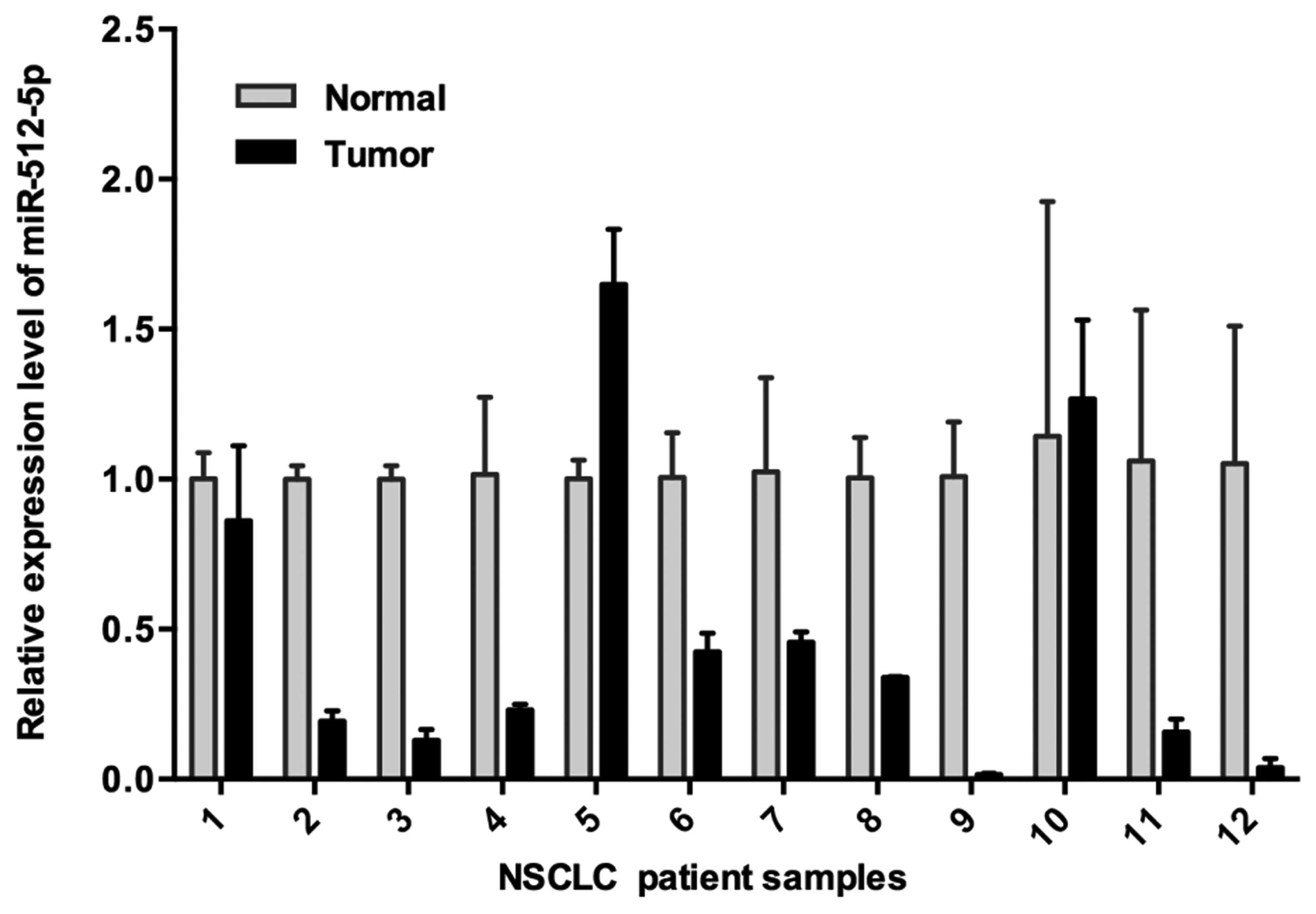
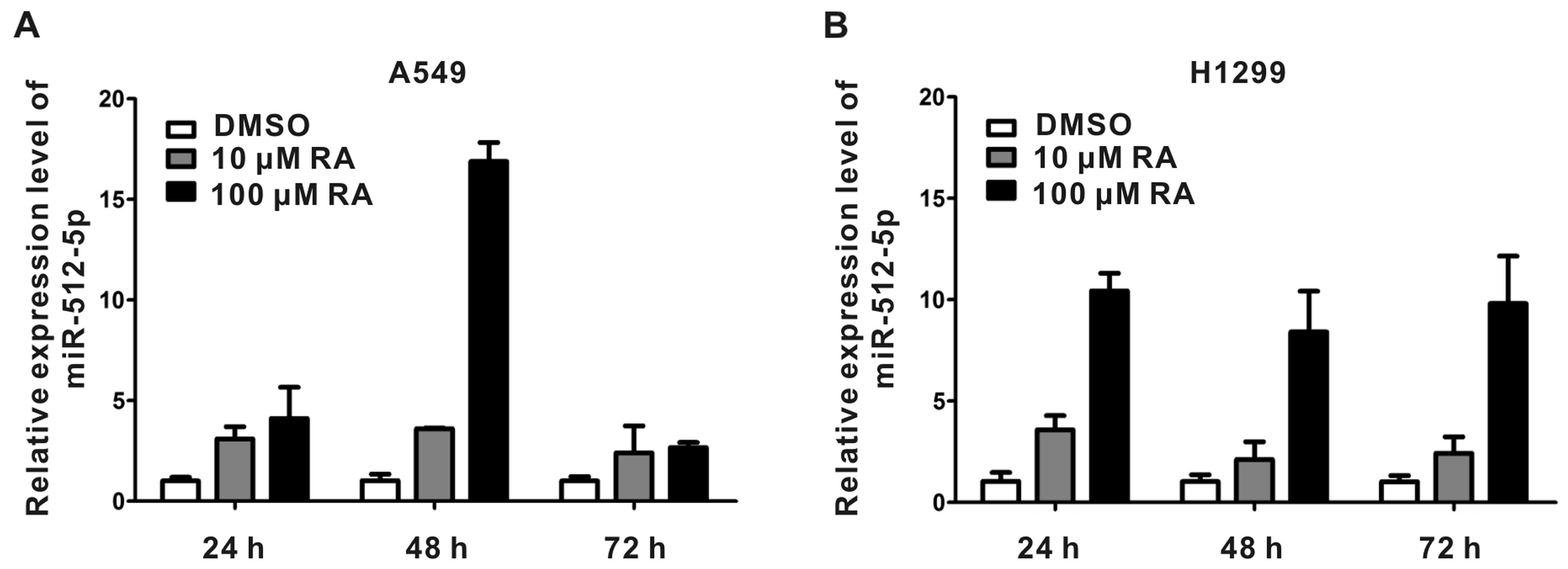
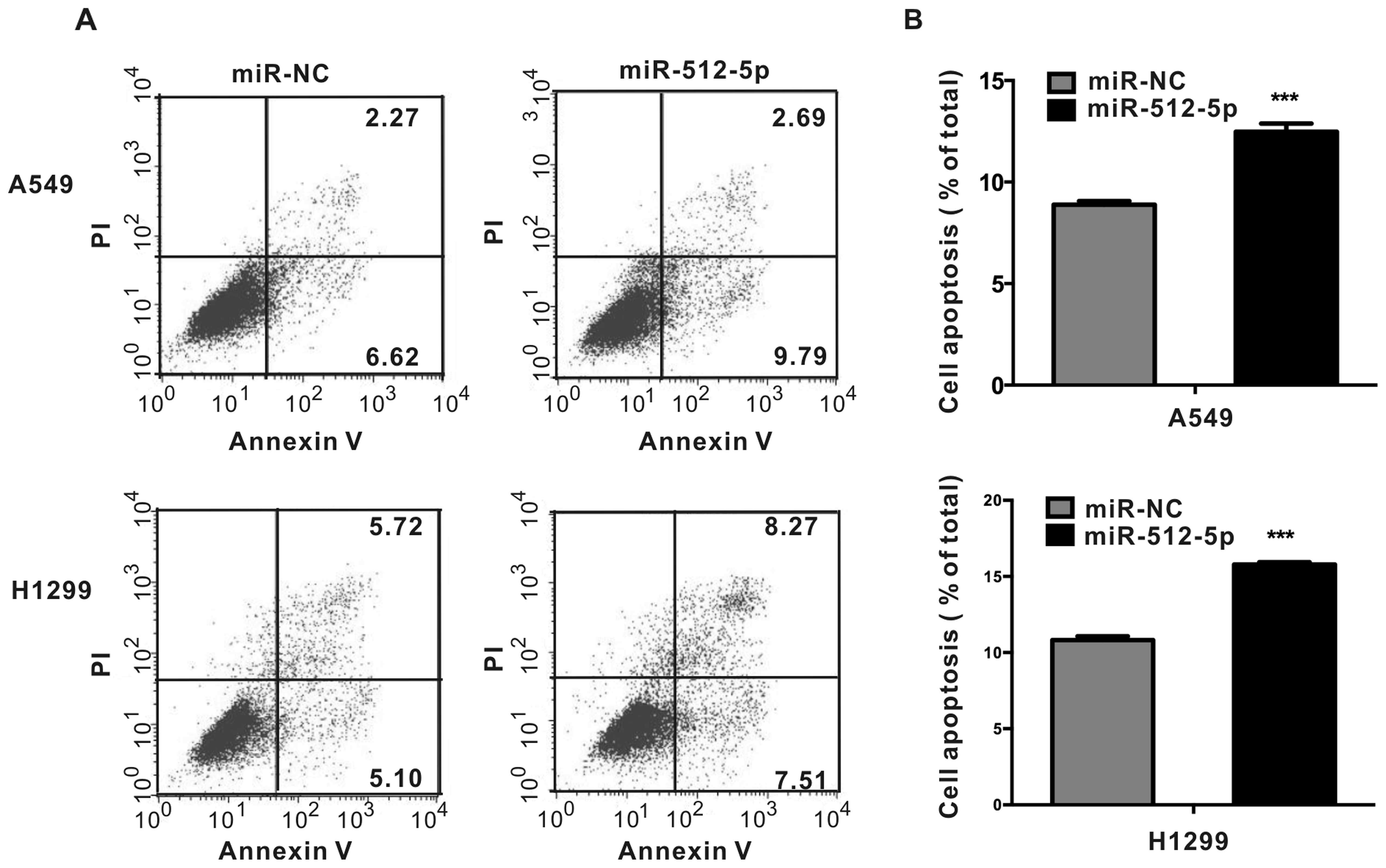
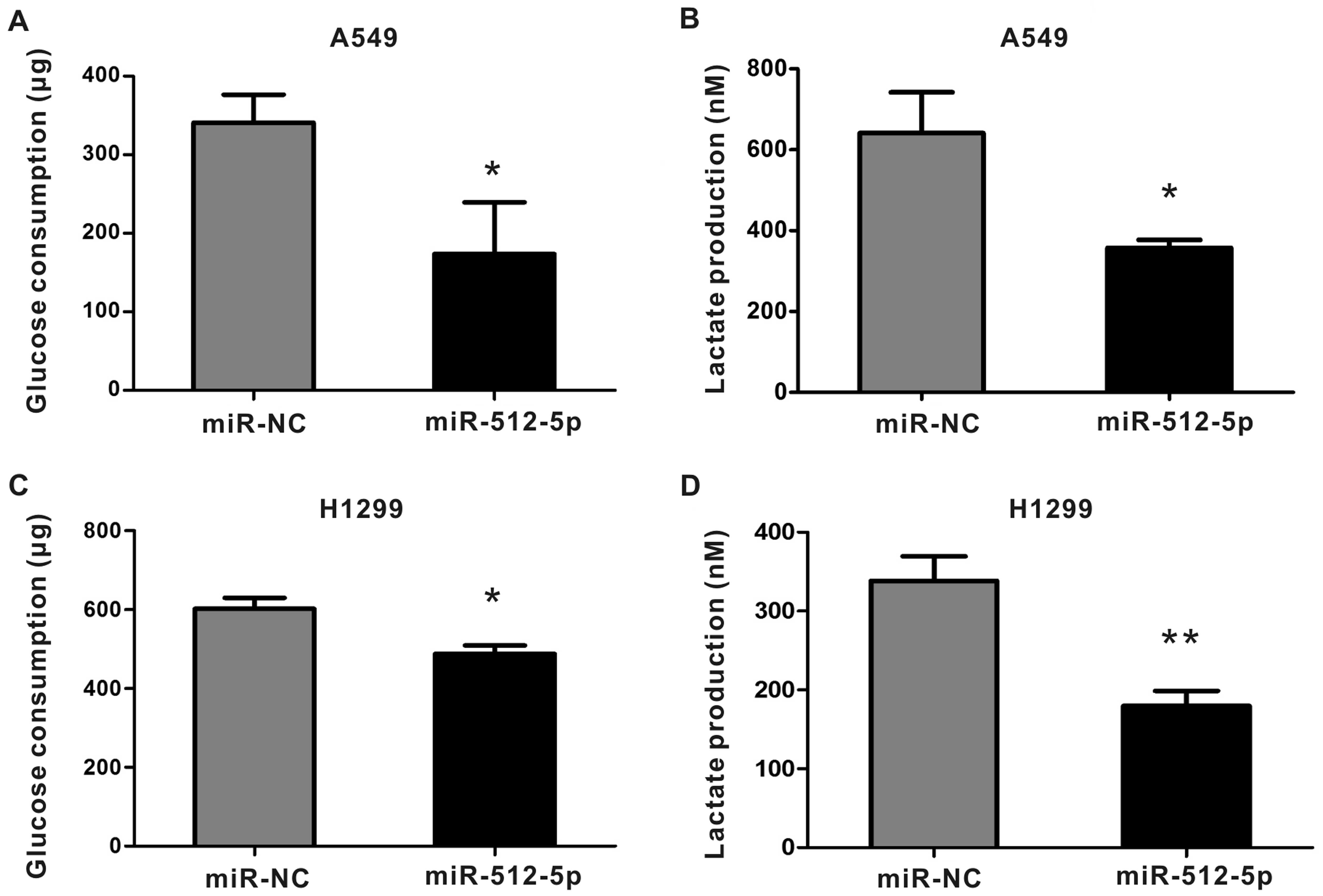
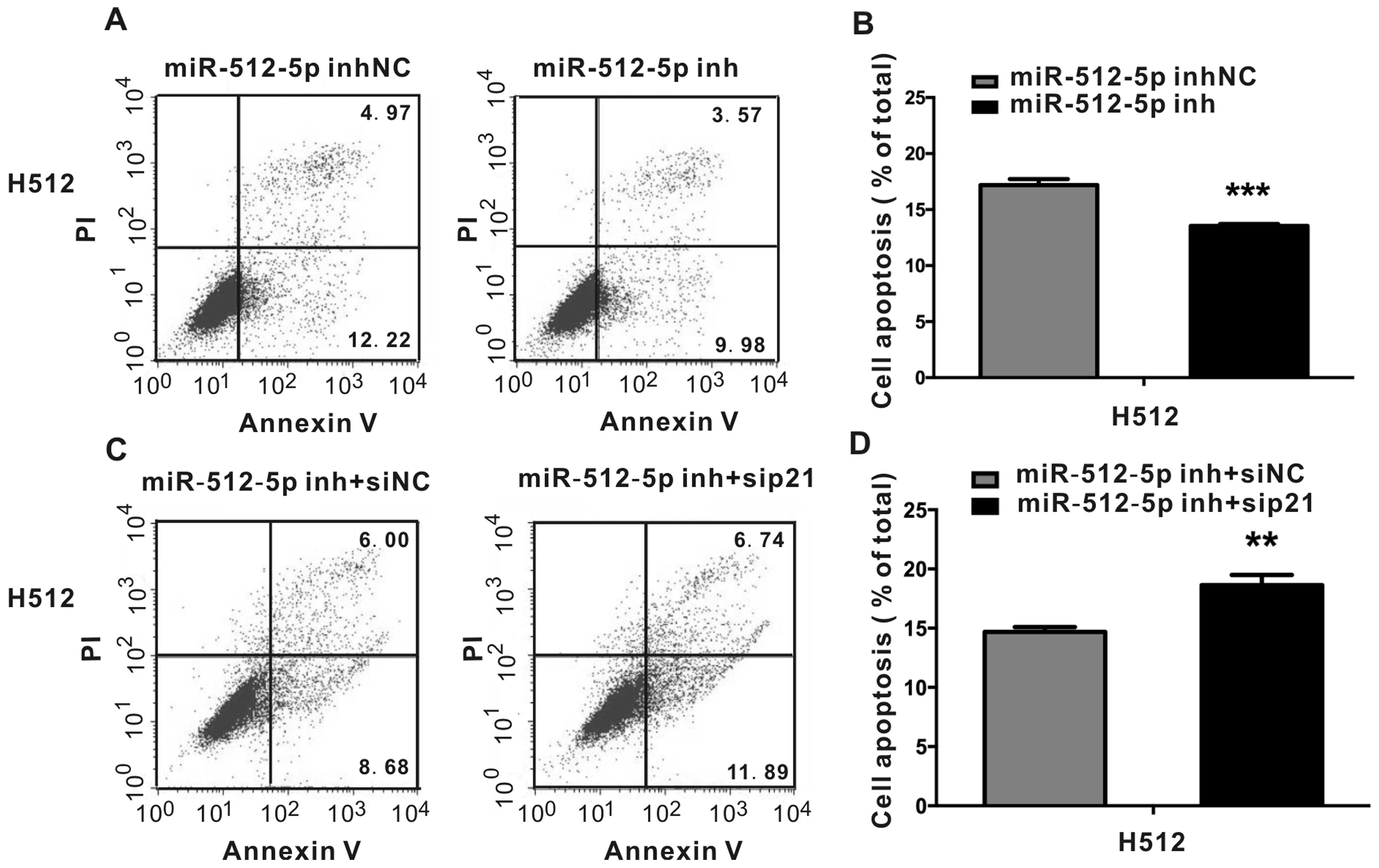
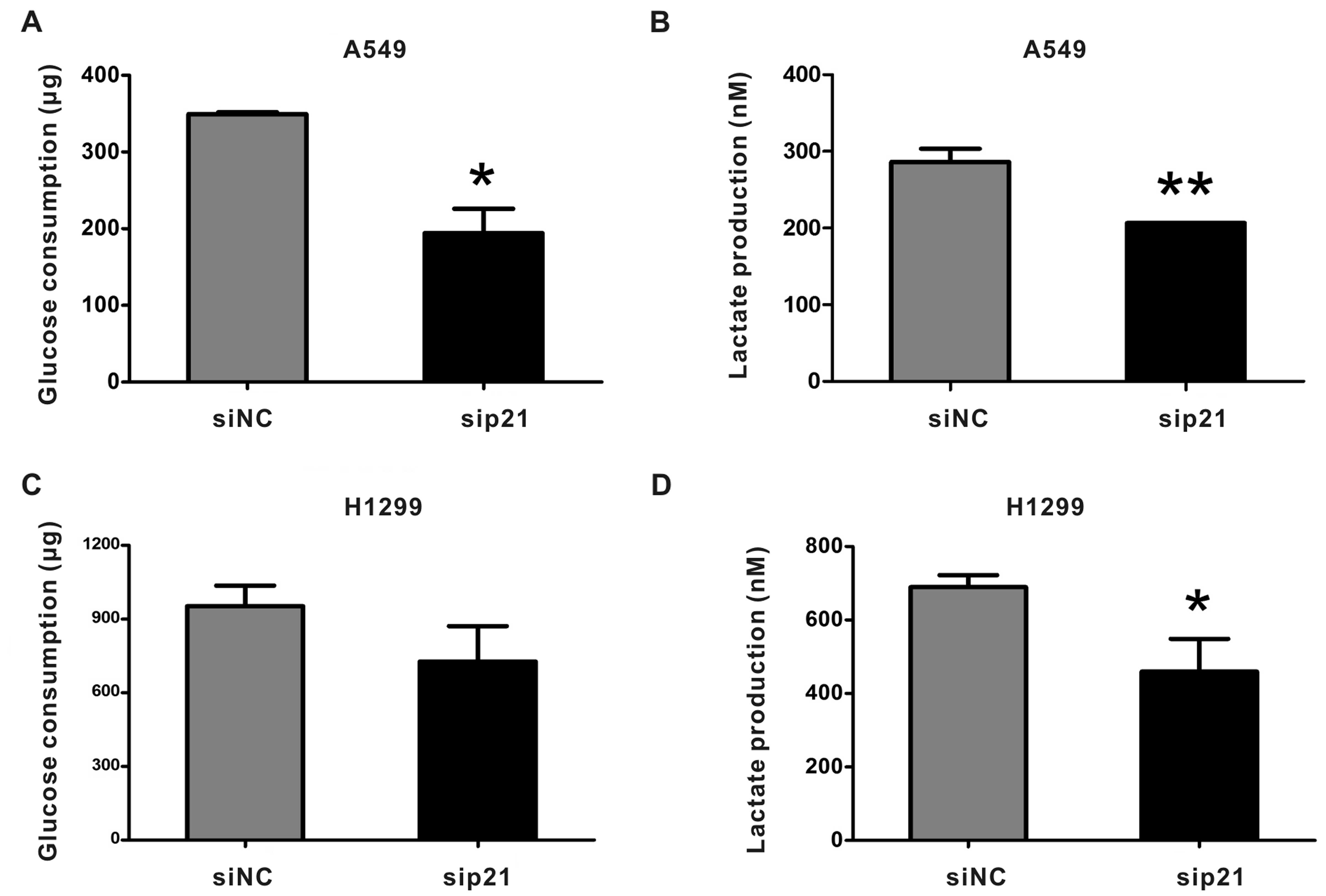
|
1
|
Torre LA, Bray F, Siegel RL, Ferlay J,
Lortet-Tieulent J and Jemal A: Global cancer statistics, 2012. CA
Cancer J Clin. 65:87–108. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Li J, Bi L, Sun Y, Lu Z, Lin Y, Bai O and
Shao H: Text mining and network analysis of molecular interaction
in non-small cell lung cancer by using natural language processing.
Mol Biol Rep. 41:8071–8079. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Joshi P, Jeon YJ, Laganà A, Middleton J,
Secchiero P, Garofalo M and Croce CM: MicroRNA-148a reduces
tumorigenesis and increases TRAIL-induced apoptosis in NSCLC. Proc
Natl Acad Sci USA. 112:8650–8655. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Li Y, Zhang D, Chen C, Ruan Z, Li Y and
Huang Y: MicroRNA-212 displays tumor-promoting properties in
non-small cell lung cancer cells and targets the hedgehog pathway
receptor PTCH1. Mol Biol Cell. 23:1423–1434. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Mendell JT: miRiad roles for the miR-17-92
cluster in development and disease. Cell. 133:217–222. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Johnson SM, Grosshans H, Shingara J, Byrom
M, Jarvis R, Cheng A, Labourier E, Reinert KL, Brown D and Slack
FJ: RAS is regulated by the let-7 microRNA family. Cell.
120:635–647. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Loo JM, Scherl A, Nguyen A, Man FY,
Weinberg E, Zeng Z, Saltz L, Paty PB and Tavazoie SF: Extracellular
metabolic energetics can promote cancer progression. Cell.
160:393–406. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Okazawa H, Shimizu J, Kamei M, Imafuku I,
Hamada H and Kanazawa I: Bcl-2 inhibits retinoic acid-induced
apoptosis during the neural differentiation of embryonal stem
cells. J Cell Biol. 132:955–968. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Burnett A, Wetzler M and Löwenberg B:
Therapeutic advances in acute myeloid leukemia. J Clin Oncol.
29:487–494. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Fisher JN, Terao M, Fratelli M, Kurosaki
M, Paroni G, Zanetti A, Gianni M, Bolis M, Lupi M, Tsykin A, et al:
MicroRNA networks regulated by all-trans retinoic acid and
Lapatinib control the growth, survival and motility of breast
cancer cells. Oncotarget. 6:13176–13200. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Khan S, Wall D, Curran C, Newell J, Kerin
MJ and Dwyer RM: MicroRNA-10a is reduced in breast cancer and
regulated in part through retinoic acid. BMC Cancer. 15:3452015.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Lichner Z, Páll E, Kerekes A, Pállinger E,
Maraghechi P, Bosze Z and Gócza E: The miR-290–295 cluster promotes
pluripotency maintenance by regulating cell cycle phase
distribution in mouse embryonic stem cells. Differentiation.
81:11–24. 2011. View Article : Google Scholar
|
|
13
|
Cheung TH, Man KN, Yu MY, Yim SF, Siu NS,
Lo KW, Doran G, Wong RR, Wang VW, Smith DI, et al: Dysregulated
microRNAs in the pathogenesis and progression of cervical neoplasm.
Cell Cycle. 11:2876–2884. 2012. View
Article : Google Scholar : PubMed/NCBI
|
|
14
|
Saito Y, Suzuki H, Tsugawa H, Nakagawa I,
Matsuzaki J, Kanai Y and Hibi T: Chromatin remodeling at Alu
repeats by epigenetic treatment activates silenced microRNA-512-5p
with down-regulation of Mcl-1 in human gastric cancer cells.
Oncogene. 28:2738–2744. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Sherr CJ and Roberts JM: CDK inhibitors:
Positive and negative regulators of G1-phase progression. Genes
Dev. 13:1501–1512. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Deng C, Zhang P, Harper JW, Elledge SJ and
Leder P: Mice lacking p21CIP1/WAF1 undergo normal development, but
are defective in G1 checkpoint control. Cell. 82:675–684. 1995.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Harper JW, Adami GR, Wei N, Keyomarsi K
and Elledge SJ: The p21 Cdk-interacting protein Cip1 is a potent
inhibitor of G1 cyclin-dependent kinases. Cell. 75:805–816. 1993.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Abbas T and Dutta A: p21 in cancer:
Intricate networks and multiple activities. Nat Rev Cancer.
9:400–414. 2009. View
Article : Google Scholar : PubMed/NCBI
|
|
19
|
Zhu X, Gao G, Chu K, Yang X, Ren S, Li Y,
Wu H, Huang Y and Zhou C: Inhibition of RAC1-GEF DOCK3 by
miR-512-3p contributes to suppression of metastasis in non-small
cell lung cancer. Int J Biochem Cell Biol. 61:103–114. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Vander Heiden MG, Cantley LC and Thompson
CB: Understanding the Warburg effect: The metabolic requirements of
cell proliferation. Science. 324:1029–1033. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Chen J and Thompson LU: Lignans and
tamoxifen, alone or in combination, reduce human breast cancer cell
adhesion, invasion and migration in vitro. Breast Cancer Res Treat.
80:163–170. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Zhang J, Attar E, Cohen K, Crumpacker C
and Scadden D: Silencing p21(Waf1/Cip1/Sdi1) expression increases
gene transduction efficiency in primitive human hematopoietic
cells. Gene Ther. 12:1444–1452. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Rao A, Coan A, Welsh JE, Barclay WW,
Koumenis C and Cramer SD: Vitamin D receptor and p21/WAF1 are
targets of genistein and 1,25-dihydroxyvitamin D3 in human prostate
cancer cells. Cancer Res. 64:2143–2147. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Adi Harel S, Bossel Ben-Moshe N, Aylon Y,
Bublik DR, Moskovits N, Toperoff G, Azaiza D, Biagoni F, Fuchs G,
Wilder S, et al: Reactivation of epigenetically silenced miR-512
and miR-373 sensitizes lung cancer cells to cisplatin and restricts
tumor growth. Cell Death Differ. 22:1328–1340. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
di Masi A, Leboffe L, De Marinis E, Pagano
F, Cicconi L, Rochette-Egly C, Lo-Coco F, Ascenzi P and Nervi C:
Retinoic acid receptors: From molecular mechanisms to cancer
therapy. Mol Aspects Med. 41:1–115. 2015. View Article : Google Scholar
|
|
26
|
Chen F, Cao Y, Qian J, Shao F,
Niederreither K and Cardoso WV: A retinoic acid-dependent network
in the foregut controls formation of the mouse lung primordium. J
Clin Invest. 120:2040–2048. 2010. View
Article : Google Scholar : PubMed/NCBI
|
|
27
|
Jones RG and Thompson CB: Tumor
suppressors and cell metabolism: A recipe for cancer growth. Genes
Dev. 23:537–548. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Muñoz-Pinedo C, El Mjiyad N and Ricci JE:
Cancer metabolism: Current perspectives and future directions. Cell
Death Dis. 3:e2482012. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
El Mjiyad N, Caro-Maldonado A,
Ramírez-Peinado S and Muñoz-Pinedo C: Sugar-free approaches to
cancer cell killing. Oncogene. 30:253–264. 2011. View Article : Google Scholar
|
|
30
|
Raina K, Agarwal C, Wadhwa R, Serkova NJ
and Agarwal R: Energy deprivation by silibinin in colorectal cancer
cells: A double-edged sword targeting both apoptotic and autophagic
machineries. Autophagy. 9:697–713. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Li R, Waga S, Hannon GJ, Beach D and
Stillman B: Differential effects by the p21 CDK inhibitor on
PCNA-dependent DNA replication and repair. Nature. 371:534–537.
1994. View
Article : Google Scholar : PubMed/NCBI
|
|
32
|
Delavaine L and La Thangue NB: Control of
E2F activity by p21Waf1/Cip1. Oncogene. 18:5381–5392. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Coqueret O and Gascan H: Functional
interaction of STAT3 transcription factor with the cell cycle
inhibitor p21WAF1/CIP1/SDI1. J Biol Chem. 275:18794–18800. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Kitaura H, Shinshi M, Uchikoshi Y, Ono T,
Iguchi-Ariga SM and Ariga H: Reciprocal regulation via
protein-protein interaction between c-Myc and p21(cip1/waf1/sdi1)
in DNA replication and transcription. J Biol Chem. 275:10477–10483.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Roninson IB: Oncogenic functions of tumour
suppressor p21(Waf1/Cip1/Sdi1): Association with cell senescence
and tumour-promoting activities of stromal fibroblasts. Cancer
Lett. 179:1–14. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Dotto GP: p21(WAF1/Cip1): More than a
break to the cell cycle? Biochim Biophys Acta. 1471:M43–M56.
2000.PubMed/NCBI
|
|
37
|
Gartel AL: The conflicting roles of the
cdk inhibitor p21(CIP1/WAF1) in apoptosis. Leuk Res. 29:1237–1238.
2005. View Article : Google Scholar : PubMed/NCBI
|