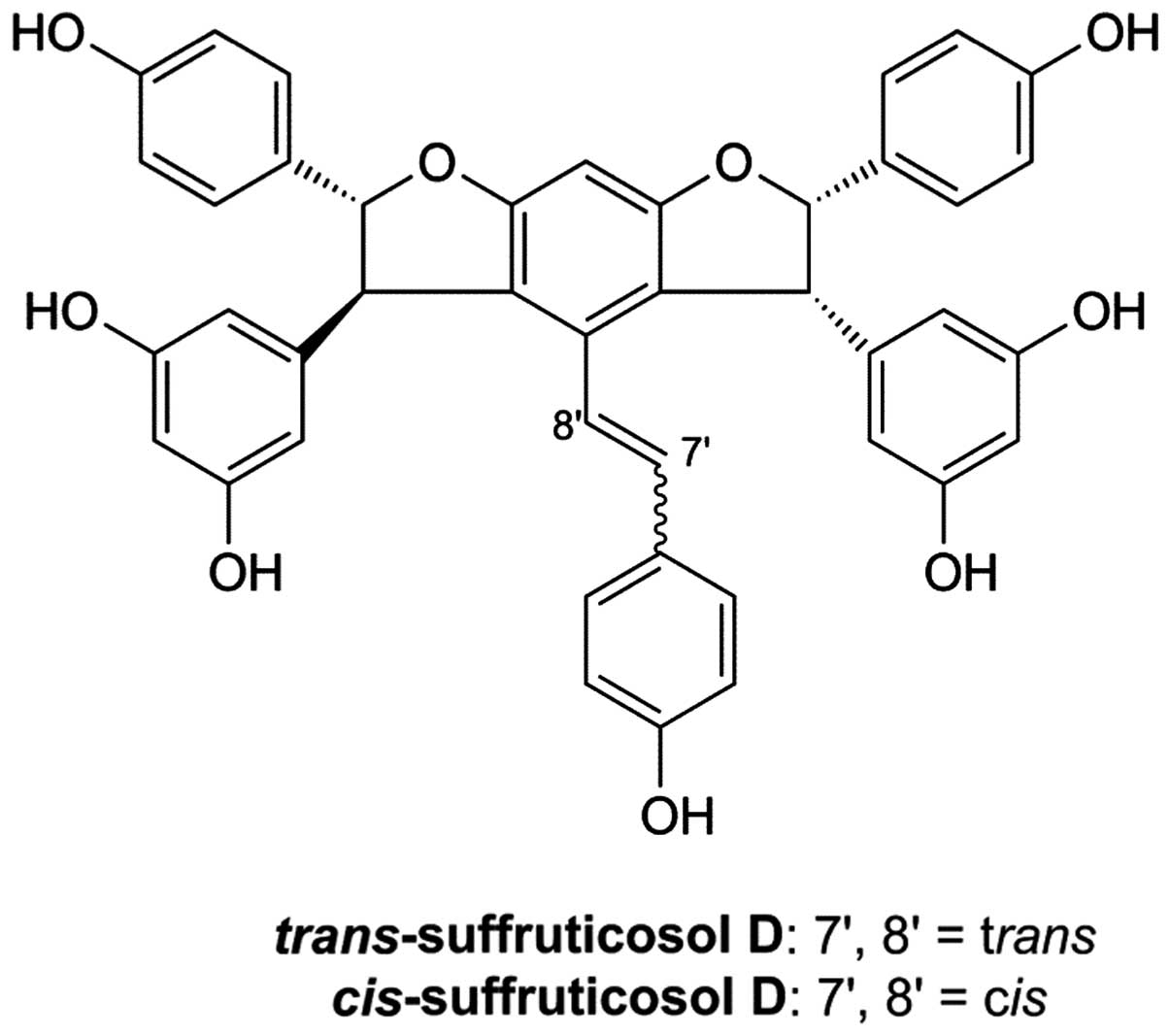
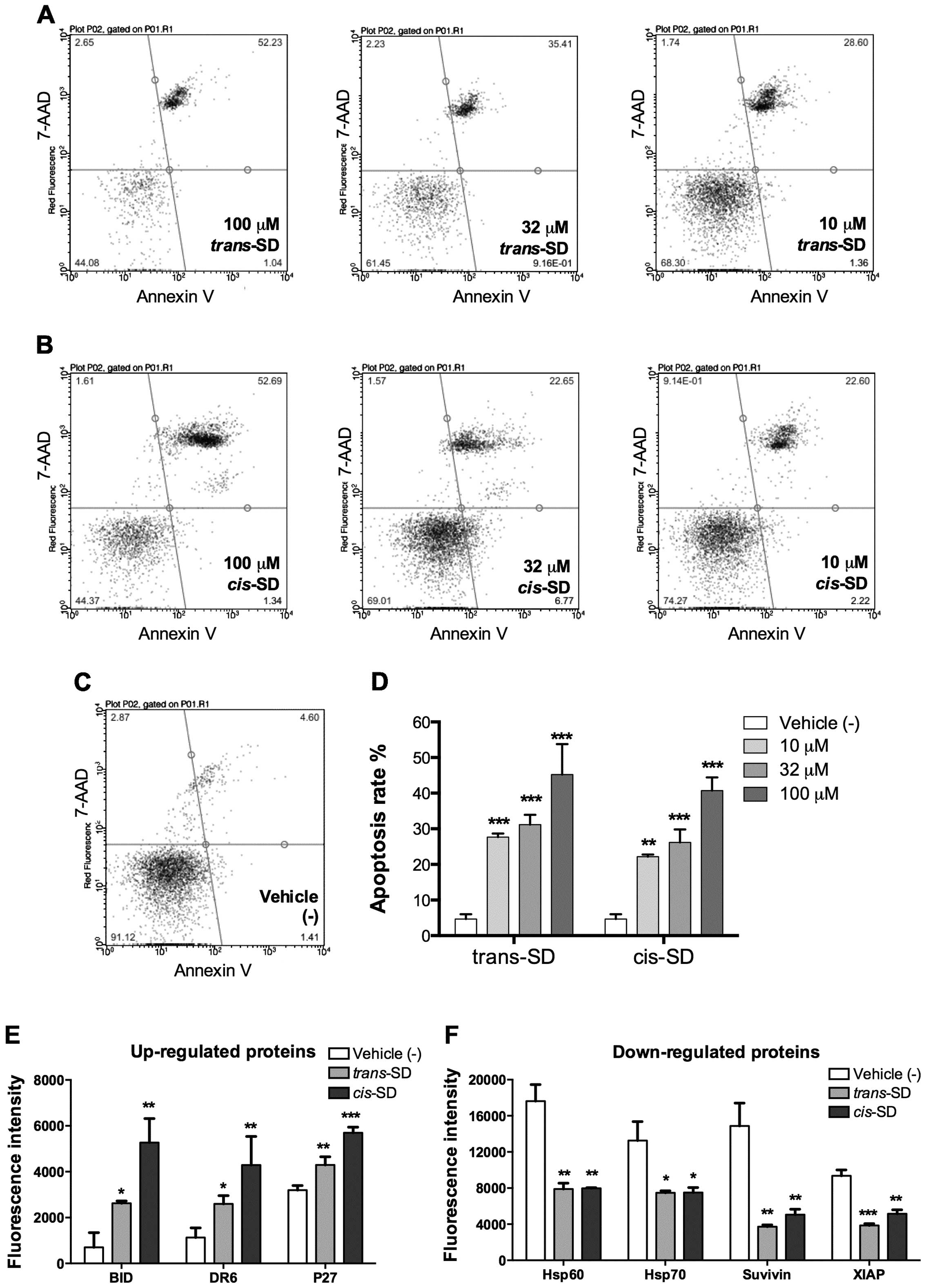
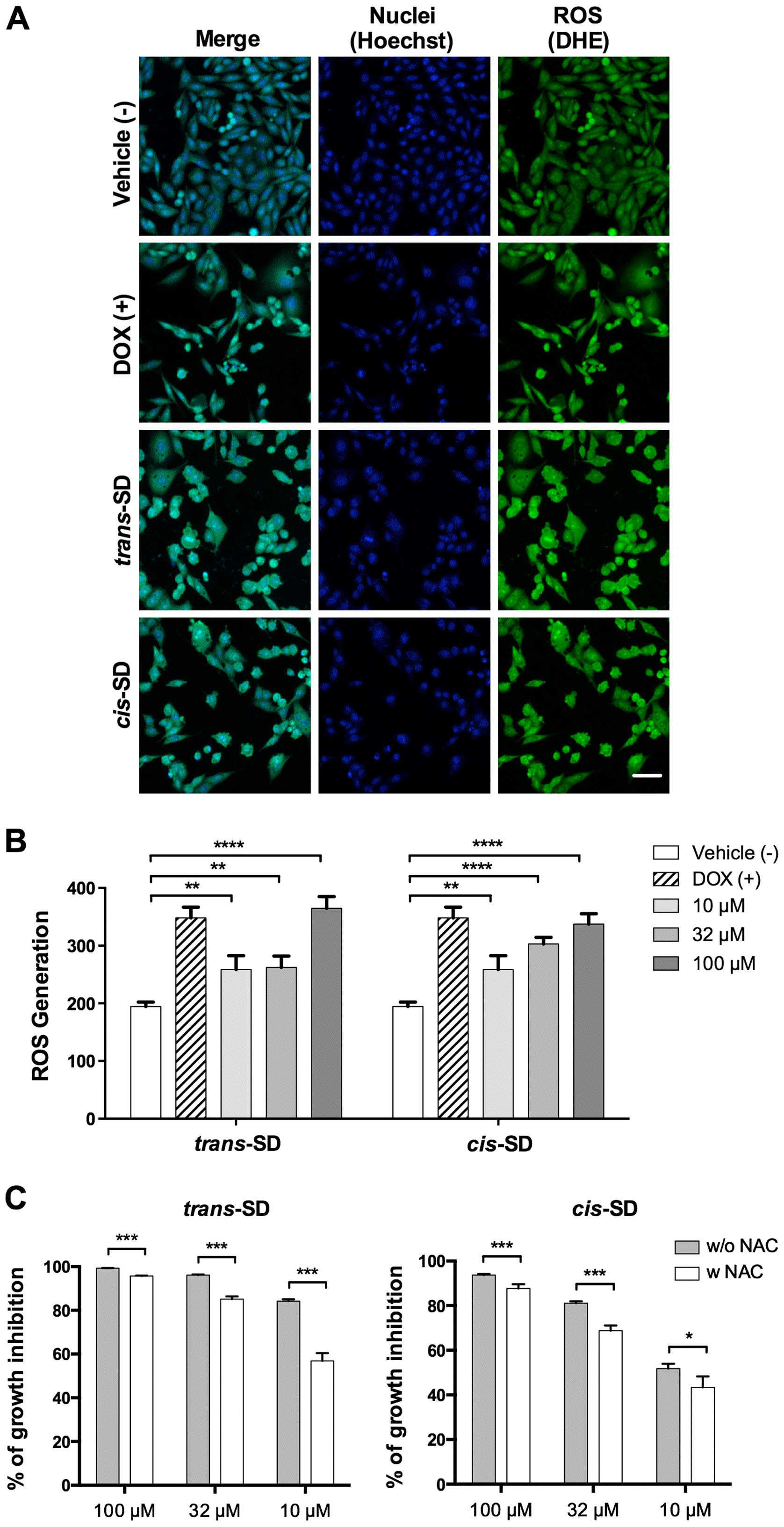
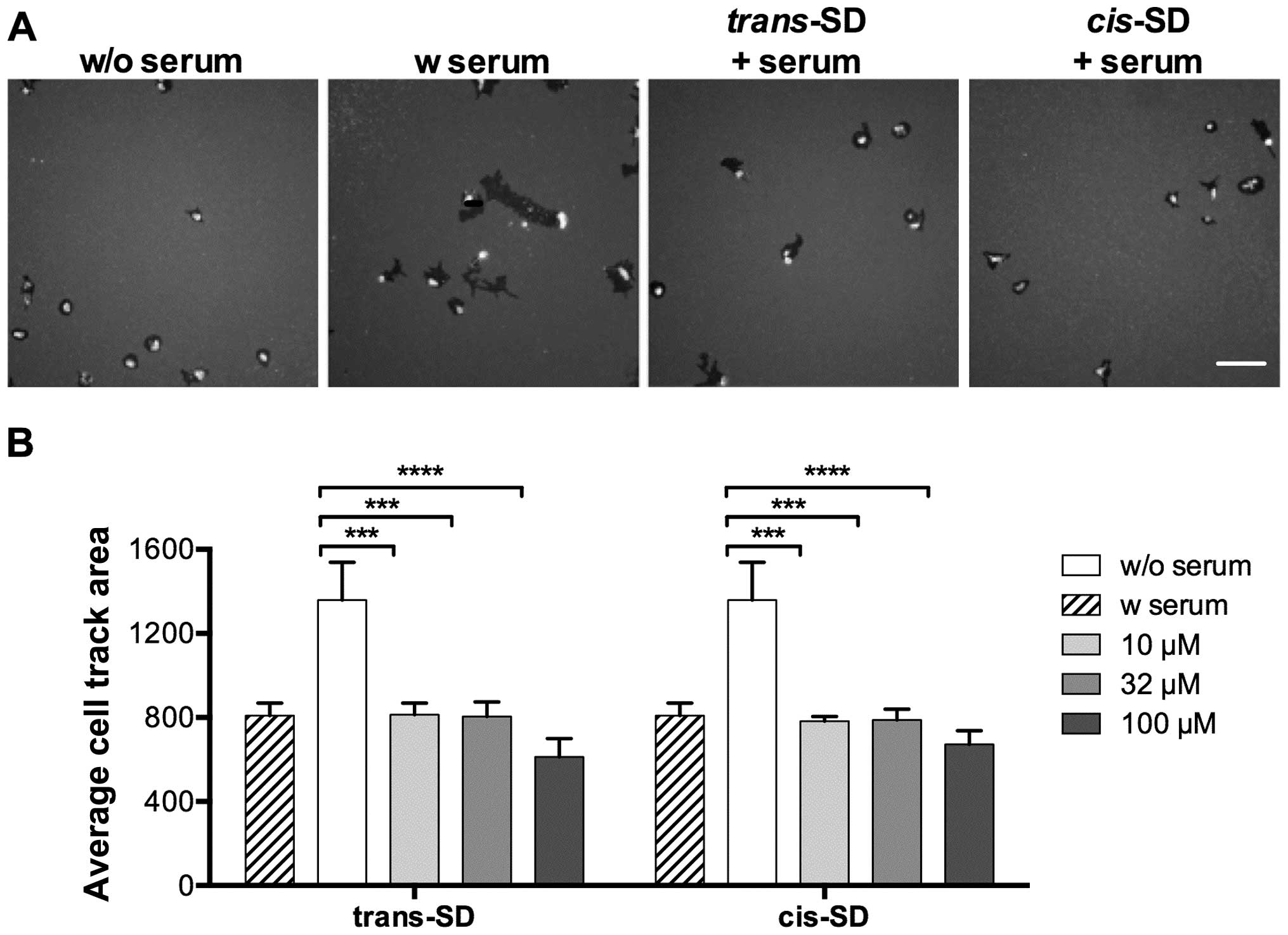
|
1
|
Cai Y, Luo Q, Sun M and Corke H:
Antioxidant activity and phenolic compounds of 112 traditional
Chinese medicinal plants associated with anticancer. Life Sci.
74:2157–2184. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Zhang Q and Gong H: Clinical Practice of
Anticancer Traditional Chinese Medicines. People's Health
Publishing House; Beijing: 1998
|
|
3
|
Bo QM, Wu ZY, Shun QS, Bao XS, Mao ZS, Ha
SQ, Lu SY and Huang JM: A Selection of the Illustrated Chinese
Anti-Cancer Herbal Medicines. Shanghai Science and Technology
Literature Press; Shanghai: 2002
|
|
4
|
Parekh HS, Liu G and Wei MQ: A new dawn
for the use of traditional Chinese medicine in cancer therapy. Mol
Cancer. 8:212009. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
He CN, Peng Y, Xu LJ, Liu ZA, Gu J, Zhong
AG and Xiao PG: Three new oligostilbenes from the seeds of Paeonia
suffruticosa. Chem Pharm Bull (Tokyo). 58:843–847. 2010. View Article : Google Scholar
|
|
6
|
Chinese Pharmacopoeia Commission. Chinese
Pharmacopoeia. China Medical Scientific and Technological Press;
Beijing: pp. 160–161. 2010
|
|
7
|
He CN, Peng Y, Wu QL, Xiao W, Peng B, Wang
Z and Xiao PG: Simultaneous determination of ten stilbenes in the
seeds of Paeonia species using HPLC-DAD. J Liquid Chromatogr Relat
Technol. 36:1708–1724. 2013.
|
|
8
|
He CN, Peng Y, Zhang YC, Xu LJ, Gu J and
Xiao PG: Phytochemical and biological studies of Paeoniaceae. Chem
Biodivers. 7:805–838. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Shen T, Xie CF, Wang XN and Lou HX:
Stilbenoids. Natural Products. Springer; pp. 1901–1949. 2013,
View Article : Google Scholar
|
|
10
|
Cai T and Cai Y: cis-Ampelopsin E, a
stilbene isolated from the seeds of Paeonia suffruticosa, inhibits
lipopolysaccharide-stimulated nitric oxide production in RAW 264.7
macrophages via blockade of nuclear factor-kappa B signaling
pathway. Biol Pharm Bull. 34:1501–1507. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Yuk HJ, Ryu HW, Jeong SH, Curtis-Long MJ,
Kim HJ, Wang Y, Song YH and Park KH: Profiling of neuraminidase
inhibitory polyphenols from the seeds of Paeonia lactiflora. Food
Chem Toxicol. 55:144–149. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Hussain S, Slevin M, Ahmed N, West D,
Choudhary MI, Naz H and Gaffney J: Stilbene glycosides are natural
product inhibitors of FGF-2-induced angiogenesis. BMC Cell Biol.
10:302009. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Simoni D, Invidiata FP, Eleopra M,
Marchetti P, Rondanin R, Baruchello R, Grisolia G, Tripathi A,
Kellogg GE, Durrant D, et al: Design, synthesis and biological
evaluation of novel stilbene-based antitumor agents. Bioorg Med
Chem. 17:512–522. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
He S, Lu Y, Jiang L, Wu B, Zhang F and Pan
Y: Preparative isolation and purification of antioxidative stilbene
oligomers from Vitis chunganeniss using high-speed counter-current
chromatography in stepwise elution mode. J Sep Sci. 32:2339–2345.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Jung M, Park WH, Jung JC, Lim E, Lee Y, Oh
S and Moon HI: Synthesis, structural characterization and
biological evaluation of novel stilbene derivatives as potential
antimalarial agents. Chem Biol Drug Des. 73:346–354. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Lee K, Lee JH, Ryu SY, Cho MH and Lee J:
Stilbenes reduce Staphylococcus aureus hemolysis, biofilm
formation, and virulence. Foodborne Pathog Dis. 11:710–717. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Shukla Y and Singh R: Resveratrol and
cellular mechanisms of cancer prevention. Ann NY Acad Sci.
1215:1–8. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Whitlock NC and Baek SJ: The anticancer
effects of resveratrol: Modulation of transcription factors. Nutr
Cancer. 64:493–502. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Pettit GR, Grealish MP, Jung MK, Hamel E,
Pettit RK, Chapuis JC and Schmidt JM: Antineoplastic agents. 465.
Structural modification of resveratrol: Sodium resverastatin
phosphate. J Med Chem. 45:2534–2542. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Kasibhatla S and Tseng B: Why target
apoptosis in cancer treatment? Mol Cancer Ther. 2:573–580.
2003.PubMed/NCBI
|
|
21
|
Cheah SC, Appleton DR, Lee ST, Lam ML,
Hadi AHA and Mustafa MR: Panduratin A inhibits the growth of A549
cells through induction of apoptosis and inhibition of NF-kappaB
translocation. Molecules. 16:2583–2598. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Ly JD, Grubb DR and Lawen A: The
mitochondrial membrane potential (Δψm) in apoptosis; an update.
Apoptosis. 8:115–128. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Kannan K and Jain SK: Oxidative stress and
apoptosis. Pathophysiology. 7:153–163. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Ozben T: Oxidative stress and apoptosis:
Impact on cancer therapy. J Pharm Sci. 96:2181–2196. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Sosa V, Moliné T, Somoza R, Paciucci R,
Kondoh H and LLeonart ME: Oxidative stress and cancer: An overview.
Ageing Res Rev. 12:376–390. 2013. View Article : Google Scholar
|
|
26
|
Suzuki Y, Nakabayashi Y, Nakata K, Reed JC
and Takahashi R: X-linked inhibitor of apoptosis protein (XIAP)
inhibits caspase-3 and −7 in distinct modes. J Biol Chem.
276:27058–27063. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Schimmer AD, Dalili S, Batey RA and Riedl
SJ: Targeting XIAP for the treatment of malignancy. Cell Death
Differ. 13:179–188. 2006. View Article : Google Scholar
|
|
28
|
Ryan BM, O'Donovan N and Duffy MJ:
Survivin: A new target for anti-cancer therapy. Cancer Treat Rev.
35:553–562. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Hu Y, Cherton-Horvat G, Dragowska V, Baird
S, Korneluk RG, Durkin JP, Mayer LD and LaCasse EC: Antisense
oligonucleotides targeting XIAP induce apoptosis and enhance
chemotherapeutic activity against human lung cancer cells in vitro
and in vivo. Clin Cancer Res. 9:2826–2836. 2003.PubMed/NCBI
|
|
30
|
He X, Khurana A, Maguire JL, Chien J and
Shridhar V: HtrA1 sensitizes ovarian cancer cells to
cisplatin-induced cytotoxicity by targeting XIAP for degradation.
Int J Cancer. 130:1029–1035. 2012. View Article : Google Scholar
|
|
31
|
Oost TK, Sun C, Armstrong RC, Al-Assaad
AS, Betz SF, Deckwerth TL, Ding H, Elmore SW, Meadows RP,
Olejniczak ET, et al: Discovery of potent antagonists of the
antiapoptotic protein XIAP for the treatment of cancer. J Med Chem.
47:4417–4426. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Mita AC, Mita MM, Nawrocki ST and Giles
FJ: Survivin: Key regulator of mitosis and apoptosis and novel
target for cancer therapeutics. Clin Cancer Res. 14:5000–5005.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Cappello F, Conway de Macario E, Marasà L,
Zummo G and Macario AJ: Hsp60 expression, new locations, functions
and perspectives for cancer diagnosis and therapy. Cancer Biol
Ther. 7:801–809. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Murphy ME: The HSP70 family and cancer.
Carcinogenesis. 34:1181–1188. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Luo X, Budihardjo I, Zou H, Slaughter C
and Wang X: Bid, a Bcl2 interacting protein, mediates cytochrome c
release from mitochondria in response to activation of cell surface
death receptors. Cell. 94:481–490. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Yamamoto H, Soh JW, Shirin H, Xing WQ, Lim
JT, Yao Y, Slosberg E, Tomita N, Schieren I and Weinstein IB:
Comparative effects of overexpression of p27Kip1 and p21Cip1/Waf1
on growth and differentiation in human colon carcinoma cells.
Oncogene. 18:103–115. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Nickeleit I, Zender S, Kossatz U and Malek
NP: p27kip1: A target for tumor therapies? Cell Div. 2:132007.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Kasof GM, Lu JJ, Liu D, Speer B, Mongan
KN, Gomes BC and Lorenzi MV: Tumor necrosis factor-alpha induces
the expression of DR6, a member of the TNF receptor family, through
activation of NF-kappaB. Oncogene. 20:7965–7975. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Yamazaki D, Kurisu S and Takenawa T:
Regulation of cancer cell motility through actin reorganization.
Cancer Sci. 96:379–386. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Olson MF and Sahai E: The actin
cytoskeleton in cancer cell motility. Clin Exp Metastasis.
26:273–287. 2009. View Article : Google Scholar
|
|
41
|
Wells A, Grahovac J, Wheeler S, Ma B and
Lauffenburger D: Targeting tumor cell motility as a strategy
against invasion and metastasis. Trends Pharmacol Sci. 34:283–289.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Levin EG: Cancer therapy through control
of cell migration. Curr Cancer Drug Targets. 5:505–518. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Monika SA, Sharma A, Suthar SK, Aggarwal
V, Lee HB and Sharma M: Synthesis of lantadene analogs with marked
in vitro inhibition of lung adenocarcinoma and TNF-α induced
nuclear factor-kappa B (NF-κB) activation. Bioorg Med Chem Lett.
24:3814–3818. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Nakanishi C and Toi M: Nuclear
factor-kappaB inhibitors as sensitizers to anticancer drugs. Nat
Rev Cancer. 5:297–309. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Reuter S, Gupta SC, Chaturvedi MM and
Aggarwal BB: Oxidative stress, inflammation, and cancer: How are
they linked? Free Radic Biol Med. 49:1603–1616. 2010. View Article : Google Scholar : PubMed/NCBI
|