Introduction
It is well-known that iron is an essential element
and plays a crucial role in cellular proliferation and DNA
synthesis. Neoplastic cells have high requirement for iron for
their significantly elevated expression of the transferrin receptor
1 (1) and the higher levels of the
Fe-containing enzyme. The iron used for biological synthesis is
from labile iron pool (LIP) that also involve in the production of
reactive oxygen species (ROS) (2).
Thus, iron depletion and stimulation of iron-dependent free radical
damage is a rapidly developing field for anticancer drug discovery
(3,4).
It has been demonstrated that the mobilization of
iron from ferritin requires the use of a suitable reductant to
chemically reduce the ferrihydrite
(Fe2O3·nH2O) phosphate mineral
core. Besides a chemical reducing agent, light or ionizing
radiation is also used to trigger the mobilization of iron ions
from ferritin (5). Small bidentate
Fe(III) chelate ligands are capable of removing iron from the
ferritin inorganic core via direct extraction followed by diffusion
of the Fe(III)-chelate out of the protein shell (6), however, to achieve significant iron
release, high ligand concentration (3.5–100 mM) is required due to
the relatively low affinity of these ligands to iron. Recently,
Bou-Abdallah et al reported that 2,6-bis[(hydroxymethyl)
amino]-1,3,5-triazine (BHT) could rapidly release iron from human
recombinant ferritin in the presence of oxygen, and proposed a
mechanism that BHT-Fe(II) mediates the regeneration of superoxide
radical, which used for reduction of ferritin (7). The finding hints that some chelators
can make contribution to content of labile iron. In thermodynamics,
the redox potential of iron is decreased once it is chelated, that
the conversion between oxidation states of iron [Fe(III)/Fe(II)] is
easily occurred. This is fundamental for catalyzing a plethora of
biochemical redox processes. However, whether the chelators that
possess antitumor activity are also involved in oxygen-catalytic
iron mobilization is less clear.
Desferoxamine (DFO) is an iron chelator and used in
clinic to treat iron overload disease and in combination therapy of
doxorubicin to reduce the acute doxorubicin-induced cardiac, renal
and hepatic toxicity. The antiproliferative effects of
desferoxamine (DFO) are mediated by an intracellular pool of iron
(8). Dp44mt is a chelator of
thiosemicarbazone of di-2-pyridylketone, exhibiting excellent
antitumor activities via multiple models of action, such ROS
induction (9), topoisomerase
inhibition (10), and lysosome
inhibition (11). Similarly the
pyridine carboxylic acid hydrazones of di-2-pyridylketone are also
iron chelators, displaying excellent antitumor activity through ROS
generation or other mechanism. The extensive mechanistic studies
support that the ROS production is involved and is labile
iron-dependent, yet the contribution of chelators to LIP is not
fully understood. We hypothesize that the ROS induction by some
tridentate chelators, such as Dp44mt, may be involved in iron
mobilization and contribute to LIP (7). To test the hypothesis, a Dp44mt
analog, di-2-pyridylketone 2-pyridine carboxylic acid hydrazone
(DPPACH) was synthesized to probe this possibility. In the study,
the iron mobilization mediated by DPPACH from ferritin at different
conditions was investigated, the results indicated that the DPPACH
at lower concentration can release iron from ferritin
oxygen-dependently, which accordingly increased ROS production. The
results from ROS assay in vitro and in vivo as well
as DNA fragmentation indicate that the chelated Fe(II) and copper
are redox active, revealing the active species may be related to
their antitumor activities. Thus, DPPACH copper complex was also
evaluated in the study. The additional DNA relaxation assay showed
that both DPPACH and its copper complex possess weaker
topoisomerase IIa (Top IIa) inhibition. The above suggested that
DPPACH and its copper complex exhibit antitumor activities via ROS
induction and Top inhibition.
Materials and methods
Materials
All reactants and solvents were AR grade. MTT,
ethidium bromide (EB), di-2-pyridylketone, RPMI-1640, horse spleen
ferritin and other chemicals were purchased from Sigma-Aldrich (St.
Louis, MO, USA). The superoxide dismutase (SOD) was obtained from
the Beyotime Institute of Biotechnology (Beijing, China).
Preparation of DPPACH
DPPACH was made by refluxing di-2-pyridylketone with
2-pyridine carboxylic acid hydrazide in absolute ethanol for 4 h.
The 2-pyridine carboxylic acid hydrazide was prepared as described
(12). 1HNMR: 15.0 (s,
NH), 8.96 (d, H, J=4 Hz), 8.753 (d, H, J=4 Hz), 8.62 (d, H, J=4
Hz), 8.62 (d, H, J=4 Hz), 8.18 (d, H, J=8 Hz), 8.10 (d, H, J=8 Hz),
8.03 (tri, H, J=12 Hz), 7.70 (tri, H, J=12 Hz), 7.64 (tri, H, J=12
Hz) and 7.50 (tri, H, J=12 Hz). ESI-MS (microTOF-Q III; Bruker
Corp., Billerica, MA, USA): m/z: 304.1176 (M+H, calcd: 304.1198).
For copper complex (m/z): 401.0104 (M+CuCl, calcd:401.0104, weak):
365.0338 (M-H+Cu, calcd: 365.0338, major peak). The structures of
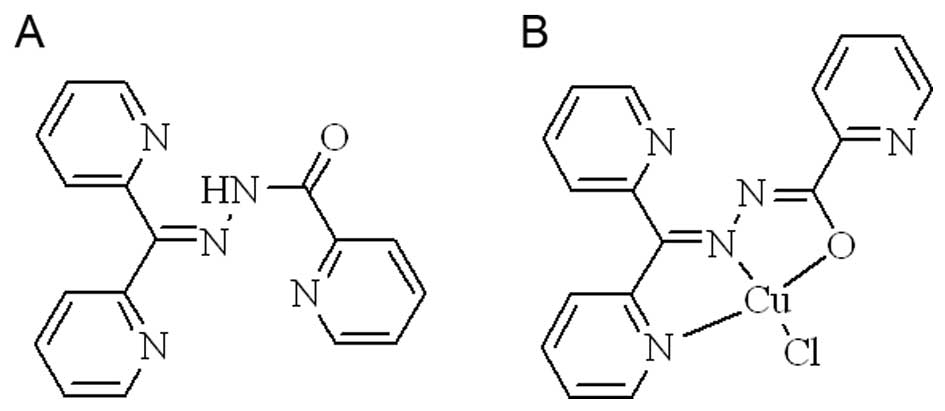
DPPACH and its copper in the study are shown below.
Determination of the mole ratio of DPPACH
to the Cu(II) complex
The stoichiometry of the reaction between DPPACH and
copper chloride was studied by Job's method of continuous
variations (13). For this method,
different volumes (0, 20, 40, 60, 80, 100, 120, 140, 160 and 180
μl) of 1 mM CuCl2 was added with different
volumes (200, 180, 160, 140, 120, 100, 80, 60, 40 and 20 μl)
of 1 mM DPPACH and diluted with water in 5 ml standard volumetric
flask. The absorbance was recorded on a Shimadzu UV-2450
spectrophotometer. The absorbance at 366 nm against the mole
fraction of the mole ratio of Cu(II) (or DPPACH) was plotted and
regressed linearly (SigmaPlot 10.0).
Iron mobilization by DPPACH
The iron release experiments were conducted in 0.05
M Tris-HCl, 50 mM NaCl, pH 7.4, 10 μl ferritin (12.5 mg/ml)
and varied concentration of DPPACH (or 3 unit SOD) in a total 1 ml
volume. The kinetics of iron release from ferritin was monitored by
the increase in the characteristic metal-to-ligand charge transfer
(MLCT) absorption bands of the corresponding Fe(II)-chelate
complexes (350 nm for DPPACH). The absorbance was measured every 5
min on a Shimadzu UV-2450 spectrophotometer with thermostatic
circulating device at 37°C.
ROS detection in vitro and in vivo
H2DCF-DA was converted to
dichlorofluorescin (DCF) according to literature (14). Briefly, 0.25 ml of 2 mM
H2DCF-DA in absolute ethanol was added to 2.0 ml of 10
mM NaOH and allowed to stand at room temperature for 30 min. The
hydrolysate was then neutralized with 10 ml of 25 mM sodium
phosphate buffer (pH 7.2), kept on ice until used. Reaction
mixtures contained either single reagent or multicomponent in 50 mM
sodium phosphate buffer (pH 7.4) with total 4 ml volume, i.e., 0.4
μM DCF, or with 6.25 μM
NH4FeSO4 (or CuCl2 or DPPACH) and
200 μM H2O2 (1 mM) for the Fenton
reactions. The fluorescence was measured on a FC-960
spectrofluorimeter (excitation at 488 and emission at 525 nm) in a
10 min time course at room temperature.
The intracellular ROS production was measured as
recommended (Beyotime Institute of Biotechnology). Approximately
106 HepG2 cells were collected and washed by PBS. The
cell pellet was re-suspended in H2DCF-DA containing
serum-free culture medium and incubated for 30 min. The stained
cells were re-collected and washed with serum-free culture medium.
Then 100 μl of the cell culture was transferred to
individual PCR tube and the test compound (or positive control) was
added, following 1-h incubation, the cell suspension was used
directly for ROS determination on a FC-960 spectrofluorimeter by
excitation at 488 and emission at 525 nm.
DNA cleavage assay
The DNA cleavage experiment was conducted by gel
electrophoresis. The reaction mixture was prepared as follows: 1
μl of pUC18 DNA (0.5 μg/μl), 5 μl of 1
mM DPPACH-Fe(II) [Cu(II); 1:1 molar ratio] in 8% dimethyl
sulphoxide (DMSO), and 1 μl of H2O2 (4
mM) followed by diluting with buffer (50 mM Tris-HCl, pH7.2 with 50
mM NaCl) to a total volume of 25 μl. The reaction mixture
was incubated at 37°C for 1 h. The 10 μl of the reaction
mixture with loading buffer were placed on 1.5% agarose gel and
subjected to electrophoresis. The bands were visualized by EB stain
and photographed on a Tocan 360 gel imager (version 3.2.1
software).
The comet assay was adapted from the literature as
described (15). HepG2 cells were
treated with or without the investigated compounds (12.5, 25
μM for DPPACH or 3, 6 μM for DPPACH-Cu complex) with
a 48-h incubation in a humidified atmosphere of 5% CO2.
The cells were harvested by centrifugation after trypsinization and
then embedded in 0.5% low melting-point agarose at a final
concentration of 104 cells/ml. Twenty microliters of
this cellular suspension was then spread onto duplicate frosted
slides that had previously been covered with 1% normal
melting-point agarose as a basal layer. Slides were allowed to
solidify for 10 min at 4°C before being placed in lysis buffer for
1 h (2.5 M NaCl, 0.1 M ethylenediaminetetraacetic acid (EDTA), 0.01
M Tris, 1% Triton X-100, 10% DMSO, pH 10.0). After lysis, the
slides were transferred into alkaline buffer for 40 min (0.001 M
EDTA, 0.3 M NaOH, pH >13.0) to allow the DNA to unwind before
migration at 0.66 V/cm and 300 mA for 30 min. All these steps were
performed in the dark. After neutralisation in 0.4 M Tris-HCl, pH
7.4, slides were stored at 4°C until analysis within 24 h. Before
analysis, the slides were stained with EB (20 μg/ml) and
covered with a cover-slip. The Images was captured using
fluorescent microscopy.
Cytotoxicity assay (MTT assay)
The stock solution of DPPACH (10 mM) was prepared in
80% DMSO, it was diluted to the required concentration with culture
when used. The copper complex was made by mixing DPPACH with high
concentration copper chloride based on 1:1 molar ratio and diluted
to required concentration with water. HCT-116 and HepG2 were
cultured in RPMI-1640 medium supplemented with 10% fetal calf serum
(FCS) and antibiotics. The cells collected during exponential-phase
(5×103/ml) were seeded equivalently into a 96-well plate
and the various amount of DPPACH (or its copper complex) was added
after the cells adhered. Following 48 h incubation at 37°C in a
humidified atmosphere of 5% CO2, 10 μl MTT
solution (5 mg/ml) was added to each well, followed by further
incubation of 4 h. The cell culture was removed by aspiration and
100 μl DMSO was added in each well to dissolve the formazan
crystals. The measurement of absorbance of the solution that was
related to the number of live cells was performed on a microplate
reader (MK3; Thermo Fisher Scientific) at 570 nm. Percent growth
inhibition was defined as the percent absorbance inhibition within
appropriate absorbance in each cell line. The assay was performed
in triplicate.
DNA Top activity assay
The nuclear extract from HepG2 cells was prepared as
previously described (14).
Nuclear extract (0.4 μg) was added to the Top reaction
mixture containing 10 mM Tris-HCl (pH 7.5), 1 mM EDTA, 150 mM NaCl,
0.1% bovine serum albumin (BSA), 5% glycerol and 0.4 μg
pUC18 and 1 μl (or 2, or 3 μl) test agent (1 mM
DPPACH or 1 mM DPPACH-Cu complex in 8% DMSO) at a final volume of
20 μl. Following incubation at 37°C for 30 min, the reaction
was terminated by adding 5 μl of stopping buffer (10% SDS,
0.025% bromophenol blue and 10% glycerol). The reaction products
were analyzed by electrophoresis on 1% agarose gel using a TBE
buffer with 0.1% SDS (89 mM Tris-HCl, 89 mM boric acid and 62 mM
EDTA) at 45 V for 3 h, stained by EB (0.5 μg/ml) and
photographed using a short wavelength UV lamp on Tocan 360 gel
scanner (Shanghai Tiancheng Technology Co., Ltd., Shanghai, China).
The assay was conducted in duplicates.
Molecular docking studies
The structure of human type II Top (3QX3) was
obtained from RCSB Protein Data Bank. The structure of DPPACH was
generated by Chemdraw (Chemdraw Ultra 8.0; CambridgeSoft,
Cambridge, MA, USA). Similarly the structure of DPPACH-Cu complex
was proposed based on stoichiometry obtained, mass spectrum and
coordination geometry of Cu(II) ion in literature (17). The energy minimization was
conducted by Chem3D (Chemdraw Ultra 8.0) (18). The resulting model was displayed in
PyMol (The PyMOL Molecular Graphics System, version 1.4.1;
Schrödinger, LLC, New York, NY, USA).
Molecular docking studies were performed by AutoDock
Vina and AutoDock Tools based on the recommended procedure
(19). Grid box was set to the
center of etoposide model, and the grid box size for Mannich base
models was set to 22, 24 and 28 for x, y and z axes, respectively.
The DPPACH or its copper complex was set as a flexible ligand by
using the default parameters of the AutoDock Tools. The optimal
conformation of the ligand was generated by AutoDock Vina.
Results
Syntheses and characterization of DPPACH
and its copper complex
To test the hypothesis that some tridentate
chelators are involved in iron mobilization from ferritin
oxygen-dependently, a tridentate chelator of DPPACH was prepared
and characterized by NMR and MS, the compound used in the study is
shown in Fig. 1A. The new chelator
is an analog of di-2-pyridylketone isonicotinoyl hydrazone and less
studied, and its metal chelating ability to copper or iron was
investigated because the metal complexes formed may be ‘active
species’ for their biological activity. The stoichiometric ratio
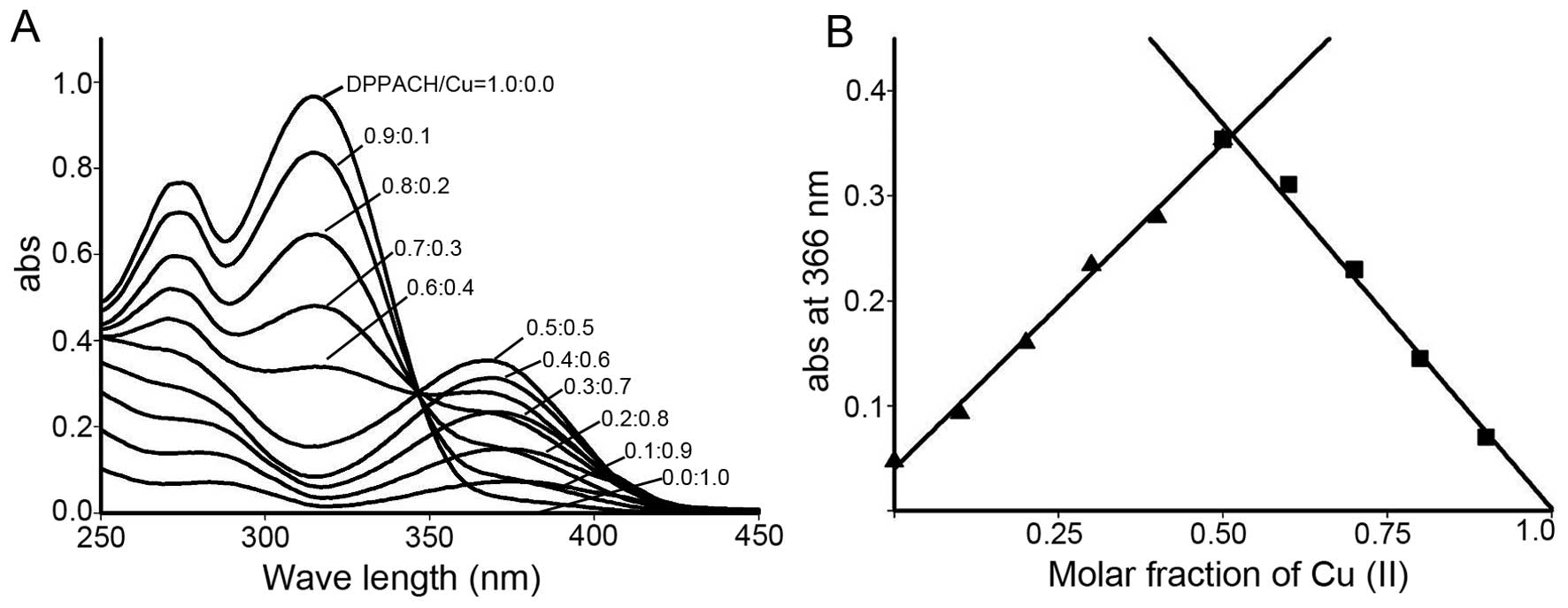
between DPPACH and copper chloride was determined by Job's method
of continuous variations. A new peak around 366 nm was due to the
chelation of the DPPACH with Cu(II). The plot of absorbance versus
mole fraction of DPPACH confirmed a new species (complex) with
ratio 1:1 between DPPACH and copper ion (Fig. 2). Further supportive evidence from
ESI-MS, indicated the predominated monomeric peak
[Cu(DPPACH-H)]+ with an m/z=365.0338 was found,
indicating the DPPACH in the copper complex was in enoled manner. A
six-fold less minor peak at m/z=401.0104 was also detected in this
mixture, suggesting a 1:1 ligand-to-copper complex was the active
species. The structure of copper complex was proposed (Fig. 1B). The stoichiometric ratio of
DPPACH/Fe(II) was not determined by the spectrometric method due to
the varied ratio, yet the new peak (350 nm) was identified to be
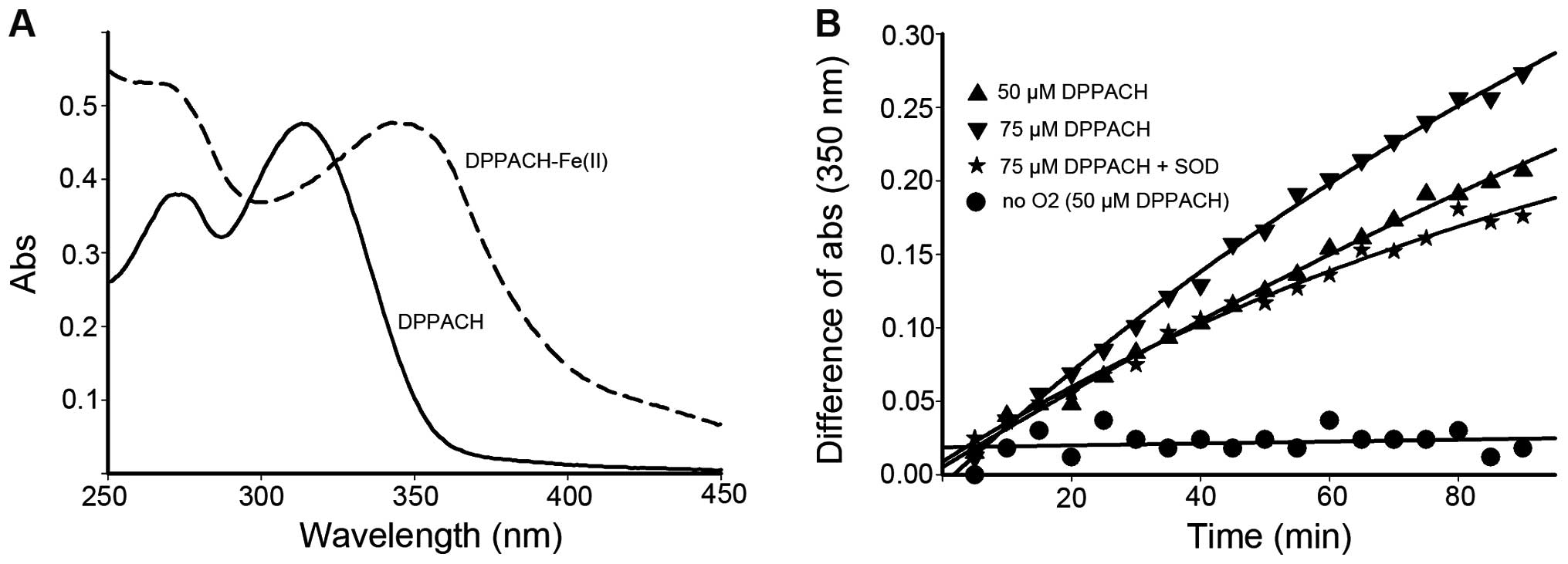
DPPACH-Fe(II) complex based on the UV-visible spectrum (Fig. 3A).
DPPACH involved in oxygen-catalytic iron
mobilization from ferritin
To test whether DPPACH was capable of removing iron
from the ferritin, the low concentration of DPPACH was used in the
assay, as the direct extraction by chelators required much higher
concentration (3.5–100 mM) to diffuse the Fe(III)-chelate out of
the protein shell. The iron release from ferritin were traced by
monitoring the increase of the characteristic MLCT absorption band
which corresponded to Fe(II)-DPPACH complexes (Fig. 3A, absorption at ~350 nm). As shown
in Fig. 3B, the iron release was
increased with increasing DPPACH in the time course, and the
profiles of iron release were in an exponential rising manner. To
determine whether superoxide radical was involved, the SOD was
added, showing that SOD inhibited significantly the iron release
thus indicating the involvement of ROS in the process of iron
removal from ferritin. To corroborate this finding, iron release
experiments under anaerobic conditions was conducted, the data
indicated almost complete inhibition of iron release by DPPACH,
suggesting that molecular oxygen is required to initiate the
generation of ROS which then reduce the mineral core and release
Fe(II), Those supporting that DPPACH could release iron from
ferritin in a oxygen-catalytic manner as previously reported
(7).
DPPACH and its copper complex induce ROS
generation
Since DPPACH was able to remove iron from ferritin,
the formed ferrous iron complex may act as either contributor to
LIP or bystander, depending on the redox characteristic of iron
DPPACH complex, thus DPPACH induction of ROS was an indicative
factor. DCF was chosen to assess ROS generation. As shown in
Fig. 4A, fluorescence intensity of
DCF was the highest in the presence of DPPACH, indicating that iron
DPPACH complex more efficiently induced ROS formation in Fenton
reaction and the complex was redox-active. In contrast to the
DPPACH-Fe(II), DPPACH-Cu(II) was also active in ROS generation, but
weaker in terms of fluorecence intensity, however, once
DPPACH-Cu(II) was partly reduced by ascorbic acid (Vc), the
DPPACH-Cu(I) was efficient in ROS generation compared to its
oxidized form. Based on the above results, we speculated that the
DPPACH-Fe(II) complex may contribute to LIP when ferritin and
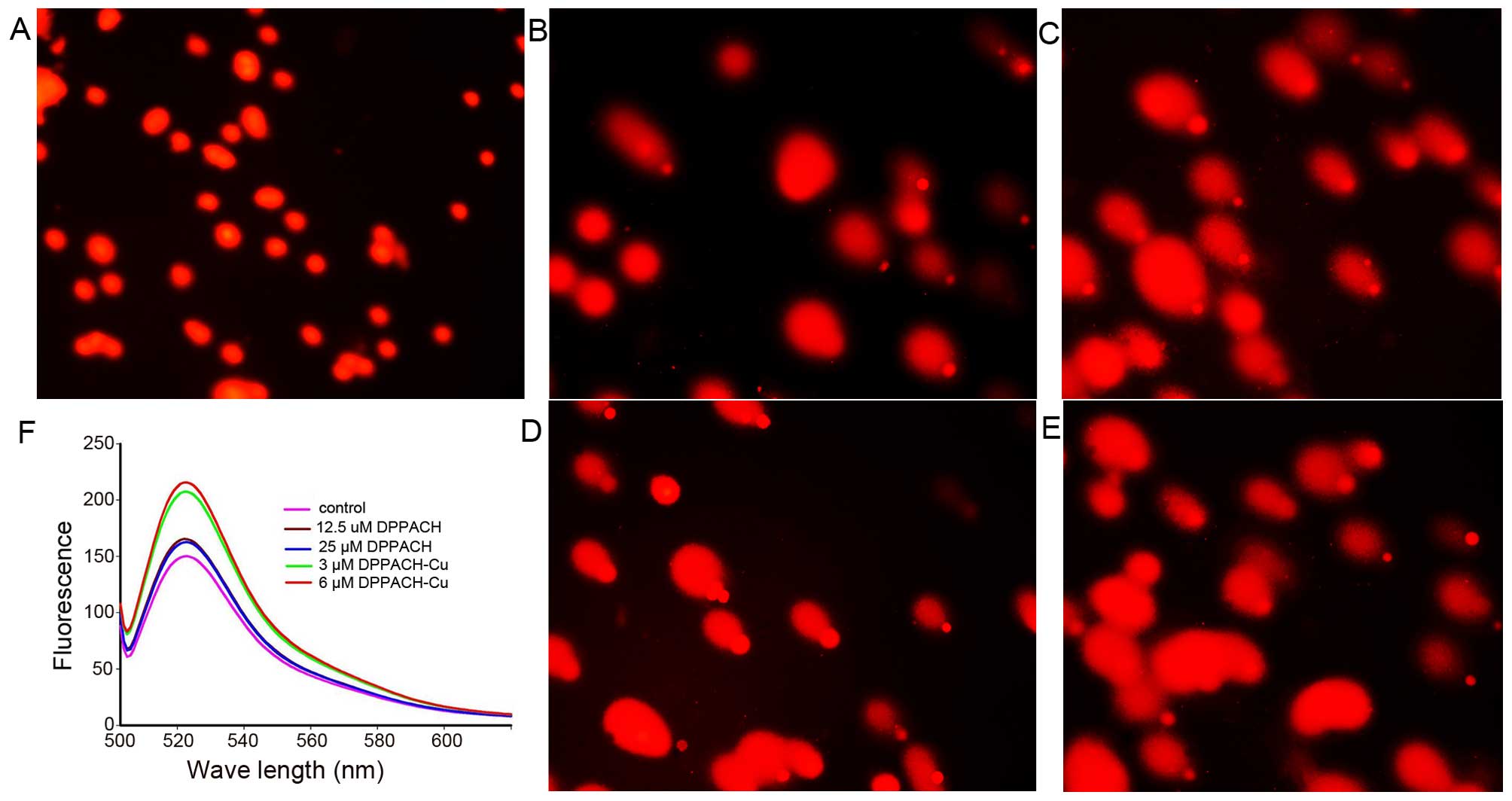
DPPACH co-existed in cells. Fig.
5F shows the ROS induction by the investigated agents in
cellular level (in vivo), both DPPACH and its copper complex
induced ROS generation in a concentration dependent manner,
indicating that the excess ROS induction occurred in vivo.
It was noted that the fluorescent intensities of DCF from copper
complex treated cells were significantly greater than that of
DPPACH, which might be related to the redox feature of
Cu2+/1+ complexes, because the Cu(I) complex is able to
also react with molecular oxygen to form the superoxide radical
except with H2O2 (20).
 | Figure 4In vitro ROS generation and
DNA cleavage caused by DPPACH and its copper complex. (A) Fenton
reaction derived ROS generation, the agents as indicated in the
figure and the ascorbic acid (Vc) used for reduction of Cu(II). The
ROS content expressed as intensity of DCF fluorescence. (B) The
Fe(II), Cu(II) and their DPPACH complexes caused DNA cleavage. Line
1, pUC18 only; line 2, 25 μM DPPACH-Cu(II); line 3, 50
μM DPPACH-Cu(II); line 4, 75 μM DPPACH-Cu(II); line
5, 25 μM DPPACH-Cu(II)+Vc; line 6, 50 μM
DPPACH-Cu(II)+Vc; line 7, 75 μM DPPACH-Cu(II)+Vc; line 8, 25
μM DPPACH-Fe(II); line 9, 50 μM DPPACH-Fe(II); line
10, 75 μM DPPACH-Fe(II). I, supercoiled; II, linear and III,
cleaved DNA. |
DNA fragmentation induced by DPPACH and
its copper complex
It has been demonstrated that ROS caused oxidative
damage of DNA, protein and lipid. The oxidative breakage of pUC18
by the investigated agents was conducted by agarose gel
electrophoresis. As expected in the assay, the supercoiled DNA
decreased, and cleaved DNA increased, with increasing
concentration, supporting that ROS is involved in DNA fragmentation
(Fig. 4B). Similarly the effect of
the agents on DNA integrity of HepG2 cells also supported the
results from that in vitro. As shown in Fig. 5A–E, a typical image of a migrated
cell nucleus with DNA strand breaks had migrated sufficiently to
form the ‘teardrop’ tail, and the greater portion of the DNA had
fragmented in concentration-dependent manner. It was noted that the
copper complex displayed stronger effect on nucleic DNA damage
compared to DPPACH (Fig. 5D and
E), showing that the copper complex more efficiently induced
DNA fragmentation, that was consistent with the results from ROS
generation in vivo (Fig.
5F), yet the underlying mechanism is not fully understood.
The cytotoxicity of the DPPACH and its
copper complex
The DPPACH and its copper complex induced ROS
generation and oxidative damage of DNA in vitro and in
vivo, this motivated us to probe their potent antitumor
activity. The inhibitory effects of the agents on the proliferation
of the HepG2 and HCT-116 cell lines was evaluated and the
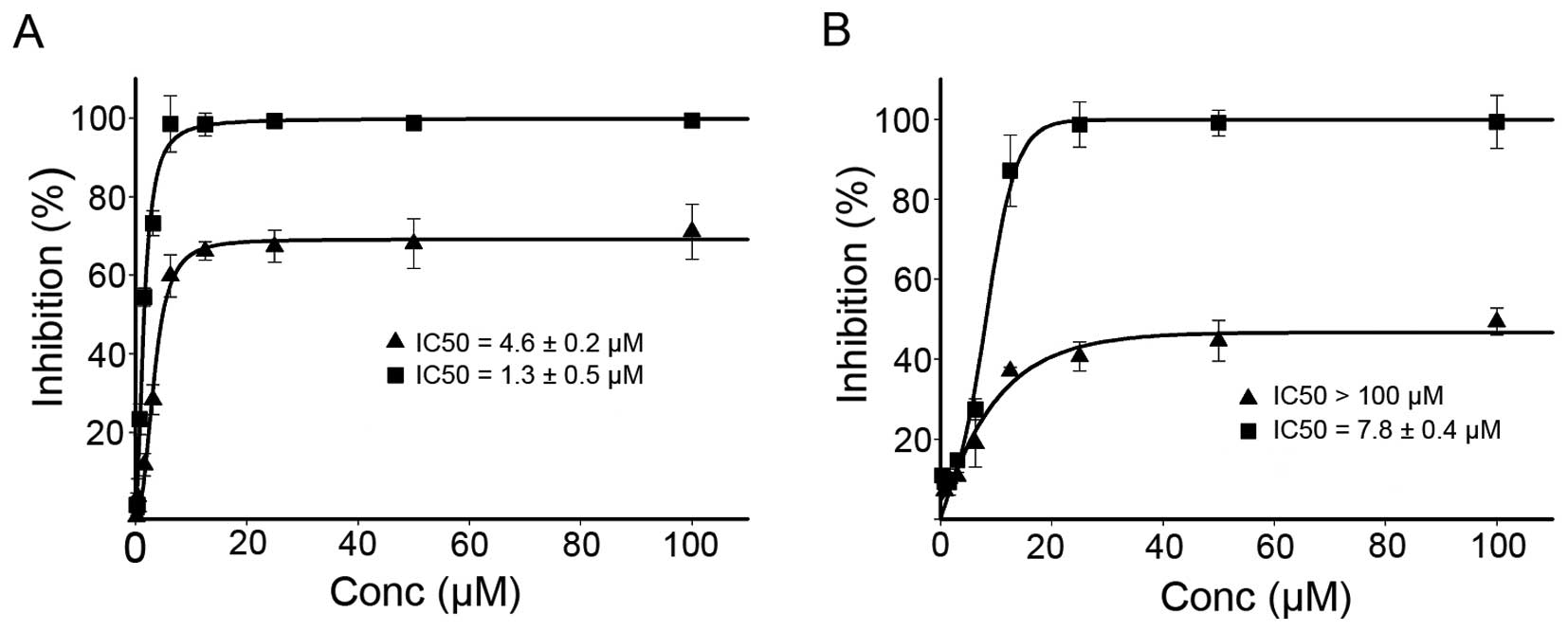
dose-response curves are depicted in Fig. 6. As shown in Fig. 6, DPPACH had significant growth
inhibition for HepG2 (IC50: 4.6±0.2 μM), but the
maximal inhibition was ~60%. It was noted that the DPPACH had poor
growth inhibition for HCT-116 cell lines (IC50: >100
μM), the maximal inhibition was only ~40%, showing a certain
degree of selectivity between the two cell lines for DPPACH. The
DPPACH copper complex exhibited excellent antitumor activity for
each cell line. The IC50 of the copper complex was
1.3±0.5 μM for HepG2 cell, an ~3-fold increase in
proliferation inhibition compared to that of DPPACH. Similar trend
against HCT-116 cell line was also observed, the IC50 of
the copper complex was 7.8±0.4 μM, an ~15-fold increase. The
data indicated that the antitumor activities exhibited by the
agents at least was partly correlated to their ability of ROS
induction in vivo.
DNA relaxation inhibition
The excellent antitumor activities for some copper
complexes have been recognized, yet the underlying mechanism was
not defined. In the present study, the antitumor activities of
copper complex was not fully correlated to its ability of ROS
generation, implying that some other mechanism occurred. Dp44mt was
reported as Top inhibitor, as its analog, the DPPACH might have
similar action. To determine whether the DPPACH and its copper
complex recapitulate such activity, pUC18 plasmid DNA was incubated
with nucleic extract in the presence of a varied concentration of
the investigated agents, and reaction products were analyzed by gel
electrophoresis. As shown in Fig.
7A, the DPPACH and its copper complex did not exhibit any
inhibition for Top I at given condition (without ATP in the assay),
while in the presence of ATP, both displayed certain degree of
inhibition based on EtB pre-stained agarose gel after 4 h
electrophoresis (Fig. 7B). As
shown in Fig. 7B, the relaxed DNA
was observed and migrated faster than the supercoiled DNA in the
control line, the cleaved DNA was increased with increasing
concentration of both reagents, and the copper complex was stronger
than DPPACH. The Top II cleavage complex was identified by
comparison with the positive control of etoposide, however in our
experiment the Top II cleavage complex was not observed. This
situation has occurred in other reported cases due to lower Top II
concentration used in the assay, but the cleaved DNA (Fig. 7B) increased with increasing of the
agents, implying that there was the possibility of catalytic or
‘poisoner’ inhibition.
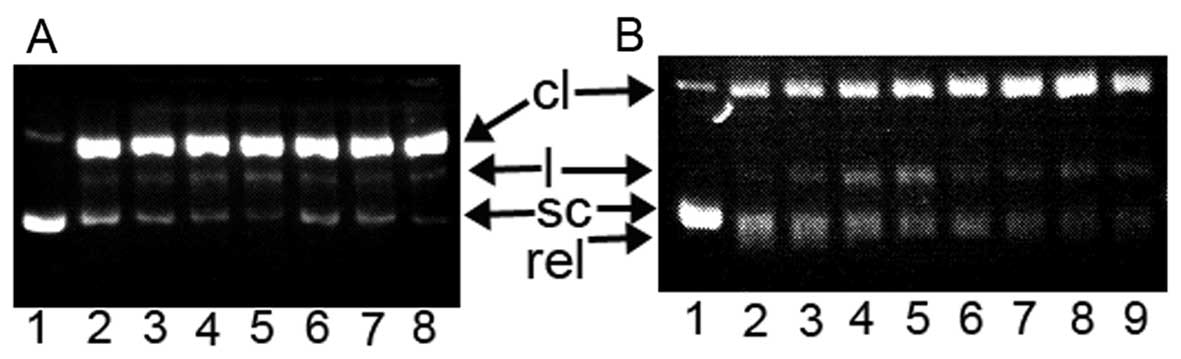 | Figure 7Topoisomerase (Top) inhibition of
DPPCAH and its copper complex. sc, supercoiled DNA; cl, cleaved
DNA; rel, relaxed DNA; and l, linear DNA. (A) Type I Top
inhibition. Lane 1, pUC18 only; lane 2, no reagent; lane 3, 150
μM DPPCAH; lane 4, 100 μM DPPCAH; lane 5, 50
μM DPPCAH; lane 6, 150 μM DPPCAH-Cu; lane 7, 100
μM DPPCAH-Cu; lane 8, 50 μM DPPCAH-Cu. (B) Type II
Top inhibition (EB prestained gel). Lane 1, pUC18 only; lane 2, no
reagent; lane 3, 150 μM DPPCAH; lane 4, 100 μM
DPPCAH; lane 5, 50 μM DPPCAH; lane 6, 150 μM
DPPCAH-Cu; lane 7, 100 μM DPPCAH-Cu; lane 8, 50 μM
DPPCAH-Cu; lane 9, 300 μM etoposide. |
Molecular docking studies
Top inhibition of the DPPACH and its copper complex
in vitro prompted us to probe the potent structural basis,
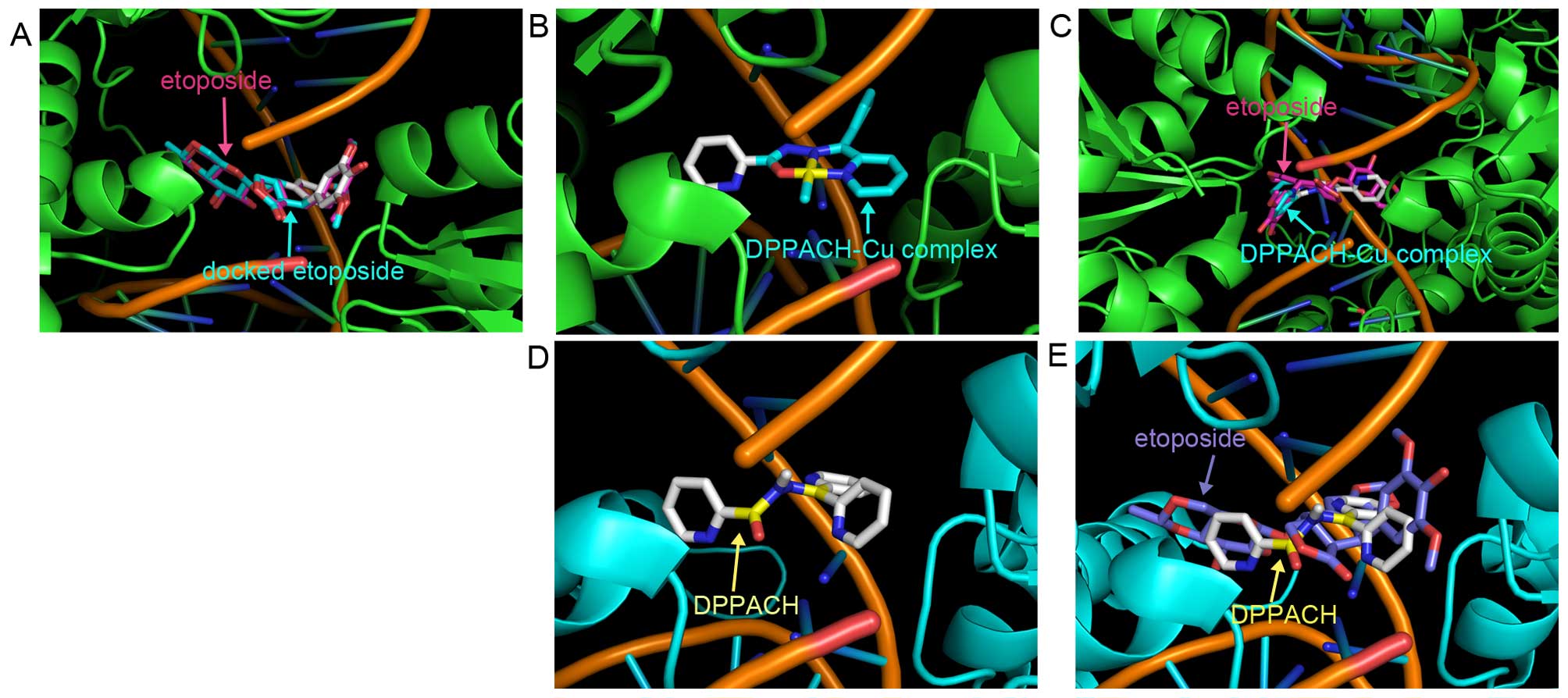
and molecular docking was conducted. The human type II Top crystal
structure (PDB ID: 3QX3) was from RCSB Protein Data Bank. In order
to evaluate the accuracy of our docking protocol, etoposide was
re-docked into the Top-DNA complexes based on a recommended
procedure (Fig. 8A). The
Top-etoposide complex derived from docking simulation showed that
the docked etoposide was almost fully superimposed on the native
co-crystallized one (Fig. 8A).
Following the same protocol DPPACH and its copper complex were
individually docked into Top II (Fig.
8B and D), the simulating affinity energies for them were −10.6
and −11.2 kcal/mol, respectively. Compared to that of docked
etoposide, −14.8 kcal/mol, the affinity energies of the DPPACH and
its copper complex with the Top were weaker than that of the
clinically used drug. The superimposition of DPPACH-Cu complex and
DPPACH on the co-crystallized etoposide are presented in Fig. 8C and E. The affinity energies from
docking simulation were correlated to their Top inhibition
activity.
Discussion
Cytosolic LIP is a pool of chelatable and
redox-active iron, which is transitory and serves as a crossroad of
cell iron metabolism (21).
Release of intracellular iron from iron-sequestering proteins
facilitates to the increases in redox-active LIP (22). Direct extraction from ferritin by
some bidentate chelators has been well documented, and in the
process the oxidative state of iron is not changed (6). Recent finding that BHT chelator is
involved in oxygen catalyzed iron mobilization from ferritin may
changevthe above concept (7),
prompting that some chelators are capable of removing iron from
ferritin of the cell, however, this contribution from chelators
that possess antitumor activity has been neglected. The
aroylhydrazones and thiosemicarbazones have been demonstrated
selective antiproliferative activity against tumor cells (23,24),
which have been attributed to their induction of cellular oxidative
stress by Fenton-type free radical generation (23). Whether the chelators are involved
in iron mobilization was not studied. We speculate some tridentate
chelators may act as iron releaser from ferritin in an
oxygen-catalytic manner, contributing to LIP and corresponding
biological activity. To address this issue, the DPPACH, an analog
of di-2-pyridylketone isonicotinoyl hydrazone was synthesized and
evaluated on iron mobilization. The results demonstrated that
DPPACH released iron from ferritin at lower concentration and was
more efficient than the reported compounds. The addition of SOD
inhibited significantly the iron release, indicating the
involvement of ROS during iron release from ferritin. ROS could be
a superoxide radical from aerobic environment, if so, the iron
mobilization would be blocked under anaerobic conditions. Our data
support a mechanism that DPPACH mobilizes iron from ferritin in an
oxygen-dependent manner, which may also contribute to its ROS
induction.
It has been demonstrated that the antiproliferative
activity of some chelators relates to their ability to enhance
redox activity of cellular iron (23). However, it is also found that there
are redox-inactive species after chelator-iron complexes formed due
to the redox potential outside the accessible range for redox
cycling (25). Thus, determination
of the redox activity of iron in the presence of the DPPACH in
vitro is required. The fluorescent intensity of DCF is widely
used for ROS detection is indicative of redox activity of the agent
(26). Our data (Fig. 3A) clearly showed that the
DPPACH-Fe(II) complex was more efficient to boost the ROS
generation among the test compounds, indicating that it was a redox
active species. The DPPACH-Cu(II) complex induced ROS production in
the absence or presence of Vc was also evaluated. In contrast to
the iron complex, the copper complex exhibited weak ability in ROS
generation, however, the addition of Vc significantly increased its
capability, indicating that the DPPACH-Cu complexes at different
oxidation state are redox-active. It was noted that DPPACH-Cu
complex at lower concentration induced efficiently ROS generation
in vivo (Fig. 5F), which
were found in many studies (27,28).
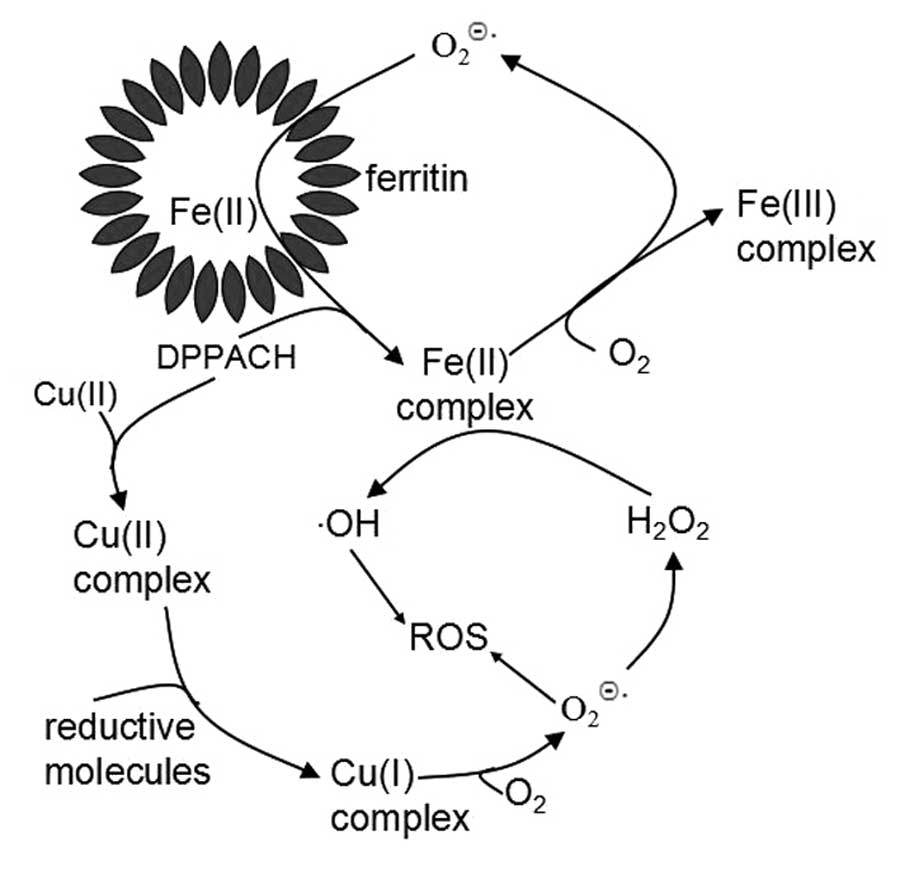
Based on above results, we propose the pathways of ROS generation
for DPPACH and its copper complex (Fig. 9), in which an oxygen-catalytic iron
mobilization from ferritin was involved for DPPACH, and the formed
ferrous complex may also participate in Fenton type ROS generation
(Fig. 9). The mechanism related to
copper complex was included in Fig.
9 to increase our understanding of the relationship between ROS
and antiproliferative activity of chelators. ROS induce chromatin
dysfunction such as single- and double-strand DNA fragmentation,
leading to cell death through apoptosis or necrosis as have been
documented (29,30). DNA cleavage caused by the
DPPACH-Fe(II) and DPPACH-Cu were fully correlated to that from ROS
assay in vitro. The difference in DNA fragmentation in the
comet assay, was noted as the DNA cleavage required lower
concentration of the copper comple. Even though slight difference
in ROS generation in vitro and in vivo was observed,
cellular DNA cleavage is consistent with their ability of ROS
induction in vivo (Fig.
5F). The data supported that DPPACH and its copper exhibited
antitumor activity via ROS generation.
Top inhibitors are important targets for drug design
to disturb growth of tumor cells. Clinically used Top inhibitors
are mostly aromatic fused organic compounds (31), including some metal complexes
(32). The iron chelator Dp44mt
was proposed to exhibit its antitumor activity via Top inhibition
although it was reported to possess paradoxical function (33). In the present study, both DPPACH
and its copper complex exhibited certain degree of Top IIα
inhibition. In a previous study, we found that the copper complex
of 2-pyridinecarboxaldehyde 2-pyridinecarboxylic acid hydrazone, an
analog of DPPACH, possessed dual inhibition of Top (34), the slight difference of ligand in
structure revealed their structure active relationship. Our data
demonstrated that the antitumor mechanism for DPPACH and its copper
complex were involved in type II Top inhibition and ROS generation,
both contributed to DNA cleavage, thus their antitumor activities
at least partly contributed to Top inhibition. Due to lack of
evidence from crystal structure, how the compounds interact with
Top, via competitive ATP binding or the DNA complex or allosteric
effect to disturb unwinding DNA helix are unknown. For insight into
the molecular mechanism, an in silico study to simulate the
potent interaction between the compound and the DNA-Top complex may
be helpful. AutoDock software is widely used to predict the binding
information of test compounds between enzyme and the inhibitor
(35). Based on the recommended
procedure, the DPPACH and its copper were docked into the structure
of human Top IIa. A square planar geometry of copper complex was
chosen based on a similar crystal structure (17). It is interesting that the affinity
energy of DPPACH was smaller than that of its copper complex, which
was consistent with the results from top inhibition.
In conclusion, DPPACH has the ability to remove iron
from ferritin in a oxygen-catalytic manner and forms a redox-active
iron complex. Moreover, the chelated iron may also be from LIP,
both contribute to redox activity of LIP, ROS generation and
antitumor activity, but the contribution of the chelator (DPPACH)
in iron mobilization from ferritin should not be neglected. The
cellular DNA fragmentation is related to both Top II inhibition and
ROS induction of DPPACH and its copper complex. However, the
contributing fraction of DPPACH-Fe(II) from ferritin to its
antitumor activity and copper complex induced ROS was not defined,
and require more study in the future.
Acknowledgements
The authors would like to thank Miss Haiyan Yu,
Yanqiu Ma, Shuang Wang and Xia Wu, undergraduates from the
Department of Pharmacy of San Quan College, Xinxiang Medical
University for their help in ROS assay. The present study was
supported by grants from the Henan Science and Technology Agency
(no. 210073), the Xinxiang Medical University (no. 505026) and the
Plan of Health Scientific and Technological Innovation Talents (no.
2109901) of Henan Province for Shaoshan Li.
References
|
1
|
Chen Z, Zhang D, Yue F, Zheng M, Kovacevic
Z and Richardson DR: The iron chelators Dp44mT and DFO inhibit
TGF-β-induced epithelial-mesenchymal transition via up-regulation
of N-Myc downstream-regulated gene 1 (NDRG1). J Biol Chem.
287:17016–17028. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Valko M, Rhodes CJ, Moncol J, Izakovic M
and Mazur M: Free radicals, metals and antioxidants in oxidative
stress-induced cancer. Chem Biol Interact. 160:1–40. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Wondrak GT: Redox-directed cancer
therapeutics: Molecular mechanisms and opportunities. Antioxid
Redox Signal. 11:3013–3069. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Crespo-Ortiz MP and Wei MQ: Antitumor
activity of artemisinin and its derivatives: from a well-known
antimalarial agent to a potential anticancer drug. J Biomed
Biotechnol. 2012:2475972012. View Article : Google Scholar
|
|
5
|
Wolszczak M and Gajda J: Iron release from
ferritin induced by light and ionizing radiation. Res Chem
Intermed. 36:549–563. 2010. View Article : Google Scholar
|
|
6
|
Sánchez P, Gálvez N, Colacio E, Miñones E
and Domínguez-Vera JM: Catechol releases iron(III) from ferritin by
direct chelation without iron(II) production. Dalton Trans.
2005:811–813. 2005. View
Article : Google Scholar
|
|
7
|
Bou-Abdallah F, McNally J, Liu XX and
Melman A: Oxygen catalyzed mobilization of iron from ferritin by
iron(III) chelate ligands. Chem Commun (Camb). 47:731–733. 2011.
View Article : Google Scholar
|
|
8
|
Saad SY, Najjar TA and Al-Rikabi AC: The
preventive role of deferoxamine against acute doxorubicin-induced
cardiac, renal and hepatic toxicity in rats. Pharmacol Res.
43:211–218. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Jansson PJ, Hawkins CL, Lovejoy DB and
Richardson DR: The iron complex of Dp44mT is redox-active and
induces hydroxyl radical formation: An EPR study. J Inorg Biochem.
104:1224–1228. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Rao VA, Klein SR, Agama KK, Toyoda E,
Adachi N, Pommier Y and Shacter EB: The iron chelator Dp44mT causes
DNA damage and selective inhibition of topoisomerase IIalpha in
breast cancer cells. Cancer Res. 69:948–957. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Lovejoy DB, Jansson PJ, Brunk UT, Wong J,
Ponka P and Richardson DR: Antitumor activity of metal-chelating
compound Dp44mT is mediated by formation of a redox-active copper
complex that accumulates in lysosomes. Cancer Res. 71:5871–5880.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Fu Y, Zhou SF, Liu YX, Yang Y, Sun X and
Li C: The cytotoxicity of benzaldehyde nitrogen mustard-2-pyridine
carboxylic acid hydrazone being involved in topoisomerase IIa
inhibition. Biomed Res Int. 2014:5270422014. View Article : Google Scholar
|
|
13
|
Likussar W and Boltz DF: Theory of
continuous variations plots and a new method for spectrophotometric
determination of extraction and formation constants. Anal Chem.
43:1265–1272. 1971. View Article : Google Scholar
|
|
14
|
Jakubowski W and Bartosz G:
2,7-dichlorofluorescin oxidation and reactive oxygen species: What
does it measure? Cell Biol Int. 24:757–760. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Singh NP, McCoy MT, Tice RR and Schneider
EL: A simple technique for quantitation of low levels of DNA damage
in individual cells. Exp Cell Res. 175:184–191. 1988. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Osheroff N, Shelton ER and Brutlag DL: DNA
topoisomerase II from Drosophila melanogaster. Relaxation of
supercoiled DNA. J Biol Chem. 258:9536–9543. 1983.PubMed/NCBI
|
|
17
|
Banerjee S, Sen S, Basak S, Mitra S,
Hughes DL and Desplanches C: Two new pseudohalide-bridged Cu(II)
complexes with a hydrazone ligand: Syntheses, crystal structures
and magnetic studies. Inorg Chim Acta. 361:2707–2714. 2008.
View Article : Google Scholar
|
|
18
|
Ok K, Jung YW, Jee JG and Byun Y: Facile
docking and scoring studies of carborane ligands with estrogen
receptor. Bull Korean Chem Soc. 34:1051–1054. 2013. View Article : Google Scholar
|
|
19
|
Trott O and Olson AJ: AutoDock Vina:
Improving the speed and accuracy of docking with a new scoring
function, efficient optimization, and multithreading. J Comput
Chem. 31:455–461. 2010.
|
|
20
|
Boukhalfa H and Crumbliss AL: Chemical
aspects of siderophore mediated iron transport. Biometals.
15:325–339. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Kakhlon O and Cabantchik ZI: The labile
iron pool: Characterization, measurement, and participation in
cellular processes(1). Free Radic Biol Med. 33:1037–1046. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Stäubli A and Boelsterli UA: The labile
iron pool in hepatocytes: Prooxidant-induced increase in free iron
precedes oxidative cell injury. Am J Physiol. 274:G1031–G1037.
1998.PubMed/NCBI
|
|
23
|
Becker EM, Lovejoy DB, Greer JM, Watts R
and Richardson DR: Identification of the di-pyridyl ketone
isonicotinoyl hydrazone (PKIH) analogues as potent iron chelators
and anti-tumour agents. Br J Pharmacol. 138:819–830. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Mylonas S and Mamalis A: Synthesis and
antitumor activity of new thiosemicarbazones of
2-acetylimidazo[4,5-b]pyridine. J Heterocycl Chem. 42:1273–1281.
2005. View Article : Google Scholar
|
|
25
|
Chaston TB, Watts RN, Yuan J and
Richardson DR: Potent antitumor activity of novel iron chelators
derived from di-2-pyridylketone isonicotinoyl hydrazone involves
fenton-derived free radical generation. Clin Cancer Res.
10:7365–7374. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Korystov YN, Shaposhnikova VV, Korystova
AF and Emel'yanov MO: Detection of reactive oxygen species induced
by radiation in cells using the dichlorofluorescein assay. Radiat
Res. 168:226–232. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Fatfat M, Merhi RA, Rahal O, Stoyanovsky
DA, Zaki A, Haidar H, Kagan VE, Gali-Muhtasib H and Machaca K:
Copper chelation selectively kills colon cancer cells through redox
cycling and generation of reactive oxygen species. BMC Cancer.
14:5272014. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Gaetke LM and Chow CK: Copper toxicity,
oxidative stress, and antioxidant nutrients. Toxicology.
189:147–163. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Jacquemin G, Margiotta D, Kasahara A,
Bassoy EY, Walch M, Thiery J, Lieberman J and Martinvalet D:
Granzyme B-induced mitochondrial ROS are required for apoptosis.
Cell Death Differ. 22:862–874. 2015. View Article : Google Scholar
|
|
30
|
Xiao D, Vogel V and Singh SV: Benzyl
isothiocyanate-induced apoptosis in human breast cancer cells is
initiated by reactive oxygen species and regulated by Bax and Bak.
Mol Cancer Ther. 5:2931–2945. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Pinar A, Yurdakul P, Yildiz I,
Temiz-Arpaci O, Acan NL, Aki-Sener E and Yalcin I: Some fused
heterocyclic compounds as eukaryotic topoisomerase II inhibitors.
Biochem Biophys Res Commun. 317:670–674. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Fu Y, Yang Y, Zhou S, Liu Y, Yuan Y, Li S
and Li C: Ciprofloxacin containing Mannich base and its copper
complex induce antitumor activity via different mechanism of
action. Int J Oncol. 45:2092–2100. 2014.PubMed/NCBI
|
|
33
|
Yalowich JC, Wu X, Zhang R, Kanagasabai R,
Hornbaker M and Hasinoff BB: The anticancer thiosemicarbazones
Dp44mT and triapine lack inhibitory effects as catalytic inhibitors
or poisons of DNA topoisomerase IIα. Biochem Pharmacol. 84:52–58.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Yang Y, Huang T, Zhou S, Fu Y, Liu Y, Yuan
Y, Zhang Q, Li S and Li C: Antitumor activity of a
2-pyridinecarboxaldehyde 2-pyridinecarboxylic acid hydrazone copper
complex and the related mechanism. Oncol Rep. 34:1311–1318.
2015.PubMed/NCBI
|
|
35
|
Huang B: MetaPocket: A meta approach to
improve protein ligand binding site prediction. OMICS. 13:325–330.
2009. View Article : Google Scholar : PubMed/NCBI
|