manufacturer's
instructions, and qPCR was performed using SuperReal PreMix (SYBR
Green) (Tiangen, Beijing, China) with an iCycler thermal cycler
(Bio-Rad, Hercules, CA, USA). The relative expression levels were
calculated using the 2−ΔΔCt method after normalization
to the expression of U6 snRNA.
MTT assay
The cells were seeded into 96-well plates
(3×103 cells/well) and incubated for 24 h. The cells
were transfected with vectors for another 24 h and then treated
with various concentrations of cisplatin. At 24, 48 and 72 h, the
cell viability was determined using the MTT solution (0.5 mg/ml)
(Sigma-Aldrich). After 4 h of incubation, the medium was discarded
and 150 μl of dimethyl sulfoxide (DMSO) (Sigma-Aldrich) was added
to each well. The plate was vortexed for 30 min, and the absorbance
at 570 nM was read using a spectrophotometer. Each experiment was
performed in triplicate.
Colony formation assay
The cells transfected with the vectors were
trypsinized to single cell suspensions and were seeded into 6-well
plates at 1,000/well. Then, after 2 weeks of culture in DMEM with
10% FBS, the cells were fixed with 4% para-formaldehyde and stained
with 0.5% crystal violet solution. Each experiment was performed in
triplicate.
Luciferase reporter assay
The 3′UTR sequence of Bcl-2 that was predicted to
interact with miR-873 was amplified and cloned into the SpeI
and HindIII sites of the pMiR-Reporter vector (Ambion, USA).
Site-directed mutagenesis of the miR-873 target site was performed
using Quik-Change™ Site-Directed Mutagenesis kit (Stratagene, USA).
The constructs were sequenced and named pBcl2-3′UTR-wt or
pBcl2-3′UTR-mut. For the reporter assays, the U87/DDP cells were
seeded into 24-well plates and transfected with either 50 ng of
pBcl2-3′UTR-wt or pBcl2-3′UTR-mut and the miR-873 or NC mimics
(RiboBio) with Lipofectamine 2000 (Invitrogen). After incubation
for 24 h, cells were harvested and assayed with the Dual-Luciferase
Reporter Assay kit (Promega, Wisconsin, MA, USA) according to the
manufacturer's instructions.
Cell migration and invasion assays
The detailed methods of cell migration and invasion
assays were as described previously (12). Cells (4×104) were seeded
in the chamber (Corning, NY, USA) for migration assay or invasion
assay. The cell number of migration rate and invasion were
calculated by photographing at five random fields.
Cell apoptosis assay
After being transfected with the vectors for 24 h,
the cells were treated with cisplatin for another 48 h, then
detached by trypsinization, centrifuged, washed in
phosphate-buffered saline(PBS), resuspended in Annexin V-binding
buffer and incubated with Annexin V-phycoerythrin (PE) and
7-amino-Actinomycin D (7AAD) (KeyGen Biotech, Nanjin, China) for 15
min. The rate of apoptosis was determined using flow cytometry.
Caspase-3/7 activity analysis
Caspase-3/7 activity was measured using the
Caspase-Glo3 assay kit (Promega) according to the manufacturer's
instructions.
Western blot assay
The cells were lysed using the mammalian protein
extraction reagent RIPA (Beyotime, Beijing, China). Approximately
100 μg of the protein sample was separated on a 12% SDS-PAGE gel
and transferred onto a PVDF membrane (Sigma-Aldrich). The membrane
was blocked with 5% skim milk for at least 2 h at room temperature.
Monoclonal antibodies against Bcl-2 (1:1,000, Cell Signaling,
Danvers, MA, USA), PARP (1:3,000, Cell Signaling), and β-actin
(1:5,000, Sigma-Aldrich) were added in 3% BSA and incubated at 4°C
overnight. The membrane was washed and then incubated with
horseradish peroxidase (HRP)-labeled secondary antibody (1:1,000,
Sigma-Aldrich) at room temperature for 1.5 h. Then, the membrane
was washed, and the proteins were visualized using an ECL kit
(Millipore, Billerica, MA, USA).
Statistical analysis
The data are shown as the means ± SD (standard
deviation) and were analyzed using SPSS 17.0 software. Statistical
significance was determined using Student's t-test, and P<0.05
was considered to be significantly different. The Spearman rank
correlation coefficients were calculated to determine the bivariate
correlations between study variables.
Results
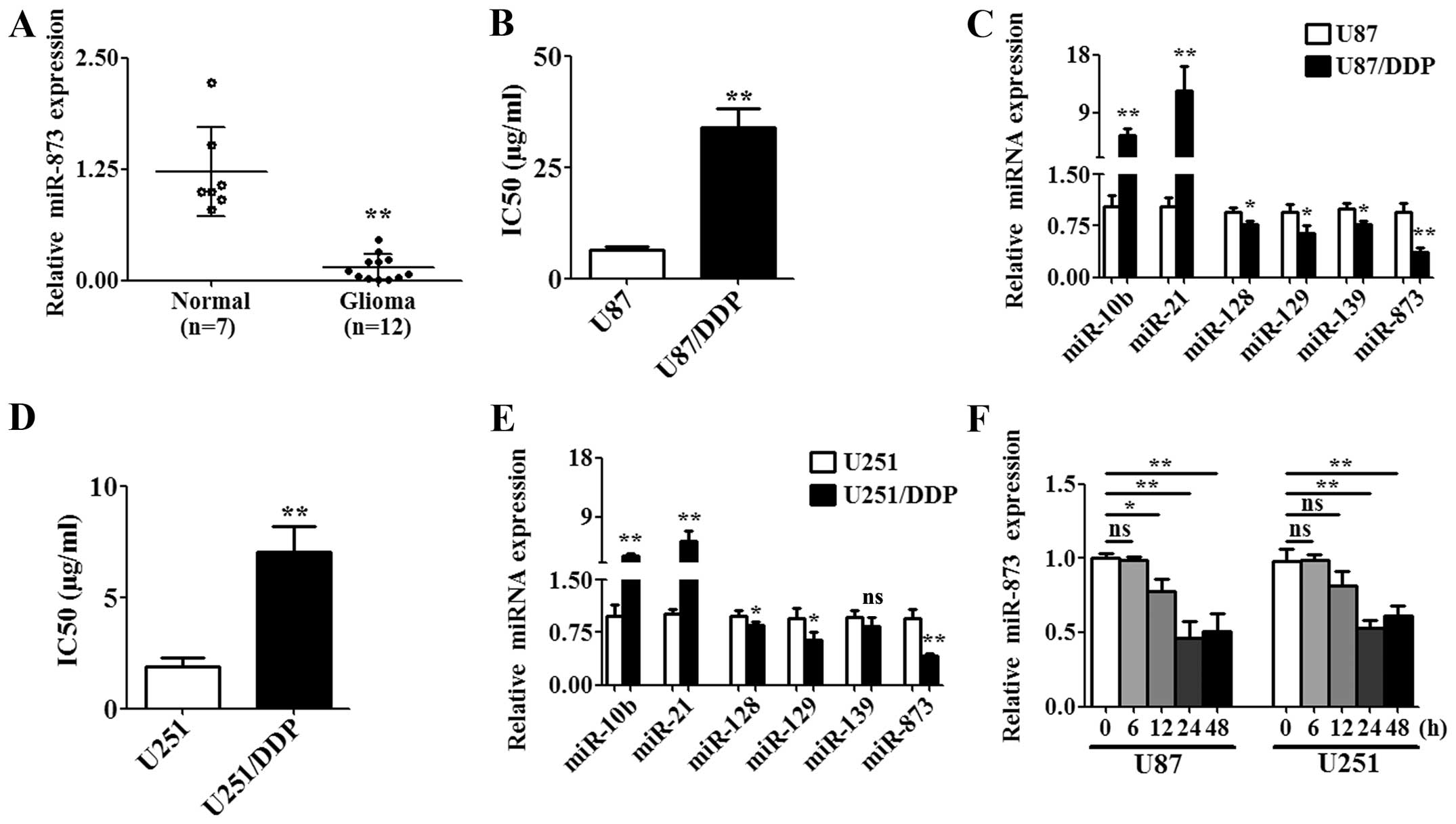
miR-873 was downregulated in glioma
tissues and cisplatin-resistant glioma cells
Cluster analysis has revealed that several miRNAs
including miR-873 are differentially expressed in glioma tissues
(17). Therefore, we compared the
expression of miR-873 in the tissues from twelve high-grade glioma
patients with those of seven normal brains by qPCR. The results
showed that miR-873 was significantly downregulated in the
high-grade glioma tissues compared with the normal tissues
(Fig. 1A). In addition,
differential expression of miRNA was frequently related to drug
resistance in gliomas (10,11,18).
Therefore, we generated the cisplatin-resistant glioma cells
designated U87/DDP and U251/DDP to determine the correlation of
dysregulated miR-873 with cisplatin resistance. We first determined
the IC50 for the wild-type cells and resistant cells. We
found that the IC50 for U87/DDP (Fig. 1B) or U251/DDP (Fig. 1D) was significantly higher than
that in the respective wild-type cells (U87 or U251). Then, we
found that several miRNAs that had been identified in the previous
cluster analysis were dysregulated in the U87/DDP or U251/DDP cells
(Fig. 1C and E). Among these,
miR-873 was significantly downregulated in U87/DDP or U251/DDP
cells compared to the respective wild-type cells (Fig. 1C and E). Next, we assessed the
effects of cisplatin on the expression of miR-873 in U87 and U251
cells. The expression of miR-873 was downregulated in a
time-dependent manner after treatment with the IC50
value of cisplatin (U87 cells were treated with 6.47 μg/ml and U251
cells were treated with 1.89 μg/ml) (Fig. 1F). These data suggested that
miR-873 might influence the susceptibility of glioma cells to
cisplatin.
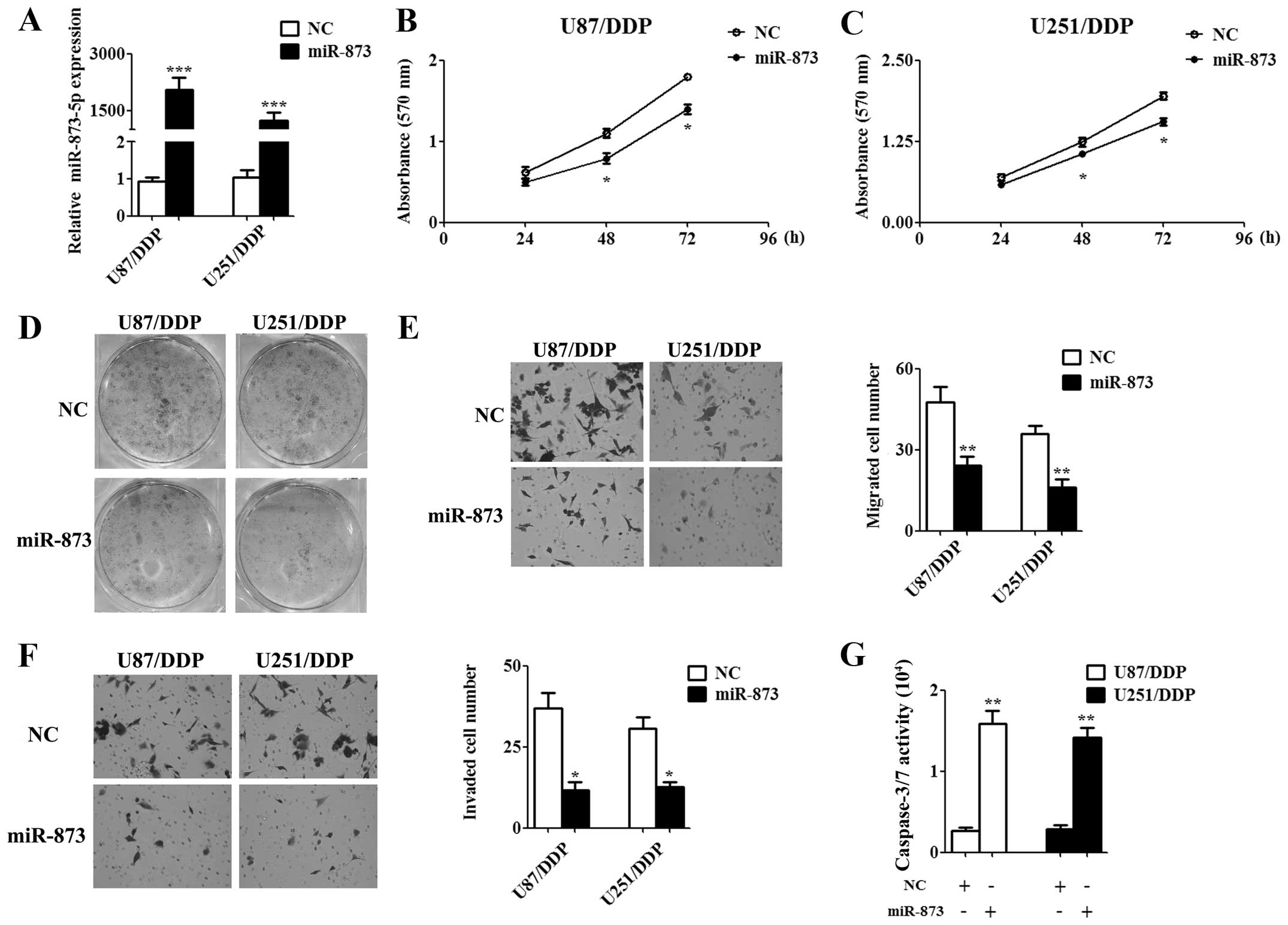
Overexpression of miR-873 attenuated the
tumorigenic properties of cisplatin-resistant glioma cells
To assess the function of miR-873 on
cisplatin-resistant glioma cells, 50 nM miR-873 mimics or 50 nM
negative control (NC) mimics, which was used as a control, was
transfected into U87/DDP and U251/DDP cells. Forty-eight hours
after the transfection, the qPCR results indicated that miR-873
expression was significantly upregulated in the miR-873 mimics
transfected cells compared to the NC mimics transfected cells
(Fig. 2A). Then, we examined cell
proliferation by MTT assay for indicated time and the results
showed that miR-873 significantly inhibited cell proliferation at
48 and 72 h (Fig. 2B and C). The
colony formation assay gave the same conclusion as the MTT assay in
the U87/DDP and U251/DDP cells (Fig.
2D). Moreover, we tested the effects of exogenous miR-873
expression on cell migration and invasion. Overexpression of
miR-873 decreased both the migration (Fig. 2E) and invasiveness (Fig. 2F) of U87/DDP and U251/DDP cells as
determined by Transwell assay. Furthermore, miR-873 obviously
incresed apoptosis of U87/DDP and U251/DDP cells as determined by
caspase-3/7 activity analysis (Fig.
2G). Collectively, these findings indicated that miR-873 acts
as a tumor suppressor and might relate to cisplatin resistance of
glioma cells.
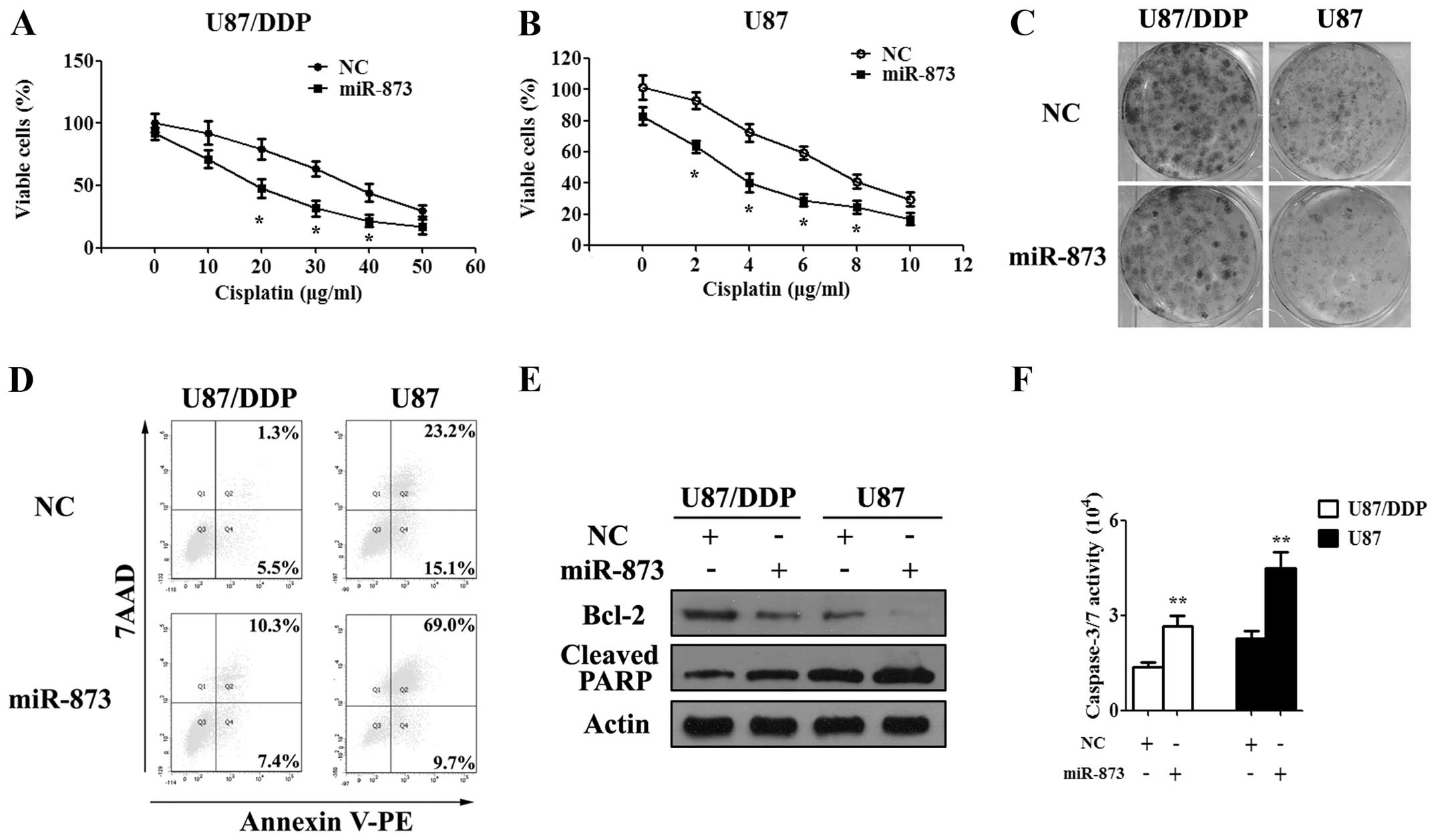
Overexpression of miR-873 sensitized
glioma cells to cisplatin
To further investigate the function of miR-873 on
the cisplatin resistance, the miR-873 or NC mimics was transfected
into U87/DDP and U87 cells. At first, we performed the MTT and
colony formation assays to determine the effect of miR-873 on the
proliferation of glioma cells. After being transfected with the
miR-873 or NC mimics for 24 h, the glioma cells were exposed to
cisplatin for another 24–72 h. Then, the absorbance of glioma cells
was measured using a microplate reader at 570 nM. The results of
the MTT assay showed that miR-873 could enhance the
anti-proliferative effect of cisplatin in both the
cisplatin-resistant and wild-type glioma cells (Fig. 3A and B). The colony formation assay
gave the same results as MTT assay in the U87/DDP and U87 cells
(Fig. 3C). Later, we used flow
cytometry to analyze the effects of miR-873 on cisplatin-induced
apoptosis of the U87/DDP and U87 cells. These results also showed
that miR-873 was able to significantly increase cisplatin-induced
apoptosis of the U87/DDP and U87 cells after 48 h of cisplatin
treatment (Fig. 3D). Moreover,
western blotting showed a decreased expression of Bcl-2 and an
increased expression of cleaved PARP, while the caspase-3/7
activity assay showed an increased activity of caspase-3/7 in the
miR-873 mimic-transfected U87/DDP and U87 cells when exposed to
cisplatin treatment (Fig. 3E and
F). Thus, overexpression of miR-873 could decrease the
resistance of the glioma cells to cisplatin.
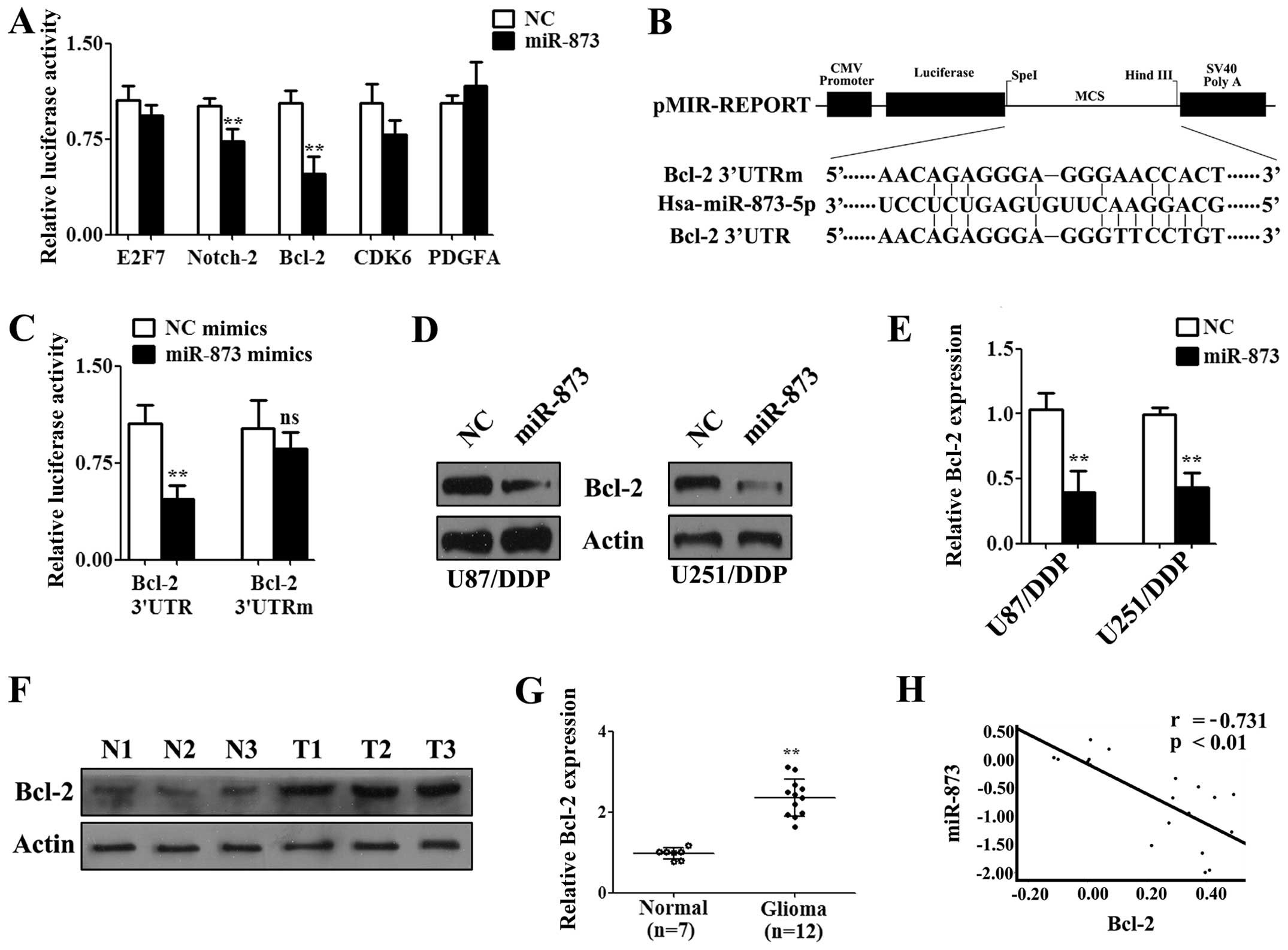
Bcl-2 is a functional target of
miR-873
It is known that miRNAs perform their functions by
regulating the expression of their target genes (19). Thus, the targets of miR-873 were
predicted by miRanda and TargetScan, two publicly available
prediction tools. We selected several putative targets that were
related to cisplatin resistance from the predictions. The 3′UTR
containing the potential binding site were cloned into
pMiR-Reporter vector and luciferase assays were performed by
transfection into U87/DDP cells. After co-transfection with 3′UTRs
and the miR-873 or NC mimics for 24 h, luciferase assays showed
that the activity of Bcl-2 3′UTR was strongest inhibited by miR-873
(Fig. 4A). Furthermore, we cloned
a mutated 3′UTR into the pMiR-Reporter vector to create a
pMiR-Bcl-2-mu vector (Fig. 4B).
After co-transfection of the U87/DDP cells with the miR-873 or NC
mimics and the pMiR-Bcl-2-wt vector or pMiR-Bcl-2-mu vector for 24
h, a luciferase assay was performed. The results showed that the
luciferase activity was decreased by transfection with the miR-873
mimic (Fig. 4C). However, no
significant difference was found when the cells were transfected
with the miR-873 or NC mimics combined with the pMiR-Bcl-2-mu
vector (Fig. 4C). Subsequently,
western blotting was performed to analyze the effect of miR-873 on
Bcl-2 protein expression. The results showed that Bcl-2 expression
was significantly lower in the cells transfected with the miR-873
mimics for 72 h compared with the NC mimics both in U87/DDP and
U251/DDP cells (Fig. 4D and E).
Furthermore, we examined the Bcl-2 levels in twelve high-grade
glioma patient tissues and those of seven normal brains tissues by
western blotting. The results showed that Bcl-2 was upregulated in
the high-grade glioma tissues (Fig. 4F
and G). There was an obvious negative correlation between these
signals (r=−0.731, P<0.01; Fig.
4H). Therefore, miR-873 could downregulate the expression of
Bcl-2 by directly targeting the Bcl-2 3′UTR.
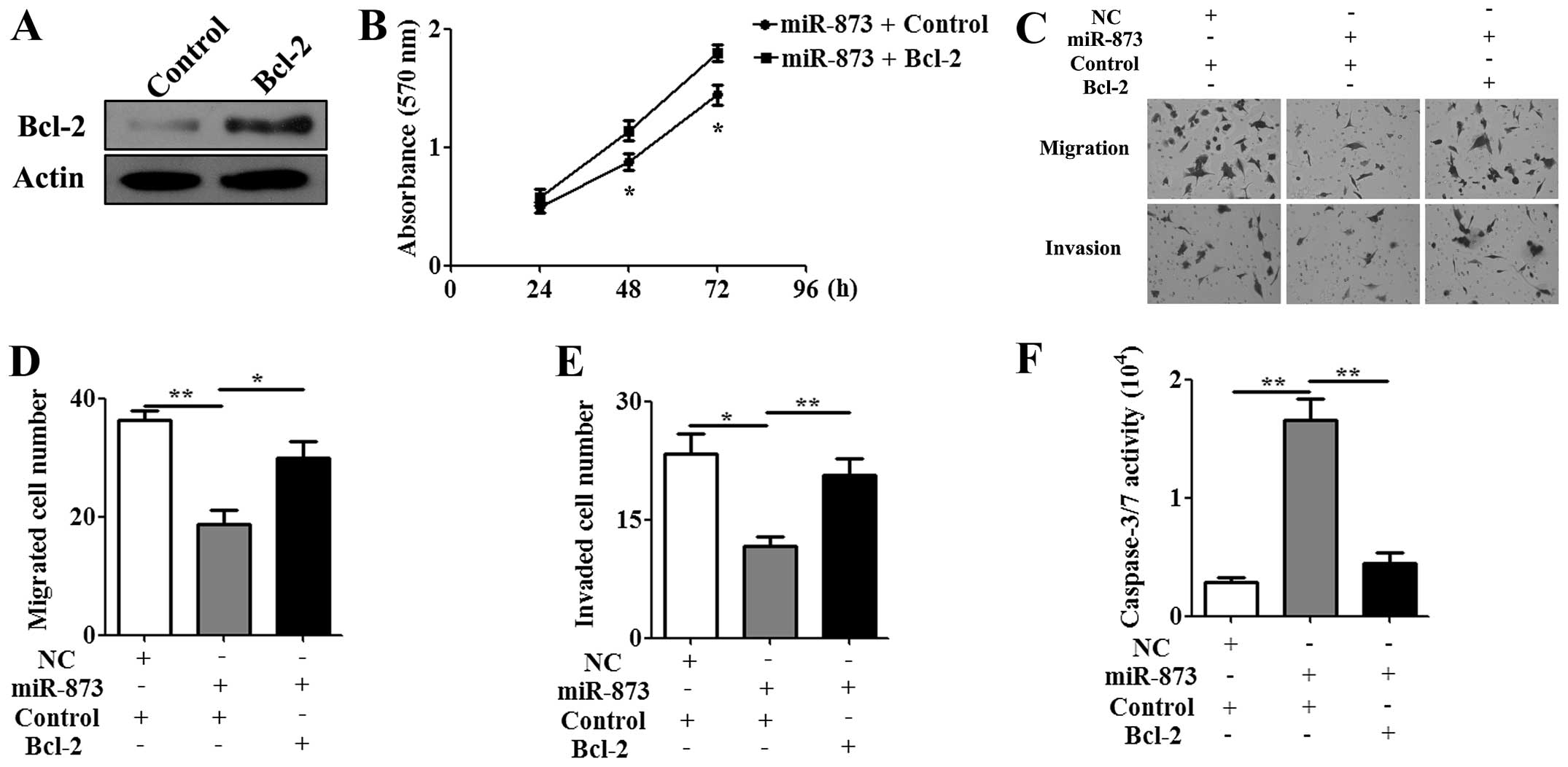
Re-expression of Bcl-2 attenuates the
effect of miR-873 on cisplatin-resistant cells
To examine whether Bcl-2 expression mediates the
phenotype associated with increased miR-873 expression, the
CMV-MCS/Bcl-2 vector or the CMV-MCS empty vector as a control was
transfected into U87/DDP cells. A western blot assay confirmed that
the expression of the Bcl-2 protein was significantly upregulated
in the U87/DDP cells (Fig. 5A).
The MTT assay showed that re-expression of Bcl-2 decreased the
antiproliferation effect of miR-873 on U87/DDP cells at 48 and 72 h
(Fig. 5B). To further investigate
the antitumor effect of miR-873 on U87/DDP cells, Transwell assay
and caspase-3/7 activity analysis were performed. Consistent with
MTT result, re-expression of Bcl-2 recovered the migration
(Fig. 5C and D) and invasive
(Fig. 5C and E) ability of U87/DDP
cells and abrogated the pro-apoptotic effect of miR-873 (Fig. 5F). These data further suggested
Bcl-2 might be a functional target of miR-873.
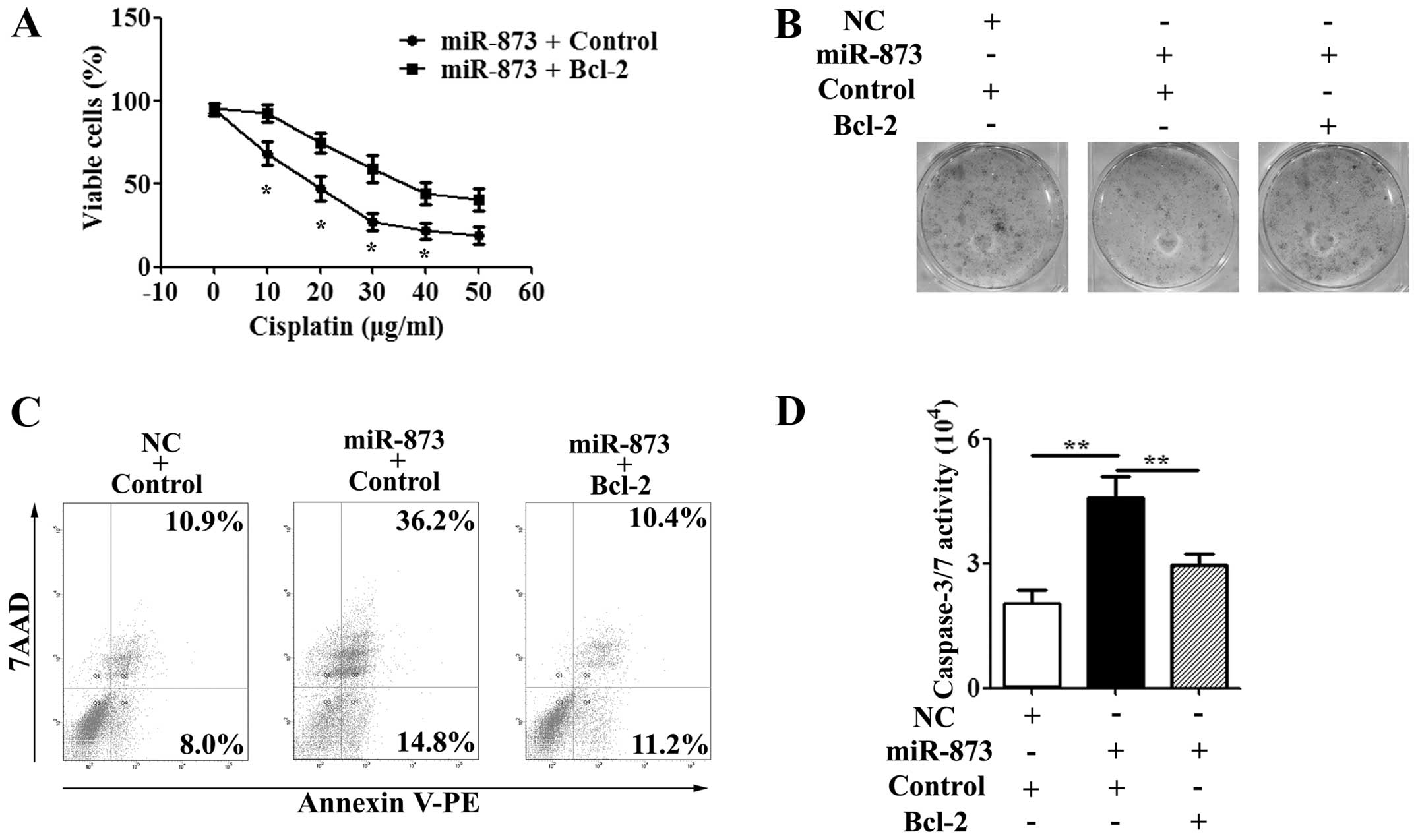
Re-expression of Bcl-2 decreases the
effect of miR-873 on cisplatin sensitivity
Having demonstrated that Bcl-2 is a direct target of
miR-873, we further investigated the role of Bcl-2 in cisplatin
sensitivity of miR-873. After being co-transfected with the miR-873
or NC mimics and the CMV-MCS/Bcl-2 or CMV-MCS vector for 24 h, the
U87/DDP cells were treated with IC50 value of cisplatin
for another 24–72 h. The MTT assay showed that the proliferation
was inhibited in the miR-873 mimic-transfected cells compared to
the NC mimic-transfected cells at 48 and 72 h (P<0.01; Fig. 6A). However, co-transfection with
the miR-873 mimics and CMV-MCS/Bcl-2 vectors decreased the
miR-873-induced sensitivity of the U87/DDP cells to cisplatin
(Fig. 6A). The colony formation
assay gave the same result as the MTT assay in the U87/DDP cells
(Fig. 6B). Furthermore, we
determined whether overexpression of Bcl-2 could impair the effect
of miR-873 on sensitivity to cisplatin using flow cytometry and the
caspase-3/7 activity assay. In the U87/DDP cells, decreases in the
caspase-3/7 activity and apoptosis rate were observed in cells
co-transfected with the miR-873 mimics and the CMV-MCS/Bcl-2 vector
cell compared to co-transfected with the miR-873 mimics and the
CMV-MCS vector after treatment with cisplatin for 48 h (Fig. 6C and D). Taken together, these
results indicated that re-expression of Bcl-2 was able to reduce
the sensitivity-enhancing effect of miR-873 to cisplatin.
Discussion
Resistance to chemotherapeutic agents is a vital
challenge in the treatment of glioma patients. In this study, we
investigated the function of miR-873 on the cisplatin resistance of
glioma cells. To our knowledge, this is the first report that
miR-873 significantly decreased tumor chemoresistance, as measured
by MTT assay, colony formation assay, flow cytometry, western
blotting and caspase-3/7 activity assay. Furthermore, we
demonstrated for the first time that miR-873 enhanced the
sensitivity of cisplatin by targeting the mRNA 3′UTR of Bcl-2 and
that miR-873 and Bcl-2 were inversely dysregulated in glioma. Thus,
these results indicated that miR-873 could serve as a molecular
target for regulating the glioma cell sensitivity to cisplatin.
Previous studies have shown that aberrant miRNA
expression was implicated in the progression of chemoresistance in
gliomas. Guo et al demonstrated that Let-7b was
significantly decreased in cisplatin-resistant glioma cells
compared to wild-type cells, and overexpression of Let-7b
significantly increased cisplatin-induced apoptosis (10). Moreover, it has been reported that
miR-106a was significantly upregulated in cisplatin-resistant and
gefitinib-resistant glioma cells and that knockdown of miR-106a
enhanced the temozolomide-induced apoptosis in glioma cells
(11). Furthermore, it has been
shown that miR-136 and miR-139 were downregulated in human glioma
tissues and cells, and overexpression of miR-136 or miR-139
promotes cisplatin-induced or temozolomide-induced apoptosis in
glioma (12,13,18).
Based on these observations, dysregulated expression of miRNA may
determine the response of glioma cells to chemotherapy. In this
study, we found that miR-873 was downregulated in high-grade glioma
tissues compared to normal tissues and deceased in
cisplatin-resistant glioma cells compared with wild-type cells.
These results indicated that miR-873 may affect the sensitivity of
glioma cells to cisplatin. However, the cause of the low expression
of miR-873 in the cisplatin-resistant glioma cells remains to be
determined in further studies.
To date, few studies have reported on the function
of miR-873. Liu et al demonstrated that when stimulated by
IL-17, miR-873 promotes the activation of NF-κB and the production
of inflammatory cytokines in human multiple sclerosis (16). It has also been reported that
miR-873 is involved in the regulation of the Hedgehog signaling and
has relevance to physiological cranial bone development (20). miR-873 is downregulated in
tamoxifen-resistant breast tumor cell lines, while overexpression
of miR-873 reverses the tamoxifen resistance by targeting
cyclin-dependent kinase 3 (CDK3) (15). More importantly, cluster analysis
revealed that miR-873 is significantly downregulated in
glioblastomas (17) and that
ectopic expression of miR-873 reduces cell proliferation, migration
and invasion of glioma cells by targeting IGF2BP1 (14). Here, we demonstrated that
overexpression of miR-873 sensitized glioma cells to cisplatin by
arresting cell proliferation and inducing apoptosis as indicated by
the results of the MTT assay, colony formation assay, flow
cytometry, western blotting and caspase-3/7 activity assay.
However, whether miR-873 could sensitize glioma cells to other
chemotherapeutic drugs remains to be determined.
Bcl-2, a key anti-apoptotic protein, functions as an
oncogene on the basis that it promotes migration and invasiveness
of glioma cells (21,22). Moreover, inhibition of Bcl-2
expression enhances the chemosensitivity of glioma cells, including
sensitivity to cisplatin (23).
Many studies have confirmed that Bcl-2 is an important target for
miRNA in the regulation of the chemosensitivity of glioma cells
(13,24,25).
In this study, we used the luciferase assay to demonstrate that
Bcl-2 was a functional target of miR-873. Ectopic expression of
Bcl-2 could offset the miR-873-induced sensitivity of the glioma
cells to cisplatin. Nevertheless, many more genes are targeted by
miR-873, and the additional target genes need to be explored
further.
In conclusion, we demonstrated that miR-873 was
down-regulated in cisplatin-resistant glioma cells compared to
wild-type cells and that miR-873 enhanced the sensitization to
cisplatin by targeting Bcl-2. Our data suggest that miR-873 might
be a potential biomarker and a promising therapeutic strategy for
cisplatin-resistant glioma cells.
Acknowledgements
This study was supported by the National Natural
Sciences Foundation of China (nos. 81171127, 81371422, 81171577 and
81371790).
References
|
1
|
Baraniskin A, Kuhnhenn J, Schlegel U,
Maghnouj A, Zöllner H, Schmiegel W, Hahn S and Schroers R:
Identification of microRNAs in the cerebrospinal fluid as biomarker
for the diagnosis of glioma. Neuro-oncol. 14:29–33. 2012.
View Article : Google Scholar :
|
|
2
|
Gabayan AJ, Green SB, Sanan A, Jenrette J,
Schultz C, Papagikos M, Tatter SP, Patel A, Amin P, Lustig R, et
al: GliaSite brachytherapy for treatment of recurrent malignant
gliomas: A retrospective multi-institutional analysis.
Neurosurgery. 58:701–709; discussion 701–709. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Rocha CR, Garcia CC, Vieira DB, Quinet A,
de Andrade-Lima LC, Munford V, Belizário JE and Menck CF:
Glutathione depletion sensitizes cisplatin- and
temozolomide-resistant glioma cells in vitro and in vivo. Cell
Death Dis. 6:e17272015. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Cai Y, Yu X, Hu S and Yu J: A brief review
on the mechanisms of miRNA regulation. Genomics Proteomics
Bioinformatics. 7:147–154. 2009. View Article : Google Scholar
|
|
5
|
Patnaik S, Mallick R, Kannisto E, Sharma
R, Bshara W, Yendamuri S and Dhillon SS: MiR-205 and MiR-375
microRNA assays to distinguish squamous cell carcinoma from
adenocarcinoma in lung cancer biopsies. J Thorac Oncol. 10:446–453.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Khan S, Wall D, Curran C, Newell J, Kerin
MJ and Dwyer RM: MicroRNA-10a is reduced in breast cancer and
regulated in part through retinoic acid. BMC Cancer. 15:3452015.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Liu K, Zhang C, Li T, Ding Y, Tu T, Zhou
F, Qi W, Chen H and Sun X: Let-7a inhibits growth and migration of
breast cancer cells by targeting HMGA1. Int J Oncol. 46:2526–2534.
2015.PubMed/NCBI
|
|
8
|
Wang YW, Chen X, Gao JW, Zhang H, Ma RR,
Gao ZH and Gao P: High expression of cAMP-responsive
element-binding protein 1 (CREB1) is associated with metastasis,
tumor stage and poor outcome in gastric cancer. Oncotarget.
6:10646–10657. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Lai NS, Wu DG, Fang XG, Lin YC, Chen SS,
Li ZB and Xu SS: Serum microRNA-210 as a potential noninvasive
biomarker for the diagnosis and prognosis of glioma. Br J Cancer.
112(Suppl): 1241–1246. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Guo Y, Yan K, Fang J, Qu Q, Zhou M and
Chen F: Let-7b expression determines response to chemotherapy
through the regulation of cyclin D1 in glioblastoma. J Exp Clin
Cancer Res. 32:412013. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Wang Q, Wang Z, Chu L, Li X, Kan P, Xin X,
Zhu Y and Yang P: The effects and molecular mechanisms of miR-106a
in multidrug resistance reversal in human glioma U87/DDP and U251/G
cell lines. PLoS One. 10:e01254732015. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Chen W, Yang Y, Chen B, Lu P, Zhan L, Yu
Q, Cao K and Li Q: MiR-136 targets E2F1 to reverse cisplatin
chemosensitivity in glioma cells. J Neurooncol. 120:43–53. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Yang Y, Wu J, Guan H, Cai J, Fang L, Li J
and Li M: MiR-136 promotes apoptosis of glioma cells by targeting
AEG-1 and Bcl-2. FEBS Lett. 586:3608–3612. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Wang RJ, Li JW, Bao BH, Wu HC, Du ZH, Su
JL, Zhang MH and Liang HQ: MicroRNA-873 (miRNA-873) inhibits
glioblastoma tumorigenesis and metastasis by suppressing the
expression of IGF2BP1. J Biol Chem. 290:8938–8948. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Cui J, Bi M, Overstreet AM, Yang Y, Li H,
Leng Y, Qian K, Huang Q, Zhang C, Lu Z, et al: MiR-873 regulates
Erα transcriptional activity and tamoxifen resistance via targeting
CDK3 in breast cancer cells. Oncogene. 22–Dec;2014.(Epub ahead of
print). View Article : Google Scholar
|
|
16
|
Liu X, He F, Pang R, Zhao D, Qiu W, Shan
K, Zhang J, Lu Y, Li Y and Wang Y: Interleukin-17 (IL-17)-induced
microRNA 873 (miR-873) contributes to the pathogenesis of
experimental autoimmune encephalomyelitis by targeting A20
ubiquitin-editing enzyme. J Biol Chem. 289:28971–28986. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Skalsky RL and Cullen BR: Reduced
expression of brain-enriched microRNAs in glioblastomas permits
targeted regulation of a cell death gene. PLoS One. 6:e242482011.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Li RY, Chen LC, Zhang HY, Du WZ, Feng Y,
Wang HB, Wen JQ, Liu X, Li XF, Sun Y, et al: MiR-139 inhibits Mcl-1
expression and potentiates TMZ-induced apoptosis in glioma. CNS
Neurosci Ther. 19:477–483. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Lynam-Lennon N, Maher SG and Reynolds JV:
The roles of microRNA in cancer and apoptosis. Biol Rev Camb Philos
Soc. 84:55–71. 2009. View Article : Google Scholar
|
|
20
|
Koufaris C, Papagregoriou G, Kousoulidou
L, Moutafi M, Tauber M, Jouret B, Kieffer I, Deltas C, Tanteles GA,
Anastasiadou V, et al: Haploinsufficiency of the miR-873/miR-876
microRNA cluster is associated with craniofacial abnormalities.
Gene. 561:95–100. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Wick W, Wagner S, Kerkau S, Dichgans J,
Tonn JC and Weller M: BCL-2 promotes migration and invasiveness of
human glioma cells. FEBS Lett. 440:419–424. 1998. View Article : Google Scholar
|
|
22
|
Wick W, Wild-Bode C, Frank B and Weller M:
BCL-2-induced glioma cell invasiveness depends on furin-like
proteases. J Neurochem. 91:1275–1283. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Zhu CJ, Li YB and Wong MC: Expression of
antisense bcl-2 cDNA abolishes tumorigenicity and enhances
chemosensitivity of human malignant glioma cells. J Neurosci Res.
74:60–66. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Wang F, Li T, Zhang B, Li H, Wu Q, Yang L,
Nie Y, Wu K, Shi Y and Fan D: MicroRNA-19a/b regulates multidrug
resistance in human gastric cancer cells by targeting PTEN. Biochem
Biophys Res Commun. 434:688–694. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Zhu W, Xu H, Zhu D, Zhi H, Wang T, Wang J,
Jiang B, Shu Y and Liu P: miR-200bc/429 cluster modulates multidrug
resistance of human cancer cell lines by targeting BCL2 and XIAP.
Cancer Chemother Pharmacol. 69:723–731. 2012. View Article : Google Scholar
|