|
1
|
Ramdass B, Duggal R, Minev B, Chowdhary A,
Ramdass B, Duggal R, Minev B, Chowdhary A and Koka P: Functional
role of solid tumor stem cells in disease etiology and
susceptibility to therapeutic interventions. J Stem Cells.
8:189–231. 2013.
|
|
2
|
Chung E and Kondo M: Role of
Ras/Raf/MEK/ERK signaling in physiological hematopoiesis and
leukemia development. Immunol Res. 49:248–268. 2011. View Article : Google Scholar
|
|
3
|
Knight T and Irving JA: Ras/Raf/MEK/ERK
pathway activation in childhood acute lymphoblastic leukemia and
its therapeutic targeting. Front Oncol. 4:1602014. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Monga SP: Role and regulation of β-catenin
signaling during physiological liver growth. Gene Expr. 16:51–62.
2014. View Article : Google Scholar
|
|
5
|
Andersson ER and Lendahl U: Therapeutic
modulation of Notch signalling: are we there yet? Nat Rev Drug
Discov. 13:357–378. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Pasillas MP, Shields S, Reilly R, Strnadel
J, Behl C, Park R, Yates JR III, Klemke R, Gonias SL and Coppinger
JA: Proteomic analysis reveals a role for Bcl2-associated
athanogene 3 and major vault protein in resistance to apoptosis in
senescent cells by regulating ERK1/2 activation. Mol Cell
Proteomics. 14:1–14. 2015. View Article : Google Scholar :
|
|
7
|
Shi X, Wu S, Yang Y, Tang L, Wang Y, Dong
J, Lü B, Jiang G and Zhao W: AQP5 silencing suppresses p38 MAPK
signaling and improves drug resistance in colon cancer cells.
Tumour Biol. 35:7035–7045. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Yu SL, Lee DC, Son JW, Park CG, Lee HY and
Kang J: Histone deacetylase 4 mediates SMAD family member 4
deacetylation and induces 5-fluorouracil resistance in breast
cancer cells. Oncol Rep. 30:1293–1300. 2013.PubMed/NCBI
|
|
9
|
Jiang AG, Yu H and Huang JA: Expression
and clinical significance of the phosphatidylinositol
3-kinase/protein kinase B signal transduction pathway in non-small
cell lung carcinoma. Oncol Lett. 8:601–607. 2014.PubMed/NCBI
|
|
10
|
Ogawa R, Ishiguro H, Kimura M, Funahashi
H, Wakasugi T, Ando T, Shiozaki M and Takeyama H: NOTCH1 expression
predicts patient prognosis in esophageal squamous cell cancer. Eur
Surg Ress. 51:101–107. 2013. View Article : Google Scholar
|
|
11
|
Chu W, Song X, Yang X, Ma L, Zhu J, He M,
Wang Z and Wu Y: Neuropilin-1 promotes epithelial-to-mesenchymal
transition by stimulating nuclear factor-kappa B and is associated
with poor prognosis in human oral squamous cell carcinoma. PLoS
One. 9:e1019312014. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Yao L, Sun B, Zhao X, Zhao X, Gu Q, Dong
X, Zheng Y, Sun J, Cheng R, Qi H, et al: Overexpression of Wnt5a
promotes angiogenesis in NSCLC. BioMed Res Int. 2014:8325622014.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Carvalho FL, Simons BW, Eberhart CG and
Berman DM: Notch signaling in prostate cancer: A moving target.
Prostate. 74:933–945. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Jamieson C, Sharma M and Henderson BR:
Targeting the β-catenin nuclear transport pathway in cancer. Semin
Cancer Biol. 27:20–29. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Gasparini C, Celeghini C, Monasta L and
Zauli G: NF-kappaB pathways in hematological malignancies. Cellular
and molecular life sciences. Cell Mol Life Sci. 71:2083–2102. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Tournier C: The 2 Faces of JNK Signaling
in Cancer. Genes Cancer. 4:397–400. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Ntziachristos P, Lim JS, Sage J and
Aifantis I: From fly wings to targeted cancer therapies: A
centennial for notch signaling. Cancer Cell. 25:318–334. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Okajima T and Irvine KD: Regulation of
notch signaling by o-linked fucose. Cell. 111:893–904. 2002.
View Article : Google Scholar
|
|
19
|
Haines N and Irvine KD: Glycosylation
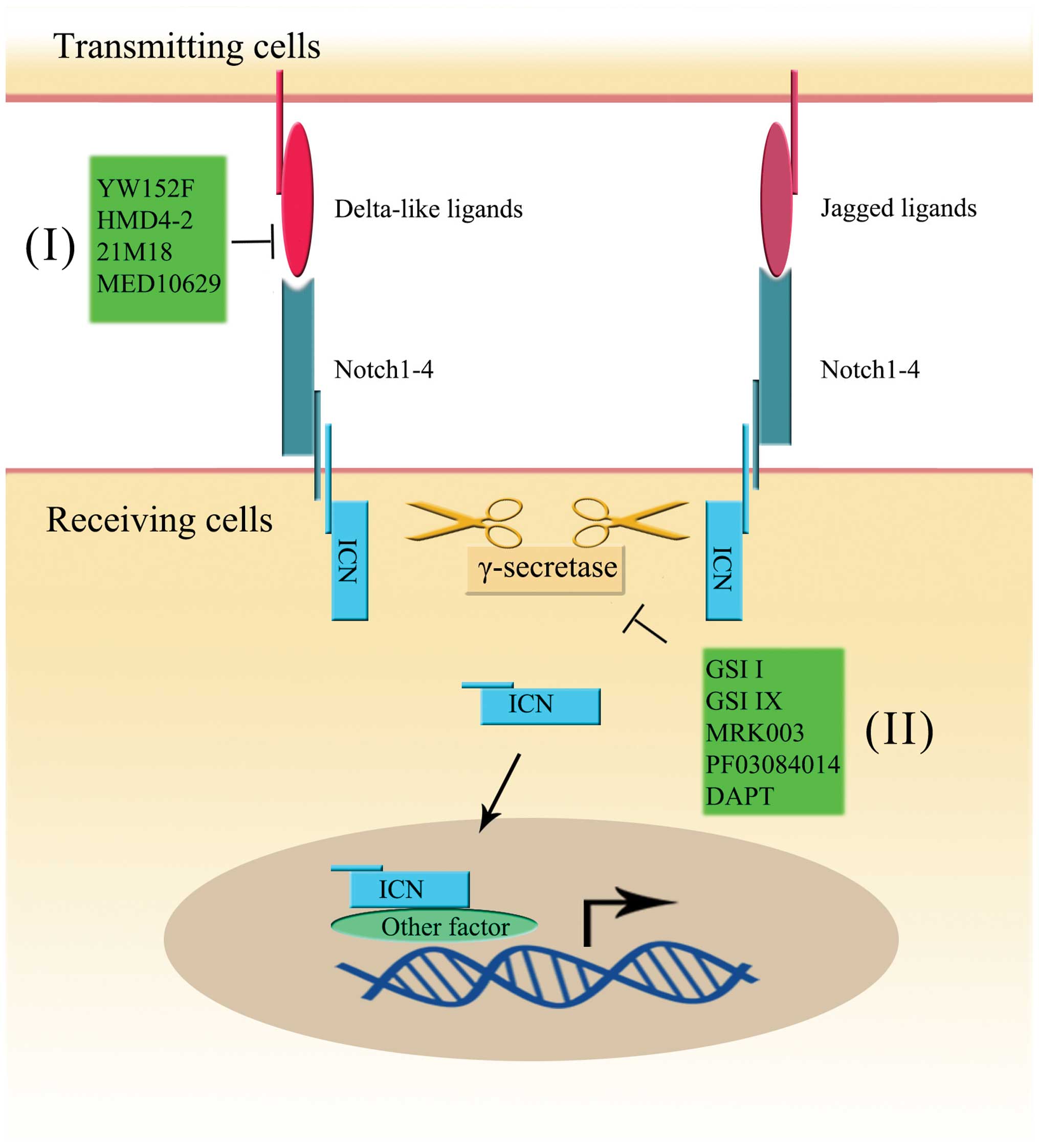
regulates Notch signalling. Nat Rev Mol Cell Biol. 4:786–797. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Guo S, Liu M and Gonzalez-Perez RR: Role
of Notch and its oncogenic signaling crosstalk in breast cancer.
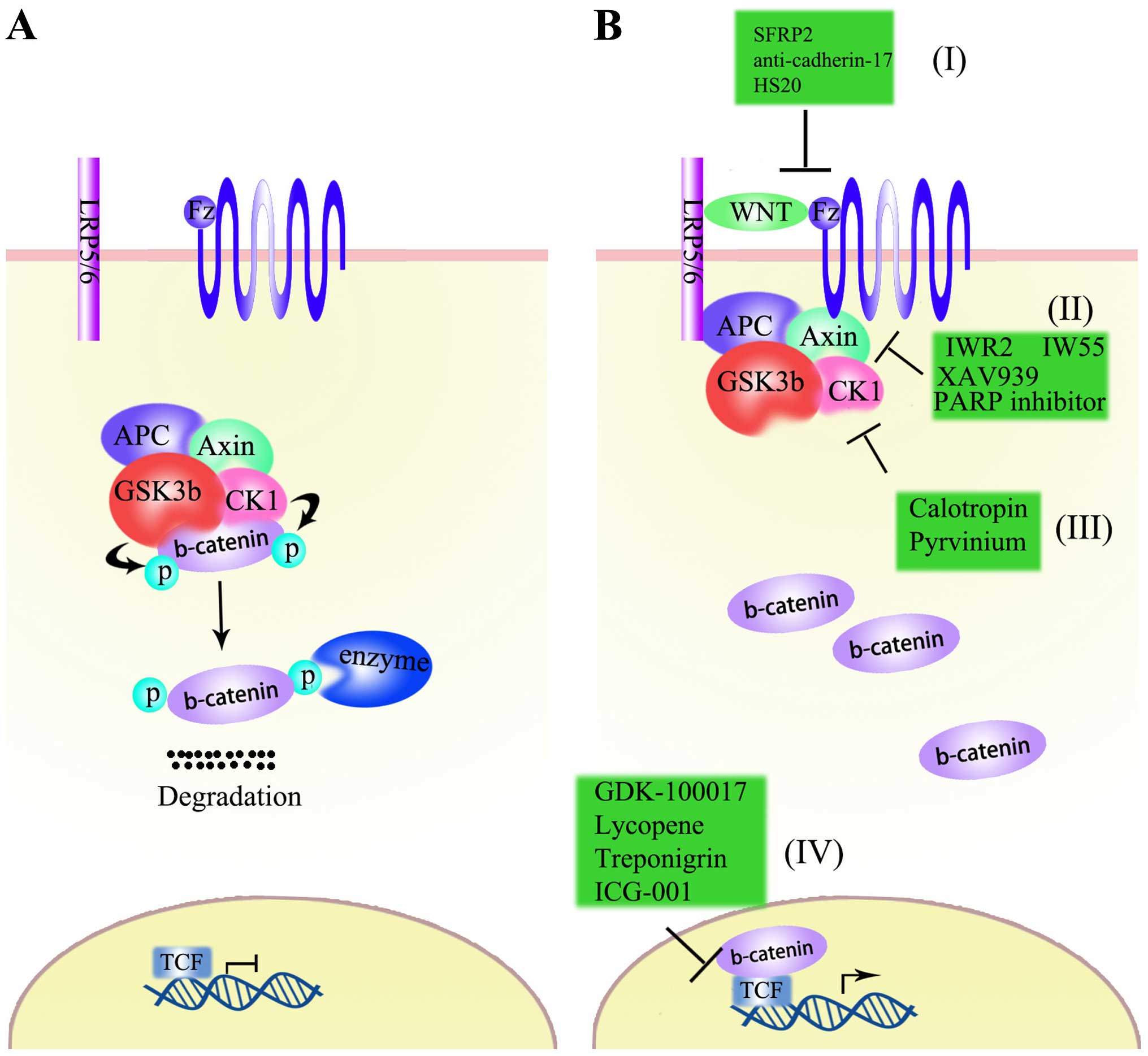
Biochim Biophys Acta. 1815:197–213. 2011.PubMed/NCBI
|
|
21
|
Lai EC: Notch signaling: Control of cell
communication and cell fate. Development. 131:965–973. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Wang Z, Li Y, Ahmad A, Azmi AS, Banerjee
S, Kong D and Sarkar FH: Targeting Notch signaling pathway to
overcome drug resistance for cancer therapy. Biochim Biophys Acta.
1806:258–267. 2010.PubMed/NCBI
|
|
23
|
Kopan R and Ilagan MX: The canonical Notch
signaling pathway: Unfolding the activation mechanism. Cell.
137:216–233. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Louvi A and Artavanis-Tsakonas S: Notch
and disease: A growing field. Semin Cell Dev Biol. 23:473–480.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Rizzo P, Osipo C, Foreman K, Golde T,
Osborne B and Miele L: Rational targeting of Notch signaling in
cancer. Oncogene. 27:5124–5131. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Wang Z, Banerjee S, Li Y, Rahman KM, Zhang
Y and Sarkar FH: Down-regulation of notch-1 inhibits invasion by
inactivation of nuclear factor-kappaB, vascular endothelial growth
factor, and matrix metalloproteinase-9 in pancreatic cancer cells.
Cancer Res. 66:2778–2784. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Wang Z, Zhang Y, Li Y, Banerjee S, Liao J
and Sarkar FH: Down-regulation of Notch-1 contributes to cell
growth inhibition and apoptosis in pancreatic cancer cells. Mol
Cancer Ther. 5:483–493. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Zavadil J, Cermak L, Soto-Nieves N and
Böttinger EP: Integration of TGF-beta/Smad and Jagged1/Notch
signalling in epithelial-to-mesenchymal transition. EMBO J.
23:1155–1165. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Sansone P, Storci G, Tavolari S, Guarnieri
T, Giovannini C, Taffurelli M, Ceccarelli C, Santini D, Paterini P,
Marcu KB, et al: IL-6 triggers malignant features in mammospheres
from human ductal breast carcinoma and normal mammary gland. J Clin
Invest. 117:3988–4002. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Patel NS, Li JL, Generali D, Poulsom R,
Cranston DW and Harris AL: Up-regulation of delta-like 4 ligand in
human tumor vasculature and the role of basal expression in
endothelial cell function. Cancer Res. 65:8690–8697. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Lawson ND, Vogel AM and Weinstein BM:
sonic hedgehog and vascular endothelial growth factor act upstream
of the Notch pathway during arterial endothelial differentiation.
Dev Cell. 3:127–136. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
South AP, Cho RJ and Aster JC: The
double-edged sword of Notch signaling in cancer. Semin Cell Dev
Biol. 23:458–464. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Ellisen LW, Bird J, West DC, Soreng AL,
Reynolds TC, Smith SD and Sklar J: TAN-1, the human homolog of the
Drosophila notch gene, is broken by chromosomal translocations in T
lymphoblastic neoplasms. Cell. 66:649–661. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Capobianco AJ, Zagouras P, Blaumueller CM,
Artavanis-Tsakonas S and Bishop JM: Neoplastic transformation by
truncated alleles of human NOTCH1/TAN1 and NOTCH2. Mol Cell Biol.
17:6265–6273. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Girard L, Hanna Z, Beaulieu N, Hoemann CD,
Simard C, Kozak CA and Jolicoeur P: Frequent provirus insertional
mutagenesis of Notch1 in thymomas of MMTVD/myc transgenic mice
suggests a collaboration of c-myc and Notch1 for oncogenesis. Genes
Dev. 10:1930–1944. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Weng AP, Ferrando AA, Lee W, Morris JP IV,
Silverman LB, Sanchez-Irizarry C, Blacklow SC, Look AT and Aster
JC: Activating mutations of NOTCH1 in human T cell acute
lymphoblastic leukemia. Science. 306:269–271. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Radtke F, Wilson A, Mancini SJ and
MacDonald HR: Notch regulation of lymphocyte development and
function. Nat Immunol. 5:247–253. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Hoyne GF: Notch signaling in the immune
system. J Leukoc Biol. 74:971–981. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Sulis ML, Williams O, Palomero T, Tosello
V, Pallikuppam S, Real PJ, Barnes K, Zuurbier L, Meijerink JP and
Ferrando AA: NOTCH1 extracellular juxtamembrane expansion mutations
in T-ALL. Blood. 112:733–740. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Breit S, Stanulla M, Flohr T, Schrappe M,
Ludwig WD, Tolle G, Happich M, Muckenthaler MU and Kulozik AE:
Activating NOTCH1 mutations predict favorable early treatment
response and long-term outcome in childhood precursor T-cell
lymphoblastic leukemia. Blood. 108:1151–1157. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Park MJ, Taki T, Oda M, Watanabe T,
Yumura-Yagi K, Kobayashi R, Suzuki N, Hara J, Horibe K and Hayashi
Y: FBXW7 and NOTCH1 mutations in childhood T cell acute
lymphoblastic leukaemia and T cell non-Hodgkin lymphoma. Br J
Haematol. 145:198–206. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Clappier E, Collette S, Grardel N, Girard
S, Suarez L, Brunie G, Kaltenbach S, Yakouben K, Mazingue F, Robert
A, et al; EORTC-CLG. NOTCH1 and FBXW7 mutations have a favorable
impact on early response to treatment, but not on outcome, in
children with T-cell acute lymphoblastic leukemia (T-ALL) treated
on EORTC trials 58881 and 58951. Leukemia. 24:2023–2031. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Weissmann S, Roller A, Jeromin S,
Hernández M, Abáigar M, Hernández-Rivas JM, Grossmann V, Haferlach
C, Kern W, Haferlach T, et al: Prognostic impact and landscape of
NOTCH1 mutations in chronic lymphocytic leukemia (CLL): A study on
852 patients. Leukemia. 27:2393–2396. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Di Ianni M, Baldoni S, Rosati E, Ciurnelli
R, Cavalli L, Martelli MF, Marconi P, Screpanti I and Falzetti F: A
new genetic lesion in B-CLL: A NOTCH1 PEST domain mutation. Br J
Haematol. 146:689–691. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Sportoletti P, Baldoni S, Cavalli L, Del
Papa B, Bonifacio E, Ciurnelli R, Bell AS, Di Tommaso A, Rosati E,
Crescenzi B, et al: NOTCH1 PEST domain mutation is an adverse
prognostic factor in B-CLL. Br J Haematol. 151:404–406. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Wickremasinghe RG, Prentice AG and Steele
AJ: p53 and Notch signaling in chronic lymphocytic leukemia: Clues
to identifying novel therapeutic strategies. Leukemia.
25:1400–1407. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Rossi D, Rasi S, Fabbri G, Spina V,
Fangazio M, Forconi F, Marasca R, Laurenti L, Bruscaggin A, Cerri
M, et al: Mutations of NOTCH1 are an independent predictor of
survival in chronic lymphocytic leukemia. Blood. 119:521–529. 2012.
View Article : Google Scholar :
|
|
48
|
Kiel MJ, Velusamy T, Betz BL, Zhao L,
Weigelin HG, Chiang MY, Huebner-Chan DR, Bailey NG, Yang DT, Bhagat
G, et al: Whole-genome sequencing identifies recurrent somatic
NOTCH2 mutations in splenic marginal zone lymphoma. J Exp Med.
209:1553–1565. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Lee SY, Kumano K, Nakazaki K, Sanada M,
Matsumoto A, Yamamoto G, Nannya Y, Suzuki R, Ota S, Ota Y, et al:
Gainof-function mutations and copy number increases of Notch2 in
diffuse large B-cell lymphoma. Cancer Sci. 100:920–926. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Rossi D, Trifonov V, Fangazio M,
Bruscaggin A, Rasi S, Spina V, Monti S, Vaisitti T, Arruga F, Famà
R, et al: The coding genome of splenic marginal zone lymphoma:
Activation of NOTCH2 and other pathways regulating marginal zone
development. J Exp Med. 209:1537–1551. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Uyttendaele H, Soriano JV, Montesano R and
Kitajewski J: Notch4 and Wnt-1 proteins function to regulate
branching morphogenesis of mammary epithelial cells in an opposing
fashion. Dev Biol. 196:204–217. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Bellavia D, Checquolo S, Campese AF, Felli
MP, Gulino A and Screpanti I: Notch3: From subtle structural
differences to functional diversity. Oncogene. 27:5092–5098. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Melchor L and Smalley MJ: Highway to
heaven: Mammary gland development and differentiation. Breast
Cancer Res. 10:3052008. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Weijzen S, Rizzo P, Braid M, Vaishnav R,
Jonkheer SM, Zlobin A, Osborne BA, Gottipati S, Aster JC, Hahn WC,
et al: Activation of Notch-1 signaling maintains the neoplastic
phenotype in human Ras-transformed cells. Nat Med. 8:979–986. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Hu C, Diévart A, Lupien M, Calvo E,
Tremblay G and Jolicoeur P: Overexpression of activated murine
Notch1 and Notch3 in transgenic mice blocks mammary gland
development and induces mammary tumors. Am J Pathol. 168:973–990.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Imatani A and Callahan R: Identification
of a novel NOTCH-4/INT-3 RNA species encoding an activated gene
product in certain human tumor cell lines. Oncogene. 19:223–231.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Reedijk M, Odorcic S, Chang L, Zhang H,
Miller N, McCready DR, Lockwood G and Egan SE: High-level
coexpression of JAG1 and NOTCH1 is observed in human breast cancer
and is associated with poor overall survival. Cancer Res.
65:8530–8537. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Zhang Z, Wang H, Ikeda S, Fahey F,
Bielenberg D, Smits P and Hauschka PV: Notch3 in human breast
cancer cell lines regulates osteoblast-cancer cell interactions and
osteolytic bone metastasis. Am J Pathol. 177:1459–1469. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Parr C, Watkins G and Jiang WG: The
possible correlation of Notch-1 and Notch-2 with clinical outcome
and tumour clinicopathological parameters in human breast cancer.
Int J Mol Med. 14:779–786. 2004.PubMed/NCBI
|
|
60
|
Baumgart A, Mazur PK, Anton M, Rudelius M,
Schwamborn K, Feuchtinger A, Behnke K, Walch A, Braren R, Peschel
C, et al: Opposing role of Notch1 and Notch2 in a Kras(G12D)-driven
murine non-small cell lung cancer model. Oncogene. 34:578–588.
2015. View Article : Google Scholar
|
|
61
|
Yang Y, Yan X, Duan W, Yan J, Yi W, Liang
Z, Wang N, Li Y, Chen W, Yu S, et al: Pterostilbene exerts
antitumor activity via the Notch1 signaling pathway in human lung
adenocarcinoma cells. PLoS One. 8:e626522013. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Licciulli S, Avila JL, Hanlon L, Troutman
S, Cesaroni M, Kota S, Keith B, Simon MC, Puré E, Radtke F, et al:
Notch1 is required for Kras-induced lung adenocarcinoma and
controls tumor cell survival via p53. Cancer Res. 73:5974–5984.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Xie M, He CS, Wei SH and Zhang L: Notch-1
contributes to epidermal growth factor receptor tyrosine kinase
inhibitor acquired resistance in non-small cell lung cancer in
vitro and in vivo. Eur J Cancer. 49:3559–3572. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Hassan KA, Wang L, Korkaya H, Chen G,
Maillard I, Beer DG, Kalemkerian GP and Wicha M: Notch pathway
activity identifies cells with cancer stem cell-like properties and
correlates with worse survival in lung adenocarcinoma. Clin Cancer
Res. 19:1972–1980. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Theys J, Yahyanejad S, Habets R, Span P,
Dubois L, Paesmans K, Kattenbeld B, Cleutjens J, Groot AJ,
Schuurbiers OC, et al: High NOTCH activity induces radiation
resistance in non small cell lung cancer. Radiother Oncol.
108:440–445. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Wael H, Yoshida R, Kudoh S, Hasegawa K,
Niimori-Kita K and Ito T: Notch1 signaling controls cell
proliferation, apoptosis and differentiation in lung carcinoma.
Lung Cancer. 85:131–140. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Huang J, Song H, Liu B, Yu B, Wang R and
Chen L: Expression of Notch-1 and its clinical significance in
different histological subtypes of human lung adenocarcinoma. J Exp
Clin Cancer Res. 32:842013. View Article : Google Scholar :
|
|
68
|
Zhou M, Jin WY, Fan ZW and Han RC:
Analysis of the expression of the Notch3 receptor protein in adult
lung cancer. Oncol Lett. 5:499–504. 2013.PubMed/NCBI
|
|
69
|
Ye YZ, Zhang ZH, Fan XY, Xu XL, Chen ML,
Chang BW and Zhang YB: Notch3 overexpression associates with poor
prognosis in human non-small-cell lung cancer. Med Oncol.
30:5952013. View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Jemal A, Bray F, Center MM, Ferlay J, Ward
E and Forman D: Global cancer statistics. CA Cancer J Clin.
61:69–90. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Yeh TS, Wu CW, Hsu KW, Liao WJ, Yang MC,
Li AF, Wang AM, Kuo ML and Chi CW: The activated Notch1 signal
pathway is associated with gastric cancer progression through
cyclooxygenase-2. Cancer Res. 69:5039–5048. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
72
|
Yao J and Qian C: Over-activated Notch-1
protects gastric carcinoma BGC-823 cells from TNFalpha-induced
apoptosis. Dig Liver Dis. 41:867–874. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
73
|
Carson C, Murdoch B and Roskams AJ: Notch
2 and Notch 1/3 segregate to neuronal and glial lineages of the
developing olfactory epithelium. Dev Dyn. 235:1678–1688. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
74
|
Sun Y, Gao X, Liu J, Kong QY, Wang XW,
Chen XY, Wang Q, Cheng YF, Qu XX and Li H: Differential Notch1 and
Notch2 expression and frequent activation of Notch signaling in
gastric cancers. Arch Pathol Lab Med. 135:451–458. 2011.PubMed/NCBI
|
|
75
|
Tseng YC, Tsai YH, Tseng MJ, Hsu KW, Yang
MC, Huang KH, Li AF, Chi CW, Hsieh RH, Ku HH, et al: Notch2-induced
COX-2 expression enhancing gastric cancer progression. Mol
Carcinog. 51:939–951. 2012. View Article : Google Scholar
|
|
76
|
Guo LY, Li YM, Qiao L, Liu T, Du YY, Zhang
JQ, He WT, Zhao YX and He DQ: Notch2 regulates matrix
metallopeptidase 9 via PI3K/AKT signaling in human gastric
carcinoma cell MKN-45. World J Gastroenterol. 18:7262–7270. 2012.
View Article : Google Scholar
|
|
77
|
Piazzi G, Fini L, Selgrad M, Garcia M,
Daoud Y, Wex T, Malfertheiner P, Gasbarrini A, Romano M, Meyer RL,
et al: Epigenetic regulation of Delta-Like1 controls Notch1
activation in gastric cancer. Oncotarget. 2:1291–1301. 2011.
View Article : Google Scholar
|
|
78
|
Pellegrinet L, Rodilla V, Liu Z, Chen S,
Koch U, Espinosa L, Kaestner KH, Kopan R, Lewis J and Radtke F:
Dll1- and dll4-mediated notch signaling are required for
homeostasis of intestinal stem cells. Gastroenterology.
140:1230–1240.e7. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
79
|
Li GG, Li L, Li C, Ye LY, Li XW, Liu DR,
Bao Q, Zheng YX, Xiang DP, Chen L, et al: Influence of
up-regulation of Notch ligand DLL4 on biological behaviors of human
gastric cancer cells. World J Gastroenterol. 19:4486–4494. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
80
|
Sun HW, Wu C, Tan HY and Wang QS:
Combination DLL4 with Jagged1-siRNA can enhance inhibition of the
proliferation and invasiveness activity of human gastric carcinoma
by Notch1/VEGF pathway. Hepatogastroenterology. 59:924–929.
2012.
|
|
81
|
Logan CY and Nusse R: The Wnt signaling
pathway in development and disease. Annu Rev Cell Dev Biol.
20:781–810. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
82
|
Rosenbluh J, Wang X and Hahn WC: Genomic
insights into WNT/β-catenin signaling. Trends Pharmacol Sci.
35:103–109. 2014. View Article : Google Scholar :
|
|
83
|
MacDonald BT, Tamai K and He X:
Wnt/beta-catenin signaling: Components, mechanisms, and diseases.
Dev Cell. 17:9–26. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
84
|
Niehrs C: The complex world of WNT
receptor signalling. Nat Rev Mol Cell Biol. 13:767–779. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
85
|
Wang JM, Huang FC, Kuo MH, Wang ZF, Tseng
TY, Chang LC, Yen SJ, Chang TC and Lin JJ: Inhibition of cancer
cell migration and invasion through suppressing the Wnt1-mediating
signal pathway by G-quadruplex structure stabilizers. J Biol Chem.
289:14612–14623. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
86
|
Lee MA, Park JH, Rhyu SY, Oh ST, Kang WK
and Kim HN: Wnt3a expression is associated with MMP-9 expression in
primary tumor and metastatic site in recurrent or stage IV
colorectal cancer. BMC Cancer. 14:1252014. View Article : Google Scholar : PubMed/NCBI
|
|
87
|
Wang SH, Li N, Wei Y, Li QR and Yu ZP:
β-catenin deacetylation is essential for WNT-induced proliferation
of breast cancer cells. Mol Med Rep. 9:973–978. 2014.PubMed/NCBI
|
|
88
|
Miao CG, Yang YY, He X, Huang C, Huang Y,
Zhang L, Lv XW, Jin Y and Li J: Wnt signaling in liver fibrosis:
Progress, challenges and potential directions. Biochimie.
95:2326–2335. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
89
|
Arend RC, Londoño-Joshi AI, Straughn JM Jr
and Buchsbaum DJ: The Wnt/β-catenin pathway in ovarian cancer: A
review. Gynecol Oncol. 131:772–779. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
90
|
Holland JD, Klaus A, Garratt AN and
Birchmeier W: Wnt signaling in stem and cancer stem cells. Curr
Opin Cell Biol. 25:254–264. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
91
|
Barbolina MV, Burkhalter RJ and Stack MS:
Diverse mechanisms for activation of Wnt signalling in the ovarian
tumour microenvironment. Biochem J. 437:1–12. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
92
|
Andersen P, Uosaki H, Shenje LT and Kwon
C: Non-canonical Notch signaling: Emerging role and mechanism.
Trends Cell Biol. 22:257–265. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
93
|
Clark CE, Nourse CC and Cooper HM: The
tangled web of non-canonical Wnt signalling in neural migration.
Neurosignals. 20:202–220. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
94
|
González-Sancho JM, Brennan KR,
Castelo-Soccio LA and Brown AM: Wnt proteins induce dishevelled
phosphorylation via an LRP5/6-independent mechanism, irrespective
of their ability to stabilize beta-catenin. Mol Cell Biol.
24:4757–4768. 2004. View Article : Google Scholar
|
|
95
|
Beier F and Loeser RF: Biology and
pathology of Rho GTPase, PI-3 kinase-Akt, and MAP kinase signaling
pathways in chondrocytes. J Cell Biochem. 110:573–580. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
96
|
Asad M, Wong MK, Tan TZ, Choolani M, Low
J, Mori S, Virshup D, Thiery JP and Huang RY: FZD7 drives in vitro
aggressiveness in Stem-A subtype of ovarian cancer via regulation
of non-canonical Wnt/PCP pathway. Cell Death Dis. 5:e13462014.
View Article : Google Scholar : PubMed/NCBI
|
|
97
|
Bernemann C, Hülsewig C, Ruckert C,
Schäfer S, Blümel L, Hempel G, Götte M, Greve B, Barth PJ, Kiesel
L, et al: Influence of secreted frizzled receptor protein 1 (SFRP1)
on neoadjuvant chemotherapy in triple negative breast cancer does
not rely on WNT signaling. Mol Cancer. 13:1742014. View Article : Google Scholar : PubMed/NCBI
|
|
98
|
Xi Y and Chen Y: Wnt signaling pathway:
Implications for therapy in lung cancer and bone metastasis. Cancer
Lett. 353:8–16. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
99
|
Nejak-Bowen KN and Monga SP: Beta-catenin
signaling, liver regeneration and hepatocellular cancer: Sorting
the good from the bad. Semin Cancer Biol. 21:44–58. 2011.
View Article : Google Scholar :
|
|
100
|
Colussi D, Brandi G, Bazzoli F and
Ricciardiello L: Molecular pathways involved in colorectal cancer:
Implications for disease behavior and prevention. Int J Mol Sci.
14:16365–16385. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
101
|
Pez F, Lopez A, Kim M, Wands JR, Caron de
Fromentel C and Merle P: Wnt signaling and hepatocarcinogenesis:
Molecular targets for the development of innovative anticancer
drugs. J Hepatol. 59:1107–1117. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
102
|
Armengol C, Cairo S, Fabre M and Buendia
MA: Wnt signaling and hepatocarcinogenesis: The hepatoblastoma
model. Int J Biochem Cell Biol. 43:265–270. 2011. View Article : Google Scholar
|
|
103
|
Gedaly R, Galuppo R, Daily MF, Shah M,
Maynard E, Chen C, Zhang X, Esser KA, Cohen DA, Evers BM, et al:
Targeting the Wnt/β-catenin signaling pathway in liver cancer stem
cells and hepatocellular carcinoma cell lines with FH535. PLoS One.
9:e992722014. View Article : Google Scholar
|
|
104
|
Hou L, Wang X, Zhou Y, Ma H, Wang Z, He J,
Hu H, Guan W and Ma Y: Inhibitory effect and mechanism of
mesenchymal stem cells on liver cancer cells. Tumour Biol.
35:1239–1250. 2014. View Article : Google Scholar
|
|
105
|
Zucchini-Pascal N, Peyre L and Rahmani R:
Crosstalk between beta-catenin and snail in the induction of
epithelial to mesenchymal transition in hepatocarcinoma: Role of
the ERK1/2 pathway. Int J Mol Sci. 14:20768–20792. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
106
|
Bustos VH, Ferrarese A, Venerando A, Marin
O, Allende JE and Pinna LA: The first armadillo repeat is involved
in the recognition and regulation of beta-catenin phosphorylation
by protein kinase CK1. Proc Natl Acad Sci USA. 103:19725–19730.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
107
|
Singh Y, Port J, Schwarz M and Braeuning
A: Genetic ablation of β-catenin inhibits the proliferative
phenotype of mouse liver adenomas. Br J Cancer. 111:132–138. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
108
|
Calderaro J, Nault JC, Bioulac-Sage P,
Laurent A, Blanc JF, Decaens T and Zucman-Rossi J: ALDH3A1 is
overexpressed in a subset of hepatocellular carcinoma characterised
by activation of the Wnt/ss-catenin pathway. Virchows Arch.
464:53–60. 2014. View Article : Google Scholar
|
|
109
|
Cheng JH, She H, Han YP, Wang J, Xiong S,
Asahina K and Tsukamoto H: Wnt antagonism inhibits hepatic stellate
cell activation and liver fibrosis. Am J Physiol Gastrointest Liver
Physiol. 294:G39–G49. 2008. View Article : Google Scholar
|
|
110
|
Li W, Zhu C, Li Y, Wu Q and Gao R: Mest
attenuates CCl4-induced liver fibrosis in rats by inhibiting the
Wnt/β-catenin signaling pathway. Gut Liver. 8:282–291. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
111
|
Tenesa A and Dunlop MG: New insights into
the aetiology of colorectal cancer from genome-wide association
studies. Nat Rev Genet. 10:353–358. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
112
|
Pandurangan AK: Potential targets for
prevention of colorectal cancer: A focus on PI3K/Akt/mTOR and Wnt
pathways. Asian Pac J Cancer Prev. 14:2201–2205. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
113
|
Curtin JC: Novel drug discovery
opportunities for colorectal cancer. Expert Opin Drug Discov.
8:1153–1164. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
114
|
Sparks AB, Morin PJ, Vogelstein B and
Kinzler KW: Mutational analysis of the APC/beta-catenin/Tcf pathway
in colorectal cancer. Cancer Res. 58:1130–1134. 1998.PubMed/NCBI
|
|
115
|
Murakami T, Mitomi H, Saito T, Takahashi
M, Sakamoto N, Fukui N, Yao T and Watanabe S: Distinct
WNT/beta-catenin signaling activation in the serrated neoplasia
pathway and the adenoma-carcinoma sequence of the colorectum. Mod
Pathol. 28:146–158. 2015. View Article : Google Scholar
|
|
116
|
Raghu D and Karunagaran D: Plumbagin
downregulates Wnt signaling independent of p53 in human colorectal
cancer cells. J Nat Prod. 77:1130–1134. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
117
|
Tai WP, Hu PJ, Wu J and Lin XC: The
inhibition of Wnt/β-catenin signaling pathway in human colon cancer
cells by sulindac. Tumori. 100:97–101. 2014.PubMed/NCBI
|
|
118
|
Tumova L, Pombinho AR, Vojtechova M,
Stancikova J, Gradl D, Krausova M, Sloncova E, Horazna M, Kriz V,
Machonova O, et al: Monensin inhibits canonical Wnt signaling in
human colorectal cancer cells and suppresses tumor growth in
multiple intestinal neoplasia mice. Mol Cancer Ther. 13:812–822.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
119
|
Bruun J, Kolberg M, Nesland JM, Svindland
A, Nesbakken A and Lothe RA: Prognostic Significance of β-catenin,
E-cadherin, and SOX9 in colorectal cancer: Results from a large
population-representative series. Front Oncol. 4:1182014.
View Article : Google Scholar
|
|
120
|
Voorham QJ, Janssen J, Tijssen M,
Snellenberg S, Mongera S, van Grieken NC, Grabsch H, Kliment M,
Rembacken BJ, Mulder CJ, et al: Promoter methylation of
Wnt-antagonists in polypoid and nonpolypoid colorectal adenomas.
BMC Cancer. 13:6032013. View Article : Google Scholar : PubMed/NCBI
|
|
121
|
Serafino A, Moroni N, Zonfrillo M,
Andreola F, Mercuri L, Nicotera G, Nunziata J, Ricci R, Antinori A,
Rasi G, et al: WNT-pathway components as predictive markers useful
for diagnosis, prevention and therapy in inflammatory bowel disease
and sporadic colorectal cancer. Oncotarget. 5:978–992. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
122
|
Abdelmaksoud-Dammak R, Miladi-Abdennadher
I, Saadallah-Kallel A, Khabir A, Sellami-Boudawara T, Frikha M,
Daoud J and Mokdad-Gargouri R: Downregulation of WIF-1 and Wnt5a in
patients with colorectal carcinoma: clinical significance. Tumour
Biol. 35:7975–7982. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
123
|
Bauer M, Bénard J, Gaasterland T, Willert
K and Cappellen D: WNT5A encodes two isoforms with distinct
functions in cancers. PLoS One. 8:e805262013. View Article : Google Scholar : PubMed/NCBI
|
|
124
|
Chai J, Modak C, Ouyang Y, Wu SY and Jamal
MM: CCN1 Induces β-catenin translocation in esophageal squamous
cell carcinoma through integrin α11. ISRN Gastroenterol.
2012:2072352012. View Article : Google Scholar
|
|
125
|
Moyes LH, McEwan H, Radulescu S,
Pawlikowski J, Lamm CG, Nixon C, Sansom OJ, Going JJ, Fullarton GM
and Adams PD: Activation of Wnt signalling promotes development of
dysplasia in Barrett's oesophagus. J Pathol. 228:99–112.
2012.PubMed/NCBI
|
|
126
|
Long A, Giroux V, Whelan KA, Hamilton KE,
Tétreault MP, Tanaka K, Lee JS, Klein-Szanto AJ, Nakagawa H and
Rustgi AK: WNT10A promotes an invasive and self-renewing phenotype
in esophageal squamous cell carcinoma. Carcinogenesis. 36:598–606.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
127
|
Yang SH, Li SL, Dong ZM and Kan QC:
Epigenetic inactivation of Wnt inhibitory factor-1 in human
esophageal squamous cell carcinoma. Oncol Res. 20:123–130. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
128
|
Ge XS, Ma HJ, Zheng XH, Ruan HL, Liao XY,
Xue WQ, Chen YB, Zhang Y and Jia WH: HOTAIR, a prognostic factor in
esophageal squamous cell carcinoma, inhibits WIF-1 expression and
activates Wnt pathway. Cancer Sci. 104:1675–1682. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
129
|
Liu K, Luo Y, Tian H, Yu KZ, He JX and
Shen WY: The tumor suppressor LKB1 antagonizes WNT signaling
pathway through modulating GSK3beta activity in cell growth of
esophageal carcinoma. Tumour Biol. 35:995–1002. 2014. View Article : Google Scholar
|
|
130
|
Tong X, Li L, Li X, Heng L, Zhong L, Su X,
Rong R, Hu S, Liu W, Jia B, et al: SOX10, a novel
HMG-box-containing tumor suppressor, inhibits growth and metastasis
of digestive cancers by suppressing the Wnt/β-catenin pathway.
Oncotarget. 5:10571–10583. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
131
|
Kuramoto T, Goto H, Mitsuhashi A, Tabata
S, Ogawa H, Uehara H, Saijo A, Kakiuchi S, Maekawa Y, Yasutomo K,
et al: Dll4-Fc, an inhibitor of Dll4-notch signaling, suppresses
liver metastasis of small cell lung cancer cells through the
downregulation of the NF-κB activity. Mol Cancer Ther.
11:2578–2587. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
132
|
Stewart KS, Zhou Z, Zweidler-McKay P and
Kleinerman ES: Delta-like ligand 4-Notch signaling regulates bone
marrow-derived pericyte/vascular smooth muscle cell formation.
Blood. 117:719–726. 2011. View Article : Google Scholar :
|
|
133
|
Ridgway J, Zhang G, Wu Y, Stawicki S,
Liang WC, Chanthery Y, Kowalski J, Watts RJ, Callahan C, Kasman I,
et al: Inhibition of Dll4 signalling inhibits tumour growth by
deregulating angiogenesis. Nature. 444:1083–1087. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
134
|
Oishi H, Sunamura M, Egawa S, Motoi F,
Unno M, Furukawa T, Habib NA and Yagita H: Blockade of delta-like
ligand 4 signaling inhibits both growth and angiogenesis of
pancreatic cancer. Pancreas. 39:897–903. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
135
|
Gurney A and Hoey T: Anti-DLL4, a cancer
therapeutic with multiple mechanisms of action. Vasc Cell.
3:182011. View Article : Google Scholar : PubMed/NCBI
|
|
136
|
Fischer M, Yen WC, Kapoun AM, Wang M,
O'Young G, Lewicki J, Gurney A and Hoey T: Anti-DLL4 inhibits
growth and reduces tumor-initiating cell frequency in colorectal
tumors with oncogenic KRAS mutations. Cancer Res. 71:1520–1525.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
137
|
Jenkins DW, Ross S, Veldman-Jones M, Foltz
IN, Clavette BC, Manchulenko K, Eberlein C, Kendrew J, Petteruti P,
Cho S, et al: MEDI0639: A novel therapeutic antibody targeting Dll4
modulates endothelial cell function and angiogenesis in vivo. Mol
Cancer Ther. 11:1650–1660. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
138
|
Liu SK, Bham SA, Fokas E, Beech J, Im J,
Cho S, Harris AL and Muschel RJ: Delta-like ligand 4-notch blockade
and tumor radiation response. J Natl Cancer Inst. 103:1778–1798.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
139
|
El Kaffas A, Nofiele J, Giles A, Cho S,
Liu SK and Czarnota GJ: Dll4-notch signalling blockade synergizes
combined ultrasound-stimulated microbubble and radiation therapy in
human colon cancer xenografts. PLoS One. 9:e938882014. View Article : Google Scholar : PubMed/NCBI
|
|
140
|
Aste-Amézaga M, Zhang N, Lineberger JE,
Arnold BA, Toner TJ, Gu M, Huang L, Vitelli S, Vo KT, Haytko P, et
al: Characterization of Notch1 antibodies that inhibit signaling of
both normal and mutated Notch1 receptors. PLoS One. 5:e90942010.
View Article : Google Scholar : PubMed/NCBI
|
|
141
|
Sharma A, Paranjape AN, Rangarajan A and
Dighe RR: A monoclonal antibody against human Notch1 ligand-binding
domain depletes subpopulation of putative breast cancer stem-like
cells. Mol Cancer Ther. 11:77–86. 2012. View Article : Google Scholar
|
|
142
|
Yan M, Callahan CA, Beyer JC, Allamneni
KP, Zhang G, Ridgway JB, Niessen K and Plowman GD: Chronic DLL4
blockade induces vascular neoplasms. Nature. 463:E6–E7. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
143
|
Rosati E, Sabatini R, De Falco F, Del Papa
B, Falzetti F, Di Ianni M, Cavalli L, Fettucciari K, Bartoli A,
Screpanti I, et al: gamma-Secretase inhibitor I induces apoptosis
in chronic lymphocytic leukemia cells by proteasome inhibition,
endoplasmic reticulum stress increase and notch down-regulation.
Int J Cancer. 132:1940–1953. 2013. View Article : Google Scholar
|
|
144
|
Palagani V, El Khatib M, Kossatz U, Bozko
P, Müller MR, Manns MP, Krech T, Malek NP and Plentz RR: Epithelial
mesenchymal transition and pancreatic tumor initiating
CD44+/EpCAM+ cells are inhibited by
γ-secretase inhibitor IX. PLoS One. 7:e465142012. View Article : Google Scholar
|
|
145
|
Schott AF, Landis MD, Dontu G, Griffith
KA, Layman RM, Krop I, Paskett LA, Wong H, Dobrolecki LE, Lewis MT,
et al: Preclinical and clinical studies of gamma secretase
inhibitors with docetaxel on human breast tumors. Clin Cancer Res.
19:1512–1524. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
146
|
López-Guerra M, Xargay-Torrent S, Rosich
L, Montraveta A, Roldán J, Matas-Céspedes A, Villamor N, Aymerich
M, López-Otín C, Pérez-Galán P, et al: The γ-secretase inhibitor
PF-03084014 combined with fludarabine antagonizes migration,
invasion and angiogenesis in NOTCH1-mutated CLL cells. Leukemia.
29:96–106. 2015. View Article : Google Scholar
|
|
147
|
Saito N, Fu J, Zheng S, Yao J, Wang S, Liu
DD, Yuan Y, Sulman EP, Lang FF, Colman H, et al: A high Notch
pathway activation predicts response to γ secretase inhibitors in
proneural subtype of glioma tumor-initiating cells. Stem Cells.
32:301–312. 2014. View Article : Google Scholar :
|
|
148
|
Groeneweg JW, Hall TR, Zhang L, Kim M,
Byron VF, Tambouret R, Sathayanrayanan S, Foster R, Rueda BR and
Growdon WB: Inhibition of gamma-secretase activity impedes uterine
serous carcinoma growth in a human xenograft model. Gynecol Oncol.
133:607–615. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
149
|
Li LC, Peng Y, Liu YM, Wang LL and Wu XL:
Gastric cancer cell growth and epithelial-mesenchymal transition
are inhibited by γ-secretase inhibitor DAPT. Oncol Lett.
7:2160–2164. 2014.PubMed/NCBI
|
|
150
|
Dahmani R, Just PA and Perret C: The
Wnt/β-catenin pathway as a therapeutic target in human
hepatocellular carcinoma. Clin Res Hepatol Gastroenterol.
35:709–713. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
151
|
Fontenot E, Rossi E, Mumper R, Snyder S,
Siamakpour-Reihani S, Ma P, Hilliard E, Bone B, Ketelsen D, Santos
C, et al: A novel monoclonal antibody to secreted frizzled-related
protein 2 inhibits tumor growth. Mol Cancer Ther. 12:685–695. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
152
|
Wang Y, Shek FH, Wong KF, Liu LX, Zhang
XQ, Yuan Y, Khin E, Hu MY, Wang JH, Poon RT, et al:
Anti-cadherin-17 antibody modulates beta-catenin signaling and
tumorigenicity of hepatocellular carcinoma. PLoS One. 8:e723862013.
View Article : Google Scholar : PubMed/NCBI
|
|
153
|
Gao W, Kim H, Feng M, Phung Y, Xavier CP,
Rubin JS and Ho M: Inactivation of Wnt signaling by a human
antibody that recognizes the heparan sulfate chains of glypican-3
for liver cancer therapy. Hepatology. 60:576–587. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
154
|
Ettenberg SA, Charlat O, Daley MP, Liu S,
Vincent KJ, Stuart DD, Schuller AG, Yuan J, Ospina B, Green J, et
al: Inhibition of tumorigenesis driven by different Wnt proteins
requires blockade of distinct ligand-binding regions by LRP6
antibodies. Proc Natl Acad Sci USA. 107:15473–15478. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
155
|
Gong Y, Bourhis E, Chiu C, Stawicki S,
DeAlmeida VI, Liu BY, Phamluong K, Cao TC, Carano RA, Ernst JA, et
al: Wnt isoform-specific interactions with coreceptor specify
inhibition or potentiation of signaling by LRP6 antibodies. PLoS
One. 5:e126822010. View Article : Google Scholar : PubMed/NCBI
|
|
156
|
Lavergne E, Hendaoui I, Coulouarn C,
Ribault C, Leseur J, Eliat PA, Mebarki S, Corlu A, Clément B and
Musso O: Blocking Wnt signaling by SFRP-like molecules inhibits in
vivo cell proliferation and tumor growth in cells carrying active
β-catenin. Oncogene. 30:423–433. 2011. View Article : Google Scholar
|
|
157
|
Wei W, Chua MS, Grepper S and So SK:
Soluble Frizzled-7 receptor inhibits Wnt signaling and sensitizes
hepatocellular carcinoma cells towards doxorubicin. Mol Cancer.
10:162011. View Article : Google Scholar : PubMed/NCBI
|
|
158
|
Amado NG, Predes D, Moreno MM, Carvalho
IO, Mendes FA and Abreu JG: Flavonoids and Wnt/β-catenin signaling:
Potential role in colorectal cancer therapies. Int J Mol Sci.
15:12094–12106. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
159
|
Ji Q, Liu X, Fu X, Zhang L, Sui H, Zhou L,
Sun J, Cai J, Qin J, Ren J, et al: Resveratrol inhibits invasion
and metastasis of colorectal cancer cells via MALAT1 mediated
Wnt/β-catenin signal pathway. PLoS One. 8:e787002013. View Article : Google Scholar
|
|
160
|
Huang SM, Mishina YM, Liu S, Cheung A,
Stegmeier F, Michaud GA, Charlat O, Wiellette E, Zhang Y, Wiessner
S, et al: Tankyrase inhibition stabilizes axin and antagonizes Wnt
signalling. Nature. 461:614–620. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
161
|
Waaler J, Machon O, Tumova L, Dinh H,
Korinek V, Wilson SR, Paulsen JE, Pedersen NM, Eide TJ, Machonova
O, et al: A novel tankyrase inhibitor decreases canonical Wnt
signaling in colon carcinoma cells and reduces tumor growth in
conditional APC mutant mice. Cancer Res. 72:2822–2832. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
162
|
Park HY, Toume K, Arai MA, Sadhu SK, Ahmed
F and Ishibashi M: Calotropin: A cardenolide from calotropis
gigantea that inhibits Wnt signaling by increasing casein kinase 1α
in colon cancer cells. Chem Bio Chem. 15:872–878. 2014. View Article : Google Scholar
|
|
163
|
Li B, Flaveny CA, Giambelli C, Fei DL, Han
L, Hang BI, Bai F, Pei XH, Nose V, Burlingame O, et al: Repurposing
the FDA-approved pinworm drug pyrvinium as a novel chemotherapeutic
agent for intestinal polyposis. PLoS One. 9:e1019692014. View Article : Google Scholar : PubMed/NCBI
|
|
164
|
Wei W, Chua MS, Grepper S and So S: Small
molecule antagonists of Tcf4/beta-catenin complex inhibit the
growth of HCC cells in vitro and in vivo. Int J Cancer.
126:2426–2436. 2010.
|
|
165
|
Lee SB, Gong YD, Park YI and Dong MS:
2,3,6-Trisubstituted quinoxaline derivative, a small molecule
inhibitor of the Wnt/beta-catenin signaling pathway, suppresses
cell proliferation and enhances radiosensitivity in A549/Wnt2
cells. Biochem Biophys Res Commun. 431:746–752. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
166
|
Preet R, Mohapatra P, Das D, Satapathy SR,
Choudhuri T, Wyatt MD and Kundu CN: Lycopene synergistically
enhances quinacrine action to inhibit Wnt-TCF signaling in breast
cancer cells through APC. Carcinogenesis. 34:277–286. 2013.
View Article : Google Scholar
|
|
167
|
Park S and Chun S: Streptonigrin inhibits
β-catenin/Tcf signaling and shows cytotoxicity in
β-catenin-activated cells. Biochim Biophys Acta. 1810:1340–1345.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
168
|
Emami KH, Nguyen C, Ma H, Kim DH, Jeong
KW, Eguchi M, Moon RT, Teo JL, Kim HY, Moon SH, et al: A small
molecule inhibitor of beta-catenin/CREB-binding protein
transcription [corrected]. Proc Natl Acad Sci USA. 101:12682–12687.
2004. View Article : Google Scholar
|
|
169
|
Yu SD, Liu FY and Wang QR: Notch
inhibitor: A promising carcinoma radiosensitizer. Asian Pac J
Cancer Prev. 13:5345–5351. 2012. View Article : Google Scholar
|
|
170
|
Wei W, Chua MS, Grepper S and So SK:
Blockade of Wnt-1 signaling leads to anti-tumor effects in
hepatocellular carcinoma cells. Mol Cancer. 8:762009. View Article : Google Scholar : PubMed/NCBI
|
|
171
|
Mazieres J, You L, He B, Xu Z, Twogood S,
Lee AY, Reguart N, Batra S, Mikami I and Jablons DM: Wnt2 as a new
therapeutic target in malignant pleural mesothelioma. Int J Cancer.
117:326–332. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
172
|
Pode-Shakked N, Harari-Steinberg O,
Haberman-Ziv Y, Rom-Gross E, Bahar S, Omer D, Metsuyanim S, Buzhor
E, Jacob-Hirsch J, Goldstein RS, et al: Resistance or sensitivity
of Wilms' tumor to anti-FZD7 antibody highlights the Wnt pathway as
a possible therapeutic target. Oncogene. 30:1664–1680. 2011.
View Article : Google Scholar : PubMed/NCBI
|