Introduction
Nasopharyngeal carcinoma (NPC) has a high incidence
rate in southern China and Southeast Asia, and its metastasis rate
is also the highest among head and neck cancers (1–3). The
close relationship of Epstein-Barr virus infection suggests NPC as
an inflammation-associated cancer (4,5).
Interleukin 8 (IL-8; alternatively known as CXCL8)
is a proinflammatory cysteine-X-cysteine (CXC) chemokine, plays
multiple roles by mediating the activation and chemotaxis of
various immune cell types, to promote immune infiltration and
angiogenesis, which in turn establishes a venue for cancer cell
local invasion, migration, and metastasis. Studies have shown that
IL-8 promotes tumor growth and metastasis in melanoma (6–9),
bladder cancer (10), and ovarian
cancer (11). We have also
previously demonstrated that the overexpression of IL-8 in
nasopharyngeal carcinoma cell line S26 cells and HONE-1 cells
activated AKT1 signaling and induced EMT (12). In recent years, it has also been
demonstrated that a link exists between IL-8 and tumor EMT, which
involve decreased expression of epithelial markers such as
E-cadherin in lung cancer (13),
hepatocellular carcinoma (14,15)
and thyroid cancer (16).
E-cadherin is a key mediator of cell-cell adhesion
in epithelial tissues, and loss of E-cadherin can promote invasive
and metastatic behavior in many epithelial tumors (17). In head and neck cancers, loss of
cell-cell adhesion resulting in stromal and vascular invasion as a
consequence of E-cadherin dysregulation is well documented
(18,19).
It has been suggested that DNA methylation plays a
major role in enhancing transcriptional silence, especially in
tumor suppressor genes (20).
E-cadherin also could be suppressed by DNA hypermethylation and has
a close relationship with tumor prognosis in head and neck squamous
cell carcinoma (21), breast
cancer (22), lung cancer
(23), bladder cancer (24).
DNA methyltransferases (DNMTs) are responsible for
the transfer of a methyl group from the universal methyl donor,
S-adenosyl-L-methionine, to the 5-position of the cytosine residue
in DNA. There are four members of the DNMT family: DNMT1, DNMT3A,
DNMT3B and DNMT3L. DNMT3A and DNMT3B encode de novo
methyltransferases, while DNMT1 encodes a maintenance
methyltransferases, which are essential for mammalian development
and reported to be associated with human tumorigenesis (25).
DNMT1 stability is regulated via various
post-translational modifications, previous studies showed that AKT1
can stabilizes DNMT1 and affects genome methylation (26,27).
IL-8 can activate AKT1 pathway to promote tumor invasion in breast
cancer (28), kidney cancer
(29), and breast cancer (30). Our previous study also showed that
in NPC, the overexpression of IL-8 can induce EMT through
activating the AKT signaling pathway (12). However, to the best of our
knowledge, there are no studies regarding the relationship between
IL-8 and DNMT1 expression, and though our previous study showed
IL-8 can reduce E-cadherin expression in NPC cell lines (12), the underlying molecular mechanisms
remain unclear. In this study, we explore the possibility that in
NPC cell lines, IL-8 can induce DNMT1 expression through AKT1
signaling then mediates silencing of E-cadherin expression which
play an important role in EMT.
Materials and methods
Cell lines and cell culture
The human low-metastasis, low endogenous IL-8
secreating level NPC cell lines CNE-2 and the CNE-2 subclones S22
and S26 have previously been established and reported (12,31),
the cells were cultured in Dulbecco's modified Eagle's medium
(DMEM) with 10% fetal bovine serum purchased from Gibco/BRL (Grand
Island, NY, USA), 100 U/ml penicillin G, 100 U of streptomycin (all
from Invitrogen, Carlsbad, CA, USA). Cells were incubated at 37°C
in a humidified atmosphere containing 5% CO2. Based on
our previous study (12),
recombinant human IL-8 (PeproTech) at a concentration of 8 ng/ml
was added into cell culture medium in experimental groups. For
vehicle controls, the cells were treated with equivalent amounts of
BSA.
Immunoblotting
Immunoblotting was performed as described previously
(12). The sources of the primary
antibodies (and their concentrations) were as follows:
anti-E-cadherin (1:1,000), anti-AKT (1:1,000), anti-phospho-AKT
(Ser473) (1:1,000), and anti-β-actin (1:1,000) these antibodies
were purchased from Cell Signaling Technology (Danvers, MA, USA);
anti-DNMT1 (1:1,000) was purchased from Santa Cruz Biotechnology
(Santa Cruz). Anti-rabbit peroxidase-conjugated secondary
antibodies were purchased from Promega. For western blot analysis
of AKT1 signaling inhibition studies, LY-294002 (the AKT1
inhibitor) (Cell Signaling Technology) (20 μM) was used one hour
prior to IL-8 stimulation in an attempt to inhibit the conventional
AKT1 pathway, for western blot analysis of DNMT1 inhibition
studies, 5-aza-2′-deoxycytidine (the inhibitor of DNA methylation)
(Sigma) (5 μM) was used in an attempt to inhibit DNMT1 function.
Cells were pretreated with 5-aza-2′-deoxycytidine for 48 h then
were treated with IL-8 for 24 h. Experiments were repeated in
triplicate.
Quantitative real-time polymerase chain
reaction
Total cellular RNA was extracted using the High Pure
RNA kit (Roche Applied Science, Penzberg, Germany). For RT-PCR, the
total RNA was quantified spectrophotometrically, and equal amounts
(1 μg) were transcribed into cDNA according to the manufacturer's
protocol (Roche Applied Science). The sequences of the PCR primers
used for amplifications of β-actin, IL-8, DNMT1, E-cadherin were as
follows: β-actin forward: 5′-CACGATGGAGGGGCCGGACTCATC-3′; β-actin
reverse, 5′-TAAAGACCTCTATGCCAACACAGT-3′. IL-8 forward,
5′-CTCCAAACCTTTCCACCCC-3′; IL-8 reverse,
5′-GATTCTTGGATACCACAGAGAATG-3′. DNMT1 forward,
5′-AGCCAAATCGGATGAGTCCATC-3′; DNMT1 reverse,
5′-CCTCCTTCAGTTTCTGTTTGGG-3′. E-cadherin forward,
5′-AACAGGATGGCTGAAGGTGA-3′; E-cadherin reverse,
5′-CCTTCCATGACAGACCCCTT-3′. Fast SYBR Green Master Mix was used to
determine the threshold cycle (Ct) value of each sample in the
CFX96 real-time PCR detection system (Bio-Rad, CA, USA). β-actin
served as the normalization gene in these studies. The relative
expression levels of the target genes were given by 2ΔCt (Ct of
β-actin minus the Ct of the target gene). PCR amplifications of
β-actin, IL-8, E-cadherin, DNMT1 were performed under the following
conditions: denaturation at 95°C for 30 sec, annealing at 58°C for
30 sec, and extension at 72°C for 30 sec; reactions were carried
out for 30 cycles. The experiments were performed in
triplicate.
Transient transfection of IL-8
For IL-8 overexpression studies, the IL-8
overexpressing vector (cat no. CH832510) and control pENTER vector
were purchased from ViGene Biosciences Inc. (Rockville, MD, USA).
These plasmids were transfected into S22, S26, CNE2 cells with
X-tremeGENE HP transfection reagent according to the manufacturer's
instructions (Roche Life Science). The cells were harvest 48 h
after transfection for immunoblot and real time-PCR analyses.
ELISA
Twenty-four hours after IL-8 overexpressing plasmids
or control pENTER vector were transfected into S22, S26, CNE2 cells
with X-tremeGENE HP transfection reagent, 2×106 of the
cells were plated into 100-mm culture plates and incubated for
another 24 h in the regular medium. The medium was then replaced
with a serum-free medium (10 ml) and the cells were incubated for
an additional 12 h. Conditioned medium was collected and subjected
to centrifugation, followed by filtration through a 0.45 μm
membrane filter to remove the debris. Secreted human IL-8
concentration in the conditioned medium was then measured using a
sandwich enzyme-linked immunosorbent assay (ELISA) kit (R&D,
MN, USA) following the manufacturer's instructions. The experiments
were performed in triplicate.
BSP (bisulfite sequencing PCR)
Bisulfite sequencing PCR was used to examine the
methylation status of E-cadherin gene promoter. Genomic DNA from
cultured cells were extracted according to the manufacturer's
protocol (Qiagen 51304), followed by bisulfite conversion using
EpiTect Bisulfite kit (Qiagen 59104). DNA amplification was
performed via PCR using primers as follows: E-cadherin-BSP-F:
5′-TGTAGGT TTTATAATTTATTTAGAT-3′; E-cadherin-BSP-R: 5′-CTCA
CAAATACTTTACAATT-3′. PCR products were cloned to pTopo TA vector
(Invitrogen), and then transfected to Top10 cells for sequencing,
at least ten clones from each sample were selected.
Statistics
Student's t-test was used to compare two independent
groups of data. A P-value <0.05 was considered statistically
significant.
Results
IL-8 treatment induces DNMT1 expression
in nasopharyngeal cancer cells
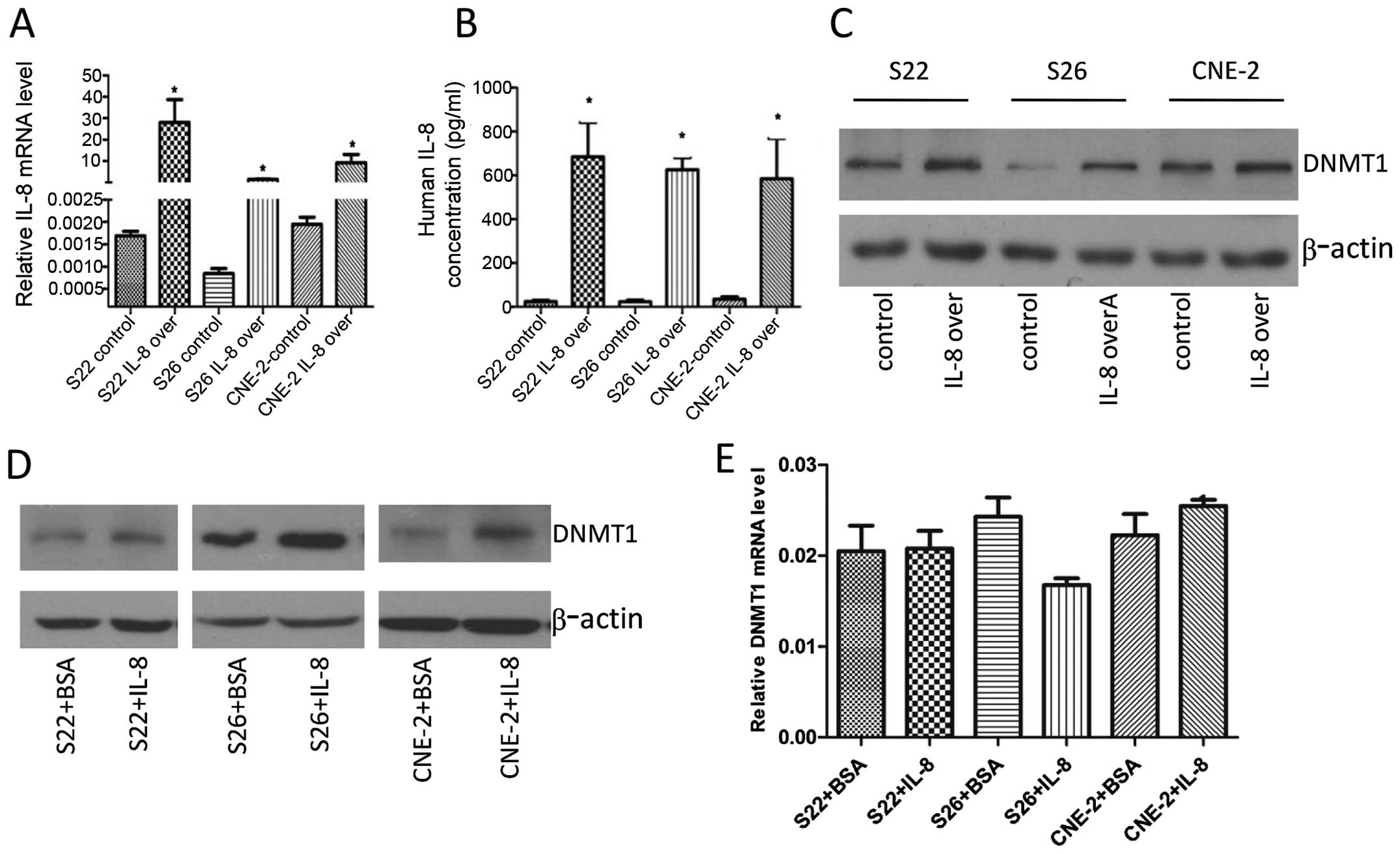
Our previous study showed that IL-8 is lowly
expressed in low-metastasis S26 cells (12). In the present study, we
investigated whether IL-8 could enhance the expression of DNMT1 by
transient transfection of IL-8 plasmid. After transient
transfection of IL-8 plasmid in S22, S26, and CNE-2 cells,
real-time PCR analysis showed that IL-8 mRNA levels were increased
greatly 48 h later (Fig. 1A), and
ELISA analysis showed high levels of IL-8 were secreted into the
culture medium by the cells (Fig.
1B). Then immunoblotting of the nuclear extracts showed an
increase in DNMT1 protein expression 48 h after transient
transfections (Fig. 1C). Next, we
used exogenous recombinant human IL-8 to verify this finding.
Consistently, DNMT1 protein level was increased in the S22, S26,
CNE-2 cells following IL-8 treatment with 8 ng/ml for 24 h
(Fig. 1D). We next analyzed DNMT1
mRNA expression using real-time PCR, and found that DNMT1 mRNA
levels were not significantly altered in these cells (Fig. 1E), suggesting that IL-8-induced
accumulation of DNMT1 protein was not due to mRNA
overexpression.
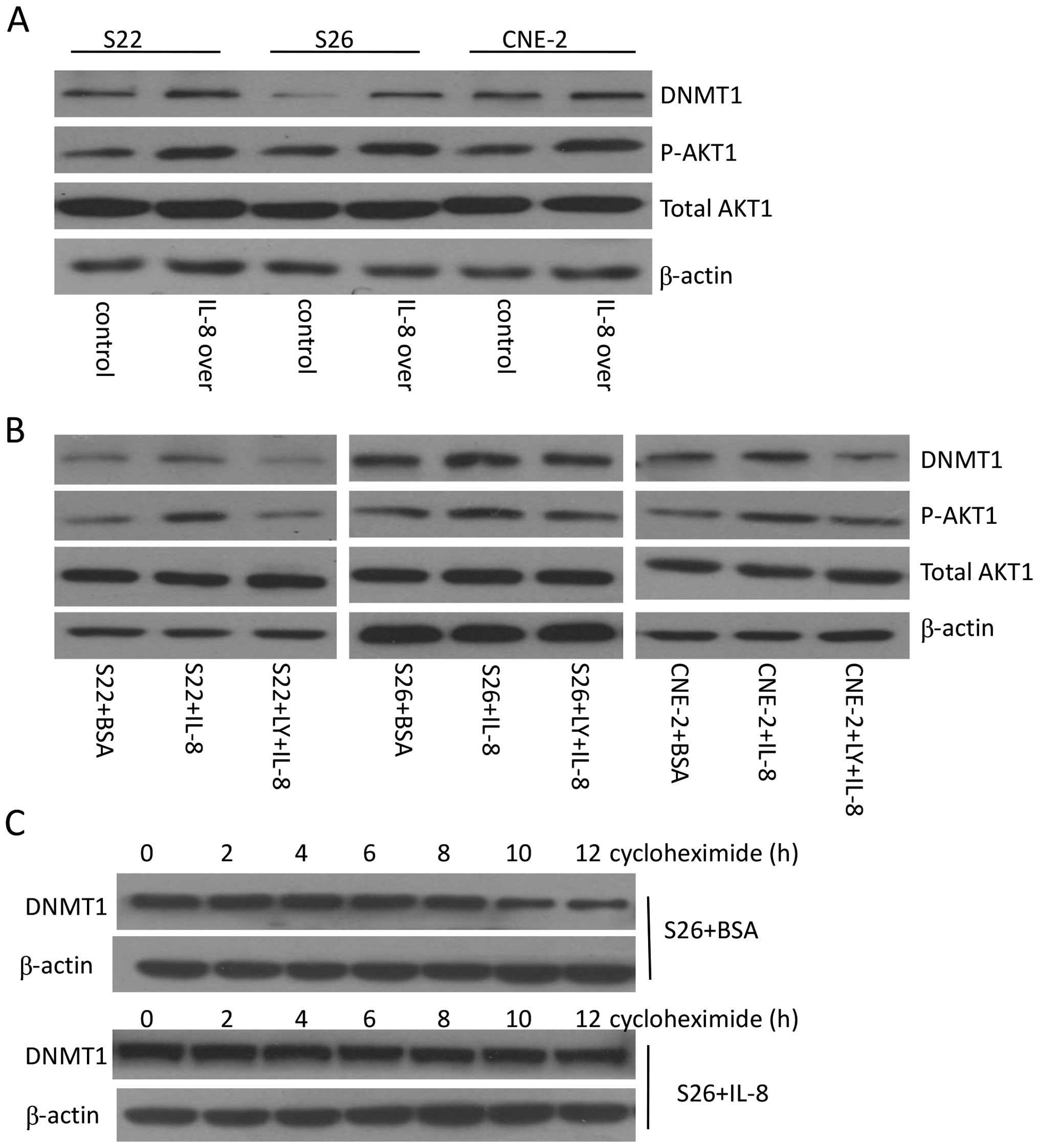
IL-8 enhances DNMT1 stabilization through
AKT1 signaling
DNMT1 protein stability can be regulated via various
post-translational modifications. It has been reported that AKT1
activity can stabilize DNMT1 protein (26,27).
Our previous study showed that IL-8 can stimulate AKT1 pathway in
NPC cells (12), therefore, we
wanted to know whether the IL-8-induced accumulation of DNMT1
protein is partly due to AKT1 signaling. After transient
transfection of IL-8, immunoblotting of nuclear extracts showed an
increase in p-AKT1 protein expression in S22, S26, and CNE-2 cells
(Fig. 2A). Then S22, S26, CNE-2
cells were treated with IL-8 at 8 ng/ml, and total AKT1 and
phosphorylated AKT1 levels were tested by immunoblotting. The
results showed that treatment of cells with IL-8 lead to activation
of the AKT1 pathway as expected. To confirm that the increase of
DNMT1 is AKT1-dependent, we treated cultured S22, S26, CNE-2 cells
with the AKT1 inhibitor LY294002 and then measured DNMT1 protein
levels. LY294002 blocked pAKT1 activation triggered by IL-8 and
resulted in reduction of total DNMT1 (Fig. 2B). To confirm DNMT1 protein
stabilization, we treated S26 cells with the protein synthesis
inhibitor cycloheximide to block the synthesis of DNMT1. Immunoblot
analysis showed that DNMT1 levels gradually decreased in 10 h
following the addition of cycloheximide to culture media without
IL-8. However, the degradation of DNMT1 was slowed down by the
addition of IL-8 to the medium, indicating that IL-8 stabilizes
DNMT1 protein, an effect persisting for ≥12 h (Fig. 2C). Therefore, we conclude that IL-8
upregulates DNMT1 protein level through activating AKT1 pathway and
enhancing DNMT1 stabilization.
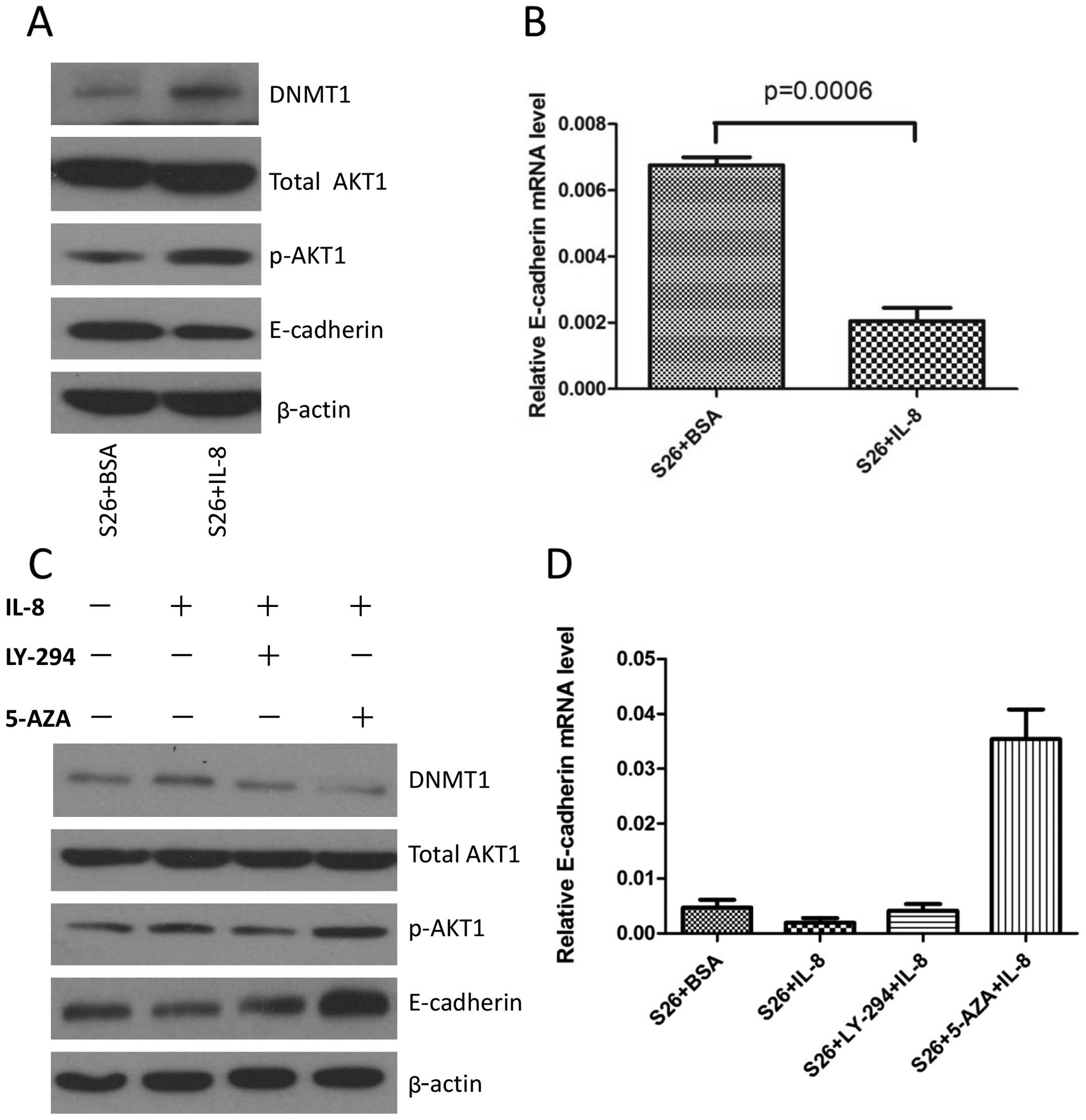
IL-8 treatment of S26 cells increases
E-cadherin level via DNMT1
Our previous study shows that overexpression of IL-8
in S26 cells can induce EMT with downregulation of E-cadherin
(12). In the present study, we
continued to explore whether this downregulation of E-cadherin
level is partly due to upregulation of DNMT1. Immunoblot analysis
showed that IL-8 inhibited the expression of E-cadherin in S26 cell
lines and also decreased the transcription of E-cadherin (Fig. 3A and B). As inhibiting AKT1 reduced
the overall DNMT1 protein levels, we expected that treating the
cells with LY294002 would result in upregulated E-cadherin
expression. Then, S26 cells were treated with IL-8 for 24 h, while
in the presence of LY-294002, an inhibitor of the AKT pathway,
which was added one hour before the addition of IL-8, immunoblot
analysis showed that LY-294002 blocked IL-8-induced pAKT1
activation and resulted in reduction of DNMT1 as well as an
increase of E-cadherin protein level. Many studies have
demonstrated that promoter methylation of E-cadherin is an
important mechanism contributing to its downregulation (33). We speculate that methylation of the
E-cadherin promoter may play an important role in IL-8 mediated
decrease of this gene. In addition, while in the presence of
5-aza-2′-deoxycytidine, an inhibitor of DNA methylation, which was
added 48 h before IL-8, and IL-8-induced downregulation of
E-cadherin was reversed (Fig. 3C).
The RNA level of E-cadherin also showed consistent changes
(Fig. 3D), suggesting that the
effect of IL-8 on E-cadherin expression is transcriptionally
regulated via DNMT1.
IL-8 downregulates E-cadherin expression
by promoting its promoter methylation via DNMT1
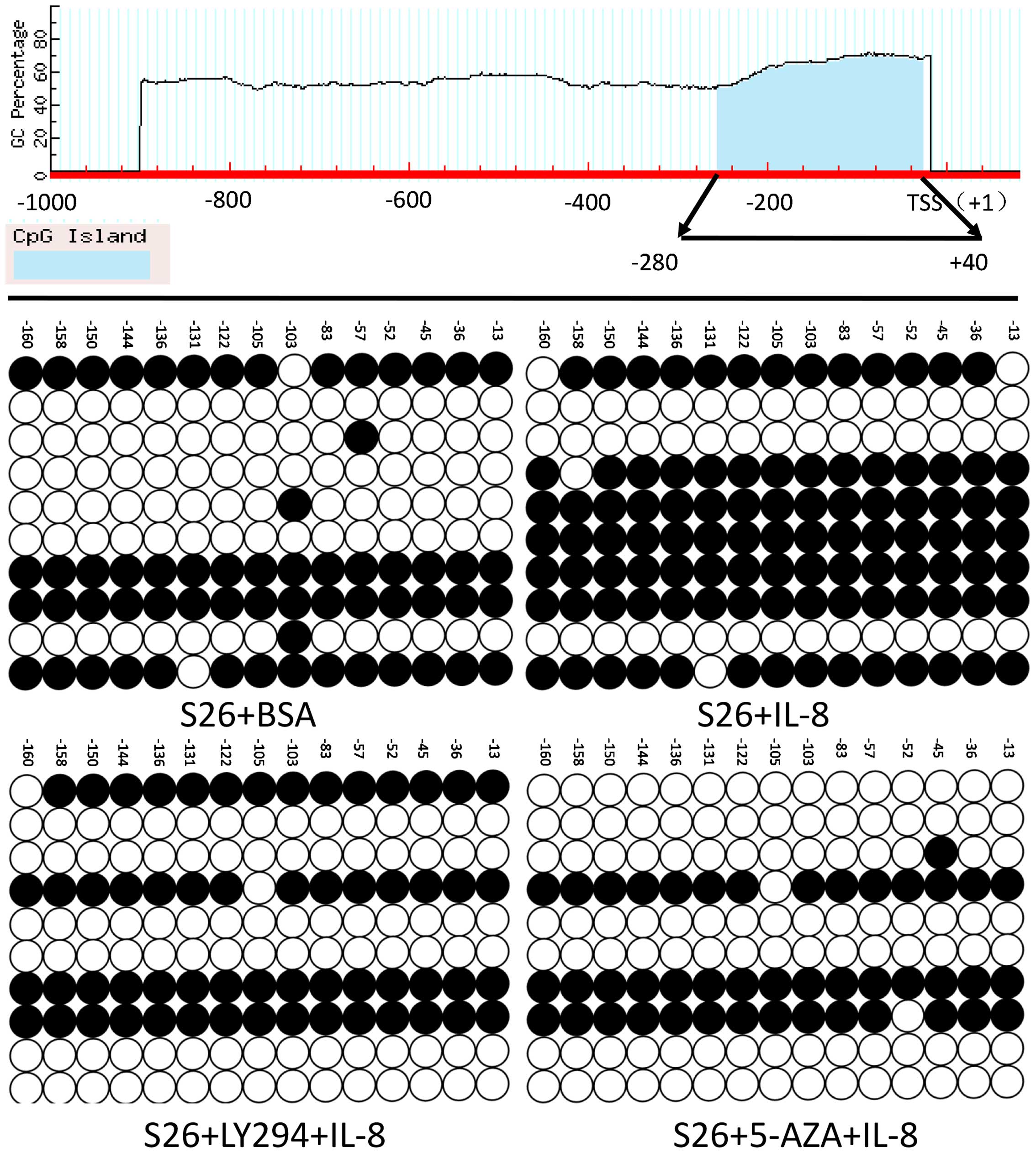
Next, in order to verify that the downregulation of
E-cadherin was partly due to its promoter methylation, the
bisulfite sequencing PCR of E-cadherin promoter analysis was
performed, the methylated cytosine residues (ranging from −280 to
+40 of E-cadherin promoter) in each clone were represented by a
solid spot as depicted (Fig. 4).
In total, 17 CpG methylation sites were detected. Bisulfite
sequencing PCR assay of the E-cadherin promoter showed that the
promoter CpG island methylation status of E-cadherin in IL-8
treated cells was markedly higher than control cells (Fig. 4). In addition, when LY-294002 or
5-aza-2′-deoxycytidine was added for 48 h together with IL-8
administration, the methylation status of E-cadherin reversed to
the control level. These data demonstrated that IL-8 could induce
hypermethylation on the specific CpG sites of E-cadherin
promoter.
Discussion
The influence of inflammation on tumorigenesis has
been intensively investigated for centuries (34). In addition, the expression of IL-8
in the tumor microenvironment is reported to be associated with
tumor progression and patient survival (35). We have previously reported that
IL-8 promotes metastasis of NPC cells in autocrine and paracrine
manner, involving activation of AKT signaling and inducing EMT in
NPC cells (12). In the present
study, we further demonstrated for the first time that IL-8
inhibited the expression of E-cadherin by inducing its promoter DNA
hypermethylation via upregulating DNMT1 protein level. Blockage of
the IL-8/AKT pathway and inhibition of DNMT1 reversed the
expression of E-cadherin. These data demonstrate a link between
inflammation and NPC progression involving epigenetic regulation of
E-cadherin.
Dysregulation of DNMT1 activity causes many human
diseases, including cancer (36).
Post-translational modifications of DNMT1 play a crucial role in
how and when it is activated. Other studies have reported that AKT
activity can inhibit DNMT1 degradation in multiple cell lines
(26,27). IL-8 signals through CXCR1 and
CXCR2, and phosphatidylinositol-3 (PI3) is a component in
CXCR1/2-signaling. The enzyme PI3-kinase (PI3K) is a principal
effector of CXCL8-mediated chemotaxis in neutrophils, and its
triggering phosphorylation results in the activation and increased
expression of AKT (28,32,37,38).
However, to our knowledge, our study is the first one to clarify
the relationship between IL-8 and DNMT1. Our findings showed that
IL-8 upregulated DNMT1 though AKT1 pathway by increasing its
stability in NPC cells. This increment of DNMT1 by IL-8 stimulation
can be eliminated by blocking AKT signaling, confirming that AKT is
a key signaling pathway by which IL-8 regulates DNMT1. Upon DNMT1
activation, both transcriptional and translational levels of
E-cadherin are reduced. We therefore confirmed E-cadherin as one of
the target genes of DNMT1 is regulated by IL-8/AKT signaling. The
important roles of E-cadherin in NPC progression relies on its
functions as an adhesion molecule regulating cell-cell contact for
tissue morphogenesis, cellular polarity and tumor invasiveness
(17). It has been reported that
loss of membranous E-cadherin expression results in enhanced cell
migration activity (39), which
significantly correlates with tumor invasion, advanced disease
stage, and tumor metastasis (40).
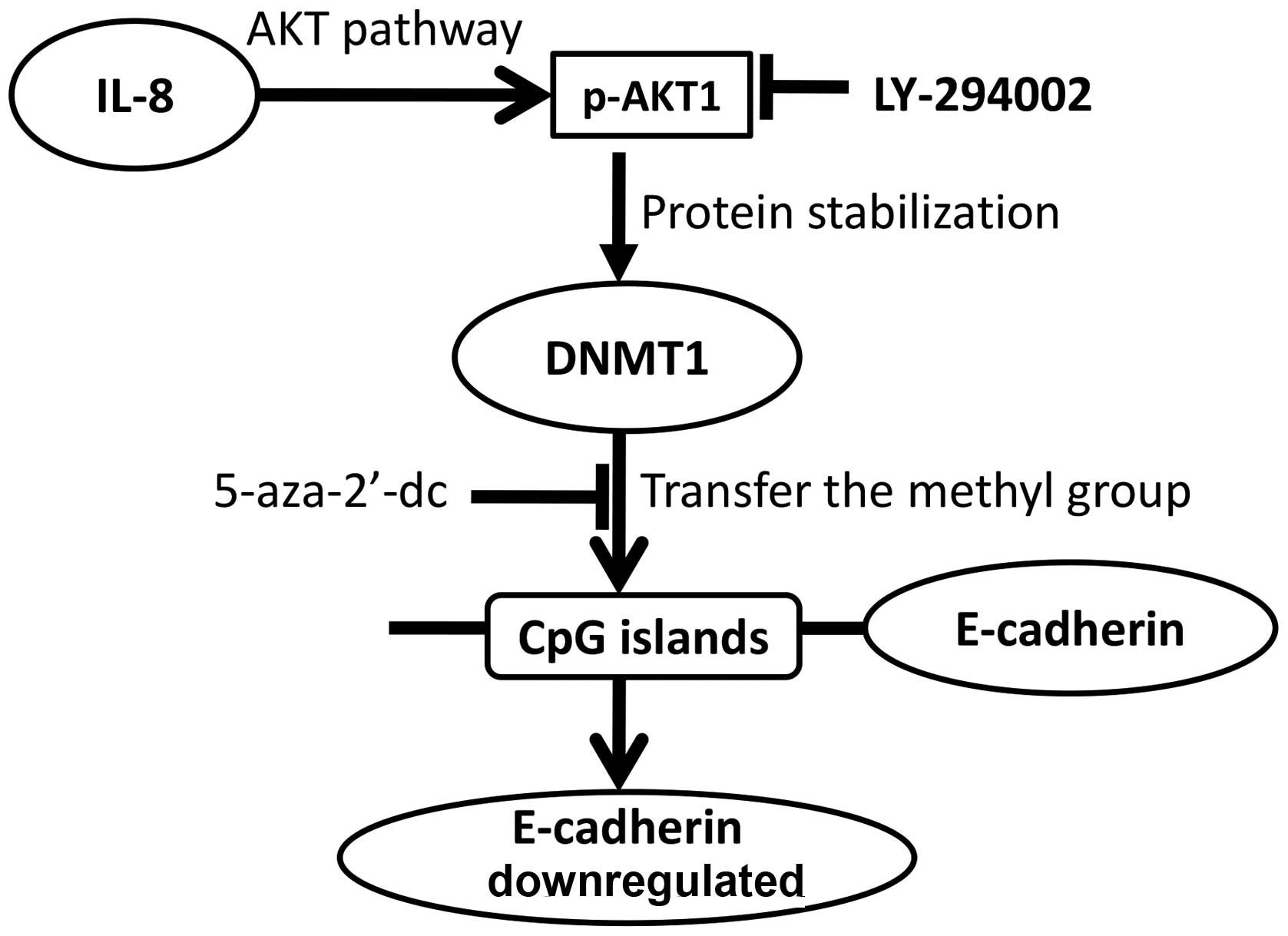
A model for this IL-8-p-AKT1-DNMT1 signaling is then
proposed based on our findings (Fig.
5) our data clearly show that the IL-8-activated AKT signaling
results in stabilization of DNMT1 and E-cadherin epigenetic
regulation. This is the first report to reveal the IL-8-mediated
E-cadherin silencing mechanism in NPC. These data suggest that
targeting IL-8 signaling is a promising approach for prevention and
treatment of NPC metastasis.
Acknowledgements
This study was supported by grants from the National
Natural Science Foundation of China (nos. 81472386, 81272340 and
81030043), the National High Technology Research and Development
Program of China (863 Program, no. 2012AA02A501), and the Urumqi
Key Laboratory of Infection and Cancer Project Foundation of China
(no. WIT-2013-04).
Abbreviations:
|
NPC
|
nasopharyngeal carcinoma
|
|
DNMT1
|
DNA methyltransferase-1
interleukin
|
|
5-aza
|
5-aza-2′-deoxycytidine
|
|
EMT
|
epithelial-mesenchymal transition
|
References
|
1
|
Wei KR, Zheng RS, Zhang SW, Liang ZH, Ou
ZX and Chen WQ: Nasopharyngeal carcinoma incidence and mortality in
China in 2010. Chin J Cancer. 33:381–387. 2014.PubMed/NCBI
|
|
2
|
Adham M, Kurniawan AN, Muhtadi AI, Roezin
A, Hermani B, Gondhowiardjo S, Tan IB and Middeldorp JM:
Nasopharyngeal carcinoma in Indonesia: epidemiology, incidence,
signs, and symptoms at presentation. Chin J Cancer. 31:185–196.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Tao CJ, Liu X, Tang LL, Mao YP, Chen L, Li
WF, Yu XL, Liu LZ, Zhang R, Lin AH, et al: Long-term outcome and
late toxicities of simultaneous integrated boost-intensity
modulated radiotherapy in pediatric and adolescent nasopharyngeal
carcinoma. Chin J Cancer. 32:525–532. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Liang FY, Sun W, Han P, Lu X, Lian YN and
Huang XM: Detecting plasma Epstein-Barr virus DNA to diagnose
postradiation nasopharyngeal skull base lesions in nasopharyngeal
carcinoma patients: a prospective study. Chin J Cancer. 31:142–149.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Wang WY, Twu CW, Lin WY, Jiang RS, Liang
KL, Chen KW, Wu CT, Shih YT and Lin JC: Plasma Epstein-Barr virus
DNA screening followed by 18F-fluoro-2-deoxy-D-glucose
positron emission tomography in detecting posttreatment failures of
nasopharyngeal carcinoma. Cancer. 117:4452–4459. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Bar-Eli M: Role of interleukin-8 in tumor
growth and metastasis of human melanoma. Pathobiology. 67:12–18.
1999. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Rofstad EK and Halsør EF: Vascular
endothelial growth factor, interleukin 8, platelet-derived
endothelial cell growth factor, and basic fibroblast growth factor
promote angiogenesis and metastasis in human melanoma xenografts.
Cancer Res. 60:4932–4938. 2000.PubMed/NCBI
|
|
8
|
Singh RK, Gutman M, Reich R and Bar-Eli M:
Ultraviolet B irradiation promotes tumorigenic and metastatic
properties in primary cutaneous melanoma via induction of
interleukin 8. Cancer Res. 55:3669–3674. 1995.PubMed/NCBI
|
|
9
|
Luca M, Huang S, Gershenwald JE, Singh RK,
Reich R and Bar-Eli M: Expression of interleukin-8 by human
melanoma cells up-regulates MMP-2 activity and increases tumor
growth and metastasis. Am J Pathol. 151:1105–1113. 1997.PubMed/NCBI
|
|
10
|
Inoue K, Slaton JW, Kim SJ, Perrotte P,
Eve BY, Bar-Eli M, Radinsky R and Dinney CP: Interleukin 8
expression regulates tumorigenicity and metastasis in human bladder
cancer. Cancer Res. 60:2290–2299. 2000.PubMed/NCBI
|
|
11
|
Shahzad MM, Arevalo JM, Armaiz-Pena GN, Lu
C, Stone RL, Moreno-Smith M, Nishimura M, Lee JW, Jennings NB,
Bottsford-Miller J, et al: Stress effects on FosB- and
interleukin-8 (IL8)-driven ovarian cancer growth and metastasis. J
Biol Chem. 285:35462–35470. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Li XJ, Peng LX, Shao JY, Lu WH, Zhang JX,
Chen S, Chen ZY, Xiang YQ, Bao YN, Zheng FJ, et al: As an
independent unfavorable prognostic factor, IL-8 promotes metastasis
of nasopharyngeal carcinoma through induction of
epithelial-mesenchymal transition and activation of AKT signaling.
Carcinogenesis. 33:1302–1309. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Desai S, Laskar S and Pandey BN: Autocrine
IL-8 and VEGF mediate epithelial-mesenchymal transition and
invasiveness via p38/JNK-ATF-2 signalling in A549 lung cancer
cells. Cell Signal. 25:1780–1791. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Fu XT, Dai Z, Song K, Zhang ZJ, Zhou ZJ,
Zhou SL, Zhao YM, Xiao YS, Sun QM, Ding ZB, et al:
Macrophage-secreted IL-8 induces epithelial-mesenchymal transition
in hepatocellular carcinoma cells by activating the
JAK2/STAT3/Snail pathway. Int J Oncol. 46:587–596. 2015.
|
|
15
|
Yu J, Ren X, Chen Y, Liu P, Wei X, Li H,
Ying G, Chen K, Winkler H and Hao X: Dysfunctional activation of
neurotensin/IL-8 pathway in hepatocellular carcinoma is associated
with increased inflammatory response in microenvironment, more
epithelial mesenchymal transition in cancer and worse prognosis in
patients. PLoS One. 8:e560692013. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Bauerle KT, Schweppe RE, Lund G, Kotnis G,
Deep G, Agarwal R, Pozdeyev N, Wood WM and Haugen BR: Nuclear
factor κB-dependent regulation of angiogenesis, and metastasis in
an in vivo model of thyroid cancer is associated with secreted
interleukin-8. J Clin Endocrinol Metab. 99:E1436–E1444. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Birchmeier W and Behrens J: Cadherin
expression in carcinomas: Role in the formation of cell junctions
and the prevention of invasiveness. Biochim Biophys Acta.
1198:11–26. 1994.PubMed/NCBI
|
|
18
|
Georgolios A, Batistatou A, Manolopoulos L
and Charalabopoulos K: Role and expression patterns of E-cadherin
in head and neck squamous cell carcinoma (HNSCC). J Exp Clin Cancer
Res. 25:5–14. 2006.PubMed/NCBI
|
|
19
|
Zhao Z, Ge J, Sun Y, Tian L, Lu J, Liu M
and Zhao Y: Is E-cadherin immunoexpression a prognostic factor for
head and neck squamous cell carcinoma (HNSCC)? A systematic review
and meta-analysis. Oral Oncol. 48:761–767. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Jin B and Robertson KD: DNA
methyltransferases, DNA damage repair, and cancer. Adv Exp Med
Biol. 754:3–29. 2013. View Article : Google Scholar :
|
|
21
|
Marsit CJ, Posner MR, McClean MD and
Kelsey KT: Hypermethylation of E-cadherin is an independent
predictor of improved survival in head and neck squamous cell
carcinoma. Cancer. 113:1566–1571. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Shargh SA, Sakizli M, Khalaj V, Movafagh
A, Yazdi H, Hagigatjou E, Sayad A, Mansouri N, Mortazavi-Tabatabaei
SA and Khorram Khorshid HR: Downregulation of E-cadherin expression
in breast cancer by promoter hypermethylation and its relation with
progression and prognosis of tumor. Med Oncol. 31:2502014.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Wang G, Hu X, Lu C, Su C, Luo S and Luo
ZW: Promoter-hypermethylation associated defective expression of
E-cadherin in primary non-small cell lung cancer. Lung Cancer.
62:162–172. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Li G, Liu Y, Yin H, Zhang X, Mo X, Tang J
and Chen W: E-cadherin gene promoter hypermethylation may
contribute to the risk of bladder cancer among Asian populations.
Gene. 534:48–53. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Jin B, Ernst J, Tiedemann RL, Xu H,
Sureshchandra S, Kellis M, Dalton S, Liu C, Choi JH and Robertson
KD: Linking DNA methyltransferases to epigenetic marks and
nucleosome structure genome-wide in human tumor cells. Cell Rep.
2:1411–1424. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Estève PO, Chang Y, Samaranayake M,
Upadhyay AK, Horton JR, Feehery GR, Cheng X and Pradhan S: A
methylation and phosphorylation switch between an adjacent lysine
and serine determines human DNMT1 stability. Nat Struct Mol Biol.
18:42–48. 2011. View Article : Google Scholar :
|
|
27
|
Hodge DR, Cho E, Copeland TD, Guszczynski
T, Yang E, Seth AK and Farrar WL: IL-6 enhances the nuclear
translocation of DNA cytosine-5-methyltransferase 1 (DNMT1) via
phosphorylation of the nuclear localization sequence by the AKT
kinase. Cancer Genomics Proteomics. 4:387–398. 2007.
|
|
28
|
Shao N, Lu Z, Zhang Y, Wang M, Li W, Hu Z,
Wang S and Lin Y: Interleukin-8 upregulates integrin β3 expression
and promotes estrogen receptor-negative breast cancer cell invasion
by activating the PI3K/Akt/NF-κB pathway. Cancer Lett. 364:165–172.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Bi LK, Zhou N, Liu C, Lu FD, Lin TX, Xuan
XJ, Jiang C, Han JL, Huang H, Zhang CX, et al: Kidney cancer cells
secrete IL-8 to activate Akt and promote migration of mesenchymal
stem cells. Urol Oncol. 32:607–612. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Wang L, Tang C, Cao H, Li K, Pang X, Zhong
L, Dang W, Tang H, Huang Y, Wei L, et al: Activation of IL-8 via
PI3K/Akt-dependent pathway is involved in leptin-mediated
epithelial-mesenchymal transition in human breast cancer cells.
Cancer Biol Ther. 16:1220–1230. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Qian CN, Berghuis B, Tsarfaty G, Bruch M,
Kort EJ, Ditlev J, Tsarfaty I, Hudson E, Jackson DG, Petillo D, et
al: Preparing the ‘soil’: The primary tumor induces vasculature
reorganization in the sentinel lymph node before the arrival of
metastatic cancer cells. Cancer Res. 66:10365–10376. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Li MQ, Luo XZ, Meng YH, Mei J, Zhu XY, Jin
LP and Li DJ: CXCL8 enhances proliferation and growth and reduces
apoptosis in endometrial stromal cells in an autocrine manner via a
CXCR1-triggered PTEN/AKT signal pathway. Hum Reprod. 27:2107–2116.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Di Croce L and Pelicci PG:
Tumour-associated hypermethylation: Silencing E-cadherin expression
enhances invasion and metastasis. Eur J Cancer. 39:413–414. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Grivennikov SI, Greten FR and Karin M:
Immunity, inflammation, and cancer. Cell. 140:883–899. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Palena C, Hamilton DH and Fernando RI:
Influence of IL-8 on the epithelial-mesenchymal transition and the
tumor microenvironment. Future Oncol. 8:713–722. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Feinberg A: DNA methylation in cancer:
Three decades of discovery. Genome Med. 6:362014. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Schraufstatter IU, Chung J and Burger M:
IL-8 activates endothelial cell CXCR1 and CXCR2 through Rho and Rac
signaling pathways. Am J Physiol Lung Cell Mol Physiol.
280:L1094–L1103. 2001.PubMed/NCBI
|
|
38
|
Lane HC, Anand AR and Ganju RK: Cbl and
Akt regulate CXCL8-induced and CXCR1- and CXCR2-mediated
chemotaxis. Int Immunol. 18:1315–1325. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Ochiai A: Dysfunction of cadherin cell
adhesion system in cancer invasion and metastasis. Gan To Kagaku
Ryoho. 26:565–571. 1999.In Japanese. PubMed/NCBI
|
|
40
|
Zheng Z, Pan J, Chu B, Wong YC, Cheung AL
and Tsao SW: Downregulation and abnormal expression of E-cadherin
and beta-catenin in nasopharyngeal carcinoma: Close association
with advanced disease stage and lymph node metastasis. Hum Pathol.
30:458–466. 1999. View Article : Google Scholar : PubMed/NCBI
|