Introduction
Colorectal cancer (CRC) is the third most common
cancer worldwide and its incidence has risen rapidly in Asian
countries during the past few decades (1,2). It
has been established that environmental and hereditary factors
contribute to the initiation and development of CRC, as indicated
by the accumulation of mutations in oncogenes (3). Chronic inflammation is regarded as an
important risk factor for the development of CRC, especially in
patients with inflammatory bowel diseases (IBD) (4). Interleukin-6 (IL-6), a
pro-inflammatory cytokine, has been shown to regulate cancer cell
growth and thereby contribute to tumor promotion and progression
(5). IL-6 has been demonstrated to
be associated with an unfavorable prognosis in patients with
various types of cancers including both sporadic and
colitis-associated CRC (6).
However, despite clear evidence implicating IL-6 in causation of
CRC, molecular mechanisms underlying this phenomenon are still
incompletely understood.
Cancer cells preferentially use glucose by aerobic
glycolysis, which is characterized by increased glycolysis and
lactate production regardless of oxygen availability. This
phenomenon, termed as the Warburg effect, is a metabolic adaptation
that promotes the proliferation of cancer cells and is added as an
emerging hallmark of cancer (7–9).
Tumor hypoxia was thought to be a major contributor in the switch
to aerobic glycolysis and hypoxia inducible factor-1α (HIF-1α) was
thought to play an important role in the increased aerobic
glycolysis in cancer cells (10).
However, cancer cells exhibit aerobic glycolysis even though tumor
cells are exposed to oxygen during tumorigenesis (11). Thus, what triggers the switch from
oxidative phosphorylation to aerobic glycolysis remains
unclear.
Fructose-2,6-bisphosphate (F2,6-BP) is a powerful
allosteric activator of phosphofructokinase 1 (PFK-1), the enzyme
that controls one of the most critical steps of glycolysis
(12). F2,6-BP is synthesised by
the family of 6-phosphofructo-2-kinase/2,6-bisphosphatase (PFKFB)
bifunctional enzymes. PFKFB3 is the most efficient isoform of this
family and is overexpressed in various types of cancers including
colon cancer (13). PFKFB3 was
demonstrated to be a hypoxia inducible gene that was stimulated
through HIF-1 interaction with the consensus hypoxia response
element site in its promoter region (14). However, the relationship between
PFKFB3 and chronic inflammation is rarely reported. In addition,
what is the function of PFKFB3 during the initiation and
development of CRC is still unclear.
In the present study, we investigated whether IL-6
promotes the development of CRC by regulating the aerobic
glycolysis and the underlying molecular mechanisms. We found that
anti-IL-6 receptor antibody decreased the expression of key genes
involved in aerobic glycolysis, whereas IL-6 treatment upregulated
PRKFB3 expression and promoted glycolysis in CRC cells. Further
analysis in human samples revealed higher PRKFB3 expression in
colorectal adenoma and adenocarcinoma tissues, which was also
associated with lymph node metastasis, intravascular cancer embolus
and TNM stage in sporadic CRC patients. Knockdown of PFKFB3 in CRC
cells also abolished IL-6 stimulated cell proliferation and
migration. Overall, our results indicate that chronic inflammation
promotes the initiation and development of CRC and IL-6 is
functioning, at least partly, through regulating PFKFB3 at early
stage of CRC.
Materials and methods
Patients specimens
Eighty-seven sporadic and 13 colitis-associated CRC
tumor tissues and their matching adjacent non-malignant tissues
were collected during surgery from patients in Zhongshan Hospital
of Fudan University. Colorectal pathological sections of patients
who were diagnosed as chronic inflammation (n=18), adenoma (n=23)
and adenocarcinoma (n=26) were collected from the Pathology
Department of Zhongshan Hospital of Fudan University. The patients
recruited to the present study had not received chemotherapy or
radiotherapy before surgery. Written informed consents were
obtained from all the patients and permission for this study was
obtained from the ethics committee of Zhongshan Hospital of Fudan
University. Fresh specimens were immediately frozen in liquid
nitrogen and stored at −80°C until further analysis.
Mouse and colitis-associated CRC
model
The colitis-associated CRC mouse model was induced
in Balb/c male mice (6–8 weeks of age) purchased from the Shanghai
Laboratory Animal Center, Chinese Academy of Sciences. Mice were
acclimatized to the environment for a week before the study
started. All animal manipulations were carried out according to the
guidelines of regulations for the use of experimental animals of
the Chinese Academy of Science. All efforts were made to minimize
animal suffering. Mice were divided into 3 groups: normal saline
control group (NS group, n=16), mouse monoclonal IgG (eBiosciences)
control group (IgG group, n=16), mouse monoclonal anti-IL-6
receptor antibody (eBiosciences) treated group (IL-6R Ab group,
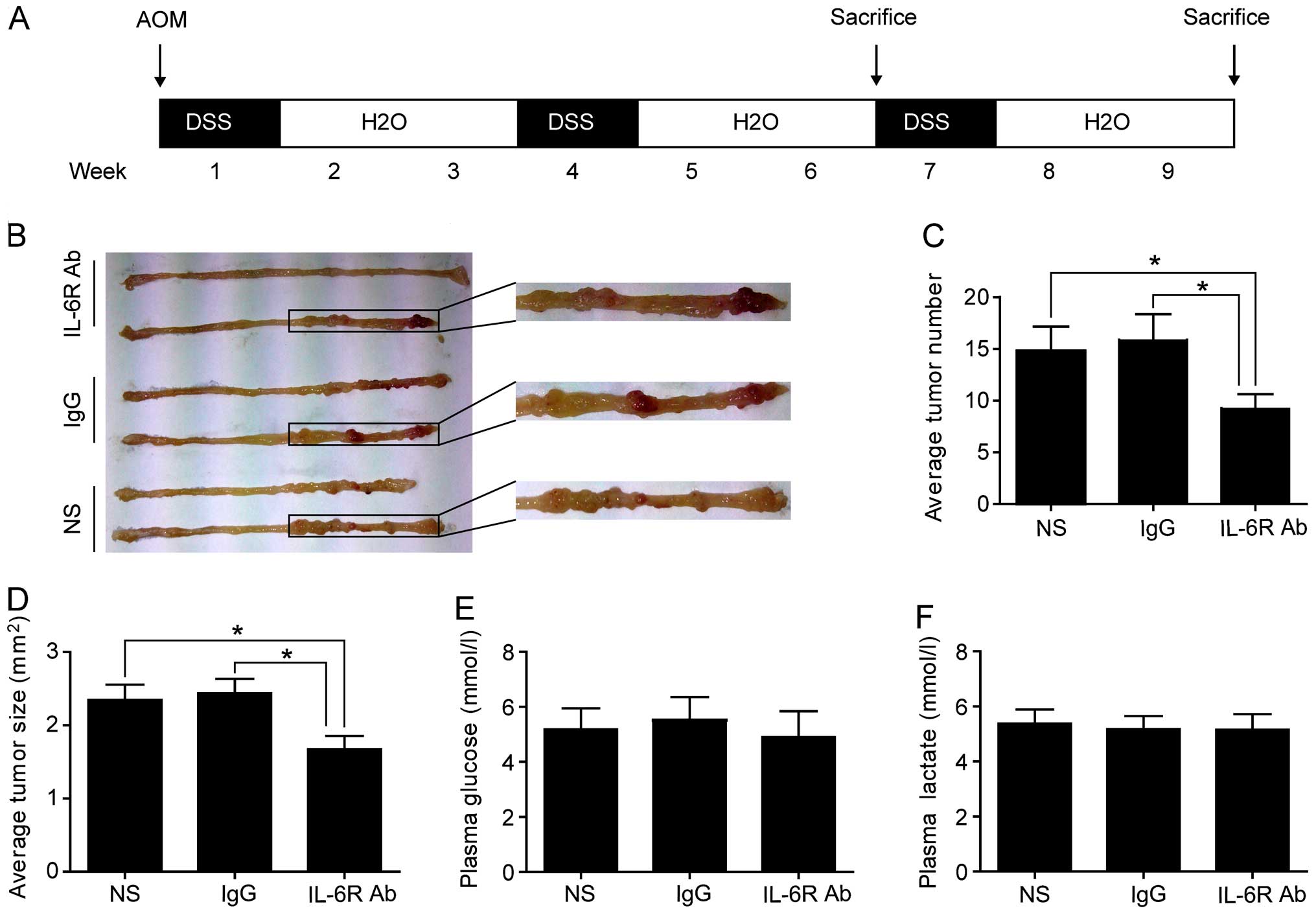
n=16). Colitis-associated CRC model protocol is shown in Fig. 1A as previously described (15). Mice were peritoneal injected with
azoxymethane (AOM, 10 mg/kg; Sigma) once at the beginning of the
first week. Then, mice were treated with 2% (w/v) dextran sulfate
sodium (DSS; Sigma) in the drinking water for a week, followed by 2
weeks of regular water for 3 cycles. In IL-6R Ab treated group,
each mouse was peritoneally injected with 10 μg monoclonal
anti-IL-6 receptor antibody diluted in 200 μl normal saline every 2
days. IgG diluted (10 μg) in 200 μl normal saline and 200 μl normal
saline were peritoneally injected into mice in the IgG and NS
groups, respectively. Eight mice in each group were sacrificed at
the end of week 6 to investigate the effect of anti-IL-6 receptor
antibody on the early stage of CRC, and 8 mice in each group were
sacrificed at the end of week 9 to evaluate the effect of anti-IL-6
receptor antibody on the CRC. Blood sample of each mouse was
collected in standard serum tubes and was immediately centrifuged
at 2400 x g for 10 min. The plasma was removed and preserved at
−80°C until further analysis. Large bowel (from the ileocecal
junction to the anal verge) of each mouse was collected and cut
open longitudinally along the main axis. Visible tumors were cut
from the mucosa and the numbers of tumors were recorded. The length
and the width of each tumor were measured by a digital micro-ruler.
The tumor size was calculated by multiplying the length and the
width. All tumors were cut into halves. One half was prepared for
histopathological analysis and immunohistochemistry, and the other
half was prepared for quantitative real-time PCR (RT-qPCR) analysis
and western blot analysis.
Histopathological analysis
One half of each tumor was fixed in 4% formalin and
embedded in paraffin. Tissue was sectioned at 4-μm thickness and
stained with hematoxylin and eosin (H&E). The slides were read
by two pathologists to determine the tumor to be colorectal adenoma
or adenocarcinoma.
Cell culture
Human SW480 and SW1116 CRC cells were obtained from
the American Type Culture Collection (ATCC; Manassas, VA, USA) and
cultured at 37°C in Leibovitz's L15 medium (Gibco, Carlsbad, CA,
USA) supplemented with 10% fetal bovine serum (FBS; Sigma), 100
units/ml of penicillin-streptomycin (Invitrogen). For inflammatory
stimulation, 20 ng/ml recombinant human IL-6 (PeproTech, Inc.,
Rocky Hill, USA) was used to treat cells.
Small interfering RNA (siRNA)
transfection
Transfection of siRNAs was carried out using
Lipofectamine RNAiMAX (Invitrogen) following the manufacturer's
instructions. Cells were 40–60% confluent at the time of the
transfection. The following siRNAs for PFKFB3 were used: PFKFB3
siRNA1 (Invitrogen; #PFKFB3HSS107862) and PFKFB3 siRNA2
(Invitrogen; #PFKFB3HSS107861). The universal control siRNA
(Invitrogen; #12935-112) was used as control. Cells were incubated
at 37°C for 48 h before harvest. For inflammatory stimulation,
cells were treated with 20 ng/ml recombinant human IL-6 for 24 h
prior to harvest.
Measurement of glucose and lactate
Mouse plasma concentration of glucose was determined
using a glucose assay kit (Sigma) and plasma concentration of
lactate was determined using the lactate assay kit (BioVision).
Glucose consumption and lactate production in CRC cells were
analyzed as previously described (16). Glucose and lactate levels in the
culture medium were also determined by using the glucose assay kit
and lactate assay kit, respectively.
Immunohistochemistry (IHC)
IHC of human and mouse colorectal tissues were
performed as previously described (17). Briefly, slides were dehydrated in
xylene and graded alcohols. Antigen retrieval was performed with
0.01 M citrate buffer at pH 6.0 at 95°C for 20 min. Slides were
incubated with diluted primary antibodies [anti-lactate
dehydrogenase isoform A (LDHA), 1:100 dilution; anti-PKKFB3, 1:150
dilution; anti-glucose transporter 1 (GLUT1), 1:250 dilution;
anti-pyruvate kinase isoform M2 (PKM2), 1:150 dilution; and
anti-hexokinase 2 (HK2), 1:400 dilution] for 12 h. Then slides were
incubated with biotinylated secondary antibody for 1 h,
peroxidase-labeled streptavidin for 15 min, and diaminobenzidine
and hydrogen peroxide chromogen substrate plus diaminobenzidine
enhancer (Dako) for 10 min, followed by counter staining with
Mayer's hematoxylin.
RT-qPCR analysis
Total RNAs were isolated from the cells and tissues
with TRIzol (Invitrogen) and cDNAs were synthesized from 1 μg total
RNA using the cDNA Synthesis kit (Takara) following the
manufacturer's protocol. RT-qPCR reactions were performed in
StepOnePlus Real-Time system (Applied Biosystems) and the
expression levels of target genes relative to β-actin were
determined by a SYBR-Green-based comparative Ct method
(2−ΔΔCt). Experiments were repeated at least 3 times.
Primers used are listed in Table
I.
 | Table IPrimer sequences of target genes and
β-actin. |
Table I
Primer sequences of target genes and
β-actin.
| Species | Genes | Sense/antisense | Sequence |
|---|
| Human | GLUT1 | Sense |
TTCACTGTCGTGTCGCTGTTTG |
| | Antisense |
TCACACTTGGGAATCAGCCCC |
| Human | HK2 | Sense |
CAAAGTGACAGTGGGTGTGG |
| | Antisense |
GCCAGGTCCTTCACTGTCTC |
| Human | PKM2 | Sense |
CCACTTGCAATTATTTGAGGAA |
| | Antisense |
GTGAGCAGACCTGCCAGACT |
| Human | PFKFB3 | Sense |
CCTCACTCGCAGCCACTTCT |
| | Antisense |
CAGTTCCTACTCAATTCCAA |
| Human | LDHA | Sense |
TTGACCTACGTGGCTTGGAAG |
| | Antisense |
GGTAACGGAATCGGGCTGAAT |
| Mouse | GLUT1 | Sense |
TATGGTAAAGAGCCGCCTAA |
| | Antisense |
GCACTGCCAGATTCAAACA |
| Mouse | HK2 | Sense |
GTGAGCCATCGTGGTTAAGC |
| | Antisense |
GCGAGGCGATCATCTTGTTG |
| Mouse | PKM2 | Sense |
TGTCTGGAGAAACAGCCAAG |
| | Antisense |
TCCTCGAATAGCTGCAAGTG |
| Mouse | PFKFB3 | Sense |
AGGTCGGCATGTTGAAGAGT |
| | Antisense |
AGAGAACAGAGCGTAGGAAG |
| Mouse | LDHA | Sense |
TGTCTCCAGCAAAGACTACTGT |
| | Antisense |
GACTGTACTTGACAATGTTGGGA |
| Human/mouse | β-actin | Sense |
CACGATGGAGGGGCCGGACTCATC |
| | Antisense |
TAAAGACCTCTATGCCAACACAGT |
Western blot analysis
Total proteins were extracted from cells or tissues
and quantified by the BCA method (Bio-Rad Laboratories). The
western blot analysis was performed as previously described
(17). For detection of PFKFB3, we
used a rabbit monoclonal anti-PFKFB3 antibody (1:6,000 dilution;
Abcam). Tubulin (1:2,000 dilution; Sigma) expression was used as an
endogenous control.
MTT cell proliferation assay
The proliferation rates of SW480 and SW1116 cells
transfected with PFKFB3 siRNAs and control siRNA with or without
IL-6 stimulation were measured by MTT assay. Cells were divided
into 4 groups: siRNA1 with IL-6 group, siRNA2 with IL-6 group,
control siRNA with IL-6 group, and control siRNA without IL-6
group. Briefly, cells were transfected with PFKFB3 siRNAs for 48 h.
Cells were then seeded at a density of 5,000 cells in 200 μl of
medium with or without IL-6 in a 96-well cell culture plate and
incubated for 24, 48 and 72 h, respectively. Cells were then
treated with 20 μl MTT (5 mg/ml; Sigma) and incubated for 4 h. The
generated formazan was dissolved in 150 μl of dimethylsulfoxide
(DMSO) after the medium was discarded. Cell viability was
determined in a microplate reader at 490 nm with subtraction of the
baseline reading.
Transwell cell migration assay
Migration ability of CRC cells was determined with a
Transwell chamber (Corning Incorporated). Briefly, cells were
treated and divided into 4 groups as described above. Cells were
resuspended in 100 μl of FBS-free medium and placed in the upper
chamber, whereas the lower chamber contained 500 μl of 10% FBS
medium with or without IL-6. After 24 h, cells remaining on the
upper side of the membrane were cleared, and the migrated cells on
the lower side of the membrane were fixed with paraformaldehyde
(PFA) and stained with 0.1% crystal violet. The number of cells
from 5 random microscopic fields was quantified for each group.
Statistical analysis
All results are presented as the means ± standard
error of the mean (SEM) unless indicated otherwise. The correlation
of PFKFB3 expression with the clinicopathological factors in
sporadic CRC patients was analyzed using the χ2 test.
The difference between two groups was analyzed using the Student's
t-test. All statistical analyses were performed using the GraphPad
Prism 5.0 software or Stata version 11.0. P<0.05 was considered
statistically significant.
Results
Anti-IL-6 receptor antibody reduces the
incidence of colitis-related CRC, but does not affect the plasma
concentrations of glucose and lactate
The AOM and DSS colitis-associated CRC mouse model
was induced as shown in Fig. 1A.
The mice were treated with anti-IL-6 receptor antibody to block
IL-6 mediated chronic inflammation or normal saline and IgG as
control. Eight mice in each group were sacrificed at the end of
week 9 to investigate the effect of anti-IL-6 receptor antibody on
the development of CRC. Mice in treated group developed fewer and
smaller macroscopic colorectal tumors (Fig. 1B–D). Plasma concentrations of
glucose and lactate were not significantly different between the
treated group and the control groups (Fig. 1E and F). These data suggested that
anti-IL-6 receptor antibody treatment could reduce the incidence of
colorectal tumors, with no significant effect on plasma
concentrations of glucose and lactate.
Anti-IL-6 receptor antibody downregulates
the key genes involved in aerobic glycolysis in colorectal
adenocarcinoma tissues
To clarify the potential mechanism by which
anti-IL-6 receptor antibody inhibits tumor formation in the
colitis-associated CRC mouse model, and to further investigate
whether this inhibition is through regulation of aerobic
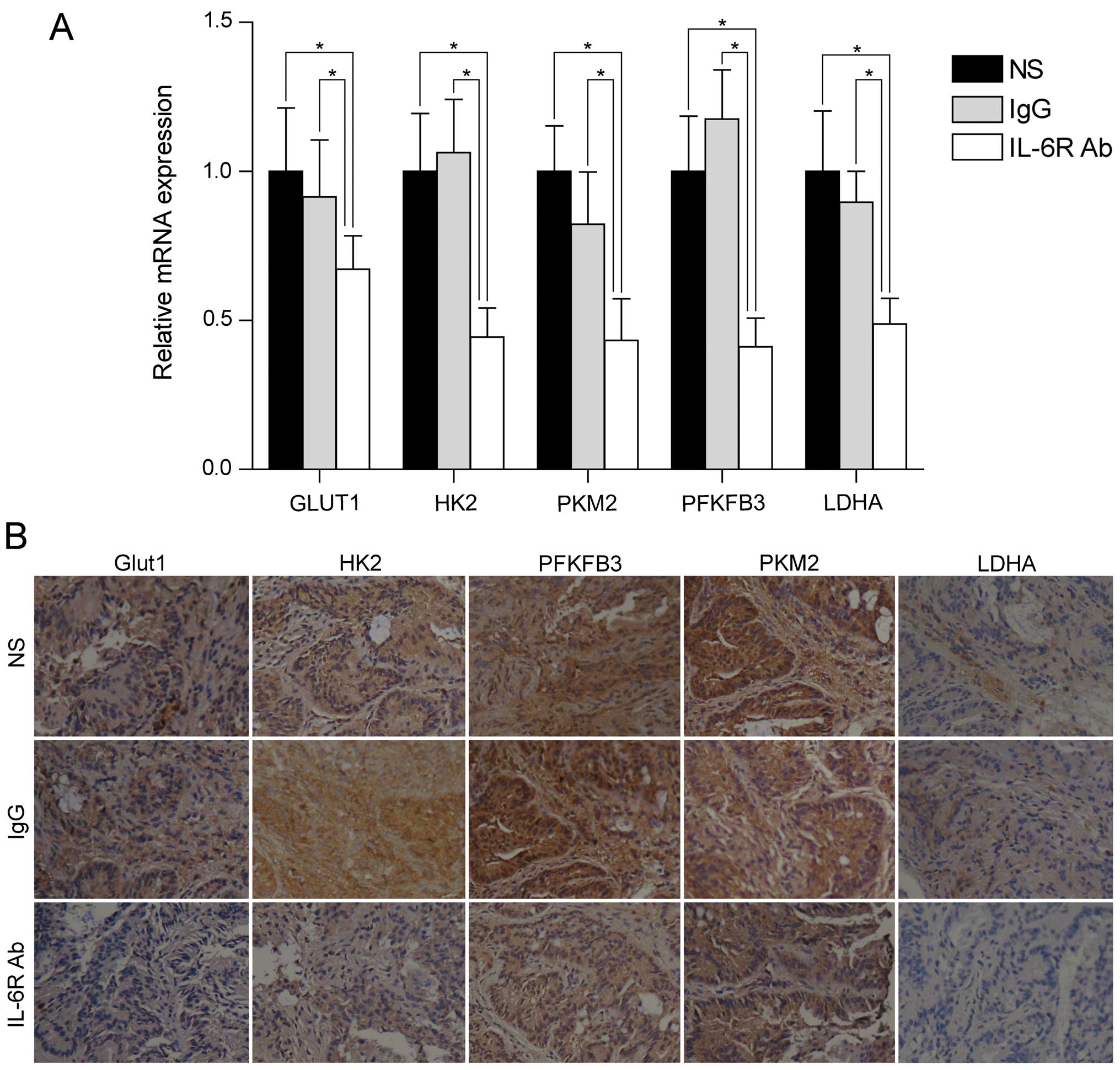
glycolysis, we examined the expression of a number of key genes
involved in aerobic glycolysis (including GLUT1, HK2, PFKFB3, PKM2
and and LDHA) in colorectal adenocarcinoma tissues. RT-qPCR
analysis showed that these genes were down-regulated after
treatment with anti-IL-6 receptor antibody (Fig. 2A). In line with the mRNA levels,
the protein levels of these genes were also downregulated after
anti-IL-6 receptor antibody treatment (Fig. 2B). These data demonstrated that
anti-IL-6 receptor antibody might inhibit colorectal adenocarcinoma
formation through regulation of aerobic glycolysis.
Anti-IL-6 receptor antibody downregulates
the key genes involved in aerobic glycolysis in colorectal adenoma
tissues
We next sought to investigate whether the change in
aerobic glycolysis genes occurred already at the early stage of
CRC. Eight mice in each group were sacrificed at the end of week 6.
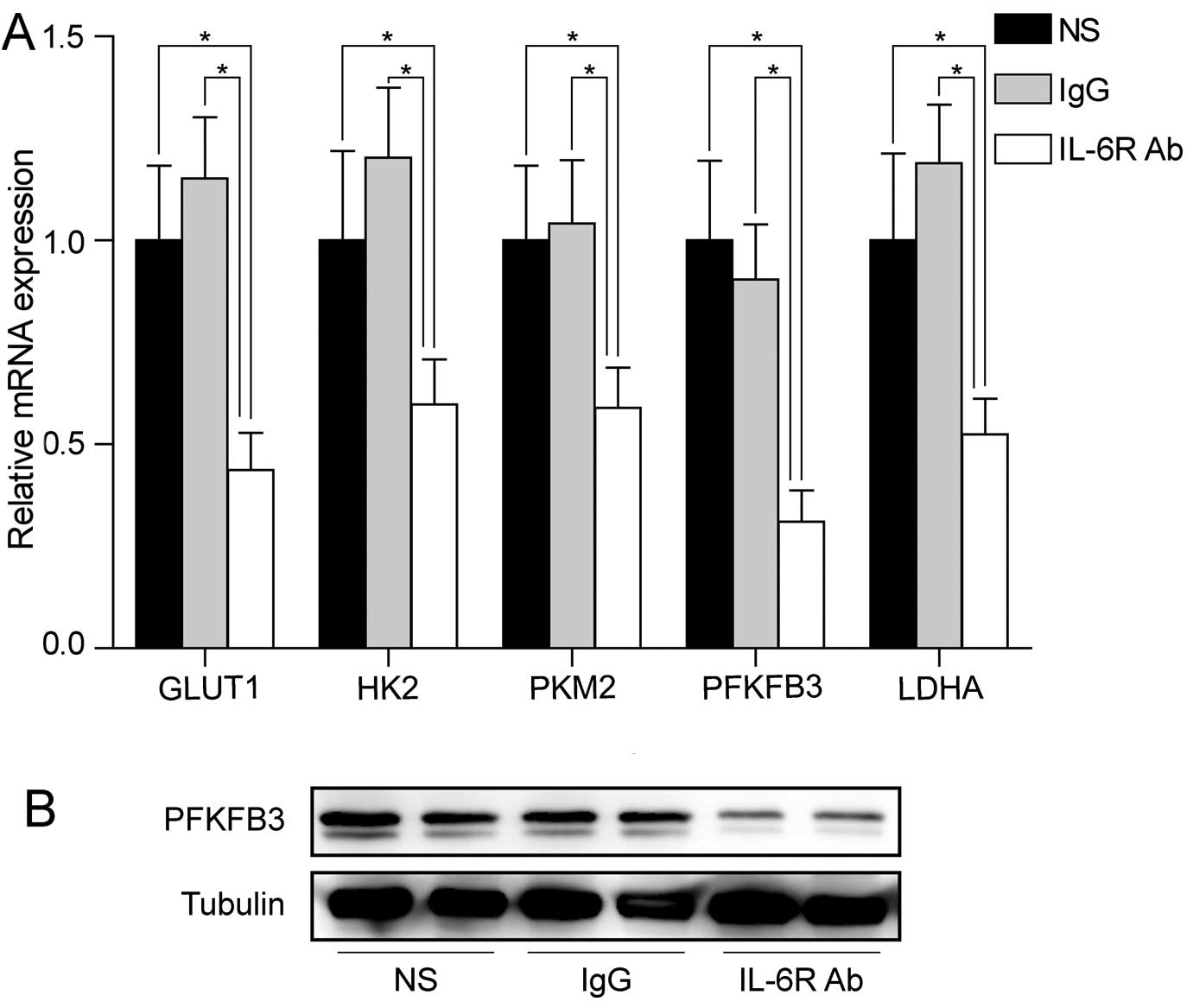
We examined the mRNA levels of key genes involved in aerobic
glycolysis in colorectal adenoma tissues. Similar mRNA levels of
downregulation of these genes were observed in colorectal adenoma
tissues after treatment with anti-IL-6 receptor antibody (Fig. 3A). Notably, PFKFB3 was the most
downregulated gene by anti-IL-6 receptor antibody in colorectal
adenoma tissues, suggesting that PFKFB3 might play an important
role at early stage of CRC. Western blot analysis also confirmed
the downregulation of PFKFB3 protein in the treated group (Fig. 3B).
PFKFB3 is overexpressed in human sporadic
and colitis-associated CRC tumor tissues and colorectal adenoma
tissues
Given PFKFB3 was the most influenced gene at early
stage of CRC in colitis-associated CRC model, we analyzed whether
PFKFB3 was differentially expressed in different human colorectal
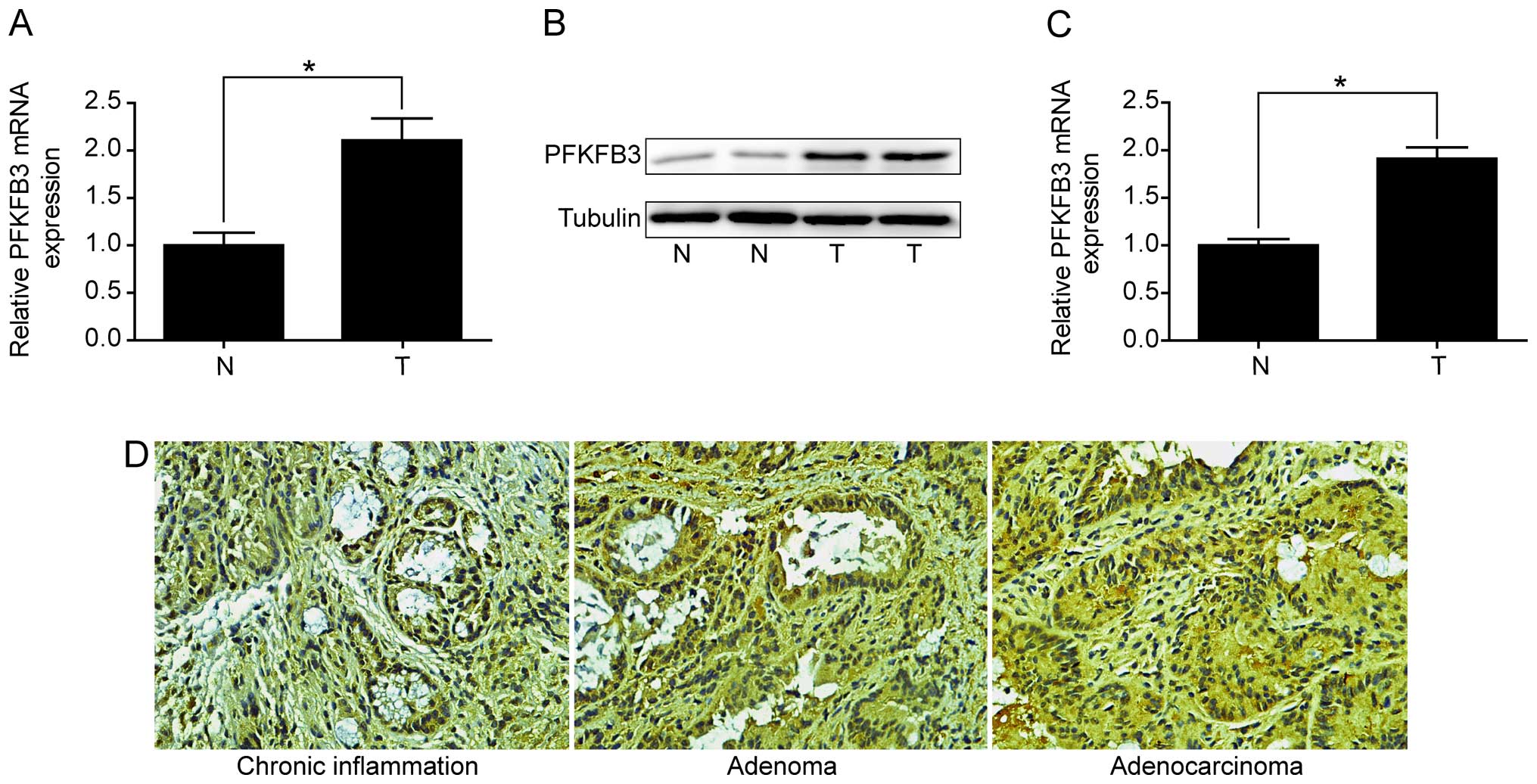
tissues. Firstly, we examined the expression of PFKFB3 in 13
colitis-associated CRC patients. The results revealed that PFKFB3
mRNA level was significantly increased in tumor tissues compared to
adjacent non-malignant tissues (Fig.
4A). Overexpression of PFKFB3 protein in tumor tissues was also
confirmed by western blot analysis (Fig. 4B). Secondly, we examined the
expression of PFKFB3 in 87 sporadic CRC patients and analyzed its
correlation with the clinicopathological factors. As shown in
Fig. 4C, RT-qPCR results validated
the overexpression of PFKFB3 in tumor tissues compared to adjacent
non-malignant tissues in sporadic CRC patients. Furthermore,
correlation analysis indicated that PFKFB3 mRNA high expression was
significantly associated with lymph node metastasis, intravascular
cancer embolus, and TNM stage in 87 sporadic CRC patients (Table II).
 | Table IIClinicopathological correlation of
PFKFB3 expression in sporadic colorectal cancer patients. |
Table II
Clinicopathological correlation of
PFKFB3 expression in sporadic colorectal cancer patients.
| PFKFB3 | | |
|---|
|
| | |
|---|
| Features | Low | Higha | χ2 | P-valueb |
|---|
| All cases | 43 | 44 | | |
| Gender | | | 0.3118 | 0.577 |
| Male | 26 | 24 | | |
| Female | 17 | 20 | | |
| Age (years) | | | 0.0975 | 0.755 |
| >65 | 23 | 25 | | |
| ≤65 | 20 | 19 | | |
| Intravascular
cancer embolus | | | 4.2548 | 0.039 |
| Present | 5 | 13 | | |
| Absent | 38 | 31 | | |
| Perineuronal
invasion | | | 1.8856 | 0.170 |
| Present | 9 | 15 | | |
| Absent | 34 | 29 | | |
| Tumor size
(cm) | | | 2.2123 | 0.137 |
| >4 | 12 | 19 | | |
| ≤4 | 31 | 25 | | |
| T stage | | | 1.046 | 0.79 |
| T1 | 3 | 2 | | |
| T2 | 11 | 9 | | |
| T3 | 11 | 10 | | |
| T4 | 18 | 23 | | |
| N stage | | | 7.622 | 0.033 |
| N0 | 18 | 8 | | |
| N1 | 13 | 14 | | |
| N2 | 12 | 22 | | |
| M stage | | | 2.591 | 0.107 |
| M0 | 38 | 33 | | |
| M1 | 5 | 11 | | |
| Tumor stage |
| I | 5 | 3 | 8.4802 | 0.037 |
| II | 16 | 6 | | |
| III | 17 | 24 | | |
| IV | 5 | 11 | | |
To clarify whether PFKFB3 expression increases
gradually in colorectal tissues from chronic inflammation to
adenoma and adenocarcinoma, we examined the PFKFB3 expression in
colorectal pathological sections of patients who were diagnosed as
colorectal chronic inflammation, adenoma and adenocarcinoma.
Notably, we found that PFKFB3 was highly expressed in colorectal
adenoma and adenocarcinoma tissues compared with chronic
inflammation tissues, but no significant difference was observed
between adenoma and adenocarcinoma tissues (Fig. 4D). The results indicated that
PFKFB3 might play an important role starting at the colorectal
adenoma stage.
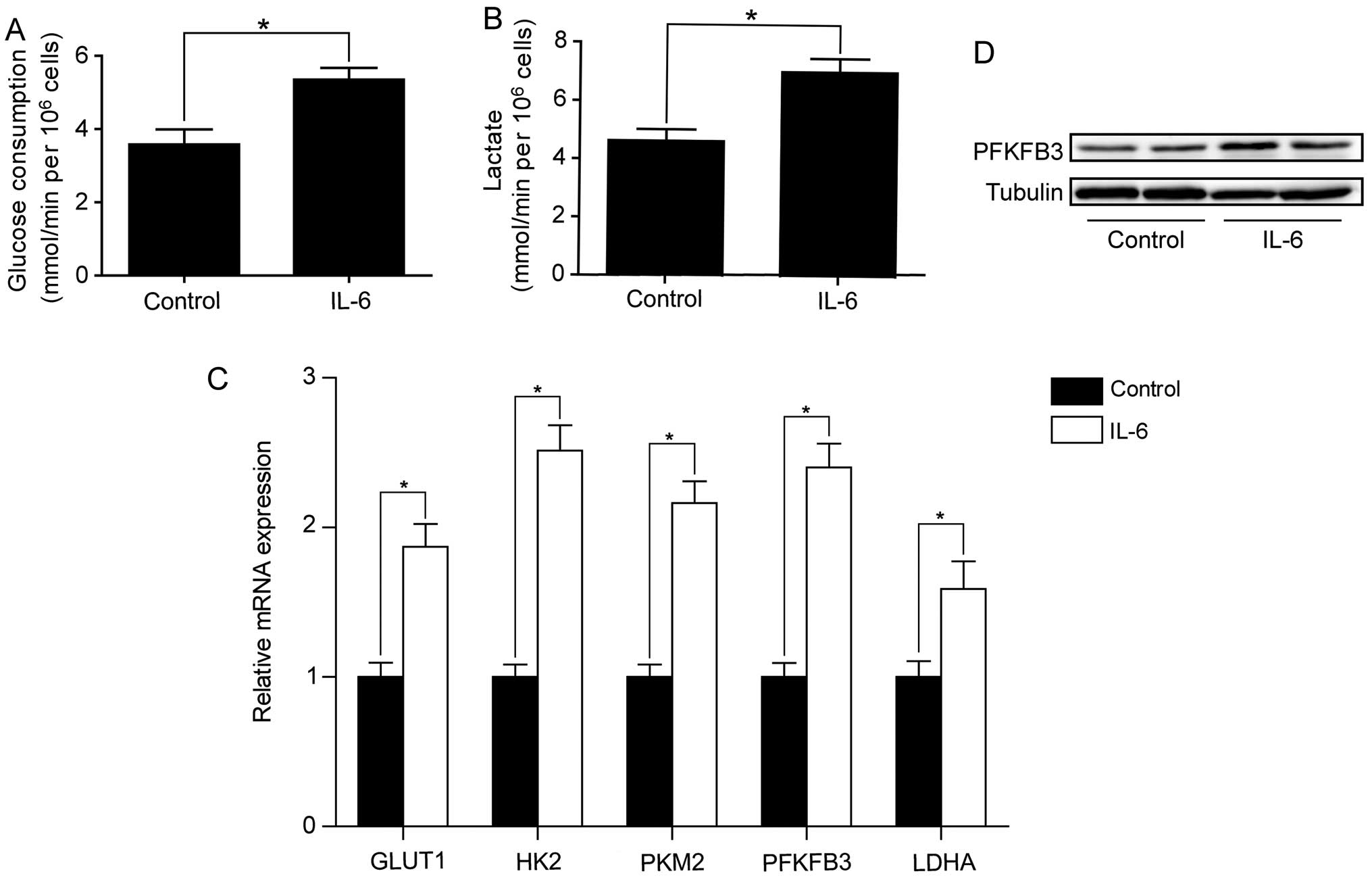
IL-6 promotes glycolysis and upregulates
PFKFB3 in SW480 and SW1116 cells
Our data thus far suggested that IL-6 promotes the
initiation and progression of CRC by regulating aerobic glycolysis.
We next sought to address the puzzle that plasma concentrations of
glucose and lactate in mice were not significantly affected after
treatment with anti-IL-6 receptor antibody. We reasoned that IL-6
might influence the local concentrations of glucose and lactate but
not the concentrations in whole blood. To this end, we first
examined the effect of IL-6 on aerobic glycolysis in SW480 and
SW1116 cells. As shown in Fig. 5A and
B, IL-6 treatment increased the rates of glucose consumption
and lactate production in SW480 cells. We also examined the effect
of IL-6 on the expression of key genes involved in aerobic
glycolysis. RT-qPCR analysis showed that these genes were
significantly upregulated by IL-6 treatment in SW480 cells
(Fig. 5C). Among these genes,
PFKFB3 was increased by 2.4-fold. In line with Q-PCR result,
western blot analysis confirmed the increased PFKFB3 protein level
after IL-6 treatment in SW480 cells (Fig. 5D). We also assessed the effect of
IL-6 on aerobic glycolysis and PFKFB3 expression in a second CRC
cell line (SW1116) and found enhanced aerobic glycolysis as well as
increased PFKFB3 expression by IL-6 treatment (data not shown).
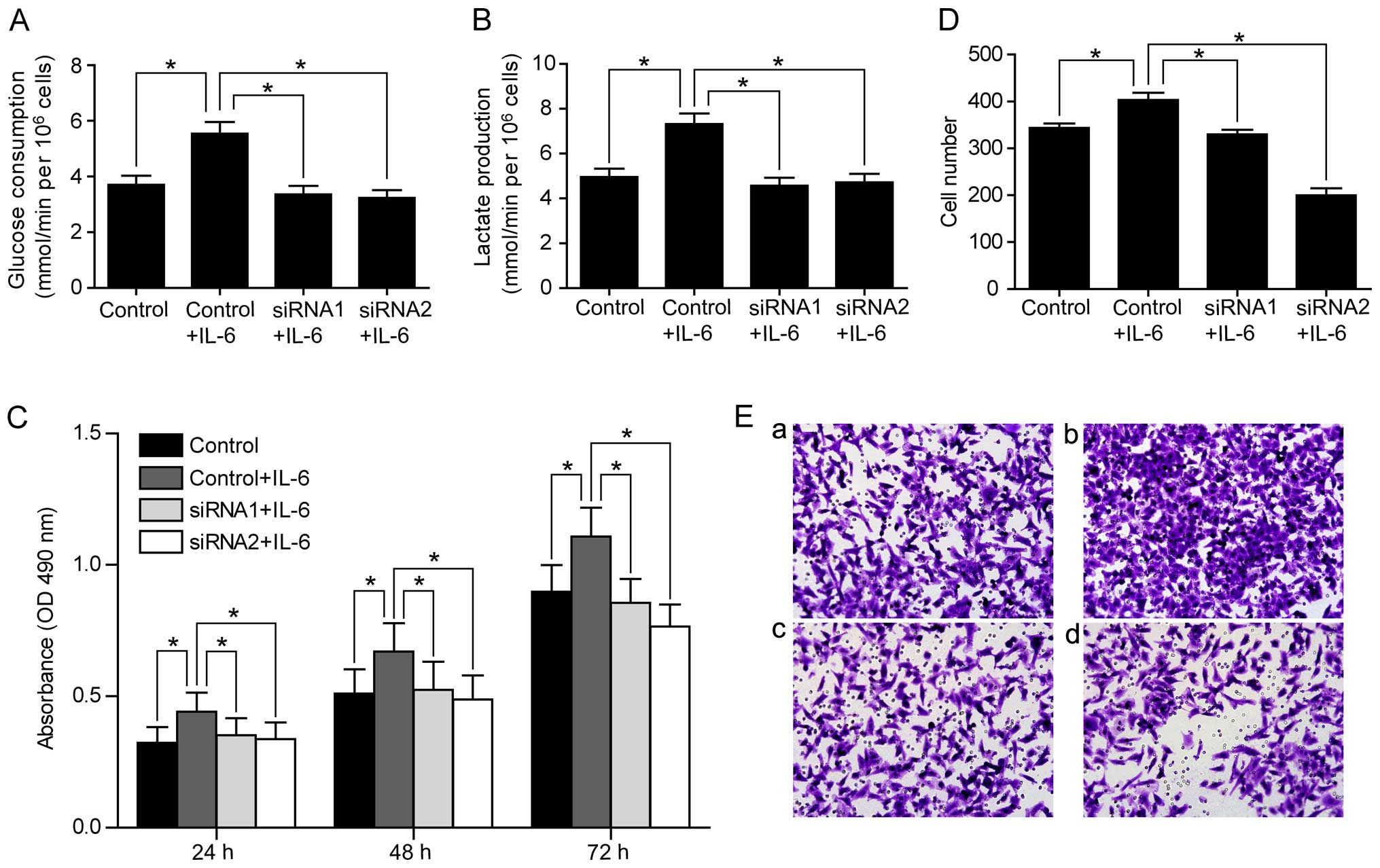
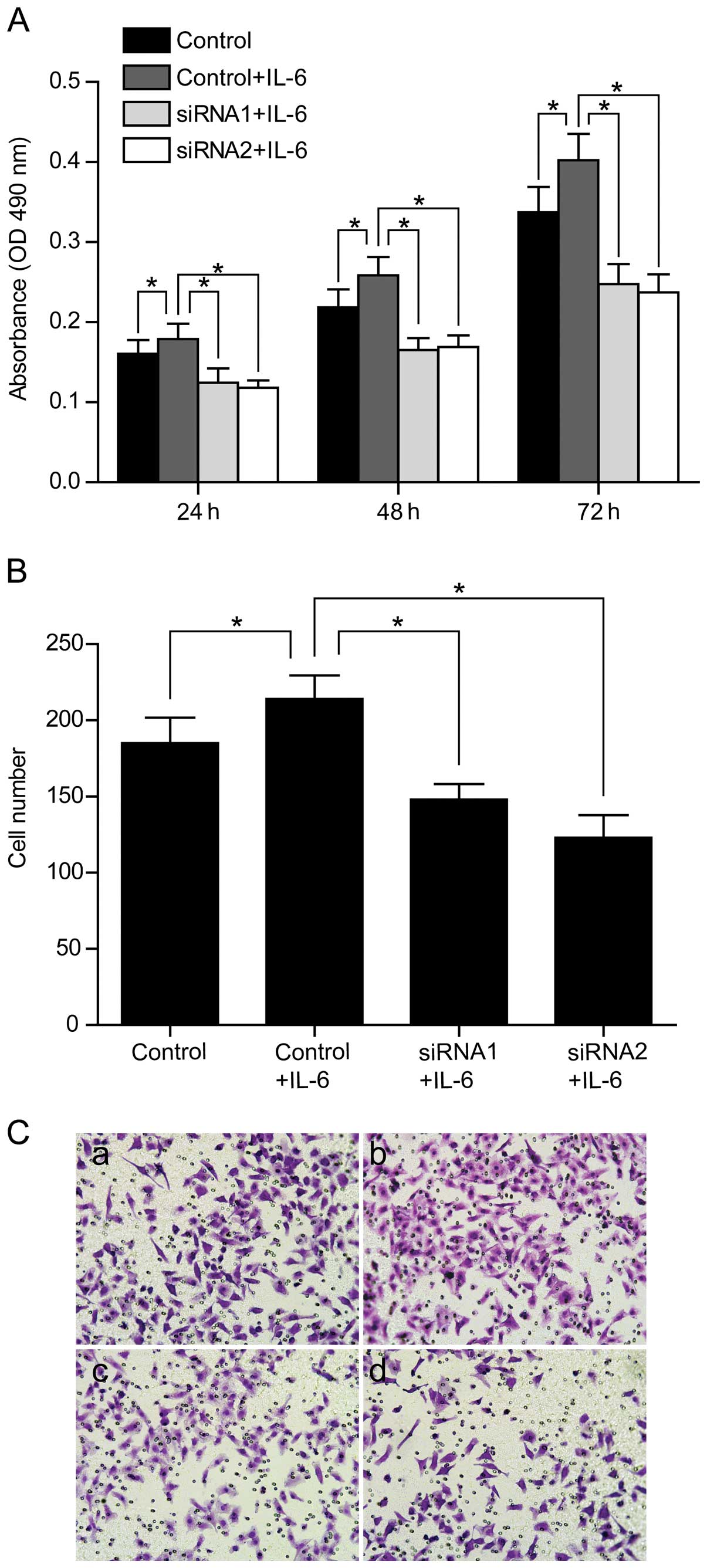
Knockdown of PFKFB3 inhibits IL-6
stimulated cell proliferation and migration
Given that IL-6 upregulated PFKFB3 expression in CRC
cells, and overexpression of PFKFB3 was associated with lymph node
metastasis and TNM stage in CRC patients, we hypothesized that IL-6
might stimulate CRC cell proliferation and migration through
regulating PFKFB3. To test this hypothesis, we examined whether the
increased rates of glucose consumption and lactate production by
IL-6 treatment could be abolished by knocking down PFKFB3. Indeed,
we found that PRKFB3 siRNAs reversed the increase of glucose
consumption and lactate production by IL-6 treatment in SW480 cells
(Fig. 6A and B). Consistently,
PFKFB3 siRNAs abolished the enhanced cell proliferation and
migration stimulated by IL-6 in SW480 cells (Fig. 6C–E), suggesting that IL-6 functions
through regulating PFKFB3. We also confirmed the inhibition effect
of PFKFB3 siRNAs on cell proliferation and migration in SW1116
cells (Fig. 7).
Discussion
Chronic inflammation is a well-known risk factor for
CRC and the aerobic glycolysis of cancer cells is also well
documented (7,18). However, whether chronic
inflammation is attributed to tumor initiation and progression of
CRC through regulating the aerobic glycolysis remains largely
unknown. In this study, we found that blocking of IL-6 function
significantly inhibited the initiation and progression of
colitis-associated CRC and decreased the expression of key genes
involved in aerobic glycolysis (especially the PFKFB3) even at
early stage of CRC. PFKFB3 was demonstrated to be overexpressed in
human colorectal adenoma and adenocarcinoma tissues, and the high
expression of PFKFB3 mRNA was associated with lymph node
metastasis, intravascular cancer embolus and TNM stage in sporadic
CRC patients. IL-6 was also demonstrated to accelerate aerobic
glycolysis and upregulate key genes involved in aerobic glycolysis
in vitro, whereas knockdown of PFKFB3 abolished the enhanced
proliferation and migration abilities stimulated by IL-6 in CRC
cells. These data indicate that IL-6 might promote the initiation
and progression of CRC by regulating PFKFB3 at early stage of
CRC.
AOM-induced CRC mouse model is widely used and can
resemble human CRC in many respects including the molecular level
(19,20). AOM and DSS-induced CRC mouse model
is a model for colitis-associated tumor development, which is
particularly applicable when the study focuses on tumor progression
driven by chronic colitis (15).
Balb/c mice were reported to be extremely sensitive to AOM and
DSS-induced colitis-associated CRC (21). In the presenr study, using the
combined treatment of one exposure of AOM and 3 cycles of DSS, we
successfully induced colitis-associated CRC in Balb/c mice. As
reported before, in the AOM and DSS-induced CRC, the tumor
promoting effect of IL-6 could be inhibited through treatment with
anti-IL-6 receptor antibody (22).
Obvious reduced tumor development was also observed in IL-6−/− mice
exposed to the AOM and DSS model (23). These previous data together with
our results indicated that IL-6 might play a key role in the
initiation and progression of colitis-associated CRC. Moreover,
blocking the IL-6 function could significantly inhibit the tumor
development of CRC.
The IL-6 dependent, signal transducer and activator
of transcription-3 (STAT3), the suppressor of cytokine signaling-3
(SOCS3), and the vascular endothelial growth factor receptor-2
(VEGFR2) were reported to be of critical importance for tumor
development in the colitis-associated CRC mouse model (23–26).
However, only few studies have investigated whether the growth
promoting effect of IL-6 in the AOM and DSS colitis-associated CRC
mouse model is realized by regulating the aerobic glycolysis. In
this study, although key genes involved in aerobic glycolysis were
significantly downregulated by anti-IL-6 receptor antibody, plasma
concentrations of glucose and lactate were not significantly
affected. However, we found the rates of glucose consumption and
lactate production were increased by treatment with IL-6 in CRC
cells. The differences might be explained by the multiple factors
that influence the plasma concentration of glucose and lactate in
the whole body.
Genetic disorders have been reported to be related
to the phenotypical changes of the morphological progression
sequence in the inflammation/adenoma/carcinoma (27). However, whether the aerobic
glycolysis is involved in inflammation/adenoma/carcinoma sequential
changes is largely unknown. In the present study, we found that key
genes of aerobic glycolysis (especially PFKFB3) were significantly
downregulated by anti-IL-6 receptor antibody treatment at early
stage of CRC. Furthermore, the expression of PFKFB3 was found to be
significantly higher in human colorectal adenoma tissue than
colorectal inflammation tissue. These results indicated that IL-6
might exert a tumor promoting effect by regulating PFKFB3 starting
at the colorectal adenoma stage. However, due to the limited number
of human tissues, the expression profile of PFKFB3 upon tumor
progression needs future study.
As far as we known, this is the first report on the
PFKFB3 expression status in a large human population with 87
sporadic CRC patients showing a close correlation between PFKFB3
expression and several clinicopathological factors. However,
whether the PFKFB3 expression can be used as an independed
prognostic factor for sporadic CRC patients needs long-term
follow-up.
The growth-promoting effect of IL-6 on CRC cells
in vitro was reported 3 decades ago (28). In addition, the aerobic glycolysis
has been proposed to support the proliferative demands of cancer
cells (7). Therefore, we examined
whether IL-6 exerts its tumor promoting effect by regulating the
aerobic glycolysis in vitro. Results showed that knockdown
of PFKFB3 not only reversed the enhanced aerobic glycolysis but
also inhibited tumor cell proliferation and migration. The
potential mechanisms of how IL-6 upregulates PFKFB3 are that IL-6
increases the expression of HIF-1 and HIF-1 activates transcription
of PFKFB3 (29). However, what is
the concrete mechanism of how IL-6 exerts its tumor promoting
effect by regulating the PFKFB3 in CRC is still under further
consideration.
In this study, the inhibitory effect of anti-IL-6
receptor antibody on the development of colitis-associated CRC was
observed. In fact, growing evidence supports a critical role for
IL-6 signaling during the development of both sporadic and
inflammation-associated CRC (5).
Therefore, new therapeutic targeting IL-6 pathway might offer a
promising option for treatment of CRC patients. To date, anti-IL-6
receptor antibody has been approved for treatment of chronic
inflammatory diseases such as rheumatoid arthritis and juvenile
idiopathic arthritis by the Food and Drug Administration (FDA)
(30). Although the therapeutic
effect of anti-IL-6 receptor antibody on CRC has not been confirmed
in clinical trials, anti-IL-6 receptor antibody provides a new
possible theoretical strategy for treatment of patients with CRC.
Therefore, further study could pay attention to the therapeutic
effect of anti-IL-6 receptor antibody on CRC, especially the
colitis-associated CRC.
In summary, the present study provides direct
evidence that chronic inflammation (IL-6) promotes the initiation
and progression of CRC by regulating aerobic glycolysis (especially
the PFKFB3). As anti-IL-6 receptor antibody has a potential
clinical usefulness for treatment of CRC, our findings provide
theoretical support for such anti-neoplasia strategy.
Acknowledgements
The present study was supported by the National
Natural Science Foundation of China (81372197).
References
|
1
|
Siegel RL, Miller KD and Jemal A: Cancer
statistics, 2015. CA Cancer J Clin. 65:5–29. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Sung JJ, Lau JY, Goh KL and Leung WK; Asia
Pacific Working Group on Colorectal Cancer. Increasing incidence of
colorectal cancer in Asia: Implications for screening. Lancet
Oncol. 6:871–876. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Cunningham D, Atkin W, Lenz HJ, Lynch HT,
Minsky B, Nordlinger B and Starling N: Colorectal cancer. Lancet.
375:1030–1047. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Ullman TA and Itzkowitz SH: Intestinal
inflammation and cancer. Gastroenterology. 140:1807–1816. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Waldner MJ, Foersch S and Neurath MF:
Interleukin-6--a key regulator of colorectal cancer development.
Int J Biol Sci. 8:1248–1253. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Li Y, de Haar C, Chen M, Deuring J,
Gerrits MM, Smits R, Xia B, Kuipers EJ and van der Woude CJ:
Disease-related expression of the IL6/STAT3/SOCS3 signalling
pathway in ulcerative colitis and ulcerative colitis-related
carcinogenesis. Gut. 59:227–235. 2010. View Article : Google Scholar
|
|
7
|
Vander Heiden MG, Cantley LC and Thompson
CB: Understanding the Warburg effect: The metabolic requirements of
cell proliferation. Science. 324:1029–1033. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Hsu PP and Sabatini DM: Cancer cell
metabolism: Warburg and beyond. Cell. 134:703–707. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Hanahan D and Weinberg RA: Hallmarks of
cancer: The next generation. Cell. 144:646–674. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Semenza GL: HIF-1: Upstream and downstream
of cancer metabolism. Curr Opin Genet Dev. 20:51–56. 2010.
View Article : Google Scholar :
|
|
11
|
Christofk HR, Vander Heiden MG, Harris MH,
Ramanathan A, Gerszten RE, Wei R, Fleming MD, Schreiber SL and
Cantley LC: The M2 splice isoform of pyruvate kinase is important
for cancer metabolism and tumour growth. Nature. 452:230–233. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Ros S and Schulze A: Balancing glycolytic
flux: The role of 6-phosphofructo-2-kinase/fructose
2,6-bisphosphatases in cancer metabolism. Cancer Metab. 1:82013.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Atsumi T, Chesney J, Metz C, Leng L,
Donnelly S, Makita Z, Mitchell R and Bucala R: High expression of
inducible 6-phosphofructo-2-kinase/fructose-2,6-bisphosphatase
(iPFK-2; PFKFB3) in human cancers. Cancer Res. 62:5881–5887.
2002.PubMed/NCBI
|
|
14
|
Obach M, Navarro-Sabaté A, Caro J, Kong X,
Duran J, Gómez M, Perales JC, Ventura F, Rosa JL and Bartrons R:
6-Phosphofructo-2-kinase (pfkfb3) gene promoter contains
hypoxia-inducible factor-1 binding sites necessary for
transactivation in response to hypoxia. J Biol Chem.
279:53562–53570. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Neufert C, Becker C and Neurath MF: An
inducible mouse model of colon carcinogenesis for the analysis of
sporadic and inflammation-driven tumor progression. Nat Protoc.
2:1998–2004. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Kawauchi K, Araki K, Tobiume K and Tanaka
N: p53 regulates glucose metabolism through an IKK-NF-kappaB
pathway and inhibits cell transformation. Nat Cell Biol.
10:611–618. 2008. View
Article : Google Scholar : PubMed/NCBI
|
|
17
|
Yuan L, Han J, Meng Q, Xi Q, Zhuang Q,
Jiang Y, Han Y, Zhang B, Fang J and Wu G: Muscle-specific E3
ubiquitin ligases are involved in muscle atrophy of cancer
cachexia: An in vitro and in vivo study. Oncol Rep. 33:2261–2268.
2015.PubMed/NCBI
|
|
18
|
Grivennikov SI and Karin M: Inflammation
and oncogenesis: A vicious connection. Curr Opin Genet Dev.
20:65–71. 2010. View Article : Google Scholar :
|
|
19
|
Takahashi M, Nakatsugi S, Sugimura T and
Wakabayashi K: Frequent mutations of the beta-catenin gene in mouse
colon tumors induced by azoxymethane. Carcinogenesis. 21:1117–1120.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Wang QS, Papanikolaou A, Sabourin CL and
Rosenberg DW: Altered expression of cyclin D1 and cyclin-dependent
kinase 4 in azoxymethane-induced mouse colon tumorigenesis.
Carcinogenesis. 19:2001–2006. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Suzuki R, Kohno H, Sugie S, Nakagama H and
Tanaka T: Strain differences in the susceptibility to azoxymethane
and dextran sodium sulfate-induced colon carcinogenesis in mice.
Carcinogenesis. 27:162–169. 2006. View Article : Google Scholar
|
|
22
|
Becker C, Fantini MC, Schramm C, Lehr HA,
Wirtz S, Nikolaev A, Burg J, Strand S, Kiesslich R, Huber S, et al:
TGF-beta suppresses tumor progression in colon cancer by inhibition
of IL-6 trans-signaling. Immunity. 21:491–501. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Grivennikov S, Karin E, Terzic J, Mucida
D, Yu GY, Vallabhapurapu S, Scheller J, Rose-John S, Cheroutre H,
Eckmann L, et al: IL-6 and Stat3 are required for survival of
intestinal epithelial cells and development of colitis-associated
cancer. Cancer Cell. 15:103–113. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Bollrath J, Phesse TJ, von Burstin VA,
Putoczki T, Bennecke M, Bateman T, Nebelsiek T, Lundgren-May T,
Canli O, Schwitalla S, et al: gp130-mediated Stat3 activation in
enterocytes regulates cell survival and cell-cycle progression
during colitis-associated tumorigenesis. Cancer Cell. 15:91–102.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Rigby RJ, Simmons JG, Greenhalgh CJ,
Alexander WS and Lund PK: Suppressor of cytokine signaling 3
(SOCS3) limits damage-induced crypt hyper-proliferation and
inflammation-associated tumorigenesis in the colon. Oncogene.
26:4833–4841. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Waldner MJ, Wirtz S, Jefremow A, Warntjen
M, Neufert C, Atreya R, Becker C, Weigmann B, Vieth M, Rose-John S,
et al: VEGF receptor signaling links inflammation and tumorigenesis
in colitis-associated cancer. J Exp Med. 207:2855–2868. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Arvelo F, Sojo F and Cotte C: Biology of
colorectal cancer. E Cancer Medical Sci. 9:5202015.
|
|
28
|
Lahm H, Petral-Malec D, Yilmaz-Ceyhan A,
Fischer JR, Lorenzoni M, Givel JC and Odartchenko N: Growth
stimulation of a human colorectal carcinoma cell line by
interleukin-1 and -6 and antagonistic effects of transforming
growth factor beta 1. Eur J Cancer. 28A:1894–1899. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Lang SA, Moser C, Gaumann A, Klein D,
Glockzin G, Popp FC, Dahlke MH, Piso P, Schlitt HJ, Geissler EK, et
al: Targeting heat shock protein 90 in pancreatic cancer impairs
insulin-like growth factor-I receptor signaling, disrupts an
interleukin-6/signal-transducer and activator of transcription
3/hypoxia-inducible factor-1alpha autocrine loop, and reduces
orthotopic tumor growth. Clin Cancer Res. 13:6459–6468. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Tanaka T, Narazaki M and Kishimoto T:
Therapeutic targeting of the interleukin-6 receptor. Annu Rev
Pharmacol Toxicol. 52:199–219. 2012. View Article : Google Scholar
|