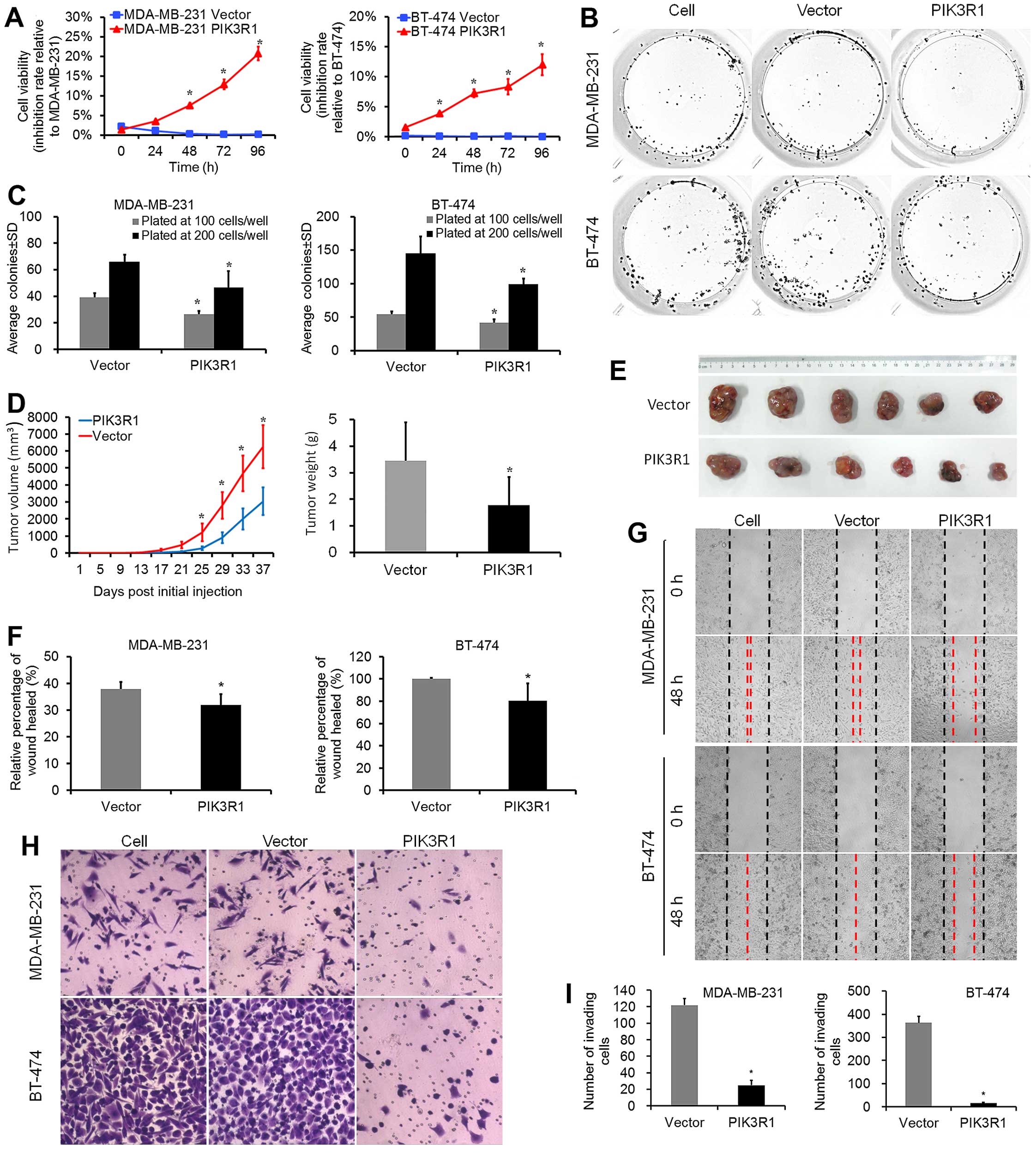
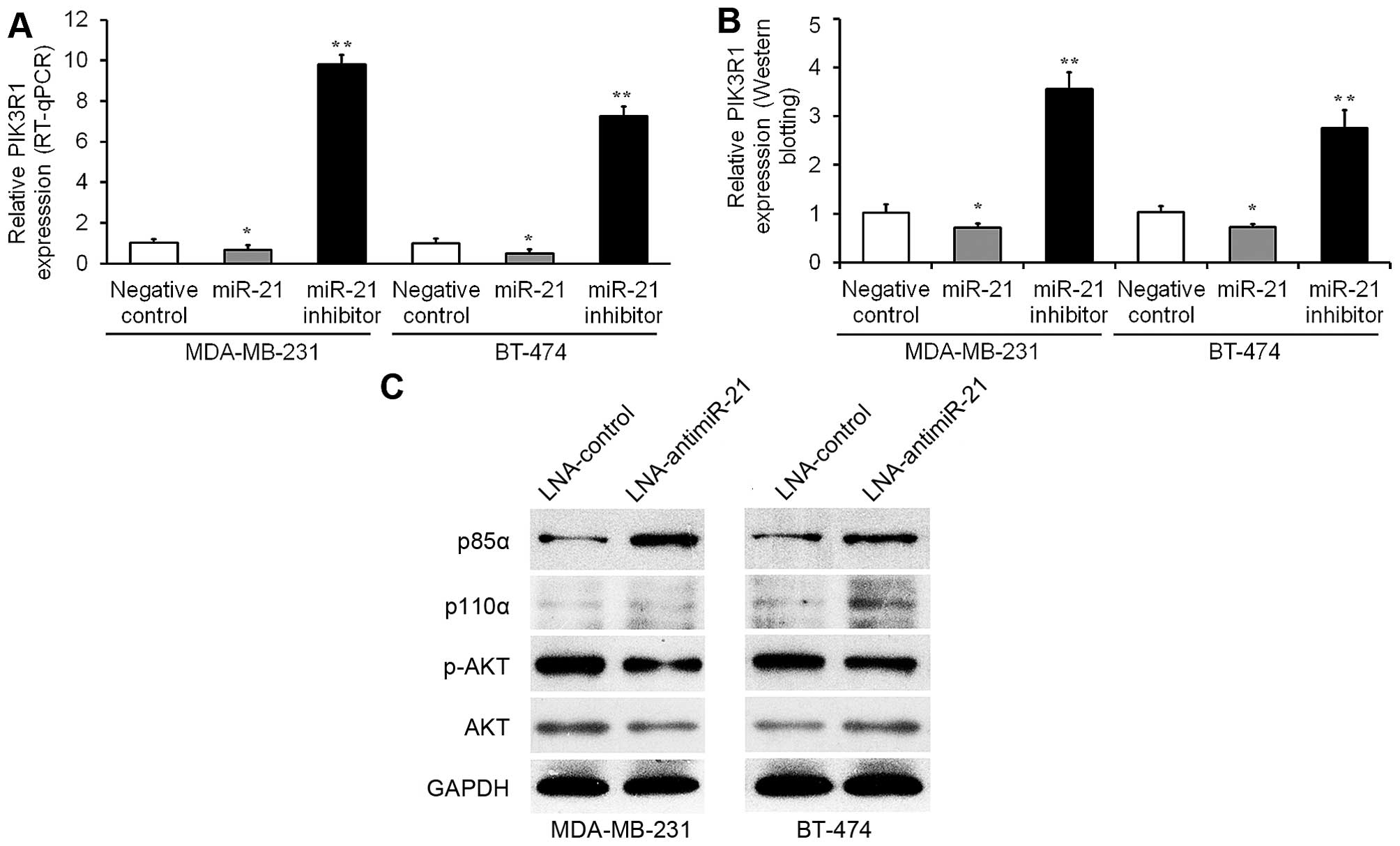
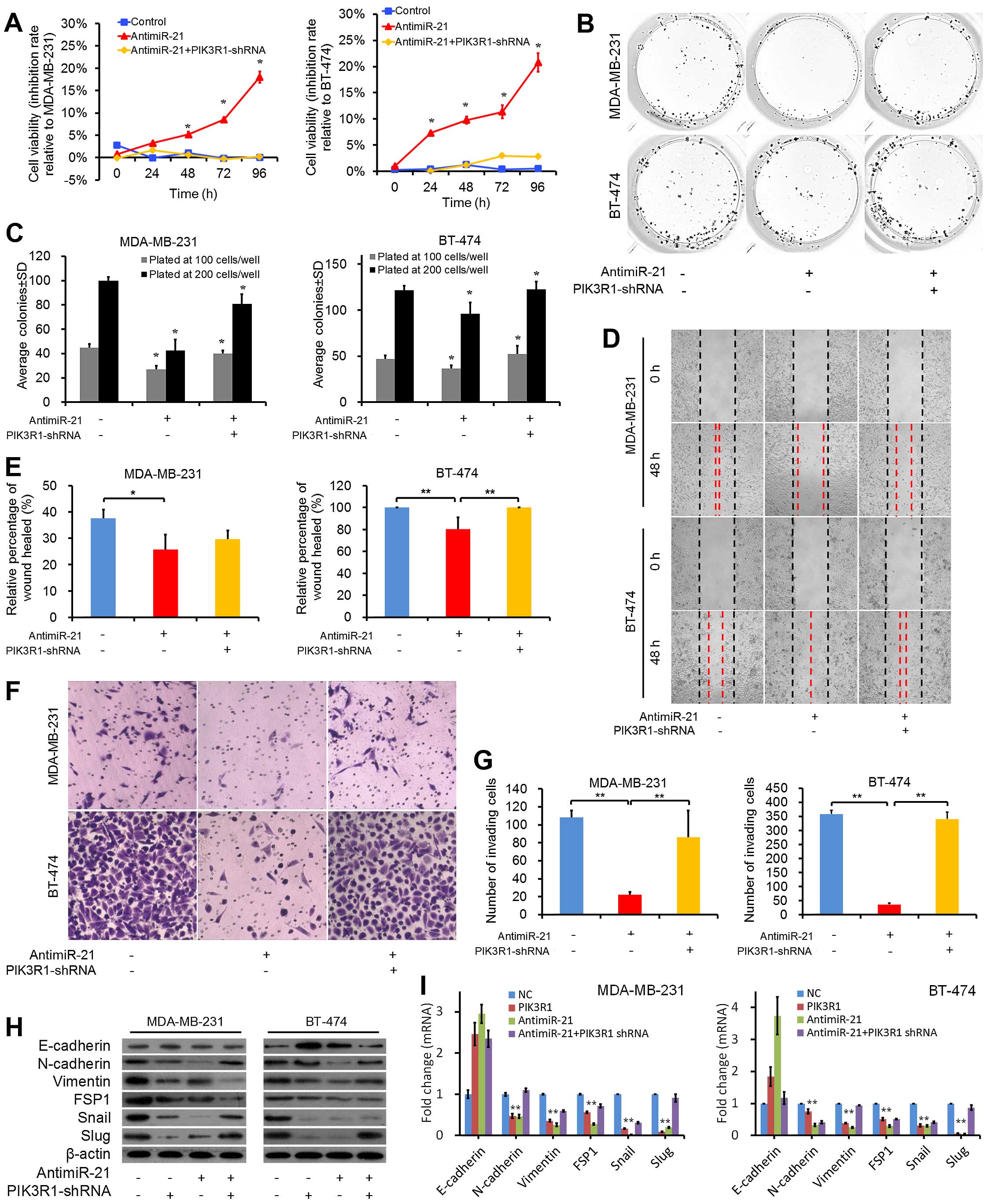
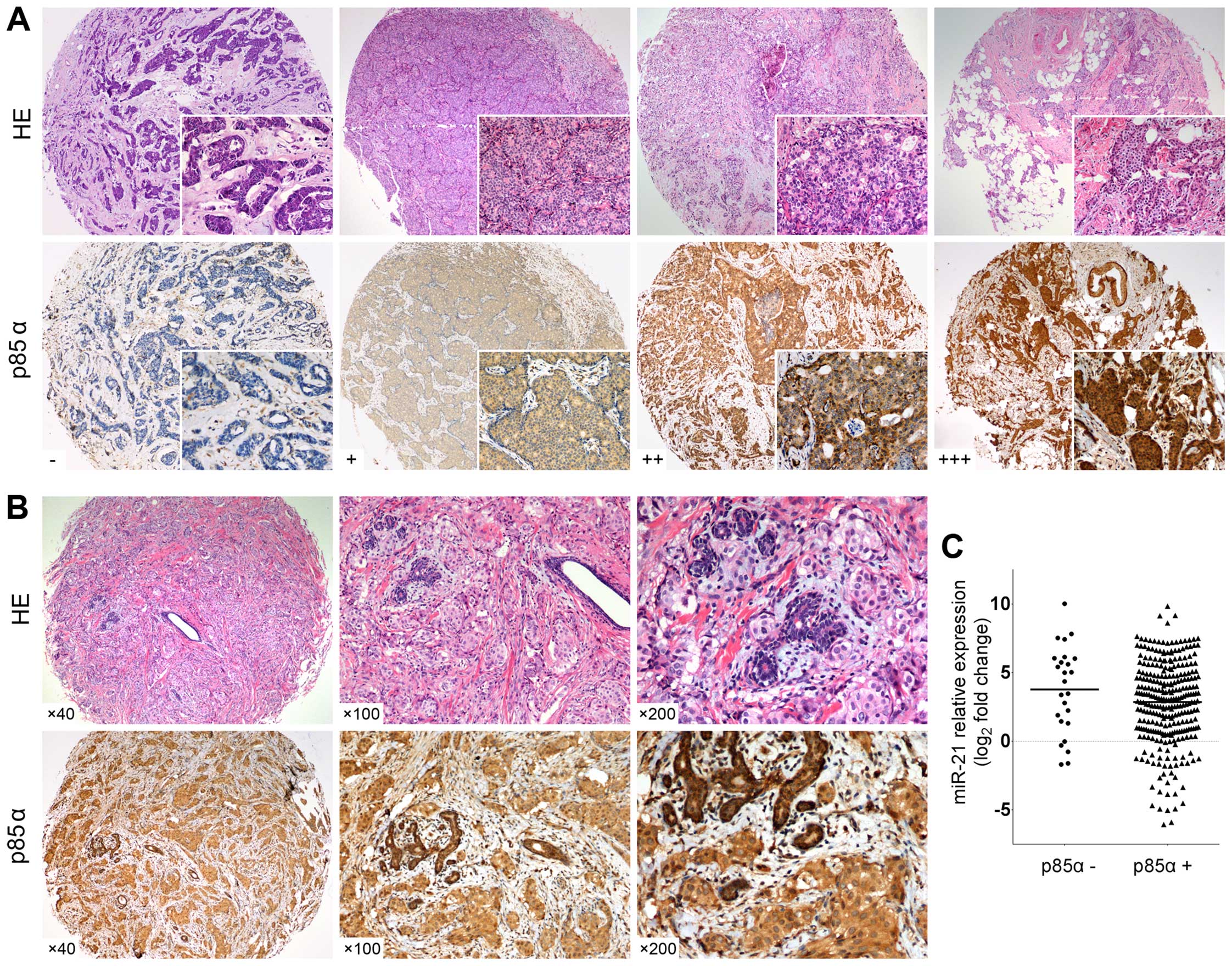
|
1
|
Polyak K: Heterogeneity in breast cancer.
J Clin Invest. 121:3786–3788. 2011. View
Article : Google Scholar : PubMed/NCBI
|
|
2
|
Banerji S, Cibulskis K, Rangel-Escareno C,
Brown KK, Carter SL, Frederick AM, Lawrence MS, Sivachenko AY,
Sougnez C, Zou L, et al: Sequence analysis of mutations and
translocations across breast cancer subtypes. Nature. 486:405–409.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Goldhirsch A, Winer EP, Coates AS, Gelber
RD, Piccart-Gebhart M, Thürlimann B, Senn HJ, Albain KS, Andre F,
Bergh J, et al: Panel members: Personalizing the treatment of women
with early breast cancer: Highlights of the St Gallen International
Expert Consensus on the Primary Therapy of Early Breast Cancer
2013. Ann Oncol. 24:2206–2223. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Elkabets M, Vora S, Juric D, Morse N,
Mino-Kenudson M, Muranen T, Tao J, Campos AB, Rodon J, Ibrahim YH,
et al: mTORC1 inhibition is required for sensitivity to PI3K p110α
inhibitors in PIK3CA-mutant breast cancer. Sci Transl Med.
5:196ra992013. View Article : Google Scholar
|
|
5
|
Myhre S, Lingjærde OC, Hennessy BT, Aure
MR, Carey MS, Alsner J, Tramm T, Overgaard J, Mills GB,
Børresen-Dale AL, et al: Influence of DNA copy number and mRNA
levels on the expression of breast cancer related proteins. Mol
Oncol. 7:704–718. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Yuan TL and Cantley LC: PI3K pathway
alterations in cancer: Variations on a theme. Oncogene.
27:5497–5510. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Uchino M, Kojima H, Wada K, Imada M, Onoda
F, Satofuka H, Utsugi T and Murakami Y: Nuclear beta-catenin and
CD44 upregulation characterize invasive cell populations in
non-aggressive MCF-7 breast cancer cells. BMC Cancer. 10:4142010.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Engelman JA: Targeting PI3K signalling in
cancer: Opportunities, challenges and limitations. Nat Rev Cancer.
9:550–562. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Hennessy BT, Smith DL, Ram PT, Lu Y and
Mills GB: Exploiting the PI3K/AKT pathway for cancer drug
discovery. Nat Rev Drug Discov. 4:988–1004. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Zhao L and Vogt PK: Class I PI3K in
oncogenic cellular transformation. Oncogene. 27:5486–5496. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Juric D, Castel P, Griffith M, Griffith
OL, Won HH, Ellis H, Ebbesen SH, Ainscough BJ, Ramu A, Iyer G, et
al: Convergent loss of PTEN leads to clinical resistance to a
PI(3)Kα inhibitor. Nature. 518:240–244. 2015. View Article : Google Scholar :
|
|
12
|
Brachmann SM, Ueki K, Engelman JA, Kahn RC
and Cantley LC: Phosphoinositide 3-kinase catalytic subunit
deletion and regulatory subunit deletion have opposite effects on
insulin sensitivity in mice. Mol Cell Biol. 25:1596–1607. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Zhao L and Vogt PK: Helical domain and
kinase domain mutations in p110alpha of phosphatidylinositol
3-kinase induce gain of function by different mechanisms. Proc Natl
Acad Sci USA. 105:2652–2657. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Araújo TG, Paiva CE, Rocha RM, Maia YC,
Sena AA, Ueira-Vieira C, Carneiro AP, Almeida JF, de Faria PR,
Santos DW, et al: A novel highly reactive Fab antibody for breast
cancer tissue diagnostics and staging also discriminates a subset
of good prognostic triple-negative breast cancers. Cancer Lett.
343:275–285. 2014. View Article : Google Scholar
|
|
15
|
Cizkova M, Vacher S, Meseure D, Trassard
M, Susini A, Mlcuchova D, Callens C, Rouleau E, Spyratos F,
Lidereau R, et al: PIK3R1 underexpression is an independent
prognostic marker in breast cancer. BMC Cancer. 13:5452013.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Visani M, de Biase D, Marucci G, Cerasoli
S, Nigrisoli E, Bacchi Reggiani ML, Albani F, Baruzzi A and Pession
A: PERNO study group: Expression of 19 microRNAs in glioblastoma
and comparison with other brain neoplasia of grades I–III. Mol
Oncol. 8:417–430. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Jamali Z, Asl Aminabadi N, Attaran R,
Pournagiazar F, Ghertasi Oskouei S and Ahmadpour F: MicroRNAs as
prognostic molecular signatures in human head and neck squamous
cell carcinoma: A systematic review and meta-analysis. Oral Oncol.
51:321–331. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Zhang F, Yang Z, Cao M, Xu Y, Li J, Chen
X, Gao Z, Xin J, Zhou S, Zhou Z, et al: MiR-203 suppresses tumor
growth and invasion and down-regulates MiR-21 expression through
repressing Ran in esophageal cancer. Cancer Lett. 342:121–129.
2014. View Article : Google Scholar
|
|
19
|
Abue M, Yokoyama M, Shibuya R, Tamai K,
Yamaguchi K, Sato I, Tanaka N, Hamada S, Shimosegawa T, Sugamura K,
et al: Circulating miR-483-3p and miR-21 is highly expressed in
plasma of pancreatic cancer. Int J Oncol. 46:539–547. 2015.
|
|
20
|
Shi Z, Zhang J, Qian X, Han L, Zhang K,
Chen L, Liu J, Ren Y, Yang M, Zhang A, et al: AC1MMYR2, an
inhibitor of dicer-mediated biogenesis of Oncomir miR-21, reverses
epithelial-mesenchymal transition and suppresses tumor growth and
progression. Cancer Res. 73:5519–5531. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Li LQ, Li XL, Wang L, Du WJ, Guo R, Liang
HH, Liu X, Liang DS, Lu YJ, Shan HL, et al: Matrine inhibits breast
cancer growth via miR-21/PTEN/Akt pathway in MCF-7 cells. Cell
Physiol Biochem. 30:631–641. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Baffa R, Fassan M, Volinia S, O'Hara B,
Liu CG, Palazzo JP, Gardiman M, Rugge M, Gomella LG, Croce CM, et
al: MicroRNA expression profiling of human metastatic cancers
identifies cancer gene targets. J Pathol. 219:214–221. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Yan LX, Huang XF, Shao Q, Huang MY, Deng
L, Wu QL, Zeng YX and Shao JY: MicroRNA miR-21 overexpression in
human breast cancer is associated with advanced clinical stage,
lymph node metastasis and patient poor prognosis. RNA.
14:2348–2360. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Yan LX, Wu QN, Zhang Y, Li YY, Liao DZ,
Hou JH, Fu J, Zeng MS, Yun JP, Wu QL, et al: Knockdown of miR-21 in
human breast cancer cell lines inhibits proliferation, in vitro
migration and in vivo tumor growth. Breast Cancer Res. 13:R22011.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Gramantieri L, Ferracin M, Fornari F,
Veronese A, Sabbioni S, Liu CG, Calin GA, Giovannini C, Ferrazzi E,
Grazi GL, et al: Cyclin G1 is a target of miR-122a, a microRNA
frequently down-regulated in human hepatocellular carcinoma. Cancer
Res. 67:6092–6099. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Gao L, Song JR, Zhang JW, Zhao X, Zhao QD,
Sun K, Deng WJ, Li R, Lv G, Cheng HY, et al: Chloroquine promotes
the anti-cancer effect of TACE in a rabbit VX2 liver tumor model.
Int J Biol Sci. 9:322–330. 2013. View Article : Google Scholar
|
|
27
|
Sun L, Li H, Chen J, Dehennaut V, Zhao Y,
Yang Y, Iwasaki Y, Kahn-Perles B, Leprince D, Chen Q, et al: A
SUMOylation-dependent pathway regulates SIRT1 transcription and
lung cancer metastasis. J Natl Cancer Inst. 105:887–898. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
De Bacco F, Luraghi P, Medico E, Reato G,
Girolami F, Perera T, Gabriele P, Comoglio PM and Boccaccio C:
Induction of MET by ionizing radiation and its role in
radioresistance and invasive growth of cancer. J Natl Cancer Inst.
103:645–661. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Sunil RL, Lan OE, Stuart JS, Puay HT and
Marc JV: WHO Classification of Tumours. Fred TB, Elain SJ, Sunil RL
and Hiroko O: IARC Press; Lyon: pp. 2402012
|
|
30
|
Taniguchi CM, Winnay J, Kondo T, Bronson
RT, Guimaraes AR, Alemán JO, Luo J, Stephanopoulos G, Weissleder R,
Cantley LC, et al: The phosphoinositide 3-kinase regulatory subunit
p85alpha can exert tumor suppressor properties through negative
regulation of growth factor signaling. Cancer Res. 70:5305–5315.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Richardson AL, Wang ZC, De Nicolo A, Lu X,
Brown M, Miron A, Liao X, Iglehart JD, Livingston DM and Ganesan S:
X chromosomal abnormalities in basal-like human breast cancer.
Cancer Cell. 9:121–132. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Toste PA, Li L, Kadera BE, Nguyen AH, Tran
LM, Wu N, Madnick DL, Patel SG, Dawson DW and Donahue TR: p85α is a
microRNA target and affects chemosensitivity in pancreatic cancer.
J Surg Res. 196:285–293. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Shekar SC, Wu H, Fu Z, Yip SC, Nagajyothi,
Cahill SM, Girvin ME and Backer JM: Mechanism of constitutive
phos-phoinositide 3-kinase activation by oncogenic mutants of the
p85 regulatory subunit. J Biol Chem. 280:27850–27855. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Luo J and Cantley LC: The negative
regulation of phosphoinositide 3-kinase signaling by p85 and it's
implication in cancer. Cell Cycle. 4:1309–1312. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Rabinovsky R, Pochanard P, McNear C,
Brachmann SM, Duke-Cohan JS, Garraway LA and Sellers WR: p85
associates with unphosphorylated PTEN and the PTEN-associated
complex. Mol Cell Biol. 29:5377–5388. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Jaiswal BS, Janakiraman V, Kljavin NM,
Chaudhuri S, Stern HM, Wang W, Kan Z, Dbouk HA, Peters BA, Waring
P, et al: Somatic mutations in p85alpha promote tumorigenesis
through class IA PI3K activation. Cancer Cell. 16:463–474. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Cheung LW, Hennessy BT, Li J, Yu S, Myers
AP, Djordjevic B, Lu Y, Stemke-Hale K, Dyer MD, Zhang F, et al:
High frequency of PIK3R1 and PIK3R2 mutations in endometrial cancer
elucidates a novel mechanism for regulation of PTEN protein
stability. Cancer Discov. 1:170–185. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Darido C, Georgy SR, Wilanowski T, Dworkin
S, Auden A, Zhao Q, Rank G, Srivastava S, Finlay MJ, Papenfuss AT,
et al: Targeting of the tumor suppressor GRHL3 by a
miR-21-dependent proto-oncogenic network results in PTEN loss and
tumorigenesis. Cancer Cell. 20:635–648. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Xiong B, Cheng Y, Ma L and Zhang C: MiR-21
regulates biological behavior through the PTEN/PI-3 K/Akt signaling
pathway in human colorectal cancer cells. Int J Oncol. 42:219–228.
2013.
|
|
40
|
Badwe R, Hawaldar R, Parmar V, Nadkarni M,
Shet T, Desai S, Gupta S, Jalali R, Vanmali V, Dikshit R, et al:
Single-injection depot progesterone before surgery and survival in
women with operable breast cancer: A randomized controlled trial. J
Clin Oncol. 29:2845–2851. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Uji K, Naoi Y, Kagara N, Shimoda M,
Shimomura A, Maruyama N, Shimazu K, Kim SJ and Noguchi S:
Significance of TP53 mutations determined by next-generation ‘deep’
sequencing in prognosis of estrogen receptor-positive breast
cancer. Cancer Lett. 342:19–26. 2014. View Article : Google Scholar
|
|
42
|
Müller V, Gade S, Steinbach B, Loibl S,
von Minckwitz G, Untch M, Schwedler K, Lübbe K, Schem C, Fasching
PA, et al: Changes in serum levels of miR-21, miR-210, and miR-373
in HER2-positive breast cancer patients undergoing neoadjuvant
therapy: A translational research project within the Geparquinto
trial. Breast Cancer Res Treat. 147:61–68. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Markou A, Yousef GM, Stathopoulos E,
Georgoulias V and Lianidou E: Prognostic significance of
metastasis-related microRNAs in early breast cancer patients with a
long follow-up. Clin Chem. 60:197–205. 2014. View Article : Google Scholar
|
|
44
|
Lee JA, Lee HY, Lee ES, Kim I and Bae JW:
Prognostic implications of MicroRNA-21 overexpression in invasive
ductal carcinomas of the breast. J Breast Cancer. 14:269–275. 2011.
View Article : Google Scholar
|
|
45
|
Donahue TR, Tran LM, Hill R, Li Y,
Kovochich A, Calvopina JH, Patel SG, Wu N, Hindoyan A, Farrell JJ,
et al: Integrative survival-based molecular profiling of human
pancreatic cancer. Clin Cancer Res. 18:1352–1363. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Fransson S, Abel F, Kogner P, Martinsson T
and Ejeskär K: Stage-dependent expression of PI3K/Akt-pathway genes
in neuroblastoma. Int J Oncol. 42:609–616. 2013.
|
|
47
|
Lu Y, Lemon W, Liu PY, Yi Y, Morrison C,
Yang P, Sun Z, Szoke J, Gerald WL, Watson M, et al: A gene
expression signature predicts survival of patients with stage I
non-small cell lung cancer. PLoS Med. 3:e4672006. View Article : Google Scholar : PubMed/NCBI
|