Introduction
Prostate cancer (PCa) is the second leading cause of
male cancer death in the Western world (1). PCa patients can be clinically
categorized into different risk groups primarily based on
histological grade (Gleason score), clinical TNM stage, and levels
of serum prostate specific antigen (2). Aggressive, poorly differentiated
high-grade PCa is incurable and potentially lethal, underscoring
the need for a greater understanding of the molecular basis of PCa
progression and improved approaches to eliminating the development
of the lethal phenotype of PCa.
Although tumorigenesis is largely acknowledged as a
cell-autonomous process involving genetically transformed cancer
cells, the importance of stromal cell types populating the tumor
microenvironment is now well established (3,4).
Reactive stroma is composed of several heterotypic cells, including
cancer associated fibroblasts (CAFs), macrophages, and endothelial
cells. CAFs are phenotypically and functionally distinguishable
from their normal counterparts based on their augmented
proliferation and differential expression of extracellular matrix
(ECM) proteins and soluble factors (5). Unlike resting fibroblasts, CAFs
acquire an activated phenotype and can be recognized by their
expression of fibroblast-specific protein 1, desmin, vimentin, and
α-smooth muscle actin (α-SMA). To date, fibroblasts in tumor
tissues are recognized as a diverse population of myofibroblastic
cells intermixed with other fibroblasts that do not express α-SMA,
although they exert tumor promoting activity (6–9).
CAFs communicate among themselves as well as with
cancer cells and inflammatory and immune cells directly through
cell contact and indirectly through paracrine/exocrine signaling,
proteases, and modulation of ECM. This complex communication
network is pivotal in providing the appropriate microenvironment to
support tumorigenesis, angiogenesis, and metastasis. Normal
fibroblasts play a helpful role in maintaining epithelial
homeostasis by suppressing proliferation of adjacent epithelia
(10–12). After epithelial neoplastic
transformation, CAFs undergo profound changes, promote tumor
growth, induce angiogenesis, and recruit bone marrow-derived
endothelial progenitor cells (13–15).
Interestingly, CAFs can even mediate resistance to antiangiogenic
therapy (16). In addition, CAFs
affect the motility of cancer cells through remodeling of the ECM
(17) and inducing epithelial to
mesenchymal transition (EMT) of adjacent carcinoma cells (7). EMT is an epigenetic transcriptional
program by which epithelial cells gain mesenchymal features as
reduced cell-cell contacts and increased motility, thereby escaping
the primary tumor and allowing dissemination of metastases to
distant locations (4,18). A hallmark of EMT is the loss of
E-cadherin expression, in correlation with the tumor grade and
stage (19–21).
Recently, Wu et al (22) reported that monoamine oxidase A
(MAOA), a mitochondrial enzyme, mediates prostate tumorigenesis and
cancer metastasis. MAOA functions to induce EMT and stabilize the
transcription factor hypoxia-inducible factor 1α (HIF-1α), which
mediates hypoxia through an elevation of reactive oxygen species
(ROS), thus enhancing the growth, invasiveness, and metastasis of
PCa cells (22). In addition, the
mammalian target of rapamycin (mTOR)-HIF-1α pathway mediates
aerobic glycolysis as a metabolic basis for trained immunity
(23). Whether MAOA and the
mTOR-HIF-1α pathway play a role in the reciprocal activation of PCa
cells and CAFs warrants further investigation.
Curcumin (diferuloylmethane) is a polyphenol derived
from the Curcuma longa plant, commonly known as turmeric.
Curcumin has been used extensively in Ayurvedic medicine for
centuries (24,25), as it is non-toxic and has a variety
of therapeutic properties including anti-oxidant, analgesic,
anti-inflammatory, and anti-septic activities (26). More recently, curcumin has been
found to possess anticancer activities via its effect on a variety
of biological pathways involved in mutagenesis, oncogene
expression, cell cycle regulation, apoptosis, tumorigenesis, and
metastasis (26). Curcumin has
exhibited an effect on targeting mTOR signaling to inhibit cancer
progression (27–29). In addition, curcumin affects a
variety of growth factor receptors and cell adhesion molecules
involved in tumor growth, angiogenesis, and metastasis (26).
Here, we investigated the role of MAOA/mTOR/HIF-1α
signaling in CAF-induced EMT and invasiveness in PCa cells and
examined the potential protective effect of curcumin on CAF-driven
PCa progression. We found that MAOA/mTOR/HIF-1α signaling mediated
CAF-induced EMT, invasion, ROS production, and CXCR4 and IL-6
receptor expression in PCa cells and curcumin suppressed
CAF-induced PCa cell invasion through MAOA/mTOR/HIF-1α
signaling.
Materials and methods
Materials
The antibodies used in this study included
polyclonal rabbit anti-human MAOA (Santa Cruz Biotechnology, Santa
Cruz, CA, USA), monoclonal rabbit anti-human anti-mTOR (Cell
Signaling Technology, Boston, MA, USA), polyclonal rabbit
anti-human HIF-1α (Bioworld, St. Louis Park, MN, USA), monoclonal
mouse anti-human E-cadherin (Cell Signaling Technology), monoclonal
mouse anti-human vimentin (Cell Signaling Technology), monoclonal
mouse anti-human α-SMA (Sigma-Aldrich, St. Louis, MO, USA),
monoclonal mouse anti-human cytokeratin (Sigma-Aldrich), and
monoclonal mouse anti-human β-actin (Santa Cruz Biotechnology).
2′,7′-Dihydrochlorofluorescein diacetate (H2DCF-DA) was
purchased from Molecular Probes (Carlsbad, CA, USA). Curcumin was
purchased from Dolcas Biotech LLC (Landing, NJ, USA).
Cell cultures
PC3 cells were obtained from the Cell Bank of the
Chinese Academy of Sciences (Shanghai, China). Human prostate CAFs
were isolated from surgically explanted cancer regions of patients
with PCa (Gleason 4+5) (30). Four
different surgical explants were used for CAF isolation. The study
protocol was approved by the relevant ethics committee of the First
Affiliated Hospital of Medical College, Xi'an Jiaotong University,
China, and the patients provided written informed consent. PC3
cells and fibroblasts were grown in Dulbecco's modified Eagle's
medium containing 10% fetal bovine serum, 10 U/ml penicillin, and
10 mg/ml streptomycin. CAFs were used for 15 passages without
significant modification of their ability to elicit EMT. Fresh
serum-free medium was added for an additional 24 h before
collection of conditioned medium (CM) in order to obtain CM free
from CAFs. PC3 cells were then incubated with the obtained CM for
72 h and used for the analyses.
Western blot analysis
PC3 cells (1×106) grown under our
experimental conditions were lysed for 20 min on ice in 300 μl
radioimmunoprecipitation assay (RIPA) lysis buffer [50 mM Tris-HCl
(pH 7.5), 150 mM NaCl, 1% Triton X-100, 2 mM EDTA, 1 mM sodium
orthovanadate, 1 mM phenyl-methanesulfonyl fluoride, 10 μg/ml
aprotinin, and 10 μg/ml leupeptin]. Total proteins (100 μg) were
loaded onto sodium dodecyl sulfate (SDS)-polyacrylamide gel
electrophoresis (PAGE) gels, separated, and transferred onto
polyvinylidene fluoride (PVDF) membranes (Roche, Penzberg,
Germany). The membranes were blocked with 5% non-fat dry milk in
Tris-buffered saline with Tween-20 [TBST; 10 mM Tris-HCl (pH 8.0),
150 mM NaCl, 0.05% Tween-20] and were subsequently incubated with
primary antibodies overnight at 4°C. After five washes of 10 min
each in TBST, the membranes were incubated with horseradish
peroxidase (HRP)-conjugated secondary antibodies (1:3,000, Cell
Signaling Technology) for 2 h and subsequently washed again. The
peroxidase reaction was performed using an enhanced
chemiluminescence detection system to visualize the immunoreactive
bands.
Cell invasion assay
A chamber-based invasion assay (Millipore,
Billerica, MA, USA) was performed to evaluate breast cancer cell
invasion. Briefly, the upper surface of the membrane was coated
with Matrigel (BD Biosciences, Franklin Lakes, NJ, USA). PC3 cells
(1×105) were resuspended in the upper chamber in
serum-free medium and allowed to migrate toward a serum gradient
(10%) in the lower chamber for 24 h. The media was aspirated from
inside the insert, and the non-invasive cells on the upper side
were removed by a cotton swab. The membrane was fixed with 4%
paraformaldehyde and stained with crystal violet. The number of
migrated cells was counted on each membrane in 10 random fields and
photographed at x100 magnification. The values reported here are
the averages of triplicate experiments.
Quantitative real-time polymerase chain
reaction assay (qRT-PCR)
Total RNAs were extracted from PC3 cells using
TRIzol reagent (Invitrogen, Carlsbad, CA, USA), and reverse
transcription was performed using the PrimeScript RT Reagent kit
(Takara, Dalian, China) according to the manufacturer's
instructions. Real-time experiments were carried out using the iQ5
Multicolor Real-Time PCR Detection System (Bio-Rad, Hercules, CA,
USA) and a SYBR Green PCR kit (Takara). The following PCR program
was used: denaturation at 95°C for 30 sec, followed by 40 cycles
consisting of denaturation at 95°C for 5 sec, annealing at 60°C for
30 sec, and extension at 72°C for 30 sec. A melting curve analysis
was applied to assess the specificity of the amplified PCR
products. The PCR primer sequences for HIF-1α was
5′-AAGTCTAGGGATGCAGCA-3′ (forward) and 5′-CAAGATCACCAGCATCATG-3′
(reverse). The PCR primer sequences for MAOA was
5′-CTGATCGACTTGCTAAGCTAC-3′ (forward) and
5′-ATGCACTGGATGTAAAGCTTC-3′ (reverse). The PCR primer sequences for
mTOR was 5′-CTGGGACTCAAATGTGTGCAGTTC-3′ (forward) and
5′-GAACAATAGGGTGAATGATCCGGG-3′ (reverse). The PCR primer sequences
for CXCR4 was 5′-TCTGTGACCGCTTCTACC-3′ (forward) and
5′-AGGATGAGGATGACTGTGG-3′ (reverse). The PCR primer sequences for
IL-6 receptor was 5′-CATTGCCATTGTTCTGAGGTTC-3′ (forward) and
5′-AGTAGTCTGTATTGCTGATGTC-3′ (reverse). The PCR primer sequences
for β-actin was 5′-TTGTTACAGGAAGTCCCTTGCC-3′ (forward) and
5′-ATGCTATCACCTCCCCTGTGTG-3′ (reverse). The amount of each target
gene was quantified by the comparative CT method using
β-actin as the normalization control (31).
Measurement of intracellular ROS
Intracellular production of
H2O2 was assayed as previously described
(32). Five minutes before the end
of the incubation, the cells were treated with 5 μg/ml
2′-7′-dichlorofluorescein diacetate (DCF-DA). After washing with
phosphate-buffered saline (PBS), the cells were lysed in 1 ml RIPA
buffer and analyzed immediately by fluorimetric analysis at 510 nm.
The data were normalized to total protein content.
Immunofluorescence microscopy
After the designated treatment, CAFs were fixed with
4% paraformaldehyde for 10 min at room temperature, permeabilized
in 0.5% Triton X-100 for 10 min, and blocked in 1% bovine serum
albumin (BSA) for 1 h. Fixed cells were then incubated with mouse
anti-human-α-SMA antibodies (1:100) or mouse anti-human-cytokeratin
antibodies (1:100) at 4°C overnight. Cells were washed and
incubated with goat anti-mouse dylight 594 (red) IgG antibody
(Qenshare Biological Inc., Xi'an, China) at 1:200 dilution for 60
min. Nuclei were stained with DAPI for 5 min. The cells were
visualized using a fluorescent microscope (Observer A1, Carl Zeiss
Microscopy GmbH, Jena, Germany) using appropriate excitation and
emission filters at x400 magnification.
RNA interference
shRNA against MAOA (Santa Cruz Biotechnology,
sc-35847-SH), shRNA against mTOR (Santa Cruz Biotechnology,
sc-35409-SH), shRNA against HIF-1α (Santa Cruz Biotechnology,
sc-35561-SH), and a negative control shRNA (Santa Cruz
Biotechnology, sc-108060) were obtained from GenePharm (Shanghai,
China). PC3 cells (2×105 per well) were seeded in 6-well
plates and transfected with shRNA. Silencing with shRNA was
performed with Lipofectamine (Invitrogen) following the
manufacturer's instructions. Silenced cells were selected by
puromycin treatment, and a pool of stably transfected cells was
generated in order to avoid clonal selection.
Statistical analysis
The data are presented as the mean ± standard
deviations (SD) values obtained from at least three independent
experiments. Statistical analysis of the data was performed by
Student's t-tests using SPSS software (version 13.0; SPSS, Chicago,
IL, USA). P-values <0.05 were considered statistically
significant.
Results
CAFs activate MAOA/mTOR/HIF-1α expression
to elicit EMT in PC3 cells
To assess the role of MAOA/mTOR/HIF-1α signaling
elicited by CAFs in the EMT of cells, we used PC3 cells, a line
isolated from a bone metastasis of human PCa and CAFs. CAFs were
obtained from the surgically explanted prostate of patients with
PCa (Gleason 4+5). Expression of E-cadherin and cytokeratin was
assessed to exclude epithelial contamination of CAFs (Fig. 1A).
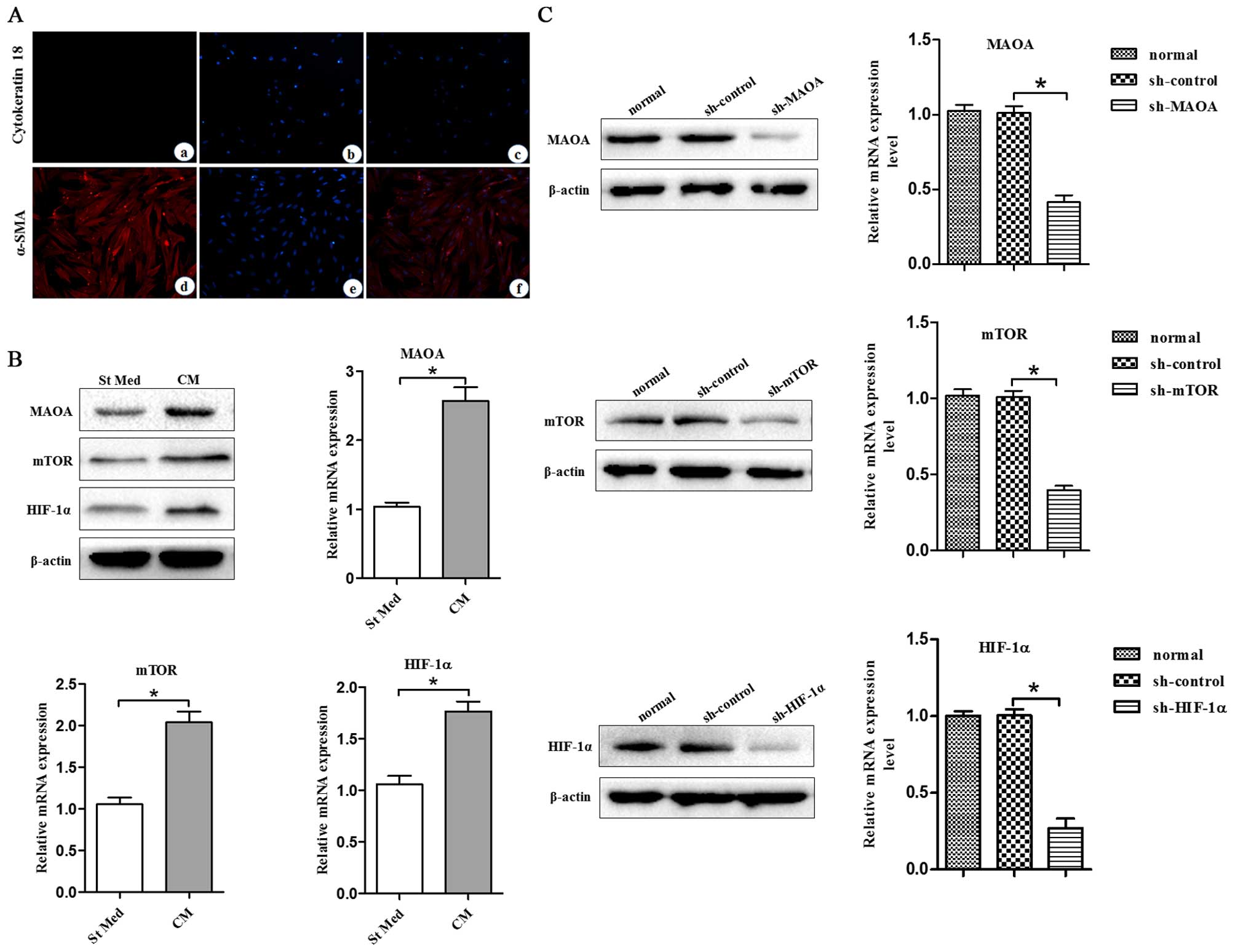 | Figure 1CAFs promote MAOA, mTOR, and HIF-1α
expression in PC3 cells. CM, conditioned media of CAFs, St Med,
starved standard media of PC3 cells. CAFs were isolated from
surgical explants from cancer regions of patients with PCa (Gleason
4+5). (A) (a–c) Cytokeratin 18 expression in CAFs was determined by
immuofluorescence microscopy analysis. (d–f) α-SMA expression in
CAFs was determined by immuofluorescence microscopy analysis. (B)
PC3 cells were incubated with or without the indicated CM and the
levels of MAOA, mTOR, HIF-1α, and β-actin were assessed by
immunoblotting and qRT-PCR. (C) MAOA, mTOR, or HIF-1α were
transiently silenced with shRNA in PC3 cells. The interfering
efficiency was determined by immunoblotting and qRT-PCR.
*P<0.05. |
In order to study the molecular pathways driving the
CAF-induced EMT response in PC3 cells, we analyzed the
MAOA/mTOR/HIF-1α pathway in PC3 cells treated with CM from CAFs.
PC3 cells exhibited CAF-induced EMT with activation of the
MAOA/mTOR pathway. In addition, CAF exposure led to activation of
HIF-1α (Fig. 1B), a known
transcription factor normally induced by hypoxia but already
reported to be activated in normoxia by inflammation in an
mTOR-dependent manner (23).
Silencing of MAOA, mTOR, or HIF-1α in PC3 cells by shRNA (Fig. 1C) after exposure to CAFs indicated
that this signaling pathway proceeds from MAOA to mTOR and then
transduction to HIF-1α (Fig. 2A).
MAOA, mTOR, or HIF-1α knockdown in PC3 cells after exposure to CAFs
revealed that this MAOA/mTOR/HIF-1α axis plays a mandatory role in
orchestrating CAF-induced EMT in PC3 cells. Indeed, silencing of
MAOA, mTOR, or HIF-1α individually reduced the invasiveness and
expression of EMT markers (E-cadherin and vimentin) among PC3 cells
(Fig. 2B–D).
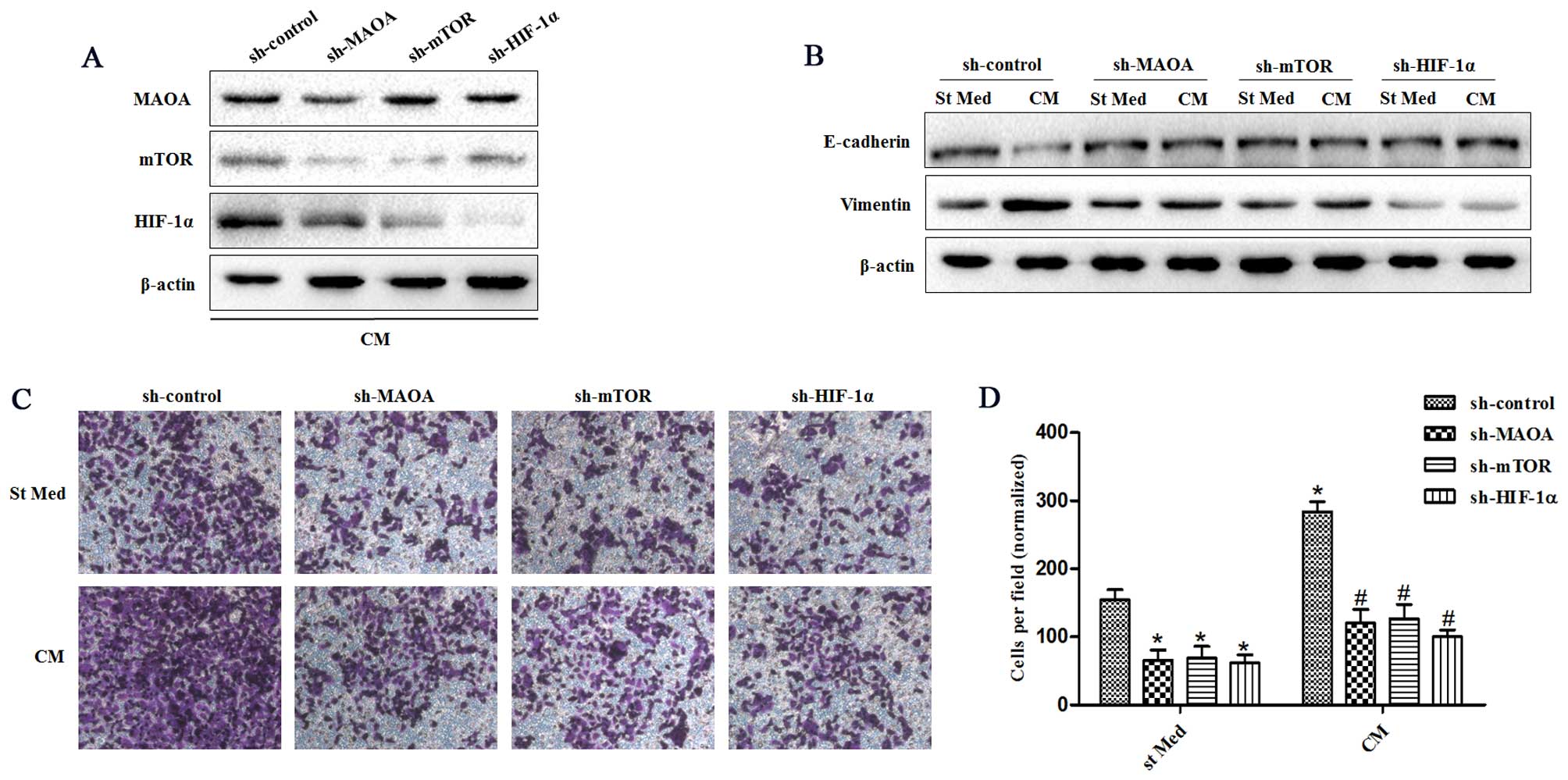 | Figure 2CAFs promote EMT by triggering a
MAOA/mTOR/HIF-1α signaling pathway. CM, conditioned media of CAFs,
St Med, starved standard media of PC3 cells. MAOA, mTOR, or HIF-1α
were transiently silenced with shRNA in PC3 cells. These cells were
incubated with or without the indicated CM after silencing, and (A)
the levels of MAOA, mTOR, HIF-1α, and β-actin were assessed by
immunoblotting. (B) PC3 cells were treated as above, and the levels
of E-cadherin, vimentin, and β-actin were assessed by
immunoblotting. (C and D) PC3 cells were treated as above (B), and
their invasion was evaluated. Invading cells were counted and a bar
graph, representative of six randomly chosen fields, is shown.
*P<0.05 versus sh-control group treated with St Med
group; #P<0.05 versus sh-control group treated with
CM. |
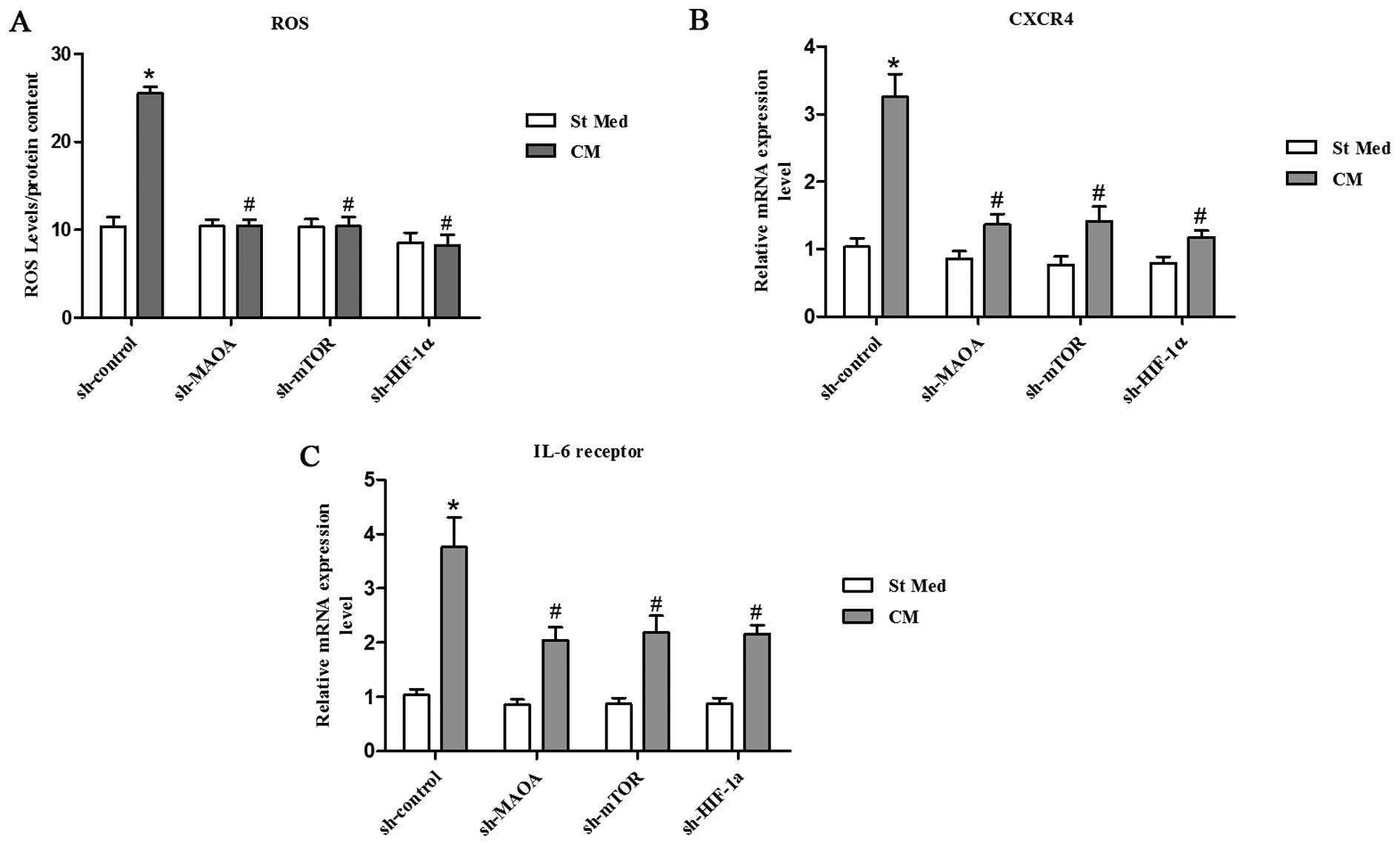
MAOA/mTOR/HIF-1α signaling regulates
CAF-induced ROS production and CXCR4 and IL-6 receptor expression
in PC3 cells
Inflammation and oxidative stress have already been
linked to cancer progression and claimed to be responsible for
enhanced malignancy (33,34). Oxidative stress, due to deregulated
intracellular ROS production, has been correlated to PCa
carcinogenesis, androgen resistance, anchorage-independence, and
resistance to anoikis (35,36).
We observed that exposure to CAFs led to an increase in the ROS
content of PC3 cells (Fig. 3A).
ROS production appears to be mainly due to MAOA/mTOR/HIF-1α
signaling, as indicated by the ability of MAOA, mTOR, or HIF-1α
knockdown to block CAF-induced ROS generation and the invasive
phenotype (Figs. 2C–D and 3A).
Moreover, culture in CM from CAFs resulted in a
significant increase in CXCR4 and IL-6 receptor expression in PC3
cells (Fig. 3B and C). However,
silencing of MAOA, mTOR, or HIF-1α by shRNA in PC3 cells abrogated
the elevated CXCR4 and IL-6 receptor expression induced by
CAF-derived CM (Fig. 3B and C),
which indicates an important role for MAOA/mTOR/HIF-1α signaling in
PC3 cells with regard to the regulation of chemotactic and
inflammation responses to CAF-derived CM.
Curcumin inhibits CAF-induced EMT in PC3
cells by suppressing MAOA/mTOR/HIF-1α signaling
Curcumin has been found to possess anticancer
activities based on its effect on a variety of biological pathways
involved in mutagenesis, oncogene expression, cell cycle
regulation, apoptosis, tumorigenesis, and metastasis; that is, the
modulation of several important molecular targets including
transcription factors (NF-κB, AP-1, β-catenin), enzymes (e.g.,
COX-2, MMPs), proinflammatory cytokines (e.g., TNF-α, IL-1β, and
IL-6), and cell surface adhesion molecules (e.g., cadherins,
integrins) (37–39). Here, we wanted to investigate
whether curcumin has a protective effect in prostate cancer via
modulation of MAOA/mTOR/HIF-1α signaling.
We observed that 25 μM curcumin [a concentration
selected according to a previous study (40)] was able to abolish the EMT response
to CAF-derived CM in PC3 cells as revealed by increased E-caderin
levels and decreased vimentin levels (Fig. 4A). However, it could not inhibit
the EMT response to CAF-derived CM in PC3 cells when MAOA, mTOR, or
HIF-1α was silenced by shRNA in PC3 cells (Fig. 4B). Similar results were obtained
for the invasiveness of PC3 cells. Curcumin abrogated the
invasiveness of PC3 cells induced by CAF-derived CM (Fig. 4C). However, it was not able to
suppress the invasiveness of PC3 cells induced by CAF-derived CM
when MAOA, mTOR, or HIF-1α was silenced by shRNA in PC3 cells
(Fig. 4D).
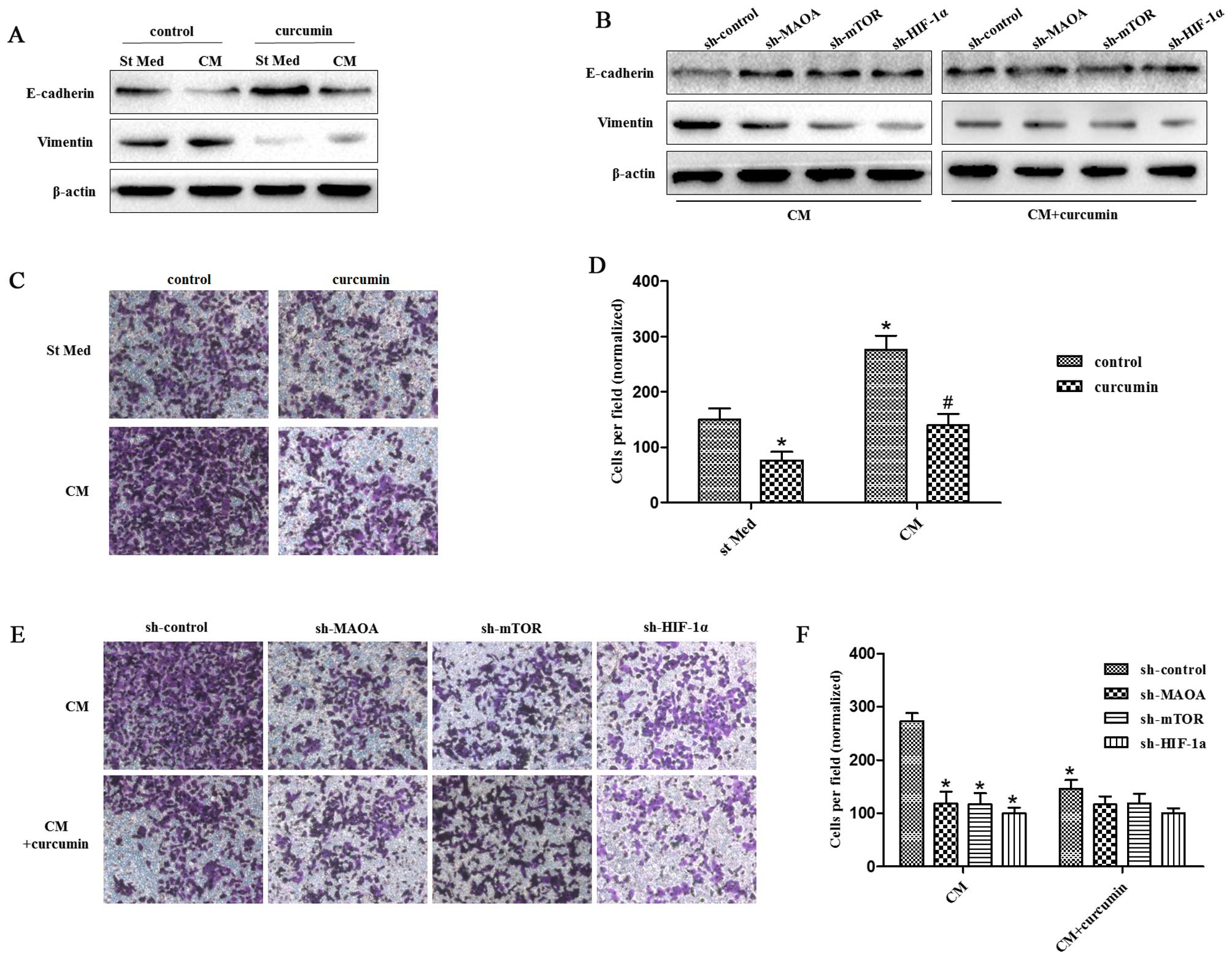 | Figure 4Curcumin suppresses CAF-induced EMT
in PC3 cells via MAOA/mTOR/HIF-1α signaling. CM, conditioned media
of CAFs, St Med, starved standard media of PC3 cells. (A) PC3 cells
were incubated with or without the indicated CM in the presence or
absence of 25 μM curcumin, and the levels of E-cadherin, vimentin,
and β-actin were assessed by immunoblotting. (B) PC3 cells in which
MAOA, mTOR, or HIF-1α was transiently silenced with shRNA, and then
were incubated with the indicated CM in the presence or absence of
25 μM curcumin, and the levels of E-cadherin, vimentin, and β-actin
were assessed by immunoblotting. (C and D) PC3 cells were treated
as in (A), and their invasion was evaluated. Invading cells were
counted and a bar graph, representative of six randomly chosen
fields, is shown. *P<0.05 versus control group
treated with St Med; #P<0.05 versus control group
treated with CM. (E and F) PC3 cells were treated as in (B), and
their invasion was evaluated. Invading cells were counted and a bar
graph, representative of six randomly chosen fields, is shown.
*P<0.05 versus sh-control group treated with CM;
#P<0.05 versus sh-control group treated with
CM+curcumin. |
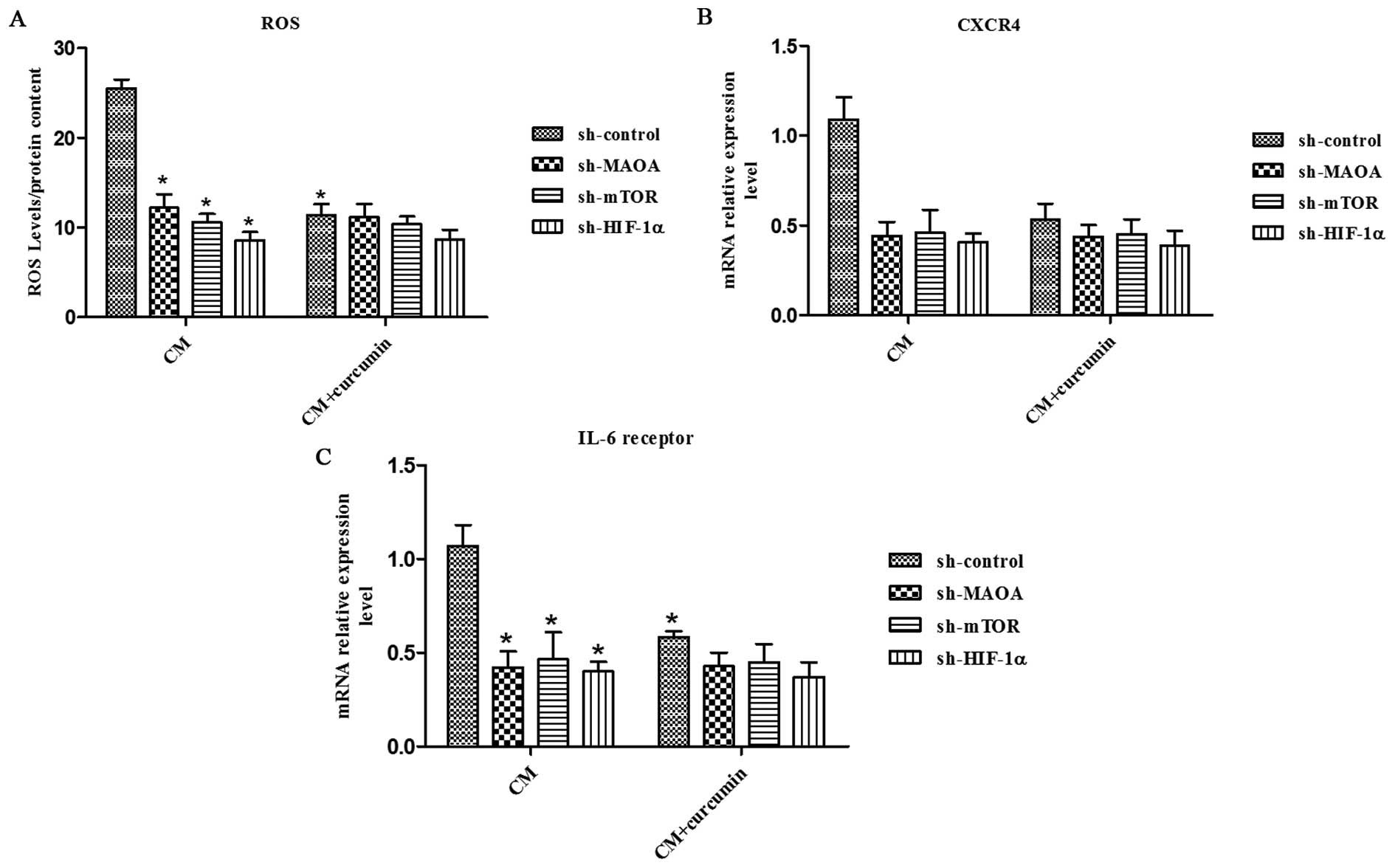
Curcumin abrogates CAF-induced ROS
production and CXCR4 and IL-6 receptor expression in PC3 cells
through MAOA/mTOR/HIF-1α signaling
Curcumin has been shown to have antioxidant activity
and anti-inflammatory activity. Here, we aimed to evaluate the
effect of curcumin on CAF-induced ROS production and CXCR4 and IL-6
receptor expression in PC3 cells. We found that curcumin abolished
the CAF-derived CM-induced ROS production and CXCR4 and IL-6
receptor expression in PC3 cells (Fig.
5). However, it could not inhibit the CAFs in CM-induced ROS
production and CXCR4 and IL-6 receptor expression in PC3 cells when
MAOA, mTOR, or HIF-1α was silenced by shRNA in PC3 cells (Fig. 5), which indicates that curcumin
inhibits CAF-induced ROS production and CXCR4 and IL-6 receptor
expression in PC3 cells by impeding MAOA/mTOR/HIF-1α signaling.
Discussion
The results presented in this report lead to three
major conclusions: i) CAFs act on cancer cells by activating
MAOA/mTOR/HIF-1α signaling, thus leading them to achieve a motile
phenotype through EMT; ii) the proinflammatory signature (elevated
CXCR4 and IL-6 receptor expression) activated in cancer cells is
mainly driven by the pathway involving MAOA, mTOR, and HIF-1α; and
iii) in response to CAF contact, cancer cells experience
MAOA/mTOR/HIF-1α signaling-mediated ROS accumulation.
MAOA has been associated with EMT activation. It has
been shown that MAOA can stabilize HIF-1α, activate the vascular
endothelial growth factor (VEGF)-A/neuropilin (NRP)1 system, and
induce the expression of TWIST1, an EMT master transcription factor
commonly associated with EMT promotion in PCa (22). Both increased MAOA expression and
EMT were observed in the same specimens of high-grade PCa, which
indicate that MAOA can drive EMT in vivo (22). Pharmacological inhibition of MAOA
in PCa cells kept basal prostatic epithelial cells from
differentiating into matured glandular structures by reorganizing
cell structures and decreasing the expression of basal cytokeratins
(41). The CAF-induced EMT process
in PCa appears to be mainly mediated by MAOA/mTOR/HIF-1α signaling,
as indicated by sensitivity of EMT suppression upon MAOA, mTOR, or
HIF-1α shRNA silencing.
Our results now include mTOR and HIF-1α
transcription factors as key players for CAF-mediated EMT
activation. Recently, many studies have demonstrated that mTOR
plays a crucial role in the regulation of cell motility, adhesion,
and invasion (42–44). mTOR regulates EMT at least in part
by downregulation of the RhoA and Rac1 signaling pathways (42) in PCa. In addition, HIF-1α has been
linked to the EMT process through the activation of both TWIST and
Snail-1 transcription factors, leading to increased invasiveness
(45,46). We report herein the activation of
HIF-1α in response to CAF contact in normoxic conditions, which is
mandatory for in vitro EMT activation. Consistent with our
findings, activation of HIF-1α also has been reported in normoxic
conditions upon treatment with several growth factors and
chemo-attractive agents (47,48).
Interestingly, EMT activation and chemotaxis surely share the
activation of a migratory cell behavior, therefore allowing a
correlation of non-hypoxic activation of HIF-1α to the induction of
a migratory behavior for PCa cells.
ROS can affect tumor progression in several ways.
They cause oxidative damage to DNA and genomic instability or alter
gene expression through modulation of transcription factors. In
addition, repeated treatment with H2O2 caused
a phenotypic conversion from mammary epithelial to fibroblast-like
as in malignant transformation (49). ROS elicited by pancreatic stellate
cells was shown to be able to induce EMT in pancreatic cancer cells
(50). In PCa cells, ROS have also
been correlated with EMT, through a MAOA-mediated delivery of
mitochondrial ROS (22). Elevated
ROS levels in MAOA-overexpressing cells contributed to increased
HIF-1α stabilization and activity, and the increased HIF-1α
expression has the potential to further induce mitochondrial
activity including the formation of specific ROS during hypoxia
(51,52), potentially programming a ‘vicious
cycle’ or feed-forward loop among MAOA, ROS, and HIF-1α to further
drive PCa tumorigenesis. We demonstrate that MAOA, mTOR, or HIF-1α
silencing abrogated the ROS production increased by CAF-derived CM
exposure, which implicates a mandatory role for MAOA/mTOR/HIF-1α
signaling in regulating CAF-induced ROS production.
CXCR4, which is involved in chemo-attraction of
cancer and endothelial cells, and IL-6, which is involved in the
organization of the pro-inflammatory response, have already been
reported to be under transcriptional control of HIF-1 (53,54).
A recent study on pancreatic cancer showed that exogenous stromal
cell-derived factor (SDF)-1 could induce CXCR4-positive pancreatic
cancer invasion and EMT (55).
Activated pancreatic cancer stellate cells secreted SDF-1 (the
ligands that binds to the pro-inflammatory chemokine receptor
CXCR4) and IL-6 to promote the pancreatic cancer EMT process. An
active IL-6R/STAT3/miR-34a loop was necessary for EMT, invasion,
and metastasis of colorectal cancer cell lines (56). Our data showed that exposure to
CAFs increased CXCR4 and IL-6 receptor expression in PCa cells.
These data indicate that PCa cells exposed to CAFs could be more
easily attracted to different locations. Active factors in this
chemo-attraction include CXCR4 and IL-6, confirming their
pleiotropic role in PCa progression. Hence, the surrounding stroma
might play a role in promoting metastasis of PCa cells from the
primary lesions to the other organs, thereby facilitating satellite
metastases.
Curcumin has been studied in multiple human
carcinomas including melanoma, head and neck, breast, colon,
pancreatic, prostate, and ovarian cancers (26). Curcumin can inhibit PCa growth and
metastasis and increase the chemopreventive effects of other
anticancer agents (57–60). Epidemiological studies attribute
the low incidence of colon cancer in India to the chemopreventive
and antioxidant properties of diets rich in curcumin (61). The mechanisms by which curcumin
exerts its anticancer effects are comprehensive and diverse,
targeting many levels of regulation in the processes of cellular
growth and apoptosis. Here, we showed that curcumin inhibited PCa
cell EMT and invasion induced by CAF-derived CM and abolished
CAF-activated CXCR4 and IL-6 receptor expression in PCa cells.
Moreover, curcumin abrogated ROS generation induced by exposure to
CAFs in PCa cells. However, shRNA-mediated downregulation of MAOA,
mTOR, or HIF-1α abolished the effects of curcumin on inhibiting PCa
EMT and invasion. These data indicate that curcumin has a
protective effect against the EMT process in the prostate
tumor-stromal interaction, which is associated with its ability to
ameliorate CAF-induced ROS production through the MAOA/mTOR/HIF-1α
signaling pathway.
Acknowledgements
This study was supported by The National Science
Foundation of China (nos. 81372736 and 81072107).
References
|
1
|
Siegel RL, Miller KD and Jemal A: Cancer
statistics, 2015. CA Cancer J Clin. 65:5–29. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Partin AW, Kattan MW, Subong EN, Walsh PC,
Wojno KJ, Oesterling JE, Scardino PT and Pearson JD: Combination of
prostate-specific antigen, clinical stage, and Gleason score to
predict pathological stage of localized prostate cancer. A
multi-institutional update. JAMA. 277:1445–1451. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Bissell MJ and Radisky D: Putting tumours
in context. Nat Rev Cancer. 1:46–54. 2001. View Article : Google Scholar
|
|
4
|
Thiery JP: Epithelial-mesenchymal
transitions in tumour progression. Nat Rev Cancer. 2:442–454. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Kalluri R and Zeisberg M: Fibroblasts in
cancer. Nat Rev Cancer. 6:392–401. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Desmoulière A, Guyot C and Gabbiani G: The
stroma reaction myofibroblast: A key player in the control of tumor
cell behavior. Int J Dev Biol. 48:509–517. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Giannoni E, Bianchini F, Masieri L, Serni
S, Torre E, Calorini L and Chiarugi P: Reciprocal activation of
prostate cancer cells and cancer-associated fibroblasts stimulates
epithelial-mesenchymal transition and cancer stemness. Cancer Res.
70:6945–6956. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Micke P and Ostman A: Tumour-stroma
interaction: Cancer-associated fibroblasts as novel targets in
anti-cancer therapy? Lung Cancer. 45(Suppl 2): S163–S175. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Sugimoto H, Mundel TM, Kieran MW and
Kalluri R: Identification of fibroblast heterogeneity in the tumor
microenvironment. Cancer Biol Ther. 5:1640–1646. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Begley LA, Kasina S, MacDonald J and
Macoska JA: The inflammatory microenvironment of the aging prostate
facilitates cellular proliferation and hypertrophy. Cytokine.
43:194–199. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Bhowmick NA, Neilson EG and Moses HL:
Stromal fibroblasts in cancer initiation and progression. Nature.
432:332–337. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Trimboli AJ, Cantemir-Stone CZ, Li F,
Wallace JA, Merchant A, Creasap N, Thompson JC, Caserta E, Wang H,
Chong JL, et al: Pten in stromal fibroblasts suppresses mammary
epithelial tumours. Nature. 461:1084–1091. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Allinen M, Beroukhim R, Cai L, Brennan C,
Lahti-Domenici J, Huang H, Porter D, Hu M, Chin L, Richardson A, et
al: Molecular characterization of the tumor microenvironment in
breast cancer. Cancer Cell. 6:17–32. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Olumi AF, Grossfeld GD, Hayward SW,
Carroll PR, Tlsty TD and Cunha GR: Carcinoma-associated fibroblasts
direct tumor progression of initiated human prostatic epithelium.
Cancer Res. 59:5002–5011. 1999.PubMed/NCBI
|
|
15
|
Orimo A, Gupta PB, Sgroi DC,
Arenzana-Seisdedos F, Delaunay T, Naeem R, Carey VJ, Richardson AL
and Weinberg RA: Stromal fibroblasts present in invasive human
breast carcinomas promote tumor growth and angiogenesis through
elevated SDF-1/CXCL12 secretion. Cell. 121:335–348. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Crawford Y and Ferrara N: Tumor and
stromal pathways mediating refractoriness/resistance to
anti-angiogenic therapies. Trends Pharmacol Sci. 30:624–630. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Pietras K, Pahler J, Bergers G and Hanahan
D: Functions of paracrine PDGF signaling in the proangiogenic tumor
stroma revealed by pharmacological targeting. PLoS Med. 5:e192008.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Friedl P: Prespecification and plasticity:
Shifting mechanisms of cell migration. Curr Opin Cell Biol.
16:14–23. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Christofori G: New signals from the
invasive front. Nature. 441:444–450. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Cowin P, Rowlands TM and Hatsell SJ:
Cadherins and catenins in breast cancer. Curr Opin Cell Biol.
17:499–508. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Francí C, Takkunen M, Dave N, Alameda F,
Gómez S, Rodríguez R, Escrivà M, Montserrat-Sentís B, Baró T,
Garrido M, et al: Expression of Snail protein in tumor-stroma
interface. Oncogene. 25:5134–5144. 2006.PubMed/NCBI
|
|
22
|
Wu JB, Shao C, Li X, Li Q, Hu P, Shi C, Li
Y, Chen YT, Yin F, Liao CP, et al: Monoamine oxidase A mediates
prostate tumorigenesis and cancer metastasis. J Clin Invest.
124:2891–2908. 2014. View
Article : Google Scholar : PubMed/NCBI
|
|
23
|
Cheng SC, Quintin J, Cramer RA, Shepardson
KM, Saeed S, Kumar V, Giamarellos-Bourboulis EJ, Martens JH, Rao
NA, Aghajanirefah A, et al: mTOR- and HIF-1α-mediated aerobic
glycolysis as metabolic basis for trained immunity. Science.
345:12506842014. View Article : Google Scholar
|
|
24
|
Aggarwal BB, Sundaram C, Malani N and
Ichikawa H: Curcumin: The Indian solid gold. Adv Exp Med Biol.
595:1–75. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Ammon HP and Wahl MA: Pharmacology of
Curcuma longa. Planta Med. 57:1–7. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Wilken R, Veena MS, Wang MB and Srivatsan
ES: Curcumin: A review of anti-cancer properties and therapeutic
activity in head and neck squamous cell carcinoma. Mol Cancer.
10:122011. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Beevers CS, Zhou H and Huang S: Hitting
the golden TORget: Curcumin's effects on mTOR signaling. Anticancer
Agents Med Chem. 13:988–994. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Beevers CS, Chen L, Liu L, Luo Y, Webster
NJ and Huang S: Curcumin disrupts the mammalian target of
rapamycin-raptor complex. Cancer Res. 69:1000–1008. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Yu S, Shen G, Khor TO, Kim JH and Kong AN:
Curcumin inhibits Akt/mammalian target of rapamycin signaling
through protein phosphatase-dependent mechanism. Mol Cancer Ther.
7:2609–2620. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Schnaitman C, Erwin VG and Greenawalt JW:
The submitochondrial localization of monoamine oxidase. An
enzymatic marker for the outer membrane of rat liver mitochondria.
J Cell Biol. 32:719–735. 1967. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Schmittgen TD and Livak KJ: Analyzing
real-time PCR data by the comparative C(T) method. Nat Protoc.
3:1101–1108. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Chiarugi P, Pani G, Giannoni E, Taddei L,
Colavitti R, Raugei G, Symons M, Borrello S, Galeotti T and Ramponi
G: Reactive oxygen species as essential mediators of cell adhesion:
The oxidative inhibition of a FAK tyrosine phosphatase is required
for cell adhesion. J Cell Biol. 161:933–944. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Mantovani A: Role of inflammatory cells
and mediators in tumor invasion and metastasis. Cancer Metastasis
Rev. 29:2412010. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Pani G, Galeotti T and Chiarugi P:
Metastasis: Cancer cell's escape from oxidative stress. Cancer
Metastasis Rev. 29:351–378. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Giannoni E, Fiaschi T, Ramponi G and
Chiarugi P: Redox regulation of anoikis resistance of metastatic
prostate cancer cells: Key role for Src and EGFR-mediated
pro-survival signals. Oncogene. 28:2074–2086. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Khandrika L, Kumar B, Koul S, Maroni P and
Koul HK: Oxidative stress in prostate cancer. Cancer Lett.
282:125–136. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Kim HI, Huang H, Cheepala S, Huang S and
Chung J: Curcumin inhibition of integrin (alpha6beta4)-dependent
breast cancer cell motility and invasion. Cancer Prev Res (Phila).
1:385–391. 2008. View Article : Google Scholar
|
|
38
|
Buhrmann C, Mobasheri A, Busch F, Aldinger
C, Stahlmann R, Montaseri A and Shakibaei M: Curcumin modulates
nuclear factor kappaB (NF-kappaB)-mediated inflammation in human
tenocytes in vitro: Role of the phosphatidylinositol 3-kinase/Akt
pathway. J Biol Chem. 286:28556–28566. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Shehzad A, Lee J and Lee YS: Curcumin in
various cancers. Biofactors. 39:56–68. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Cheng TS, Chen WC, Lin YY, Tsai CH, Liao
CI, Shyu HY, Ko CJ, Tzeng SF, Huang CY, Yang PC, et al:
Curcumin-targeting peri-cellular serine protease matriptase role in
suppression of prostate cancer cell invasion, tumor growth, and
metastasis. Cancer Prev Res (Phila). 6:495–505. 2013. View Article : Google Scholar
|
|
41
|
Zhao H, Nolley R, Chen Z, Reese SW and
Peehl DM: Inhibition of monoamine oxidase A promotes secretory
differentiation in basal prostatic epithelial cells.
Differentiation. 76:820–830. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Chen X, Cheng H, Pan T, et al: mTOR
regulate EMT through RhoA and Rac1 pathway in prostate cancer. Mol
Carcinog. 54:1086–1095. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Liu L, Li F, Cardelli JA, Martin KA,
Blenis J and Huang S: Rapamycin inhibits cell motility by
suppression of mTOR-mediated S6K1 and 4E-BP1 pathways. Oncogene.
25:7029–7040. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Gulhati P, Bowen KA, Liu J, Stevens PD,
Rychahou PG, Chen M, Lee EY, Weiss HL, O'Connor KL, Gao T, et al:
mTORC1 and mTORC2 regulate EMT, motility, and metastasis of
colorectal cancer via RhoA and Rac1 signaling pathways. Cancer Res.
71:3246–3256. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Cannito S, Novo E, Compagnone A, Valfrè di
Bonzo L, Busletta C, Zamara E, Paternostro C, Povero D, Bandino A,
Bozzo F, et al: Redox mechanisms switch on hypoxia-dependent
epithelial-mesenchymal transition in cancer cells. Carcinogenesis.
29:2267–2278. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Yang MH and Wu KJ: TWIST activation by
hypoxia inducible factor-1 (HIF-1): Implications in metastasis and
development. Cell Cycle. 7:2090–2096. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Calvani M, Rapisarda A, Uranchimeg B,
Shoemaker RH and Melillo G: Hypoxic induction of an HIF-1
alpha-dependent bFGF autocrine loop drives angiogenesis in human
endothelial cells. Blood. 107:2705–2712. 2006. View Article : Google Scholar
|
|
48
|
Stasinopoulos I, O'Brien DR and Bhujwalla
ZM: Inflammation, but not hypoxia, mediated HIF-1alpha activation
depends on COX-2. Cancer Biol Ther. 8:31–35. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Mori K, Shibanuma M and Nose K: Invasive
potential induced under long-term oxidative stress in mammary
epithelial cells. Cancer Res. 64:7464–7472. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Lei J, Huo X, Duan W, Xu Q, Li R, Ma J, Li
X, Han L, Li W, Sun H, et al: α-Mangostin inhibits hypoxia-driven
ROS-induced PSC activation and pancreatic cancer cell invasion.
Cancer Lett. 347:129–138. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Chandel NS, McClintock DS, Feliciano CE,
Wood TM, Melendez JA, Rodriguez AM and Schumacker PT: Reactive
oxygen species generated at mitochondrial complex III stabilize
hypoxia-inducible factor-1 alpha during hypoxia: A mechanism of
O2 sensing. J Biol Chem. 275:25130–25138. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Chandel NS, Maltepe E, Goldwasser E,
Mathieu CE, Simon MC and Schumacker PT: Mitochondrial reactive
oxygen species trigger hypoxia-induced transcription. Proc Natl
Acad Sci USA. 95:11715–11720. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Semenza GL: Oxygen homeostasis. Wiley
Interdiscip Rev Syst Biol Med. 2:336–361. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Youn SW, Lee SW, Lee J, Jeong HK, Suh JW,
Yoon CH, Kang HJ, Kim HZ, Koh GY, Oh BH, et al: COMP-Ang1
stimulates HIF-1α-mediated SDF-1 overexpression and recovers
ischemic injury through BM-derived progenitor cell recruitment.
Blood. 117:4376–4386. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Li X, Ma Q, Xu Q, Liu H, Lei J, Duan W,
Bhat K, Wang F, Wu E and Wang Z: SDF-1/CXCR4 signaling induces
pancreatic cancer cell invasion and epithelial-mesenchymal
transition in vitro through non-canonical activation of Hedgehog
pathway. Cancer Lett. 322:169–176. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Rokavec M, Öner MG, Li H, Jackstadt R,
Jiang L, Lodygin D, Kaller M, Horst D, Ziegler PK, Schwitalla S, et
al: IL-6R/STAT3/miR-34a feedback loop promotes EMT-mediated
colorectal cancer invasion and metastasis. J Clin Invest.
124:1853–1867. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Zhou DY, Ding N, Du ZY, Cui XX, Wang H,
Wei XC, Conney AH, Zhang K and Zheng X: Curcumin analogues with
high activity for inhibiting human prostate cancer cell growth and
androgen receptor activation. Mol Med Rep. 10:1315–1322.
2014.PubMed/NCBI
|
|
58
|
Dorai T, Diouri J, O'Shea O and Doty SB:
Curcumin inhibits prostate cancer bone metastasis by up-regulating
bone morphogenic protein-7 in vivo. J Cancer Ther. 5:369–386. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Eom DW, Lee JH, Kim YJ, et al: Synergistic
effect of curcumin on epigallocatechin gallate-induced anticancer
action in PC3 prostate cancer cells. BMB Rep. 48:461–466. 2015.
View Article : Google Scholar :
|
|
60
|
Wang P, Wang B, Chung S, Wu Y, Henning SM
and Vadgama JV: Increased chemopreventive effect by combining
arctigenin, green tea polyphenol and curcumin in prostate and
breast cancer cells. RSC Advances. 4:35242–35250. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Mohandas KM and Desai DC: Epidemiology of
digestive tract cancers in India. V. Large and small bowel. Indian
J Gastroenterol. 18:118–121. 1999.PubMed/NCBI
|